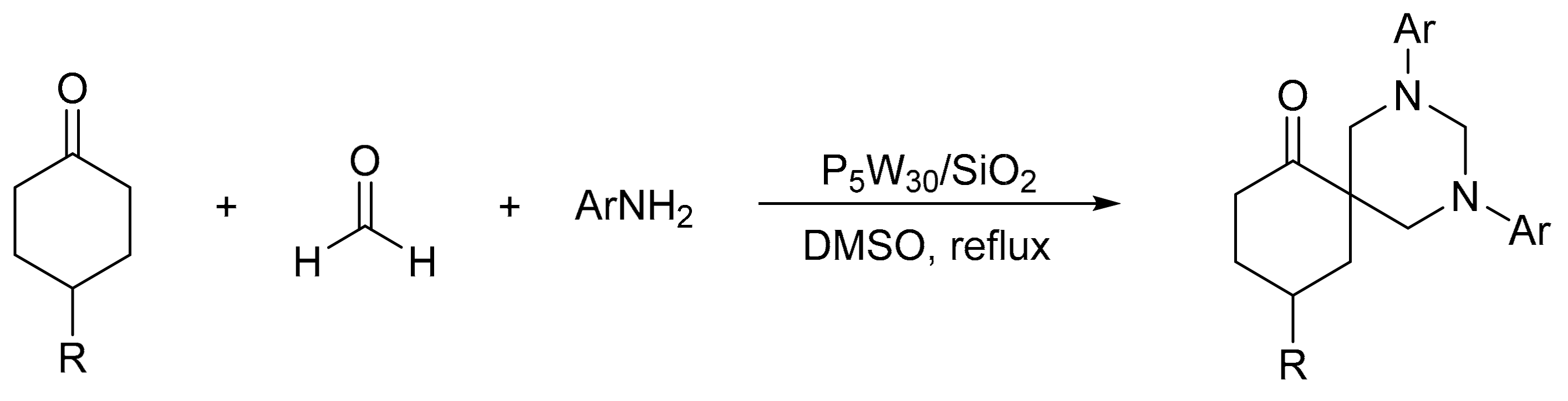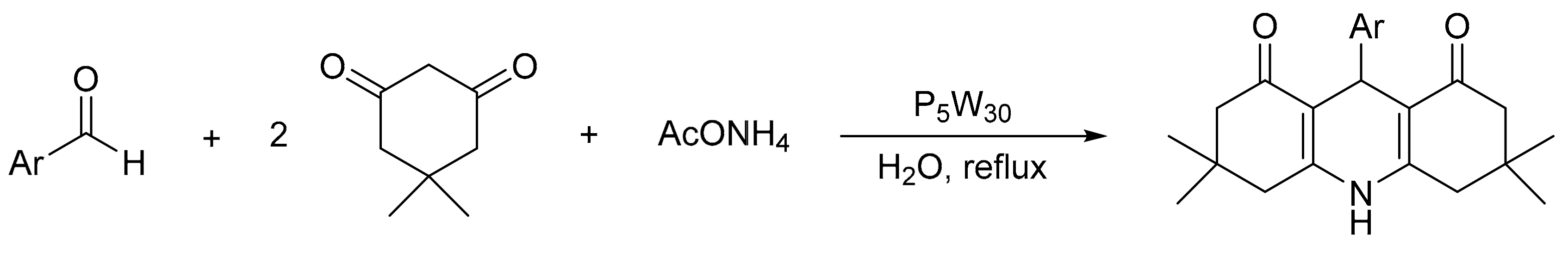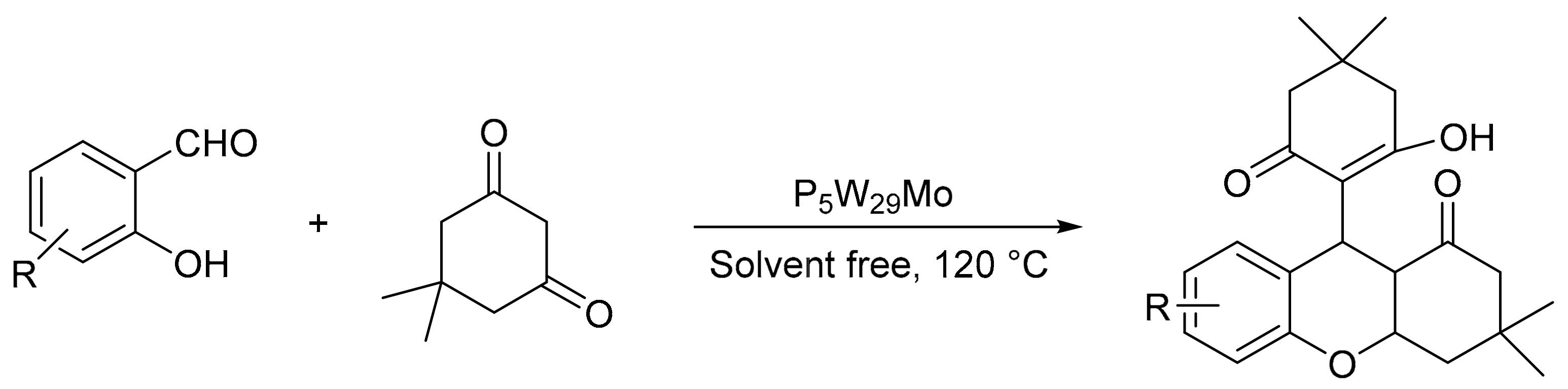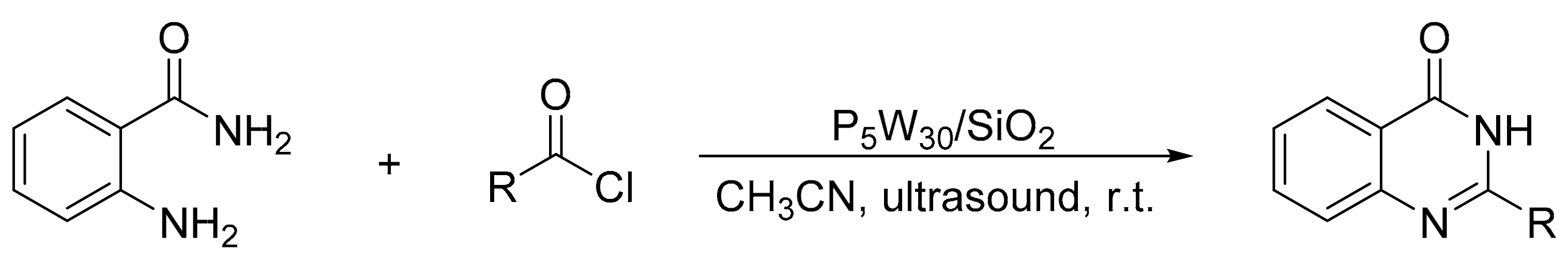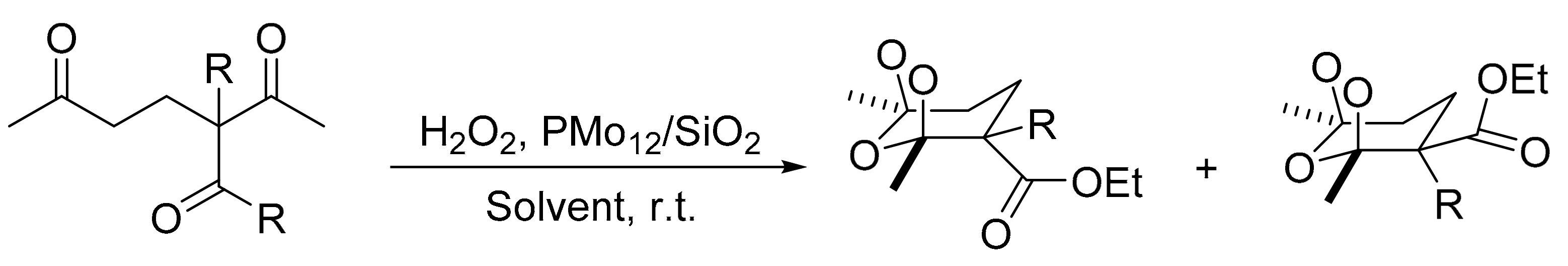Abstract
Over the past two decades, polyoxometalates (POM) have received considerable attention as solid catalysts, due to their unique physicochemical characteristics, since, first, they have very strong Bronsted acidity, approaching the region of a superacid, and second, they are efficient oxidizers that exhibit rapid redox transformations under fairly mild conditions. Their structural mobility is also highlighted, since they are complex molecules that can be modified by changing their structure or the elements that compose them to model their size, charge density, redox potentials, acidity, and solubility. Finally, they can be used in substoichiometric amounts and reused without an appreciable loss of catalytic activity, all of which postulate them as versatile, economic and ecological catalysts. Therefore, in 2009, we wrote a review article highlighting the great variety of organic reactions, mainly in the area of the synthesis of bioactive heterocycles in which they can be used, and this new review completes that article with the contributions made in the same area for the period 2010 to 2020. The synthesized heterocycles to be covered include pyrimidines, pyridines, pyrroles, indoles, chromenes, xanthenes, pyrans, azlactones, azoles, diazines, azepines, flavones, and formylchromones, among others.
1. Introduction
Environmental problems and social pressure currently poses the chemical industry with one of the greatest challenges throughout its history, which is to redesign existing industrial processes and design new ones in compliance with the basic rules of sustainability [1]. Although it is true that the chemical industry has made an enormous contribution to the development of humanity, there are also quite a few unfortunate events in which it has caused serious problems to public health and the environment. There are many examples of this, but they all have in common that they were designed to solve a certain problem, without a more extensive analysis of its possible side effects, its impact on the environment, or its long-term life span. All this forced the chemical sector to carry out a critical self-analysis that led to the development of what we now know as Green Chemistry [2]. Subsequently, the chemical industry coined another important term “Technology/Sustainable Development”, which can be summarized as “sustainability is the goal” and “green chemistry is the means to achieve it” [3].
From its beginnings in the 1990s to the present, the field of Green Chemistry has grown in many ways, in which heterogeneous catalysis has been considered one of its most important pillars. Although heterogeneous processes have been used since 1930, its development was more conscious, with a careful vision of the future, and it was strengthened from the establishment of the principles of Green Chemistry, which promotes the use of catalytic over stoichiometric processes, and where homogeneous catalysis still presents several problems that must be addressed, since conventional catalysts such as H2SO4, HCl, HF, AlCl3, BF3, and ZnCl2 pose handling, containment, disposal and regeneration risks due to their corrosive and toxic nature [4,5]. However, heterogeneous catalysis has advantages such as easy separation of the reaction medium and reuse, and, in most cases, it is more selective, reducing the generation of unwanted by-products [6].
On the other hand, in organic synthesis, numerous reactions are carried out in an acid medium, which translates into an important use of acid catalysts both at the laboratory and industrial levels, and this leads to more and more procedures being investigated in which the homogeneous acid catalyst can be replaced by a heterogeneous one, due to the ecological advantages already mentioned [7]. So in recent years, organic transformations catalyzed by inorganic solids have gained importance throughout the world, and within these solids, heteropolyacids (HPA) and related compounds stand out [8]. This is demonstrated in the large number of publications on basic and applied research in fine chemical processes, since, thanks to their ionic structure, they have several available protons, which makes them extremely promising in catalysis because it gives them a very strong Brønsted acidity and suitable redox properties, which are modifiable, changing the chemical composition of the polyoxometalate. Because of this, HPAs have been used in a large number of highly selective organic reactions including esterification, dehydration, cyclization, oxidation of amines, epoxidation of olefins, and other processes such as photocatalysis, water decontamination, biomass conversion, and oxidative desulfurization of fuels, among others. Furthermore, they have been used in both heterogeneous and homogeneous systems [9,10,11,12,13,14,15,16,17]. All these reactions in which they have been used can find wide applications in fine chemical production such as fragrances, pharmaceuticals, and food [18,19].
HPAs are a large family of compounds made up of nanometric transition metal-oxygen octahedral anions, which form a stable and compact backbone of polymeric oxoanions as basic structural units and present a great variety of structures. The most researched and used HPAs are those with a Keggin-type structure, especially as acid catalysts, due to their availability and chemical stability [20]. Their primary structure is represented by the formula [XM12O40]x−8, where (X) is the central atom, with P5+ and Si4+ being the most common and (M) the metal ion or heteroatom, Mo6+ and W6+ being the most used. One or more M ions can be replaced by other metal ions, for example, V5+, Co2+, Zn2+, etc., to change their properties such as acidity, redox potential, or solubility [21,22].
Recently, other HPA structures have been a major focus of attention, such as the Wells–Dawson and Preyssler structures [23,24]. The general formula of the Wells–Dawson heteropolyanion is [(Xn+)2M18O62](16−2n)−, where Xn+ represents a central atom, such as phosphorous (V), arsenic (V), sulfur (VI), and fluorine, surrounded by a cage of M addenda atoms, such as tungsten (VI), molybdenum (VI) or a mixture of elements, each of them composing MO6 (M-oxygen) octahedral units. The Preyssler polyanion consists of a cyclic assembly of five PW6O22 units, each derived from the Keggin anion, [PW12O40]3−, by eliminating two sets of shared WO6 octahedra with three corners [16].
On the other hand, heterocyclic compounds are important because they have wide applications in pharmaceutical chemistry and in the fine chemical industry, which is evidenced in that more than 90% of new drugs contain heterocyclic rings, and this generates great interest in synthetic chemists for redesigning the classic syntheses towards cleaner technologies and better yields, as well as obtaining new skeletons and studying their activities [25].
Therefore, this review is the continuation of a previous review published in 2009 and brings together the reports on the advances and new designs of catalysts based on heteropolyacids and their application in the catalytic synthesis of het-erocycles [26], updated with the contributions made in the same area for the for the period 2010 to 2020.
2. Pyrimidines
Nitrogen-containing heterocycles are widely distributed in nature and in living substances, and perform a vital function in a large number of biochemical and metabolic reactions. Within this group are pyrimidines, which are a class of six-membered heterocycles that contain two nitrogen atoms in positions 1 and 3 and are an important source for the discovery of many drugs due to their versatile properties such as anticancer, antiviral, antibacterial and insecticide activity [27,28,29,30,31,32]. This molecule can be functionalized with other biologically important molecule residues to improve its bioactivity, as in the case of 3,4-dihydropyrimidine-2 (1H) -ones (thiones), an important compound in organic synthesis due to its bioactivity [33]. The most suitable procedure for their preparation is the Biginelli synthesis, a multicomponent condensation reaction in an acid medium that involves an aldehyde, a β-dicarbonylcompound and urea or thiourea [34]. The relevance of this procedure has encouraged the search for more environmentally friendly conditions in the last years.
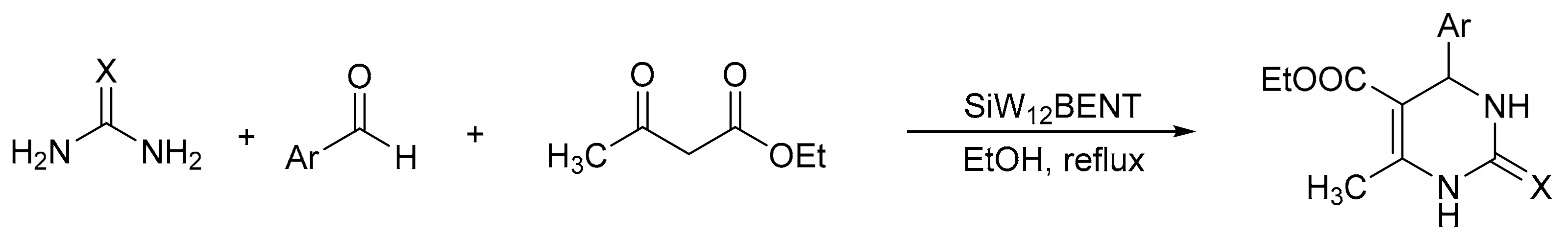
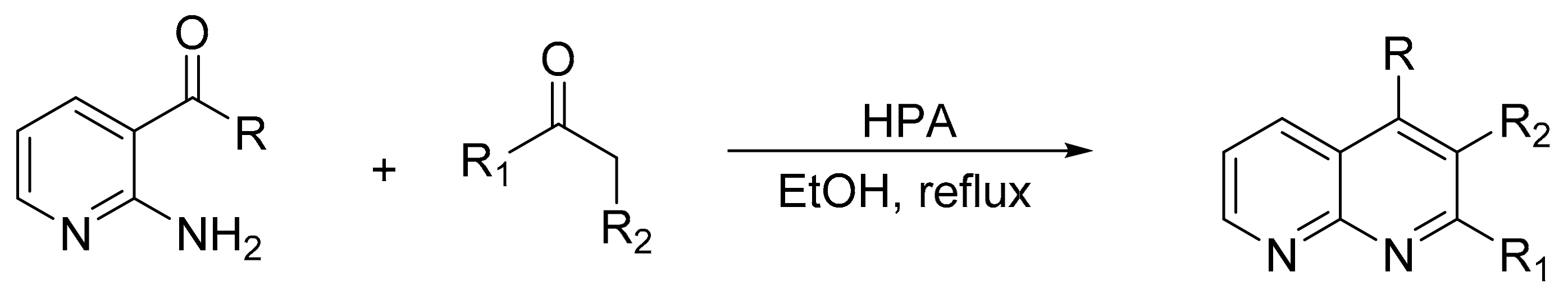
V. Chopda demonstrated the use of 12-tungstosilicic acid (H4[SiW12O40]) over natural bentonite (SiW12BENT) to synthetize 14 pyrimidinones with yields between 81 and 92%, in a procedure that involves the three-component condensation reaction (urea, ethyl acetoacetate, and aldehydes) in ethanol reflux and a catalyst (Scheme 1) [5]. The optimal reaction conditions for the synthesis of dihydropyrimidinones were 0.09 g of SiW12BENT (10% of active phase over the bentonite), aldehydes (2 mmol), ethylacetoacetate (2 mmol), and urea (2.4 mmol), which were refluxed in ethanol (25 mL) for 5 h. The catalyst was recovered and reused for five cycles without significant loss of activity (89–87%).
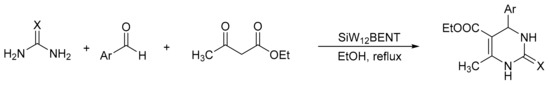
Scheme 1.
General procedure for Biginelli derivative synthesis.
The same reaction was carried out by the group of R. Tayebee et al. [35] using an organic–inorganic hybrid material based on Santa Bárbara Amorphous #15 (SBA-15) modified with piperazine as an anchoring compound to immobilize H5PW10V2O40 (PW10V2), as catalyst. The material was named PW10V2/Pip-SBA-15. The general procedure to synthesize 3,4-dihydropyrimidin-2 (1H) -ones consisted of heating a mixture of ethyl acetoacetate (2 mmol), aldehyde (2 mmol), urea (2.5 mmol), and PW10V2/Pip-SBA-15 (0.6 g of catalyst corresponding to 2 mol% of PW10V2) at 100 °C in solvent-free conditions, for 0.33–4 h, and the yields were between 47 and 90%.
Freitas et al. [7] carried out the Biginelli synthesis catalyzed by the joint use of HPA and ionic liquids. The new materials catalyzed the synthesis of different 3,4-dihydropyrimidine-2(1H)-ones without solvent with yields between 70 and 99%, for a total of ten compounds. The ionic liquids caused a positive effect because the yield of the reaction increased, and the synthesis time decreased. The optimal reaction conditions were: 1 mmol of each reagent, catalyst: 28% SiW12/Y (50 mg), ionic liquid: 1-n-butyl-3-methylimidazolium hexafluorophosphate (BMI·PF6, 0.5 mL), and 100 °C for 1 h.
D’alessandro et al. [36] reported a similar procedure for the synthesis of dihydropyrimidinones. In this work, molybdophosphoric acid was modified by partially exchanging molybdenum atoms for vanadium, bismuth, or vanadium–bismuth atoms (PMo11V, PMo11Bi, and PMo10VBi) and the resulting acids were tested in the Biginelli synthesis of 3,4-dihydropyrimidin-2-(1H)-ones under solvent-free conditions. Although V-doped HPAs are more used in oxidation reactions than as acid catalysts, there are reports on their use in the acylation of alcohols and the nitration of phenol, which led to their evaluation in these reactions. It was found that the inclusion of V, Bi, and Bi–V in the structure of PMox notably improved the catalyst activity, presenting a greater number of acidic sites in the following order: PMo10BiV > PMo11V > PMo11Bi > PMo12, and this was correlated with the performance of the reaction. The general procedure for the synthesis of 3,4-dihydropyrimidin-2(1H)-one consisted of a mixture of ethyl acetoacetate (2 mmol), 4-chlorobenzaldehyde (2 mmol), urea (3 mmol), and bulk HPA (1 mol%) without solvent and heating at 80 °C for 1 h. Under these conditions, the most active catalyst was PMo10BiV, and 12 compounds were synthesized with yields between 80 and 98% and high selectivity. The catalyst was reused without a significant decrease in activity.
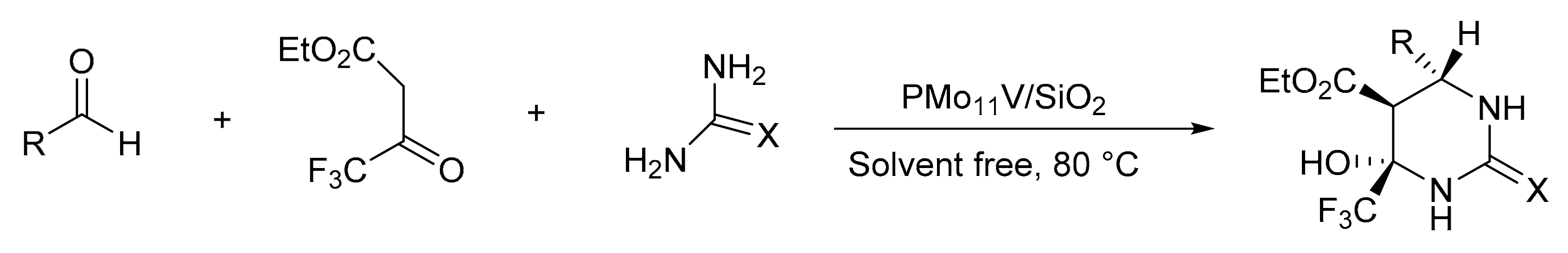
Our research group reported a Biginelli reaction variant. In this case, we used ethyl trifluoromethyl acetoacetate instead of ethyl acetoacetate. For this, Keggin H3PMo11VO40 (PMo11V) HPAs were synthesized by the hydrothermal synthesis method and were later included in silica by the sol-gel method [37]. The materials were adequately characterized and their catalytic activity was evaluated by the synthesis of a series of highly substituted hexahydropyrimidines. A mixture of ethyltrifluoroacetoacetate (1 mmol), aldehyde (1 mmol), urea (1.2 mmol), and the selected catalyst was heated at 80 °C for 1.5 h under solvent-free conditions. In total, 11 compounds were synthesized with yields between 75 and 90%. The catalyst was reused in four cycles without significant loss of activity (Scheme 2).
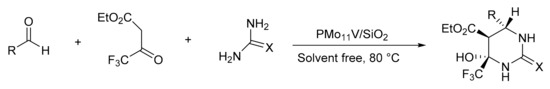
Scheme 2.
Synthesis of fluorinated hexahydropyrimidine.
Similarly, Preyssler heteropolyacid was included in a silica sol-gel matrix, and under similar reaction conditions, 17 trifluoromethyl-hexahydropyrimidine derivatives were obtained with yields between 87 and 97%. Among the benefits of this method, a low environmental impact, excellent yields, high diastereoselectivity (only one diastereoisomer was obtained, see Scheme 2), shorter synthesis times, and easy recovery of the catalyst by filtration stand out [24].
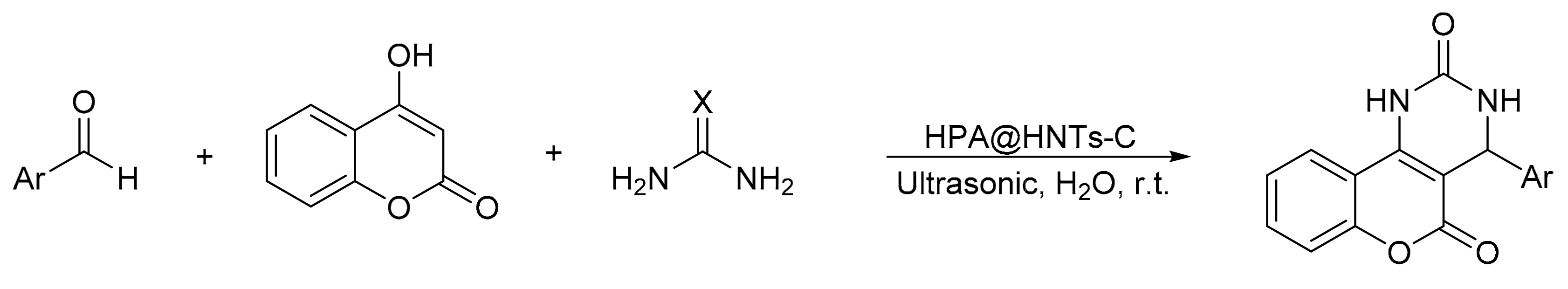
S. Sadjadi et al. [9] reported a modification of the Biginelli reaction, using 4-hydroxicoumarin as a reaction substrate. They prepared a novel hybrid catalyst based on the incorporation of phosphomolybdic acid (H3PMo12O40) into creatin-functionalized halloysite clay nanotubes (HNTs-C) designated (HPA@HNTs-C), then they prepared a series of benzopyranopyrimidines under ultrasonic irradiation in aqueous media (Scheme 3). The method involves the suspension of an equimolar amount of aldehyde, urea or thiourea, 4-hydroxicoumarin, and 0.03 g of the catalyst in water. The suspension was subjected to ultrasonic irradiation (200 W) at 20 °C for a reaction time between 4 and 22 min. Overall, seven compounds of benzopyranopyrimidines were obtained with yields between 80 and 95%. By means of hot filtration, the solid product could be recovered, and it was verified that there was no HPA leaching in the hybrid materials, which shows that they are heterogeneous and reusable catalysts.
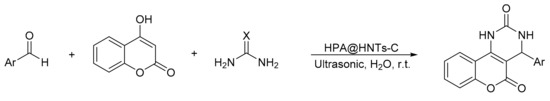
Scheme 3.
Synthesis of benzopyranopyrimidine derivatives.
