Guerbet Reactions for Biofuel Production from ABE Fermentation Using Bifunctional Ni-MgO-Al2O3 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
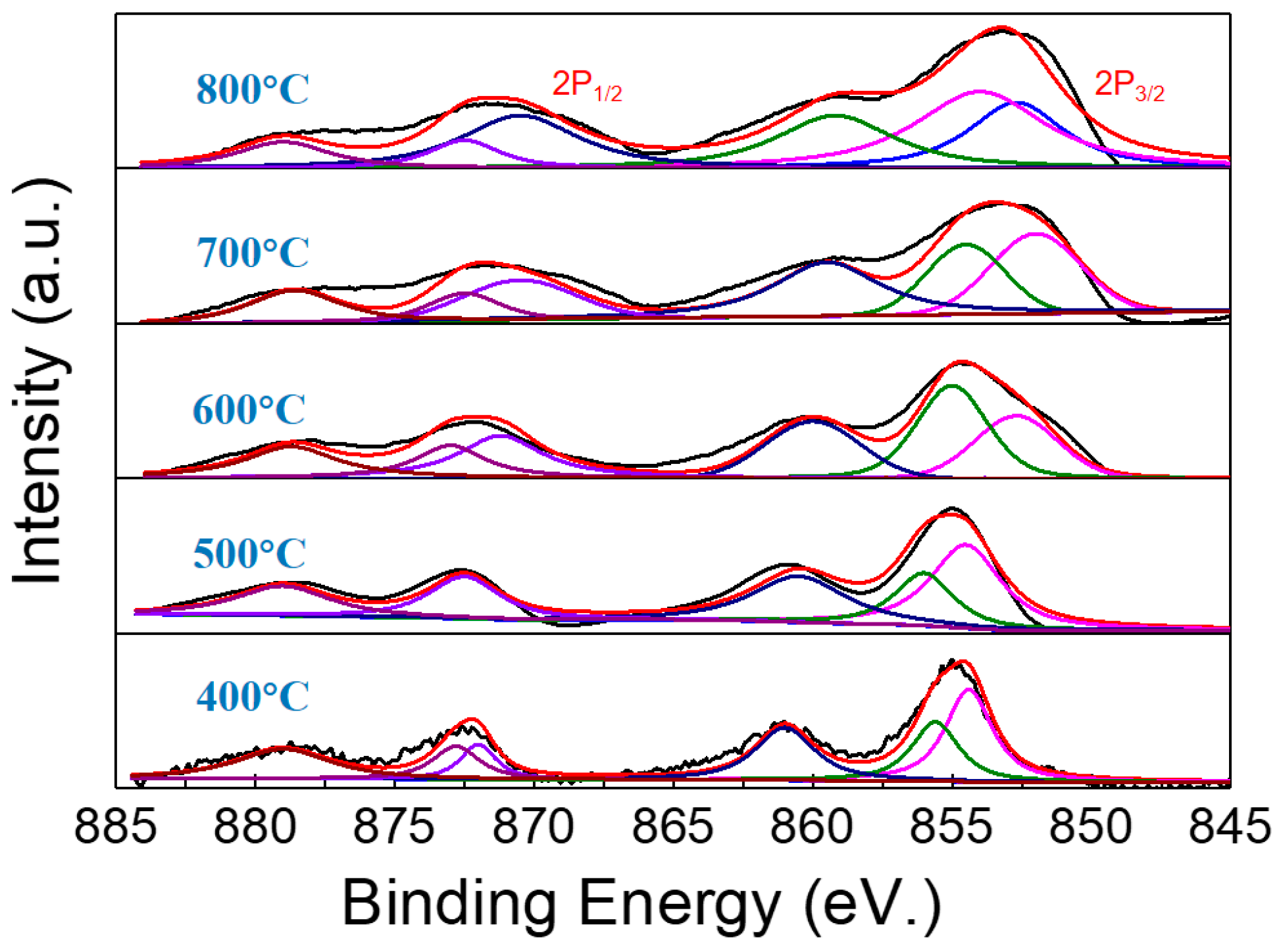
2.1. Characterization of the as-Prepared Catalyst
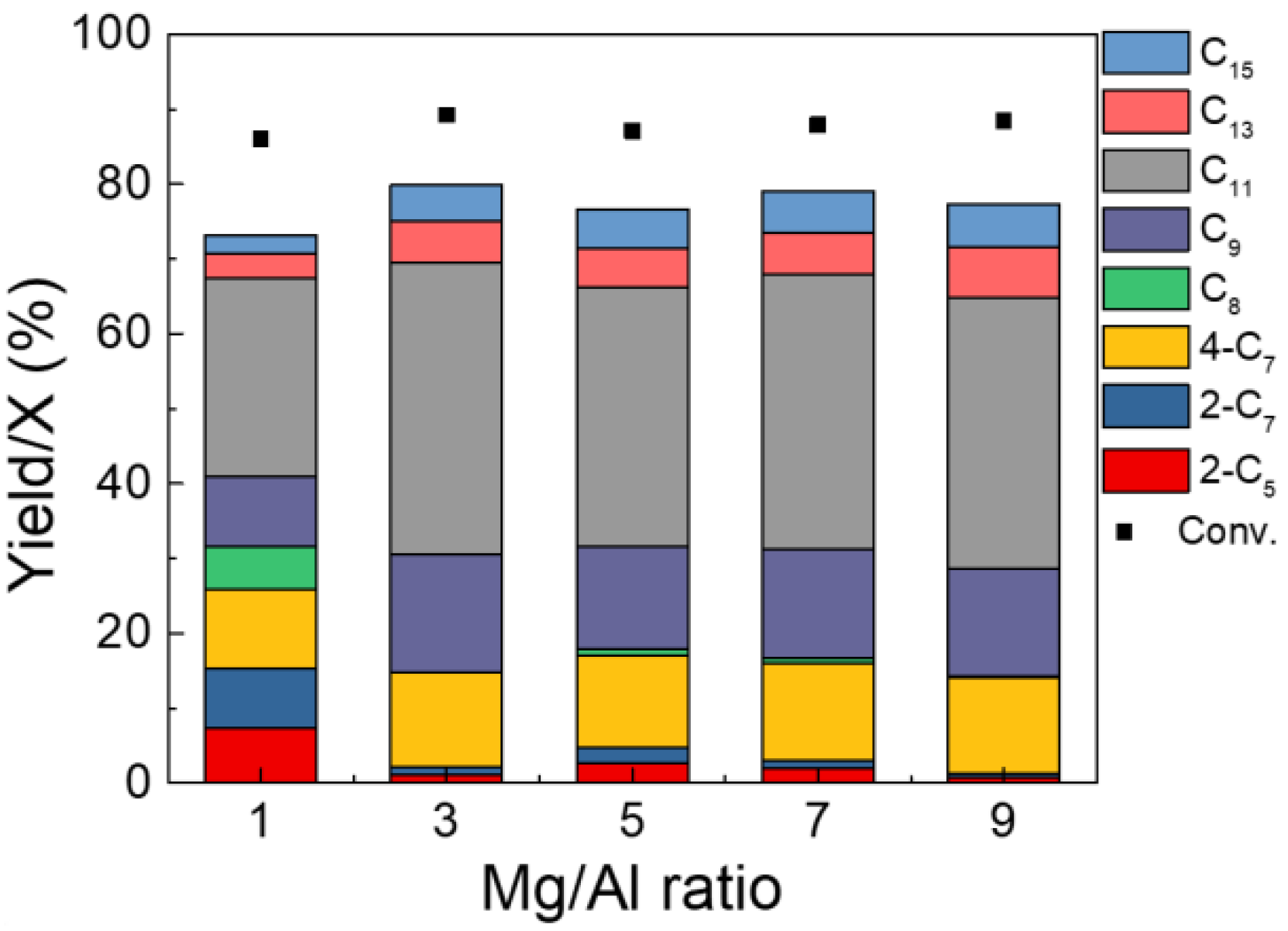
2.2. Catalytic Upgrading of ABE Mixture
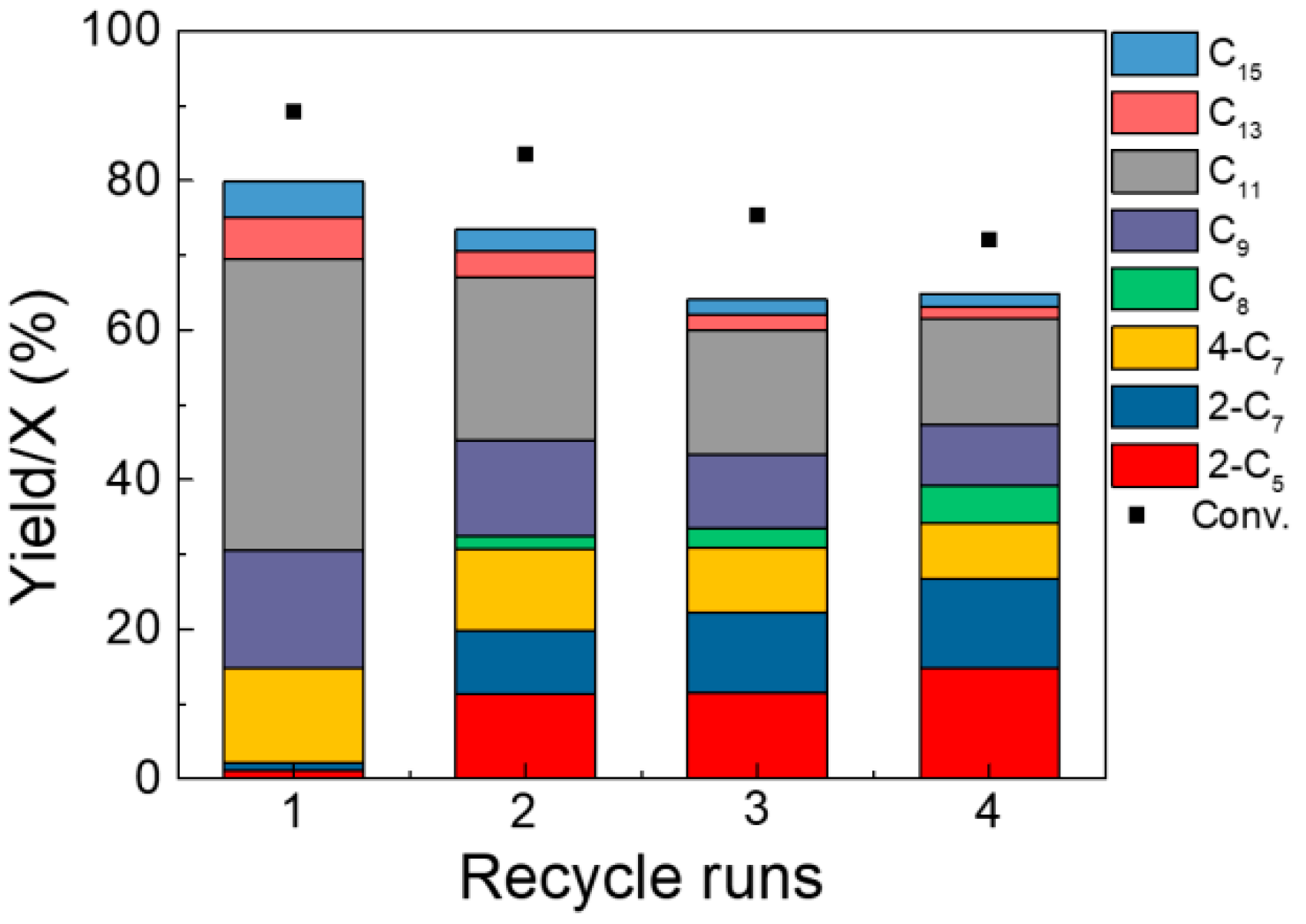
2.3. Regeneration Performance
3. Experimental Section
3.1. Materials
3.2. Preparation of the Catalyst
3.3. Characterization Techniques
3.4. Catalytic Conversion of Feedstock to Biofuel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic Conversion of Biomass to Biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Caes, B.R.; Teixeira, R.E.; Knapp, K.G.; Raines, R.T. Biomass to Furanics: Renewable Routes to Chemicals and Fuels. ACS Sustain. Chem. Eng. 2015, 3, 2591–2605. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z. Catalytic Conversion of Biomass into Chemicals and Fuels over Magnetic Catalysts. ACS Catal. 2016, 6, 326–338. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, Z.; Shao, Y.; Gong, X.; Wang, H.; Liu, X.; Parker, S.F.; Han, X.; Wang, Y. Direct hydrodeoxygenation of raw woody biomass into liquid alkanes. Nat. Commun. 2016, 7, 11162. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, M.F. Biorefineries for biofuel upgrading: A critical review. Appl. Energy 2009, 86, S151–S161. [Google Scholar] [CrossRef]
- Ghosh, S.; Chowdhury, R.; Bhattachary, P. Sustainability of cereal straws for the fermentative production of second generation biofuels: A review of the efficiency and economics of biochemical pretreatment processes. Appl. Energy 2017, 198, 284–298. [Google Scholar] [CrossRef]
