Catalysis on Nanostructured Indium Tin Oxide Surface for Fast and Inexpensive Probing of Antibodies during Pandemics
Abstract
:1. Introduction
2. Results and Discussion
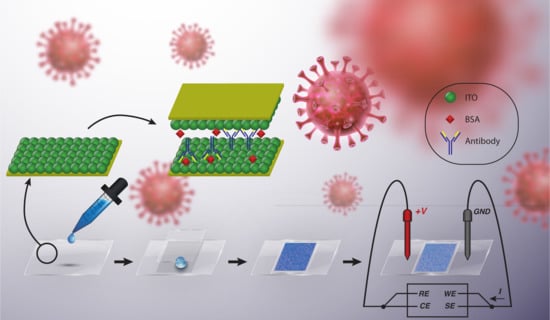
2.1. Biosensor Fabrication
2.2. ITO Nanostructure
2.3. Electronic Circuit
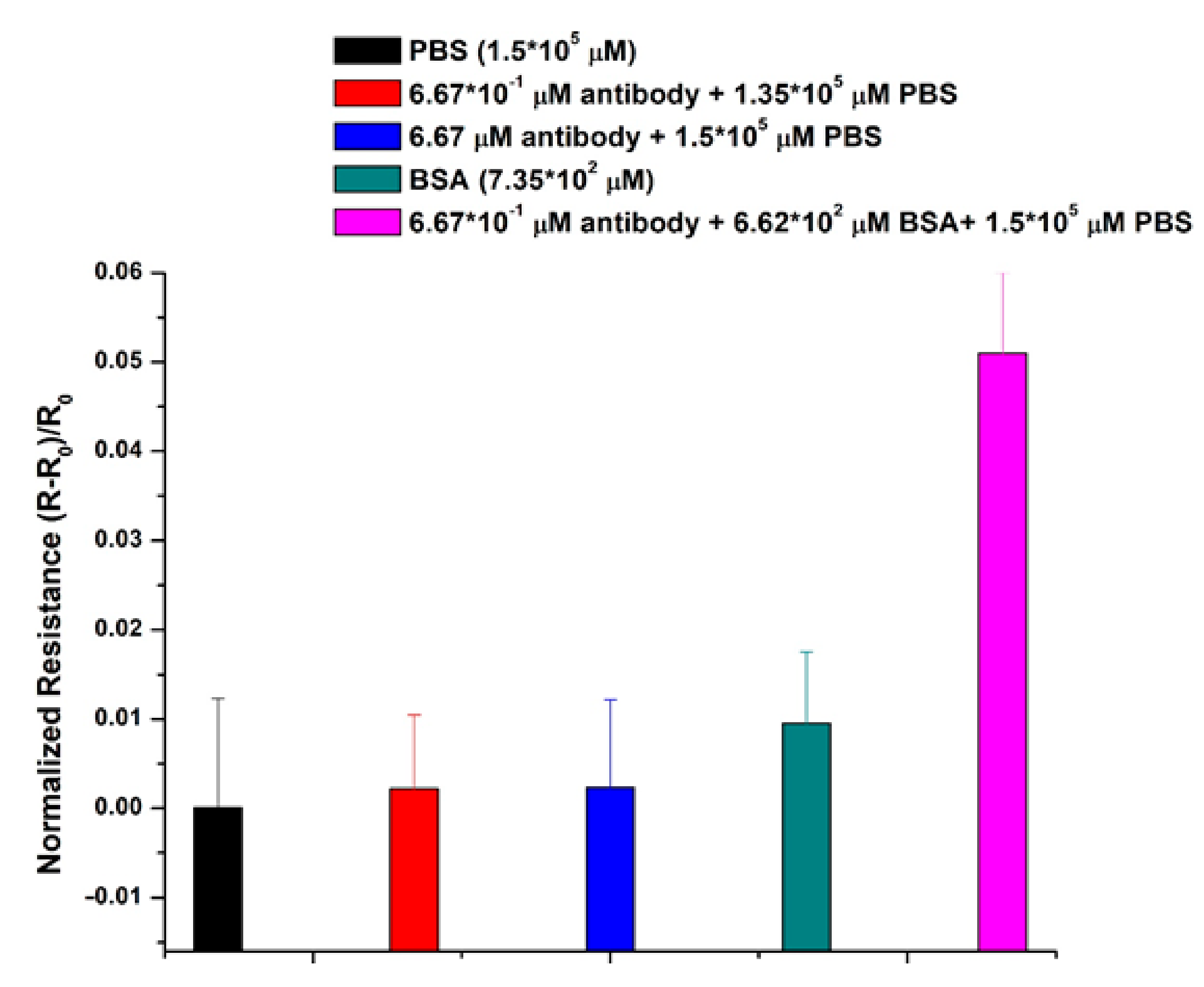
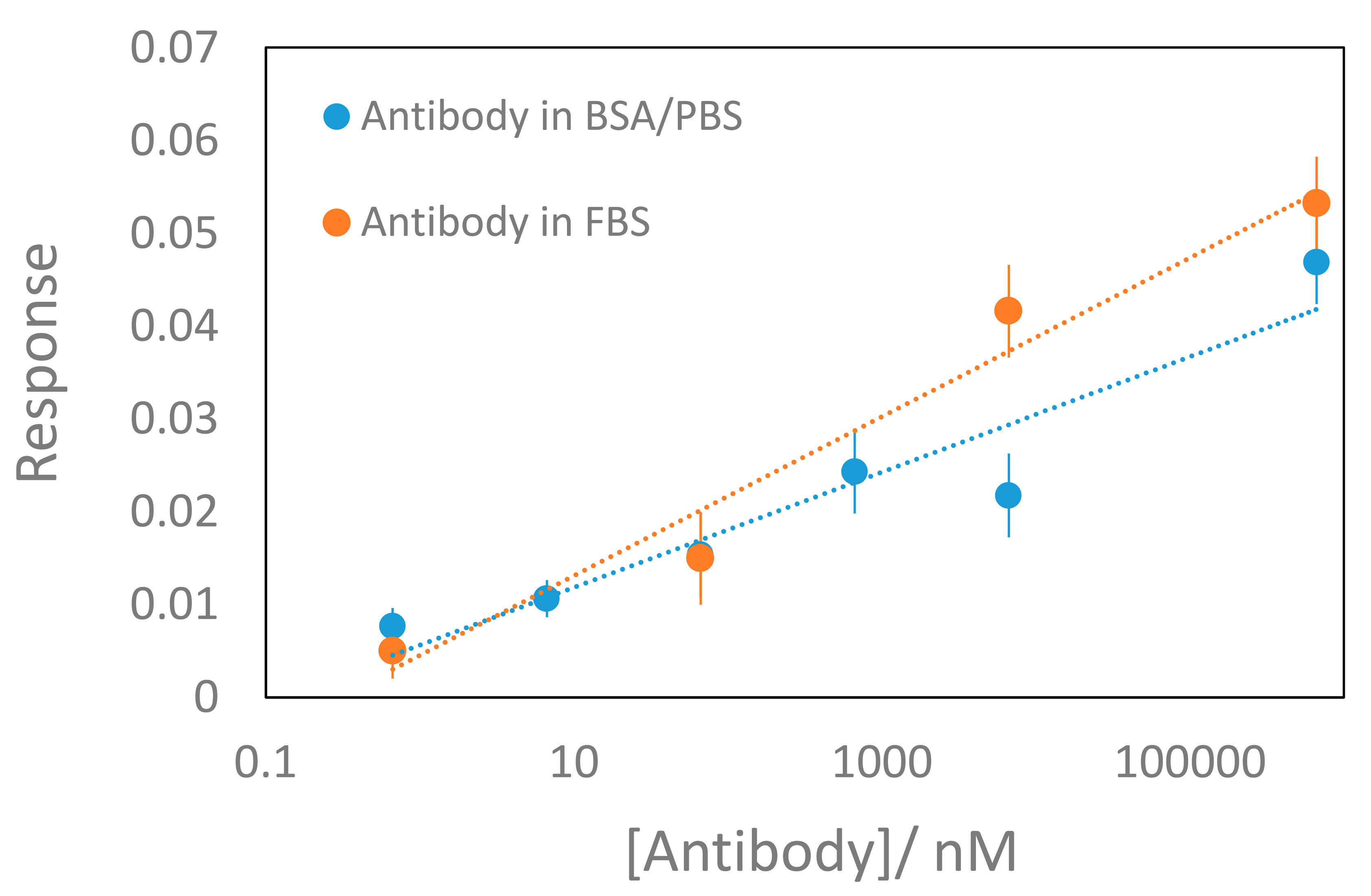
2.4. Antibody Measurements
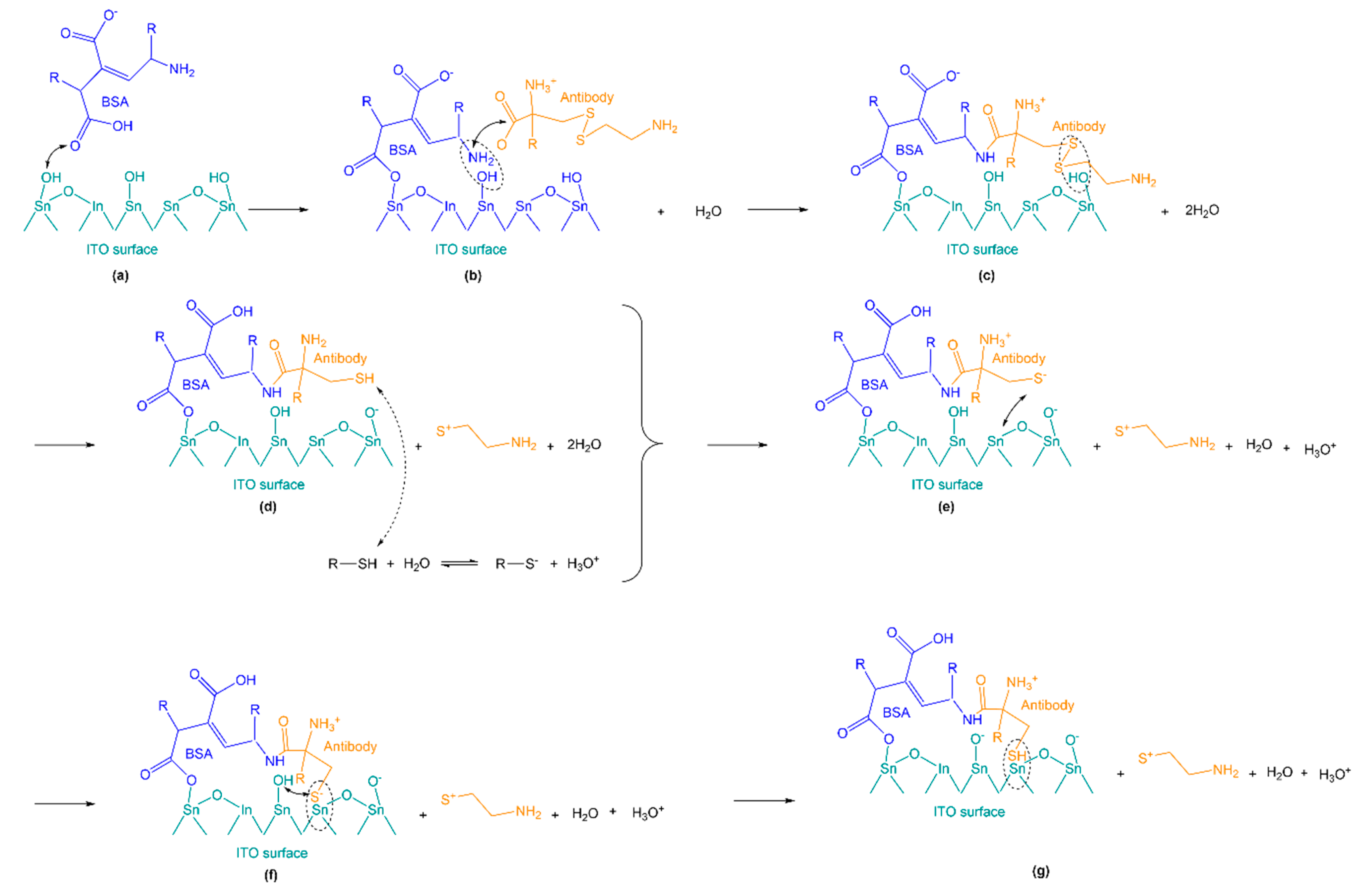
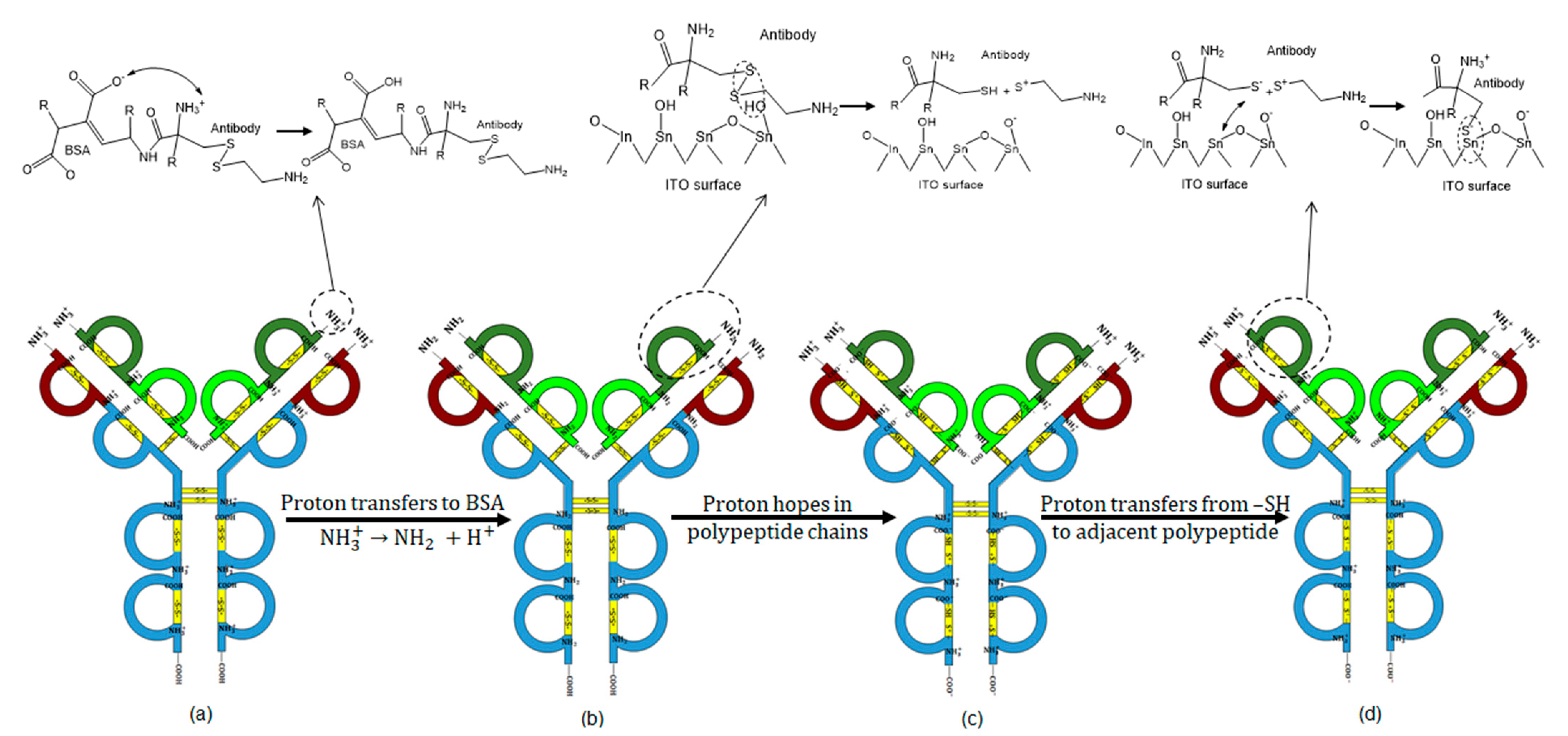
2.5. Catalytic Effects of the Indium Tin Oxide Substrate
2.5.1. XPS Characterization
2.5.2. Charge Transport Mechanism
3. Materials and Methods
3.1. Sample Preparation
3.2. Sensor Fabrication
3.3. Sensor Functionalization and Sample Immobilization
3.4. Thickness Measurement Using UV-SR Filmetrics-40
3.5. XPS Analysis
3.6. Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stadlbauer, D.; Amanat, F.; Chromikova, V.; Jiang, K.; Strohmeier, S.; Asthagiri