Nanocomposite Cathode Catalysts Containing Platinum Deposited on Carbon Nanotubes Modified by O, N, and P Atoms
Abstract
1. Introduction
- High specific surface area;
- Predominantly mesoporous structure ensuring the transport of reagents;
- High stability in alkaline and acidic environments under the operating conditions of FCs;
- High proton and electronic conductivity: the electrically conductive material of the support provides a path through which the transfer of electrons between the support and the deposited metal occurs;
- Strong interaction between the nanoparticles of the metal and support material, which increases the stability of the catalyst.
2. Results and Discussion
2.1. Determination of the Electrical Conductivity of Monoplatinum Catalysts
2.2. Determination of the Electrical Conductivity of Monoplatinum Catalysts
2.3. Corrosion Stability of Pt-Catalysts
3. Materials and Methods
3.1. CNT Modification Methods
3.2. The Polyol Synthesis of Monoplatinum Catalysts
3.3. Electrochemical Methods
3.4. Methodology for Measuring Resistance and Calculating Electrical Conductivity
3.5. Structural Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.T.; Hor, T.A.; Zong, Y.; Liu, Z. Oxygen reduction in alkaline media: From mechanisms to recent advances of catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Li, L.; Hu, L.; Li, J.; We, Z. Enhanced stability of Pt nanoparticles electrocatalysts for fuel cells. Nano Res. 2015, 3, 418–440. [Google Scholar] [CrossRef]
- Dubau, L.; Castanheira, L.; Maillard, F.; Chatenet, M.; Lottin, O.; Maranzana, G.; Dillet, J.; Lamibrac, A.; Perrin, J.C.; Moukheiber, E. A review of PEM fuel cell durability: Materials degradation, local heterogeneities of aging and possible mitigation strategies. WIREs Energy Environ. 2014, 3, 540–560. [Google Scholar] [CrossRef]
- Capelo, A.; Esteves, M.A.; de Sa, A.I.; Silva, R.A.; Cangueiro, L.; Almeida, A.; Vilar, R.; Rangel, C.M. Stability and durability under potential cycling of Pt/C catalyst with new surface-functionalized carbon support. Int. J. Hydrogen Energy 2016, 41, 12962–12975. [Google Scholar] [CrossRef]