- Ennaert, T.; Aelst, J.V.; Dijkmans, J.; Clercq, R.D.; Schutyser, W.; Dusselier, M.; Verboekend, D.; Sels, B.F. Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem. Soc. Rev. 2016, 45, 584–611. [Google Scholar] [CrossRef]
- Teng, J.J.; Ma, H.; Wang, F.R.; Wang, L.F.; Li, X.H. Catalytic Fractionation of Raw Biomass to Biochemicals and Organosolv Lignin in a Methyl Isobutyl Ketone/H2O Biphasic System. ACS Sustain. Chem. Eng. 2016, 4, 2020–2026. [Google Scholar] [CrossRef]
- Pileidis, F.D.; Titirici, M.M. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef]
- Pang, J.F.; Zheng, M.Y.; Sun, R.Y.; Wang, A.Q.; Wang, X.D.; Zhang, T. Synthesis of ethylene glycol and terephthalic acid from biomass for producing PET. Green Chem. 2016, 18, 342–359. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Bi, P.Y.; Wang, J.C.; Jiang, P.W.; Wu, X.P.; Xue, H.; Liu, J.X.; Zhou, X.G.; Li, Q.X. Production of jet and diesel biofuels from renewable lignocellulosic biomass. Appl. Energy 2015, 150, 128–137. [Google Scholar] [CrossRef]
- Han, J.; Sen, S.M.; Alonso, D.M.; Dumesic, J.A.; Maravelias, C.T. A strategy for the simultaneous catalytic conversion of hemicellulose and cellulose from lignocellulosic biomass to liquid transportation fuels. Green Chem. 2014, 16, 653–661. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.S.; Cai, C.Y.; Wang, S.H.; Pei, H.S.; Zhang, J.S. Corn stover pretreatment by inorganic salts and its effects on hemicellulose and cellulose degradation. Bioresour. Technol. 2009, 100, 5865–5871. [Google Scholar] [CrossRef]
- Liao, Y.H.; Liu, Q.Y.; Wang, T.J.; Long, J.X.; Ma, L.L.; Zhang, Q. Zirconium phosphate combined with Ru/C as a highly efficient catalyst for the direct transformation of cellulose to C6 alditols. Green Chem. 2014, 16, 3305–3312. [Google Scholar] [CrossRef]
