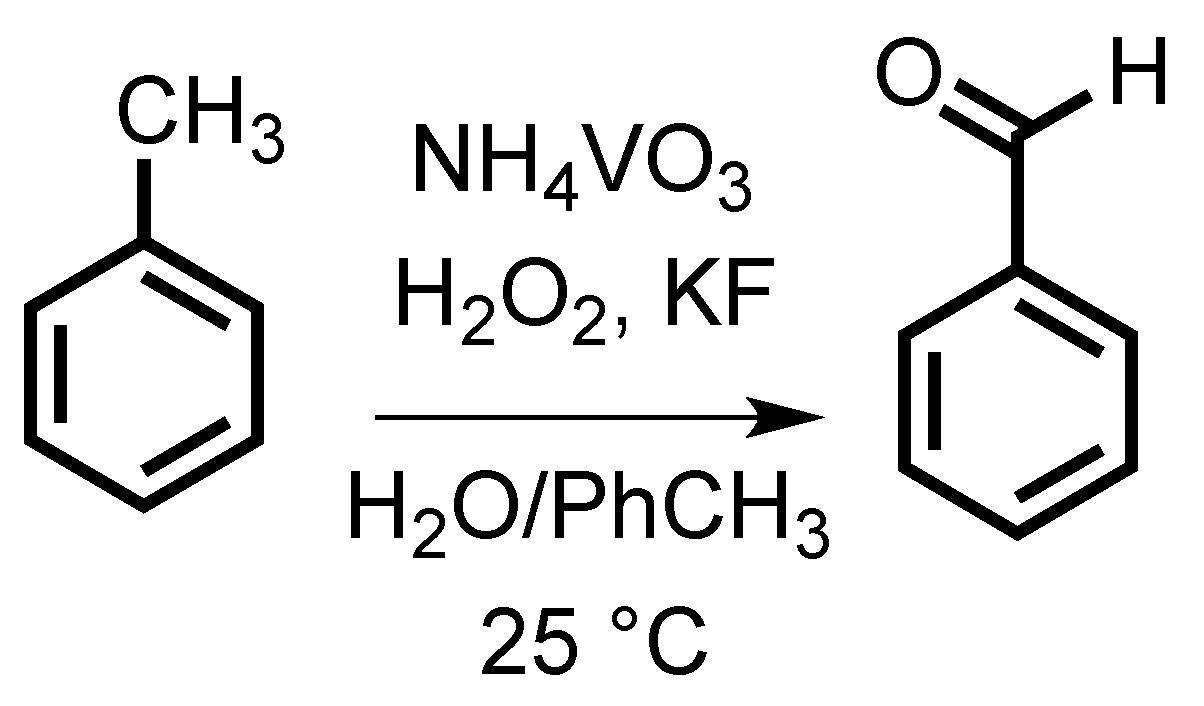
Sustainable Highly Selective Toluene Oxidation to Benzaldehyde
Abstract
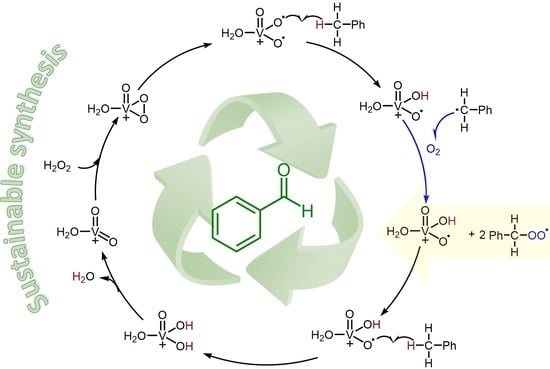
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Reactions in Biphasic System (PhCH3:H2O 1:1 v/v)
3.2. Reactions in H2O with a Stoichiometric Amount of Toluene
3.3. Reactions in Biphasic System (Organic Solvent:H2O 1:1 v/v)
3.4. Reactions in Biphasic System (Ionic Liquid:H2O 1:10 v/v)
3.5. Reactions in Biphasic System (PhCH3:H2O 1:1 v/v): Inorganic Salt Screening
3.6. Reactions in Biphasic System (PhCH3:H2O 1:1 v/v) under O2 Atmosphere
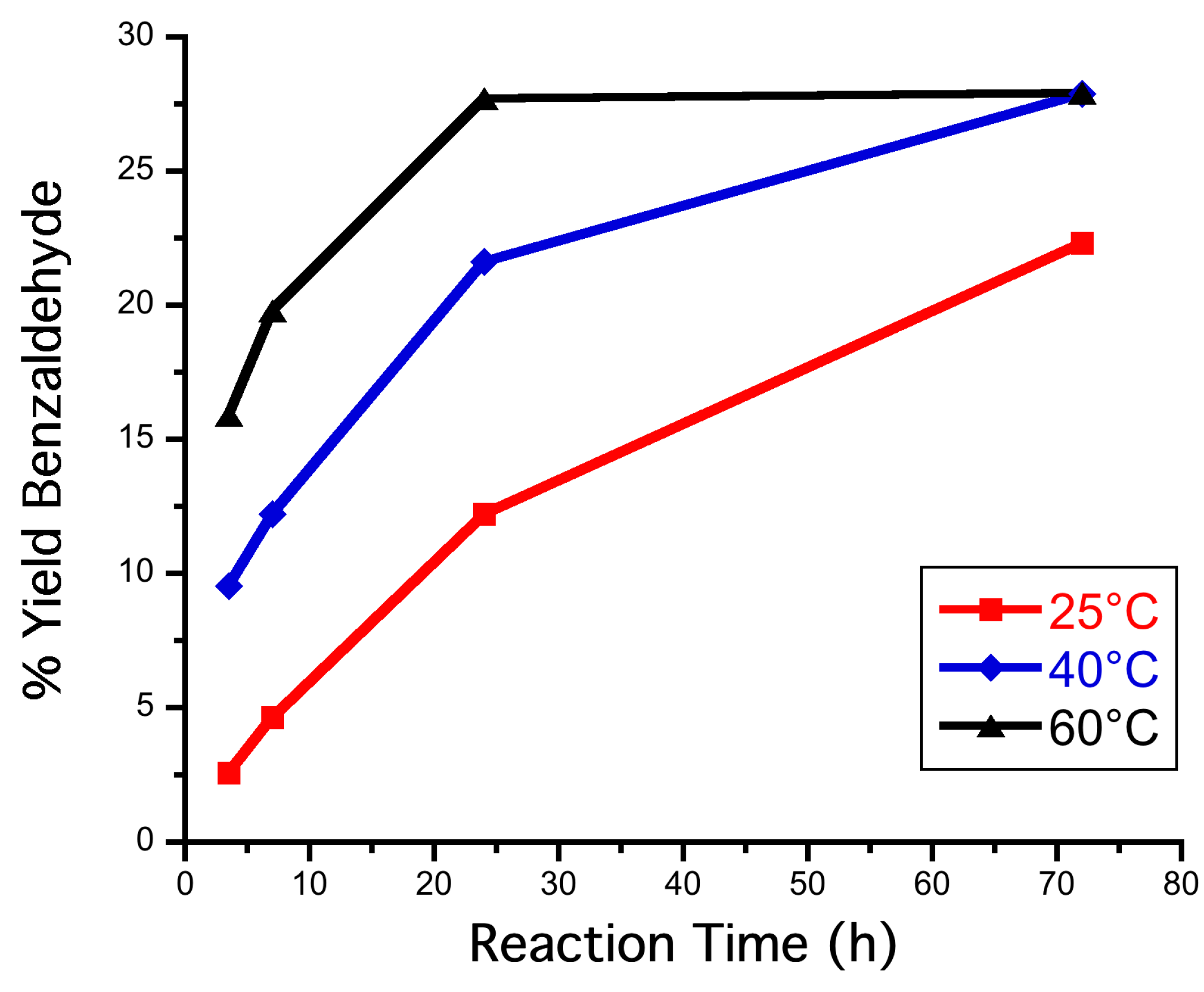
3.7. Reactions in Biphasic System (PhCH3:H2O 1:1 v/v) at Different Temperatures
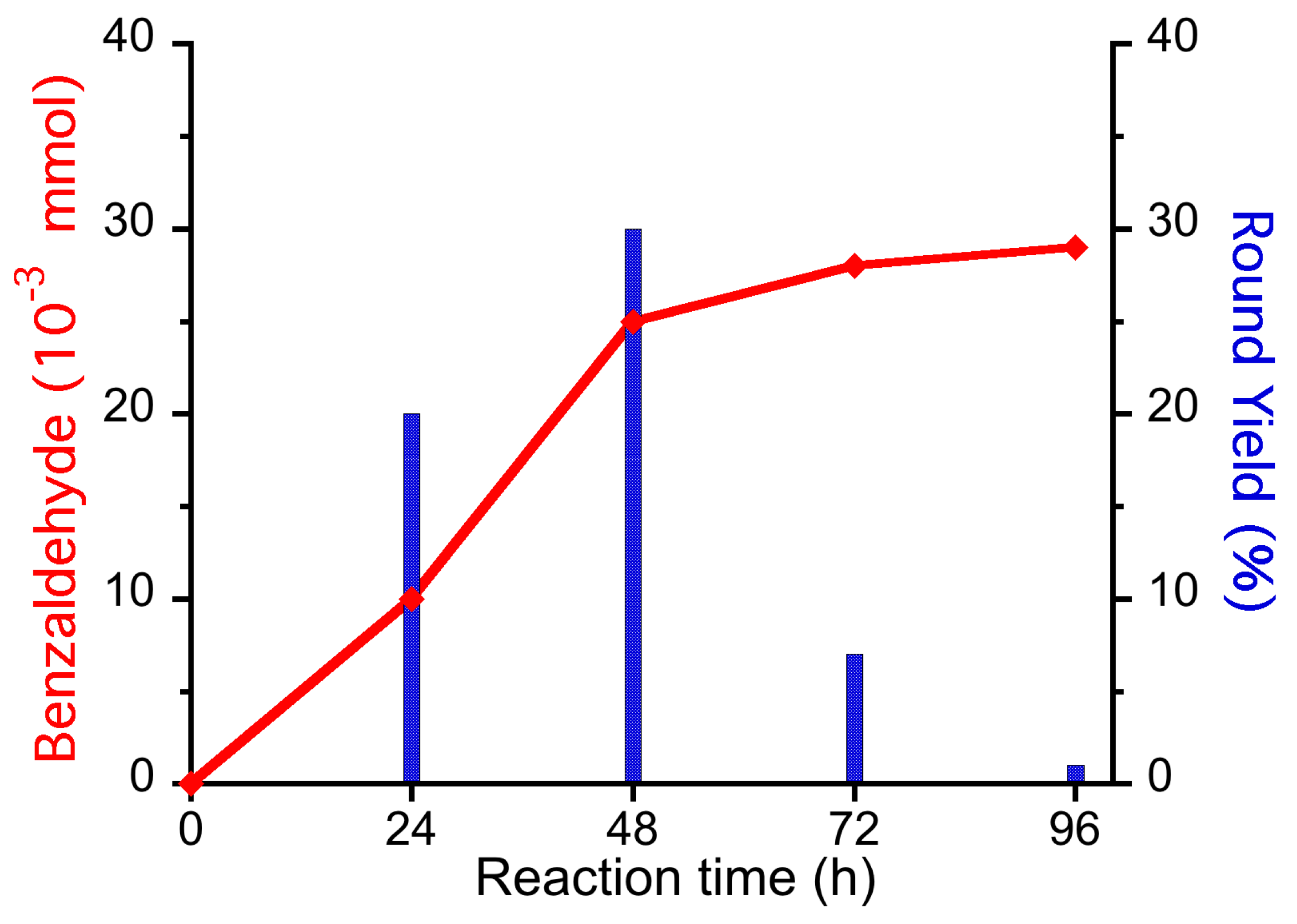
3.8. Reactions Reported in Biphasic System (PhCH3:H2O 1:1 v/v) at 60 °C
3.9. Reactions in Biphasic System (PhCH3:H2O 1:1 v/v) at 60 °C, under O2 Atmosphere
3.10. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kesavan, L.; Tiruvalam, R.; Rahim, M.H.A.; Bin Saiman, M.I.; Enache, D.I.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Taylor, S.H.; Knight, D.W. Solvent-Free Oxidation of Primary Carbon-Hydrogen Bonds in Toluene Using Au-Pd Alloy Nanoparticles. Science 2011, 331, 195–199. [Google Scholar] [CrossRef]
- Acharyya, S.S.; Ghosh, S.; Tiwari, R.; Sarkar, B.; Kumar, R.; Pendem, C.; Sasaki, T.; Bal, R. Preparation of CuCr2O4 Spinel Nanoparticles Catalyst for Selective Oxidation of Toluene to Benzaldehyde. Green Chem. 2014, 16, 2500–2508. [Google Scholar] [CrossRef]
- Mal, D.D.; Khilari, S.; Pradhan, D. Efficient and Selective Oxidation of Toluene to Benzaldehyde on Manganese Tungstate Nanobars: A Noble Metal-Free Approach. Green Chem. 2018, 20, 2279–2289. [Google Scholar] [CrossRef]