- Cheng, K.; He, D.; Peng, T.; Lv, H.; Mu, P.; Shichun, M. Porous graphene supported Pt catalysts for proton exchange membrane fuel cells. Electrochim. Acta 2014, 132, 356–363. [Google Scholar] [CrossRef]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Progress in modified carbon support materials for Pt and Pt-alloy cathode catalysts in polymer electrolyte membrane fuel cells. Prog. Mater. Sci. 2016, 82, 445–498. [Google Scholar] [CrossRef]
- Melchionna, M.; Marchesan, S.; Prato, M.; Fornasiero, P. Carbon nanotubes and catalysis: The many facets of a successful marriage. Catal. Sci. Technol. 2015, 5, 3859–3875. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, C.; Dou, S.; Liu, D.; Wang, S. Oxidized carbon nanotubes as an efficient metal free electrocatalyst for the oxygen reduction reaction. RSC Adv. 2015, 5, 41901–41904. [Google Scholar] [CrossRef]
- Sang, Y.; Fu, A.; Li, H.; Zhang, J.; Li, Z.; Li, H.; Zhao, X.S.; Guo, P. Experimental and theoretical studies on the effect of functional groups on carbon nanotubes to its oxygen reduction reaction activity. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 476–484. [Google Scholar] [CrossRef]
- Othman, S.H.; Ritter, U.; McCarthy, E.K.; Fernandes, D.; Kelarakis, A.; Tsierkezos, N.G. Synthesis and electrochemical characterization of nitrogen-doped and nitrogen–phosphorus-doped multi-walled carbon nanotubes. Ionics 2017, 23, 2025–2035. [Google Scholar] [CrossRef]
- Mohammadi, F.; Tavakol, H. Synthesis of phosphorus doped carbon nanotubes using chemical vapor deposition. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 218–225. [Google Scholar] [CrossRef]
- Zhu, J.; He, G.; Tian, Z.; Liang, L.; Shen, P.K. Facile synthesis of boron and nitrogen-dual-doped graphene sheets anchored platinum nanoparticles for oxygen reduction reaction. Electrochim. Acta 2016, 194, 276–282. [Google Scholar] [CrossRef]