- Liao, Y.H.; Liu, Q.Y.; Wang, T.J.; Long, J.X.; Zhang, Q.; Zhang, Q.; Ma, L.L.; Zhang, Q.; Liu, Y.; Li, Y.P. Promoting Hydrolytic Hydrogenation of Cellulose to Sugar Alcohols by Mixed Ball Milling of Cellulose and Solid Acid Catalyst. Energy Fuels 2018, 28, 5778–5784. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, S.; Pillinger, M.; Valente, A.A. Modified versions of sulfated zirconia as catalysts for the conversion of xylose to furfural. Catal. Lett. 2007, 114, 151–160. [Google Scholar] [CrossRef]
- Mascal, M.; Dutta, S.; Gandarias, I. Hydrodeoxygenation of the Angelica Lactone Dimer, a Cellulose-Based Feedstock: Simple, High-Yield Synthesis of Branched C7–C10 Gasoline-like Hydrocarbons. Angew. Chem. Int. Ed. 2014, 53, 1854–1857. [Google Scholar] [CrossRef]
- Wang, T.J.; Weng, Y.J.; Qiu, S.B.; Long, J.X.; Chen, L.G.; Li, K.; Liu, Q.Y.; Zhang, Q.; Ma, L.L. Gasoline Production by One-pot Catalytic Conversion of Lignocellulosic Biomass Derived Sugar/Polyol. Energy Procedia 2015, 75, 773–778. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.G.; Wang, T.J.; Zhang, Q.; Wang, C.G.; Yan, J.Y.; Ma, L.L. One-Pot Catalytic Conversion of Raw Lignocellulosic Biomass into Gasoline Alkanes and Chemicals over LiTaMoO6 and Ru/C in Aqueous Phosphoric Acid. ACS Sustain. Chem. Eng. 2015, 3, 1745–1755. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, X.H.; Hu, B.C.; Lu, G.Z.; Wang, Y.Q. Efficient catalytic conversion of lignocellulosic biomass into renewable liquid biofuels via furan derivatives. RSC Adv. 2014, 4, 31101–31107. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Li, S.; Wang, W.; Li, L.; Wang, A.; Wang, X.; Yu, C.; Zhang, T. Synthesis of diesel and jet fuel range alkanes with furfural and ketones from lignocellulose under solvent free conditions. Green Chem. 2014, 16, 4879. [Google Scholar] [CrossRef]
- Sheng, X.; Li, N.; Li, G.; Wang, W.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Direct synthesis of gasoline and diesel range branched alkanes with acetone from lignocellulose. Green Chem. 2016, 18, 3707–3711. [Google Scholar] [CrossRef]
- Xia, Q.N.; Cuan, Q.; Liu, X.H.; Gong, X.Q.; Lu, G.Z.; Wang, Y.Q. Pd/NbOPO4 Multifunctional Catalyst for the Direct Production of Liquid Alkanes from Aldol Adducts of Furans. Angew. Chem. Int. Ed. 2014, 53, 9755–9760. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Chang, Z.; Gao, L.L.; Chen, C.J.; Niu, Y.P.; Qin, P.Y.; Wang, Z.; Tan, T.W. Acetone–butanol–ethanol (ABE) fermentation integrated with simplified gas stripping using sweet sorghum bagasse as immobilized carrier. Chem. Eng. J. 2015, 277, 176–185. [Google Scholar] [CrossRef]
- Anbarasan, P.; Baer, Z.C.; Sreekumar, S.; Gross, E.; Binder, J.B.; Blanch, H.W.; Toste, D. Integration of chemical catalysis with extractive fermentation to produce fuels. Nature 2012, 491, 235–239. [Google Scholar] [CrossRef]
- Xu, G.Q.; Li, Q.; Feng, J.G.; Liu, Q.; Zhang, Z.J.; Wang, X.C.; Zhang, X.Y.; Mu, X.D. Direct a-Alkylation of Ketones with Alcohols in Water. ChemSusChem 2014, 7, 105–109. [Google Scholar] [CrossRef]
- Xue, C.; Liu, M.; Guo, X.; Hudson, E.P.; Chen, L.; Bai, F.; Liu, F.; Yang, S. Bridging chemica and bio-catalysis: High-value liquid transportation fuel production from renewable agricultural residues. Green Chem. 2017, 19, 660–669. [Google Scholar] [CrossRef]