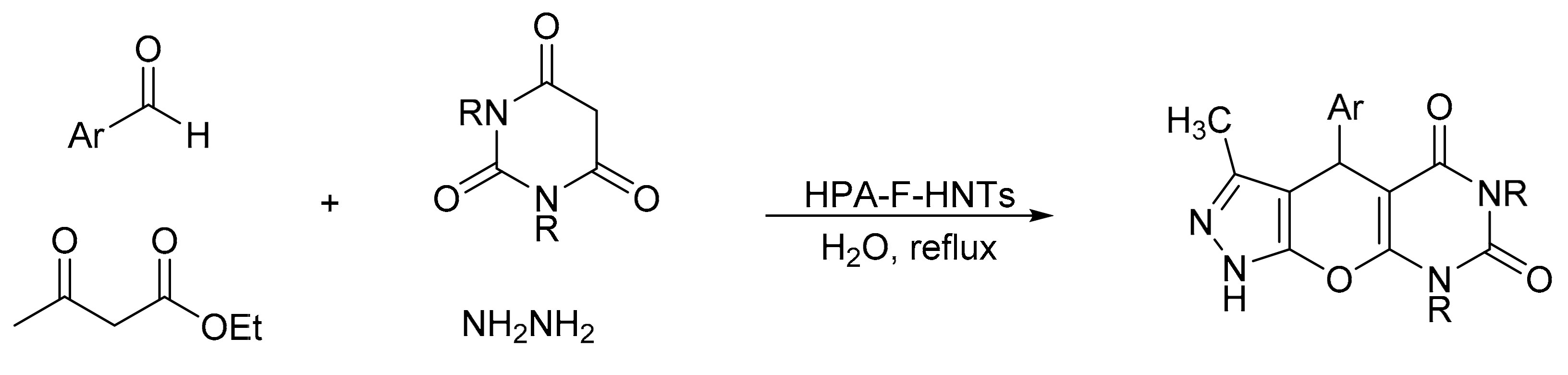
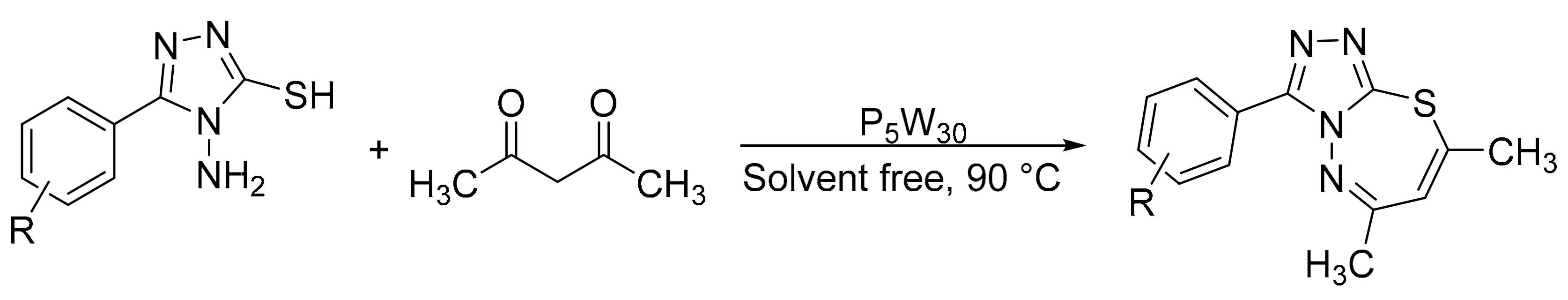
Heravi et al. [11] reported a new hybrid material based on the Keggin-type heteropolyacid, tungstophosphoric acid, supported on amine-functionalized halloysite nanoclay (HPA-F-HNTs), and its use in the green synthesis of pyrazolopyranopyrimidines by a four-component domino process (Scheme 4). The reaction involves the mixture of barbituric acid (1 mmol), hydrazine hydrate (1.1 mmol), ethyl acetoacetate (1 mmol), and aldehydes (1 mmol) in the presence of a catalytic amount of new materials (0.03 g), and with water as solvent at reflux conditions. The data obtained show that the materials are efficient in the synthesis of the desired compounds with yields older than 90% and short reaction times (35–50 min). The heterogeneous catalyst was reused for three cycles without significant loss of activity.
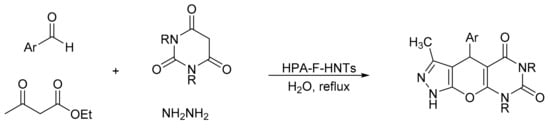
Scheme 4.
Synthesis of pyrazolopyranopyrimidines.
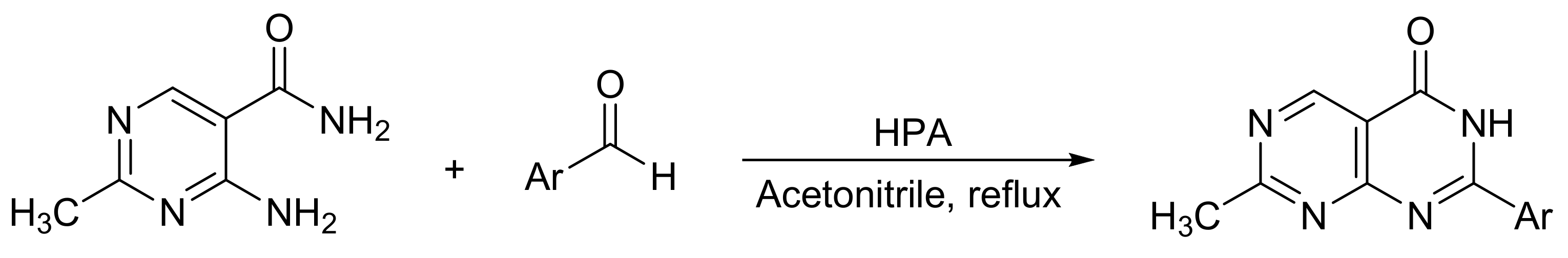
Fang et al. [38] reported a variant of Biginelli reaction for dihydropyrimidine synthesis. In this research, the catalytic activity of three bulk Keggin HPAs (H3PMo12O40, PMo12; H3PW12O40, PW12; and H5PMo10V2O40, PMo10V2) was evaluated in the synthesis of 7-methylpyrimido[4,5-d]pyrimidin-4(3H)-ones 2-substituted (Scheme 5). The reaction procedure consisted of mixing 4-amino-2-methylpyrimidine-5-carboxamide (2 mmol), aromatic aldehyde (2 mmol), and HPA (0.02 mmol) in acetonitrile (20 mL) under reflux conditions for 2 h. In this way, 14 compounds were obtained with yields between 79 and 93%, PMo10V2 being the most active catalyst. The procedure stands out because it is carried out under mild ration conditions, it is simple, and high yields are achieved.
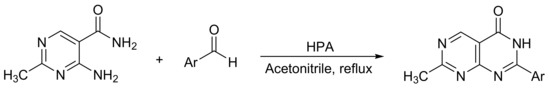
Scheme 5.
Synthesis of 2-substituted-7-methylpyrimido[4,5-d]pyrimidin-4(3H)-ones.
Derikvand et al. [39] reported a procedure in solvent-free conditions for the synthesis of 4,6-diarylpyrimidin-2(1H)-ones using H3PMo12O40 (PMo12) as catalyst (Scheme 6). A mixture of ketone (1 mmol), aldehyde (1 mmol), urea (1.5 mmol), trimethylsilyl chloride (1 mmol), and bulk PMo12 (2 mmol%) was stirred at 70 °C for 15–25 min. All compounds were synthesized whit yields between 90 and 95% and without the need for recrystallization.
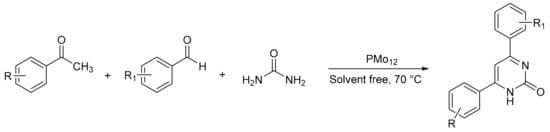
Scheme 6.
Synthesis of 4,6-diarylpyrimidin-2(1H)-ones.
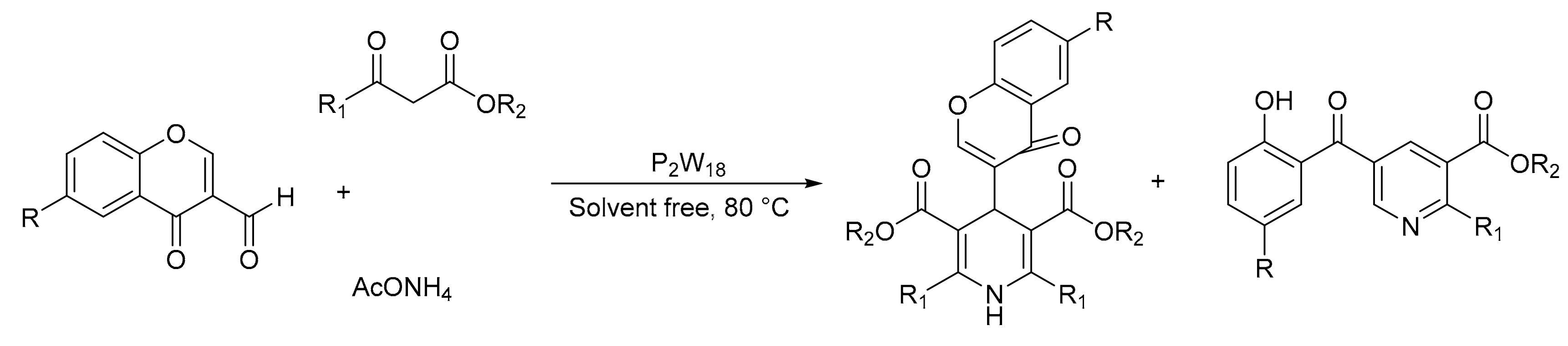
Similar results were obtained by H. Heravi et al. [40], who reported the synthesis of 4,6-diarylpyrimidin-2(1H)-ones, under similar reaction conditions, using a catalytic amount of Wells–Dawson type-heteropolyacid in bulk form (H6P2W18O62.18H2O, P2W18), under solvent-free conditions, reporting yields between 90 and 95% and times between 5 and 10 min.
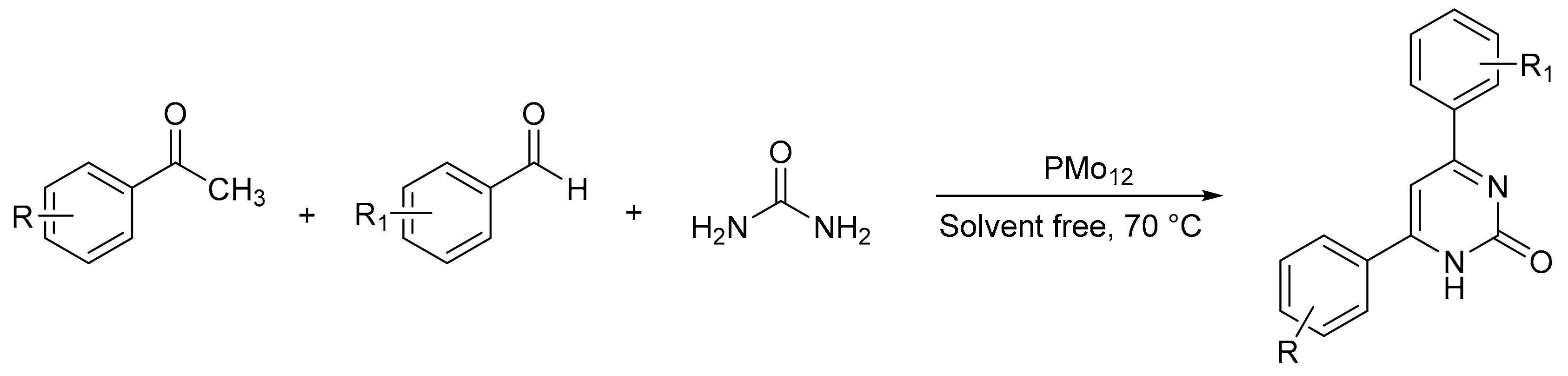
Heravi et al. [41] prepared catalysts based on Preyssler HPAs supported on silica nanoparticles (H14[NaP5W30O110])/SiO2, P5W30/SiO2), and evaluated them in the synthesis of 1,3-diaryl-5-spirohexahydropyrimidines by means of a one-pot condensation reaction. A mixture of ketone (1 mmol), aniline (3 mmol), formaldehyde (6 mmol), and a catalytic amount of P5W30SiO2 was refluxed in 5 mL of DMSO for times between 15 and 35 min. In total, eight compounds were obtained with yields between 90 and 98% (Scheme 7). The catalyst was easily recovered by filtration and reused for five times with very low yield reduction.
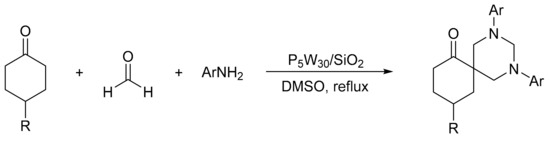
Scheme 7.
Synthesis of 1,3-diaryl-5-spirohexahydropyrimidines.
3. Pyridines
Heterocyclic systems bearing 1,4-dihydropyridines are compounds of great relevance in organic synthesis due to their pharmacological and biological activities. In fact, nifedipine is one of the most representative compounds of this group, since it is the principal calcium channel blocker and is used as a medicine for reducing vascular resistance and arterial pressure [42,43]. They also have antioxidant properties, protecting against oxidative stress and related disorders [44]. The hydropyridine core is present in other heterocyclic systems and has relevant bioactivities including neuroprotective [45] and chemosensitizing [46], anti-inflammatory [47], antitubercular [48], anti-ischemic [49,50], hepatoprotective, cardiovascular [51,52], antidiabetic [53], and antianginal activities [54], among others.
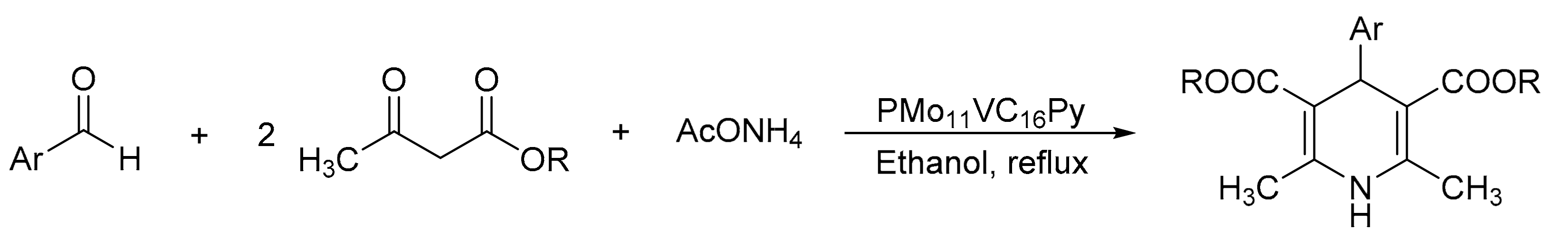
Palermo et al. [18] reported the synthesis of new micellar Keggin heteropolyacid catalysts (HPA-C16Py) using hexadecyltrimethylammonium bromide (cetyltrimethylammonium bromide-CTAB), 1-hexadecylpyridinium chloride, and Keggin heteropolyacids H3PMo12O40 and H4PMo11VO40 as precursors (PMo12C16Py and PMo11VC16Py). They were appropriately characterized and used in the preparation of a series of bioactive 1,4-dihydropyridine derivatives, such as nifedipine and nemadipine B (Scheme 8). The general procedure consisted of a mixture of methylacetoacetate (2 mmol), 2-nitrobenzaldehyde (1 mmol), ammonium acetate (1.3 mmol), and catalyst (30 mg, 1.5 mmol%), under reflux conditions in ethanol (8 mL) for 8 h. Under these conditions, a series of seven 1,4-dihydropyridine derivatives were obtained with yields of 70 to 81% using the most active catalyst (PMo11VC16Py, 30 mg, 20% w/w). Using the same material, 1,4-asymmetric dihydropyridines such as nitrendipine can be obtained by a sequence of steps with very good yield (78%). The materials were used for five cycles without a significant loss of activity (78–75%).
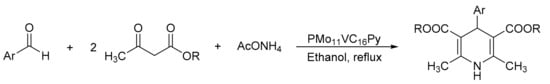
Scheme 8.
Synthesis of 1,4-dihydropyridines (general procedure for Hantzsch derivative synthesis).
Gharib et al. [55] reported the synthesis of 1,8-dioxodecahydroacridines (polyfunctionalized derivatives of fused 1,4-dihydropyridines) for the subsequent multicomponent synthesis between dimedone, β-dicarbonyl compounds, and ammonium acetate as substrates, using bulk Preyssler HPA as catalyst (H14[NaP5W30O110], P5W30) (Scheme 9). The Preyssler catalyst is easily synthesized, stable up to 300 °C, reusable, efficient, green, and inexpensive. The procedure involves the use of an equimolecular amount of substrates (the test was performed using 10 mmol of aldehyde, 0.01 g of catalyst, and 10 mL of water). The suspension was stirred at reflux for a time period between 130 and 180 min. Overall, seven acridine derivatives were obtained with yields between 82 and 94%, and the catalyst was recycled and reused five times with a small loss of activity (85–80%, using 4-florobenzaldehyde as a substrate).
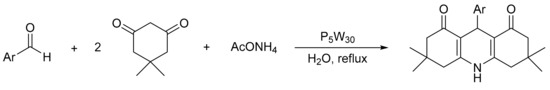
Scheme 9.
Hantzsch reaction for acridine synthesis.
A similar procedure for 1,8-dioxodecahydroacridines was reported by Baradaran-Sirjani et al. [56], using two Preyssler heteropolyacids, H14[NaP5W29MoO110] (P5W30) and H14[NaP5W30O110] (P5W29Mo). The general procedure consisted of a mixture of dimedone (2 mmol), aromatic aldehydes (1 mmol), ammonium acetate (1 mmol), and 0.03 g of catalyst at 110 °C without solvent. In total, eight compounds were prepared in a short time period of 20 min with yields between 80 and 95%. The authors studied the structure of the new molecule both experimentally and theoretically, by evaluating fluorescence properties in different solvents and temperatures, and density functional theory (DFT) calculations using the theoretical level B3LYP/6-31+G (d, p), respectively.
The Hantzsch condensation reaction makes it possible to obtain different heterocyclic compounds with biological activity from an aldehyde, a β-dicarbonyl compound and a source of ammonia. Our research group studied this reaction by adding 3-formylchromones as a substrate instead of a simple aldehyde, with the idea of obtaining dihydropyridines functionalized in the 4-position with the 3-chromonyl substituent. For this we uses heteropolyacids of the Wells–Dawson type (H6P2W18O62·24H2O, P2W18, 1 mol%) as catalyst, 3-formylchromones as an aldehyde component (1 mmol), a β-ketoester (1 mmol), and ammonium acetate (1 mmol), under solvent-free conditions at 80 °C. However, although the desired products were synthesized, functionalized pyridines were the largest compound obtained and became the alternative route for the formation of the dihydropyridine ring (Scheme 10). In total, 13 polysubstituted pyridines were obtained in short times between 15 and 30 min with yields between 60 and 99%, also with easy separation and recovery of catalyst to be reused [57].
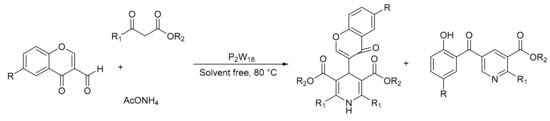
Scheme 10.
3-Formylcromones as substrate in the Hantzsch-like multicomponent condensation.
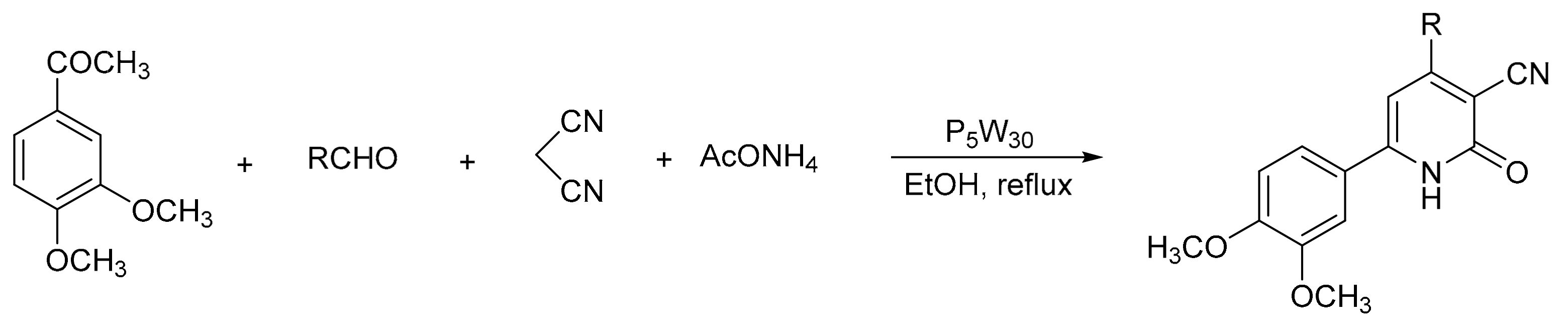
Heravi et al. [58] reported an easy and eco-friendly route to the preparation of 3-cyanopyridines via a one-pot multicomponent reaction, in the presence of Preyssler HPA as a catalyst with excellent results (Scheme 11). The procedure consisted of a mixture of 3,4-dimethoxyacetophenone (1 mmol), malononitrile (1 mmol), the appropriate aldehyde (1 mmol), ammonium acetate (8 mmol), and heteropolyacid (2 mol%) in ethanol (5 mL) under reflux conditions for 3 h. In these conditions 10 compounds of 3-cyanopyridines were obtained with yields between 90 and 94%, using the most active catalyst (H14[NaP5W30O110]).
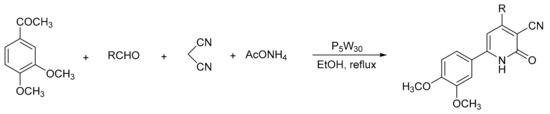
Scheme 11.
Synthesis of 3-cyanopyridines.
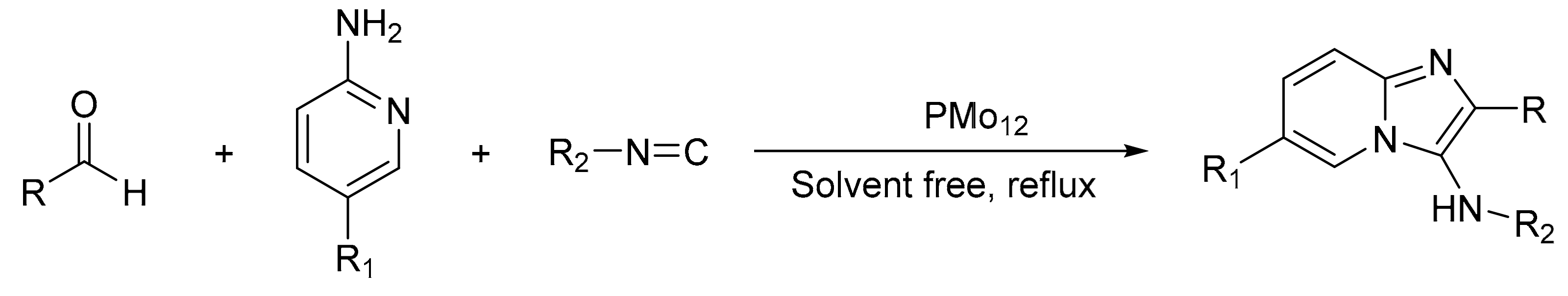
Oskooie et al. [59] presented the one-pot synthesis of 3-aminoimidazo [1,2-a] pyridines with H3PMo12O40 as a catalyst for the synthesis of different derivatives of 3-aminoimidazo[1,2-a]pyridine with excellent results (Scheme 12). In a typical experiment for the preparation of 6-bromo-N-cyclohexyl-2-phenylimidazo[1,2-a]pyridin-3-amine, a mixture of 2-amino-5-bromopyridine (0.17 g, 1 mmol), benzaldehyde (0.13 g, 1.2 mmol), cyclohexyl isonitrile (0.12 g, 1.1 mmol) and H3PMo12O40 (0.03 g) was stirred for 20 min at room temperature, in solvent-free conditions. In these conditions, 10 compounds were prepared with yields between 90 and 98%.
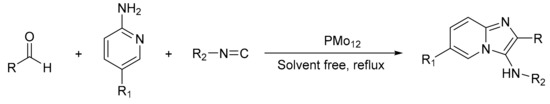
Scheme 12.
Synthesis of 3-aminoimidazo[1,2-a] pyridine.
4. Pyrroles
Pyrrole and its derivatives are nitrogen-containing heterocyclic compounds of great importance because they are highly distributed in of natural origin such as porphyrins, bacteriochlorins, porphyrinogens, chlorophyll, vitamin B12, bile pigments, such as bilirubin and biliverdin, and alkaloids isolated from marine sources [60]. In organic synthesis they are an outstanding building block to obtain bioactive compounds useful in medical chemistry and pharmacology. Thus a wide variety of activities of these compounds have been reported, such as inhibition of the HIV virus, antibacterial and antifungal, antioxidant, antitumor, anticancer, antimalarial, and anticonvulsant effects [61,62,63,64]. There are many conventional methods for the synthesis of pyrrole derivatives including Friedel–Crafts acylations and alkylations, Michael additions, Heck couplings, Knorr synthesis, Paal–Knorr condensation, Hantzsch synthesis, hydroarylations, carbenoid insertions, and cyclizations [65,66]. However, in recent years, multicomponent reactions have aroused much interest as they are an environmentally friendly and cheaper method.
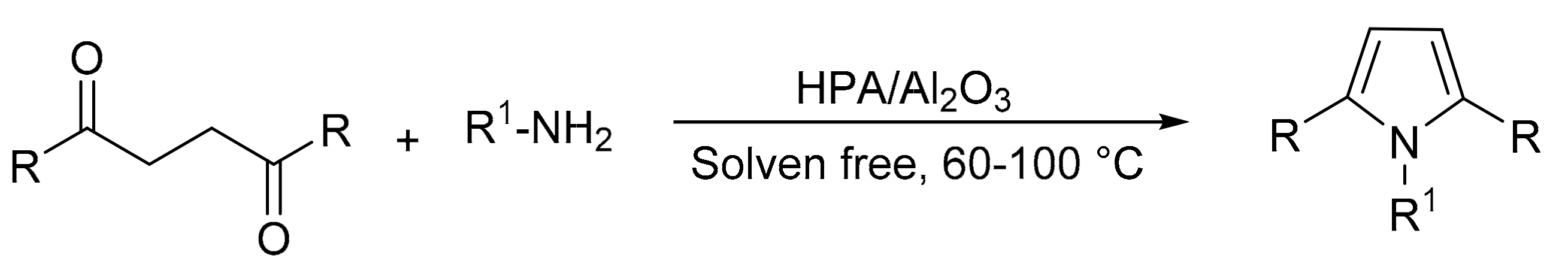
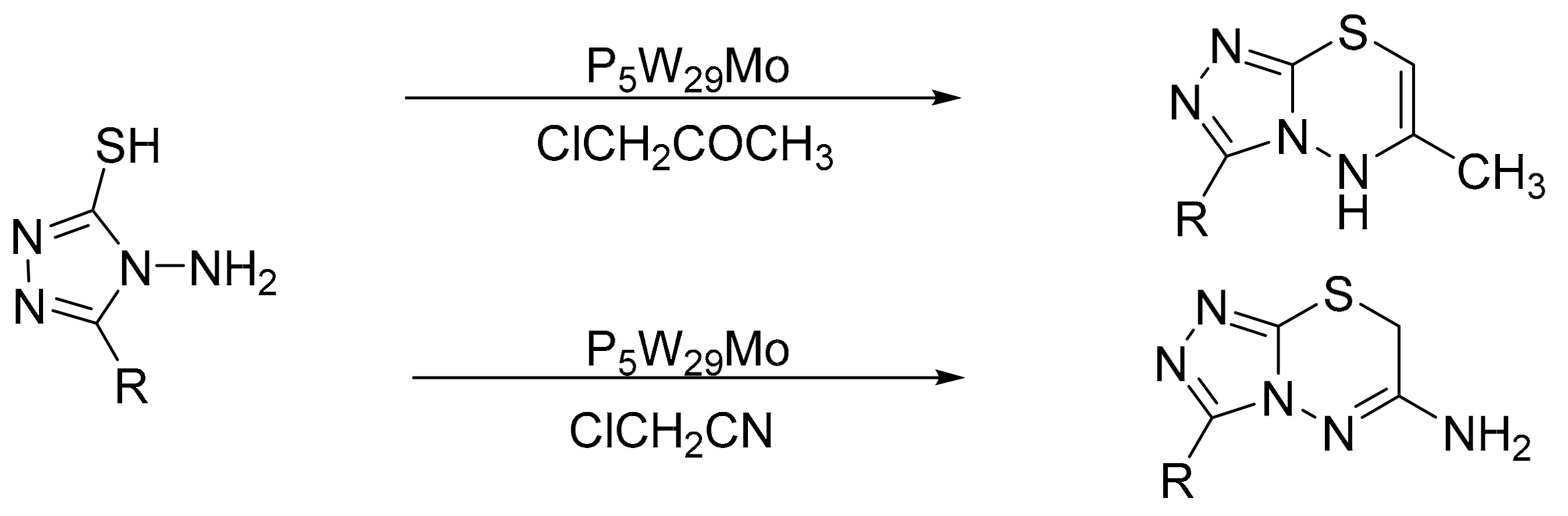
Our research group reported a suitable method for pyrrole synthesis using the Paal–Knorr methodology [67]. For this, catalysts based on Preyssler-type HPA (H14[NaP5W30O110], P5W30; H14[NaP5W29MoO110], P5W29Mo) supported on commercial mesoporous alumina (CATALOX SBa-90®) were synthesized (P5W30/Al2O3 and P5W29Mo/Al2O3) with the aim of preparing materials with acid-base properties. The materials were synthesized by the water impregnation method, and the characterization determined that the intact structure was preserved. The activity of both the supported and bulk catalysts was evaluated, and in total 12 compounds were obtained, in solvent-free reactions, short reaction times (0.5–3 h), and temperatures between 60 and 100 °C (Scheme 13). There were no significant differences between the yields achieved with the supported and the bulk (53–98%) catalysts, although the amount of active phase used in the former was ten times less, which helps to minimize costs. The supported catalyst was used for three cycles without noticeable loss of activity.
