Supported-Metal Catalysts in Upgrading Lignin to Aromatics by Oxidative Depolymerization
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Key Reaction Parameters
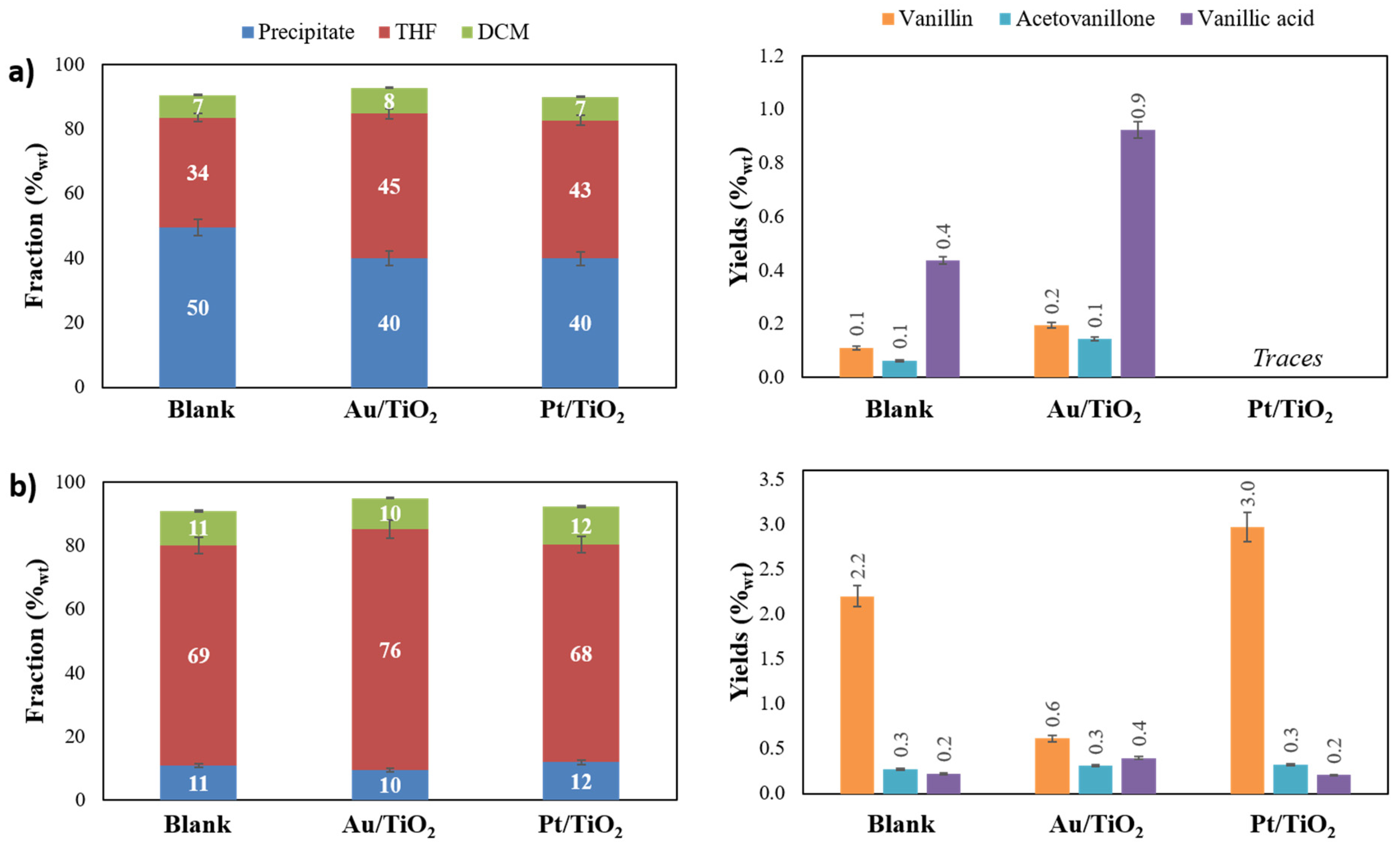
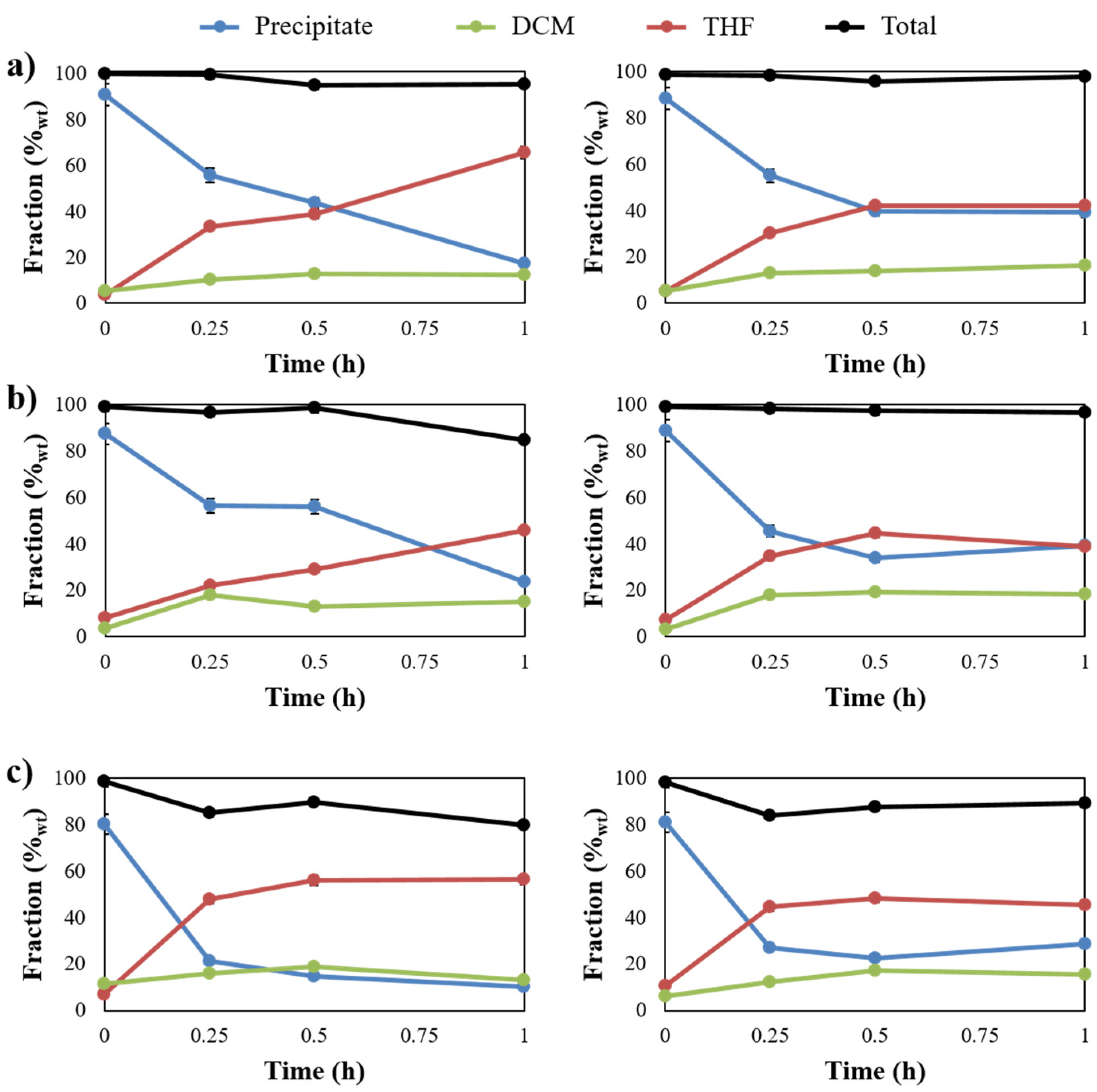