- Dionisio, A.P.; Molina, G.; Souza de Carvalho, D.; dos Santos, R.; Bicas, J.L.; Pastore, G.M. Natural Food Additives, Ingredients and Flavourings, Chapter 11. In Natural Flavourings from Biotechnology for Foods and Beverages; Woodhead Publishing: Shaston, UK, 2012. [Google Scholar]
- Pugh, S.; McKenna, R.; Halloum, I.; Nielsen, D.R. Engineering Escherichia Coli for Renewable Benzyl Alcohol Production. Metab. Eng. Commun. 2015, 2, 39–45. [Google Scholar] [CrossRef]
- Wheeler, O.H. Étard reaction: I. its scope and limitation with substituted toluenes. Can. J. Chem. 1958, 36, 667–671. [Google Scholar] [CrossRef]
- Partenheimer, W. Methodology and Scope of Metal/Bromide Autoxidation of Hydrocarbons. Catal. Today 1995, 23, 69–158. [Google Scholar] [CrossRef]
- Partenheimer, W. The High Yield Synthesis of Benzaldehydes from Benzylic Alcohols Using Homogeneously Catalyzed Aerobic Oxidation in Acetic Acid. Adv. Synth. Catal. 2006, 348, 559–568. [Google Scholar] [CrossRef]
- Lu, B.; Cai, N.; Sun, J.; Wang, X.; Li, X.; Zhao, J.; Cai, Q. Solvent-Free Oxidation of Toluene in an Ionic Liquid with H2O2 as Oxidant. Chem. Eng. J. 2013, 225, 266–270. [Google Scholar] [CrossRef]
- Guajardo, N.; Carlesi, C.; Aracena, Á. Toluene Oxidation by Hydrogen Peroxide in Deep Eutectic Solvents. ChemCatChem 2015, 7, 2451–2454. [Google Scholar] [CrossRef]
- Pembere, A.M.S.; Cui, C.; Anumula, R.; Wu, H.; An, P.; Liang, T.; Luo, Z. A Hexagonal Ni6 Cluster Protected by 2-Phenylethanethiol for Catalytic Conversion of Toluene to Benzaldehyde. Phys. Chem. Chem. Phys. 2019, 21, 17933–17938. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Chen, Y.; Dai, L. Synergistic Effect between Cu–Cr Bimetallic Oxides Supported on g-C3N4 for the Selective Oxidation of Toluene to Benzaldehyde. Catal. Sci. Technol. 2019, 9, 4441–4450. [Google Scholar] [CrossRef]
