2.1. Acetone Uptake on TiO2 and CeO2
Adsorbed phase monitoring: In order to evaluate the individual uptakes of acetone on TiO
2 and CeO
2, the materials were successively exposed to 200 ppm of acetone inside the diffuse reflectance infrared Fourier transform device (DRIFT) cell (setup (b) on
Figure 1).
Figure 2a,c respectively reports the DRIFT spectra of TiO
2 and CeO
2 surfaces upon acetone uptake at 294 K using 20% O
2 in Ar as bath gas. Spectra are recorded in the range 3200–1000 cm
−1 with subtraction of the unexposed fresh material spectra as backgrounds.
The presence of surface species on both metal oxides once acetone is introduced in the DRIFT cell is attested by the growth of various absorption bands with time on their respective DRIFT spectra. Noticeably the DRIFT spectra of acetone uptake on CeO2 and TiO2 exhibited similarities and contrasts. In order to interpret these infrared absorption patterns, literature data were collected to assign the bands observed to the corresponding groups.
Acetone uptake on TiO
2: Typical absorption bands were observed at 2972, 2937, 2921, 2869, 1705, 1697, 1667, 1595, 1469, 1450, 1422, 1380, 1365 and 1236 cm
−1 (
Figure 2a). The νC=O stretching vibration at 1705 and 1697 cm
−1, the δCH
3 at 1422–1365 cm
−1 and the δC-C bending at 1236 cm
−1 attest of the presence of adsorbed acetone species [
19]. The temporal evolution of acetone adsorbed on TiO
2 surface is reported in
Figure 2b from the integration of its characteristic band of absorption at 2972 cm
−1. As the uptake of acetone was initiated, the intensity of the characteristic bands sharply increased during the first 10 min of exposure. It suggest that the adsorption equilibrium of acetone was reached rapidly; nonetheless, a gradual decrease in the surface coverage of acetone was noticed beyond 10 min of exposure while the inlet concentration of acetone was kept constant. This behavior suggests possible consumption of acetone on the surface of TiO
2 once a significant surface coverage is reached.
Conversion of adsorbed acetone on TiO
2: Indeed, other surface absorption bands continuously increase on the DRIFT spectra in
Figure 2a. Interestingly, these bands located at 1667, 1595 and 1450 cm
−1 can be assigned to the typical vibration modes of mesityl oxide (MO) [
20]. The temporal evolution of MO surface coverage is displayed along with acetone in
Figure 2b. It is retrieved after integration of the absorption band at 1450 cm
−1. The observation of MO on TiO
2 surface has formerly been attributed to surface condensation and dehydration reactions of acetone molecules by Coronado et al. [
21], El-Maazawi et al. [
17], Xu et al. [
16] and Barakat et al. [
13]. The temporal evolution of MO on the TiO
2 surface is reported in
Figure 2b. Between acetone and MO temporal profiles, a noticeable delay is visible on
Figure 2b. Barakat et al. [
13] and El-Maazawi et al. [
17] interpret delay as a surface coverage threshold in acetone to initiate the formation of MO. Our observations confirm the existence of a threshold to induce acetone condensation and dehydration. Assuming that saturation of the surface is reached when acetone reaches its maximum at t = 8 min, we estimate the surface coverage threshold at 0.2, in accordance with former values reported at ca. 0.3. It has to be noted that diacetone alcohol (DAA) was identified and reported as a reaction intermediate of acetone condensation to form MO, but it was not observed on the DRIFT spectra recorded along acetone uptake on TiO
2. This suggests that the conversion rate of DAA into MO on TiO
2 was high enough not to allow any accumulation on the surface.
Acetone uptake on CeO
2: The introduction of acetone in the DRIFT cell containing CeO
2 initially resulted in the formation of specific bands at 2962, 2930, 1695, 1425, 1369 and 1234 cm
−1 (
Figure 2c). These bands were respectively assigned to νs-CH
3, ν
as-CH
3, νC=O, δ
as-CH
3, δ
s-CH
3 and νC-C vibration modes of adsorbed acetone. In the course of adsorption, at approximately 5 min, the band at 1695 cm
−1 started splitting into two bands, at 1709 and 1676 cm
−1, along with new increasing bands at 1630, 1577, 1556, 1441, 1202, 1122 and 989 cm
−1 (
Figure 2c), attesting of other adsorbed species than acetone [
22].