Arunkumar, G.; Krammer, F. SARS-CoV-2 seroconversion in humans: A detailed protocol for a serological assay to detect antigen production and test setup. Curr. Protoc. Microbiol. 2020, 57, 100–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D. Severe acute respiratory syndrome coronavirus 2− specific antibody responses in coronavirus disease patients. Emerg. Infect. Dis. 2020, 26, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020, 19, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, L.L.; Ransegnola, B.P.; Jin, D.K.; Muecksch, F.; Weisblum, Y.; Bao, W.; George, P.J.; Rodriguez, M.; Tricoche, N.; Schmidt, F.; et al. Serological Assays Estimate Highly Variable SARS-CoV-2 Neutralizing Antibody Activity in Recovered COVID-19 Patients. J. Clin. Microbiol. 2020, 58, 2005–2020. [Google Scholar] [CrossRef] [PubMed]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Starr, T.N.; Greaney, A.J.; Addetia, A.; Hannon, W.W.; Choudhary, M.C.; Dingens, A.S.; Li, J.Z.; Bloom, J.D. Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. bioRxiv 2020, 30, 405472–405499. [Google Scholar] [CrossRef]
- Meyerowitz-Katz, G.; Merone, L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int. J. Infect. Dis. 2020, 101, 138–148. [Google Scholar] [CrossRef]
- Erikstrup, C.; Hother, C.E.; Pedersen, O.B.V.; Mølbak, K.; Skov, R.L.; Holm, D.K.; Sækmose, S.; Nilsson, A.C.; Brooks, P.T.; Boldsen, J.K.; et al. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. medRxiv 2020, 24, 849–857. [Google Scholar] [CrossRef]
- Walls, A.C.; Fiala, B.; Schäfer, A.; Wrenn, S.; Pham, M.N.; Murphy, M.; Tse, L.V.; Shehata, L.; O’Connor, M.A.; Chen, C.; et al. Elicitation of Potent Neutralizing Antibody Responses by Designed Protein Nanoparticle Vaccines for SARS-CoV-2. Cell 2020, 183, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Odinsen, O.; Owusu-Ofori, S.; Dompreh, A.; Sarkodie, F.; Opare-Sem, O.; Parker, D.; Allain, J.