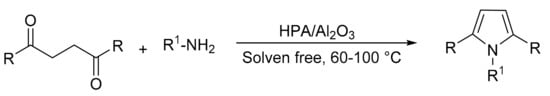
Scheme 13.
Paal–Knorr pyrrole synthesis.
In this study, the sustainability parameters of the procedure and recyclability of the catalyst were also analyzed, determining that the proposed methodology is eco-friendly compared to the processes commonly used. It is also highlighted that only with the supported materials could more complex pyrroles be synthesized through tandem one-pot reactions that result in the synthesis of 2-amino-3-cyano-4-H-chromenes and their subsequent conversion into pyrrole derivatives (Scheme 14). This is due to the acid-base properties of the supported materials.
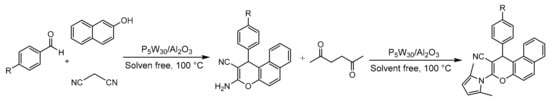
Scheme 14.
Synthesis of chromene-pyrrole derivatives.
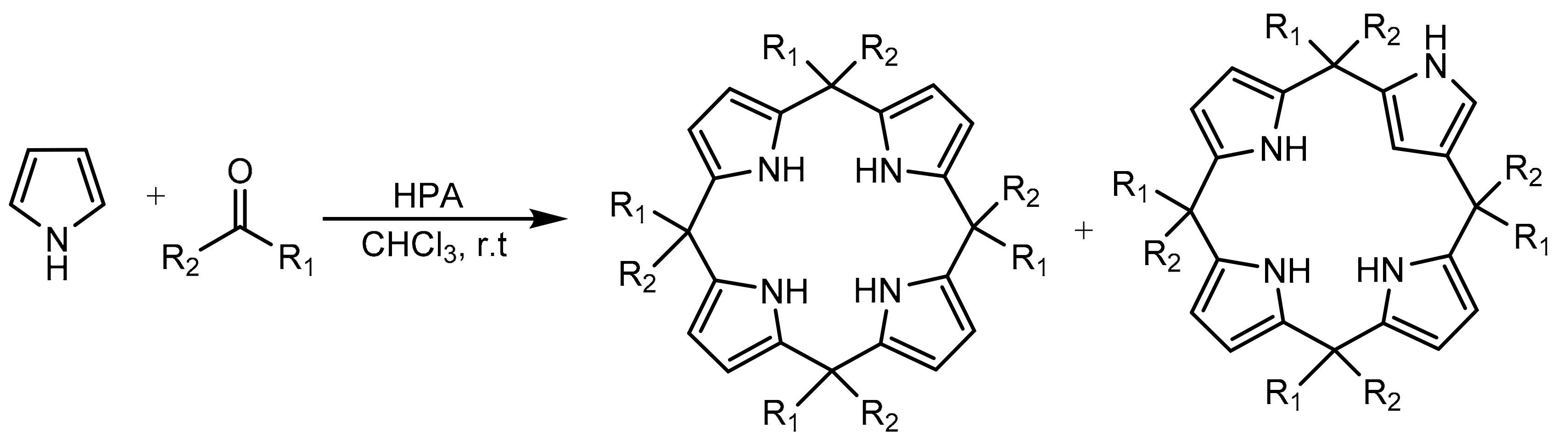
Other molecules containing the pyrrole core as substructure can be synthesized by a simplified methodology. Gharib et al. [68,69] reported a catalytic method for calix[4]pyrroles using Preyssler (H14[NaP5W30O110]) and Wells–Dawson (H6[P2W18O62]) heteropolyacids in bulk form. Calixarenes are an important type of macrocyclic compounds that are widely employed as ligands in supramolecular chemistry. The methodology is appropriate to prepare calix[4]pyrroles and N-confused calix[4]pyrroles by reaction of dialkyl or cycloalkyl ketones with acidic catalysts. Good yields of different calixarenes were obtained under mild and environmentally friendly conditions. In a model experiment, a mixture of pyrrole (8 mmol), acetone (8 mmol), and heteropolyacid catalyst (0.04 mmol) in chloroform (10 mL) was stirred for 6 h at room temperature. In total, eight calix[4]pyrroles were obtained with yields between 22% and 85%. The authors reported better yields in the synthesis of all compounds with the Preyssler catalyst compared to the Wells–Dawson one under the same conditions, which they attributed to the higher number of acidic protons present in the Preyssler catalyst. Still, the results obtained with the Wells–Dawson catalyst were also good. In some experiments, confused calix[4]pyrroles were detected as secondary product (10–12%) (Scheme 15). Similarly, the Preyssler catalyst was recovered and reused for three cycles without appreciable loss of catalytic activity.
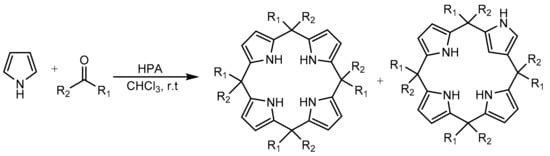
Scheme 15.
Synthesis of calix[4]pyrroles and confused calix[4]pyrroles.
5. Indoles
The indole core is a near-ubiquitous component in biologically active natural heterocyclic compounds and an important building block for the development of bioactive synthetic molecules because indole derivatives have the unique property of mimicking the structure of peptides and to bind reversibly to enzymes. This makes them of great interest for medicinal chemistry and the pharmaceutical industry, for the discovery of novel drugs, which currently report several thousand new specific derivatives annually. Among the many biological activities reported, the following stand out antitumor, antiviral, anti-inflammatory effects, and they are also used as dopamine agonists, antidepressants, among other applications [70,71,72,73,74].
The Fischer indole synthesis is the most widely investigated synthesis of indole and carbazole derivatives, and different acid catalysts have been used to assist this transformation. Beheshtiha and coworkers [75] reported the preparation of indole and carbazole with excellent yields using phenylhydrazine derivatives and different carbonyl compounds as substrates in the presence of a catalytic amount of Keggin-type heteropolyacid (H3PW12O40, PW12). Scheme 16 shows Fisher’s synthesis for carbazole derivatives.
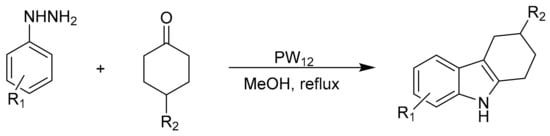
Scheme 16.
Synthesis of carbazole.
The reaction conditions to obtain indole derivatives consist in the preparation of a solution of phenylhydrazine derivatives (1 mmol), carbonyl compounds (1 mmol), and heteropolyacid (0.05 g) in MeOH (5 mL). The reaction mixture was heated at reflux for a time between 30 and 45 min. Overall, 18 indoles were prepared with yields between 65 and 90%, free of secondary products. The catalyst was reused for four cycles with minor loss of activity.
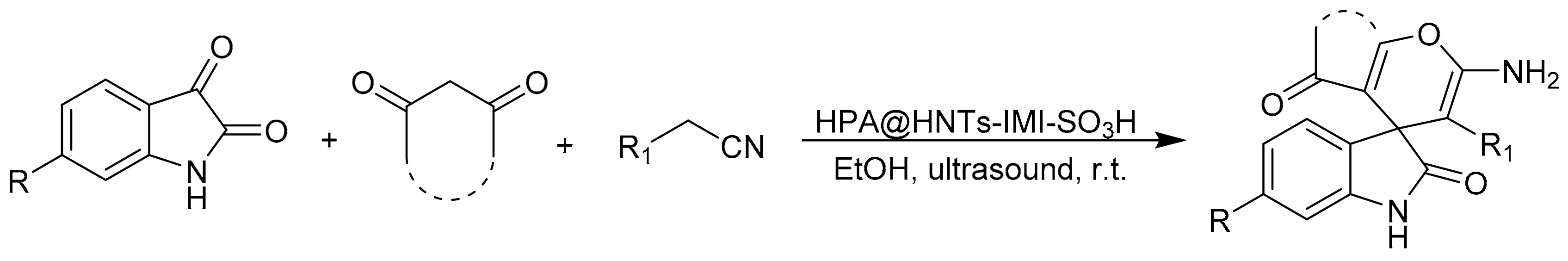
The same group reported a multicomponent reaction to prepare spirooxindoles [76]. In this work, the authors prepared a novel heterogeneous catalyst, HPA@HNTs-IMI-SO3H, synthesized based on the functionalization of halloysite nanotubes with ionic liquid and subsequent incorporation of heteropolyacid. Then, the catalytic activity of new materials was studied in the multicomponent reaction of malononitrile or cyanoacetic esters, isatines, and 1,3-dicarbonyl compounds assisted by ultrasonic irradiation. The reaction conditions were: isatines (1 mmol), malononitrile or cyanoacetic esters (1 mmol), 1,3-dicarbonyl compounds (1 mmol), and HPA@HNTs-IMI-SO3H as catalyst (0.025 g) in H2O/EtOH (8:2, 10 mL). This mixture was then sonicated at 80 W at 20 °C for a reaction time between 5 and 90 min. In these conditions, 10 spirooxindoles were obtained with yields between 70 and 98% (Scheme 17).
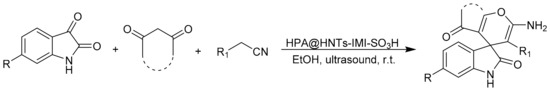
Scheme 17.
Synthesis of spirooxindole.
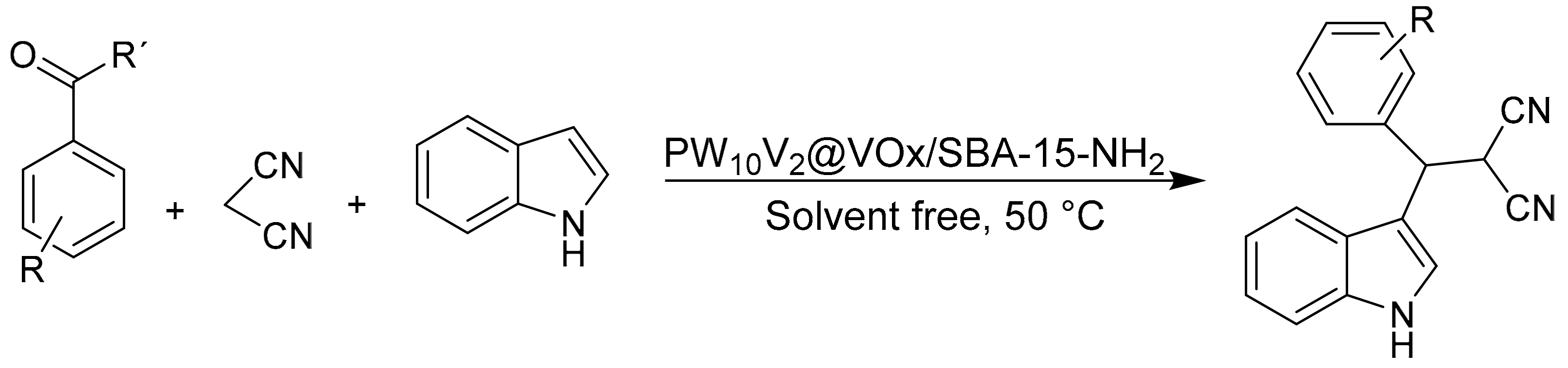
Mahdizadeh Ghohe et al. [77] synthesized vanadium-doped SBA-15 (VOx/SBA-15) materials by sol-gel synthesis; subsequently the surface of the resulting VOx/SBA-15 material was modified by adding 3-(triethoxysilyl) propylamine as a linker compound to finally non-covalently support the heteropolyacid H5PW10V2O40, thus obtaining a new inorganic–organic nanoporous material named PW10V2@VOx/SBA-15-NH2. The materials were extensively characterized, determining that the Keggin-type structure was preserved after immobilization of the HPA and that they presented a solid Lewis–Brønsted acid character. The materials were efficient in the one-pot synthesis of 3-substituted indoles under mild solvent-free reaction conditions. The general procedure consisted of a mixture of aldehyde (1 mmol), indole (1 mmol), malononitrile (1 mmol), and PW10V2@VOx/SBA-15-NH2 (30 mg) at 50 °C, in times between 15 and 40 min and with yields between 73 and 95% (Scheme 18). The immobilization of the HPA component through electrostatic interactions suppresses the leaching of the catalyst and therefore improves the recyclability without apparent loss of catalytic activity.
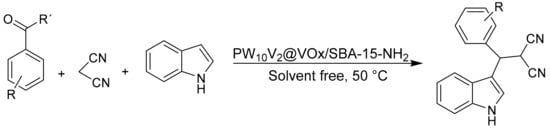
Scheme 18.
One-pot synthesis of 3-substituted indoles.
The same group developed materials similar to the previous ones; on this occasion the heteropolyacid H5PW10V2O40 was anchored on SBA-15 modified with pyridine (HPA/TPI-SBA-15) [78]. The catalytic activity of the materials was evaluated in the preparation of bis-(indolyl)-methanes. The procedure consisted of a mixture of carbonyl compound (1 mmol), indole (2 mmol) and PW10V2/TPI-SBA-15 (0.02 g) heated to 100 °C under solvent-free conditions for a time that varied between 15 min and 2 h. In total, 10 compounds were obtained with yields between 65 and 95% (Scheme 19). The authors highlighted that the advantages of the supported HPA cluster over the bulk HPA were the greater reactivity of the supported material, the easy separation of the reaction medium, the high reuse (10 cycles without a considerable loss of activity) and the rapid recovery of the material.
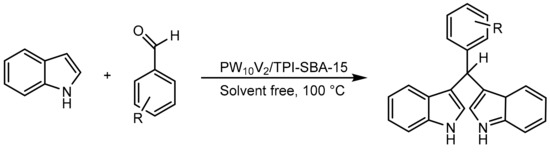
Scheme 19.
Synthesis of bis-(indolyl)-methanes.
Rahimi et al. [22] reported an easy process for the synthesis of bis (indole) derivatives by the Michael reaction in which indole reacts with different electron-deficient alkenes. To achieve this, they used tungstophosphoric acid (PW12) as a catalyst in an aqueous medium and the reaction was assisted by ultrasound. Ultrasound is a powerful technique in organic synthesis as higher reaction rates and high yields are achieved compared to traditional methods. The general procedure consisted of mixing indole (1 mmol), electron-deficient alkenes (0.5 mmol), and PW12 (3 mol%) in water (4 mL) and heating to reflux under ultrasonic irradiation. They also carried out the reaction under the same conditions but with conventional heating, finding great differences in the reaction time between the two procedures to obtain similar yields (10–20 min when irradiated with ultrasound vs. 8–12 h with conventional heating). They obtained 13 compounds with yields between 77 and 93% (Scheme 20).
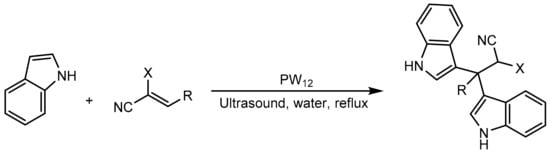
Scheme 20.
Synthesis of bis (indole) derivatives.
6. Chromenes and Xanthenes
Chromenes, also named benzopyrans, are heterocyclic compounds made up of a benzene ring fused to a pyran nucleus. This nucleus is important because it is present in an extensive variety of natural products and in synthetic compounds with important biological applications, so it is associated with pharmacological diversity. In particular, the possible anticancer activities have been the most investigated, but biological activities such as antimicrobial, antiviral, anti-inflammatory, antioxidant properties, treatment of skin disorders, and diabetes mellitus, among others, have also been reported. They are also increasingly used for the therapy of neurodegenerative diseases such as Down syndrome, Parkinson’s disease, Alzheimer’s disease, schizophrenia, and AIDS-related insanity [79,80,81,82,83,84].
In addition to presenting the biological activities of chromenes, xanthenes have many applications such as phosphorescent dyes, laser technologies, fluorescent material for the visualization of biomolecules that allow numerous advanced biological imaging experiments, which include photoactivated localization microscopy (PALM) and related super-resolution imaging techniques. Additionally, in recent years, there has been increasing clinical interest in the development of xanthenes and their derivatives as possible biomarkers to detect and quantify key analytes within cells and thus diagnose and treat diseases [85,86,87,88,89]. Although chromenes and xanthenes have been synthesized by different methods, including the Friedel–Crafts reaction, they suffer from drawbacks such as the use of harmful chemicals, catalysts and solvents, multistep manipulation, long reaction time, low yields, and highly complex treatment procedure [90,91]. This has increased the interest in these reactions using procedures that include the principles of green chemistry.
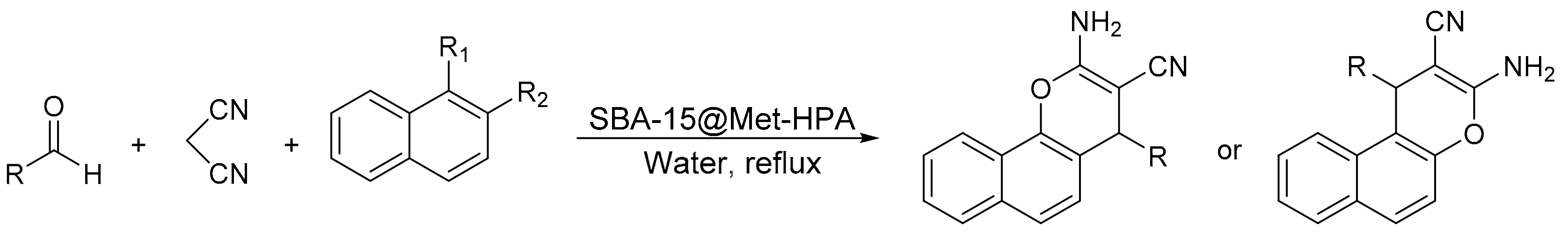
S. Sadjadi et al. [10] reported the synthesis of a novel hybrid catalyst based on the modification of Cl-functionalized SBA-15 with methenamine and subsequent incorporation of a Keggin heteropolyacid (SBA-15@Met-HPA). The catalyst was tested in the multicomponent reaction between an aldehyde, malononitrile, and 1-naphthol or 2-naphthol, using water at 100 °C as solvent reaction. In a representative experiment a mixture of a benzaldehyde (1 mmol), malononitrile (1 mmol), 1-or 2-naphthol (1 mmol), and SBA-15@Met-HPA (0.03 g) in water (5 mL) was refluxed for 15 min. In these conditions 14 4H-chromenes were obtained with yields between 89 and 97%. The catalyst was recycled and reused for five cycles without loss of activity (96–92%) (Scheme 21).

Scheme 21.
Synthesis of 2-amino-4H-chromene derivatives.
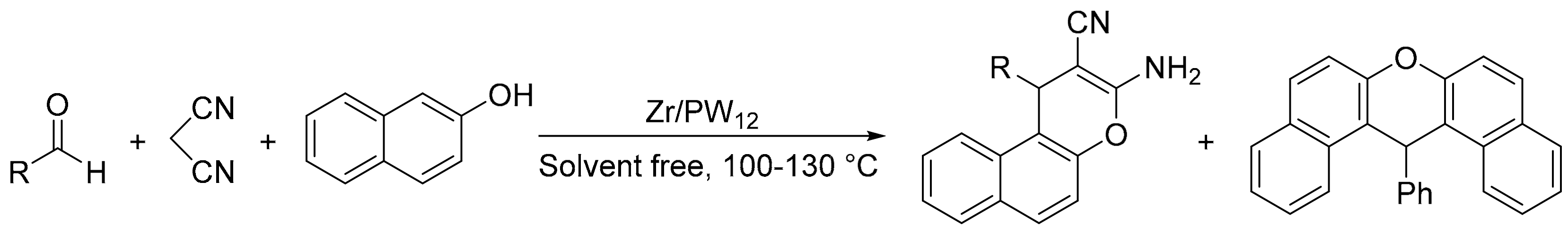
Our research group carried out the same reaction using zirconia/phosphotungstic acid composites (Zr/PW12) in solvent-free conditions [92]. Several reaction conditions were tested, including temperature, catalyst amount, and substrate ratio. In a typical experiment, a mixture of benzaldehyde (1.3 mmol), malononitrile (1 mmol), 2-naphthol (1 mmol), and catalyst (85 mg: 0.009 or 0.018 mmol of active phase) was heated at 100–130 °C for 3 h. In all cases two products were detected, 2-amino-3-cyano-4H-chromene (4) and the unexpected 14-aryl-14H-dibenzo[a,j]xanthene (5) depending on the reaction conditions (Scheme 22). The formation of 14-aryl-14H-dibenzo[a,j]xanthene is attributable to a pseudo multicomponent reaction between two molecules of naphthol and one molecule of aldehyde. Modification of the reaction conditions can change the selectivity towards one or the other product.
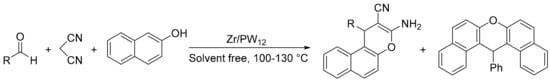
Scheme 22.
Chromene vs. xanthene synthesis.
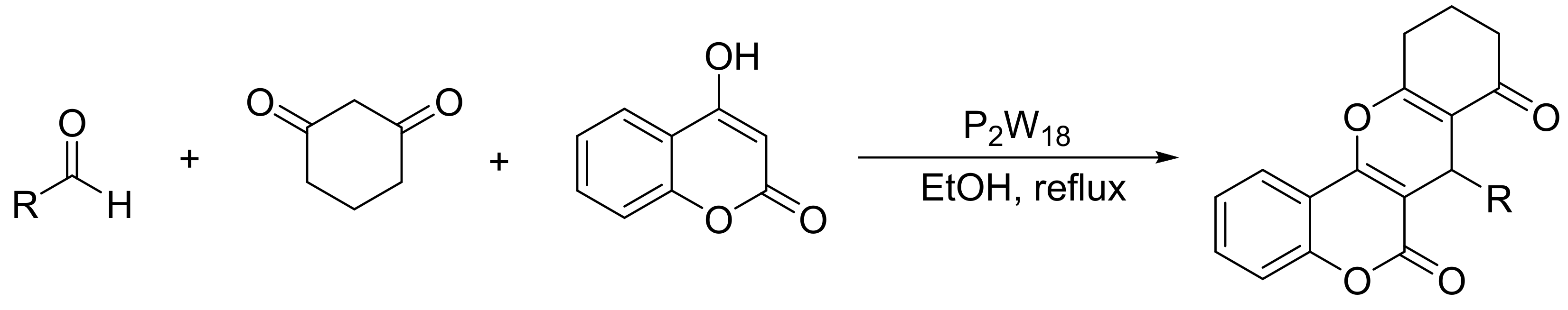
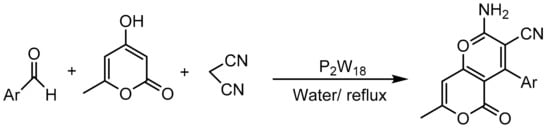
In a recent paper, Motamedi et al. [93] reported the synthesis of other chromene derivatives, specifically benzopyrano[3,2-c]chromene-6,8-diones. These compounds can be synthesized by a three-component one-pot cyclocondensation of 4-hydroxycoumarin (1 mmol), aldehydes (1 mmol), and 1,3-cyclohexadione (1 mmol) using a catalytic amount of Wells–Dawson heteropolyacids (P2W18, 0.04 mmol) in boiling ethanol (50 mL). Under these conditions and in a short reaction time (4 min), 12 benzopyrano[3,2-c]chromene-6,8-dione derivatives were obtained with yields between 79 and 90% (Scheme 23).
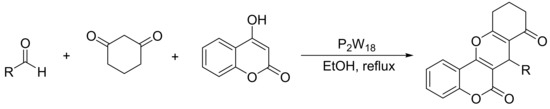
Scheme 23.
Synthesis of benzopyrano[3,2-c]chromene-6,8-diones.
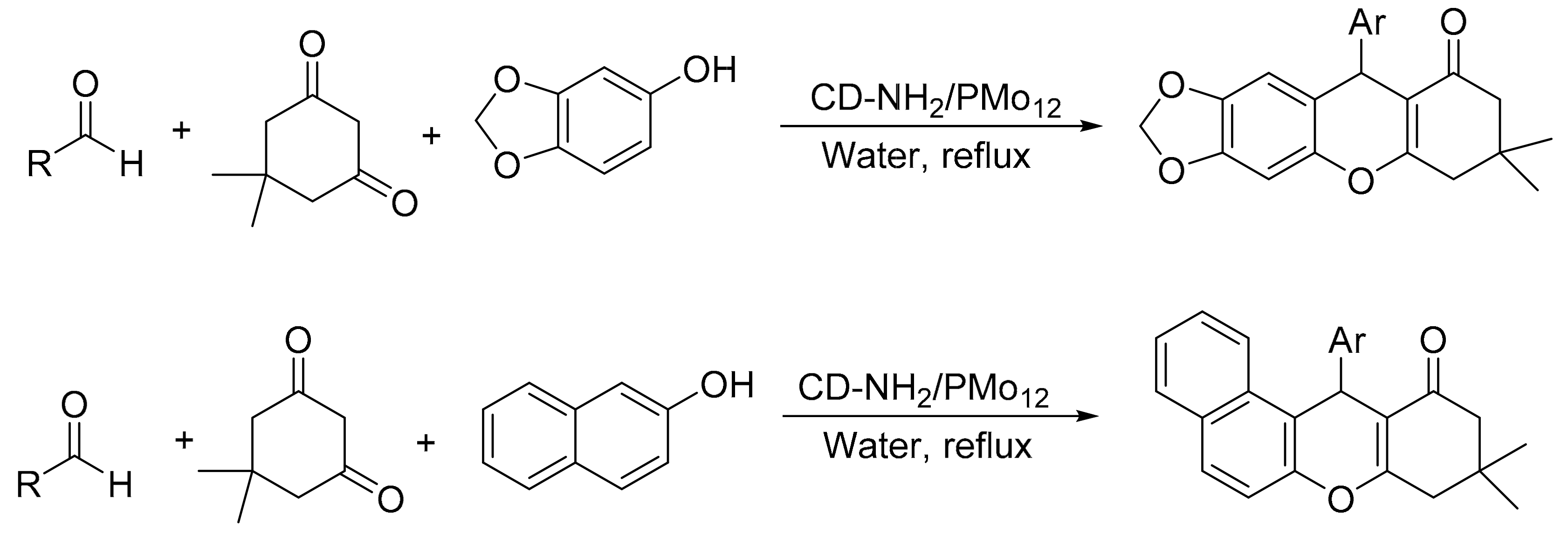
Heravi’s group reported the design of new cyclodextrin nanosponge (which was previously functionalized with an amine precursor prior to immobilization of the Keggin heteropolyacids through wet impregnation method) [12]. The procedure gave a suitable material that did not undergo relevant leaching after the different catalytic cycles. The material provides an effective catalyst for accelerating the synthesis of xanthene derivatives in aqueous media. The reaction was performed using dimedone (1 mmol), 3,4-methhylene-dioxyphenol or 2-naphthol (1 mmol), and aldehyde (1 mmol) in the presence of 0.02 g of CD-NH2/PMo12 in water (5 mL), at 100 °C for a time period of 1.5–3.2 h. In this condition seven compounds of xanthenes derived from 2-naphthol (87–92%) and nine compounds of 3,4-methhylene-dioxyphenol (88–95%) were obtained (Scheme 24).
