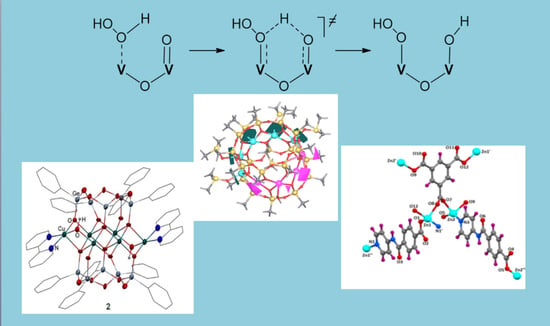
Oxidation of Organic Compounds with Peroxides Catalyzed by Polynuclear Metal Compounds
Abstract
:1. Introduction
2. Oxidations Catalyzed by Soluble Polynuclear Compounds
2.1. Oxidation Catalyzed by Iron Complexes
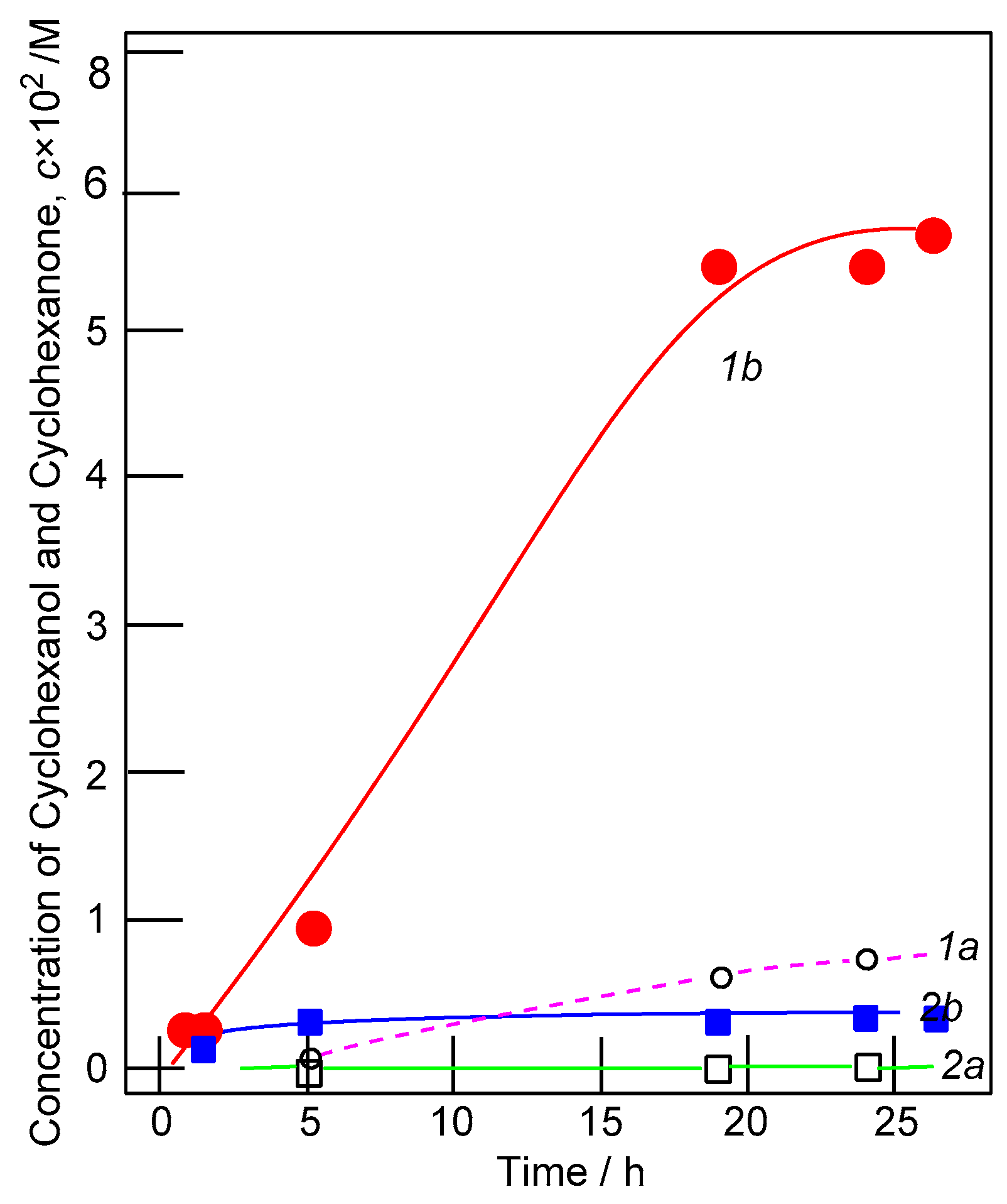
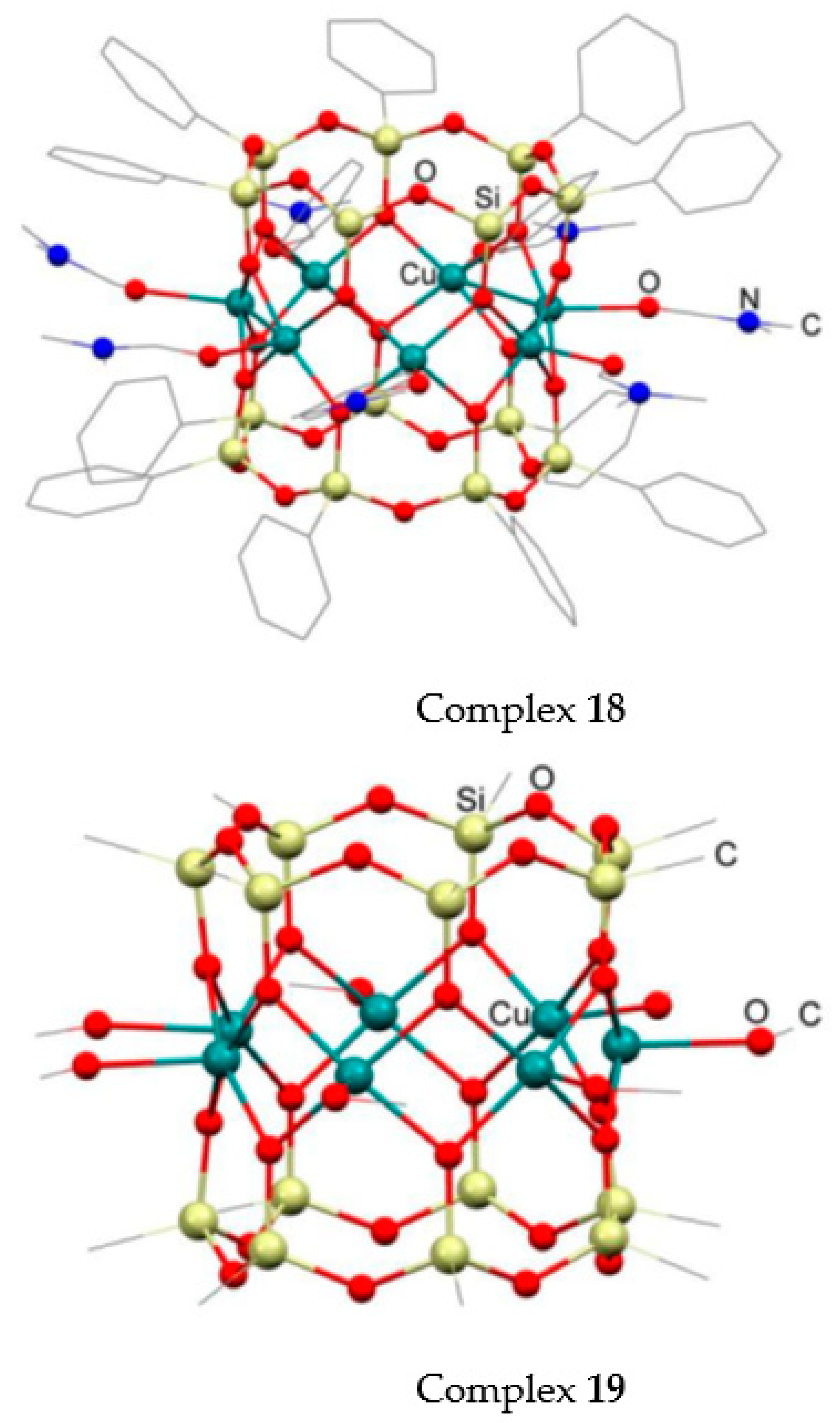
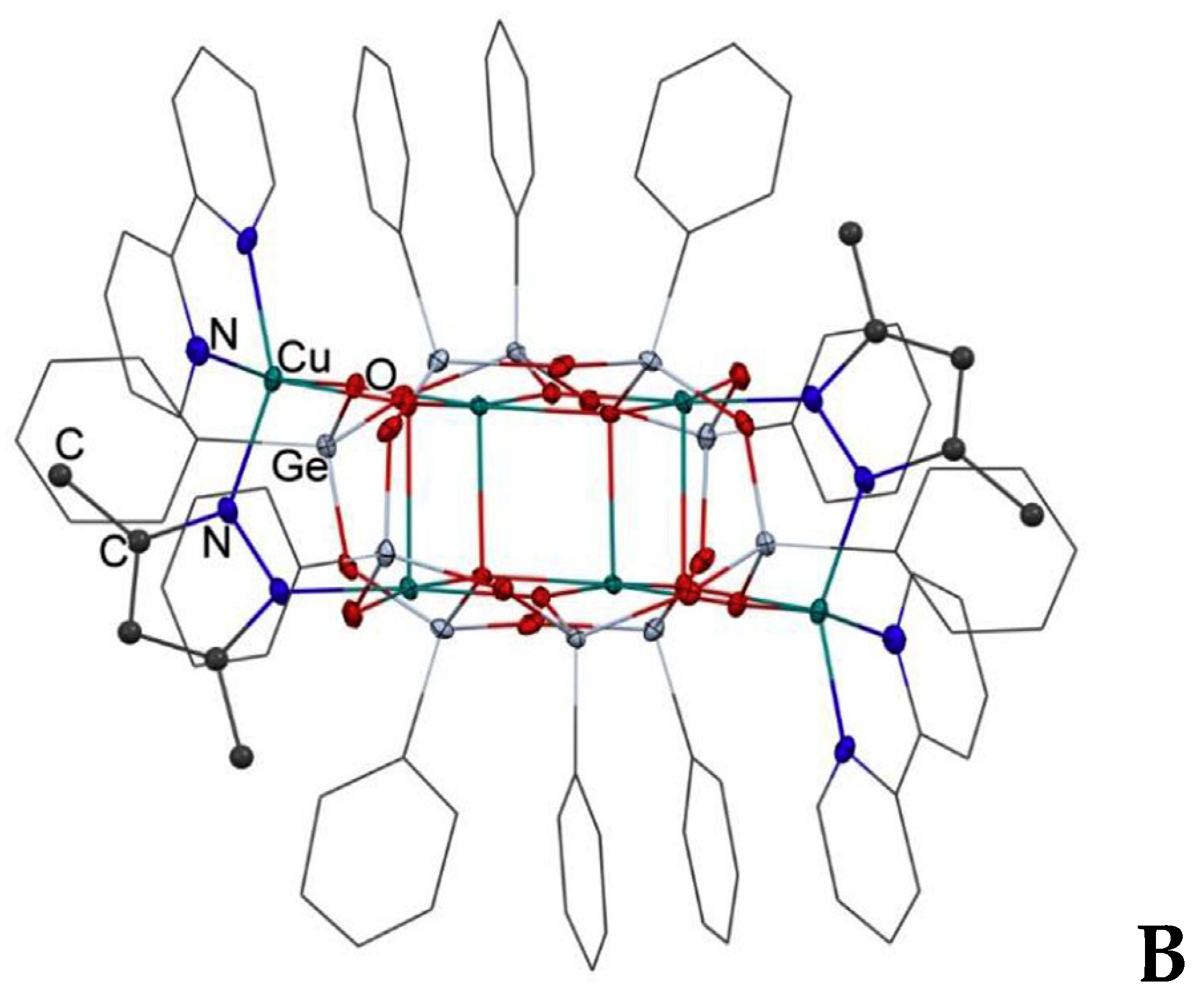
2.2. Oxidation Catalyzed by Copper Complexes
2.3. Polymanganese Complexes in Oxidations with H2O2
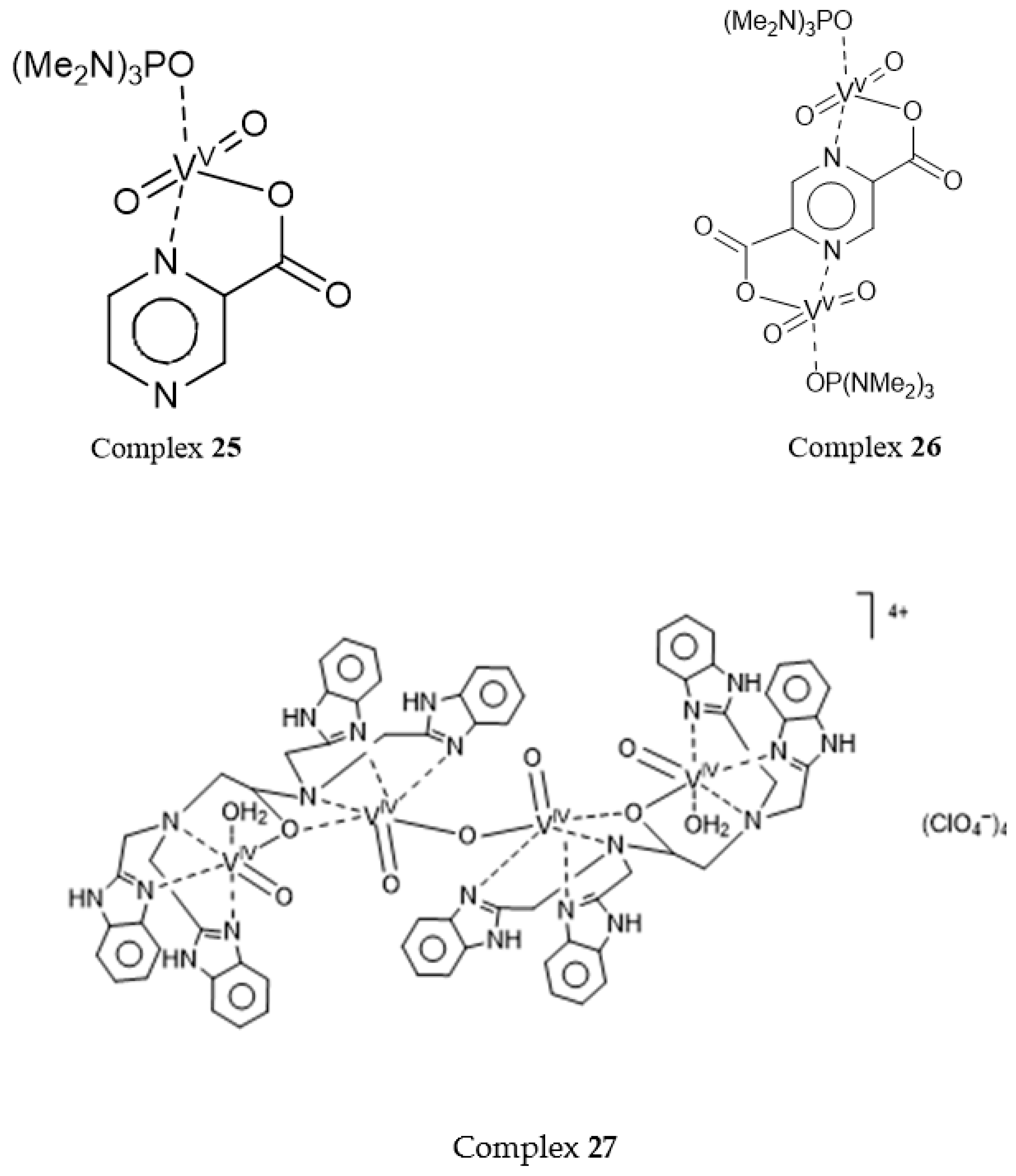
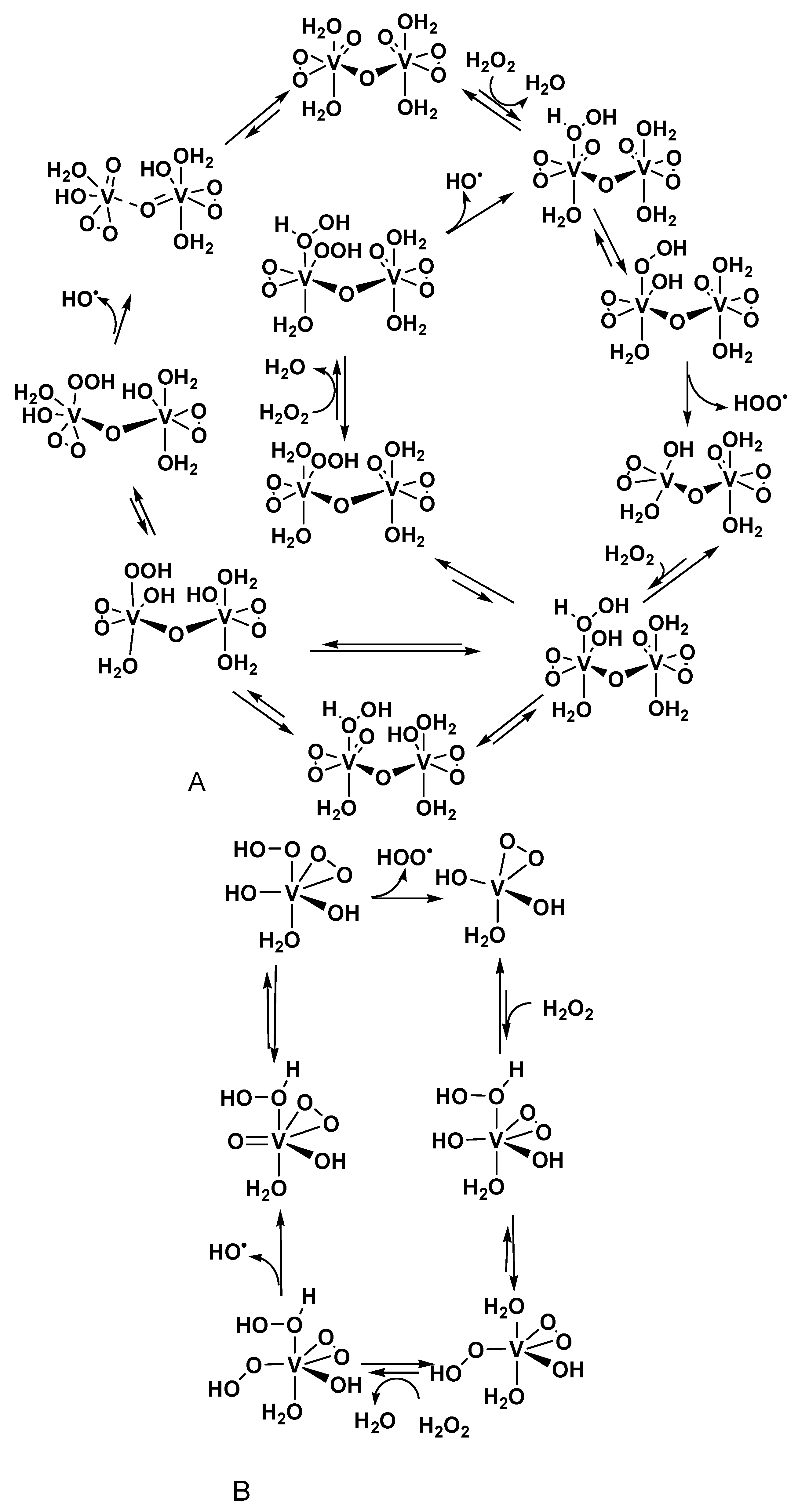
2.4. Oxidation Catalyzed by Polyvanadate Ions
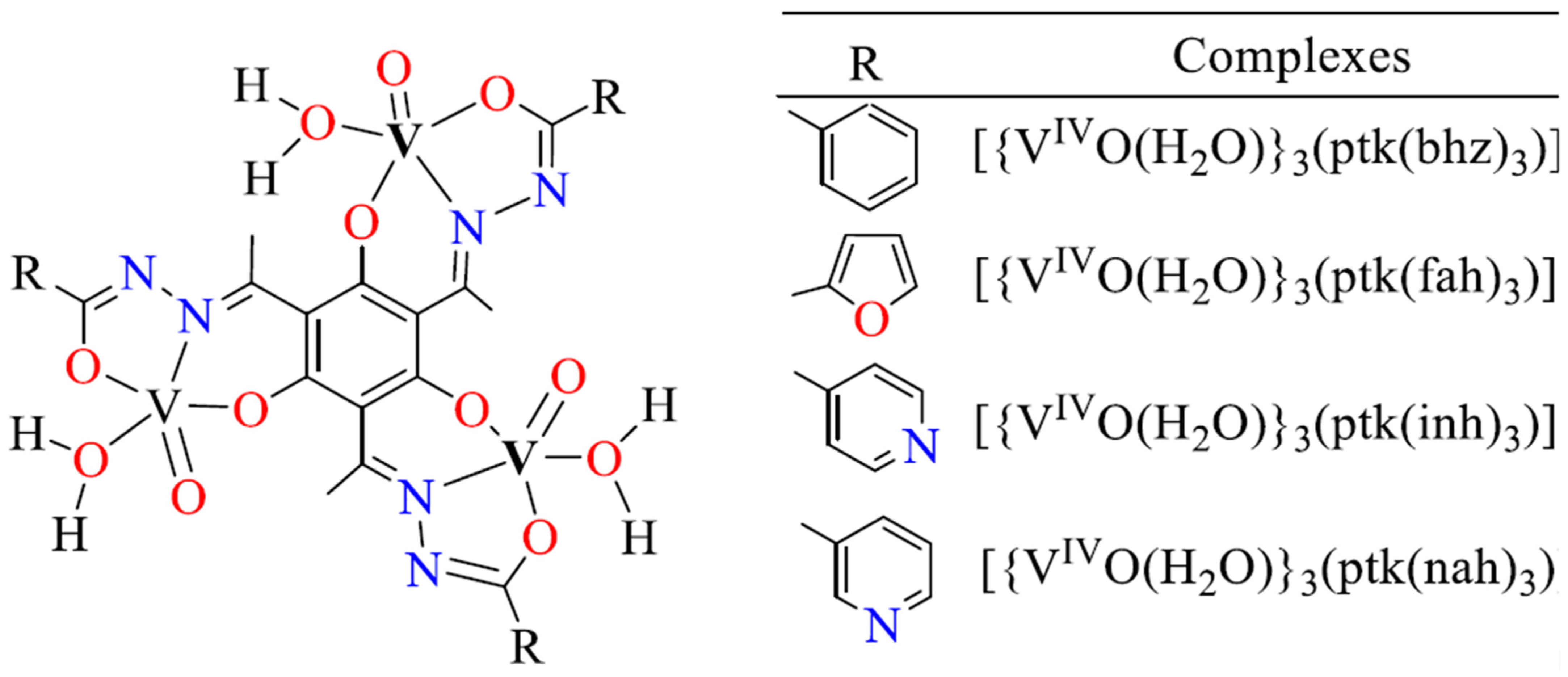
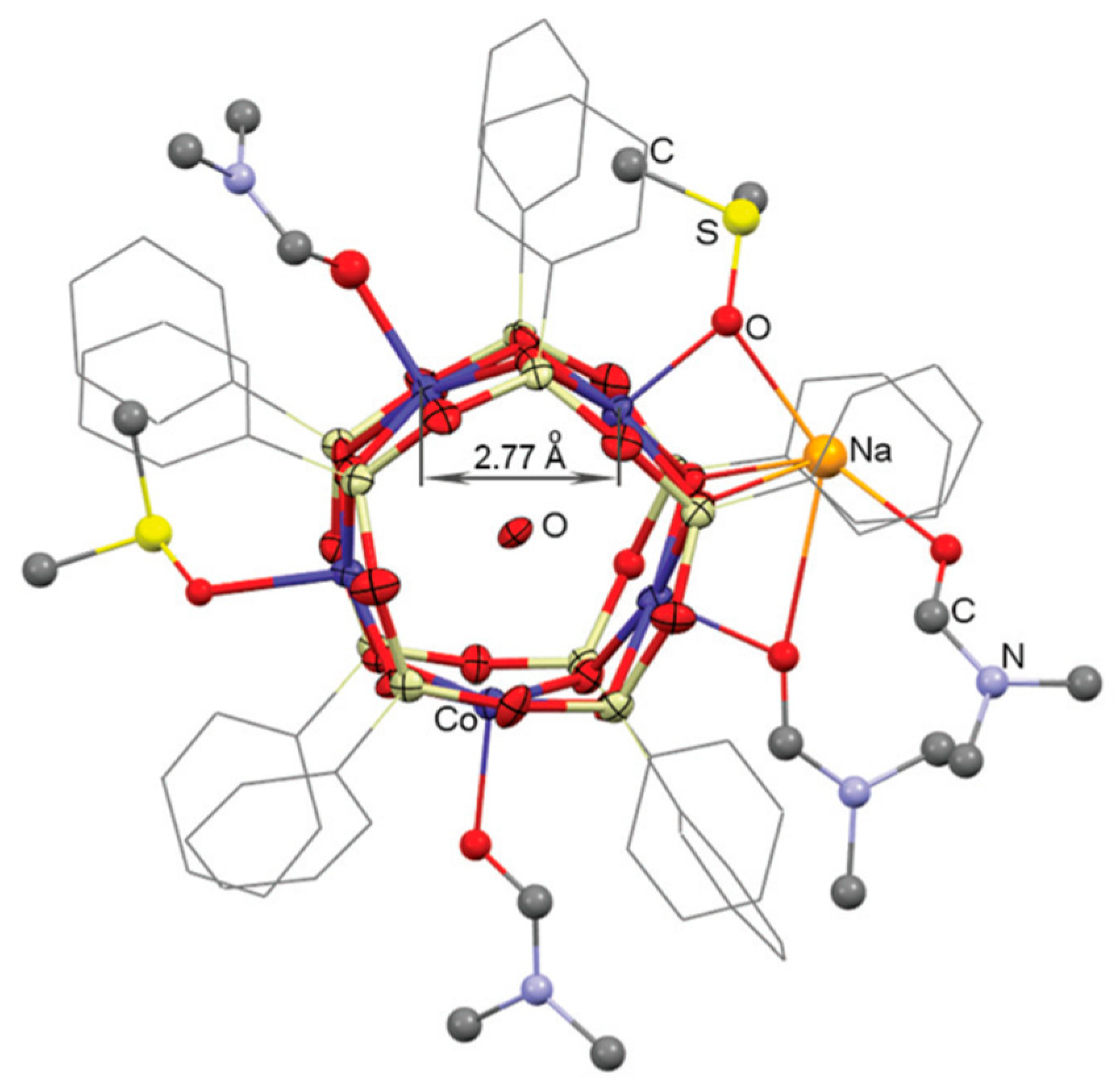
2.5. Oxidation Catalyzed by Other Metal Complexes
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; Taylor & Francis Group: Abingdon, UK, 2005; ISBN 0-8247-5356-9. [Google Scholar]
- Shilov, A.E.; Shul’pin, G.B. Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer Academic Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2002; p. 548. ISBN 978-0-306-46945-9. [Google Scholar]
- Ma, Z.; Mahmudov, K.T.; Aliyeva, V.A.; Gurbanov, A.V.; Pombeiro, A.J.L. TEMPO in metal complex catalysis. Coord. Chem. Rev. 2020, 423, 213482. [Google Scholar] [CrossRef]
- Beller, M.; Bolm, C. (Eds.) Transition Metals for Organic Synthesis, 2nd ed.; Wiley–VCH: Weinheim, Germany; New York, NY, USA, 2004; ISBN 3-527-30613-7. [Google Scholar]
- Shul’pin, G.B. Selectivity in C–H functionalizations. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, J., Poeppelmeier, K., Casella, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Chapter 6.04; Volume 6, pp. 79–104. [Google Scholar] [CrossRef]
- Pombeiro, A.J.L. (Ed.) Advances in Organometallic Chemistry and Catalysis; Wiley: Hoboken, NJ, USA, 2014; Chapter 1; pp. 3–14. ISBN 978-1-118-51014-8. [Google Scholar] [CrossRef]
- Pombeiro, A.J.L.; Guedes da Silva, F.C. (Eds.) Alkane Functionalization; Wiley: Hoboken, NJ, USA, 2018; ISBN 978-1-119-37924-9. [Google Scholar]
- Bryliakov, K. (Ed.) Frontiers of Green Catalytic Selective Oxidations; Springer-Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Shilov, A.E.; Shul’pin, G.B. Activation of C–H Bonds by Metal Complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxidations. Comptes Rendus Chim. 2003, 6, 163–178. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Hydrocarbon Oxygenations with Peroxides Catalyzed by Metal Compounds. Mini-Rev. Org. Chem. 2009, 6, 95–104. [Google Scholar] [CrossRef]
- Shul’pin, G.B. New Trends in Oxidative Functionalization of Carbon–Hydrogen Bonds: A Review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S. Metal Complexes Containing Redox-active Ligands in Oxidation of Hydrocarbons and Alcohols: A Review. Catalysts 2019, 9, 1046. [Google Scholar] [CrossRef] [Green Version]
- Levitsky, M.M.; Bilyachenko, A.N.; Shul’pin, G.B. Oxidation of C-H compounds with peroxides catalyzed by polynuclear transition metal complexes in Si- or Ge-sesquioxane frameworks: A review. J. Organomet. Chem. 2017, 849–850, 201–218. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Selectivity enhancement in functionalization of C–H bonds: A review. Org. Biomol. Chem. 2010, 8, 4217–4228. [Google Scholar] [CrossRef]
- Shul’pin, G.B. C–H functionalization: Thoroughly tuning ligands at a metal ion, a chemist can greatly enhance catalyst’s activity and selectivity, Perspective. Dalton Trans. 2013, 42, 12794–12818. [Google Scholar] [CrossRef]
- Kozlov, Y.N.; Nadezhdin, A.D.; Purmal, A.P. Mechanism of initiation in the Fe(3+) + H2O2 system. Kinet. Catal. 1973, 14, 141–148. [Google Scholar]
- Shul’pin, G.B.; Nizova, G.V.; Kozlov, Y.N.; Gonzalez Cuervo, L.; Süss-Fink, G. Hydrogen peroxide oxygenation of alkanes including methane and ethane catalyzed by iron complexes in acetonitrile. Adv. Synth. Catal. 2004, 346, 317–332. [Google Scholar] [CrossRef] [Green Version]
- Nizova, G.V.; Krebs, B.; Süss-Fink, G.; Schindler, S.; Westerheide, L.; Gonzalez Cuervo, L.; Shul’pin, G.B. Hydroperoxidation of methane and other alkanes with H2O2 catalysed by a dinuclear iron complex and an amino acid. Tetrahedron 2002, 58, 9231–9237. [Google Scholar] [CrossRef]
- Parrilha, G.L.; Ferreira, S.S.; Fernandes, C.; Silva, G.C.; Carvalho, N.M.F.; Antunes, O.A.C.; Drago, V.; Bortoluzzid, A.J.; Horn, A., Jr. Properties of (m-Oxo)di-iron Complexes and Catalytic Activity Toward Cyclohexane Oxidation. J. Braz. Chem. Soc. 2010, 21, 603–613. [Google Scholar] [CrossRef]
- Mayilmurugan, R.; Stoeckli-Evans, H.; Suresh, E.; Palaniandavar, M. Chemoselective and biomimetic hydroxylation of hydrocarbons by non-heme l-oxo-bridged diiron(III) catalysts using m-CPBA as oxidant. Dalton Trans. 2009, 5101–5114. [Google Scholar] [CrossRef]
- Sorokin, A.B.; Kudrik, E.V.; Bouchub, D. Bio-inspired oxidation of methane in water catalyzed by N-bridged diiron phthalocyanine complex. Chem. Commun. 2008, 2562–2564. [Google Scholar] [CrossRef]
- Kudrik, E.V.; Afanasiev, P.; Alvarez, L.X.; Dubourdeaux, P.; Cle’mancey, M.; Latour, J.-M.; Blondin, G.; Bouchu, D.; Albrieux, F.; Nefedov, S.E.; et al. An N-bridged high-valent diiron–oxo species on a porphyrin platform that can oxidize methane. Nat. Chem. 2012, 4, 1024–1029. [Google Scholar] [CrossRef]
- Groves, J.T.; Haushalter, R.C.; Nakamura, M.; Nemo, T.E.; Evans, B.J. High-valent iron-porphyrin complexes related to peroxidase and cytochrome P-450. J. Am. Chem. Soc. 1981, 103, 2884–2886. [Google Scholar] [CrossRef]
- Seo, M.S.; Kim, N.H.; Cho, K.-B.; So, J.E.; Park, S.K.; Clémancey, M.; Garcia-Serres, R.; Latour, J.-M.; Shaik, S.; Nam, W. A mononuclear non-heme iron(IV)-oxo complex which is more reactive than cytochrome P450 model compound I. Chem. Sci. 2011, 2, 1039–1045. [Google Scholar] [CrossRef]
- Karslyan, E.E.; Shul’pina, L.S.; Kozlov, Y.N.; Pombeiro, A.J.L.; Shul’pin, G.B. Oxygenation of saturated and aromatic hydrocarbons with H2O2 catalyzed by the carbonyl thiophenolate iron complex (OC)3Fe(PhS)2Fe(CO)3. Catal. Today 2013, 218–219, 93–98. [Google Scholar] [CrossRef]