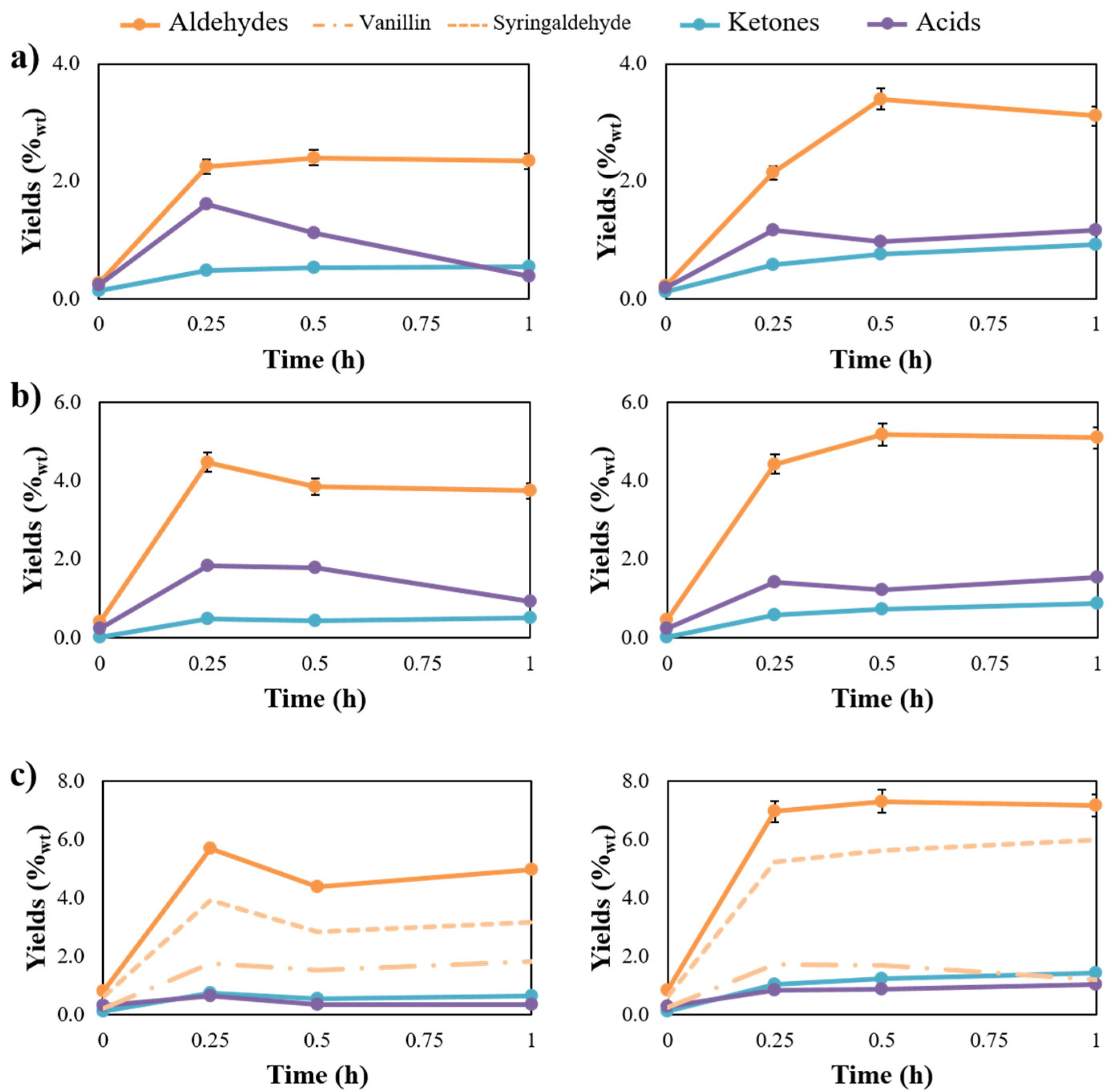
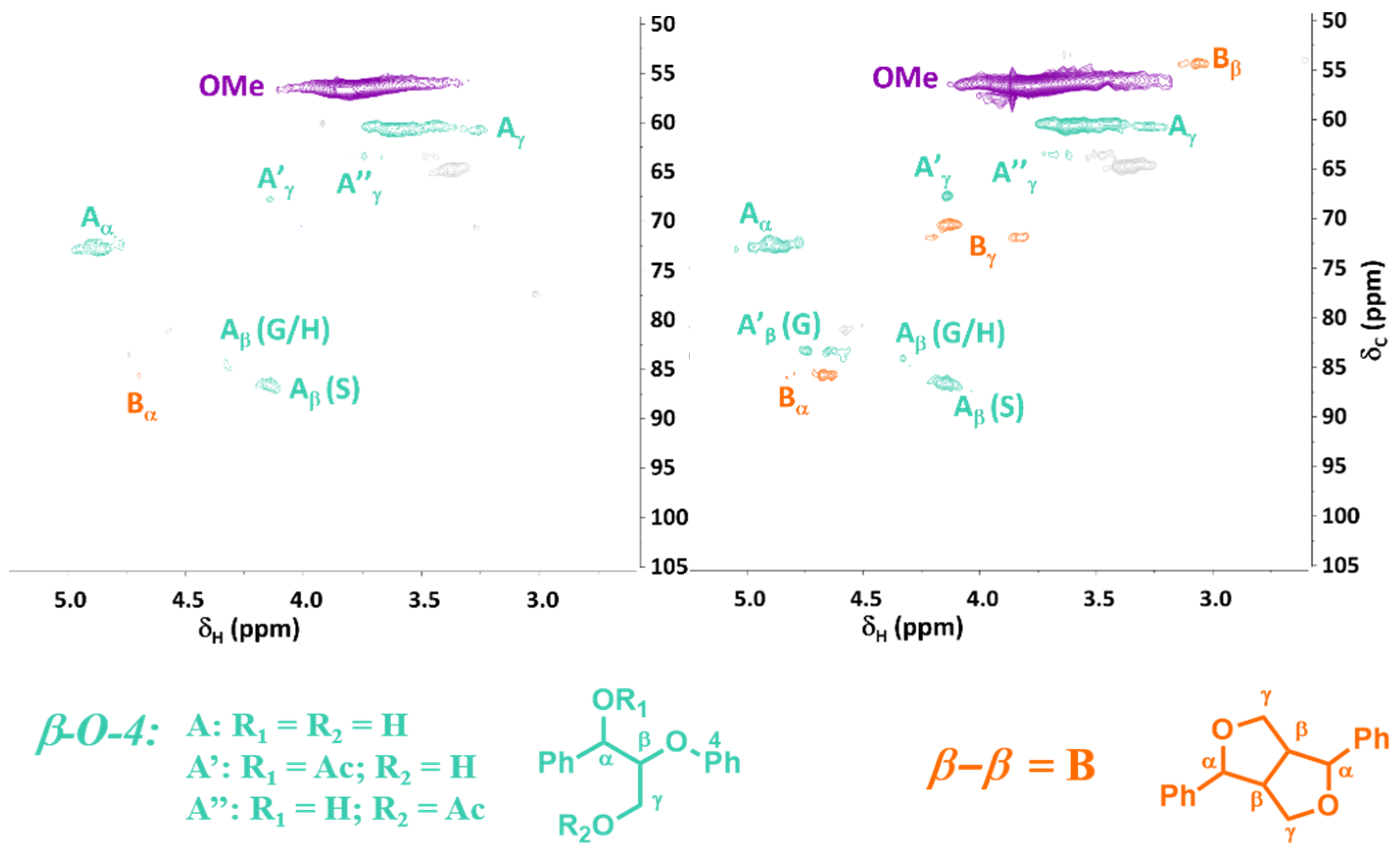