- Shea, Y.; Chen, J.; Zhang, C.; Lu, Z.; Ni, M.; Sit, P.H.-L.; Leung, M.K.H. Nitrogen-doped graphene derived from ionic liquid as metal-free catalyst for oxygen reduction reaction and its mechanisms. Appl. Energy 2018, 225, 513–521. [Google Scholar] [CrossRef]
- Sibul, R.; Kibena-Põldsepp, E.; Mäeorg, U.; Merisalu, M.; Kikas, A.; Kisan, V.; Treshchalov, A.; Sammelselg, V.; Tammeveski, K. Sulphur and nitrogen co-doped graphene-based electrocatalysts for oxygen reduction reaction in alkaline medium. Electrochem. Commun. 2019, 109, 106603. [Google Scholar] [CrossRef]
- Zhou, Y.; Neyerlin, K.; Olson, T.S.; Pylypenko, S.; Bult, J.; Dinh, H.N. Enhancement of Pt and Pt-alloy fuel cell catalyst activity and durability via nitrogen-modified carbon supports. Energy Environ Sci. 2010, 3, 1437–1446. [Google Scholar] [CrossRef]
- Brandiele, R.; Durante, C.; Zerbetto, M.; Vicentini, N.; Kosmala, T.; Badocco, D.; Pastore, P.; Rizzi, G.A.; Isse, A.A.; Gennaro, A. Probing the correlation between Pt-support interaction and oxygen reduction reaction activity in mesoporous carbon materials modified with Pt-N active sites. Electrochim. Acta 2018, 277, 287–300. [Google Scholar] [CrossRef]
- Perini, L.; Durante, C.; Favaro, M.; Perazzolo, V.; Agnoli, S.; Schneider, O.; Granozzi, G.; Gennaro, A. Metal-support interaction in platinum and palladium nanoparticles loaded on nitrogen-doped mesoporous carbon for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2015, 7, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, X.; Yang, L.; Wang, F.; Yin, J. Unprotected Pt nanoclusters anchored on ordered mesoporous carbon as an efficient and stable catalyst for oxygen reduction reaction. Electrochim. Acta 2019, 297, 539–544. [Google Scholar] [CrossRef]
- Li, D.; Wang, C.; Strmcnik, D.S.; Tripkovic, D.V.; Sun, X.; Kang, Y.; Chi, M.; Snyder, J.D.; Vliet, D.; Tsai, Y.; et al. Functional links between Pt single crystal morphology and nanoparticles with different size and shape: The oxygen reduction reaction case. Energy Environ. Sci. 2014, 7, 4061–4069. [Google Scholar] [CrossRef]