-P. Antibody detection and kinetics of antibody production during early stages of immunization with hepatitis B virus vaccine. Clin. Vaccine Immunol. 2007, 14, 1623–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, P.K.S.; Ng, K.-C.; Chan, R.C.W.; Lam, R.K.Y.; Chow, V.C.Y.; Hui, M.; Wu, A.; Lee, N.; Yap, F.H.Y.; Cheng, F.W.T.; et al. Immunofluorescence assay for serologic diagnosis of SARS. Emerg. Infect. Dis. 2004, 10, 530–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Li, W.; Drabek, D.; Okba, N.M.A.; van Haperen, R.; Osterhaus, A.D.M.E.; van Kuppeveld, F.J.M.; Haagmans, B.L.; Grosveld, F.; Bosch, B.-J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020, 11, 2251–2257. [Google Scholar] [CrossRef]
- Liu, I.-J.; Chen, P.-J.; Yeh, S.-H.; Chiang, Y.-P.; Huang, L.-M.; Chang, M.-F.; Chen, S.-Y.; Yang, P.-C.; Chang, S.-C.; Wang, W.-K.; et al. Immunofluorescence Assay for Detection of the Nucleocapsid Antigen of the Severe Acute Respiratory Syndrome (SARS)-Associated Coronavirus in Cells Derived from Throat Wash Samples of Patients with SARS. J. Clin. Microbiol. 2005, 43, 2444–2448. [Google Scholar] [CrossRef] [Green Version]
- Ejazi, S.A.; Ghosh, S.; Ali, N. Antibody detection assays for COVID-19 diagnosis: An early overview. Immunol. Cell Biol. 2020, 98, 530–538. [Google Scholar] [CrossRef]
- Kaushik, A.K.; Dhau, J.S.; Gohel, H.; Mishra, Y.K.; Kateb, B.; Kim, N.-Y.; Goswami, D.Y. Electrochemical SARS-CoV-2 Sensing at Point-of-Care and Artificial Intelligence for Intelligent COVID-19 Management. ACS Appl. Bio Mater. 2020, 3, 7306–7325. [Google Scholar] [CrossRef]
- Alafeef, M.; Dighe, K.; Moitra, P.; Pan, D. Rapid, Ultrasensitive, and Quantitative Detection of SARS-CoV-2 Using Antisense Oligonucleotides Directed Electrochemical Biosensor Chip. ACS Nano 2020. [Google Scholar] [CrossRef]
- Rashed, M.Z.; Kopechek, J.A.; Priddy, M.C.; Hamorsky, K.T.; Palmer, K.E.; Mittal, N.; Valdez, J.; Flynn, J.; Williams, S.J. Rapid detection of SARS-CoV-2 antibodies using electrochemical impedance-based detector. Biosens. Bioelectron. 2021, 171, 112709–112715. [Google Scholar] [CrossRef]
- de Eguilaz, M.R.; Cumba, L.R.; Forster, R.J. Electrochemical detection of viruses and antibodies: A mini review. Electrochem. Commun. 2020, 116, 106762–106769. [Google Scholar] [CrossRef]
- Brown, R.C.H.; Kelly, D.; Wilkinson, D.; Savulescu, J. The scientific and ethical feasibility of immunity passports. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Phelan, A.L. COVID-19 immunity passports and vaccination certificates: Scientific, equitable, and legal challenges. Lancet 2020, 395, 1595–1598. [Google Scholar] [CrossRef]
- “Immunity Passports” in the Context of COVID-19. Available online: https://www.who.int/publications/i/item/WHO/2019nCoV/Sci_Brief/Immunity_passport/2020.1 (accessed on 24 April 2020).