Conversion of adsorbed acetone on CeO
2: The band at 1630 was assigned to νC=O and δCH vibrations in -CH
2-C=O groups of diacetone alcohol-like species (DAA) [
20]. Furthermore, the band at 1676 cm
−1 was characteristic of the νC=O and νC=C vibration modes of MO. A very broad band, appearing in the range 3500–3600 cm
−1 (not displayed in
Figure 2c), accompanied them. It is assigned to dissociatively adsorbed H
2O, resulting from the dehydration of DAA molecule upon its conversion into MO. The peak at 1556 cm
−1 also attested a contribution from the νOCO vibration of the formate species, which could be expected on the CeO
2 surface.
The adsorption of acetone on CeO
2 led to molecularly adsorbed acetone. It is gradually transformed into three condensation products: DAA, MO and formates. Their time evolution is reported in
Figure 2d by monitoring the normalized peak areas of the different species: νC=O of DAA at 1630 cm
−1, νC=C of MO at 1676 cm
−1 and ν
asCOO of formates at 1556 cm
−1.
According to the evolution of products adsorbed on CeO
2 surface reported in
Figure 2d, in the first five minutes, no condensation products were yet observed. During this time lapse, acetone gradually accumulated on CeO
2 surface until a maximum. Then it gradually decreased concomitantly with the formation of MO, DAA and formates. The increase in MO was faster than that of DAA, the intermediate between acetone condensation and MO. This could be consistent with a fast initial consumption of the intermediate, hindering its accumulation on the surface. Indeed, DAA was highly reactive and rapidly dehydrated to MO. As the surface became gradually covered, the formation of DAA was slowed down as the likelihood of condensation of two acetone molecules diminishes. Formates also gradually accumulated on the CeO
2 surface. In accordance with former observations reported for IPA adsorption on CeO
2, acetone was converted into carboxylate species, formates (HCOO
−), via a mechanism certainly involving lattice oxygen [
20]. This type of reactive adsorption is observed on transition metal oxides, with readily accessible multiple oxidation states [
23,
24]. On these oxide surfaces, the condensation of acetone into MO is reported to start beyond a surface coverage of 0.28 as for TiO
2. Profiles reported in
Figure 2d supported the existence of a coverage threshold regarding acetone conversion to MO.
Reactive adsorption pathway of acetone: Based on the work of Zaki et al. [
20] the following steps of acetone reactive adsorption on CeO
2 were proposed. In most of the metal oxides, Mn
+ sites typically act as Lewis acids and the O
2– ions act as Lewis bases. First, acetone initially adsorbs on the surface of Lewis acid sites (Ce
4+). If no basic site is available in the vicinity, acetone remains adsorbed on the metal center. If basic sites (-OH
– or O
2–) are available, the C-H bond may be activated for α-hydrogen abstraction and the consequent formation of an anionic enolate-type ion. This allows the reaction of the enolate species with another acetone molecule, if the surface coverage of acetone is high enough, to lead to a DAA intermediate. Finally, dehydration of DAA leads to adsorbed MO and H
2O
(g) or H
2O
(ads). MO can be considered as the final product of acetone reactive uptake on CeO
2 and on TiO
2. If intermediate species, namely formates and DAA are observed on CeO
2, this is not the case on TiO
2. Assuming that similar reaction steps occur on both metal oxides, intermediates are likely to be formed on the TiO
2 surface as well, but directly consumed, suggesting of a higher surface reaction kinetic on TiO
2 for that step. According to the proposed mechanism, Lewis acid sites are essential for anchoring acetone molecules to the surface. Coexisting base sites catalyze the condensation of the acetone molecules into mesityl oxide surface species, via formation and subsequent decomposition of enolate and diacetone alcohol species.
The Lewis acid and base sites generate pairs of particularly efficient adsorption capacity toward condensation products thus formed. Consequently, before any plasma exposure of the metal oxides with adsorbed acetone, surface reactions are already initiated, changing the nature of the organics in the adsorbed phase on CeO2 and TiO2.
Gas phase analysis: a quantitative approach of acetone uptakes: To improve the characterization of acetone adsorption on the TiO
2 and CeO
2 surface, adsorbed amounts of acetone are determined using experimental set up (a) allowing the breakthrough curve methodology as reported by Jia et al. [
6].