- Perazzolo, V.; Brandiele, R.; Durante, C.; Zerbetto, M.; Causin, V.; Rizzi, G.A.; Cerri, I.; Granozzi, G.; Gennaro, A. Density Functional Theory (DFT) and experimental evidences of metalesupport interaction in platinum nanoparticles supported on nitrogen- and sulfur-doped mesoporous carbons: Synthesis, activity, and stability. ACS Catal. 2018, 8, 1122–1137. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Liu, H.; Li, R.; Sun, X.; Ye, S. Enhanced stability of Pt electrocatalysts by nitrogen doping in CNTs for PEM fuel cells. Electrochem. Commun. 2009, 11, 2071–2076. [Google Scholar] [CrossRef]
- Bogdanovskaya, V.; Vernigor, I.; Radina, M.; Andreev, V.; Korchagin, O.; Novikov, V. Carbon Nanotube Modified by (O, N, P) Atoms as Effective Catalysts for Electroreduction of Oxygen in Alkaline Media. Catalysts 2020, 10, 892. [Google Scholar] [CrossRef]
- Wei, Q.; Tong, X.; Zhang, G.; Qiao, J.; Gong, Q.; Sun, H. Nitrogen-Doped Carbon Nanotube and Graphene Materials for Oxygen Reduction Reactions. Catalysts 2015, 5, 1574–1602. [Google Scholar] [CrossRef]
- Contreras, E.; Dominguez, D.; Tiznado, H.; Guerrero-Sanchez, J.; Takeuchi, N.; Alonso-Nunez, G.; Contreras, O.E.; Oropeza-Guzmán, M.T.; Romo-Herrer, J.M. N-Doped carbon nanotubes enriched with graphitic nitrogen in a buckypaper configuration as efficient 3D electrodes for oxygen reduction to H2O2. Nanoscale 2019, 11, 2829–2839. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ali, F.; Irfan, M.; Muhammad, F.; Glowacz, A.; Antonino-Daviu, J.A.; Caesarendra, W.; Qamar, S. Mechanical Pressure Characterization of CNT-Graphene Composite Material. Micromachines 2020, 11, 1000. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, S.; Wang, Y.; Li, H.; Zhou, H.; Chen, B.; Zhang, X.; Chen, H.; Qu, K.; Zhao, J. One Simple Strategy towards Nitrogen and Oxygen Codoped Carbon Nanotube for Efficient Electrocatalytic Oxygen Reduction and Evolution. Catalysts 2019, 9, 159. [Google Scholar] [CrossRef]