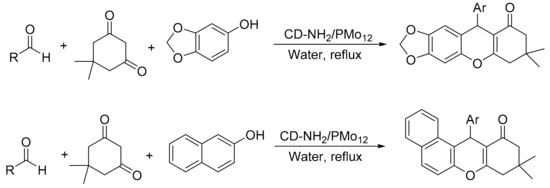
Scheme 24.
Synthesis of xanthene derivatives.
Similarly, Rivera et al. [94,95] reported a green method to prepare xanthene derivatives from naphthol using tungstophosphoric acid/zirconia (Zr/PW12) composites prepared by the sol-gel method. In total, 10 compounds of 14-aryl-14H-dibenzo[a,j]xanthenes were obtained with yields between 78 and 99, using aldehyde (1.2 mmol), 2-naphthol (2 mmol), and catalyst (0.01 mmol%), at a temperature of 130 °C in a reaction time between 1 and 5 h.
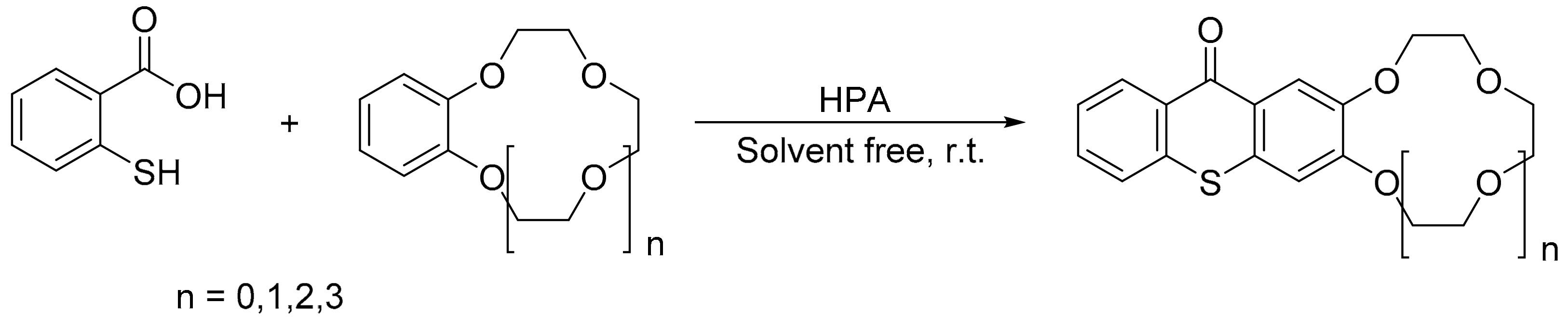
Gharib et al. reported a convenient process to catalytically obtain thioxanthone crown ethers with Wells–Dawson H6[P2W18O62] and Preyssler H14[NaP5W30O110] as catalysts [96]. The reaction involves the mixture of thiosalicylic acid (1 mmol), the, respective, crown ether (5 mmol), and catalyst (5 mmol%), and the reaction conditions were: 20 °C for 1.5 h. Overall, four thioxanthone crown ethers were obtained with yields between 72 and 94% using the Preyssler catalyst (H14[NaP5W30O110]) (Scheme 25).
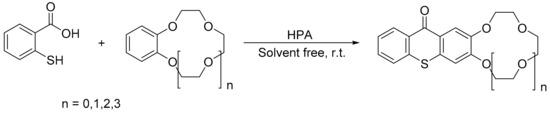
Scheme 25.
Synthesis of thioxanthone crown ethers.
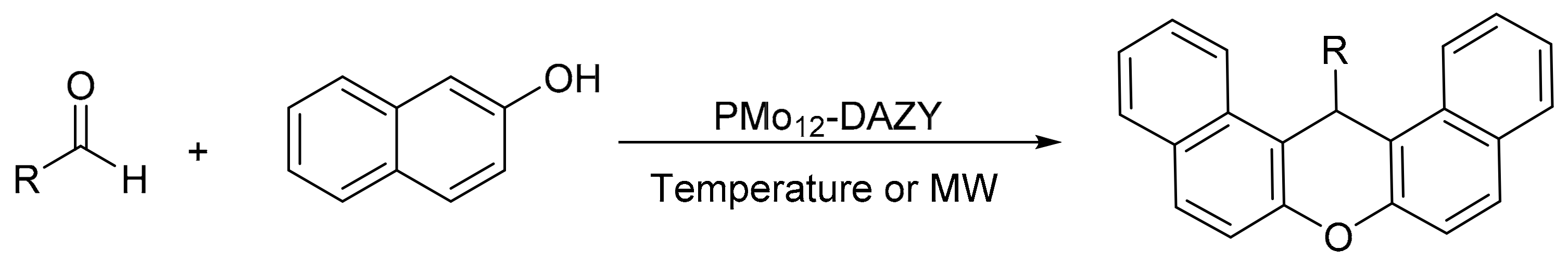
Majid Moghadam et al. [85] encapsulated molybdophosphoric acid in dealuminated zeolites-Y (PMo12-DAZY) by hydrothermal treatment. The dealumination of zeolite must be carried out to prevent the decomposition of PMo12 due to the basicity of zeolite, and it also increases the stability of zeolite and produces a system of secondary pores in the matrix that favors the deposition of higher amounts of PMo12. The materials were evaluated in the synthesis of xanthene derivatives under solvent-free conditions and by two different methods, conventional heating and under microwave irradiation (Scheme 26). The general procedure consisted of mixing 2-naphthol (2 mmol), aldehyde (1 mmol), and catalyst (300 mg, 0.0125 mmol) and heating by conventional method at 100 °C for 80–150 min or irradiating at 800 W for 2–35 min. In total, 13 compounds were synthesized with yields between 75 and 95% in the two procedures, the main difference being the saving of time when the reaction was assisted by microwaves. The catalyst was reused in up to four cycles with small loss of yield (93–85%).
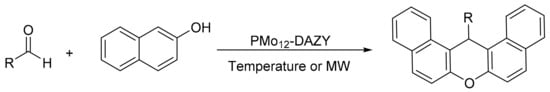
Scheme 26.
Synthesis of 14-substituted-14H-dibenzo[a,j]xanthenes.
7. Pyrans
Pyrans, in particular benzopyran, constitute an important class of 6-membered heterocycles with one oxygen atom and are an outstanding scaffold for synthesizing a wide variety of compounds with pharmaceutical applications with many already approved drugs circulating on the market. An example of this is that the structural characteristics of the pyran present in the tetrahydropyran rings are responsible for the antibacterial activity of commercial antibiotics such as erythromycin and streptomycin. More complex structures containing pyrans such as cyclodextrins have also been studied and have received attention in recent years for innovative biomedical applications. Other recently reported applications include pyran dyes used as promising photoredox catalysts, materials for solar cells and sensors, and activity as acid corrosion inhibitors [97,98,99,100].
M. Zakeri et al. [101] reported a pseudo-multicomponent procedure for benzopyran derivatives for the reaction between salicylaldehydes and 5,5-dimethyl-1,3-cyclohexanedione in the presence of a catalytic amount of Preyssler-type heteropolyacid H14[NaP5W 29MoO110] in solvent-free conditions. The experimental conditions were: a mixture of substituted salicylaldehyde (2 mmol), substituted 1, 3-hexanedione (4 mmol), and heteropolyacid (0.03 mmol) heated at 120 °C for 18–40 min. In these conditions, 10 benzopyrans were obtained with yields between 85 and 95% (Scheme 27).
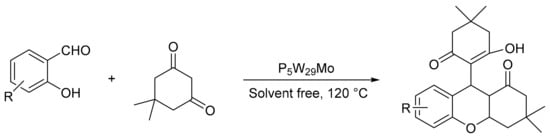
Scheme 27.
Synthesis of benzopyrans.
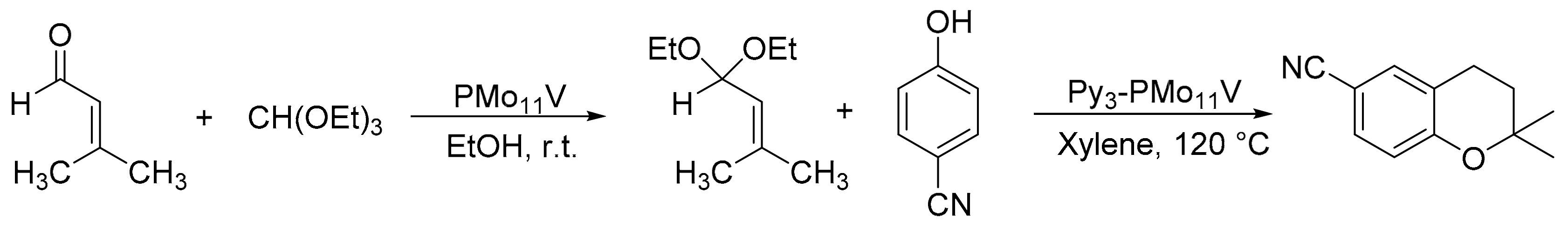
Our research group reported a green method for the synthesis of 6-cyano-2,2-dimethyl-2-H-1-benzopyran (6-CN-2,2-DMB) and its subsequent enantioselective epoxidation by the use of the heteropolyacids H4PMo11VO40 (PMo11V) and (PyH)3HPMo11VO40 (Py3-PMo11V), and homogeneous Jacobsen catalysts (Scheme 28) [33]. The reaction was carried out in three steps with a yield of 35%. In the first step, 1,1-diethoxy-3-methyl-2-butene was synthesized from triethyl orthoformate (18 mmol), 3-methyl-2-butenal (18 mmol), and PMo11V (1 mmol%) in absolute ethanol (5 mL) at 20 °C for 1h with a yield of 89%. For the second step, 6-CN-2,2-DMB was synthesized through the use of Py3-PMo11V (1 mmol%) as a catalyst, by adding 1,1-diethoxy-3-methyl-2-butene (17 mmol) previously synthesized and 4-cyanophenol (13 mmol) in p-xylene (20 mL) as solvent at 120 °C for 6 h. The catalyst was recovered by centrifugation and reused. Finally, 6-cyano-2,2-dimethyl-2-H-1-benzopyran epoxide was obtained using Jacobsen-type catalysts and dimethyldioxirane (DMD) generated in situ as oxidizing agent. In the presence of 4-phenylpyridine N-oxide (4-PPNO) at 4 °C, enantioselectivities of 87% were obtained for 3S, 4S-epoxide, and 68% for 3R, 4R-epoxide with S,S and R,R-Jacobsen catalysts, respectively. The overall yield was approximately 17% for 3S, 4S-epoxide.
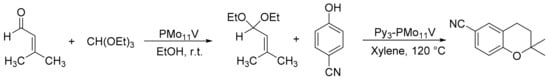
Scheme 28.
Synthesis of 6-cyano-2,2-dimethyl-2-H-1-benzopyran.
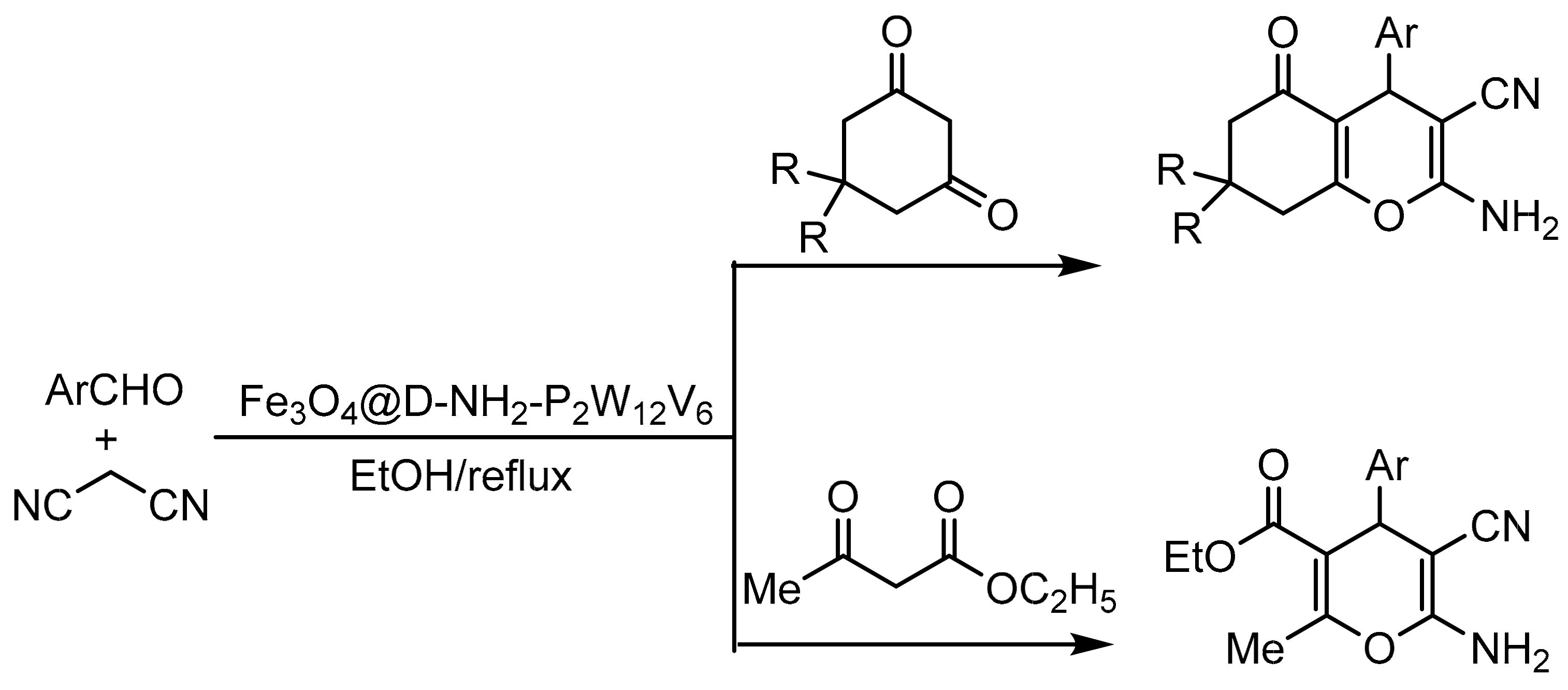
Ali Jamshidi et al. [102] prepared and broadly characterized a dendrimer-functionalized iron magnetic nanoparticle catalyst (symmetric star-shaped branched macromolecules, nanometric in size with a defined, homogeneous, and monodispersed structure) as an anchor point to support the Wells–Dawson HPA substituted with vanadium H9[H3P2W12V6O62]·18H2O through electrostatic bonds. The catalyst was named (Fe3O4@D-NH2-P2W12V6), and its catalytic activity was studied in the one pot synthesis of tetrahydrobenzo[b]pyrans and 2-amino-3-cyano-4H-pyrans (Scheme 29). The general procedure consisted of a mixture of aldehydes (1 mmol), malononitrile (1.2 mmol), 1,3-cyclohexanedione or ethyl acetoacetate (1 mmol), and Fe3O4@D-NH2-P2W12V6 (0.02 g) at reflux conditions in ethanol (2 mL) for a time between 4 and 30 min. They synthesized 21 compounds with yields between 82 and 96%. The catalyst was reused for 4 cycles with a small loss in yield (92–85%).
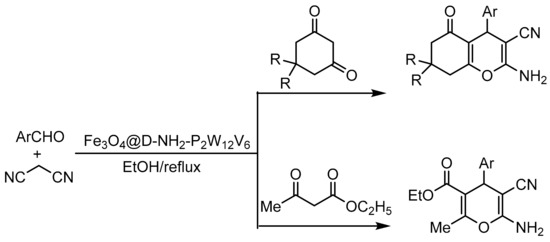
Scheme 29.
Synthesis of highly substituted pyran derivatives.
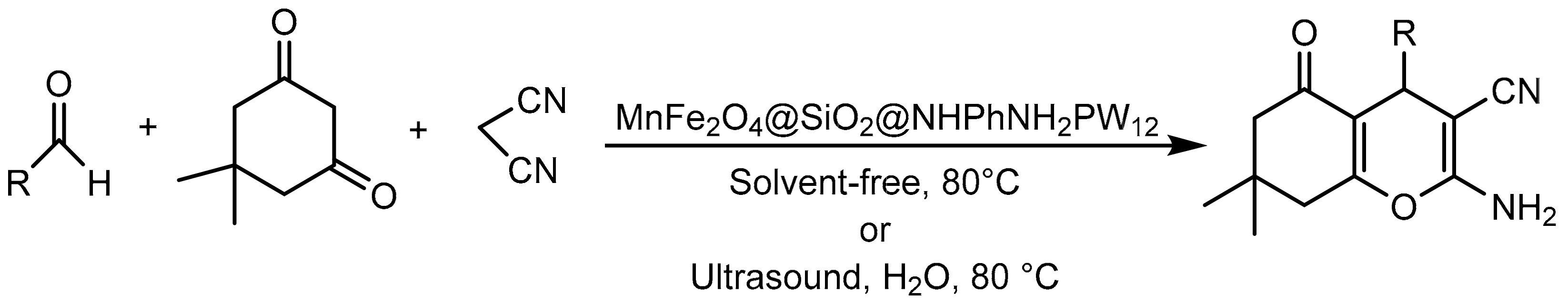
Roya Mozafari et al. [103] also immobilized HPA on magnetic materials; on this occasion, they supported tungstophosphoric acid on magnesium ferrite nanoparticles coated with silicon oxide and functionalized with diamine as an anchor point (MnFe2O4@SiO2@NHPhNH2PW12). The materials were extensively characterized and evaluated in the synthesis of tetrahydrobenzo[b]pyran derivatives by two different methods: conventional heating without solvent and ultrasonic irradiation (Scheme 30). The procedure consisted of a mixture of the aromatic aldehyde (1 mmol), malononitrile (1.2 mmol), dimedone (1 mmol), and MnFe2O4@SiO2@NHPhNH2PW12 (0.040 g) heated under solvent-free conditions at 80 °C for 25–35 min or under sonication at 80 °C in the presence of H2O for 8–12 min. In both procedures, nine compounds were synthesized with yields between 85 and 95%. The difference between the two methods was in considerable time savings when ultrasound was applied.
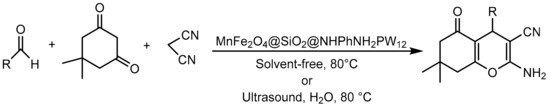
Scheme 30.
Synthesis of tetrahydrobenzo[b]pyran derivatives.
Continuing with HPA supported on magnetic materials, Behrooz Maleki et al. [104] synthesized NiFe2O4 magnetic nanoparticles coated with silica and supported them on H3PW12O40 (NiFe2O4@SiO2–H3PW12O40, NFS-PW12). The catalyst was studied in the one-pot synthesis of tetrahydrobenzo[b]pyran and pyrano[2,3-c]pyrazole derivatives. The procedure consisted of mixing aldehyde (1.1 mmol), malononitrile or ethyl cyanoacetate (2.2 mmol), 1,3-dicarbonyl compound (1,3-cyclohexanedione or 5,5-dimethyl-1,3-cyclohexanedione) (3.1 mmol), or 3-methyl-1-phenyl-2-pyrazoline-5-one (5.1 mmol) and NFS–PW12 (0.02 g) in ethanol (3 mL) under reflux conditions for a time of 5–45 min with yields between 80 and 90%. The catalyst was reused in six cycles with yields between 94 and 86%.
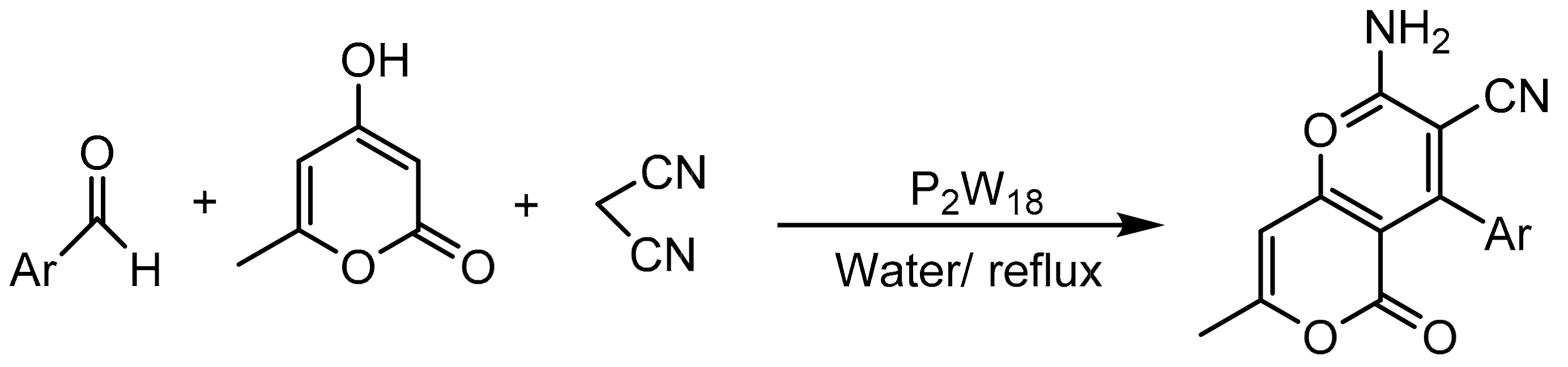
On the other hand, Deepika Rajguru et al. [105] synthesized pyrano[4,3-b]pyran derivatives via a one-pot reaction, using H6P2W18O62·18H2O (P2W18) as catalyst. A mixture of 4-hydroxy-6-methylpyran-2-one (1 mmol), aromatic aldehyde (1 mmol), malononitrile (1.2 mmol), and P2W18 (1 mol %) was stirred in 20 mL of water in reflux conditions for 45–60 min. They synthesized 12 compounds with yields between 87 and 95% (Scheme 31). The catalyst was recovered and reused by filtering the reaction product, and the aqueous filtrate containing the P2W18 catalyst was used for the next reaction series. This procedure was repeated for four cycles with a small decrease in activity (94–84%).
Scheme 31.
Synthesis of 2-amino-4-aryl-3-cyano-5-oxo-4H,5H-pyrano[4,3-b]pyrans.
8. Azlactones
Azlactones (also known as oxazol-5-(4H)-ones) are molecules with a five-membered ring highly substituted by reactive sites, which makes them an important building block for the synthesis of different compounds of pharmacological and industrial interest. From them, oxazoles can be obtained thanks to their acidic nature or cycloaddition to produce heterocycles. Moreover, the electrophilic nature of carbonyl allows nucleophilic attack to open the ring [106]. In recent years, they have been investigated as a starting material for the synthesis of functional chiral amino acids and their corresponding derivatives, biologically active peptides, biosensors, herbicides, fungicides, and agrochemical intermediates. They have been used as antihypertensive, anticancer, and antitumor agents. The most common technique for the production of azlactone is the Erlenmeyer method, which is based on the condensation of benzaldehydes with hippuric acid using sodium acetate as catalyst and acetic anhydride as a dehydrating agent. However, in recent years, with the rise of green chemistry, eco-friendly procedures such as the use of heterogeneous catalysts and solvent-free conditions have been studied [107,108,109,110].
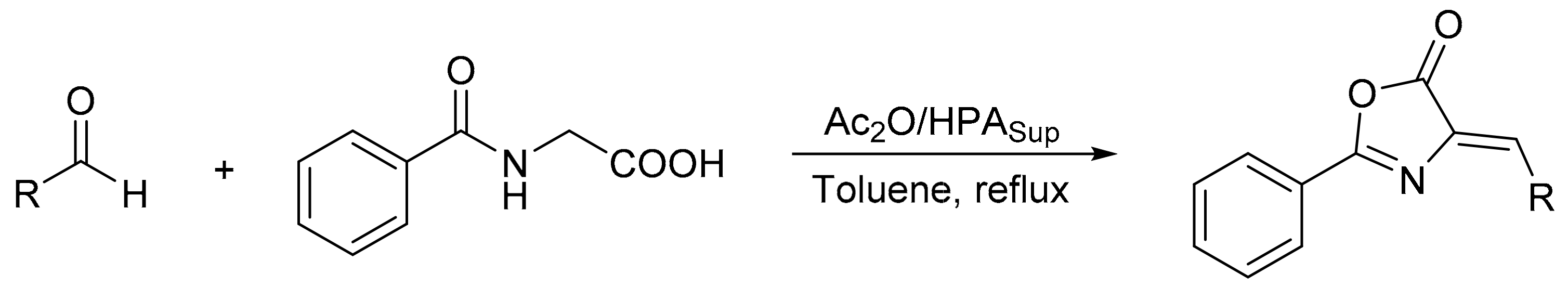
Blanco et al. [111] prepared phosphomolybdic and tungstophosphoric acid catalysts supported on a silica–alumina material, using the sol-gel method, and their catalytic activity was evaluated in the synthesis of azlactones (4-benzylidene-2-phenyloxazolin-5-ones and 4-alkylidene-2-phenyloxazolin-5-ones) by the Erlenmeyer method. In a common experiment, the aldehyde or cyclohexanone (1 mmol), hippuric acid (1 mmol), and the catalyst (0.0013 or 0.0065 mmol) were dissolved in toluene (2 mL) and heated to reflux conditions for 5 min. Then acetic anhydride (1 mmol) was added to the hot solution, and the mixture was refluxed for another 1 h. In total, 11 compounds were obtained with high conversion and selectivity (87–96%), with the exception of the azlactones synthesized from 2-nitrobenzaldehyde and cyclohexanone, which gave yields between 70 and 80%. The catalysts were used for several cycles without significant loss of activity (Scheme 32).
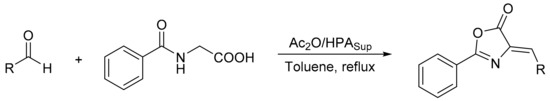
Scheme 32.
Synthesis of 4-benzylidene-2-phenyloxazolin-5-ones and 4-alkylidene-2-phenyloxazolin-5-ones by Erlenmeyer method.
Similarly, Ruiz et al. [112] reported the synthesis of Well–Dawson (H6P2W18O62.24H2O) catalysts supported on silica (P2W18/Sop) for azlactone synthesis. The reaction conditions were: benzaldehyde (1 mmol), hippuric acid (1 mmol), acetic anhydride (1.5 mmol), and P2W18/Sop (0.4 mmol) in toluene (3 mL) at 110 °C for 60 min. In these conditions nine azlactones were obtained with yields between 70 and 93%. The catalyst was recycled and reused without loss of catalytic activity.
In this same reaction and under solvent-free conditions at 80 °C, M. Rostami et al. [108] evaluated organic–inorganic hybrid materials, based on Keggin-type polyoxometalate PW12O40 and Na2WO4 salts with 1-butyl-3-methylimidazolium ([bmim][Cl]) ([bmim]3PW12O40, [bmim]4W10). These hybrid materials have different properties compared to the organic and inorganic components separately, which makes their study interesting. The authors reported 15 compounds obtained in 5–75 min with yields between 89 and 95%. The catalysts were reused for five cycles with yields between 95 and 84%, which shows a small decrease in the catalytic activity of the materials.
9. Azoles
Azoles are a family of five-membered heterocyclic compounds containing a nitrogen atom and at least one other non-carbon atom (i.e., nitrogen, sulfur, or oxygen) as part of the ring [113]. Their most prominent biological activity is antifungal activity, with proven efficacy in the treatment of fungal pathogens in humans, animals, and plants [114,115]. In fact, there are commercial azole fungicides on the market such as myclobutanil, propiconazole, and tebuconazole [116]. They are widely used in personal care products, UV stabilizers, and in aircraft for their anticorrosion properties. They have also been studied as proton conducting materials and have been applied in various polymeric electrolyte membranes (PEM) due to the efficient transfer of protons that their structure allows [117,118].