- Paul, P.; Ghosh, A.; Chatterjee, S.; Bera, A.; Alam, S.M.; Islam, S.M. Development of a Polymer Embedded Reusable Heterogeneous Oxovanadium(IV) Catalyst for Selective Oxidation of Aromatic Alkanes and Alkenes Using Green Oxidant. Inorg. Chim. Acta 2019, 492, 198–212. [Google Scholar] [CrossRef]
- Shi, G.; Xu, S.; Bao, Y.; Xu, J.; Liang, Y. Selective Aerobic Oxidation of Toluene to Benzaldehyde on Immobilized CoOx on SiO2 Catalyst in the Presence of N-Hydroxyphthalimide and Hexafluoropropan-2-ol. Catal. Commun. 2019, 123, 73–78. [Google Scholar] [CrossRef]
- Song, L.-N.; Ding, F.; Yang, Y.-K.; Ding, D.; Chen, L.; Au, C.-T.; Yin, S.-F. Synthesis of TiO2 /Bi2 MoO6 Composite for Partial Oxidation of Aromatic Alkanes under Visible-Light Illumination. ACS Sustain. Chem. Eng. 2018, 6, 17044–17050. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, B.; Wang, Z.; Lu, S.; Li, J.; Liu, Y.; Li, C. Photocatalytic Aerobic Oxidation of Toluene and Its Derivatives to Aldehydes on Pd/Bi2WO6. Chin. J. Catal. 2017, 38, 440–446. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Y.; Zeng, H.; Chen, Z.; Little, R.D.; Ma, C. A Promising Electro-Oxidation of Methyl-Substituted Aromatic Compounds to Aldehydes in Aqueous Imidazole Ionic Liquid Solutions. J. Electroanal. Chem. 2015, 751, 105–110. [Google Scholar] [CrossRef]
- Silva, G.C.; Carvalho, N.M.F.; Horn, A.; Lachter, E.R.; Antunes, O.A.C. Oxidation of Aromatic Compounds by Hydrogen Peroxide Catalyzed by Mononuclear Iron(III) Complexes. J. Mol. Catal. A Chem. 2017, 426, 564–571. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lv, M.; Zhou, X.-T.; Wang, J.-X.; Han, Q.; Ji, H.-B. A Novel System Comprising Metalloporphyrins and Cyclohexene for the Biomimetic Aerobic Oxidation of Toluene. Catal. Commun. 2018, 109, 76–79. [Google Scholar] [CrossRef]