- Bogdanovskaya, V.; Kuzov, A.; Radina, M.; Filimonov, V.; Sudarev, G.; Osina, M. Stability against Degradation and Activity of Catalysts with Different Platinum Load Synthesized at Carbon Nanotubes. Russ. J. Electrochem. 2020, 56, 969–983. [Google Scholar] [CrossRef]



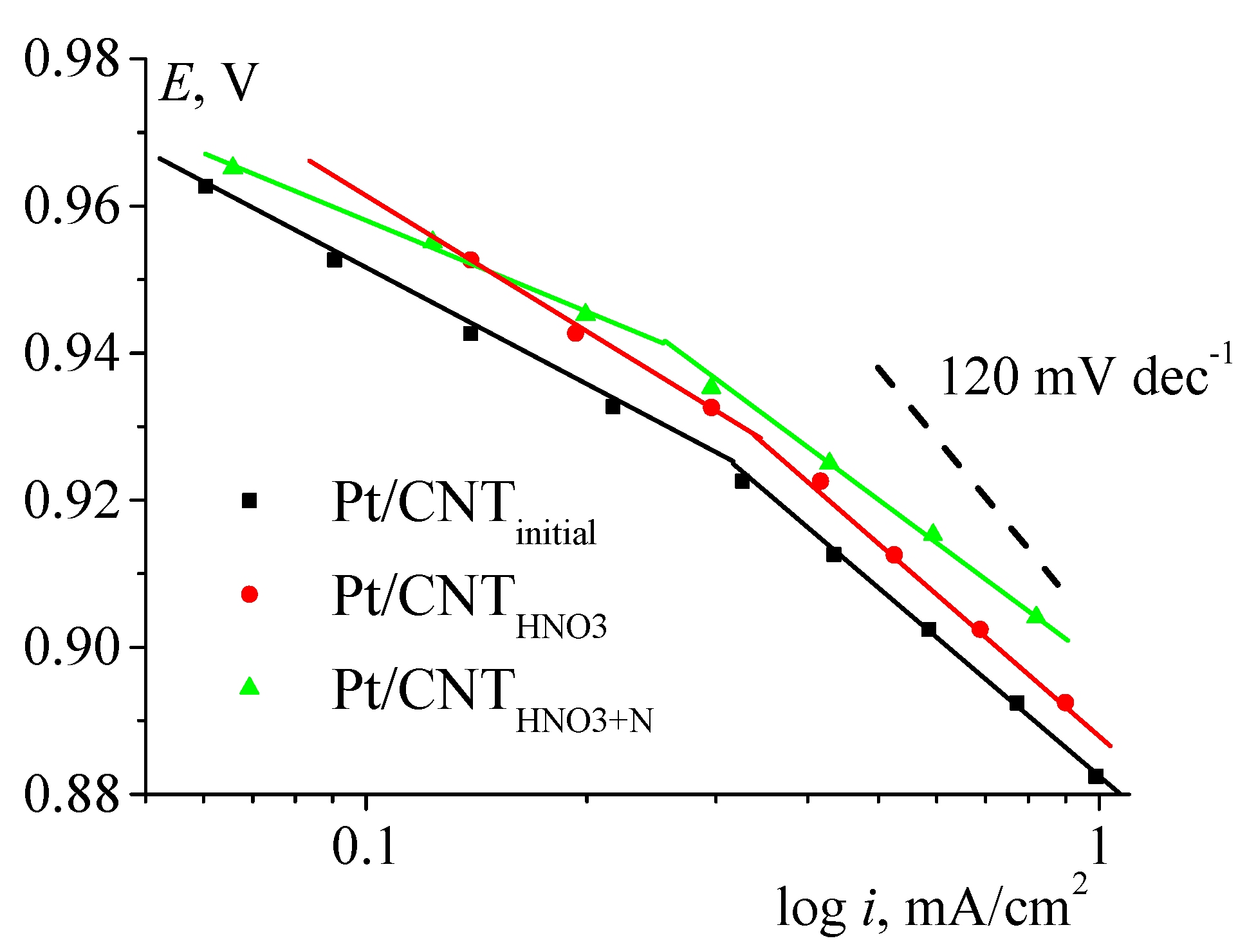
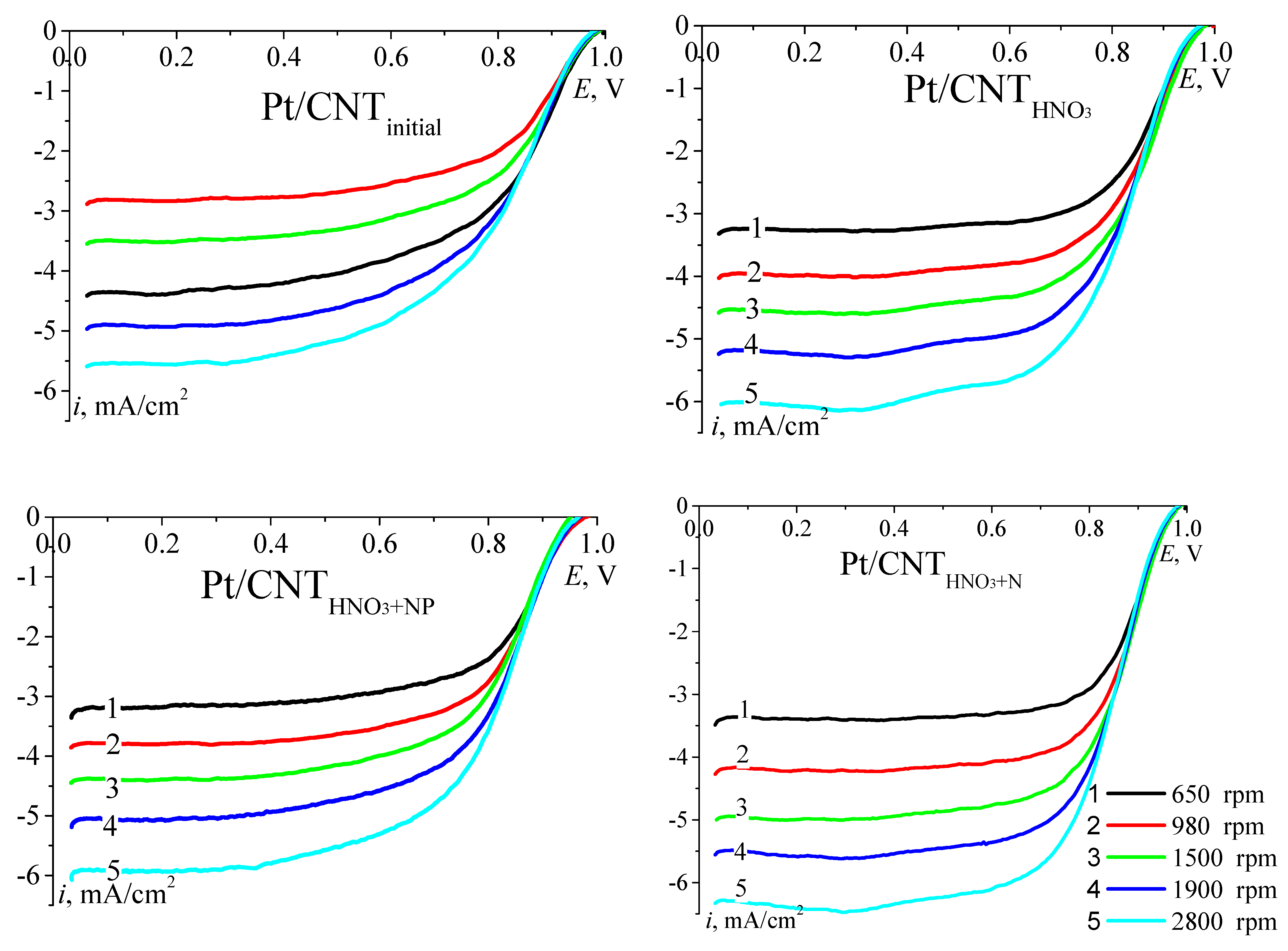

| No. | Catalyst | SBET, m2/g | Vpore, cm3/g | Sme, m2/g |
|---|---|---|---|---|
| 1 | 16.3 wt.% Pt/CNTinitial | 171 | 0.59 | 130 |
| 2 | 18.75 wt.% Pt/CNTHNO3 | 192 | 0.89 | 178 |
| 3 | 21.2 wt.% Pt/CNTHNO3+N | 213 | 0.51 | 119 |
| 4 | 17.8 wt.% Pt/CNTHNO3+P | 161 | 0.99 | 161 |
| 5 | 15.83 wt.% Pt/CNTHNO3+NP | 184 | 0.62 | 126 |
| No. | Catalyst | Element/at.% in CNT | Element/at.% in Pt/CNT |
|---|---|---|---|
| 1 | Pt/CNTinitial | O/0.46 | - |
| 2 | Pt/CNTHNO3 | O/15.4 | O/11.9 |
| N/0.75 | |||
| N/1.2 | Pt/1.78 | ||
| 3 | Pt/CNTHNO3+N | O/12.84 | O/8.36 |
| N/1.6 | |||
| N/1.98 | Pt/1.1 | ||
| 4 | Pt/CNTHNO3+P | O/10.8 | - |
| N/1.0 | |||
| P/0.2 | |||
| 5 | Pt/CNTHNO3+NP | O/10.8 | - |
| N/1.55 | |||
| P/0.4 |
| No. | Catalyst | Specific Resistance R, Ω cm | Density, g/cm3 | Specific Conductance κ, S/cm |
|---|---|---|---|---|
| 1 | Pt/CNTinitial | 5.9 | 1.69 | 0.169 |
| 2 | Pt/CNTHNO3 | 4.5 | 1.12 | 0.223 |
| 3 | Pt/CNTHNO3+N | 6.28 | 1.96 | 0.159 |
| 4 | Pt/CNTHNO3+P | 4.7 | 1.00 | 0.212 |
| 5 | Pt/CNTHNO3+NP | 5.5 | 1.61 | 0.182 |
| Material/No., as in Table 3 | Pt, wt% | 0.5 M H2SO4 | 0.1 M KOH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SEAS Pt, m2/g | E1/2, V | ikin, mA /cm2 | jmass, mA/mgPt | SEAS Pt, m2/g | E1/2, V | ikin, mA /cm2 | jmass, mA/mgPt | ||
| at 0.95 V | at 0.90 V | at 0.95 V | at 0.90 V (n) | ||||||
| 1500 rpm | 1500 rpm | ||||||||
| Pt/C*/1 | 20 | 48.5 | - | - | - | 51.7 | 0.88 | 0.50 | 55.7 |
| (3.2) | |||||||||
| Pt/CNTinitial/2 | 16.3 | 41.0 | 0.83 | 0.3 | 57.75 | 38.3 | 0.85 | 0.36 | 51 |
| (3.1) | |||||||||