- Landry, C.J.; Burns, F.P.; Baerlocher, F.; Ghandi, K. Novel Solid-State Microbial Sensors Based on ZnO Nanorod Arrays. Adv. Funct. Mater. 2018, 28, 1706309–1706318. [Google Scholar] [CrossRef]
- Ghandi, K. Polymorph purity, monitoring and associated compositions. U.S. Patent 2015/0126409 A1, 7 May 2015. [Google Scholar]
- Hlubina, P.; Lunackova, M.; Lunacek, J. Simple method for determination of the thickness of a nonabsorbing thin film using spectral reflectance measurement. Appl. Opt. 2009, 48, 985–989. [Google Scholar] [CrossRef]
- Pluk, H.; Stokes, D.J.; Lich, B.; Wieringa, B.; Fransen, J. Advantages of indium–tin oxide-coated glass slides in correlative scanning electron microscopy applications of uncoated cultured cells. J. Microsc. 2009, 233, 353–363. [Google Scholar] [CrossRef]
- Shah, S.S.; Howland, M.C.; Chen, L.-J.; Silangcruz, J.; Verkhoturov, S.V.; Schweikert, E.A.; Parikh, A.N.; Revzin, A. Micropatterning of proteins and mammalian cells on indium tin oxide. ACS Appl. Mater. Interfaces 2009, 1, 2592–2601. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.B.; Pastor, N.C.; Soler, E.P.; Soltani, A.; Othmane, A. A Comparative Study on Surface Treatments in the Immobilization Improvement of Hexahistidine-tagged Protein on the Indium Tin Oxide Surface. J. Nanomed. Nanotechnol. 2016, 7, 56984–56996. [Google Scholar] [CrossRef] [Green Version]
- Leng, X.; Bollinger, A.T.; Božović, I. Purely electronic mechanism of electrolyte gating of indium tin oxide thin films. Sci. Rep. 2016, 6, 31239–31248. [Google Scholar] [CrossRef] [Green Version]
- Blinov, L.M.; Lazarev, V.V.; Palto, S.P.; Yudin, S.G. Electric fields and surface charge in a thin-film ferroelectric-dielectric switchable structure that were determined with a spectral probe. J. Exp. Theor. Phys. 2014, 118, 990–994. [Google Scholar] [CrossRef]
- Pirkanniemi, K.; Sillanpää, M. Heterogeneous water phase catalysis as an environmental application: A review. Chemosphere 2002, 48, 1047–1060. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, P.; Shao, T.; Wang, J.; Jin, L.; Li, X. Different nanostructured In2O3 for photocatalytic decomposition of perfluorooctanoic acid (PFOA). J. Hazard. Mater. 2013, 260, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.; He, K.; Duan, L.; Li, J.; Wang, L. Air pollution and its control in China. Front. Environ. Sci. Eng. China 2007, 1, 129–142. [Google Scholar] [CrossRef]
- Li, J.; Fu, H.; Fu, L.; Hao, J. Complete Combustion of Methane over Indium Tin Oxides Catalysts. Environ. Sci. Technol. 2006, 40, 6455–6459. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-F.; Chiou, B.-S. Effect of oxygen concentration in the sputtering ambient on the microstructure, electrical and optical properties of radio-frequency magnetron-sputtered indium tin oxide films. Semicond. Sci. Technol. 1996, 11, 196–202. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. X-ray photoelectron spectroscopic characterization of chemically modified electrodes used as chemical sensors and biosensors: A review. Chemosensors 2015, 3, 70–117. [Google Scholar] [CrossRef] [Green Version]