- Xia, H.; Liu, Z.; Xu, Y.; Zuo, J.; Qin, Z. Highly Efficient V-Mo-Fe-O Catalysts for Selective Oxidation of Toluene to Benzaldehyde. Catal. Commun. 2016, 86, 72–76. [Google Scholar] [CrossRef]
- Deng, W.; Wan, Y.; Jiang, H.; Luo, W.-P.; Tan, Z.; Jiang, Q.; Guo, C.-C. Solvent-Free Aerobic Oxidation of Toluene over Metalloporphyrin/NHPI/CTAB: Synergy and Mechanism. Catal. Lett. 2014, 144, 333–339. [Google Scholar] [CrossRef]
- Maurya, M.R.; Sarkar, B.; Kumar, A.; Ribeiro, N.; Miliute, A.; Pessoa, J.C. New Thiosemicarbazide and Dithiocarbazate Based Oxidovanadium (IV) and Dioxidovanadium (V) Complexes. Reactivity and Catalytic Potential. New J. Chem. 2019, 43, 17620–17635. [Google Scholar] [CrossRef]
- Wang, X.; Cao, X.; Hu, X.; Li, G.; Zhu, L.; Hu, C. Effect of Zirconium Addition on Vanadium-Catalyzed Toluene Oxidation by H2O2 in CH3COOH. J. Mol. Catal. A Chem. 2012, 357, 1–10. [Google Scholar] [CrossRef]
- Wu, X.; Deng, Z.; Yan, J.; Zhang, F.; Zhang, Z. Effect of Acetic Anhydride on the Oxidation of Toluene to Benzaldehyde with Metal/Bromide Catalysts. Ind. Eng. Chem. Res. 2014, 53, 14601–14606. [Google Scholar] [CrossRef]
- Feng, J.-B.; Wu, X.-F. Transition Metal-Catalyzed Oxidative Transformations of Methylarenes: Transition Metal-Catalyzed Oxidative Transformations of Methylarenes. Appl. Organometal. Chem. 2015, 29, 63–86. [Google Scholar] [CrossRef]
- Gaster, E.; Kozuch, S.; Pappo, D. Selective Aerobic Oxidation of Methylarenes to Benzaldehydes Catalyzed by N -Hydroxyphthalimide and Cobalt(II) Acetate in Hexafluoropropan-2-ol. Angew. Chem. Int. Ed. 2017, 56, 5912–5915. [Google Scholar] [CrossRef]
- Galloni, P.; Mancini, M.; Floris, B.; Conte, V. A Sustainable Two-Phase Procedure for V-Catalyzed Toluene Oxidative Bromination with H2O2–KBr. Dalton Trans. 2013, 42, 11963–11970. [Google Scholar] [CrossRef]