- Rabe, V.; Frey, W.; Baro, A.; Laschat, S.; Bauer, M.; Bertagnolli, H.; Rajagopalan, S.; Asthalter, T.; Roduner, E.; Dilger, H.; et al. Syntheses, Crystal Structures, Spectroscopic Properties, and Catalytic Aerobic Oxidations of Novel Trinuclear Non-Heme Iron Complexes. Eur. J. Inorg. Chem. 2009, 4660–4674. [Google Scholar] [CrossRef]
- Romakh, V.B.; Therrien, B.; Süss-Fink, G.; Shul’pin, G.B. Synthesis, molecular structure and catalytic potential of the tetrairon complex [Fe4(N3O2-L)4(μ-O)2]4+ (L = 1-carboxymethyl-4,7-dimethyl-1,4,7-triazacyclononane). Inorg. Chem. 2007, 46, 3166–3175. [Google Scholar] [CrossRef] [PubMed]
- Jarenmark, M.; Turitsyna, E.A.; Haukka, M.; Shteinman, A.A.; Nordlander, E. A monocarboxylate-bridged diiron(III) l-oxido complex that catalyzes alkane oxidation by hydrogen peroxide. New J. Chem. 2010, 34, 2118–2121. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Chygorin, E.N.; Kokozay, V.N.; Bon, V.V.; Bocča, R.; Kozlov, Y.N.; Shul’pina, L.S.; Jezierska, J.; Ozarowski, A.; Pombeiro, A.J.L.; et al. Heterometallic CoIII4FeIII2 Schiff Base Complex: Structure, Electron Paramagnetic Resonance, and Alkane Oxidation Catalytic Activity. Inorg. Chem. 2012, 51, 9110–9122. [Google Scholar] [CrossRef]
- Quesne, M.G.; Senthilnathan, D.; Singh, D.; Kumar, D.; Maldivi, P.; Sorokin, A.B.; de Visser, S.P. Origin of the enhanced reactivity of μ-nitrido-bridged diiron(IV)-oxo porphyrinoid complexes over cytochrome P450 Compound I. ACS Catal. 2016, 6, 2230–2243. [Google Scholar] [CrossRef] [Green Version]
- Gomez, L.; Canta, M.; Font, D.; Prat, I.; Ribas, X.; Costas, M. Regioselective Oxidation of Nonactivated Alkyl C–H Groups Using Highly Structured Non-Heme Iron Catalysts. J. Org. Chem. 2013, 78, 1421–1433. [Google Scholar] [CrossRef]
- Olivo, G.; Cussy, O.; Borrell, M.; Costas, M. Oxidation of alkane and alkene moieties with biologically inspired nonheme iron catalysts and hydrogen peroxide: From free radicals to stereoselective transformations. J. Biol. Inorg. Chem. 2017, 22, 425–452. [Google Scholar] [CrossRef]
- Karmakar, A.; Martins, L.M.; Yahorava, Y.; Guedes da Silva, F.; Pombeiro, A.J.L. Synthesis, Structures, Electrochemistry, and Catalytic Activity towards Cyclohexanol Oxidation of Mono-, Di-, and Polynuclear Iron(III) Complexes with 3-Amino-2-Pyrazinecarboxylate. Appl. Sci. 2020, 10, 2692. [Google Scholar] [CrossRef]
- Lyakin, K.P.; Talsi, E.P. Non-heme oxoiron(V) intermediates in chemo-, regio- and stereoselective oxidation of organic substrates. Coord. Chem. Rev. 2019, 384, 126–139. [Google Scholar] [CrossRef]
- Ouyang, J.; Haotian, S.; Liang, Y.; Commisso, A.; Li, D.; Xu, R.; Yu, D. Recent Progress in Metal-containing Silsesquioxanes: Preparation and Application. Curr. Org. Chem. 2017, 21, 2829–2848. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shubina, E.S. Cagelike metallagermanates and metallagermoxanes: Synthesis, structures and functional properties. A review. Coord. Chem. Rev. 2019, 386, 209–239. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Levitsky, M.M.; Yalymov, A.I.; Korlyukov, A.A.; Vologzhanina, A.V.; Kozlov, Y.N.; Shul’pina, L.S.; Nesterov, D.S.; Pombeiro, A.J.L.; Lamaty, F.; et al. A heterometallic (Fe6Na8) cage-like silsesquioxane: Synthesis, structure, spin glass behavior and high catalytic activity. RSC Adv. 2016, 6, 48165–48180. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Levitsky, M.M.; Yalymov, A.I.; Korlyukov, A.A.; Khrustalev, V.N.; Vologzhanina, A.V.; Shul’pina, L.S.; Ikonnikov, N.S.; Trigub, A.E.; Dorovatovsky, P.V.; et al. Cage-like Fe,Na-Germsesquioxanes: Structure, Magnetism, and Catalytic Activity, English Version. Angew. Chem. Int. Ed. 2016, 55, 15360–15363. [Google Scholar] [CrossRef] [PubMed]
- Yalymov, A.I.; Bilyachenko, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Shul’pina, L.S.; Dorovatovskii, P.V.; Es’kova, M.A.; Lamaty, F.; Bantreil, X.; et al. High Catalytic Activity of Heterometallic (Fe6Na7 and Fe6Na6) Cage Silsesquioxanes in Oxidations with Peroxides. Catalysts 2017, 7, 101. [Google Scholar] [CrossRef]
- Collins, T.J.; Ryabov, A.D. Targeting of High-Valent Iron-TAML Activators at Hydrocarbons and Beyond. Chem. Rev. 2017, 117, 9140–9162. [Google Scholar] [CrossRef]
- Fürstner, A. Iron Catalysis in Organic Synthesis: A Critical Assessment of What It Takes to Make This Base Metal a Multitasking Champion. ACS Cent. Sci. 2016, 2, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, J.; Huang, X.; Sun, C. Recent Advances in Iron-Catalyzed C-H Bond Activation Reactions. Curr. Inorg. Chem. 2012, 2. [Google Scholar] [CrossRef]
- Schreder, K.; Junge, K.; Bitterlich, B.; Beller, M. Fe-Catalyzed Oxidation Reactions of Olefins, Alkanes, and Alcohols: Involvement of Oxo- and Peroxo Complexes. In a Book Iron Catalysis; Plietker, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 33, pp. 83–109. ISBN 978-3-642-14670-1. [Google Scholar] [CrossRef]
- Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Direct C-H transformation via iron catalysis. Chem. Rev. 2011, 111, 1293–1314. [Google Scholar] [CrossRef]
- Lee1, C.; Sedlak, D.L. A novel homogeneous Fenton-like system with Fe(III)–phosphotungstate for oxidation of organic compounds at neutral pH values. J. Mol. Catal. A Chem. 2009, 311, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mitra, M.; Nimir, H.; Hrovat, D.A.; Shteinman, A.A.; Richmond, M.G.; Costas, M.; Nordlander, E. Catalytic C-H Oxidations by Nonheme Mononuclear Fe(II) Complexes of Two Pentadentate Ligands: Evidence for an Fe(IV) Oxo Intermediate. J. Mol. Catal. 2016, 426, 350–356. [Google Scholar] [CrossRef]