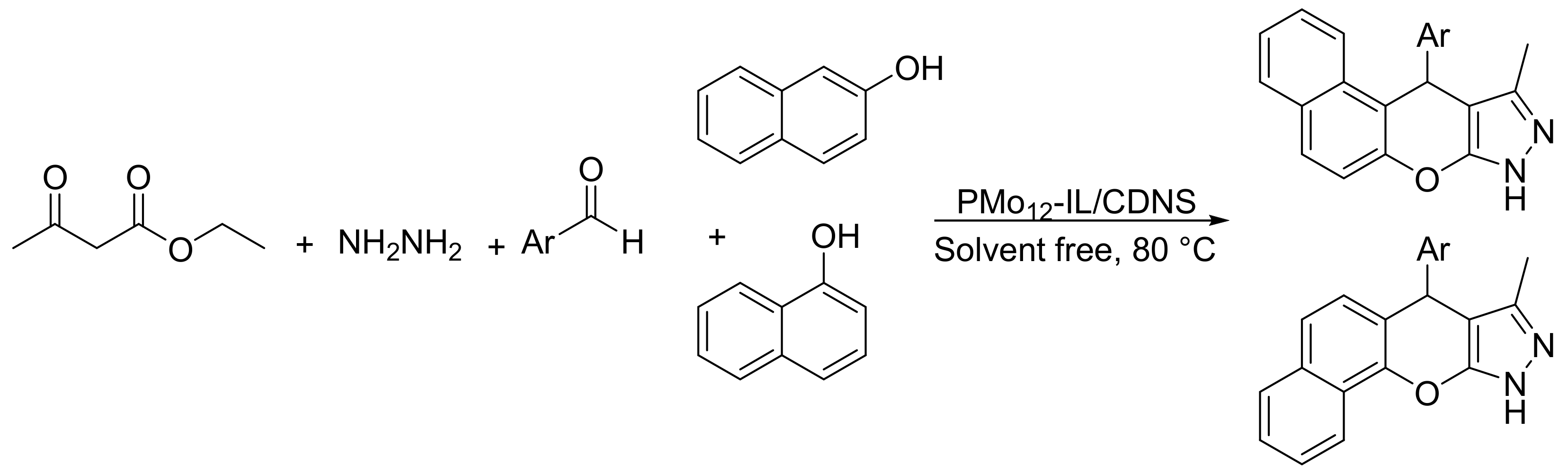
Heravi’s group reported the preparation of a novel hybrid catalytic material formed by phosphomolybdic acid (PMo12) immobilized on ionic liquid (IL) decorated cyclodextrinnanosponges (CDNS) [119]. The new materials were used as efficient catalyst for the suitable synthesis of benzochromeno-pyrazole through a cascade reaction. The reaction conditions were: hydrazine hydrate (1.1 mmol), ethylacetoacetate (1 mmol), benzaldehyde (1 mmol), β-naphthol or α-naphthol (1 mmol), and PMo12-IL/CDNS (0.04 g), at 80 °C, for 2–3 h. In these conditions 10 benzochromeno-pyrazoles were obtained with yields between 84 and 95% (Scheme 33). The catalyst was reused four times without appreciable loss of catalytic activity.
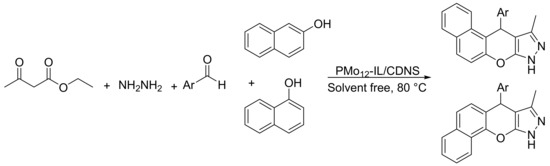
Scheme 33.
Synthesis of benzochromeno-pyrazole derivatives.
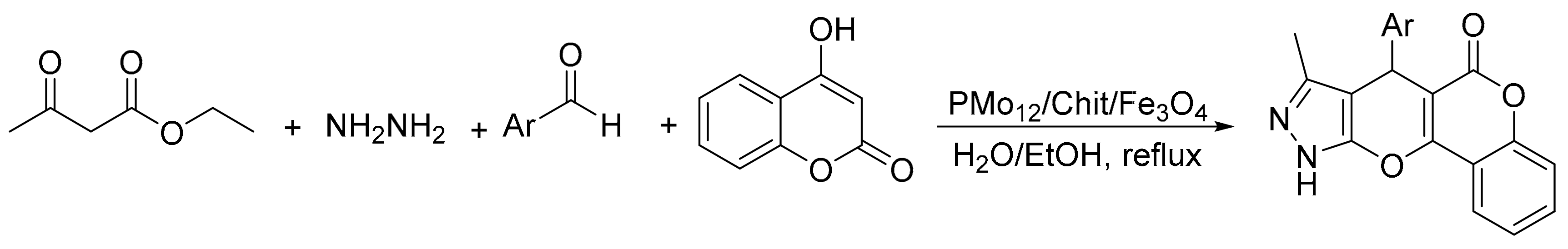
Similarly, Ayati el al. [14] reported the synthesis of a magnetic material formed by H3PMo12O40 immobilized chitosan/Fe3O4. In an experiment a solution containing ethyl acetoacetate (1 mmol), hydrazine hydrate (1 mmol), 4-hydroxy coumarin (1 mmol), aromatic aldehyde (1 mmol), and PMo12/chit/Fe3O4 (0.02 g) was dissolved in a mixture of H2O (2 mL) and EtOH (2 mL), and was stirred under reflux conditions for 2.5–4.5 h. In total, eight compounds were obtained with yields of 90 to 96% (Scheme 34). The catalyst is recyclable and could be reused without significant loss of activity.
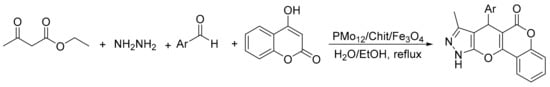
Scheme 34.
Synthesis of pyrano-pyrazole.
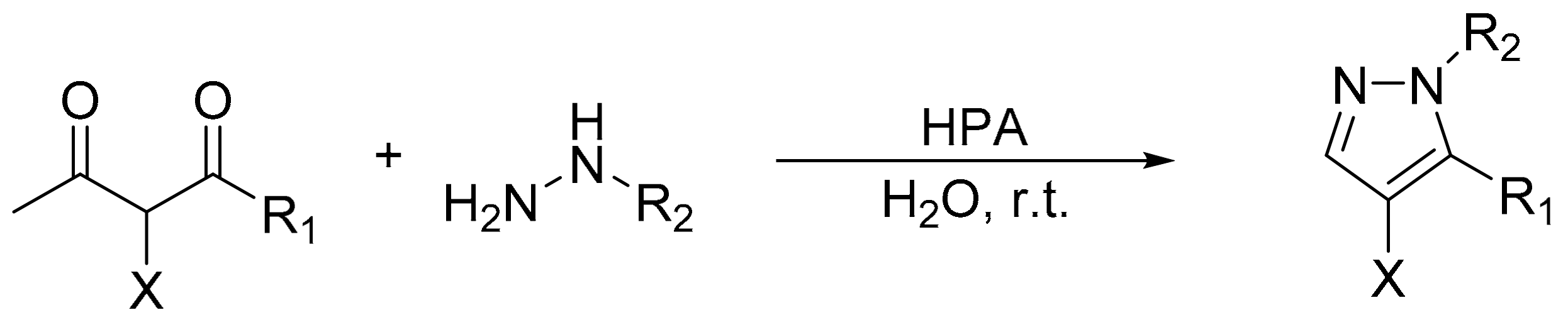
Gharib and coworkers reported the catalytic activity of Preyssler, H14[NaP 5W30O110], Wells–Dawson, H6[P2W18O62], and Keggin, H3[PW12O40] and H4 [PMo11VO40], heteropolyacids in the preparation of pyrazoles by condensation of hydrazines with various 1,3-diketones [120]. The highest yields were achieved using H14[NaP5W30O110]. The reaction conditions were a mixture of 1,3-diketone (2 mmol), hydrazines (2.2 mmol), and HPA catalysts (0.03 mmol) in water at room temperature, for 5 min (Scheme 35). By this procedure 14 pyrazoles were obtained with very yields between 77 and 96%. The authors reported that catalysts can be reused several times without any appreciable loss of activity.
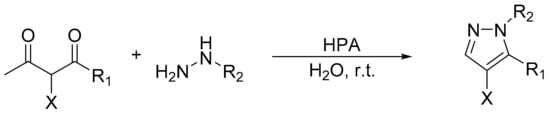
Scheme 35.
Synthesis of pyrazoles.
One of the most important azoles are imidazoles. They are a class of azoles that are characterized by having important therapeutic properties, where their anti-inflammatory, antiviral, antibacterial, antiallergenic, and antitumor activities stand out. In addition, the antihypertensive drugs Losartan and Olmesartan containing the imidazole moiety are marketed [121,122].
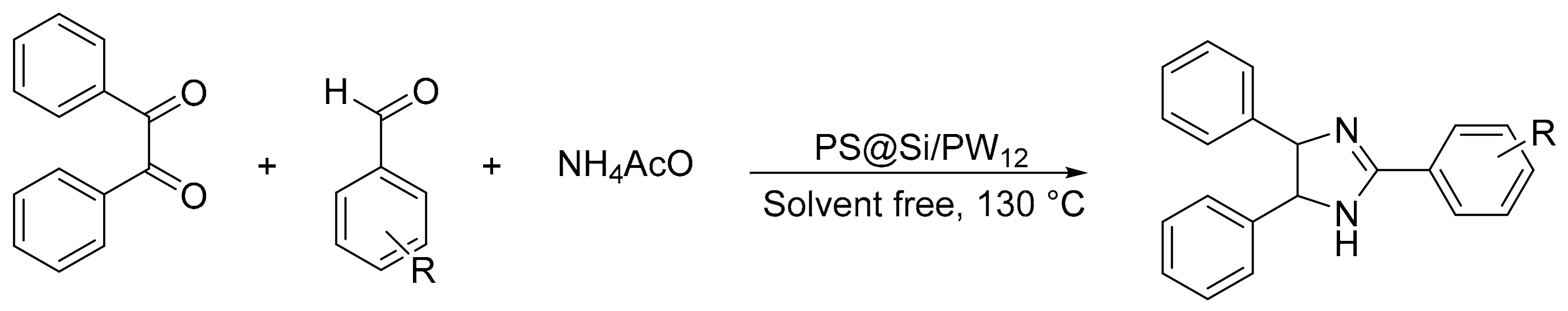
Our research group reported the synthesis of new materials based on tungstophosphoric acid supported on core-shell polystyrene-silica microspheres or hollow silica spheres (PS@Si/PW12) [123]. The catalysts were used in the synthesis of 2,4,5-triphenyl-1H-imidazole from a benzyl: benzaldehyde: ammonium acetate molar ratio of 1:1:1.2, 1 mmol% of catalyst, at 130 °C, for 90 min, under solvent-free conditions. In total, four compounds were obtained with a yield of 78 to 93% (Scheme 36).
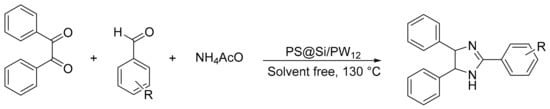
Scheme 36.
Synthesis of trisubstituted imidazole.
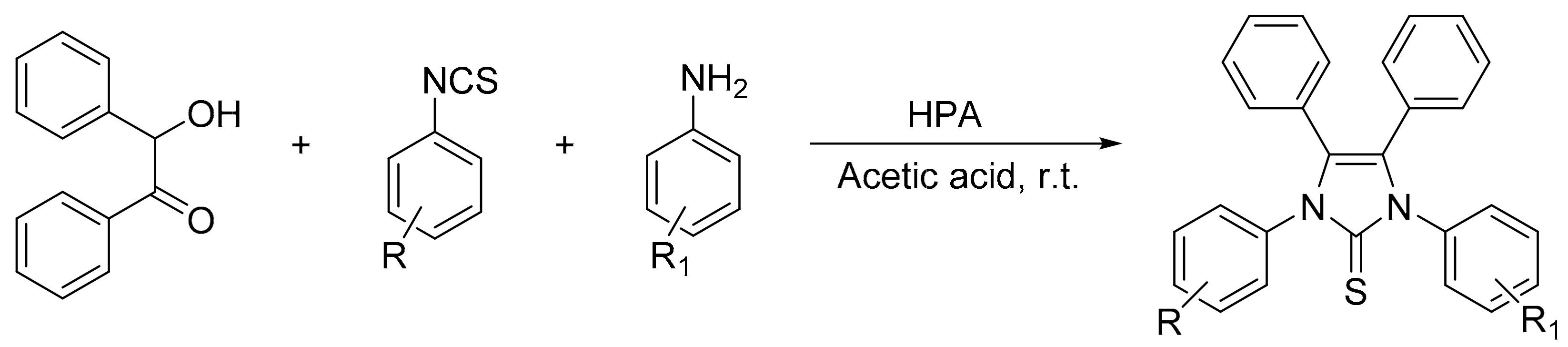
Shaker et al. [13] reported a facile one-pot three-component procedure for the preparation of new 1,3,4,5-tetrasubstituted-1H-imidazole-2(3H)-thiones assisted by bulk Preyssler and Keggin heteropolyacids using acetic acid as reaction solvent. The reaction between benzoin (1 mmol), isothiocyanates (1 mmol), and aromatic amines (1 mmol) in the presence of HPA (0.1 g) and glacial acetic acid (6 mL) at room temperature leads to the facile formation of six 1,3,4,5-tetrasubstituted-1H-imidazole-2(3H)-thiones 4a–f, although they had a great difference in the time and yield of the reaction between the HPA H3[PMo12O40] and H14[NaP5W30O110] (30 min/80–92% and 90 min/67–80%, respectively) (Scheme 37). Overall, five of them had not been previously reported in the literature.
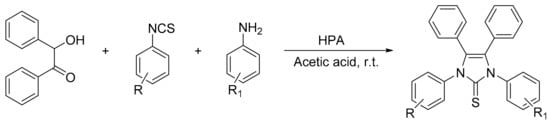
Scheme 37.
Synthesis of 1,3,4,5-tetrasubstituted-1H-imidazole-2(3H)-thiones.
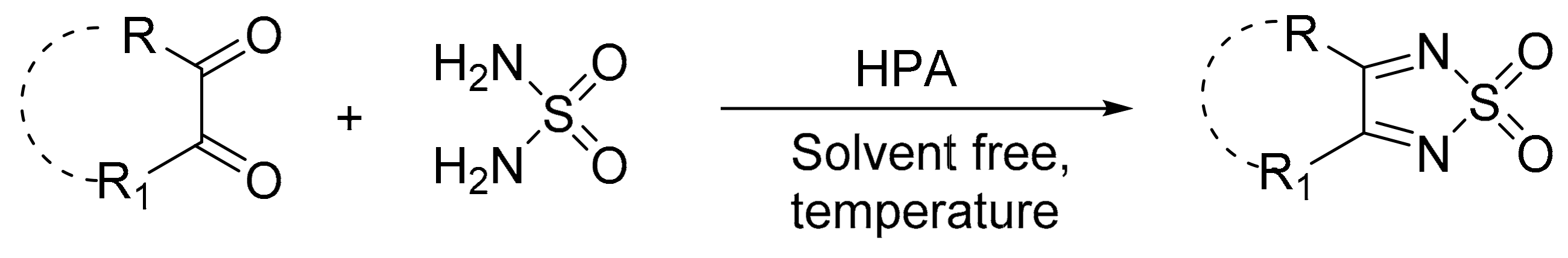
Mirifico et al. [19] obtained 1,2,5-thiadiazole 3,4-disubstituted 1,1-dioxide-derived compounds from the condensation reaction of 1,2-dicarbonyl compounds with sulfonamide, catalyzed by a bulk Keggin-type acid and supported on nanosized silica (H3PMo12O40; PMo12, PMo12supp) (Scheme 38). In the study, some conditions such as the use or absence of solvent, temperature, and molar ratio of reagents were varied to compare the results under different experimental conditions. Under suitable experimental conditions, eight compounds were obtained with yields between 49 and 93%.
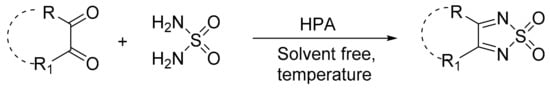
Scheme 38.
Synthesis of 3,4-disubstituted 1,2,5-thiadiazole 1,1-dioxide derivatives.
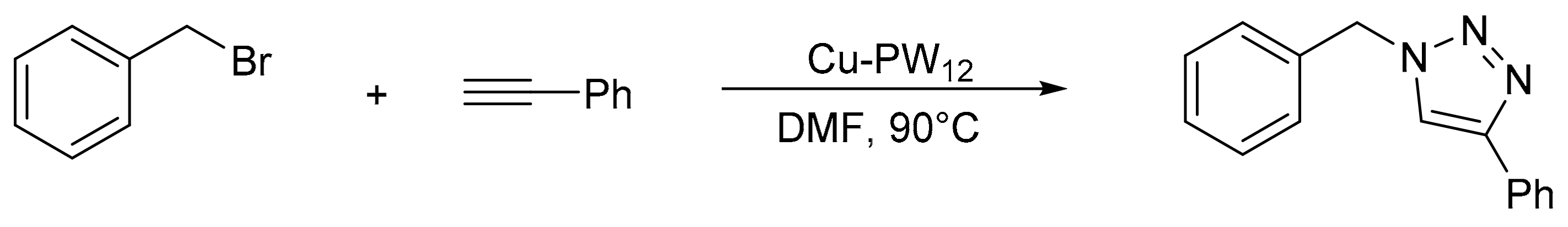
Purnima et al. [124] reported an elegant procedure for the synthesis of substituted triazoles using a catalyst of copper-exchanged phosphotungstic acid (Cu-PW12). The procedure consisted in treating a mixture of benzyl bromide (1.47 mmol) with sodium azide (1.47 mmol), phenyl acetylene (0.98 mmol), triethylamine (1.96 mmol), and Cu-PW12 (10 mol%) in the presence of dimethyl furan (DMF) (5 mL). The reaction was completed in 8–10 h at 90 °C, although some products were synthesized in less time (20–30 min). Overall, 20 compounds were obtained with yields between 77 and 93%. The catalyst was recovered and reused for three to four cycles with a minimal decrease in activity (Scheme 39).
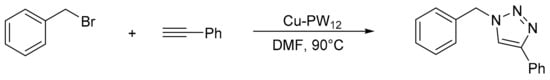
Scheme 39.
Synthesis of substituted triazoles.
10. Diazines
Diazines are heterocyclic compounds formed by a benzene ring containing two nitrogen atoms. There are three diazine systems called pyridazine (1,2-diazine), pyrimidine (1,3-diazine), and pyrazines (1,4-diazine). Their benzo analogs are also very relevant molecules and include phthalazine, cinnoline, quinazoline, pteridine, quinoxaline, and phenazine. This important family of heterocycles compounds can be found in almost every area of chemistry and biochemistry. Similarly, related six-membered heterocycles can contain only one nitrogen atom and other heteroatoms such as oxygen and sulfur [125]. Currently, new diazine-based natural substance products continue to be isolated and studied, and both they and synthetic derivatives are frequently used as flavorings or pharmaceuticals. Their diverse properties due to their structure give them a wide range of applications, ranging from medical chemistry to electrochemistry [126,127,128].
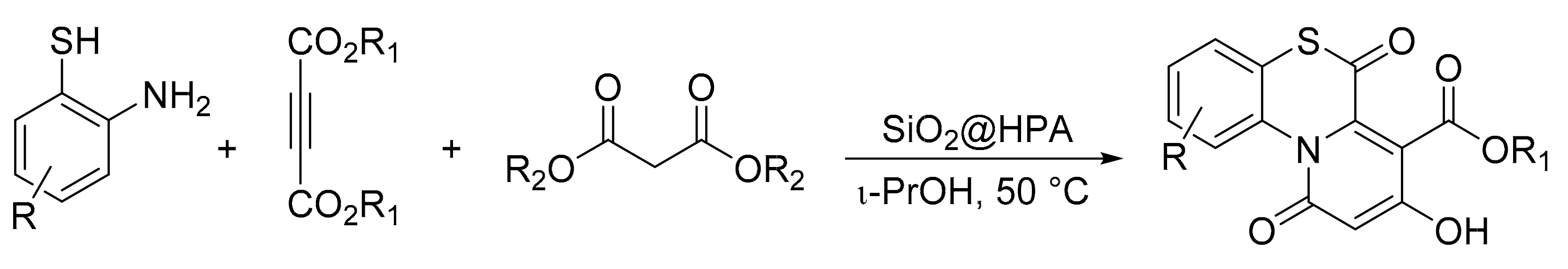
Samzadeh-Kermani et al. [15] reported a multicomponent reaction catalyzed by heteropolyacid effective for the synthesis of 1,4-benzothiazine derivatives. It was reported that H3PW12O40 was used both in bulk and supported on silica as a catalyst in a reaction involving 2-aminobenzenethiols (1 mmol), acetylenic esters (1 mmol), malonate esters (3 mmol), and HPA (0.1 mmol or 100 mg of supported HPA) in i-PrOH (3 mL) at 50 °C for 7 h. In total, 11 compounds were obtained with yields of 81 to 92% (Scheme 40).
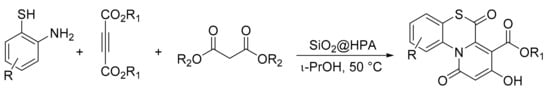
Scheme 40.
Synthesis of 1,4-benzothiazines.
Hakimi et al. [129] studied the synthesis of [3,4-b][1,3,4]thiadiazines from the condensation of 4-amino-6-methyl-3-thioxo-1,2,4-triazine-5(2H)-one (AMTTO) or 4-amino-1,4-dihydro-5-methylene-1,2,4-triazole-5-thione (AMTT) with phenacyl bromide in the presence of a catalytic amount of various Keggin-type heteropolyacids, including H3[PW12O40], H4[SiW12O40], K7[PMo2W9O40], and H3[PW12O40]·6H2O-SiO2 under reflux conditions. A mixture of AMTTO or AMTT (2 mmol), phenacyl bromide (2 mmol) and 0.04 g of heteropolyacid was added to a solution of sodium (2 mmol) in ethanol (30 mL). The reaction mixture was stirred for an appropriate time under reflux conditions. The best yields were achieved with the supported catalyst PW12/SiO2 (0.04) in 45 min with yields of 95 and 93% (Scheme 41).
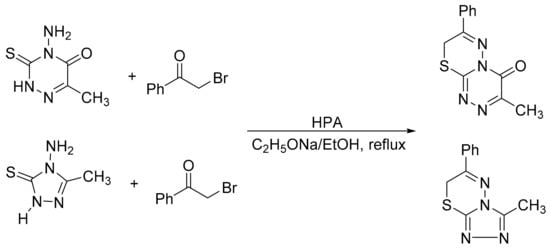
Scheme 41.
Synthesis of [3,4-b][1,3,4]thiadiazines.
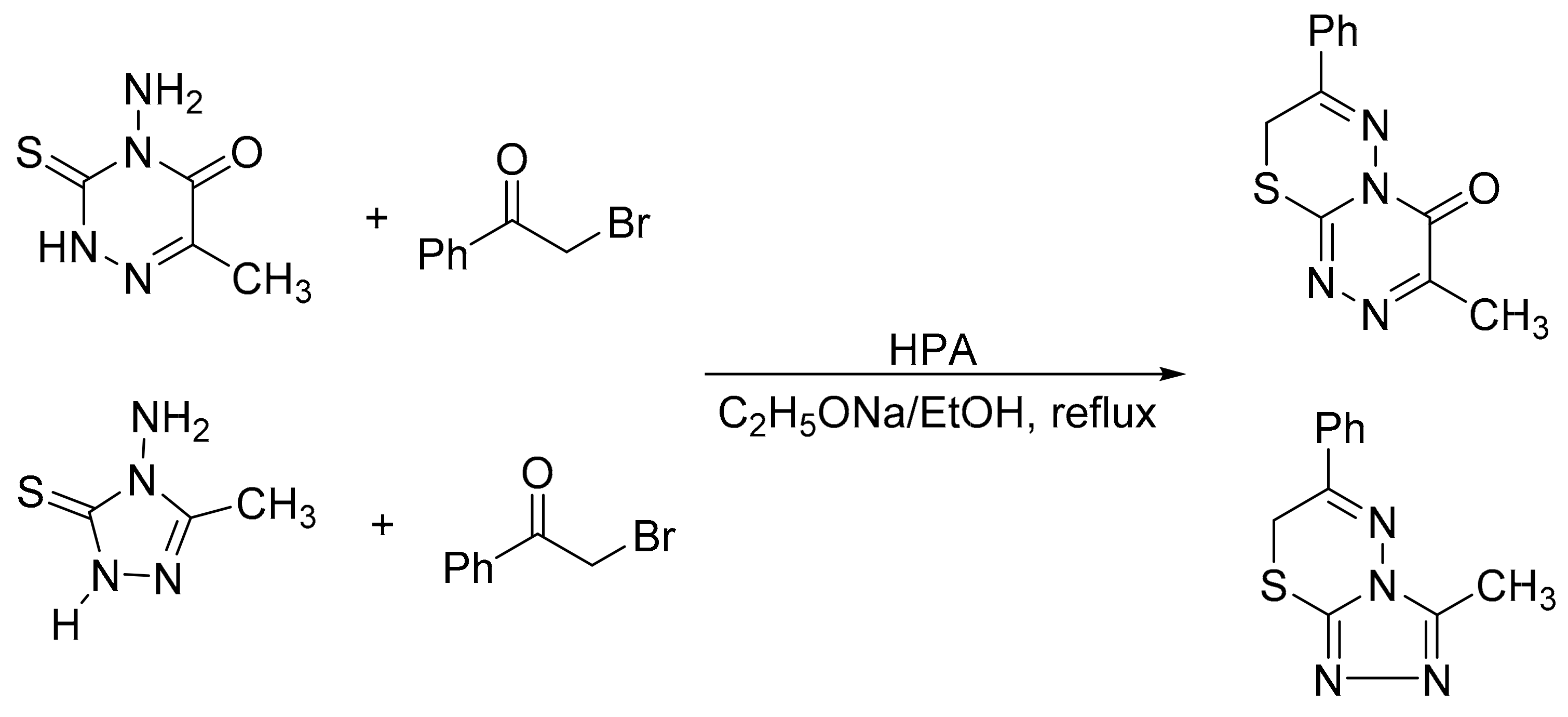
Motamedi et al. [130] reported a one-pot synthesis of [1,2,4]triazolo[3,4-b][1,3,4]thiadiazines and 3,7-dimethyl-4H-[1,2,4]triazino [3,4-b][1,3,4]thiadiazin-6-one by cyclocondensation reaction with α-chloroacetonitrile and α-haloketones with HPA as catalyst. The highest yields were obtained with Preyssler catalyst, H14[NaP5W29MoO110]. A mixture of 4-amino-5-substituted-1,2,4-triazole-3-thiones or 4-amino-6-methyl-1,2,4-triazine-3(2H)-thion-5-one (0.9 mmol) and HPA (0.04 mmol in acetic acid (10 mL) was refluxed for 10 min/3 h depending on the substrate. Seven compounds were obtained with yields of 83 to 98% (Scheme 42).
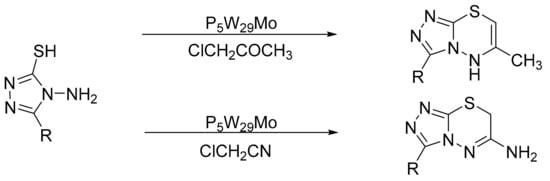
Scheme 42.
Synthesis of thiadiazines.
Motamedi et al. [131] also reported a regioselective procedure for the synthesis of 2-substituted [1,2,4]triazolo[5,1-b][1,3] thiazin-7-ones assisted by heteropolyacids. A mixture of 5-substituted-3-(4H-[1,2,4]triazol-3-ylsulfanyl)-acrylic acids and the appropriate Preyssler heteropolyacid (0.04 mmol) in acetic acid (20 mL) was stirred for 10–15 min at room temperature. Three compounds were obtained with yields between 78 and 90% (Scheme 43).