- Rehder, D. The role of vanadium in biology. Metallomics 2015, 7, 730–742. [Google Scholar] [CrossRef]
- Kaim, W.; Schwederski, B.; Klein, A. Bioinorganic Chemistry—Inorganic Elements in the Chemistry of Life: An Introduction and Guide, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Conte, V.; Floris, B. Vanadium Catalyzed Oxidation with Hydrogen Peroxide. Inorg. Chim. Acta 2010, 363, 1935–1946. [Google Scholar] [CrossRef]
- Conte, V.; Floris, B. Vanadium and Molybdenum Peroxides: Synthesis and Catalytic Activity in Oxidation Reactions. Dalton Trans. 2011, 40, 1419–1436. [Google Scholar] [CrossRef] [PubMed]
- Floris, B.; Sabuzi, F.; Coletti, A.; Conte, V. Sustainable Vanadium-Catalyzed Oxidation of Organic Substrates with H2O2. Catal. Today 2017, 285, 49–56. [Google Scholar] [CrossRef]
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic Applications of Vanadium: A Mechanistic Perspective. Chem. Rev. 2019, 119, 2128–2191. [Google Scholar] [CrossRef]
- Sabuzi, F.; Pomarico, G.; Conte, V.; Galloni, P. Peroxo-Vanadium Complexes as Sustainable Catalysts in Oxidations, Halogenations and Other Organic Transformations. In Vanadium Catalysis; Sutradhar, M., da Silva, J.A.L., Pombeiro, A.J.L., Eds.; Royal Society of Chemistry: London, UK, 2021; Chapter 5; pp. 97–110. [Google Scholar]
- Sabuzi, F.; Pomarico, G.; Floris, B.; Valentini, F.; Galloni, P.; Conte, V. Sustainable Bromination of Organic Compounds: A Critical Review. Coordin. Chem. Rev. 2019, 385, 100–136. [Google Scholar] [CrossRef]
- Penín, L.; Gigli, M.; Sabuzi, F.; Santos, V.; Galloni, P.; Conte, V.; Parajó, J.C.; Lange, H.; Crestini, C. Biomimetic Vanadate and Molybdate Systems for Oxidative Upgrading of Iono- and Organosolv Hard- and Softwood Lignins. Processes 2020, 8, 1161. [Google Scholar] [CrossRef]