- Schreder, K.; Join, B.; Amali, A.J.; Junge, K.; Ribas, X.; Costas, M.; Beller, M. A Biomimetic Iron Catalyst for the Epoxidation of Olefins with Molecular Oxygen at Room Temperature. Angew. Chem. Int. Ed. 2011, 50, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Retcher, B.; Costa, J.S.; Tanga, J.; Hage, R.; Gamez, P.; Reedijk, J. Unexpected high oxidation of cyclohexane by Fe salts and dihydrogen peroxide in acetonitrile. J. Mol. Catal. A Chem. 2008, 286, 1–5. [Google Scholar] [CrossRef]
- Nam, W.; Lee, Y.-M.; Fukuzumi, S. Tuning Reactivity and Mechanism in Oxidation Reactions by Mononuclear Nonheme Iron(IV)-Oxo Complexes. Acc. Chem. Res. 2014, 47, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Bilis, G.; Louloudi, M. The Catalytic Function of Nonheme Iron (III) Complex for Hydrocarbon Oxidation. Bioinorg. Chem. Appl. 2010, 2010, 861892. [Google Scholar] [CrossRef] [Green Version]
- Gomez, L.; Garcia-Bosch, I.; Company, A.; Benet-Buchholz, J.; Polo, A.; Sala, X.; Ribas, X.; Costas, M. Stereospecific C–H Oxidation with H2O2 Catalyzed by a Chemically Robust Site-Isolated Iron Catalyst. Angew. Chem. Int. Ed. 2009, 48, 5720–5723. [Google Scholar] [CrossRef]
- England, J.; Gondhia, R.; Bigorra-Lopez, L.; Petersen, A.R.; White, A.J.P.; Britovsek, G.J.P. Towards robust alkane oxidation catalysts: Electronic variations in non-heme iron(II) complexes and their effect in catalytic alkane oxidation. Dalton Trans. 2009, 5319–5334. [Google Scholar] [CrossRef] [Green Version]
- Stanje, B.; Traar, P.; Schachner, J.; Belaj, F.; Mösch-Zanetti, N.C. Iron catalyzed oxidation of benzylic alcohols to benzoic acids. Dalt. Trans. 2018, 47, 6412–6420. [Google Scholar] [CrossRef]
- Jaafar, H.; Vileno, B.; Thibon, A.; Mandon, D. Tuning the conversion of cyclohexane into cyclohexanol/one by molecular dioxygen, protons and reducing agents at a single non-porphyrinic iron centre and chemical versatility of the tris(2-pyridylmethyl)amine TPAFeIICl2complex in mild oxidation chemistry. Dalton Trans. 2011, 40, 92–106. [Google Scholar] [CrossRef]
- Bitterlich, B.; Anilkumar, G.; Gelalcha, G.F.; Spilker, B.; Grotevendt, A.; Jackstell, R.; Kin Tse, M.; Beller, M. Development of a General and Efficient Iron-Catalyzed Epoxidation with Hydrogen Peroxide as Oxidant. Chem. Asian J. 2007, 2, 521–529. [Google Scholar] [CrossRef]
- Garcia-Bosch, I.; Codol, Z.; Prat, I.; Ribas, X.; Lloret-Fillol, J.; Costas, M. Iron-Catalyzed C–H Hydroxylation and Olefin cis-Dihydroxylation Using a Single-Electron Oxidant and Water as the Oxygen-Atom Source. Chem. Eur. J. 2012, 18. [Google Scholar] [CrossRef]
- Yang, Z.; Qian, J.; Yu, A.; Pan, B. Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement. Proc. Natl. Acad. Sci. USA 2019, 116, 6659–6664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes de Araújo, M.; Correia, G.A.; Carvalho, W.A.; Shul’pina, L.S.; Kozlov, Y.N.; Shul’pin, G.B.; Mandelli, D. The effect of additives (pyrazine, pyrazole and their derivatives) in the oxidation with FeCl3–H2O2 in aqueous solutions. Catal. Today 2020. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kirillova, M.V.; Pombeiro, A.J.L. Multicopper complexes and coordination polymers for mild oxidative functionalization of alkanes. Coord. Chem. Rev. 2012, 256, 2741–2759. [Google Scholar] [CrossRef]
- Calero, R.; Vega, A.; María García, A.; Spodine, E.; Manzur, J. Oxidation and Catalytic Properties of a Binuclear Copper(I) Complex with a Meta-Xylyl Spacer Ligand. J. Chil. Chem. Soc. 2003, 48. [Google Scholar] [CrossRef]
- Wurtele, C.; Sander, O.; Lutz, V.; Waitz, T.; Tuczek, F.; Schindler, S. Aliphatic C-H Bond Oxidation of Toluene Using Copper Peroxo ComplexeThat Are Stable at Room Temperature. J. Am. Chem. Soc. 2009, 131, 7544–7545. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, A.M.; Kirillova, M.V.; Shul’pina, L.S.; Figiel, P.J.; Gruenwald, K.R.; Guedes da Silva, M.F.C.; Haukka, M.; Pombeiro, A.J.L.; Shul’pin, G.B. Mild oxidative functionalization of alkanes and alcohols catalyzed by new mono- and dicopper(II) aminopolyalcoholates. J. Mol. Catal. A Chem. 2011, 350, 26–34. [Google Scholar] [CrossRef]
- Song, X.; Yan, Y.; Wang, Y.; Hu, D.; Xiao, L.; Yu, J.; Zhang, W.; Jia, M. Hybrid compounds assembled from copper-triazole complexes and phosphomolybdic acid as advanced catalysts for the oxidation of olefins with oxygen. Dalton Trans. 2017, 47, 16655–16662. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Karabach, Y.Y.; Kirillova, M.V.; Haukka, M.; Pombeiro, A.J.L. New diamondoid-like [Cu3B(l-O)6] core self-assembled from Bis-Tris biobuffer for mild hydrocarboxylation of alkanes to carboxylic acids. Dalton Trans. 2011, 40, 6378. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, C.B.A.E.; Guedes da Silva, M.F.C.; Liu, C.-M.; Pombeiro, A.J.L. Peroxidative Oxidation of Alkanes and Alcohols under Mild Conditions by Di- and Tetranuclear Copper (II) Complexes of Bis (2-Hydroxybenzylidene) Isophthalohydrazide. Molecules 2018, 23, 2699. [Google Scholar] [CrossRef] [Green Version]
- Nandi, M.; Roy, P. Peroxidative oxidation of cycloalkane by di-, tetra- and polynuclear copper (II) complexes. Indian J. Chem. 2013, 52, 1263–1268. Available online: http://hdl.handle.net/123456789/21507.
- Saha, M.; Vyas, M.K.; Martins, L.M.D.R.S.; Martins, N.M.R.; Pombeiro, A.J.L.; Mobin, S.M.; Bhattacherjee, D.; Bhabak, K.P.; Mukhopadhyay, S. Copper(II) tetrazolato complexes: Role in oxidation catalysis and protein binding. Polyhedron 2017, 132, 53–63. [Google Scholar] [CrossRef]
- Sutradhar, M.; Roy Barman, T.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Aroylhydrazone Schiff Base Derived Cu(II) and V(V) Complexes: Efficient Catalysts towards Neat Microwave-Assisted Oxidation of Alcohols. Int. J. Mol. Sci. 2020, 21, 2832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiota, Y.; Yoshizawa, K. Comparison of the Reactivity of Bis(μ-oxo)CuIICuIII and CuIIICuIII Species to Methane. Inorg. Chem. 2009, 48. [Google Scholar] [CrossRef] [PubMed]