- Goulas, K.A.; Gunbas, G.; Dietrich, P.J.; Sreekumar, S.; Gippo, A.; Chen, J.P.; Gokhale, A.A.; Toste, F.D. ABE Condensation over Monometallic Catalysts: Catalyst Characterization and Kinetics. ChemCatChem 2017, 9, 677–688. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Sacia, E.R.; Sreekumar, S.; Gunbas, G.; Gokhale, A.A.; Scown, C.D.; Toste, F.D.; Bell, A.T. Novel pathways for fuels and lubricants from biomass optimized using life-cycle greenhouse gas assessment. Proc. Natl. Acad. Sci. USA 2015, 112, 7645–7649. [Google Scholar] [CrossRef]
- Vo, H.T.; Yeo, S.M.; Dahnum, D.; Jae, J.; Hong, C.S.; Lee, H. Pd/C-CaO-catalyzed α-alkylation and hydrodeoxygenation of an acetone-butanol-ethanol mixture for biogasoline synthesis. Chem. Eng. J. 2017, 313, 1486–1493. [Google Scholar] [CrossRef]
- Sreekumar, S.; Baer, Z.C.; Gross, E.; Padmanaban, S.; Goulas, K.; Gunbas, G.; Alayoglu, S.; Blanch, H.W.; Clark, D.S.; Toste, F.D. Chemocatalytic upgrading of tailored fermentation products toward biodiesel. ChemSusChem 2014, 7, 2445–2448. [Google Scholar] [CrossRef] [PubMed]
- Goulas, K.A.; Sreekumar, S.; Song, Y.; Kharidehal, P.; Gunbas, G.; Dietrich, P.J.; Toste, F.D. Synergistic Effects in Bimetallic Palladium Copper Catalysts Improve Selectivity in Oxygenate Coupling Reactions. J. Am. Chem. Soc. 2016, 138, 6805–6812. [Google Scholar] [CrossRef] [PubMed]
- Onyestyák, G.; Novodarszki, G.; Barthos, R.; Klebert, S.; Wellisch, A.F.; Pilbath, A. Acetone alkylation with ethanol over multifunctional catalysts by a borrowing hydrogen strategy. RSC Adv. 2015, 5, 99502–99509. [Google Scholar] [CrossRef]