Figure 3 reports the respective values of specific surface areas of both oxides and the irreversibly adsorbed amounts of acetone expressed in µmol/g respectively for TiO
2 and CeO
2. The specific surface of TiO
2 (45 ± 5 m
2/g) was lower than the one of CeO
2 (75 ± 5 m
2/g). However, the adsorption capacity of TiO
2 and CeO
2 should be considered from a surface density of acetone taken up, expressed in µmol/m
2. The surface density of acetone molecules adsorbed on TiO
2 was 2.64 µmol/m² and that of CeO
2 was 2.13 µmol/m². This result shows that both metal oxides provide a surface density of sorption sites for acetone in the same order of magnitude. The experimental value determined for TiO
2 exceeded that of CeO
2 by 20%, which might promote condensation reactions between acetone molecule and could possibly be consistent with a higher kinetic in MO formation on TiO
2.
2.2. Interaction of OZONE with TiO2 and CeO2: Impact of Adsorbed Acetone
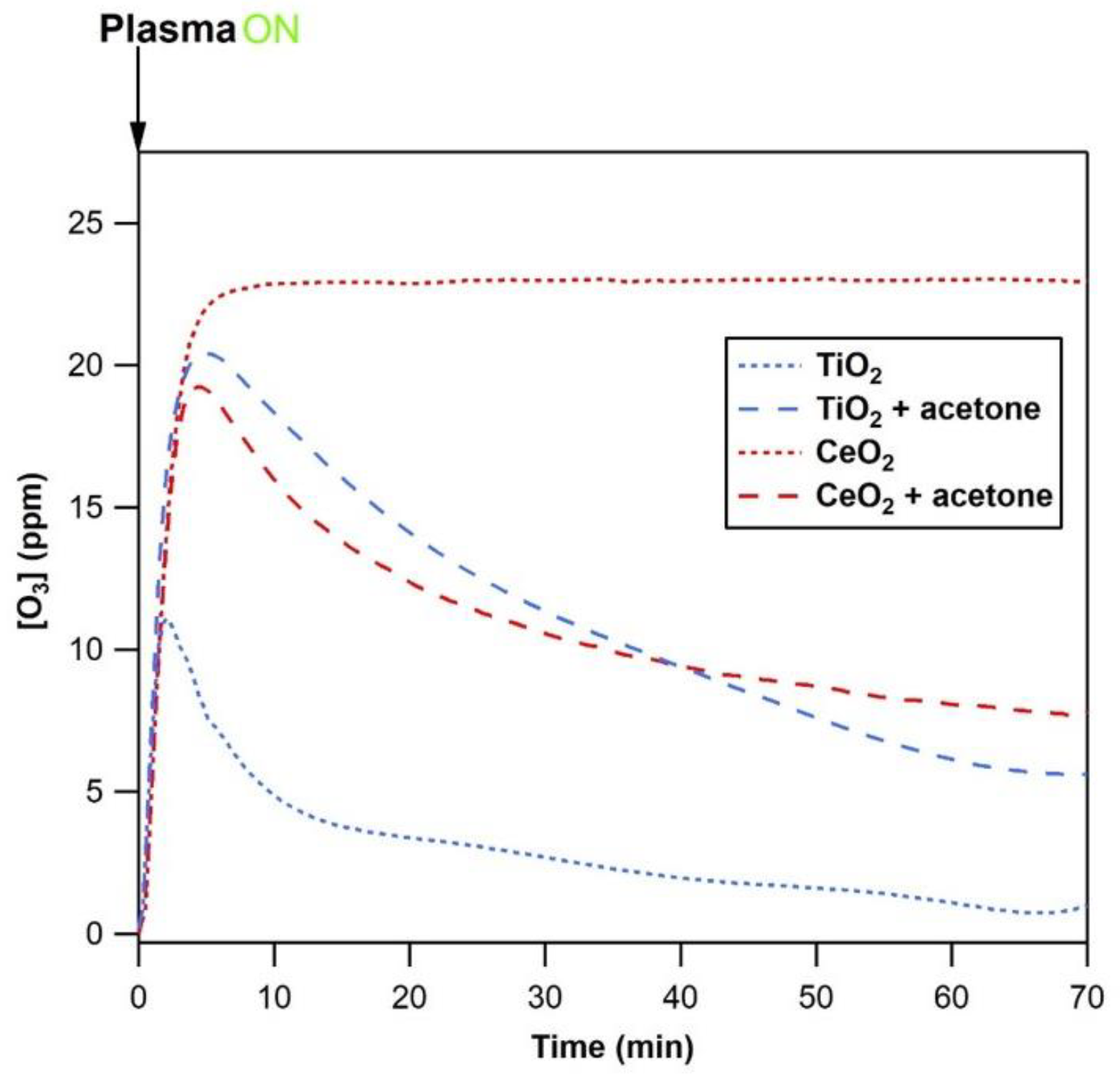
The aim of the present section was to understand the similarities and differences of the interaction of ozone produced by plasma with CeO
2 or TiO
2 under different surface states, i.e., clean surface vs. covered by acetone. This approach addressed qualitatively and quantitatively the reactive behavior of ozone on both oxides. The breakthrough curves of ozone were monitored using the set up (a) (U-shape reactor and FT-IR) dedicated to gas phase monitoring and described in
Figure 1. Once set in the U-shape reactor, both materials were treated first under dry air at 400 °C. Then two different cases were compared. First, exposure of the clean CeO
2 or TiO
2 to a 22 or 24 ppm ozone flow of 750 mL/min in order to monitor the uptake of ozone. Second, the materials were preliminarily exposed to 200 ppm of acetone for 60 min, as described in
Section 2.1, then flushed using dry air, and subsequently exposed to a 22 or 24 ppm and 750 mL/min ozone flow. In the second case, the ozone interacts with a surface equilibrated with acetone. Note that the exact ozone concentration was respectively 22 ppm and 24 ppm for TiO
2 and CeO
2.
Since, it is not experimentally achievable to have simultaneously the same BET surface for both metal oxides, the same masses of materials and the same absolute amount of acetone taken up on each material, the following compromise was defined. The mass of material and BET surface were 200 mg, i.e., 9 m