- Shul’pina, L.S.; Vinogradov, M.M.; Kozlov, Y.N.; Nelyubina, Y.V.; Ikonnikov, N.S.; Shul’pin, G.B. Copper complexes with 1,10-phenanthrolines as efficient catalysts for oxidation of alkanes by hydrogen peroxide. Inorg. Chem. Acta 2020, 512, 119889. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Dronova, M.S.; Yalymov, A.I.; Korlyukov, A.A.; Shul’pina, L.S.; Arkhipov, D.E.; Shubina, E.S.; Levitsky, M.M.; Kirilin, A.D.; Shul’pin, G.B. New binuclear cage-like copper(II) silsesquioxane (“cooling tower”); its high catalytic activity in oxidation of benzene and alcohols. Eur. J. Inorg. Chem. 2013, 30, 5240–5246. [Google Scholar] [CrossRef]
- Dronova, M.S.; Bilyachenko, A.N.; Yalymov, A.I.; Kozlov, Y.N.; Shul’pina, L.S.; Korlyukov, A.A.; Arkhipov, D.E.; Levitsky, M.M.; Shubina, E.S.; Shul’pin, G.B. Solvent-controlled synthesis of tetranuclear cage-like copper(II) silsesquioxanes. Remarkable features of the cage structures and their high catalytic activity in oxidation with peroxides. Dalton Trans. 2014, 43, 872–882. [Google Scholar] [CrossRef]
- Vinogradov, M.M.; Kozlov, Y.N.; Bilyachenko, A.N.; Nesterov, D.S.; Shul’pina, L.S.; Zubavichus, Y.V.; Pombeiro, A.J.L.; Levitsky, M.M.; Yalymov, A.I.; Shul’pin, G.B. Alkane oxidation with peroxides catalyzed by cage-like copper(II) silsesquioxanes. New J. Chem. 2015, 39, 187–199. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Dronova, M.S.; Yalymov, A.I.; Lamaty, F.; Bantreil, X.; Martinez, J.; Bizet, C.; Shul’pina, L.S.; Korlyukov, A.A.; Arkhipov, D.E.; et al. Cage-like Copper(II) Silsesquioxanes: Transmetalation Reactions, Structural, Quantum Chemical and Catalytic Studies. Chem. Eur. J. 2015, 21, 8758–8770. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Kulakova, A.N.; Levitsky, M.M.; Petrov, A.A.; Korlyukov, A.A.; Shul’pina, L.S.; Khrustalev, V.N.; Dorovatovskii, P.V.; Vologzhanina, A.V.; Tsareva, U.S.; et al. Unusual Tri-, Hexa- and Nonanuclear Organosilicon Copper Clusters: Synthesis, Structures and Catalytic Activity in Oxidations with Peroxides. Inorg. Chem. 2017, 56, 4093–4103. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Kulakova, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Vologzhanina, A.V.; Titov, A.A.; . Dorovatovskii, P.V.; Shul’pina, L.S.; Lamaty, F.; et al. Ionic Complexes of Tetra- and Nonanuclear Cage Copper(II) Phenylsilsesquioxanes: Synthesis and High Activity in Oxidative Catalysis. ChemCatChem 2017, 9, 4437–4447. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Korlyukov, A.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Lamaty, F.; Bantreil, X.; Villemejeanne, B.; et al. Si10Cu6N4 Cage Hexacoppersilsesquioxanes Containing N-Ligands: Synthesis, Structure, and High Catalytic Activity in Peroxide Oxidations. Inorg. Chem. 2017, 56, 15026–15040. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Shubina, E.S.; Shul’pin, G.B. Mild and Regioselective Hydroxylation of Methyl Group in Neocuproine: Approach to an N,O-Ligated Cu6 Cage Phenylsilsesquioxane”. Organometallics 2018, 37, 168–171. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Shubina, E.S.; Vologzhanina, A.V.; Shul’pin, G.B. Heptanuclear Cage CuII-Silsesquioxanes: Synthesis, Structure and Catalytic Activity. Eur. J. Inorg. Chem. 2018, 22, 2505–2511. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Kulakova, A.N.; Shul’pina, L.S.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Tsareva, U.S.; Shubina, E.S.; et al. Family of penta- and hexanuclear metallasilsesquioxanes: Synthesis, structure and catalytic properties in oxidations. J. Organometal. Chem. 2018, 867, 133–141. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Bilyachenko, A.N.; Korlyukov, A.A.; Levitsky, M.M.; Shul’pina, L.S.; Bantreil, X.; Lamaty, F.; Vologzhanina, A.V.; Shubina, E.S.; Dorovatovskii, P.V.; et al. High cluster (Cu9) cage silsesquioxanes. Synthesis, structure and catalytic activity. Inorg. Chem. 2018, 57, 11524–11529. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Korlyukov, A.A.; Shul’pina, L.S.; Bantreil, X.; Lamaty, F.; Shubina, E.S.; Levitsky, M.M.; Ikonnikov, N.S.; Shul’pin, G.B. A new “bicycle helmet”-like copper(II),sodiumphenylsilsesquioxane. Synthesis, structure and catalytic activity. Dalton Trans. 2018, 47, 15666–15669. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Bilyachenko, A.N.; Levitsky, M.M.; Shul’pina, L.S.; Korlyukov, A.A.; Zubavichus, Y.V.; Khrustalev, V.N.; Vologzhanina, A.V.; Shubina, E.S.; Dorovatovskii, P.V.; et al. Coordination Affinity of Cu(II)-Based Silsesquioxanes toward N,N-Ligands and Associated Skeletal Rearrangements: Cage and Ionic Products Exhibiting a High Catalytic Activity in Oxidation Reactions. Inorg. Chem. 2020, 59, 4536–4545. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Shubina, E.S.; Levitsky, M.M.; Ikonnikov, N.S.; Bilyachenko, A.N.; Kozlov, Y.N.; Shul’pin, G.B. Palanquin-like Cu4Na4 Silsesquioxane. Synthesis (via oxidation of 1,1-bis(diphenyphosphino)methane), structure and catalytic activity in akane or alcohol oxidation with peroxides. Catalysts 2019, 9, 154. [Google Scholar] [CrossRef] [Green Version]
- Kulakova, A.N.; Bilyachenko, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Shul’pina, L.S.; Bantreil, X.; Lamaty, F.; Shubina, E.S.; Levitsky, M.M.; et al. Cu42Ge24Na4—A Giant Trimetallic Sesquioxane Cage: Synthesis, Structure, and Catalytic Activity. Catalysts 2018, 8, 484. [Google Scholar] [CrossRef] [Green Version]
- Kulakova, A.N.; Korlyukov, A.A.; Zubavichus, Y.V.; Khrustalev, V.N.; Bantreil, X.; Shul’pina, L.S.; Levitsky, M.M.; Ikonnikov, N.S.; Shubina, E.S.; Lamaty, F.; et al. Hexacoppergermsesquioxanes as complexes with N-ligands: Synthesis, structure and catalytic properties. J. Organometal. Chem. 2019, 884, 17–28. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Sedykh, E.E.; Levitsky, M.M.; Dorovatovskii, P.V.; Khrustalev, V.N.; Shul’pina, L.S.; Shubina, E.S.; Kozlov, Y.N.; Ikonnikov, N.S.; Bilyachenko, A.N.; et al. The first tris-heteroleptic copper cage, ligated by germsesquioxanes, 2,2′-bipyridines and 3,5-dimethylpyrazolates. Synthesis, structure and unique catalytic activity in oxidation of alkanes and alcohols with peroxides. J. Organometal. Chem. 2019, 899, 120911. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Rout, L. Recent advances in copper-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2008, 252, 134–154. [Google Scholar] [CrossRef]