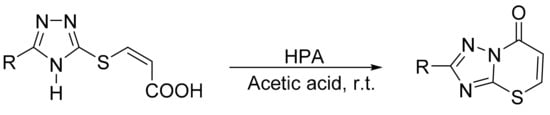
Scheme 43.
Synthesis of thiazinones.
Gharib et al. [132] presented the synthesis of polysubstituted quinolines in the presence of silica-supported Preyssler nanoparticles, H14[NaP5W30O110]/SiO2 (SiO2/P5W30). A mixture of 2-aminoaryl ketone (1.0 mmol), α-methylene ketone (1 mmol) and heteropolyacid as catalyst (0.05 mmol) and water (1.0 mL) was refluxed for 2 h. In these conditions 29 substituted quinolines were obtained with yields of 89 to 96% (Scheme 44). The catalyst is recyclable and reusable without loss of catalytic activity (five cycles, 94–97%).
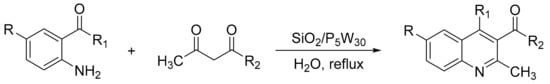
Scheme 44.
Synthesis of polysubstituted quinolines.
Similarly, our research group reported a green procedure for the synthesis of polysubstituted quinolines based on the use of tungstophosphoric acid included in a commercial polymeric matrix of polyacrylamide (APTPOL60; PW12/Pol60). The procedure consisted in the reaction of a variety of 2-aminoaryl ketones (1 mmol), methyl acetoacetate (1.2 mmol), and PW12/Pol60 (100 mg) in absolute ethanol (5 mL), at 78 °C. The catalyst did not leach after several reaction cycles. Seven compounds were synthesized with yields between 89 and 99% [133].
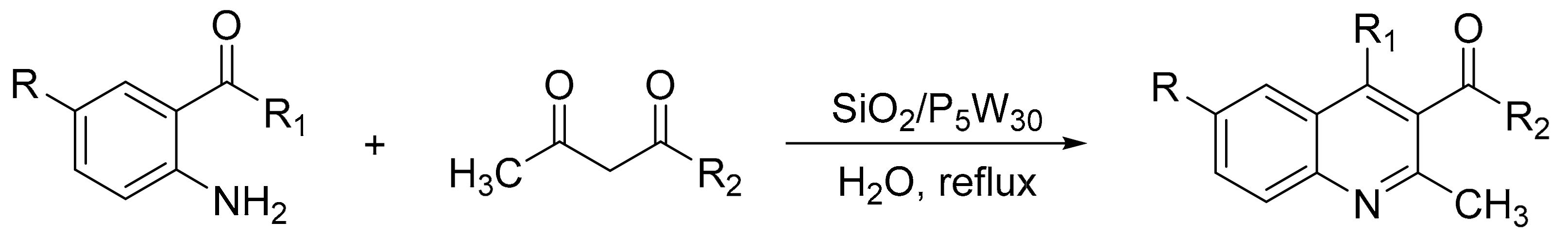
Bentarzi et al. [134] reported the synthesis of compounds structurally related to quinolines using the Friedlander methodology. HPAs exhibit high yields, in particular the silicates, SiW12 and SiMo12, with yield between 73 and 93%. In a typical procedure a mixture of 2-aminocotinaldehyde (1 mmol), α-methylene carbonyl compound (1 mmol), and catalyst (0.2 g) was refluxed in ethanol (15 mL). Overall, six products were obtained (Scheme 45).
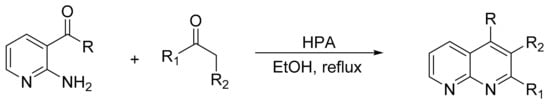
Scheme 45.
Synthesis of quinoline derivatives.
Heravi’s group reported new conditions for the Friedländer synthesis of quinolines using Wells–Dawson catalyst. A mixture of 2-amino acetophenone (1 mmol), ethyl acetoacetate (1.2 mmol) and H6[P2W18O62] (9x10−3 mmol) was stirred at 70 °C under solvent-free conditions for 2–5 h. In total, seven compounds were obtained with yields of 75 to 93% free of secondary products. The catalyst was reused for five cycles (92–90%) [135].
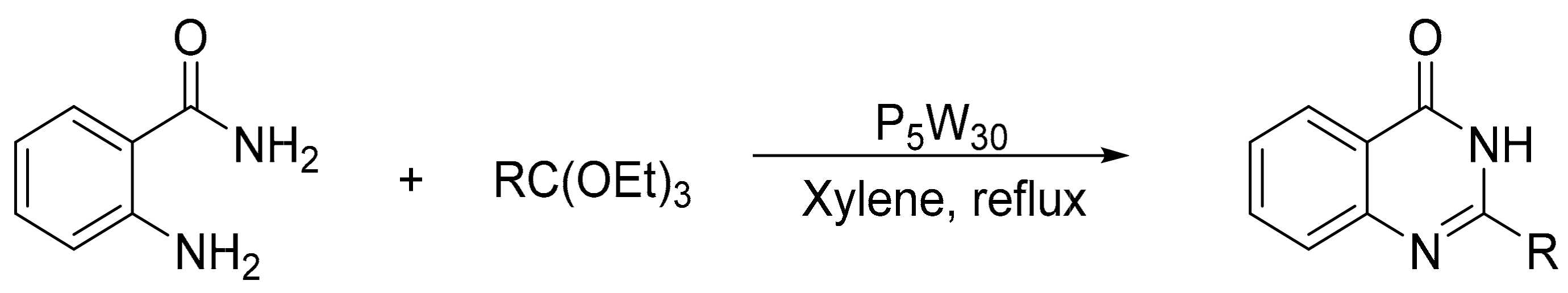
Allameh et al. [136] studied a highly efficient procedure for the synthesis of quinazolin-4(3H)-ones from the condensation of 2-aminobenzamide (0.01 mol), triethylorthoesters (0.015 mol), and heteropolyacids (0.15 mol%) as catalyst. The mixture was refluxed in various solvents for 18–40 min. The most effective catalysts were H14[NaP5W30O110], and the ideal solvent, xylene. Three compounds of quinazolinones were obtained with yields above 92% (Scheme 46).
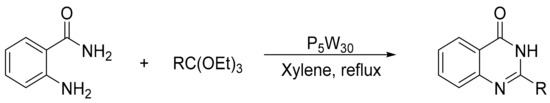
Scheme 46.
Synthesis of quinazolin-4(3H)-ones.
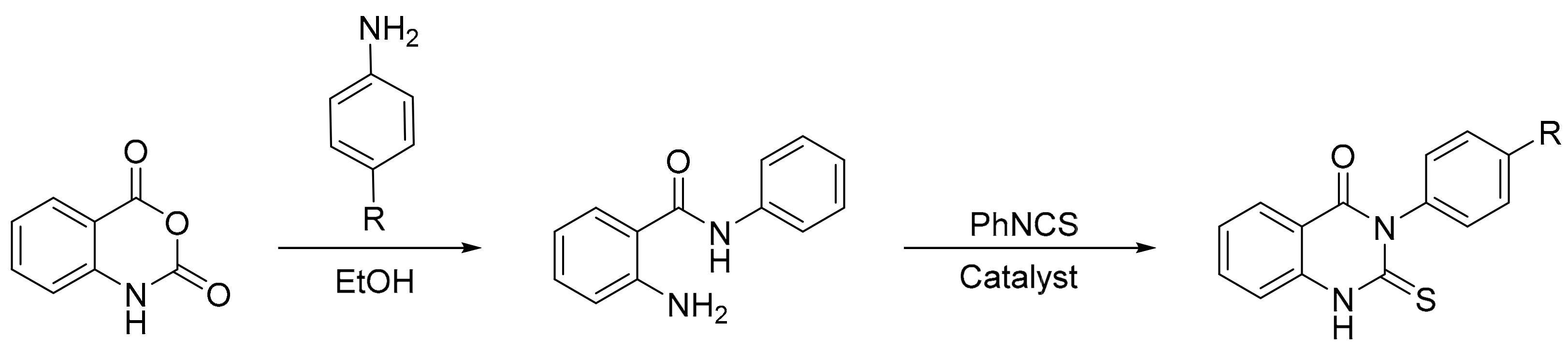
The same authors reported a procedure involving the reaction of isatoic anhydride with aniline derivatives that gave 2-amino-N-(aryl)benzamides. The products reacted with phenylisothiocyanate in the presence of Preyssler heteropolyacids afforded 3-(aryl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one derivatives (Scheme 47). A mixture of 2-amino-N-(aryl)benzamides (0.01 mol), phenylisothiocyanate (0.01 mol), and an appropriate amount of heteropolyacid (0.15 mol%) was refluxed in various solvents for 36–45 min. The best reaction condition obtained was using xylene as solvent, and three new compounds were synthesized with yields above 84% [137].
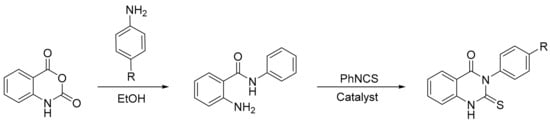
Scheme 47.
Synthesis of 3-(aryl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one derivatives.
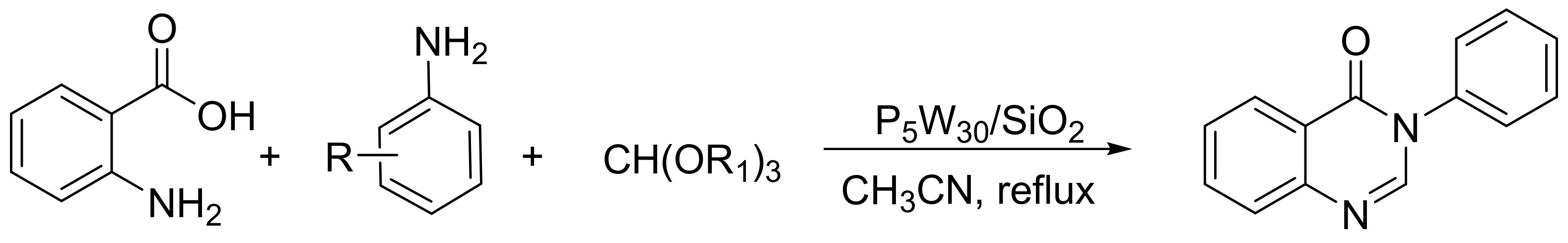
Heravi et al. [138] reported a new type of Preyssler heteropolyacid based on its inclusion in silica nanoparticles and its use in the synthesis of 4(3H)-quinazolinones (Scheme 48). A mixture of anthranilic acid (10 mmol), orthoester (10 mmol), substituted aniline (10 mmol), and silica-supported Preyssler nanoparticles (0.03 mmol) was refluxed in 5 mL of acetonitrile for 15 min. In total, 12 compounds were obtained with yields of 88 to 98%. The catalyst was used five times without appreciable loss of catalytic activity (98–91%).
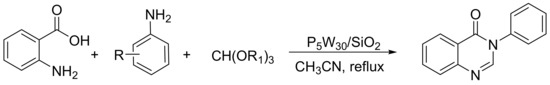
Scheme 48.
Synthesis of 4(3H)-quinazolinones.
The same authors presented a similar study about the use of Keggin heteropolyacids in the synthesis of twelve 4-arylaminoquinazolines with yields between 82 and 95%. The most active catalyst was a Keggin heteropolyacid doped with vanadium (H6[PMo9V3O40]) [139]. This research group also studied a rapid and efficient synthesis of 4(3H)-quinazolinones under ultrasonic irradiation using silica-supported Preyssler nanoparticles (H14[NaP5W30O110])/SiO2; P5W30/SiO2) for the reaction between 2-aminobenzamide and acyl chlorides (Scheme 49). A mixture of 2-amino-benzamide (10 mmol), acylchlorides (10 mmol) and a catalytic amount of P5W30/SiO2 (0.03 mmol) in CH3CN (10 mL) was irradiated by ultrasound at room temperature for 5–10 min. In total, five 4(3H)-quinazolinones were obtained with yields of 88% to 95%. The reaction requires 35 to 65 min to achieve comparable yields without the use of ultrasound. The catalyst reusability (three cycles, 95–91%), and easy workup procedure are other advantages of this methodology [140].
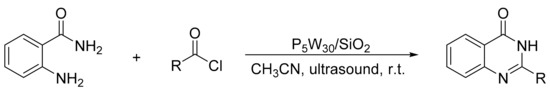
Scheme 49.
Synthesis of 4(3H)-quinazolinones.
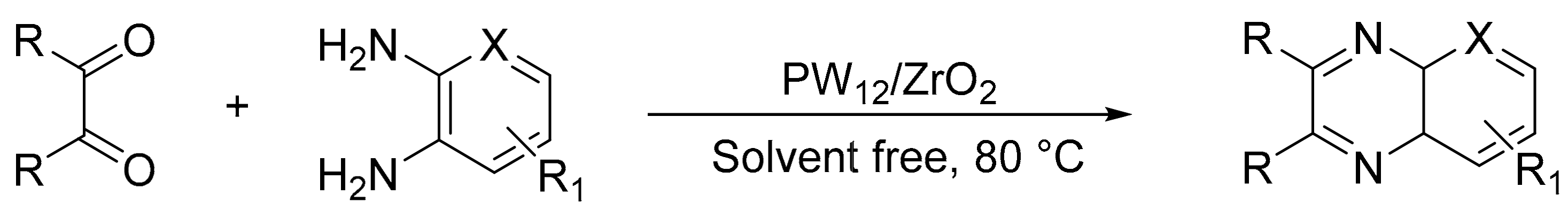
Our research group obtained a heterogeneous catalyst where tungstophosphoric acid was supported on zirconia oxide, and it was evaluated in the synthesis of quinoxaline derivatives by a condensation of 1,2-diamines with 1,2-dicarbonyl compounds, in a solvent-free conditions and with conventional heating [140,141]. A mixture of the corresponding benzyl (1.2 mmol), diamine, and PW12/ZrO2 (1 mmol%, approx. 30 mg) was stirred at 80 °C, generally for a short time period (5–20 min). Quinoxaline derivatives (11 compounds) were formed with yields between 65 and 100%. The advantages of the procedure include the easy recovery and washing of the catalyst, as well as multiple uses without a significant loss of its activity (Scheme 50).
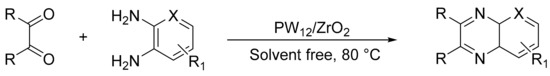
Scheme 50.
Synthesis of quinoxalines.
Huang et al. [142] reported a similar procedure using Keggin heteropolyacid in bulk form. A representative experiment involves a mixture of 1,2-diamine 1 (1 mmol), 1,2-diketone 2 (1 mmol), and H4SiW12O40 (1 mol %) in water (3 mL). The mixture was stirred at room temperature for 1–1.5 h (eight compounds, 90–96%). The less reactive aromatic diamines such as 4-nitro, gave the desired product in low yield under the same reaction conditions.
11. Azepines
Azepines are seven-membered heterocycles with a nitrogen atom in their structure. This scaffold is found in the molecular skeleton of some natural products, as well as in many pharmaceutical products, such as cetiedil and mecillinam [143]. Azepines exist in four tautomeric forms, such as 1H-, 2H-, 3H-, and 4H-azepines, and of these 3H-azepines are the most stable system. Like all fully unsaturated seven-membered heterocycles, they are antiaromatic because they do not meet the (4n + 2)π electron criteria for aromaticity and are mostly highly unstable. When two nitrogen atoms are present in the structure, they are called diazepines and exist in five tautomeric forms. These heterocycles can be fused to other rings generating molecular diversity, the nests with one or two benzene rings being the most studied and generally are more stable than their parental monocyclic molecules, namely, benzazepine. Similarly, diazepines fused with one or two benzene rings are called benzodiazepines and dibenzodiazepines, respectively [144]. Derivatives of benzodiazepines are of pharmacological importance especially in the treatment of psychiatric and neurological disorders as potential tranquilizers, sedatives, central nervous system depressants, anti-inflammatories, anticonvulsants, muscle relaxants, antispasmodics, antipsychotics, and hypnotics. In fact, some of the most widely used anxiolytics contain benzodiazepines such as chlordiazepoxide and diazepam, while clozapines are used mainly for schizophrenia [145,146,147,148,149].
Kaoua et al. [150] reported a simple method to synthetize 1,4 and 1,5 benzodiazepine derivatives via the reaction of ketimine intermediates with aldehydes in the presence of Keggin-type heteropolyacids. In a representative experiment, a mixture of (E)-3-(1-((2-amino-2-methylpropyl)imino)ethyl)-4-hydroxy-6-methyl-2H-pyran-2-one or (E)-3-(1-((2-aminophenyl)imino)ethyl)-4-hydroxy-6-methyl-2H-pyran-2-one (10 mmol) and aldehyde (10 mmol) in the presence of Keggin acid catalyst (1% mmol) dissolved in ethanol (15 mL) was refluxed with stirring. High yields (88–92%) and short reaction times (15–30 min) using the most active catalyst (H5PW10V2O40, PW10V2) were obtained for both electron-releasing and electron-withdrawing substituted 1,4-diazepine and 1,5-benzodiazepine derivatives (Scheme 51).
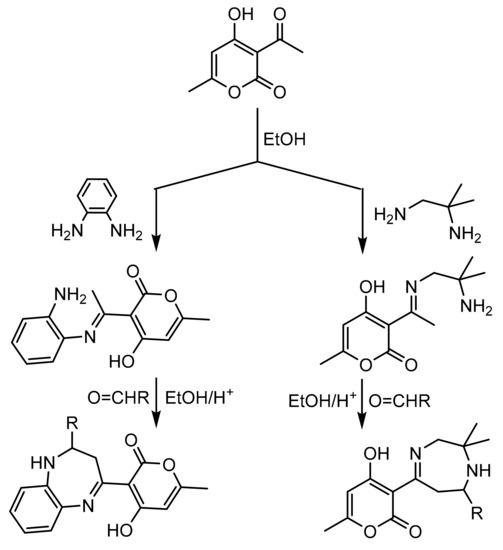
Scheme 51.
Synthesis of 1,4-diazepine and 1,5-benzodiazepine derivatives.
Our research group reported a suitable method for 1,5-benzodiazepine synthesis by condensing different o-phenylenediamines and 1,3-aryl-1,3-propanodiones in the presence of Preyssler catalyst (NaH14P5W30O110; P5W30) (Scheme 52) [151]. A mixture of 1.3-diaryl-1,3-propanodione, 1,2-phenylenediamine, and 1 mmol% of HPA (approx. 57 mg) was heated at 120 °C. The process presented simple operating conditions and good to excellent performance for the synthesis of 1,5-benzodiazepines, with the synthesis of 10 compounds and yields between 53 and 93%. The catalyst was used in five cycles without appreciable loss of its activity (93–88%).
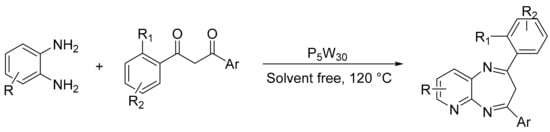
Scheme 52.
Synthesis of 1,5-benzodiazepines.
Our group also prepared and characterized new and robust materials based on the immobilization of H3PW12O40 on silica with organized multimodal porous structure (MESSI-2PTA) that were evaluated in a reaction similar to the previous one (Scheme 52). These materials were used as highly active, selective and reusable catalysts in the solventless synthesis of 3H-1,5-benzodiazepines from substituted ophenylenediamine (0.5 mmol), 1,3-diphenyl-1,3-propanedione (0.25 mmol) and MESSI-2PTA (1 mmol%), at 90 °C for 1 h [152].
Using a similar condensation procedure Hekmatshoar et al. [153] reported a suitable method to prepare 6,8-dimethyl-3-aryl-[1,2,4]triazolo[3,4-b][1,3,4]thiazepine by cyclodehydration of 3-aryl-4-amino-5-mercapto-1,2,4-triazole with 2,4-pentanedione using Preyssler as catalyst, obtaining very good yields (Scheme 53). A mixture of 3-aryl-4-amino-5-mercapto-1,2,4-triazole (1 mmol), 2,4-pentanedione (1 mmol), and catalyst (0.01 mmol) was refluxed at 90 °C for 1.5 h in solvent-free conditions. In total, five compounds were obtained with a yield of 78 to 93%.
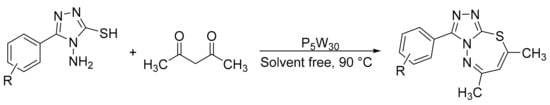
Scheme 53.
Synthesis of thiazepine.
12. Flavones and Formylchromones
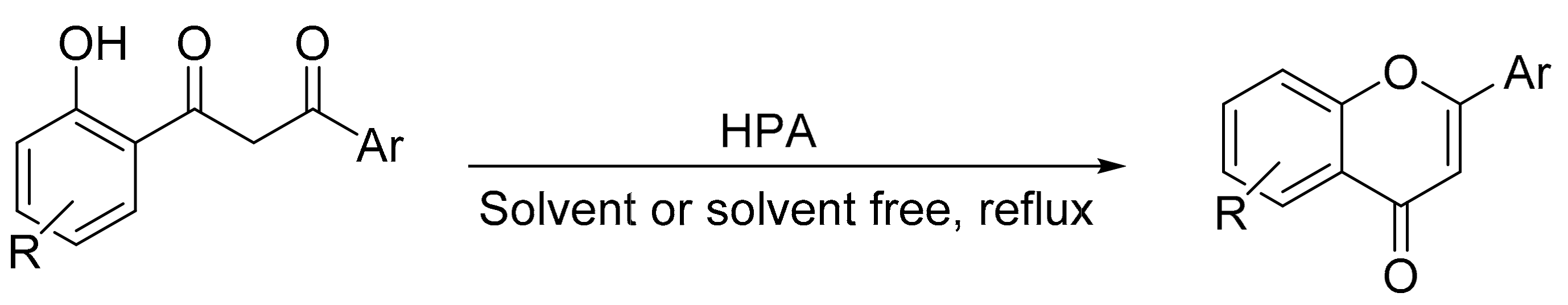
Flavones, also known as 2-phenylchromones, are polyphenolic heterocyclic systems present in various plants and in foods such as cereals, legumes, and honey, so they are considered as nutraceuticals because they are part of the human diet and provide great health benefits. Thus, the structure of the flavones (oxo group in C-4 and conjugated B and C rings) present in cereals is associated with anti-inflammatory properties, improvement in insulin sensitivity, and a greater interaction with transporters of the intestinal membrane [154,155]. Similarly, neuropharmacological actions have been attributed to flavonoids such as chrysin present in honey [156], and there are many studies on the anticancer activity of natural flavonoid compounds [157]. This has led to consider chromones as the central backbone in a number of functional organic compounds, and in the development of new drugs. Other properties identified in chromone-based compounds are optical and chelating functions, which allow their wide application in the designation of organic materials. These biological activities are attributed to their structure and multiple substituents both on the heteroaryl ring and on the phenyl fragments of the central backbone [158]. The numerous synthetic methods of flavones have been categorically classified based on the different synthetic approaches in the following manner: (i) carbonylative cyclization, (ii) cyclodehydration of 1-(2-hydroxyaryl)-3-arylpropane-1,3-dione, (iii) oxa-Michael-oxidative annulation, (iv) oxidative coupling/CeH activation, (v) oxidative dehydrogenation of flavanones [159].
Gharib et al. [160] reported the use of Preyssler and Keggin heteropolyacids in bulk (H14[NaP5W30O110], (P5W30); H14[NaP5W29MoO110]; P5W29Mo), and silica-supported (P5W29Mo/SiO2) form as catalyst for obtaining substituted chromones and flavones by direct cyclization-dehydration of 1-(2-hydroxyphenyl)-3-aryl-1,3-propanediones (Scheme 54). The reactions were performed using chloroform as solvent at reflux temperature conditions and in the absence of solvent, at 110 °C. The test in the absence of solvent involves stirred of a mixture of 1,3-diketone (0.7 mmol) and a catalytic amount of bulk Preyssler and Keggin acid (H3[PW12O40], PW12) at 110 °C for a time of 30–50 min. Overall, 11 flavones and chromones were obtained under these conditions, and although the yields were similar, the highest yield was achieved with the catalyst (P5W30), with yields between 90 and 97%. Similar results were obtained using chloroform as reaction solvent and Preyssler heteropolyacids supported on silica (P5W29Mo/SiO2 (50, 40, and 30% active phase)). The synthetic method in solvent-free conditions is a simple, clean, and environmentally friendly alternative for synthesizing substituted flavones and chromones.
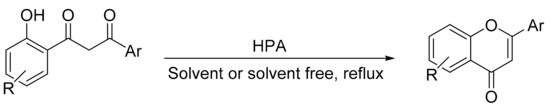
Scheme 54.
Synthesis of chromones and flavones.
Our research group developed other materials for the synthesis of these heterocyclic systems that include the use of mesoporous titania and tungstophosphoric acid (TiO2/PW12) composites [161] and silica supported Wells–Dawson heteropolyacid (H6P2W18O62·24H2O, SiO2/P2W18) [23]. Booth materials are effective catalysts for the synthesis of flavones and chromones with yields between 82 and 87% (Scheme 54).
Finally, Migliorero et al. [8] reported a similar method to prepare flavones and chromones using glycerol as suitable solvent. Glycerol is usually produced as a by-product of the transesterification of a triglyceride in the production of natural fatty acid derivatives. In addition, glycerol, which is a biodegradable, innocuous, recyclable, and reusable liquid manufactured from renewable sources, shows similar properties to an ionic liquid and has a potential use as green solvent for suitable organic transformation [162,163,164,165]. The best reactions conditions were: 1-(2-hydroxyphenyl)-3-aryl-1,3-propanodione, 1 mmol; glycerol, 30 mmol; HPA, 5% mmol; 120 °C; and 30–40 min, and in these conditions twelve flavones and chromones were prepared with yields of 82 to 98%.
13. Miscellanea
Although so far we have presented different examples of low environmental impact synthesis of the most common heterocyclic systems, to end this review we present other interesting examples of not so common heterocyclic systems. Recent contributions in this area are indicated below.
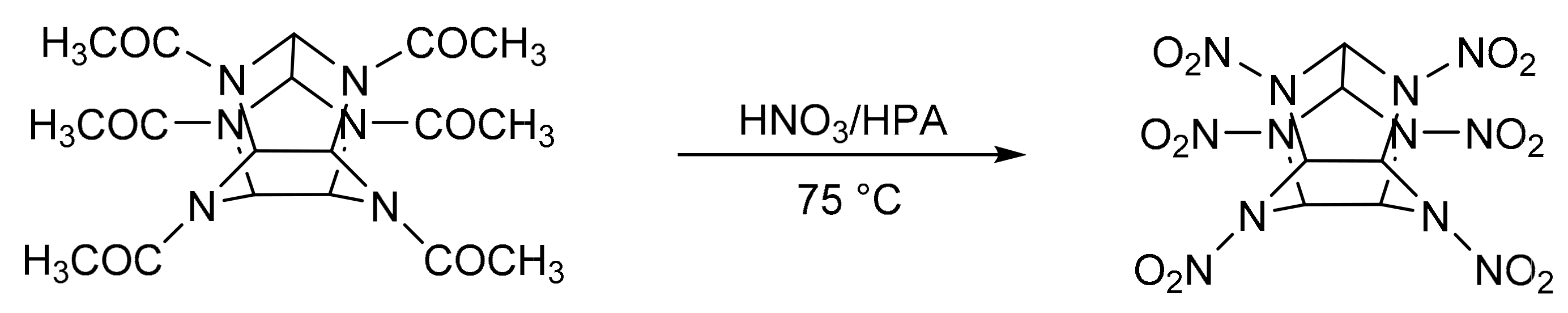
2, 4, 6, 8, 10, 12-Hexanitro-2, 4, 6, 8, 10, 12-hexaazaisowurtzitane (CL-20) is an energetic component in propellant formulations. It is prepared via nitration of precursors in the presence of concentrated mineral acids [166]. A. Ramazani et al. [167] reported the green synthesis of CL-20 in the presence of clean nitrating agents such as heteropolyacids (Scheme 55). The optimized conditions were: substrate, 1 mmol; 98% of concentrated nitric acid (10 mL) and a Keggin-type heteropolyacids, for example, H3PW12040, 100 mg, a reaction temperature of 75 °C, in a reaction time of 2 h. In this condition, a practically quantitative yield of CL-20 was obtained.