- Onyestyák, G.; Novodárszki, G.; Wellisch, F.; Pilbáth, A. Upgraded biofuel from alcohol-acetone feedstocks over a two-stage flow-through catalytic system. Catal. Sci. Technol. 2016, 6, 4516–4524. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Shen, C.; Jie, W.; Tan, T.W. Upgrade of solvent-free acetone-butanol-ethanol mixture to high-value biofuels over Ni-containing Mgo-SiO2 catalysts with greatly improved water-resistance. ACS Sustain. Chem. Eng. 2017, 5, 8181–8191. [Google Scholar] [CrossRef]
- Carlini, C.; Marchionnab, M.; Noviello, M.; Gallettia, A.M.R.; Sbranaa, G.; Basiled, F.; Vaccarid, A. Guerbet condensation of methanol with n-propanol to isobutyl alcohol over heterogeneous bifunctional catalysts based on Mg-Al mixed oxides partially substituted by different metal components. J. Mol. Catal. A Chem. 2005, 232, 13–20. [Google Scholar] [CrossRef]
- Horaa, L.; Kelbichováa, V.; Kikhtyanina, O.; Bortnovskiyb, O.; Kubicka, D. Aldol condensation of furfural and acetone over Mg Al layered double hydroxides and mixed oxides. Catal. Today 2014, 223, 138–147. [Google Scholar] [CrossRef]
- Abello, S.; Medina, F.; Tichit, D.; Prez-Ramrez, J.; Groen, J.C.; Sueiras, J.E.; Salagre, P.; Cesteros, Y. Aldol Condensations Over Reconstructed Mg-Al Hydrotalcites: Structure-Activity Relationships Related to the Rehydration Method. Chem. Eur. J. 2005, 11, 728–739. [Google Scholar] [CrossRef]
- Pupovac, K.; Palkovits, R. Cu/MgAl2O4 as Bifunctional Catalyst for AldolCondensation of 5-Hydroxymethylfurfural and SelectiveTransfer Hydrogenation. ChemSusChem 2013, 6, 2103–2110. [Google Scholar] [CrossRef]