- Sabbatini, A.; Martins, L.M.D.R.S.; Mahmudov, K.T.; Kopylovich, M.N.; Drew, M.G.B.; Pettinari, C.; Pombeiro, A.J.L. Microwave-assisted and solvent-free peroxidative oxidation of 1-phenylethanol to acetophenone with a CuII-TEMPO catalytic system. Catal. Com. 2014, 48, 69–72. [Google Scholar] [CrossRef]
- Perraud, O.; Sorokin, A.B.; Dutasta, J.-P.; Martinez, A. Oxidation of cycloalkanes by H2O2 using a copper–hemicryptophane complex as a catalyst. Chem. Commun. 2013, 49, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Ünver, H.; Kani, I. Homogeneous oxidation of alcohols in water catalyzed with Cu(II)-triphenyl acetate/bipyridyl complex. Polyhedron 2017, 134, 257–262. [Google Scholar] [CrossRef]
- Czerwińska, K.; Machura, B.; Kula, S.; Krompiec, S.; Erfurt, K.; Roma-Rodrigues, C.; Fernandes, A.R.; Shul’pina, L.S.; Ikonnikov, N.S.; Shul’pin, G.B. Copper(II) complexes of functionalized 2,2′:6′,2″-terpyridines and 2,6-di(thiazol-2-yl)pyridine: Structure, spectroscopy, cytotoxicity and catalytic activity. Dalton Trans. 2017, 46, 9591–9604. [Google Scholar] [CrossRef] [Green Version]
- Gurbanov, A.V.; Martins, L.M.D.R.S.; Kopylovich, M.N.; Sutradhar, M.; Zubkov, F.I.; Mahmudov, K.T.; Pombeiro, A.J.L. Mechanochemical and Conventional Synthesis of Copper(II)Coordination Polymers Bearing Arylhydrazone of Acetoacetanilide and Their Catalytic Activityin Conversion of Acetone to Acetic Acid. ChemistrySelect 2020, 5, 923–7927. [Google Scholar] [CrossRef]
- Choroba, K.; Machura, B.; Kula, S.; Raposo, L.R.; Fernandes, A.R.; Kruszynski, R.; Erfurt, K.; Shul’pina, L.S.; Kozlov, Y.N.; Shul’pin, G.B. Copper(II) complexes with 2,2′:6′,2”-terpyridine, 2,6-di(thiazol-2-yl)pyridine and 2,6-di(pyrazin-2-yl)pyridine substituted with quinolines. Synthesis, structure, antiproliferative activity, and catalytic activity in oxidation of alkanes and alcohols with peroxides. Dalton Trans. 2019, 48, 12656–12673. [Google Scholar] [CrossRef]
- Brinksma, J.; Rispens, M.T.; Hage, R.; Feringa, B.L. New manganese catalysts for alcohol oxidation. Inorg. Chim. Acta 2002, 337, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Steen, J.D.; Stepanovic, S.; Parvizian, M.; de Boer, J.W.; Hage, R.; Chen, J.; Swart, M.; Gruden, M.; Browne, W.R. Lewis versus Bronsted Acid Activation of a Mn(IV) Catalyst for Alkene Oxidation. Inorg. Chem. 2019, 58, 14924–14930. [Google Scholar] [CrossRef] [Green Version]
- Pinto, M.F.; Olivares, M.; Vivancos, A.; Guisado-Barrios, G.; Albrecht, M.; Royo, B. (Di)Triazolylidene Manganese Complexes in Catalytic Oxidation of Alcohols to Ketones and Aldehydes. Catal. Sci. Technol. 2019, 9, 2421–2425. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Lindsay-Smith, J.R. Oxidations by the reagent ‘H2O2–manganese(IV) complex–carboxylic acid’. Part 1. Oxidation of saturated hydrocarbons with peroxy acids and hydrogen peroxide. Russ. Chem. Bull. 1998, 47, 2379–2386. [Google Scholar] [CrossRef]
- Lindsay Smith, J.R.; Shul’pin, G.B. Efficient stereoselective oxygenation of alkanes by peroxyacetic acid or hydrogen peroxide and acetic acid catalysed by a manganese(IV) 1,4,7-trimethyl-1,4,7-triazacyclononane complex. Tetrahedron Lett. 1998, 39, 4909–4912. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Süss-Fink, G.; Lindsay Smith, J.R. Oxidations by the system “Hydrogen Peroxide–Manganese(IV) Complex–Acetic Acid”, Part II. Hydroperoxidation and hydroxylation of alkanes in acetonitrile. Tetrahedron 1999, 55, 5345–5358. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Süss-Fink, G.; Shul’pina, L.S. Oxidations by the system “hydrogen peroxide–manganese(IV) complex–carboxylic acid”. Part 3. Oxygenation of ethane, higher alkanes, alcohols, olefins and sulfides. J. Mol. Catal. A Chem. 2001, 170, 17–34. [Google Scholar] [CrossRef]
- Mandelli, D.; Woitiski, C.B.; Schuchardt, U.; Shul’pin, G.B. Hydrogen-peroxide epoxidation of natural olefins catalyzed by a dinuclear manganese complex. Chem. Natur. Comp. 2002, 38, 243–245. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nizova, G.V.; Kozlov, Y.N.; Pechenkina, I.G. Oxidations by the “hydrogen peroxide–manganese(IV) complex–carboxylic acid” system. Part 4. Efficient acid-base switching between catalase and oxygenase activities of a dinuclear manganese(IV) complex in the reaction with H2O2 and an alkane. New J. Chem. 2002, 26, 1238–1245. [Google Scholar] [CrossRef]
- Nizova, G.V.; Bolm, C.; Ceccarelli, S.; Pavan, C.; Shul’pin, G.B. Hydrocarbon oxidations with hydrogen peroxide catalyzed by a soluble polymer-bound manganese(IV) complex with 1,4,7-triazacyclononane. Adv. Synth. Catal. 2002, 344, 899–905. [Google Scholar] [CrossRef]
- Kozlov, Y.N.; Mandelli, D.; Woitiski, C.B.; Shul’pin, G.B. Mechanism of the oxidation of olefins and alkanes with a H2O2–dimeric Mn(IV) complex–acetic acid system. Russ. J. Phys. Chem. 2004, 78, 370–374. [Google Scholar]
- Woitiski, C.B.; Kozlov, Y.N.; Mandelli, D.; Nizova, G.V.; Schuchardt, U.; Shul’pin, G.B. Oxidations by the system “hydrogen peroxide–dinuclear manganese(IV) complex–carboxylic acid”. Part 5. Epoxidation of olefins including natural terpenes. J. Mol. Catal. A Chem. 2004, 222, 103–119. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nizova, G.V.; Kozlov, Y.N.; Arutyunov, V.S.; dos Santos, A.C.M.; Ferreira, A.C.T.; Mandelli, D. Oxidations by the system ‘‘hydrogen peroxide–[Mn2L2O3][PF6]2 (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)–oxalic acid’’. Part 6. Oxidation of methane and other alkanes and olefins in water. J. Organometal. Chem. 2005, 690, 4498–4504. [Google Scholar] [CrossRef]