- Sabuzi, F.; Churakova, E.; Galloni, P.; Wever, R.; Hollmann, F.; Floris, B.; Conte, V. Thymol Bromination—A Comparison between Enzymatic and Chemical Catalysis. Eur. J. Inorg. Chem. 2015, 3519–3525. [Google Scholar] [CrossRef]
- Coletti, A.; Sabuzi, F.; Floris, B.; Galloni, P.; Conte, V. Efficient and Sustainable V-Catalyzed Oxidative Desulfurization of Fuels Assisted by Ionic Liquids. J. Fuel Chem. Technol. 2018, 46, 1121–1129. [Google Scholar] [CrossRef]
- Floris, B.; Sabuzi, F.; Galloni, P.; Conte, V. The Beneficial Sinergy of MW Irradiation and Ionic Liquids in Catalysis of Organic Reactions. Catalysts 2017, 7, 261. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, J.; Huang, C.; Lei, Z. Ionic Liquids in Selective Oxidation: Catalysts and Solvents. Chem. Rev. 2017, 117, 6929–6983. [Google Scholar] [CrossRef] [PubMed]
- Ceres, G.; Conte, V.; Mirruzzo, V.; Kolar, J.; Strlič, M. Imidazolium-Based Ionic Liquids for the Efficient Treatment of Iron Gall Inked Papers. ChemSusChem 2008, 1, 921–926. [Google Scholar] [CrossRef]
- Bonchio, M.; Conte, V.; Di Furia, F.; Modena, G.; Moro, S.; Edwards, J.O. Nature of the Radical Intermediates in the Decomposition of Peroxovanadium Species in Protic and Aprotic Media. Inorg. Chem. 1994, 33, 1631–1637. [Google Scholar] [CrossRef]
- Bortolini, O.; Conte, V. Vanadium (V) Peroxocomplexes: Structure, Chemistry and Biological Implications. J. Inorg. Biochem. 2005, 99, 1549–1557. [Google Scholar] [CrossRef]
- Conte, V.; Coletti, A.; Floris, B.; Licini, G.; Zonta, C. Mechanistic Aspects of Vanadium Catalysed Oxidations with Peroxides. Coord. Chem. Rev. 2011, 255, 2165–2177. [Google Scholar] [CrossRef]
- Ohkubo, K.; Mizushima, K.; Iwata, R.; Souma, K.; Suzuki, N.; Fukuzumi, S. Simultaneous Production of p-Tolualdehyde and Hydrogen Peroxide in Photocatalytic Oxygenation of p-Xylene and Reduction of Oxygen with 9-Mesityl-10-Methylacridinium Ion Derivatives. Chem. Commun. 2010, 46, 601–603. [Google Scholar] [CrossRef]