- Morgiela, J.; Szlezyngera, M.; Pomorskaa, M.; Marszałek, K.; Mani, R.; Marszałekb, K.; Mania, R. In-situ TEM heating of Ni/Al multilayers. Int. J. Mater. Res. 2015, 106, 703–710. [Google Scholar] [CrossRef]
- Gong, Y.H.; Shen, C.; Wang, J.; Tan, W.T. Improved Selectivity of Long-Chain Products from Aqueous Acetone Butanol Ethanol Mixture over High Water Resistant Catalyst Based on Hydrophobic SBA-16. ACS Sustain. Chem. Eng. 2019, 7, 10323–10331. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; He, L.; Li, L.; Pan, X.; Wang, A.; Wang, X.; Zhang, T. Upgrading ethanol to n-butanol over highly dispersed Ni–MgAlO catalysts. J. Catal. 2016, 344, 184–193. [Google Scholar] [CrossRef]
- Carvalhoa, D.L.; Avillezb, R.R.; Rodriguesc, M.T.; Borgesa, L.; Appelc, L.G. Mg and Al mixed oxides and the synthesis of n-butanol from ethanol. Appl. Catal. A Gen. 2012, 415–416, 96–100. [Google Scholar] [CrossRef]
- Xia, K.; Lang, W.; Li, P.; Yan, X.; Guo, Y. The properties and catalytic performance of Pt-In/Mg(Al)O catalysts for the propane dehydrogenation reaction: Effects of pH value in preparing Mg(Al)O supports by the co-precipitation method. J. Catal. 2016, 338, 104–114. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Physical Electronics: Chanhassan, MN, USA, 1995. [Google Scholar] [CrossRef]
- Dries, G.; Willinton, Y.H.; Bert, S.; Pascal, V.D.V. Review of catalytic systems and thermodynamics for the Guerbet condensation reaction and challenges for biomass valorization. Catal. Sci. Technol. 2015, 5, 3876–3902. [Google Scholar] [CrossRef]

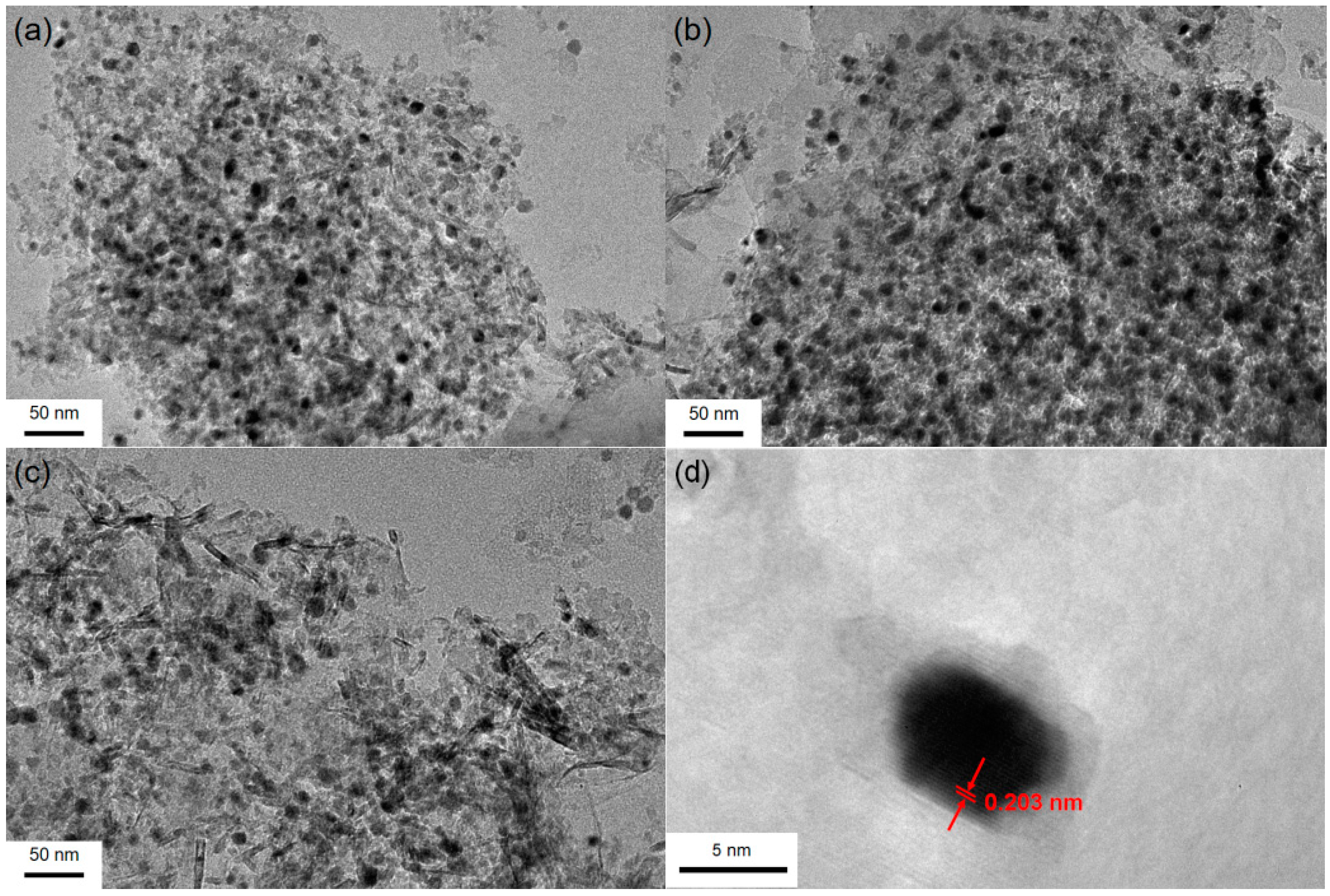
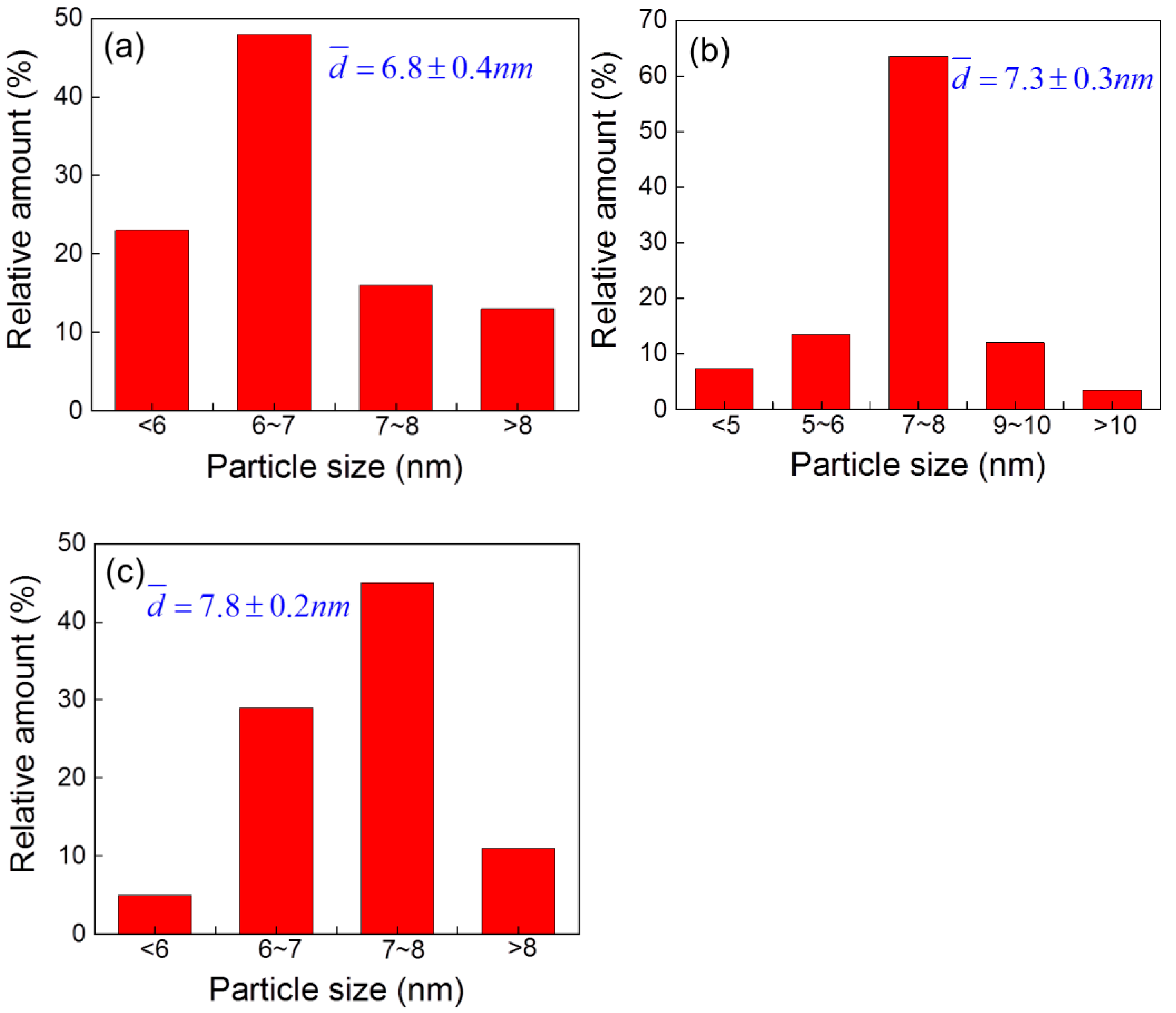
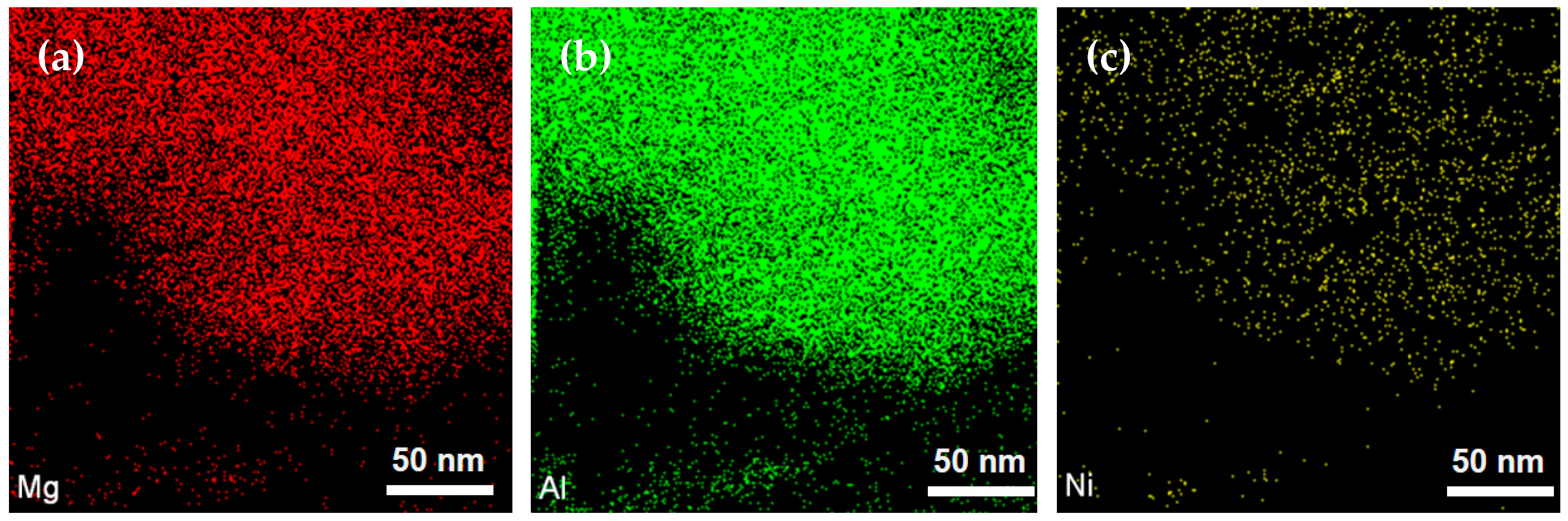
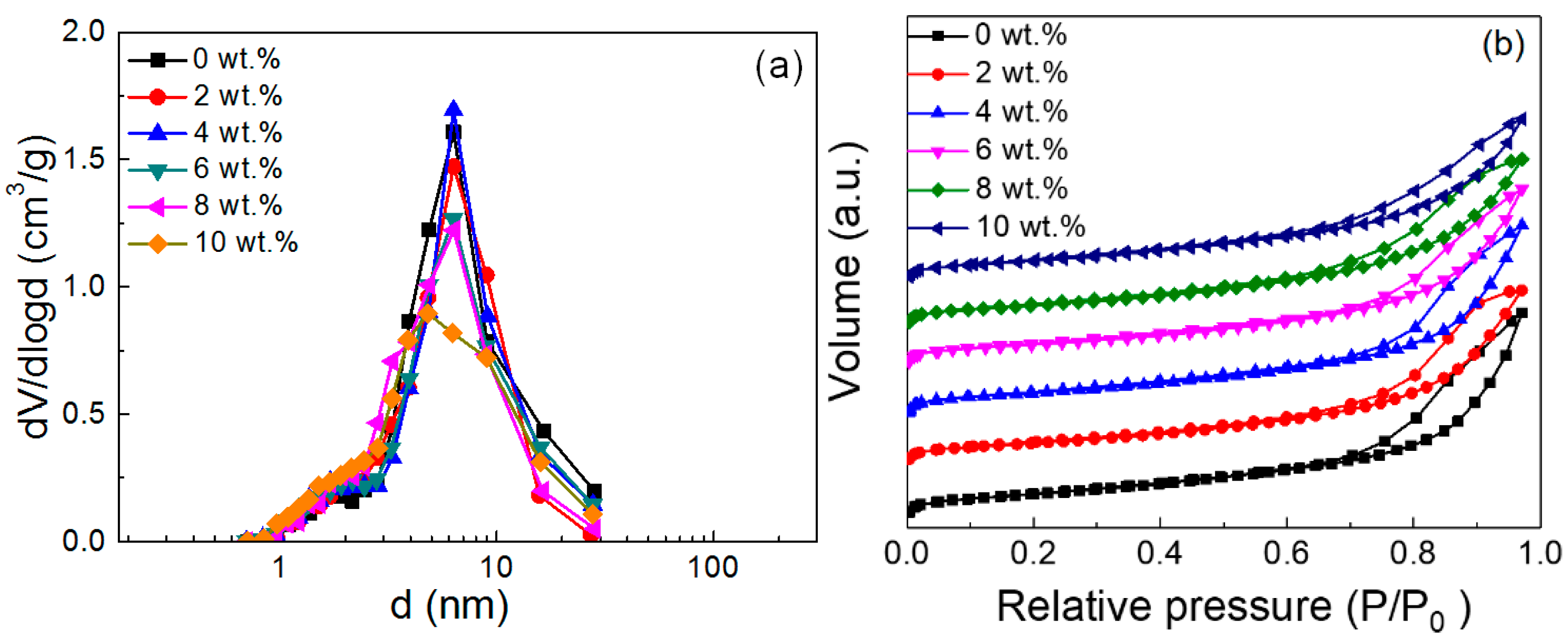
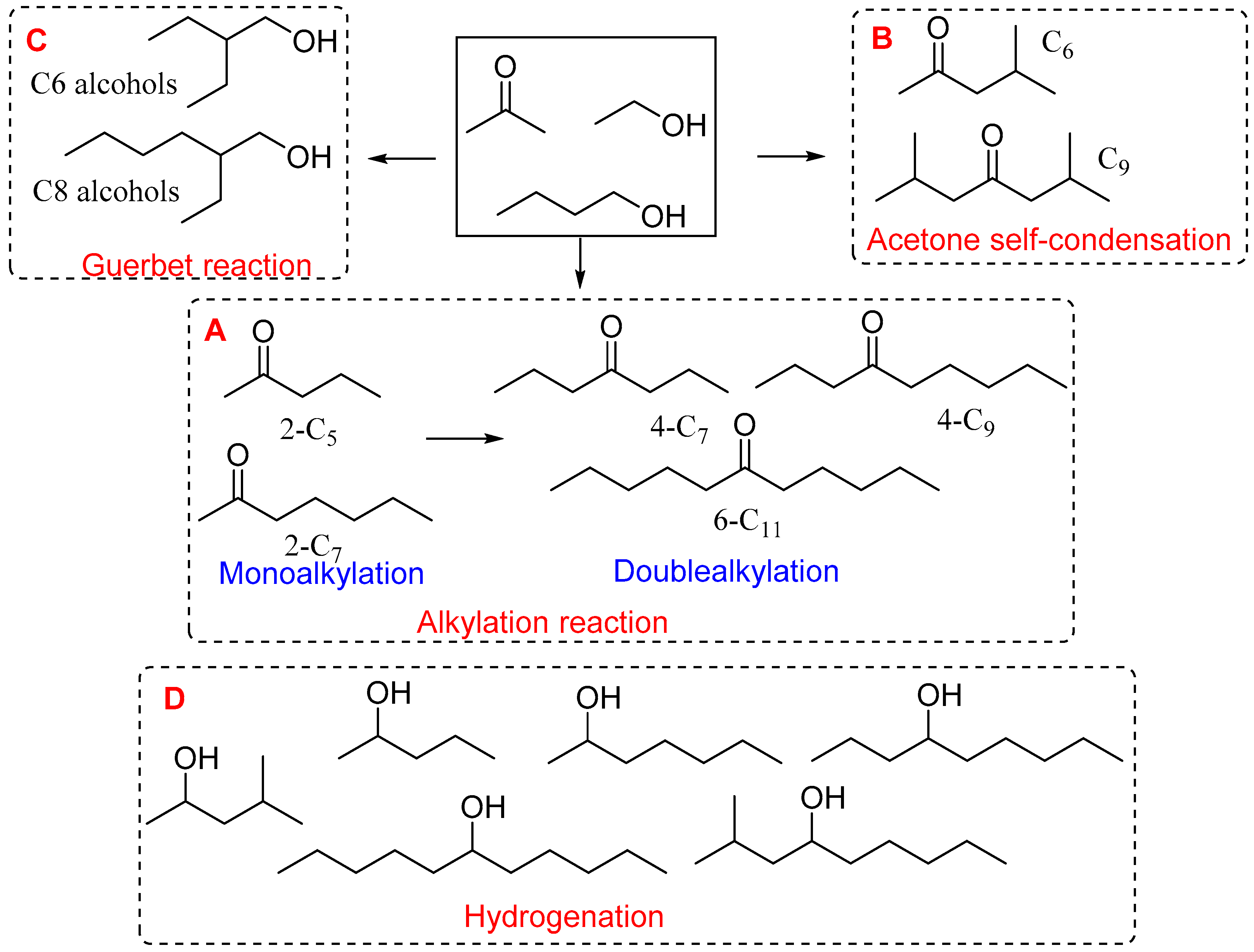