- Mandelli, D.; Steffen, R.A.; Shul’pin, G.B. Carvone epoxidation by the system “Hydrogen peroxide–[Mn2L2O3][PF6]2 (L =1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid”: A combinatorial approach to the process optimization” <Part 7 from the series “Oxidations by the system hydrogen peroxide–[Mn2L2O3][PF6]2 (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid. React. Kinet. Catal. Lett. 2006, 88, 165–174. [Google Scholar]
- Romakh, V.B.; Therrien, B.; Karmazin-Brelot, L.; Labat, G.; Stoeckli-Evans, H.; Shul’pin, G.B.; Süss-Fink, G. Dinuclear manganese complexes containing 1,4-dimethyl-1,4,7-triazacyclononane ligands as well as carboxylato and oxo bridges. Inorg. Chim. Acta 2006, 359, 1619–1626. [Google Scholar] [CrossRef] [Green Version]
- Romakh, V.B.; Therrien, B.; Süss-Fink, G.; Shul’pin, G.B. Dinuclear manganese complexes containing chiral 1,4,7-triazacyclononane-derived ligands and their catalytic potential for the oxidation of olefins, alkanes, and alcohols. Inorg. Chem. 2007, 46, 1315–1331. [Google Scholar] [CrossRef] [PubMed]
- Nizova, G.V.; Shul’pin, G.B. A unique rate-accelerating effect of certain amino acids in the H2O2 oxidation of alkanes catalyzed by a dinuclear manganese complex containing 1,4,7-trimethyl-1,4,7-triazacyclononane” <Part 9 from the series “Oxidations by the system hydrogen peroxide–[Mn2L2O3][PF6]2 (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid”>. Tetrahedron 2007, 63, 7997–8001. [Google Scholar] [CrossRef]
- Kozlov, Y.N.; Nizova, G.V.; Shul’pin, G.B. Alkane oxidation by the system ‘tert-butyl hydroperoxide–[Mn2L2O3][PF6]2 (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid. J. Phys. Org. Chem. 2008, 21, 119–126. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Matthes, M.G.; Romakh, V.B.; Barbosa, M.I.F.; Aoyagi, J.L.T.; Mandelli, D. Oxidations by the system ‘hydrogen peroxide–[Mn2L2O3][PF6]2 (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid’. Part 10: Co-catalytic effect of different carboxylic acids in the oxidation of cyclohexane, cyclohexanol, and acetone. Tetrahedron 2008, 64, 2143–2152. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Kholuiskaya, S.N.; Plieva, M.I. Oxidations by the system ‘hydrogen peroxide–[Mn2L2O3]2+ (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)–oxalic acid’. Part 11. Degradation of dye Rhodamine 6G and oxygenation of cyclohexene. J. Mol. Catal. A Chem. 2009, 299, 77–87. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Strelkova, T.V.; Mandelli, D. Oxidation of Reactive Alcohols with Hydrogen Peroxide Catalyzed by Manganese Complexes” <Part 12 from the series “Oxidations by the system hydrogen peroxide–[Mn2L2O3]2+ (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid”>. Catal. Lett. 2010, 138, 193–204. [Google Scholar] [CrossRef]
- Kozlov, Y.N.; Shul’pina, L.S.; Strelkova, T.V.; Shul’pin, G.B. Kinetics and Mechanism of 1-Phenylethanol Oxidation by the System Hydrogen Peroxide–Manganese(IV) Binuclear Complex–Oxalic Acid. Russ. J. Phys. Chem. A 2010, 84, 1502–1505. [Google Scholar] [CrossRef]
- Mandelli, D.; Kozlov, Y.N.; Carvalho, W.A.; Shul’pin, G.B. Oxidations by the system ‘hydrogen peroxide–[Mn2L2O3]2+ (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid’. Part 13. Epoxidation of methyl oleate in acetonitrile solution. Catal. Commun. 2012, 26, 93–97. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nesterov, D.S.; Shul’pina, L.S.; Pombeiro, A.J.L. A hydroperoxo-rebound mechanism of alkane oxidation with hydrogen peroxide catalyzed by binuclear manganese(IV) complex in the presence of an acid with involvement of atmospheric dioxygen <Part 14 from the series “Oxidations by the system hydrogen peroxide–[Mn2L2O3]2+ (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid”>. Inorg. Chim. Acta 2017, 455, 666–676. [Google Scholar] [CrossRef]
- Conte, V.; Coletti, A.; Floris, B.; Licini, G.; Zonta, C. Mechanistic aspects of vanadium catalysed oxidations with peroxides. Coord. Chem. Rev. 2011, 255, 2165–2177. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Attanasio, D.; Suber, L. Efficient H2O2 oxidation of alkanes and arenes to alkyl peroxides and phenols catalyzed by the system vanadate–pyrazine-2-carboxylic acid. J. Catal. 1993, 142, 147–152. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Attanasio, D.; Suber, L. Oxidations by a H2O2–VO3––pyrazine-2-carboxylic acid reagent. 1. Oxidations of alkanes in CH3CN to produce alkyl peroxides. Russ. Chem. Bull. 1993, 42, 55–59. [Google Scholar]
- Kirillov, A.M.; Shul’pin, G.B. Pyrazinecarboxylic acid and analogs: Highly efficient co-catalysts in the metal-complex-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2013, 257, 732–754. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Druzhinina, A.N.; Nizova, G.V. Oxidation with the H2O2–VO3––pyrazine-2-carboxylic acid reagent. 2. Oxidation of alcohols and aromatic hydrocarbons. Russ. Chem. Bull. 1993, 42, 1327–1329. [Google Scholar]
- Nizova, G.V.; Shul’pin, G.B. Oxidation by a H2O2–vanadium complex–2-pyrazinecarboxylic acid reagent. 3. Evidence for hydroxyl radical formation. Russ. Chem. Bull. 1994, 43, 1146–1148. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Süss-Fink, G. Oxidations by the reagent “H2O2–vanadium complex–pyrazine-2-carboxylic acid”. Part 4. Oxidation of alkanes, benzene and alcohols by an adduct of H2O2 with urea. J. Chem. Soc. Perkin Trans. 2 1995, 7, 1459–1463. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Drago, R.S.; Gonzalez, M. Oxidations by a “H2O2-vanadium complex–pyrazine-2-carboxylic acid” reagent. 5. Oxidation of lower alkanes with the formation of carbonyl compounds. Russ. Chem. Bull. 1996, 45, 2386–2388. [Google Scholar] [CrossRef]
- Guerreiro, M.C.; Schuchardt, U.; Shul’pin, G.B. Oxidation with the “O2–VO3––pyrazine-2-carboxylic acid” reagent. Part 6. Oxidation of n-heptane and cyclohexane. Direct determination of alkyl hydroperoxides by gas-liquid chromatography. Russ. Chem. Bull. 1997, 46, 749–754. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Guerreiro, M.C.; Schuchardt, U. Oxidations by the reagent O2-H2O2-vanadium complex-pyrazine-2-carboxylic acid. Part 7. Hydroperoxidation of higher alkanes. Tetrahedron 1996, 52, 13051–13062. [Google Scholar] [CrossRef]