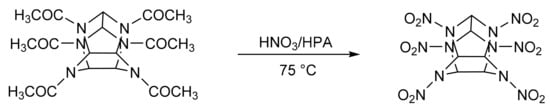
Scheme 55.
Synthesis of 2, 4, 6, 8, 10, 12-hexanitro-2, 4, 6, 8, 10, 12-hexaazaisowurtzitane (CL-20).
One interesting work for the synthesis of ozonides and tetraoxanes under heterogeneous conditions was reported by L. Yaremenko et al. [168]. H3PMo12O40 supported on SiO2 (PMo12/SiO2) was developed for the peroxidation reaction of 1,3-and 1,5-diketones with hydrogen peroxide to obtain 1,2,4,5-tetraoxanes and 1,2,4-trioxolanes with high yield and selectivity (up to 86 and 90%, respectively) under heterogeneous conditions. The optimized conditions were: substrate, 1 mmol; 7.4 M ethereal solution of H2O2 (1–3 mmol); PMo12/SiO2 (0.016 mmol); temperature, 20 °C; and reaction time, 1 h. In these conditions 11 compounds were obtained. Scheme 56 represents the several ozonides (1,2,4-trioxolanes). The new compounds exhibit antifungal activity, in some cases superior to commercial fungicides.
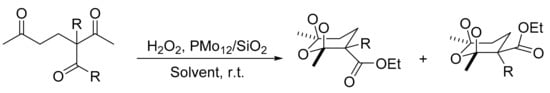
Scheme 56.
Synthesis of oxonides.
Other reactions of great importance in recent years are those for obtaining compounds of industrial and pharmacological interest from biomass derivatives, as a replacement for petrochemical derivatives as raw material. An example of this is the catalytic transformation of 5-hydroxymethylfurfural (5-HMF) obtained from biomass into high-value chemical products. Following this idea, Jihong Lan et al. [17] evaluated the performance of different vanadium-substituted Keggin-type heteropolyacid catalysts (H4PMo11VO40, H5PMo10V2O40, and H6PMo9V3O40), in the selective oxidation of 5-HMF to maleic anhydride (MA) and maleic acid (MAC), with O2 as an oxidizing agent (Scheme 57). In the procedure for the oxidation of 5-HMF to MA, PMoxVx (0.02 mmol) was dissolved in 2 mL of acetonitrile, later 1.3 mL of acetic acid, 2.4 mmol of 5-HMF, and 10 atm of oxygen were added. The mixture was heated at 90 °C for 8 h. Subsequently, for the synthesis of MAC by dehydration, the previous mixture was allowed to cool, 5 mL of acetonitrile was added, 2.5 mL of the solution was taken, 5 mL of acetic anhydride was added, and it was heated under reflux for 2 h. In this way, the generated MA was fully converted to MAC. With the H5PMo10V2O40 catalyst, the highest yield was achieved (64% the total yields of MA and MAC).

Scheme 57.
Synthesis of maleic anhydride and maleic acid.
14. Conclusions
It is well known that heterocyclic wide range of pharmaceutical and biological properties, new structures are expected to be developed in the following years. Since the techniques used to synthesize this kind of compound are versatile, the obtained structures could be very varied. Due to their wide range of biological activity in synthetic and industrial applications, the synthesis of these compounds has recently received a great deal of attention for the discovery of improved protocols towards clean, milder and high yielding approaches.
Catalysis by heteropolyacids (HPA) and related compounds is a field of increasing importance worldwide. Numerous developments are being carried out in basic research, as well as in fine chemistry processes. HPAs possess, a very strong acidity and, on the other hand, appropriate redox properties, which can be changed by varying the chemical composition of heteropolyanion.
As can be seen in this review, these catalysts were excellent since most of the products are obtained with quantitative yields free of secondary products. High atomic economy multicomponent transformations can be carried out under very mild, solvent-free reaction conditions. In addition, both bulk and supported catalysts are often used and recycled without appreciable loss of catalytic activity.
Author Contributions
Conceptualization, G.P.R. and R.L.; bibliographic search, A.M.E. and G.B.; writing—original draft preparation, A.M.E. and G.P.R.; writing—review and editing, R.L. and G.B.; supervision, G.P.R.; project administration, G.P.R.; funding acquisition, G.P.R. and G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was supported by CONICET (PIP 0084), Agencia Nacional de Promoción Científica y Técnica ANPCyT (0157), UNLP and Comisión de Investigaciones Científicas Provincia de Buenos Aires CICPBA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank to Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Universidad Nacional de La Plata (UNLP) for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, T.L.; Kim, H.; Pan, S.Y.; Tseng, P.C.; Lin, Y.P.; Chiang, P.C. Implementation of green chemistry principles in circular economy system towards sustainable development goals: Challenges and perspectives. Sci. Total Environ. 2020, 716, 136998. [Google Scholar] [CrossRef] [PubMed]
- Török, B.; Dransfield, T. Green chemistry: Historical perspectives and basic concepts. In Green Chemistry; Török, B., Timothy, D., Eds.; Elsevier: Boston, MA, USA, 2018; pp. 3–16. ISBN 9780128095492. [Google Scholar]
- Mallesham, B.; Raikwar, D.; Shee, D. The role of catalysis in green synthesis of chemicals for sustainable future. In Advanced Functional Solid Catalysts for Biomass Valorization; Hussain, C.M., Sudarsanam, P., Eds.; Elsevier: Boston, MA, USA, 2020; pp. 1–37. ISBN 978-0-12-820236-4. [Google Scholar]
- Kokel, A.; Schäfer, C. Application of green chemistry in homogeneous catalysis. In Green Chemistry; Török, B., Dransfield, T., Eds.; Elsevier: Boston, MA, USA, 2018; pp. 375–414. ISBN 9780128095492. [Google Scholar]
- Chopda, L.V.; Dave, P.N. 12-tungstosilicic acid H4[W12SiO40] over natural bentonite as a heterogeneous catalyst for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones. ChemistrySelect 2020, 5, 2395–2400. [Google Scholar] [CrossRef]
- Pálinkó, I. Heterogeneous catalysis: A fundamental pillar of sustainable synthesis. In Green Chemistry; Török, B., Dransfield, T., Eds.; Elsevier: Boston, MA, USA, 2018; pp. 415–447. ISBN 9780128095492. [Google Scholar]
- Freitas, E.F.; Souza, R.Y.; Passos, S.T.A.; Dias, J.A.; Dias, S.C.L.; Neto, B.A.D. Tuning the Biginelli reaction mechanism by the ionic liquid effect: The combined role of supported heteropolyacid derivatives and acidic strength. RSC Adv. 2019, 9, 27125–27135. [Google Scholar] [CrossRef]
- Migliorero, M.B.C.; Palermo, V.; Durango, A.A.E.; Luz Villa, H.A.; Vazquez, P.G.; Sathicq, G.A.; Romanelli, P.G. Green and efficient synthesis of flavones and chromones using heteropolyacids as catalyst in glycerol. Lett. Org. Chem. 2018, 15, 826–832. [Google Scholar] [CrossRef]
- Sadjadi, S.; Majid, M.; Heravi, M.M. Heteropolyacid creatin-halloysite clay and heterogeneous catalyst for the synthesis of benzopyranopyrimidines. Res. Chem. Intermed. 2017, 43, 6701–6717. [Google Scholar] [CrossRef]
- Sadjadi, S.; Heravi, M.M.; Zadsirjan, V.; Ebrahimizadeh, M. SBA-15 methenamine-HPA: A novel, simple, and efficient catalyst for one-pot three-component synthesis of 2-amino-4H-chromene derivatives in aqueous medium. Res. Chem. Intermed. 2017, 43, 5467–5483. [Google Scholar] [CrossRef]
- Sadjadi, S.; Heravi, M.M.; Daraie, M. Heteropolyacid supported on amine-functionalized halloysite nano clay as an efficient catalyst for the synthesis of pyrazolopyranopyrimidines via four-component domino reaction. Res. Chem. Intermed. 2016, 43, 2201–2214. [Google Scholar] [CrossRef]
- Sadjadi, S.; Heravi, M.M.; Daraie, M. Cyclodextrin nanosponges: A potential catalyst and catalyst support for synthesis of xanthenes. Res. Chem. Intermed. 2017, 43, 843–857. [Google Scholar] [CrossRef]
- Shaker, M.; Davoodnia, A.; Vahedi, H.; Lari, J.; Roshani, M.; Mallaeke, H. Synthesis of some new 1,3,4,5-tetrasubstituted-1H-imidazole-2(3H)-thiones via a facile one-pot three-component reaction in the presence of solvent and heteropolyacids. J. Heterocycl. Chem. 2017, 54, 313–317. [Google Scholar] [CrossRef]
- Ayati, A.; Heravi, M.M.; Daraie, M.; Tanhaei, B.; Bamoharram, F.F.; Sillanpaa, M. H3PMo12O40 immobilized chitosan/Fe3O4 as a novel efficient, green and recyclable nanocatalyst in the synthesis of pyrano-pyrazole derivatives. J. Iran. Chem. Soc. 2016, 13, 2301–2308. [Google Scholar] [CrossRef]
- Samzadeh-Kermani, A. Yield of benzothiazine derivatives and catalysis by heteropolyacids. J. Sulfur Chem. 2016, 37, 692–701. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Li, S.; Jin, Q.; Zhao, J. Review on oxidative desulfurization of fuel by supported heteropolyacid catalysts. J. Ind. Eng. Chem. 2020, 82, 1–16. [Google Scholar] [CrossRef]
- Lan, J.; Lin, J.; Chen, Z.; Yin, G. Transformation of 5-hydroxymethylfurfural (HMF) to maleic anhydride by aerobic oxidation with heteropolyacid catalysts. ACS Catal. 2015, 5, 2035–2041. [Google Scholar] [CrossRef]
- Palermo, V.; Sathicq, Á.G.; Constantieux, T.; Rodríguez, J.; Vázquez, P.G.; Romanelli, G.P. First report about the use of micellar keggin heteropolyacids as catalysts in the green multicomponent synthesis of nifedipine derivatives. Catal. Lett. 2016, 146, 1634–1647. [Google Scholar] [CrossRef]
- Arroyo, N.R.; Rozas, M.F.; Vázquez, P.; Romanelli, G.P.; Mirífico, M.V. Solvent-free condensation reactions to synthesize five-membered heterocycles containing the sulfamide fragment. Synthesis 2016, 48, 1344–1352. [Google Scholar] [CrossRef]
- Benferrah, N.; Hammadi, M.; Dokari, H.; Villemin, D. Tungstosilicic acid (H4SiWO) have efficient catalysts for synthesis of lawsone. Der Pharma Chem. 2016, 8, 6–9. [Google Scholar]
- Védrine, J.C. Importance, features and uses of metal oxide catalysts in heterogeneous catalysis. Chin. J. Catal. 2019, 40, 1627–1636. [Google Scholar] [CrossRef]
- Rahimi, S.; Amrollahi, M.A.; Kheilkordi, Z. An efficient ultrasound-promoted method for the synthesis of bis(indole) derivatives. Comptes Rendus Chim. 2015, 18, 558–563. [Google Scholar] [CrossRef]
- Bennardi, D.O.; Romanelli, G.P.; Sathicq, Á.G.; Autino, J.C.; Baronetti, G.T.; Thomas, H.J. Wells-Dawson heteropolyacid as reusable catalyst for sustainable synthesis of flavones. Appl. Catal. A Gen. 2011, 404, 68–73. [Google Scholar] [CrossRef]
- Sathicq, Á.G.; Ruiz, D.M.; Constantieux, T.; Rodriguez, J.; Romanelli, G.P. Preyssler heteropoly acids encapsulated in a silica framework for an âefficient preparation of fluorinated hexahydropyrimidine derivatives under solvent-free conditions. Synlett 2014, 25, 881–883. [Google Scholar] [CrossRef]
- Balci, M. Recent advances in the synthesis of fused heterocycles with new skeletons via alkyne cyclization. Tetrahedron Lett. 2020, 61, 151994. [Google Scholar] [CrossRef]
- Romanelli, G.; Autino, J. Recent applications of heteropolyacids and related compounds in heterocycles synthesis. Mini. Rev. Org. Chem. 2009, 6, 359–366. [Google Scholar] [CrossRef]
- He, Z.-X.; Zhao, T.-Q.; Gong, Y.-P.; Zhang, X.; Ma, L.-Y.; Liu, H.-M. Pyrimidine: A promising scaffold for optimization to develop the inhibitors of ABC transporters. Eur. J. Med. Chem. 2020, 200, 112458. [Google Scholar] [CrossRef]
- Upadhyay, A.; Gopal, M.; Srivastava, C.; Pandey, N.D. Synthesis and insecticidal activity of 3,4-dihydropyrimidine-2(1H)-thiones against the pulse beetle, Callosobruchus chinensis. J. Pestic. Sci. 2011, 36, 467–472. [Google Scholar] [CrossRef]
- Sekhar, T.; Thriveni, P.; Krishna, H.M.; Ramesh, K.; Jasmine, S.M.; Allam, U.S. Synthesis and antibacterial activity of novel (4-Fluorophenyl)(4-(naphthalen-2-yl)-6-aryl-2-thioxo-2,3-dihydropyrimidin-1(6 H)-yl)methanone derivatives. J. Heterocycl. Chem. 2019, 56, 44–50. [Google Scholar] [CrossRef]
- Bhat, M.; Al-Dhfyan, A.; Al-Omar, M. Targeting cancer stem cells with novel 4-(4-Substituted phenyl)-5-(3,4,5-trimethoxy/3,4-dimethoxy)-benzoyl-3,4-dihydropyrimidine-2(1H)-one/thiones. Molecules 2016, 21, 1746. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Huber, A.D.; Casey, M.C.; Kirby, K.A.; Wilson, D.J.; Kankanala, J.; Xie, J.; Parniak, M.A.; Sarafianos, S.G.; et al. 6-Arylthio-3-hydroxypyrimidine-2,4-diones potently inhibited HIV reverse transcriptase-associated RNase H with antiviral activity. Eur. J. Med. Chem. 2018, 156, 652–665. [Google Scholar] [CrossRef]
- Pathania, S.; Rawal, R.K. Pyrrolopyrimidines: An update on recent advancements in their medicinal attributes. Eur. J. Med. Chem. 2018, 157, 503–526. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, G.; Sathicq, Á.; Vázquez, P.; Villa, A.; Alarcón, E.; Grajales, E.; Cubillos, J. Green synthesis of 6-cyano-2,2-dimethyl-2-H-1-benzopyran and its subsequent enantioselective epoxidation. J. Mol. Catal. A Chem. 2015, 398, 11–16. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, N.; Xu, J.; Zhang, W.; Kong, F. Microwave-assisted and iodine-catalyzed synthesis of dihydropyrimidin-2-thiones via Biginelli reaction under solvent-free conditions. Synth. Commun. 2013, 43, 139–146. [Google Scholar] [CrossRef]
- Tayebee, R.; Amini, M.M.; Ghadamgahi, M.; Armaghan, M. H5PW10V2O40/Pip-SBA-15: A novel reusable organic-inorganic hybrid material as potent Lewis acid catalyst for one-pot solvent-free synthesis of 3,4-dihydropyrimidinones. J. Mol. Catal. A Chem. 2013, 366, 266–274. [Google Scholar] [CrossRef]
- D’alessandro, O.; Sathicq, Á.; Palermo, V.; Sanchez, L.; Thomas, H.; Vazquez, P.; Constantieux, T.; Romanelli, G. Doped keggin heteropolyacids as catalyst in the solvent-free, multicomponent synthesis of substituted 3,4-dihydropyrimidin-2-(1H)-ones. Curr. Org. Chem. 2012, 16, 2763–2769. [Google Scholar] [CrossRef]
- Palermo, V.; Sathicq, Á.; Constantieux, T.; Rodríguez, J.; Vázquez, P.; Romanelli, G. New vanadium keggin heteropolyacids encapsulated in a silica framework: Recyclable catalysts for the synthesis of highly substituted hexahydropyrimidines under suitable conditions. Catal. Lett. 2015, 145, 1022–1032. [Google Scholar] [CrossRef]
- Fang, Z.D.; Fang, D.; Tan, Y.Y. Synthesis of 2-substituted 7-methylpyrimido[4,5-d]pyrimidin-4(3H)-ones catalyzed by heteropolyacids. Res. Chem. Intermed. 2013, 41, 1203–1211. [Google Scholar] [CrossRef]
- Pourghobadi, Z.; Derikvand, F. H3PMo12O40 catalyzed three-component one-pot synthesis of 4,6-diarylpyrimidin-2(1H)-ones under solvent-free conditions. Chin. Chem. Lett. 2010, 21, 269–272. [Google Scholar] [CrossRef]
- Heravi, M.M.; Derikvand, F.; Ranjbar, L.; Bamoharram, F.F. H6P2W18O62-18H2O, a green and reusable catalyst for the three-component, one-pot synthesis of 4,6-diarylpyrimidin-2(1H)-ones under solvent-free conditions. Synth. Commun. 2010, 40, 1256–1263. [Google Scholar] [CrossRef]
- Heravi, M.M.; Sadjadi, S.; Sadjadi, S.; Oskooie, H.A.; Shoar, R.H.; Bamoharram, F.F. Supported preyssler nanoparticles in synthesis of 1,3-diaryl-5-spirohexahydropyrimidines. J. Chin. Chem. Soc. 2009, 56, 246–250. [Google Scholar] [CrossRef]
- Sanchez, L.; Sathicq, A.; Thomas, H.; Romanelli, G. Synthesis of dihydropyridines: Patented catalysts and biological applications. Recent Pat. Catal. 2012, 1, 119–128. [Google Scholar] [CrossRef]
- Shaldam, M.A.; Elhamamsy, M.H.; Esmat, E.A.; El-Moselhy, T.F. 1,4-dihydropyridine calcium channel blockers: Homology modeling of the receptor and assessment of structure activity relationship. ISRN Med. Chem. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Velena, A.; Zarkovic, N.; Troselj, G.K.; Bisenieks, E.; Krauze, A.; Poikans, J.; Duburs, G. 1,4-dihydropyridine derivatives: Dihydronicotinamide analogues—Model compounds targeting oxidative stress. Oxid. Med. Cell. Longev. 2016, 2016, 1–35. [Google Scholar] [CrossRef]
- Klusa, V. Atypical 1,4-dihydropyridine derivatives, an approach to neuroprotection and memory enhancement. Pharmacol. Res. 2016, 113, 754–759. [Google Scholar] [CrossRef]
- Godhani, D.R.; Dobariya, P.B.; Jogel, A.A.; Sanghani, A.M.; Mehta, J.P. An efficient synthesis, characterization, and antimicrobial screening of tetrahydropyrimidine derivatives. Med. Chem. Res. 2014, 23, 2417–2425. [Google Scholar] [CrossRef]
- Idhayadhulla, A.; Kumar, R.S.; Nasser, A.J.A.; Kavimani, S.; Indhumathy, S. Anti-inflammatory activity of new series of 1,4-dihydropyridine derivatives. Pharm. Chem. J. 2015, 49, 463–466. [Google Scholar] [CrossRef]
- Sirisha, K.; Achaiah, G.; Reddy, V.M. Facile synthesis and antibacterial, antitubercular, and anticancer activities of novel 1,4-dihydropyridines. Arch. Pharm. 2010, 343, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Dhanjal, J.K.; Goyal, S.; Tyagi, C.; Hamid, R.; Grover, A. Development of dual inhibitors against Alzheimer’s disease using fragment-based QSAR and molecular docking. Biomed. Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malek, R.; Maj, M.; Wnorowski, A.; Jóźwiak, K.; Martin, H.; Iriepa, I.; Moraleda, I.; Chabchoub, F.; Marco-Contelles, J.; Ismaili, L. Multi-target 1,4-dihydropyridines showing calcium channel blockade and antioxidant capacity for Alzheimer’s disease therapy. Bioorg. Chem. 2019, 91, 103205. [Google Scholar] [CrossRef]
- Swarnalatha, G.G.; Prasanthi, N.; Sirisha, C.M.C. 1,4-dihydropyridines: A multtifunctional molecule—A review. Int. J. ChemTech Res. 2011, 3, 75–89. [Google Scholar]
- Khedkar, S.; Auti, P. 1,4-dihydropyridines: A class of pharmacologically important molecules. Mini Rev. Med. Chem. 2014, 14, 282–290. [Google Scholar] [CrossRef]
- Praveenkumar, E.; Gurrapu, N.; Kolluri, K.P.; Yerragunta, V.; Kunduru, R.B.; Subhashini, N.J.P. Synthesis, anti-diabetic evaluation and molecular docking studies of 4-(1-aryl-1H-1,2,3-triazol-4-yl)-1,4-dihydropyridine derivatives as novel 11-βhydroxysteroid dehydrogenase-1 (11β-HSD1) inhibitors. Bioorg. Chem. 2019, 90, 103056. [Google Scholar] [CrossRef]
- Duburs, G.; Vigante, B.; Plotniece, A.; Krauze, A.; Sobolevs, A.; Briede, J.; Klusa, V.; Velena, A. Dihydropyridine derivatives as bioprotectors. Chem. Today 2008, 26, 68–70. [Google Scholar]
- Gharib, A.; Jahangir, M.; Roshani, M.; Scheeren, J.W. Catalytic synthesis of fused 1,4-dihydropyridines and 1,4-dihydropyridine derivatives using preyssler heteropolyacids catalyst. Synth. Commun. 2012, 42, 3311–3320. [Google Scholar] [CrossRef]
- Baradaran-Sirjani, Z.; Roshani, M.; Khashi, M.; Beyramabadi, A.S. Catalytic performance of Preyssler heteropolyacids and synthesis, experimental, theoretical characterizations, fluorescence properties of 1,8-dioxodecahydroacridine derivative. Indian J. Chem. Sect. B Org. Med. Chem. 2018, 57B, 715–721. [Google Scholar]
- Sanchez, L.M.; Sathicq, Á.G.; Jios, J.L.; Baronetti, G.T.; Thomas, H.J.; Romanelli, G.P. Solvent-free synthesis of functionalized pyridine derivatives using Wells-Dawson heteropolyacid as catalyst. Tetrahedron Lett. 2011, 52, 4412–4416. [Google Scholar] [CrossRef]
- Heravi, M.M.; Beheshtia, Y.S.; Khorshidi, M.; Baghernejad, B.; Bamoharram, F.F. Application of heteropolyacids as heterogeneous and recyclable catalysts for one-pot synthesis of 3-cyanopyridine derivatives. Chin. J. Chem. 2009, 27, 569–572. [Google Scholar] [CrossRef]
- Oskooie, H.A.; Amini, M.; Heravi, M.M.; Bamoharram, F.F. One-pot synthesis of 3-aminoimidazo[1,2-a]pyridines catalyzed by heteropolyacids. Chin. J. Chem. 2010, 28, 299–302. [Google Scholar] [CrossRef]
- Azad, I.; Hassan, F.; Saquib, M.; Ahmad, N.; Khan, R.A.; Al-Sehemi, G.A.; Nasibullah, M. A critical review on advances in the multicomponent synthesis of pyrroles. Orient. J. Chem. 2018, 34, 1670–1700. [Google Scholar] [CrossRef]
- Lu, N.-N.; Weng, Z.-Y.; Chen, Q.-Y.; Boison, D.; Xiao, X.-X.; Gao, J. Evaluation on the inhibition of pyrrol-2-yl ethanone derivatives to lactate dehydrogenase and anticancer activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 165, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- Teixeira, C.; Barbault, F.; Rebehmed, J.; Liu, K.; Xie, L.; Lu, H.; Jiang, S.; Fan, B.; Maurel, F. Molecular modeling studies of N-substituted pyrrole derivatives—Potential HIV-1 GP41 inhibitors. Bioorg. Med. Chem. 2008, 16, 3039–3048. [Google Scholar] [CrossRef]
- Thiel, O. Heterocyclic Chemistry in Drug Discovery; Li, J.J., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2013; Volume 52, ISBN 978-1118148907. [Google Scholar]
- Olivier, W.J.; Smith, J.A.; Bissember, A.C. Methods for the synthesis of annulated pyrroles via cyclisation strategies. Org. Biomol. Chem. 2018, 16, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Tzankova, D.; Vladimirova, S.; Peikova, L.; Georgieva, M. Synthesis of pyrrole and substituted pyrroles (review). J. Chem. Technol. Metall. 2018, 53, 451–464. [Google Scholar]
- Portilla-Zúñiga, O.; Sathicq, Á.; Martínez, J.; Rojas, H.; De Geronimo, E.; Luque, R.; Romanelli, G.P. Novel bifunctional mesoporous catalysts based on preyssler heteropolyacids for green pyrrole derivative synthesis. Catalysts 2018, 8, 419. [Google Scholar] [CrossRef]
- Gharib, A.; Jahangir, M.; Roshani, M. A facile synthesis of calix[4]pyrroles using heteropolyacids as green, eco-friendly, reusable and recyclable catalyst. Bulg. Chem. Commun. 2012, 44, 113–117. [Google Scholar]
- Gharib, A.; Jahangir, M.; Scheeren, J. Novel catalytic method synthesis of calix[4]pyrroles using Preyssler and Wells-Dawson heteropolyacids. Pol. J. Chem. Technol. 2011, 13, 70–73. [Google Scholar] [CrossRef]
- Jarak, I.; Kralj, M.; Piantanida, I.; Šuman, L.; Žinić, M.; Pavelić, K.; Karminski-Zamola, G. Novel cyano-and amidino-substituted derivatives of thieno[2,3-b]-and thieno[3,2-b]thiophene-2-carboxanilides and thieno[3′,2′:4,5]thieno-and thieno[2′,3′:4,5]thieno [2,3-c]quinolones: Synthesis, photochemical synthesis, DNA binding, and antitumor evalu. Bioorg. Med. Chem. 2006, 14, 2859–2868. [Google Scholar] [CrossRef]
- Kaushik, N.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.; Verma, A.; Choi, E. Biomedical importance of indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kumar, P.; Pathak, D. Biological importance of the indole nucleus in recent years: A comprehensive review. J. Heterocycl. Chem. 2010, 47, 491–502. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Chen, Q.; Yang, G.-F. A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 2015, 89, 421–441. [Google Scholar] [CrossRef]
- Shirinzadeh, H.; Neuhaus, E.; Erguc, E.I.; Aliyev, T.A.; Gurer-Orhan, H.; Suzen, S. New indole-7-aldehyde derivatives as melatonin analogues; synthesis and screening their antioxidant and anticancer potential. Bioorg. Chem. 2020, 104, 104219. [Google Scholar] [CrossRef]
- Beheshtiha, Y.S.; Heravi, M.M.; Saeedi, M.; Fallah, A.; Bamoharram, F.F. Heteropolyacid catalyzed synthesis of indole derivatives via Fischer indole synthesis. Gazi Univ. J. Sci. 2011, 24, 709–714. [Google Scholar]
- Sadjadi, S.; Heravi, M.M.; Malmir, M.; Masoumi, B. HPA decorated halloysite nanoclay: An efficient catalyst for the green synthesis of spirooxindole derivatives. Appl. Organomet. Chem. 2018, 32, 1–12. [Google Scholar] [CrossRef]
- Ghohe, N.M.; Tayebee, R.; Amini, M.M.; Osatiashtiani, A.; Isaacs, M.A.; Lee, A.F. H5PW10V2O40-VOx/SBA-15-NH2 catalyst for the solventless synthesis of 3-substituted indoles. Tetrahedron 2017, 73, 5862–5871. [Google Scholar] [CrossRef]