2 for TiO
2, and 100 mg, i.e., 7.5 m
2 for CeO
2. Hence, the amount of acetone irreversibly adsorbed before ozone exposure was 23 µmol on TiO
2 and 16.5 µmol on CeO
2. Therefore, we reached a surface density of 2.6 µmol/m
2 for TiO
2 and 2.2 µmol/m
2 for CeO
2, consistently with the previous section.
Figure 4 and
Figure 5 respectively reported the temporal profiles of the ozone concentration at the outlet of reactor, and the corresponding concentration of the ozone consumed.
Without preadsorbed acetone, the clean ceria is a highly effective ozone decomposer compared to clean TiO2. In the case of ceria, during 60 min of 22 ppm O3 exposure, no ozone was detected downstream the U-shape reactor. At the opposite, ozone breaks through TiO2 beyond 40 min of exposure only. TiO2 transiently induced the decomposition of a fraction of ozone through its molecular and dissociative adsorption on Lewis acid sites (Ti3+ and Ti4+). Ti is a Group IV transition metal and Ce is a lanthanide. Beyond the electronic structure, the polarity, the coordination of the cation, the surface lattice and the corresponding surface vacancies are drivers of the surface properties of the solids. For metal oxides, the polarity of the surface and the degree of coordinative saturation of the metal cations are known as the driving forces of adsorption. The presence of a dipole moment increases the surface Gibbs energy, making a polar surface more unstable and more reactive than a non-polar one. However, more prominently, the coordinatively unsaturated surface atoms are central for adsorption and reactivity. With a lower number of nearest neighbors than their corresponding ions in the bulk, metal cations have underpopulated d orbitals and become active in bonding with adsorbates in order to increase their coordination number and lower their surface energy through chemisorption. In metal oxides, the coordination unsaturated M+ sites behave like Lewis acids and the O2– ions behave like Lewis bases. Acetone, and ketones in general, initially adsorb molecularly on metal oxides through a σ bond between the metal atom of the solid and the carbonyl oxygen atom of the molecule. The electron rich carbonyl oxygen donates an electron pair to the surface acting as a Lewis base, and the surface metal center acts as a Lewis acid by accepting the electron pair.