- Arndt, B.; Sellschopp, K.; Creutzburg, M.; Grånäs, E.; Krausert, K.; Vonk, V.; Müller, S.; Noei, H.; Feldbauer, G.B.V.; Stierle, A. Carboxylic acid induced near-surface restructuring of a magnetite surface. Commun. Chem. 2019, 2, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Hirunsit, P.; Toyao, T.; Siddiki, S.M.A.H.; Shimizu, K.; Ehara, M. Origin of Nb2O5 Lewis Acid Catalysis for Activation of Carboxylic Acids in the Presence of a Hard Base. ChemPhysChem 2018, 19, 2848–2857. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; May, K. Disulfide bond structures of IgG molecules: Structural variations, chemical modifications and possible impacts to stability and biological function. MAbs 2012, 4, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Fass, D.; Thorpe, C. Chemistry and Enzymology of Disulfide Cross-Linking in Proteins. Chem. Rev. 2018, 118, 1169–1198. [Google Scholar] [CrossRef]
- Sela, M.; Lifson, S. On the reformation of disulfide bridges in proteins. Biochim. Biophys. Acta 1959, 36, 471–478. [Google Scholar] [CrossRef]
- Battistoni, C.; Gastaldi, L.; Lapiccirella, A.; Mattogno, G.; Viticoli, S. Octahedral vs tetrahedral coordination of the co(II) ion in layer compounds: CoxZn1−xIn2S4(O⩽x⩽0.46) solid solution. J. Phys. Chem. Solids 1986, 47, 899–903. [Google Scholar] [CrossRef]
- DeCollo, T.V.; Lees, W.J. Effects of Aromatic Thiols on Thiol−Disulfide Interchange Reactions That Occur during Protein Folding. J. Org. Chem. 2001, 66, 4244–4249. [Google Scholar] [CrossRef] [PubMed]
- Servagent-Noinville, S.; Revault, M.; Quiquampoix, H.; Baron, M.-H. Conformational Changes of Bovine Serum Albumin Induced by Adsorption on Different Clay Surfaces: FTIR Analysis. J. Colloid Interface Sci. 2000, 221, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhu, D.; Zhu, J.; Nussinov, R.; Ma, B. Local and global anatomy of antibody-protein antigen recognition. J. Mol. Recognit. 2018, 31, 2693. [Google Scholar] [CrossRef] [PubMed]
- Weinkauf, R.; Schanen, P.; Yang, D.; Soukara, S.; Schlag, E.W. Elementary Processes in Peptides: Electron Mobility and Dissociation in Peptide Cations in the Gas Phase. J. Phys. Chem. 1995, 99, 11255–11265. [Google Scholar] [CrossRef]
- Sheu, S.-Y.; Yang, D.-Y.; Selzle, H.L.; Schlag, E.W. Charge transport in a polypeptide chain. Eur. Phys. J. D At. Mol. Opt. Plasma Phys. 2002, 20, 557–563. [Google Scholar] [CrossRef]
- Schlag, E.W.; Sheu, S.-Y.; Yang, D.-Y.; Selzle, H.L.; Lin, S.H. Theory of Charge Transport in Polypeptides. J. Phys. Chem. B 2000, 104, 7790–7794. [Google Scholar] [CrossRef]
- Cordes, M.; Jacques, O.; Köttgen, A.; Jasper, C.; Boudebous, H.; Giese, B. Development of a Model System for the Study of Long Distance Electron Transfer in Peptides. Adv. Synth. Catal. 2008, 350, 1053–1062. [Google Scholar] [CrossRef]
- Heiland, G. Surface conductivity of semiconductors and its variation by adsorption, transverse electric fields and irradiation. Discuss. Faraday Soc. 1959, 28, 168–182. [Google Scholar] [CrossRef]
- Rao, S.N.; Manissero, D.; Steele, V.R.; Pareja, J. A Systematic Review of the Clinical Utility of Cycle Threshold Values in the Context of COVID-19. Infect. Dis. Ther. 2020, 9, 573–586. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattahi, A.; Afaghi, P.; Ghandi, K. Catalysis on Nanostructured Indium Tin Oxide Surface for Fast and Inexpensive Probing of Antibodies during Pandemics. Catalysts 2021, 11, 191. https://doi.org/10.3390/catal11020191
Fattahi A, Afaghi P, Ghandi K. Catalysis on Nanostructured Indium Tin Oxide Surface for Fast and Inexpensive Probing of Antibodies during Pandemics. Catalysts. 2021; 11(2):191. https://doi.org/10.3390/catal11020191
Chicago/Turabian StyleFattahi, Arash, Pooya Afaghi, and Khashayar Ghandi. 2021. "Catalysis on Nanostructured Indium Tin Oxide Surface for Fast and Inexpensive Probing of Antibodies during Pandemics" Catalysts 11, no. 2: 191. https://doi.org/10.3390/catal11020191