- Tayebee, R.; Amini, M.M.; Nehzat, F.; Sadeghi, O.; Armaghan, M. H5PW10V2O40/pyridino-SBA-15 as a highly recyclable, robust and efficient inorganic-organic hybrid material for the catalytic preparation of bis(indolyl)methanes. J. Mol. Catal. A Chem. 2013, 366, 140–148. [Google Scholar] [CrossRef]
- Pratap, R.; Ram, V.J. Natural and synthetic chromenes, fused chromenes, and versatility of dihydrobenzo[h]chromenes in organic synthesis. Chem. Rev. 2014, 114, 10476–10526. [Google Scholar] [CrossRef]
- Il’ina, I.; Mikhalchenko, O.; Pavlova, A.; Korchagina, D.; Tolstikova, T.; Volcho, K.; Salakhutdinov, N.; Pokushalov, E. Highly potent analgesic activity of monoterpene-derived (2S,4aR,8R,8aR)-2-aryl-4,7-dimethyl-3,4,4a,5,8,8a-hexahydro-2H-chromene-4,8-diols. Med. Chem. Res. 2014, 23, 5063–5073. [Google Scholar] [CrossRef]
- Oliveira-Pinto, S.; Pontes, O.; Baltazar, F.; Costa, M. In vivo efficacy studies of chromene-based compounds in triple-negative breast cancer—A systematic review. Eur. J. Pharmacol. 2020, 887, 173452. [Google Scholar] [CrossRef] [PubMed]
- Van Kiem, P.; Nhiem, X.N.; Anh, H.N.; Yen, T.H.D.; Cuong, T.N.; Tai, H.B.; Yen, H.P.; Nam, H.N.; Van Minh, C.; Chinh, P.T.; et al. Enantiomeric chromene derivatives with anticancer effects from Mallotus apelta. Bioorg. Chem. 2020, 104268. [Google Scholar] [CrossRef]
- Dinparast, L.; Hemmati, S.; Alizadeh, A.A.; Zengin, G.; Kafil, H.S.; Bahadori, M.B.; Dastmalchi, S. An efficient, catalyst-free, one-pot synthesis of 4H-chromene derivatives and investigating their biological activities and mode of interactions using molecular docking studies. J. Mol. Struct. 2020, 1203, 127426. [Google Scholar] [CrossRef]
- Nasri, S.; Bayat, M. One pot synthesis of new heterocyclic systems: Polysubstituted pyrano[3,2-c]chromene and benzo[g]chromene derivatives. J. Mol. Struct. 2018, 1164, 77–83. [Google Scholar] [CrossRef]
- Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Mohammadpoor-Baltork, I.; Moosavifar, M. Host (nanocavity of dealuminated zeolite-Y)-guest (12-molybdophosphoric acid) nanocomposite material: An efficient and reusable catalyst for synthesis of 14-substituted-14-H-dibenzo[a,j] xanthenes under thermal and microwave irradiation conditions. Comptes Rendus Chim. 2011, 14, 489–495. [Google Scholar] [CrossRef]
- Wysocki, L.M.; Grimm, J.B.; Tkachuk, A.N.; Brown, T.A.; Betzig, E.; Lavis, L.D. Facile and general synthesis of photoactivatable xanthene dyes. Angew. Chem. Int. Ed. 2011, 50, 11206–11209. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.M.; Miguel, D.; Resa, S.; Gonzalez-Garcia, M.C.; Diaz-Torres, Y.; Cuerva, J.M.; Crovetto, L. Design, synthesis and photophysical studies of improved xanthene dye to detect acetate. J. Photochem. Photobiol. A Chem. 2019, 371, 300–305. [Google Scholar] [CrossRef]
- Puente-Muñoz, V.; Paredes, J.M.; Resa, S.; Ortuño, A.M.; Talavera, E.M.; Miguel, D.; Cuerva, J.M.; Crovetto, L. Efficient acetate sensor in biological media based on a selective Excited State Proton Transfer (ESPT) reaction. Sens. Actuators B Chem. 2017, 250, 623–628. [Google Scholar] [CrossRef]
- Gong, J.; Liu, C.; Jiao, X.; He, S.; Zhao, L.; Zeng, X. Novel mitochondria-targeted viscosity probe based on a fluorescent rotatable xanthene-hemicyanine dyad. Microchem. J. 2020, 158, 105191. [Google Scholar] [CrossRef]
- Harichandran, G.; Parameswari, P.; Shanmugam, P. An efficient solvent free Amberlite IRA-400 Cl resin mediated multicomponent synthesis and photophysical properties of fluorescent 4 H -chromene derivatives. Dye. Pigment. 2017, 139, 541–548. [Google Scholar] [CrossRef]
- Kumar, S.L.M.; Singh, J.; Manna, S.K.; Maji, S.; Konwar, R.; Panda, G. Diversity oriented synthesis of chromene-xanthene hybrids as anti-breast cancer agents. Bioorg. Med. Chem. Lett. 2018, 28, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Palermo, V.; Sosa, A.A.; Rivera, T.S.; Pizzio, L.R.; Romanelli, G.P. Unexpected result in the catalytic solvent-free multicomponent synthesis of 2-amino-3-cyano-4H-chromene. Org. Prep. Proced. Int. 2019, 51, 443–455. [Google Scholar] [CrossRef]
- Motamedi, R.; Baghbani, S.; Bamoharram, F.F. Catalytic method for synthesis of benzopyrano[3,2-c]chromene-6,8-dione derivatives by heteropoly acids. Synth. Commun. 2012, 42, 1604–1612. [Google Scholar] [CrossRef]
- Rivera, T.S.; Sosa, A.; Romanelli, G.P.; Blanco, M.N.; Pizzio, L.R. Tungstophosphoric acid/zirconia composites prepared by the sol-gel method: An efficient and recyclable green catalyst for the one-pot synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes. Appl. Catal. A Gen. 2012, 443–444, 207–213. [Google Scholar] [CrossRef]
- Rivera, T.S.; Blanco, M.N.; Pizzio, L.R.; Romanelli, G.P. Green catalytic synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes using recyclable mesoporous zirconia modified with tungstophosphoric acid. Green Chem. Lett. Rev. 2012, 5, 433–437. [Google Scholar] [CrossRef]
- Gharib, A.; Jahangir, M.; Roshani, M.; Scheeren, J.W. A method for catalytic synthesis of convenient thioxanthone crown ethers using Wells-Dawson, H6[P2W18O62] and Preyssler H14[NaP5W30O110]. Bulg. Chem. Commun. 2010, 42, 217–221. [Google Scholar]
- Su, S.; Yin, P.; Li, J.; Chen, G.; Wang, Y.; Qu, D.; Li, Z.; Xue, X.; Luo, X.; Li, M. In vitro and in vivo anti-biofilm activity of pyran derivative against Staphylococcus aureus and Pseudomonas aeruginosa. J. Infect. Public Health 2020, 13, 791–799. [Google Scholar] [CrossRef]
- Kaldre, D. Pyrans and benzo derivatives: Applications. Ref. Modul. Chem. Mol. Sci. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Zhang, X.; Zeng, D.; Luo, Y.; Duan, X.; Gao, X.; Cai, P.; Zhang, Q.; Zhang, J.; et al. A new fluorinated pyran-bridged A-D-A type small molecular acceptor for organic solar cells. Dye. Pigment. 2020, 175, 108165. [Google Scholar] [CrossRef]
- Saranya, J.; Benhiba, F.; Anusuya, N.; Subbiah, R.; Zarrouk, A.; Chitra, S. Experimental and computational approaches on the pyran derivatives for acid corrosion. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125231. [Google Scholar] [CrossRef]
- Zakeri, M.; Heravi, M.M.; Oskooie, H.A.; Bamoharram, F.F. Catalytic performance of heteropolyacids as efficient and eco-friendly catalysts for the one-pot synthesis of benzopyran derivatives. Synth. React. Inorg. Met. Nano Met. Chem. 2010, 40, 916–921. [Google Scholar] [CrossRef]
- Jamshidi, A.; Maleki, B.; Zonoz, F.M.; Tayebee, R. HPA-dendrimer functionalized magnetic nanoparticles (Fe3O4@D-NH2-HPA) as a novel inorganic-organic hybrid and recyclable catalyst for the one-pot synthesis of highly substituted pyran derivatives. Mater. Chem. Phys. 2018, 209, 46–59. [Google Scholar] [CrossRef]
- Mozafari, R.; Heidarizadeh, F. Phosphotungstic acid supported on SiO2@NHPhNH2 functionalized nanoparticles of MnFe2O4 as a recyclable catalyst for the preparation of tetrahydrobenzo[b]pyran and indazolo[2,1-b]phthalazine-triones. Polyhedron 2019, 162, 263–276. [Google Scholar] [CrossRef]
- Maleki, B.; Eshghi, H.; Khojastehnezhad, A.; Tayebee, R.; Ashrafi, S.S.; Kahoo, G.E.; Moeinpour, F. Silica coated magnetic NiFe2O4 nanoparticle supported phosphomolybdic acid; synthesis, preparation and its application as a heterogeneous and recyclable catalyst for the one-pot synthesis of tri-and tetra-substituted imidazoles unde. RSC Adv. 2015, 5, 64850–64857. [Google Scholar] [CrossRef]
- Rajguru, D.; Keshwal, B.S.; Jain, S. H6P2W18O6218H2O: A green and reusable catalyst for one-pot synthesis of pyrano[4,3-b]pyrans in water. Chin. Chem. Lett. 2013, 24, 1033–1036. [Google Scholar] [CrossRef]
- Hewlett, N.; Hupp, C.; Tepe, J. Reactivity of Oxazol-5-(4H)-ones and their application toward natural product synthesis. Synthesis 2009, 2009, 2825–2839. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, X.; Xu, J.; Jiang, X.; Cai, X. Construction of allylic amino acid derivatives through a catalytic asymmetric allylic alkylation of azlactones with vinyl cyclopropanes. Tetrahedron Lett. 2020, 61, 151694. [Google Scholar] [CrossRef]
- Rostami, M.; Khosropour, A.; Mirkhani, V.; Moghadam, M.; Tangestaninejad, S.; Mohammadpoor-Baltork, I. Organic-inorganic hybrid polyoxometalates: Efficient, heterogeneous and reusable catalysts for solvent-free synthesis of azlactones. Appl. Catal. A Gen. 2011, 397, 27–34. [Google Scholar] [CrossRef]
- Moghanian, H.; Shabanian, M.; Jafari, H. Microwave-assisted efficient synthesis of azlactone derivatives using TsCl/DMF under solvent-free conditions. Comptes Rendus Chim. 2012, 15, 346–349. [Google Scholar] [CrossRef]
- Conway, P.A.; Devine, K.; Paradisi, F. A simple and efficient method for the synthesis of Erlenmeyer azlactones. Tetrahedron 2009, 65, 2935–2938. [Google Scholar] [CrossRef]
- Romanelli, G.; Autino, J.C.; Vázquez, P.; Pizzio, L.; Blanco, M.; Cáceres, C. A suitable synthesis of azlactones (4-benzylidene-2-phenyloxazolin-5-ones and 4-alkylidene-2-phenyloxazolin-5-ones) catalyzed by silica-alumina supported heteropolyacids. Appl. Catal. A Gen. 2009, 352, 208–213. [Google Scholar] [CrossRef]
- Ruiz, M.D.; Baronetti, T.G.; Thomas, J.H.; Romanelli, P.G. H6P2W12O6224H2O supported on silica: A powerful and reusable catalyst for the synthesis of 4-arylidene-2-phenyl-5(4)-oxazolones (azlactones). Curr. Catal. 2012, 1, 67–72. [Google Scholar] [CrossRef]
- Eicher, T.; Hauptmann, S.; Speicher, A. Five-Membered Heterocycles: Sections 5.22–5.36. In The Chemistry of Heterocycles; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 122–184. ISBN 978-3-527-32747-8. [Google Scholar]
- Shafiei, M.; Peyton, L.; Hashemzadeh, M.; Foroumadi, A. History of the development of antifungal azoles: A review on structures, SAR, and mechanism of action. Bioorg. Chem. 2020, 104, 104240. [Google Scholar] [CrossRef] [PubMed]
- Murumkar, P.R.; Ghuge, R.B. Vicinal Diaryl Oxadiazoles, Oxazoles, and Isoxazoles. In Vicinal Diaryl Substituted Heterocycles; Yadav, M.R., Ghuge, R.B., Murumkar, P.R., Eds.; Elsevier: Vadodara, India, 2018; pp. 277–303. ISBN 9780081022498. [Google Scholar]
- Nong, Q.-Y.; Liu, Y.-A.; Qin, L.-T.; Liu, M.; Mo, L.-Y.; Liang, Y.-P.; Zeng, H.-H. Toxic mechanism of three azole fungicides and their mixture to green alga Chlorella pyrenoidosa. Chemosphere 2021, 262, 127793. [Google Scholar] [CrossRef]
- Jang, J.; Kim, D.-H.; Min, C.-M.; Pak, C.; Lee, J.-S. Azole structures influence fuel cell performance of phosphoric acid-doped poly(phenylene oxide) with azoles on side chains. J. Memb. Sci. 2020, 605, 118096. [Google Scholar] [CrossRef]
- Bhagat, J.; Singh, N.; Nishimura, N.; Shimada, Y. A comprehensive review on environmental toxicity of azole compounds to fish. Chemosphere 2020, 262, 128335. [Google Scholar] [CrossRef]
- Sadjadi, S.; Heravi, M.M.; Daraie, M. A novel hybrid catalytic system based on immobilization of phosphomolybdic acid on ionic liquid decorated cyclodextrin-nanosponges: Efficient catalyst for the green synthesis of benzochromeno-pyrazole through cascade reaction: Triply green. J. Mol. Liq. 2017, 231, 98–105. [Google Scholar] [CrossRef]
- Gharib, A.; Jahangir, M.; Scheeren, J.H.W. Catalytic synthesis of pyrazoles and diazepines under green conditions at room temperature using heteropolyacids catalysts. Synth. Commun. 2013, 43, 309–325. [Google Scholar] [CrossRef]
- Timmerman, H.; van der Goot, H. Histamine receptors and their ligands: Mechanisms and applications. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Elsevier: San Diego, CA, USA, 2009; pp. 1149–1166. ISBN 9780080450469. [Google Scholar]
- Leurs, R.; Vischer, H.F.; Wijtmans, M.; de Esch, I.J.P. En route to new blockbuster anti-histamines: Surveying the offspring of the expanding histamine receptor family. Trends Pharmacol. Sci. 2011, 32, 250–257. [Google Scholar] [CrossRef]
- Gorsd, M.; Sathicq, G.; Romanelli, G.; Pizzio, L.; Blanco, M. Tungstophosphoric acid supported on core-shell polystyrene-silica microspheres or hollow silica spheres catalyzed trisubstituted imidazole synthesis by multicomponent reaction. J. Mol. Catal. A Chem. 2016, 420, 294–302. [Google Scholar] [CrossRef]
- Purnima, K.V.; Sreenu, D.; Bhasker, N.; Nagaiah, K.; Lingaiah, N.; Reddy, B.V.S.; Yadav, J.S. Copper salt of 12-tungstophosphoric acid: An efficient and reusable heteropoly acid for the click chemistry. Chin. J. Chem. 2013, 31, 534–538. [Google Scholar] [CrossRef]
- Miller, M.M.; DelMonte, A.J. Six-Membered Ring Systems. In Progress in Heterocyclic Chemistry; Gribble, G.W., Joule, J.A., Eds.; Elsevier: San Diego, CA, USA, 2011; pp. 371–402. ISBN 978-0-08-096805-6. [Google Scholar]
- Verbitskiy, E.V.; Kvashnin, Y.A.; Baranova, A.A.; Khokhlov, K.O.; Chuvashov, R.D.; Schapov, I.E.; Yakovleva, Y.A.; Zhilina, E.F.; Shchepochkin, A.V.; Makarova, N.I.; et al. Synthesis and characterization of linear 1,4-diazine-triphenylamine-based selective chemosensors for recognition of nitroaromatic compounds and aliphatic amines. Dye. Pigment. 2020, 178, 108344. [Google Scholar] [CrossRef]
- Rinderspacher, K.A. Six-Membered Ring Systems: Diazines and Benzo Derivatives. In Progress in Heterocyclic Chemistry; Gribble, G.W., Joule, J.A., Eds.; Elsevier: New York, NY, USA, 2020; pp. 465–504. ISBN 978-0-12-819962-6. [Google Scholar]
- Achelle, S.; Robin-le Guen, F. Emission properties of diazines chromophores: Structure-properties relationship. J. Photochem. Photobiol. A Chem. 2017, 348, 281–286. [Google Scholar] [CrossRef]
- Hakimi, F.; Hassanabadi, A.; Tabatabaee, M.; Heravi, M.M. Heteropolyacides as green and reusable catalysts for the synthesis of [3,4-b][1,3,4] thiadiazines. Bull. Chem. Soc. Ethiop. 2014, 28, 67. [Google Scholar] [CrossRef]
- Motamedi, R.; Monfared, A.; Nezamabadi, Z.G.; Bamoharram, F.F. Facile one-pot synthesis of [1,2,4]triazolo[3,4-b][1,3,4]thiadiazines and 3,7-dimethyl-4H-[1,2,4]triazino[3,4-b][1,3,4]thiadiazin-6-one using heteropolyacid catalysts. J. Heterocycl. Chem. 2011, 48, 604–607. [Google Scholar] [CrossRef]
- Motamedi, R.; Heravi, M.M.; Nazari, Z.; Bamoharram, F.F. Regioselective synthesis of 2-substituted [1,2,4]Triazolo[5,1-b][1,3] thiazin-7-ones by heteropolyacids. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 1672–1675. [Google Scholar] [CrossRef]
- Gharib, A.; Khorasani, B.R.H.; Jahangir, M.; Roshani, M.; Bakhtiari, L.; Mohadeszadeh, S.; Ahmadi, S. Preyssler heteropolyacid supported on nano-SiO2, H14[NaP5W30O110]/SiO2: A green and reusable catalyst in the synthesis of polysubstituted quinolines. Bulg. Chem. Commun. 2014, 46, 223–232. [Google Scholar]
- Bennardi, O.D.; Blanco, N.M.; Pizzio, R.L.; Autino, J.C.; Romanelli, G.P. An efficient and green catalytic method for Friedländer quinoline synthesis using tungstophosphoric acid included in a polymeric matrix. Curr. Catal. 2015, 4, 65–72. [Google Scholar] [CrossRef]
- Bentarzi, Y.; Benadji, S.; Bennamane, N.; Rabia, C.; Nedjar-Kolli, B. An efficient heteropoly acid-catalysed procedure for synthesis of quinolines and naphthyridines. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 971–979. [Google Scholar]
- Heravi, M.M.; Haj, N.M.; Baghernejad, B.; Beheshtia, Y.S.; Bamoharram, F.F. Application of heteropoly acids as heterogeneous and recyclable catalysts for friedländer synthesis of quinolines. eJournal Chem. 2010, 7, 875–881. [Google Scholar] [CrossRef][Green Version]
- Allameh, S.; Hashemi, M.M.; Heravi, M.M.; Bamoharram, F.F. Synthesis of quinazolin-4(3H)-one derivatives using heteropolyacids as heterogeneous and recyclable catalysts. Asian J. Chem. 2011, 23, 1588–1590. [Google Scholar]
- Allameh, S.; Heravi, M.M.; Hashemi, M.M.; Bamoharram, F.F. Synthesis of 3-(aryl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one derivatives using heteropolyacids as green, heterogeneous and recyclable catalysts. Chin. Chem. Lett. 2011, 22, 131–134. [Google Scholar] [CrossRef]
- Heravi, M.M.; Sadjadi, S.; Sadjadi, S.; Oskooie, H.A.; Shoar, R.H.; Bamoharram, F.F. Silica-supported preyssler nanoparticles as new catalysts in the synthesis of 4(3H)-quinazolinones. S. Afr. J. Chem. 2009, 62, 1–4. [Google Scholar]
- Heravi, M.M.; Sadjadi, S.; Mokhtari Haj, N.; Oskooie, H.A.; Shoar, R.H.; Bamoharram, F.F. A novel multi-component synthesis of 4-arylaminoquinazolines. Tetrahedron Lett. 2009, 50, 943–945. [Google Scholar] [CrossRef]
- Heravi, M.M.; Sadjadi, S.; Sadjadi, S.; Oskooie, H.A.; Bamoharram, F.F. Rapid and efficient synthesis of 4(3H)-quinazolinones under ultra sonic irradiation using silica-supported Preyssler nano particles. Ultrason. Sonochem. 2009, 16, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Sosa, A.A.; Rivera, T.S.; Blanco, M.N.; Pizzio, L.R.; Romanelli, G.P. Tungstophosphoric acid supported on zirconia: A recyclable catalyst for the green synthesis on quinoxaline derivatives under solvent-free conditions. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 1071–1079. [Google Scholar] [CrossRef]
- Huang, T.K.; Shi, L.; Wang, R.; Guo, X.Z.; Lu, X.X. Keggin type heteropolyacids-catalyzed synthesis of quinoxaline derivatives in water. Chin. Chem. Lett. 2009, 20, 161–164. [Google Scholar] [CrossRef]
- Gholamzadeh, P. The Pictet-Spengler reaction: A powerful strategy for the synthesis of heterocycles. In Avances en Química Heterocíclica; Scriven, E.F., Ramsden, C.A., Eds.; Elsevier: Cambridge, UK, 2019; pp. 153–226. ISBN 978-0-12-817149-3. [Google Scholar]
- Ji Ram, V.; Sethi, A.; Nath, M.; Pratap, R. Seven-Membered Heterocycles. In The Chemistry of Heterocycles; Elsevier: Delhi, Indiai, 2019; pp. 393–425. ISBN 9780128192108. [Google Scholar]
- Ghalib, R.M.; Mehdi, S.H.; Malla, A.M.; Bogdanović, G.A.; Trifunovic, S.R.; Alam, M.G. Synthesis of new seven membered heterocyclic rings: An easy access to indeno-benzo[1,4]diazepines. Arab. J. Chem. 2020. [Google Scholar] [CrossRef]
- Miyanaga, S.; Sakurai, H.; Saiki, I.; Onaka, H.; Igarashi, Y. Anti-invasive and anti-angiogenic activities of naturally occurring dibenzodiazepine BU-4664L and its derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 963–965. [Google Scholar] [CrossRef]
- Petrascheck, M.; Ye, X.; Buck, L.B. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature 2007, 450, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Dulawa, S.C.; Hen, R. Recent advances in animal models of chronic antidepressant effects: The novelty-induced hypophagia test. Neurosci. Biobehav. Rev. 2005, 29, 771–783. [Google Scholar] [CrossRef]
- Wille, S.M.R.; Cooreman, S.G.; Neels, H.M.; Lambert, W.E.E. Relevant issues in the monitoring and the toxicology of antidepressants. Crit. Rev. Clin. Lab. Sci. 2008, 45, 25–89. [Google Scholar] [CrossRef] [PubMed]
- Kaoua, R.; Bennamane, N.; Bakhta, S.; Benadji, S.; Rabia, C.; Nedjar-Kolli, B. Synthesis of substituted 1,4-diazepines and 1,5-benzodiazepines using an efficient heteropolyacid-catalyzed procedure. Molecules 2010, 16, 92–99. [Google Scholar] [CrossRef]
- Pasquale, G.A.; Ruiz, D.M.; Jios, J.L.; Autino, J.C.; Romanelli, G.P. Preyssler catalyst-promoted rapid, clean, and efficient condensation reactions for 3H-1,5-benzodiazepine synthesis in solvent-free conditions. Tetrahedron Lett. 2013, 54, 6574–6579. [Google Scholar] [CrossRef]
- Morales, D.M.; Frenzel, R.A.; Romanelli, G.P.; Pizzio, L.R. Synthesis and characterization of nanoparticulate silica with organized multimodal porous structure impregnated with 12-phosphotungstic acid for its use in heterogeneous catalysis. Mol. Catal. 2020, 481, 110210. [Google Scholar] [CrossRef]
- Hekmatshoar, R.; Sadjadi, S.; Sadjadi, S.; Heravi, M.M.; Bamoharram, F.F. H14[NaP5W30O110] as a heterogeneous recyclable catalyst for the synthesis of 6,8-dimethyl-3-aryl-[1,2,4]triazolo[3,4-b][1,3,4]thiazepine derivatives. Synth. Commun. 2010, 40, 1778–1783. [Google Scholar] [CrossRef]
- Ravisankar, S.; Queiroz, V.A.V.; Awika, J.M. Rye flavonoids—Structural profile of the flavones in diverse varieties and effect of fermentation and heat on their structure and antioxidant properties. Food Chem. 2020, 324, 126871. [Google Scholar] [CrossRef]
- Awika, J.M.; Rose, D.J.; Simsek, S. Complementary effects of cereal and pulse polyphenols and dietary fiber on chronic inflammation and gut health. Food Funct. 2018, 9, 1389–1409. [Google Scholar] [CrossRef] [PubMed]
- German-Ponciano, L.J.; Costa, D.B.P.; Feitosa, L.M.; Campos, K.d.S.; da Silva, N.S.C.; Cueto-Escobedo, J.; Lima-Maximino, M.; Rodríguez-Landa, J.F.; Maximino, C. Chrysin, but not flavone backbone, decreases anxiety-like behavior in animal screens. Neurochem. Int. 2020, 140, 104850. [Google Scholar] [CrossRef]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef]
- Tian, S.; Luo, T.; Zhu, Y.; Wan, J.-P. Recent advances in the diversification of chromones and flavones by direct C-H bond activation or functionalization. Chin. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- Kshatriya, R.; Jejurkar, V.P.; Saha, S. In memory of Prof. Venkataraman: Recent advances in the synthetic methodologies of flavones. Tetrahedron 2018, 74, 811–833. [Google Scholar] [CrossRef]
- Gharib, A.; Jahangir, M.; Roshani, M.; Scheeren, J.W. Effective catalytic synthesis of substituted flavones and chromones using Preyssler and heteropolyacids (HPAs) as catalysts. Bulg. Chem. Commun. 2010, 42, 210–216. [Google Scholar]
- Pérez, M.E.; Ruiz, D.M.; Autino, J.C.; Blanco, M.N.; Pizzio, L.R.; Romanelli, G.P. Mesoporous titania/tungstophosphoric acid composites: Suitable synthesis of flavones. J. Porous Mater. 2013, 20, 1433–1440. [Google Scholar] [CrossRef]
- Díaz-Álvarez, A.; Cadierno, V. Glycerol: A promising green solvent and reducing agent for metal-catalyzed transfer hydrogenation reactions and nanoparticles formation. Appl. Sci. 2013, 3, 55–69. [Google Scholar] [CrossRef]
- Anastas, P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; ISBN 0198506988. [Google Scholar]
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on solvent use in the pharmaceutical industry. Org. Process Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Shotland, Y. Glycerol as a green solvent for high product yields and selectivities. Environ. Chem. Lett. 2007, 5, 67–71. [Google Scholar] [CrossRef]
- Bayat, Y.; Mokhtari, J.; Farhadian, N.; Bayat, M. Heteropolyacids: An efficient catalyst for synthesis of CL-20. J. Energ. Mater. 2012, 30, 124–134. [Google Scholar] [CrossRef]
- Ramazani, A.; Azizkhani, V.; Joo, S.W. Heteropolyacids: As efficient catalysts for the nitration of 2,4,6,8,10,12-hexaacetyl-2,4,6,8,10,12-hexaazaisowurtzitane. Rev. Roum. Chim. 2019, 64, 569–575. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Radulov, P.S.; Belyakova, Y.Y.; Demina, A.A.; Fomenkov, D.I.; Barsukov, D.V.; Subbotina, I.R.; Fleury, F.; Terent’ev, A.O. Catalyst development for the synthesis of ozonides and tetraoxanes under heterogeneous conditions: Disclosure of an unprecedented class of fungicides for agricultural application. Chem. A Eur. J. 2020, 26, 4734–4751. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).