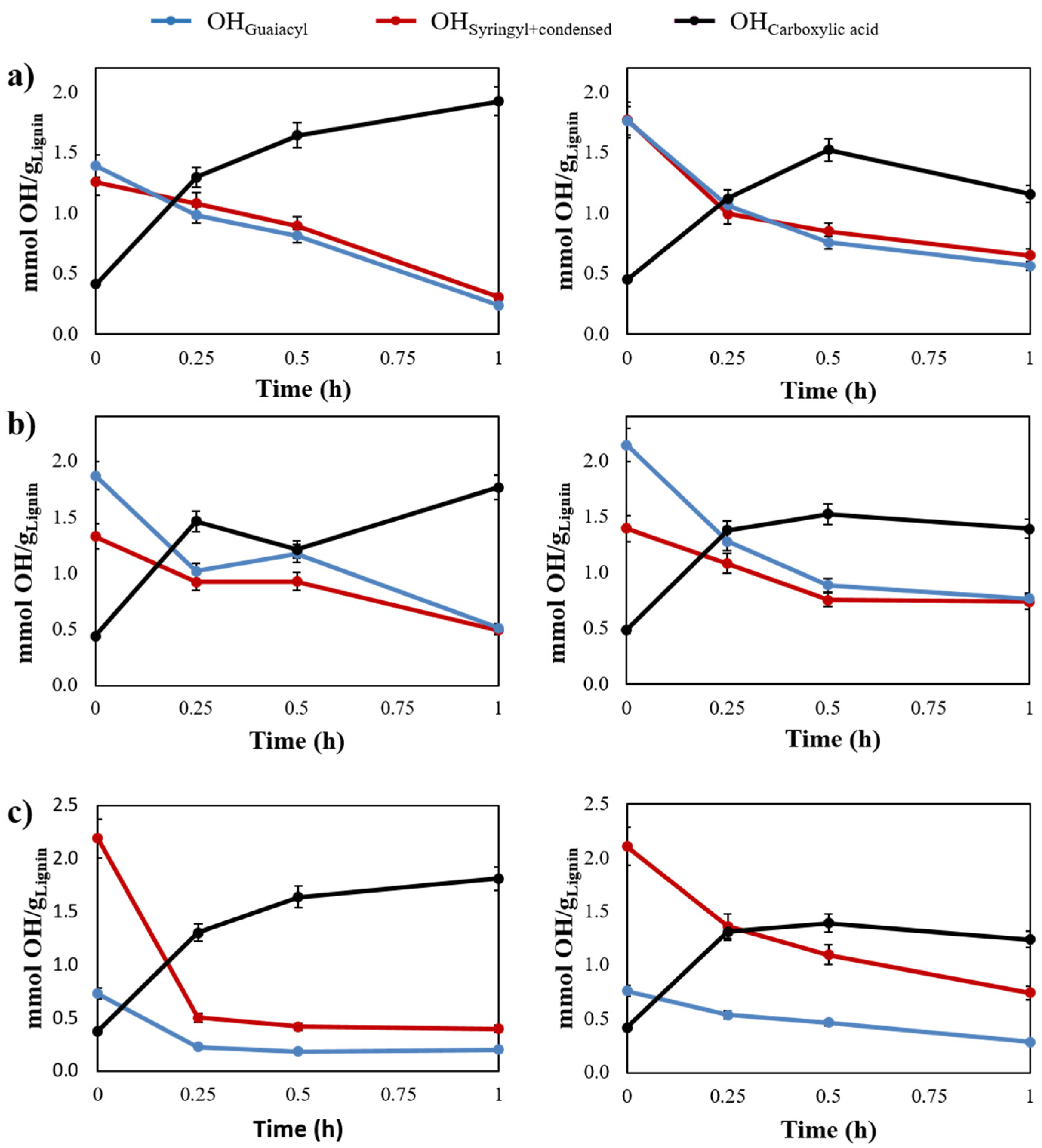
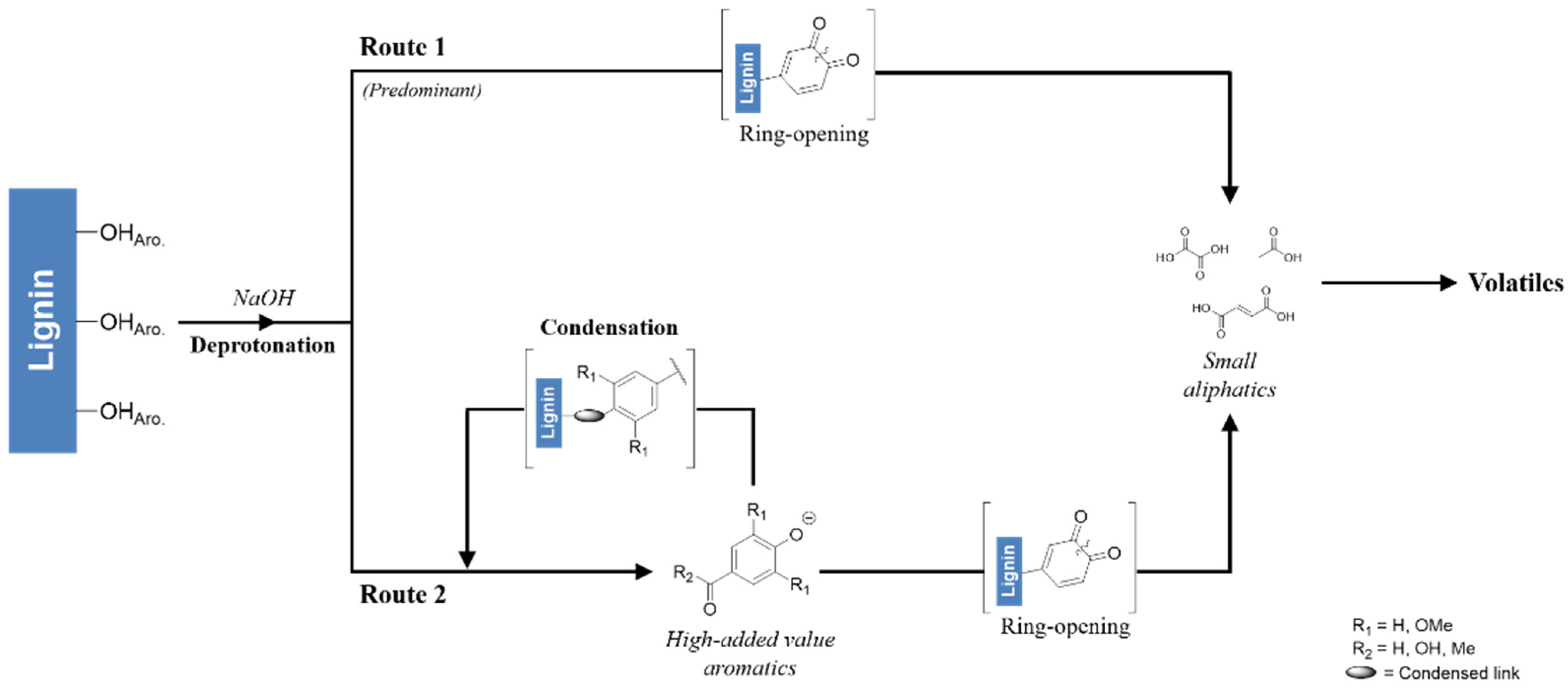
