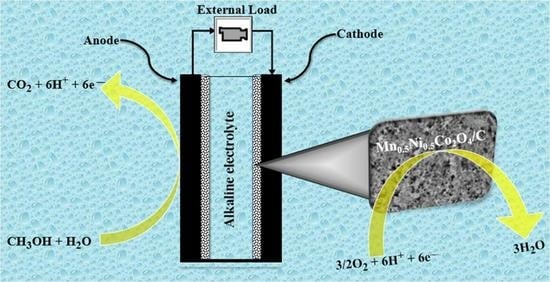
Mn-Ni-Co-O Spinel Oxides towards Oxygen Reduction Reaction in Alkaline Medium: Mn0.5Ni0.5Co2O4/C Synergism and Cooperation
Abstract
Share and Cite
Matthews, T.; Dolla, T.H.; Gwebu, S.S.; Mashola, T.A.; Dlamini, L.T.; Carleschi, E.; Ndungu, P.; Maxakato, N.W. Mn-Ni-Co-O Spinel Oxides towards Oxygen Reduction Reaction in Alkaline Medium: Mn0.5Ni0.5Co2O4/C Synergism and Cooperation. Catalysts 2021, 11, 1059. https://doi.org/10.3390/catal11091059
Matthews T, Dolla TH, Gwebu SS, Mashola TA, Dlamini LT, Carleschi E, Ndungu P, Maxakato NW. Mn-Ni-Co-O Spinel Oxides towards Oxygen Reduction Reaction in Alkaline Medium: Mn0.5Ni0.5Co2O4/C Synergism and Cooperation. Catalysts. 2021; 11(9):1059. https://doi.org/10.3390/catal11091059
Chicago/Turabian StyleMatthews, Thabo, Tarekegn Heliso Dolla, Sandile Surprise Gwebu, Tebogo Abigail Mashola, Lihle Tshepiso Dlamini, Emanuela Carleschi, Patrick Ndungu, and Nobanathi Wendy Maxakato. 2021. "Mn-Ni-Co-O Spinel Oxides towards Oxygen Reduction Reaction in Alkaline Medium: Mn0.5Ni0.5Co2O4/C Synergism and Cooperation" Catalysts 11, no. 9: 1059. https://doi.org/10.3390/catal11091059
APA StyleMatthews, T., Dolla, T. H., Gwebu, S. S., Mashola, T. A., Dlamini, L. T., Carleschi, E., Ndungu, P., & Maxakato, N. W. (2021). Mn-Ni-Co-O Spinel Oxides towards Oxygen Reduction Reaction in Alkaline Medium: Mn0.5Ni0.5Co2O4/C Synergism and Cooperation. Catalysts, 11(9), 1059. https://doi.org/10.3390/catal11091059