- Chakravorti, M.C.; Saricar, A.R. Studies on Oxoperoxofluorovanadates (V). J. Fluor. Chem 1976, 8, 255–262. [Google Scholar] [CrossRef]
- Schwendt, P.; Joniakova, D. Fluorooxoperoxo Complexes of Vanadium (V). Polyhedron 1984, 3, 287–290. [Google Scholar] [CrossRef]
- Damoyi, N.E. A DFT Mechanistic Study of the ODH of n-Hexane over Isolated H3VO4. Mol. Catal. 2018, 452, 83–92. [Google Scholar] [CrossRef]
- Bagno, A.; Conte, V.; Di Furia, F.; Moro, S. Ab Initio Calculations on Water−Peroxovanadium Clusters, VO(O2)(H2O)n+ (n = 1−5). Implications for the Structure in Aqueous Solution. J. Phys. Chem. A 1997, 101, 4637–4640. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
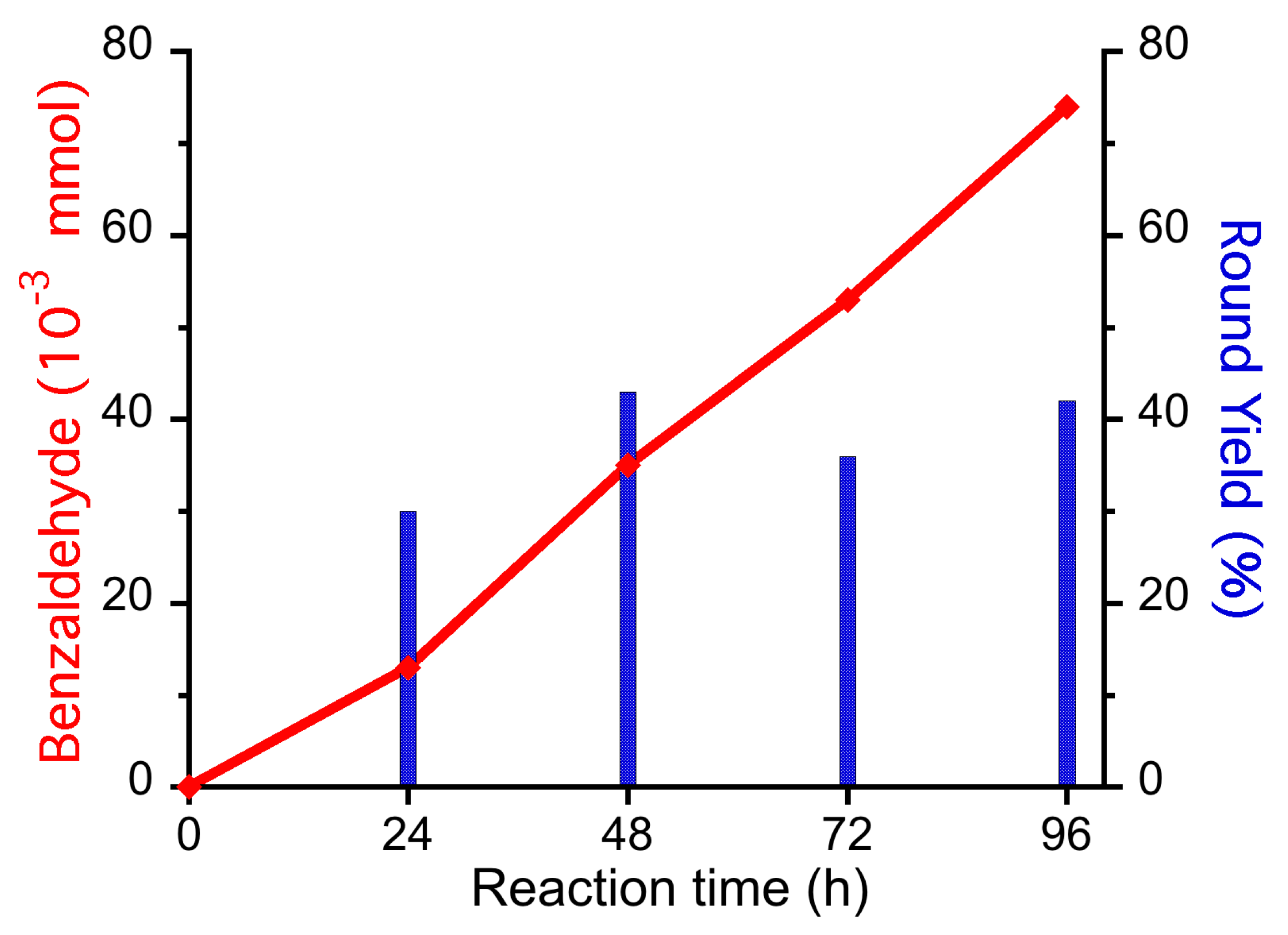


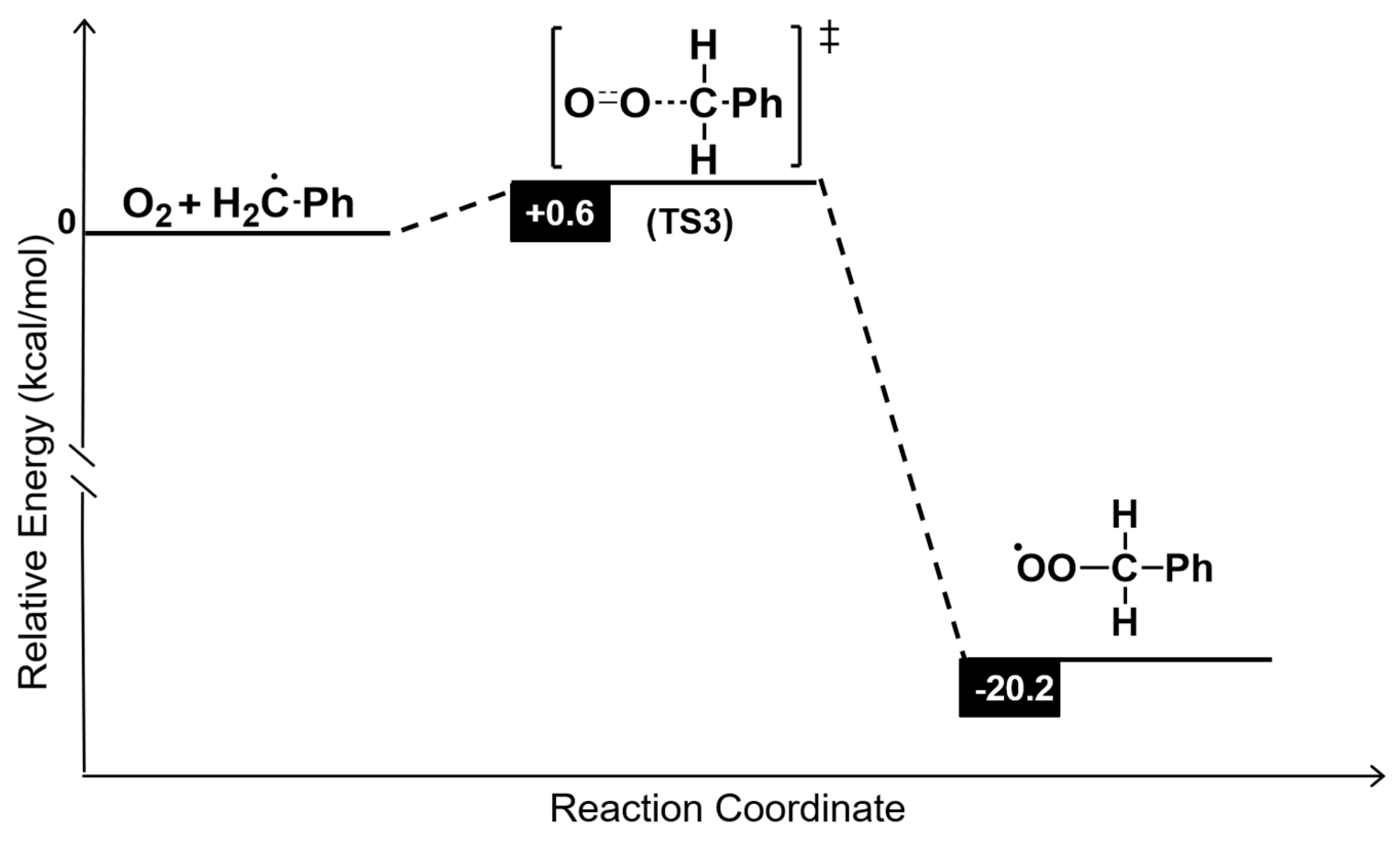
| Entry | H2O2 (mmol) | VO3− (mmol) | KF (mmol) | Benzaldehyde (*10−3 mmol) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 0.2 | 0.02 | 0.25 | 5.0 | 3 |
| 2 | 0.1 + 0.1 1 | 0.02 | 0.25 | 3.0 | 2 |
| 3 | 0.2 | 0.10 | 0.25 | 20.0 | 10 |
| 4 | 0.1 + 0.1 1 | 0.10 | 0.25 | 22.0 | 11 |
| 5 | 0.2 | 0.20 | 0.25 | 4.0 | 2 |
| 6 | 0.05 | 0.05 | 0.25 | 7.5 | 15 2 |
| 7 | 0.05 | 0.025 | 0.25 | 3.0 | 8 |
| 8 | 0.05 | 0.05 | - | - | - |
| Entry | Toluene (mmol) | H2O2 (mmol) | VO3− (mmol) | KF (mmol) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 0.05 | 0.05 | 0.05 | 0.25 | 1 |
| 2 | 0.5 | 0.05 | 0.05 | 0.25 | 1 |
| 3 | 0.5 | 0.5 | 0.05 | 0.25 | 1 |
| 4 | 0.5 | 0.05 | 0.05 | 0.25 | 12 1 |
| 5 2 | 0.5 | 0.05 | 0.05 | 0.25 | 0 |
| 6 3 | 0.5 | 0.05 | 0.05 | 0.25 | 0.5 |
| 7 4 | 0.5 | 0.05 | 0.05 | 0.25 | 24 |
| Entry | Salt | Yield (%) |
|---|---|---|
| 1 | KF | 15 |
| 2 | LiF | 12 |
| 3 | NaF | 10 |
| 4 | CsF | 11 |
| 5 | TBAF | 10 |
| 6 | NaCl | 3 |
| 7 | KCl | 4 |
| 8 | KBr | 19 |