2.2. Evaluating the Influence of Catalytic Lignin Oxidation
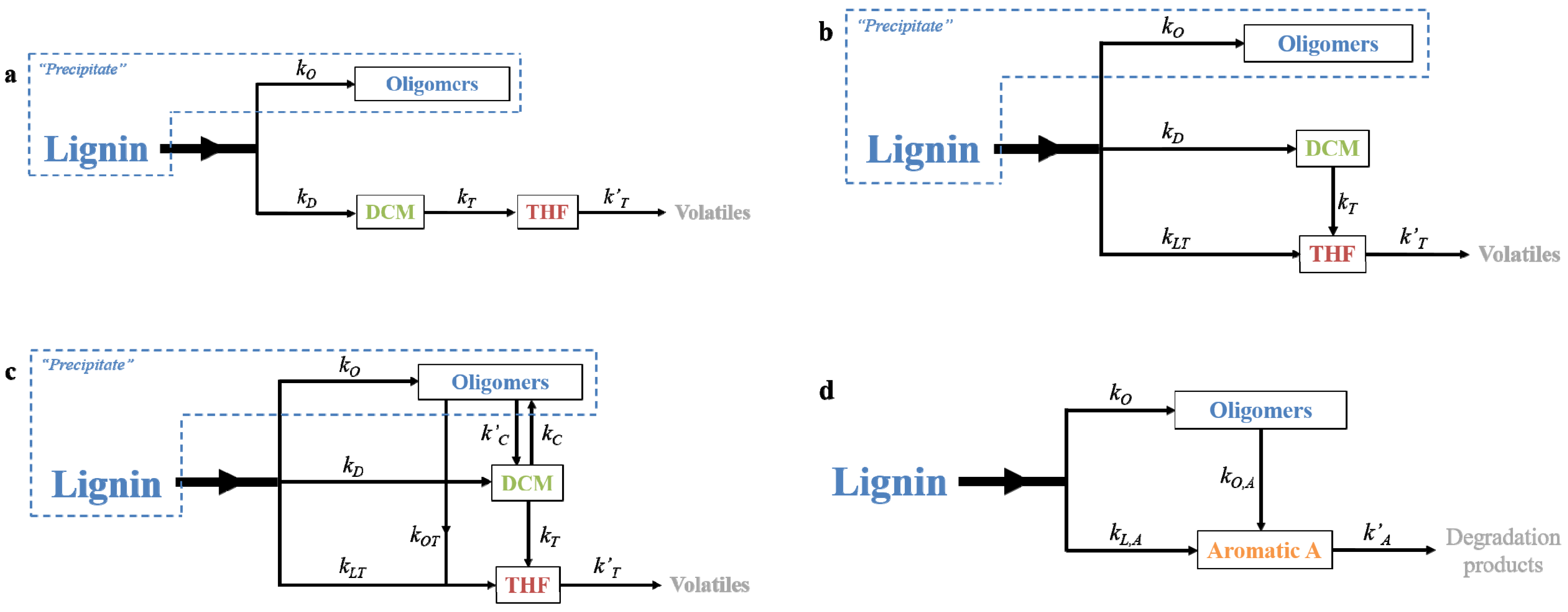