| Pt/CNTHNO3/3 | 18.7 | 40.7 | 0.82 | 0.22 | 56.2 | 41.2 | 0.86 | 0.43 | 51.5 |
| (3.3) | |||||||||
| Pt/CNTHNO3+N/4 | 21.2 | 46.7 | 0.83 | 0.27 | 55.4 | 47.1 | 0.88 | 0.61 | 73.0 |
| (3.6) | |||||||||
| Pt/CNTHNO3+P/5 | 17.8 | 54.6 | 0.85 | 0 | 61.7 | 50.3 | 0.87 | 0.53 | 61 |
| (3.1) | |||||||||
| Pt/CNTHNO3+NP/6 | 15.8 | 55.5 | 0.82 | 0.08 | 61 | 54.0 | 0.87 | 0.43 | 65 |
| (3.4) | |||||||||
| Catalyst | Initial Data | After 500 Cycles | After 1000 Cycles | |||
|---|---|---|---|---|---|---|
| SPt, m2/gPt | E½, V | SPt, m2/gPt | E½, V | SPt, m2/gPt | E½, V | |
| Pt/C | 60 | 0.88 | 34 | 0.87 | 25 | 0.86 |
| Pt/CNTinitial | 38.3 | 0.85 | 33.1 | 0.84 | 24.6 | 0.84 |
| Pt/CNTHNO3 | 41.2 | 0.86 | 27.8 | 0.85 | 15.4 | 0.85 |
| Pt/CNTHNO3+N | 47.1 | 0.89 | 33.7 | 0.87 | 25.1 | 0.86 |
| Pt/CNTHNO3+P | 50.3 | 0.88 | 34.3 | 0.88 | 18.9 | 0.87 |
| Pt/CNTHNO3+NP | 54 | 0.87 | 37 | 0.86 | 26 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanovskaya, V.; Vernigor, I.; Radina, M.; Andreev, V.; Korchagin, O. Nanocomposite Cathode Catalysts Containing Platinum Deposited on Carbon Nanotubes Modified by O, N, and P Atoms. Catalysts 2021, 11, 335. https://doi.org/10.3390/catal11030335
Bogdanovskaya V, Vernigor I, Radina M, Andreev V, Korchagin O. Nanocomposite Cathode Catalysts Containing Platinum Deposited on Carbon Nanotubes Modified by O, N, and P Atoms. Catalysts. 2021; 11(3):335. https://doi.org/10.3390/catal11030335
Chicago/Turabian StyleBogdanovskaya, Vera, Inna Vernigor, Marina Radina, Vladimir Andreev, and Oleg Korchagin. 2021. "Nanocomposite Cathode Catalysts Containing Platinum Deposited on Carbon Nanotubes Modified by O, N, and P Atoms" Catalysts 11, no. 3: 335. https://doi.org/10.3390/catal11030335
APA StyleBogdanovskaya, V., Vernigor, I., Radina, M., Andreev, V., & Korchagin, O. (2021). Nanocomposite Cathode Catalysts Containing Platinum Deposited on Carbon Nanotubes Modified by O, N, and P Atoms. Catalysts, 11(3), 335. https://doi.org/10.3390/catal11030335