- Nizova, G.V.; Süss-Fink, G.; Shul’pin, G.B. Oxidations by the reagent «O2-H2O2-vanadium complex-pyrazine-2-carboxylic acid»-8. Efficient oxygenation of methane and other lower alkanes in acetonitrile. Tetrahedron 1997, 53, 3603–3614. [Google Scholar] [CrossRef]
- Schuchardt, U.; Guerreiro, M.C.; Shul’pin, G.B. Oxidation with the ‘O2–H2O2–vanadium complex–pyrazine-2-carboxylic acid’ reagent. 9. Oxidation of cyclohexene and decalin. Russ. Chem. Bull. 1998, 47, 247–252. [Google Scholar] [CrossRef]
- Süss-Fink, G.; Nizova, G.V.; Stanislas, S.; Shul’pin, G.B. Oxidations by the reagent ‘O2–H2O2–vanadate anion–pyrazine-2-carboxylic acid’. Part 10. Oxygenation of methane in acetonitrile and water. J. Mol. Catal. A Chem. 1998, 13, 163–170. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Ishii, Y.; Sakaguchi, S.; Iwahama, T. Oxidations with the “O2-H2O2-vanadiumcomplex-pyrazine-2-carboxylic acid” reagent. 11. Oxidation of styrene, phenylacetylene, and their derivatives with the formation of benzaldehyde and benzoic acid. Russ. Chem. Bull. 1999, 48, 887–890. [Google Scholar]
- Süss-Fink, G.; Stanislas, S.; Shul’pin, G.B.; Nizova, G.V.; Stoeckli-Evans, H.; Neels, A.; Bobillier, C.; Claude, S. Oxidative functionalisation of alkanes: Synthesis, molecular structure and catalytic implications of anionic vanadium(V) oxo and peroxo complexes containing bidentate N,O ligands. J. Chem. Soc. Dalton Trans. 1999, 18, 3169–3175. [Google Scholar] [CrossRef] [Green Version]
- Shul’pin, G.B.; Kozlov, Y.N.; Nizova, G.V.; Süss-Fink, G.; Stanislas, S.; Kitaygorodskiy, A.; Kulikova, V.S. Oxidations by the reagent “O2-H2O2-vanadium derivative–pyrazine-2-carboxylic acid” Part 12. Main features, kinetics and mechanism of alkane hydroperoxidation. J. Chem. Soc. Perkin Trans. 2 2001, 2, 1351–1371. [Google Scholar] [CrossRef]
- Kozlov, Y.N.; Nizova, G.V.; Shul’pin, G.B. The mechanism of hydrogen peroxide-induced aerobic oxidation of alkanes in catalysis by a vanadium complex and pyrazine-2-carboxylic acid. Russ. J. Phys. Chem. 2001, 75, 770–774. [Google Scholar]
- Süss-Fink, G.; Gonzalez, L.; Shul’pin, G.B. Alkane oxidation with hydrogen peroxide catalyzed homogeneously by vanadium-containing polyphosphomolybdates. Appl. Catal. A General. 2001, 217, 111–117. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N. Kinetics and mechanism of alkane hydroperoxidation with tert-butyl hydroperoxide catalysed by a vanadate ion. Org. Biomol. Chem. 2003, 1, 2303–2306. [Google Scholar] [CrossRef] [PubMed]
- Süss-Fink, G.; Hong, Y.; Nizova, G.V.; Stanislas, S.; Shul’pin, G.B. Oxygenation of methane with atmospheric oxygen in aqueous solution promoted by H2O2 and catalyzed by a vanadate ion–pyrazine-2-carboxylic acid system. Russ. Chem. Bull. 1997, 46, 1801–1803. [Google Scholar] [CrossRef]
- Nizova, G.V.; Kozlov, Y.N.; Shul’pin, G.B. Effect of acetonitrile on the catalytic decomposition of hydrogen peroxide by vanadium ions and conjugated oxidation of alkanes. Russ. Chem. Bull. 2004, 53, 2330–2333. [Google Scholar] [CrossRef]
- Kozlov, Y.N.; Nizova, G.V.; Shul’pin, G.B. Oxidations by the reagent “O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid”. Part 14. Competitive oxidation of alkanes and acetonitrile (solvent). J. Mol. Catal. A Chem. 2005, 227, 247–253. [Google Scholar] [CrossRef]
- De la Cruz, M.H.C.; Kozlov, Y.N.; Lachter, E.R.; Shul’pin, G.B. Oxidations by the reagent “O2-H2O2-vanadium derivative-pyrazine-2-carboxylic acid. Part 13. Kinetics and mechanism of the benzene hydroxylation. New J. Chem. 2003, 27, 634–638. [Google Scholar] [CrossRef]
- Jannini, M.J.D.M.; Shul’pina, L.S.; Schuchardt, U.; Shul’pin, G.B. Oxidation of alkanes with hydrogen peroxide catalyzed by the “vanadate-ion–pyrazine-2-carboxylic acid” system in the presence of pyridine (Part 15 of the series “Oxidations by the reagent O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid”). Petrol. Chem. 2005, 45, 413–418. [Google Scholar]
- Khaliullin, R.Z.; Bell, A.T.; Head-Gordon, M. A density functional theory study of the mechanism of free radical generation in the system vanadate/PCA/H2O2. J. Phys. Chem. B 2005, 109, 17984–17992. [Google Scholar] [CrossRef]
- Kozlov, Y.N.; Romakh, V.B.; Kitaygorodskiy, A.; Buglyó, P.; Süss-Fink, G.; Shul’pin, G.B. Oxidation of 2-propanol and cyclohexane by the reagent “Hydrogen peroxide-vanadate anion-pyrazine-2-carboxylic acid”: Kinetics and mechanism. J. Phys. Chem. A 2007, 111, 7736–7752. [Google Scholar] [CrossRef]
- Romakh, V.B.; Süss-Fink, G.; Shul’pin, G.B. Vanadate ion-catalyzed oxidation of methane with hydrogen peroxide in an aqueous solution. Petrol. Chem. 2008, 48, 440–443. [Google Scholar] [CrossRef] [Green Version]
- Bolm, C. Vanadium-catalyzed asymmetric oxidations. Coord. Chem. Rev. 2003, 237, 245–256. [Google Scholar] [CrossRef]
- Gusevskaya, E.V.; Menini, L.; Parreira, L.A.; Mesquita, R.A.; Kozlov, Y.N.; Shul’pin, G.B. Oxidation of isoeugenol to vanillin by the “H2O2-vanadate-pyrazine-2-carboxylic acid” reagent. J. Mol. Catal. A 2012, 363–364, 140–147. [Google Scholar] [CrossRef]
- Sutradhar, M.; Shvydkiy, N.V.; Guedes da Silva, M.F.C.; Kirillova, M.V.; Kozlov, Y.N.; Pombeiro, A.J.L.; Shul’pin, G.B. New binuclear oxovanadium(V) complex as a catalyst in combination with pyrazinecarboxylic acid (PCA) for efficient alkane oxygenation by H2O2. Dalton Trans. 2013, 42, 11791–11803. [Google Scholar] [CrossRef] [PubMed]
- Licini, G.; Conte, V.; Coletti, A.; Mba, M.; Zont, C. Recent advances in vanadium catalyzed oxygen transfer reactions. Coord. Chem. Rev. 2011, 255, 2345–2357. [Google Scholar] [CrossRef] [Green Version]