| Entry | H2O2 (mmol) | VO3− (mmol) | KF (mmol) | Benzaldehyde (*10−3 mmol) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 0.05 | 0.05 | 0.05 | 10.0 | 20 |
| 2 | 0.05 | 0.025 | 0.05 | 5.4 | 11 |
| 3 | 0.05 | 0.025 | 0.025 | 9.4 | 19 |
| 4 | 0.05 | 0.01 | 0.01 | 1.5 | 4 |
| Entry | H2O2 (mmol) | VO3− (mmol) | KF (mmol) | Benzaldehyde (*10−3 mmol) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 0.05 | 0.05 | 0.05 | 13.0 | 33 |
| 2 | 0.05 | 0.05 | - | 19.4 | 40 |
| 3 | 0.05 | 0.025 | - | 15.0 | 30 |
| 4 | 0.05 | 0.01 | 1.7 | 5 | |
| 5 | - | 0.05 | - | - | - |
| Entry | Atmosphere | H2O2 (mmol) | VO3− (mmol) | KF (mmol) | Benzaldehyde (*10−3 mmol) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | O2 | 0.05 | 0.025 | - | 15.4 | 31 |
| 2 | air | 0.05 | 0.025 | 0.025 | 16.3 | 33 |
| 3 | O2 | 0.05 | 0.01 | - | 14.0 | 28 |
| 4 | air | 0.05 | 0.01 | 0.01 | 14.6 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentini, F.; Ferracci, G.; Galloni, P.; Pomarico, G.; Conte, V.; Sabuzi, F. Sustainable Highly Selective Toluene Oxidation to Benzaldehyde. Catalysts 2021, 11, 262. https://doi.org/10.3390/catal11020262
Valentini F, Ferracci G, Galloni P, Pomarico G, Conte V, Sabuzi F. Sustainable Highly Selective Toluene Oxidation to Benzaldehyde. Catalysts. 2021; 11(2):262. https://doi.org/10.3390/catal11020262
Chicago/Turabian StyleValentini, Francesca, Giacomo Ferracci, Pierluca Galloni, Giuseppe Pomarico, Valeria Conte, and Federica Sabuzi. 2021. "Sustainable Highly Selective Toluene Oxidation to Benzaldehyde" Catalysts 11, no. 2: 262. https://doi.org/10.3390/catal11020262
APA StyleValentini, F., Ferracci, G., Galloni, P., Pomarico, G., Conte, V., & Sabuzi, F. (2021). Sustainable Highly Selective Toluene Oxidation to Benzaldehyde. Catalysts, 11(2), 262. https://doi.org/10.3390/catal11020262