Bulanin et al. [
25] showed that there are three main modes for ozone adsorption on TiO
2: (i) weakly bonded molecules that form hydrogen bonds with OH groups and are physically adsorbed on the surface; (ii) molecules adsorbed on the weaker Lewis acid sites and (iii) ozone interaction with strong Lewis acid sites. However, only ozone adsorbed on strong Lewis sites leads to the dissociation of the molecule. The continuous increase in concentration at the outlet of the sorbent reactor indicates that the decomposition efficiency of ozone decreases with time. This behavior is consistent with an increasing coverage of strong Lewis acid sites, making them gradually less available for further ozone adsorption. In 1998, Bulanin et al. [
26] also reported the uptake of O
3 on CeO
2. Using electron-paramagnetic resonance, IR and kinetic studies, they show the formation of a surface ozonide species generated by the reaction of ozone with the electron rich surface sites on CeO
2. Alike TiO
2, they revealed two kinds of Lewis acid sites, where ozone can either adsorb. They also show that ozone can form weak hydrogen bonds with the most acidic OH groups on the surface. On acidic oxides such as alumina and titanium dioxide, O
3 activation and decomposition was shown to occur via dissociation on strong Lewis sites [
23]. In the case of ceria, however, another non-dissociative mechanism of O
3 decomposition, involving unstable ozonide formation on surface basic oxygen sites was also observed. The enhanced dissociation in the case of CeO
2, as compared to TiO
2, could thus be attributed to this additional decomposition mechanism. However, does ozone chiefly interact with the material surface when organics are involved?
With preadsorbed acetone, the behavior of ozone is strongly altered on both oxides. On the one hand, the presence of acetone greatly inhibits the ozone uptake in the case of ceria. On the other hand, it is markedly enhanced in the case of TiO
2. The inhibition observed in the case of ceria may be due to the blockage of basic sites responsible for ozone dissociation by acetone decomposition products [
24]. However, it is worth noticing that the ozone outlet concentration (
Figure 4) and ozone consumption (
Figure 5) are very similar for both materials covered by taking up acetone with equivalent surface densities of acetone. This result indicates that, the behavior of ozone on materials covered by organics did not mainly depend on the nature of the materials, but it was driven by the presence of the organics. It could also be noted that during the first minutes of exposure to ozone, if the uptake was close to 100%, it gradually decreased to 20% for precovered TiO
2 and 40% for precovered CeO
2.
The similar trends in ozone behavior observed for TiO2 and CeO2, preliminarily exposed to acetone indicate that, at high surface coverages, the adsorbed organics drive the uptake and reactivity of ozone, rather than the material itself. In order to get a better understanding of surface oxidation processes, CO and CO2 production in the gas phase, and the evolution of adsorbed intermediates were investigated.
2.3. Ozonation of Acetone Adsorbed on TiO2 and CeO2
Monitoring of surface organics during ozonation of acetone exposed TiO2 and CeO2:
In the following, ozonation of TiO
2 and CeO
2, preliminarily exposed to 200 ppm acetone was monitored deploying the DRIFT technique (
Figure 1, setup (b)). After the adsorption step, the reversible fraction of acetone was flushed under dry air during 60 min. As ozone was sent on the surface of TiO
2 or CeO
2, the reversible fraction with acetone adsorbed was removed by flushing and only the irreversible fraction of organics remained on the adsorbed phase of metal oxides. Note that the surface reaction involving adsorbed acetone molecules and leading to surface byproducts was still effective along the flushing step. The conversion of acetone taken up still contributed to the modification of the organic phase during that step.
Figure 6a,c respectively reports DRIFT spectra collected during the ozonation of irreversibly adsorbed acetone on TiO
2 (a) and CeO
2 (c) surfaces in 20% O
2/80% Ar gas mixture at 294 K. DRIFT spectra are displayed in the range 3200–1000 cm
−1 with subtraction of the spectra of the respective unexposed materials as backgrounds.
Figure 6a,c evidenced that the oxidation of the surface organics by ozone occurred on TiO
2 and on CeO
2. If formates are observed in both cases, each material shows a different reactivity. A specific analysis of each case is described in the following.
Ozonation of TiO
2 preliminarily exposed to acetone: DRIFT monitoring: As discussed in the previous section, acetone adsorption on TiO
2 results in molecularly adsorbed acetone and a main condensation product, MO. Exposing the saturated surface to ozone results in the oxidation of the organics at the expense of a new adsorbed product: formates. DRIFT spectra and temporal evolution of adsorbed organics are reported in
Figure 6a,b respectively. Acetone was monitored through the evolution of the narrow band at 2976 cm
−1 to avoid peak overlapping with the products formed. As the discharge was turned on, the peak at 1667 cm
−1, corresponding to the νC=C of MO was broadened, probably because of formates gradually accumulating on the TiO
2 surface. The presence of surface formates was attested by the increasing band between 1567 and 1556 cm
−1. It is confirmed by a new peak at 1734 cm
−1, attributed to the νC=O vibration of carboxylic structure.