- Sutradhar, M.; da Silva, J.A.L.; Pombeiro, A.J.L. (Eds.) Vanadium Catalysis; RSC: London, UK, 2020; Chapter 4; pp. 72–96. ISBN 978-1-83916-088-2. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Talsi, E.P.; Stas’ko, S.N.; Kholdeeva, O.A.; Popov, S.A.; Tkachev, A.V. Stereoselective oxidation of linalool with tert-butyl hydroperoxide, catalyzed by a vanadium(V) complex with a chiral terpenoid ligand. J. Mol. Catal. A Chem. 2003, 194, 79–88. [Google Scholar] [CrossRef]
- Fomenko, I.S.; Vincendeau, S.; Manoury, E.; Poli, R.; Abramov, P.A.; Nadolinny, V.A.; Sokolov, M.N.; Gushchin, A.L. An oxidovanadium(IV) complex with 4,4′-di-tert-butyl-2,2′-bipyridine ligand: Synthesis, structure and catalyzed cyclooctene epoxidation. Polyhedron 2020, 177, 114305. [Google Scholar] [CrossRef]
- Süss-Fink, G.; Gonzalez Cuervo, L.; Therrien, B.; Stoeckli-Evans, H.; Shul’pin, G.B. Mono and oligonuclear vanadium complexes as catalysts for alkane oxidation: Synthesis, molecular structure and catalytic potential. Inorg. Chim. Acta 2004, 357, 475–484. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kuznetsov, M.L.; Romakh, V.B.; Shul’pina, L.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L.; Shul’pin, G.B. Mechanism of oxidations with H2O2 catalyzed by vanadate anion or oxovanadium(V) triethanolaminate (vanadatrane) in combination with pyrazine-2-carboxylic acid (PCA): Kinetic and DFT studies. J. Catal. 2009, 267, 140–157. [Google Scholar] [CrossRef]
- Shul’pina, L.S.; Kirillova, M.V.; Pombeiro, A.J.L.; Shul’pin, G.B. Alkane oxidation by the H2O2–NaVO3–H2SO4 system in acetonitrile and water. Tetrahedron 2009, 65, 2424–2429. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kuznetsov, M.L.; Kozlov, Y.N.; Shul’pina, L.S.; Kitaygorodskiy, A.; Pombeiro, A.J.L.; Shul’pin, G.B. Participation of Oligovanadates in Alkane Oxidation with H2O2 Catalyzed by Vanadate Anion in Acidified Acetonitrile: Kinetic and DFT Studies. ACS Catal. 2011, 1, 1511–1520. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.; Roy Barman, T.; Kuznetsov, M.L.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Vanadium complexes of different nuclearities in the catalytic oxidation of cyclohexane and cyclohexanol—An experimental and theoretical investigation. New J. Chem. 2019, 43, 17557–17570. [Google Scholar] [CrossRef]
- Maurya, M.R.; Tomar, R.; Avecilla, F.; Ribeiro, N.; Carvalho, M.F.N.N.; Kuznetsov, M.L.; Correia, I.; Pessoa, J.C. Trinuclear vanadium(iv) and vanadium(v) complexes derived from 2,4,6-triacetylphloroglucinol and study of their peroxidase mimicking activity. Dalton Trans. 2020, 49, 2589–2609. [Google Scholar] [CrossRef] [PubMed]
- Nagataki, T.; Ishii, K.; Tachi, Y.; Itoh, S. Ligand effects on NiII-catalysed alkane-hydroxylation with m-CPBA. Dalton Trans. 2007, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Bandoh, H.; Nagatomo, S.; Kitagawa, T.; Fukuzumi, S. Aliphatic hydroxylation by a bis(_-oxo)dinickel(III) complex. J. Am. Chem. Soc. 1999, 121, 8945–8946. [Google Scholar] [CrossRef]
- Kunishita, A.; Doi, Y.; Kubo, M.; Ogura, T.; Sugimoto, H.; Itoh, S. Ni(II)/H2O2 reactivity in bis[(pyridin-2-yl)methyl] amine tridentate ligand system. Aromatic hydroxylation reaction by bis(_-oxo)dinickel(III)complex. Inorg. Chem. 2009, 48, 4997–5004. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.I.; Shul’pina, L.S.; Mandelli, D.; Korlyukov, A.A.; Vologzhanina, A.V.; Es’kova, M.A.; Shubina, E.S.; Levitsky, M.M.; Shul’pin, G.B. Novel Cage-Like Hexanuclear Nickel(II) Silsesquioxane. Synthesis, Structure, and Catalytic Activity in Oxidations with Peroxides. Molecules 2016, 21, 665. [Google Scholar] [CrossRef] [Green Version]
- Bilyachenko, A.N.; Yalymov, A.I.; Levitsky, M.M.; Korlyukov, A.A.; Es’kova, M.A.; Long, J.; Larionova, J.; Guari, Y.; Shul’pina, L.S.; Ikonnikov, N.S.; et al. First cage-like pentanuclear Co(II)-silsesquioxane. Dalton Trans. 2016, 35, 13663–13666. [Google Scholar] [CrossRef]
- Paul, A.; Martins, L.M.D.R.S.; Karmakar, A.; Kuznetsov, M.L.; Novikov, A.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Invironmentally benign benzyl alcohol oxidation and C-C coupling catalysed by amide functionalized 3D Co(II) and Zn(II) metal organic frameworks. J. Catal. 2020, 385, 324–337. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Petrovskiy, P.V. Oxidation of alkanes and alcohols with hydrogen peroxide catalyzed by complex Os3(CO)10(mu-H)2. Appl. Organometal. Chem. 2010, 24, 464–472. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Kudinov, A.R.; Mandelli, D. Extremely Efficient Alkane Oxidation by a New Catalytic Reagent H2O2/Os3(CO)12/Pyridine. Inorg. Chem. 2009, 48, 10480–10482. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kudinov, A.R.; Shul’pina, L.S.; Petrovskaya, E.A. Oxidations catalyzed by osmium compounds. Part 1: Efficient alkane oxidation with peroxides catalyzed by an olefin carbonyl osmium(0) complex. J. Organometal. Chem. 2006, 691, 837–845. [Google Scholar] [CrossRef]
- Shul’pina, L.S.; Kudinov, A.R.; Petrovskaya, E.A.; Strelkova, T.V.; Shul’pin, G.B. Hydrogen peroxide oxidation of alkanes catalyzed by the osmium complex Os3(CO)11(eta2-PhCOCH=CHCOPh). Petrol. Chem. 2006, 46, 164–166. [Google Scholar] [CrossRef]





























Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shul’pin, G.B.; Shul’pina, L.S. Oxidation of Organic Compounds with Peroxides Catalyzed by Polynuclear Metal Compounds. Catalysts 2021, 11, 186. https://doi.org/10.3390/catal11020186
Shul’pin GB, Shul’pina LS. Oxidation of Organic Compounds with Peroxides Catalyzed by Polynuclear Metal Compounds. Catalysts. 2021; 11(2):186. https://doi.org/10.3390/catal11020186
Chicago/Turabian StyleShul’pin, Georgiy B., and Lidia S. Shul’pina. 2021. "Oxidation of Organic Compounds with Peroxides Catalyzed by Polynuclear Metal Compounds" Catalysts 11, no. 2: 186. https://doi.org/10.3390/catal11020186
APA StyleShul’pin, G. B., & Shul’pina, L. S. (2021). Oxidation of Organic Compounds with Peroxides Catalyzed by Polynuclear Metal Compounds. Catalysts, 11(2), 186. https://doi.org/10.3390/catal11020186