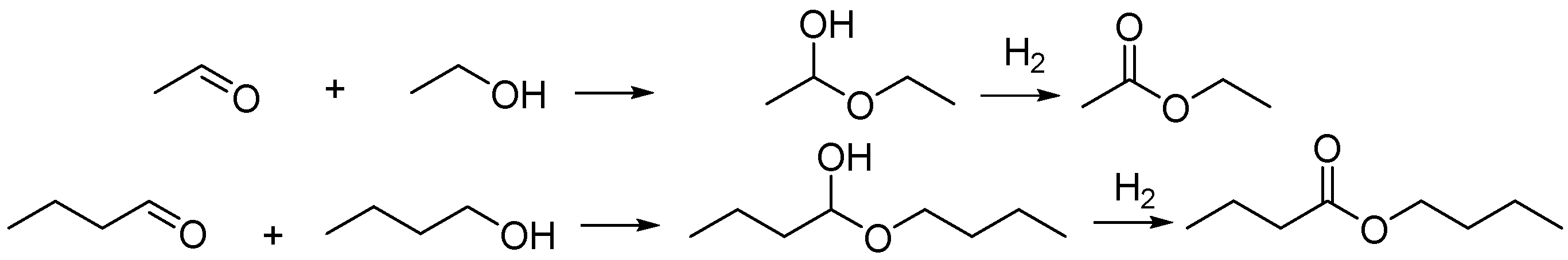

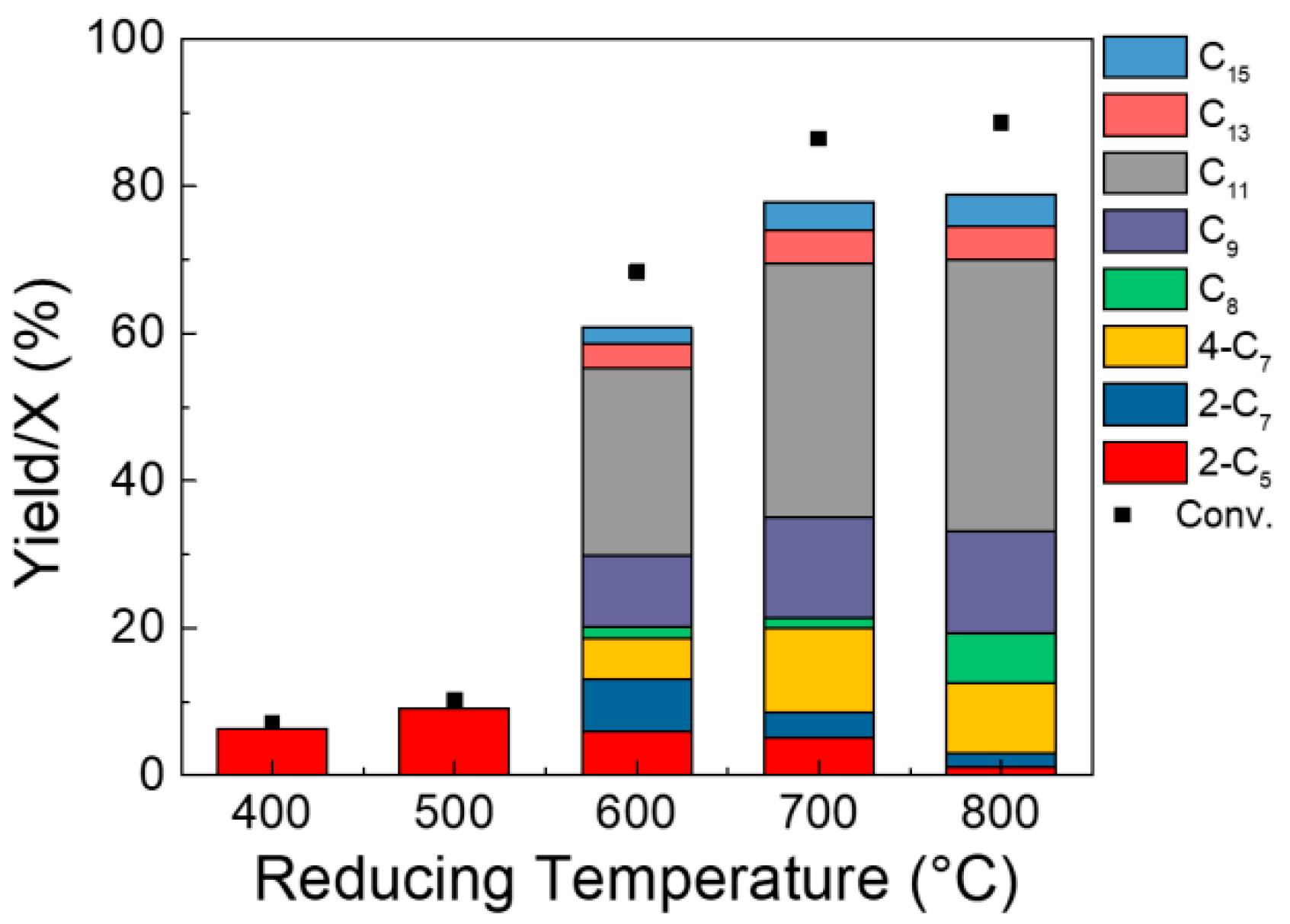

| Ni Loading (wt.%) | Surface Area (m2/g) | Pore Volume (cm3/g) | Mean Pore Diameter (nm) | Crystalline Size (nm) |
|---|---|---|---|---|
| 0 | 267.1 | 0.8 | 6 | / |
| 2 | 256.1 | 0.74 | 5.8 | 6.7 |
| 4 | 238 | 0.69 | 5.7 | 7.1 |
| 6 | 237.5 | 0.68 | 5.7 | 7.5 |
| 8 | 237.4 | 0.65 | 5.5 | 7.9 |
| 10 | 227.2 | 0.63 | 5.5 | 8.5 |
| Temperature (°C) | Ni0 Amount (%) | Ni2+ Amount (%) | Ni0/Ni2+ Molar Ratio |
|---|---|---|---|
| 400 | 7 | 93 | 0.07 |
| 500 | 17.4 | 82.6 | 0.21 |
| 600 | 55.7 | 44.3 | 1.28 |
| 700 | 78 | 22 | 3.55 |
| 800 | 87 | 13 | 6.69 |
| Mg/Al | Acid Sites (µmol/g) | Basic Sites (µmol/g) Total Basic Sites (mmol/g) | ||
|---|---|---|---|---|
| Weak Basic Sites (mmol/g) | Moderate Basic Sites (mmol/g) | Total Basic Sites | ||
| 1:1 | 0.41 | 0.69 | 0.40 | 1.09 |
| 3:1 | 0.40 | 0.68 | 0.56 | 1.24 |
| 5:1 | 0.37 | 0.65 | 0.60 | 1.25 |
| 7:1 | 0.32 | 0.63 | 0.76 | 1.39 |
| 9:1 | 0.27 | 0.61 | 0.84 | 1.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Wang, P.; Wang, J.; Tan, T. Guerbet Reactions for Biofuel Production from ABE Fermentation Using Bifunctional Ni-MgO-Al2O3 Catalysts. Catalysts 2021, 11, 414. https://doi.org/10.3390/catal11040414
Wu Z, Wang P, Wang J, Tan T. Guerbet Reactions for Biofuel Production from ABE Fermentation Using Bifunctional Ni-MgO-Al2O3 Catalysts. Catalysts. 2021; 11(4):414. https://doi.org/10.3390/catal11040414
Chicago/Turabian StyleWu, Zhiyi, Pingzhou Wang, Jie Wang, and Tianwei Tan. 2021. "Guerbet Reactions for Biofuel Production from ABE Fermentation Using Bifunctional Ni-MgO-Al2O3 Catalysts" Catalysts 11, no. 4: 414. https://doi.org/10.3390/catal11040414