Ozonation of CeO
2 preliminarily exposed to acetone: DRIFT monitoring: DRIFT spectra and temporal profiles corresponding to the ozonation of CeO
2, preliminarily exposed to acetone, are reported on
Figure 6c,d. At t = 0, the surface consisted of three other compounds than acetone: MO (νC=C = 1676 cm
−1), DAA (νC=O = 1630 cm
−1) and formate species (ν
asCOO) at 1556 cm
−1 (
Figure 2c). Exposing the surface to ozone resulted in a decrease in the intensities of the methyl stretching and bending frequencies of MO and DAA. The band 1693 cm
−1 could be ascribed to formates, interacting with surface basic sites [
27]. Meanwhile, the bands at 1556, 1373 and 1359 cm
−1 could be assigned to the νCOO stretch of formate species upon acetone adsorption, and become more intense.
Figure 6d shows that MO, a condensation product present on the surface after the adsorption and flushing steps, decreased at the expense of formates, gradually accumulating on the CeO
2 surface.
Figure 5 shows that ozone decomposition on the surface in the first five minutes was concomitant with the significant MO loss and formate production
Figure 6d. The surface coverage of formates remained relatively constant throughout the ozonation period. It suggests that the surface had reached a steady state, determined either by saturation of the adsorption sites and/or by the dynamic equilibrium between the carboxylates formation and consumption.
Mineralization and carbon balance during ozonation of acetone exposed TiO2 and CeO2.
The production of CO and CO
2 in the gas phase during the ozonation of acetone exposed TiO
2 and CeO
2 was investigated using the U shape reactor coupled to the 10 m optical-path white-cell (
Figure 1, setup (a)).
Figure 7 reports the temporal profiles of CO and CO
2 monitored in the gas phase during ozone exposure of TiO
2 and CeO
2 preliminarily exposed to 200 ppm acetone.
Figure 8 reports the corresponding mineralization percentage defined as the ratio of the total number of moles of CO and CO
2 produced divided by the irreversibly adsorbed number of moles of acetone according to Equation (1). In that Equation,
X(
t) represents the mineralization percentage as a function of time
t, (
Ace)
0 represents the number of mole of acetone irreversibly adsorbed before ozonation starts, [CO] and [CO
2] represent the concentration in ppm of CO and CO
2, and Φ is the total molar flow corresponding to 750 mL/min and equals to 5.13 × 10
−4 mol/s.
Comparing the temporal profile of CO2 and CO monitored at the downstream of TiO2 vs. CeO2 reactors emphasizes contrasted mineralization regimes. First, for CeO2, concentrations of CO remained below the detection limits of the instrument (i.e., 10 ppb) while in the case of TiO2 from 0.2 to 0.4 ppm CO were produced on the whole investigated time span. CO2 profiles confirmed contrasted behaviors. In the case of CeO2, CO2 production exhibited a characteristic transient regime while in the case of TiO2, CO2 production was lower (ca. 1 ppm) but remained rather constant on the experimental time.
The contrasted CO
2 dynamics may sound unexpected since: (i) the absolute amounts of acetone taken up on TiO
2 (23 µmol) exceeded the one taken up on CeO
2 (16 µmol); while (ii) the amounts of O
3 consumed were equivalent between both systems. One hypothesis to explain these contrasted behaviors relies on the fact that the oxidation pathway differs between TiO
2 and CeO
2, not directly because of their different chemical natures, but because they induce differences in the chemical nature of the adsorbed organics. More precisely, the high and transient formation of CO
2 using CeO
2 (
Figure 7) suggests that, as the ozonation is initiated, a significant fraction of the organic phase adsorbed on CeO
2 is more compliant with the ozone reaction than organics adsorbed on TiO
2. In spite of the fact that TiO
2 and CeO
2 have preliminarily adsorbed the same initial VOC, namely acetone, they induce a significant conversion of acetone on their surface into surface intermediates. This reactive adsorption is effective on both oxides, with similar pathways, but with contrasted kinetics, leading to the presence of similar (mesithyl oxide) and different (DAA and formate) oxidation intermediates.
Consequently, the higher mineralization percentage observed in
Figure 8 for CeO
2 was not due to a higher consumption of ozone or a higher amount of organics present on its surface, but rather to a difference in the nature of organic adsorbates compared to TiO
2. From that point of view, the mineralization ability of a metal oxide under post-plasma exposure is driven by its ability to induce reactive adsorption of the primary VOC and conversion to organics readily mineralized.