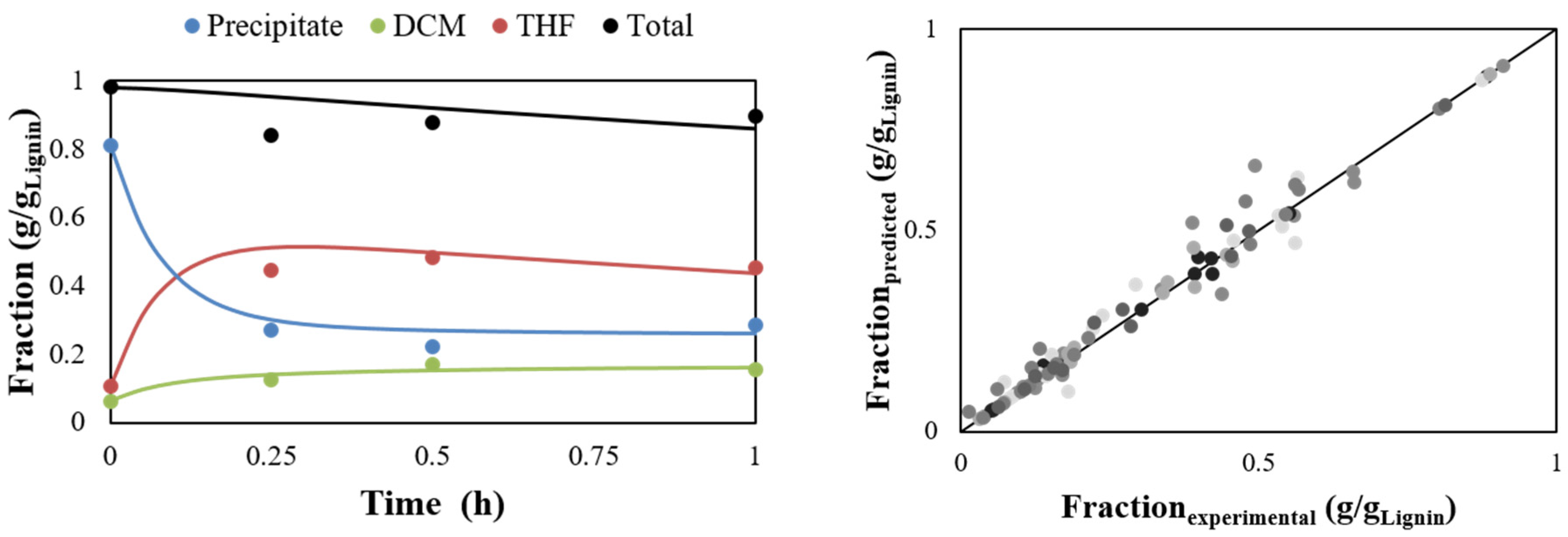
2.3. Development of A Kinetic Model
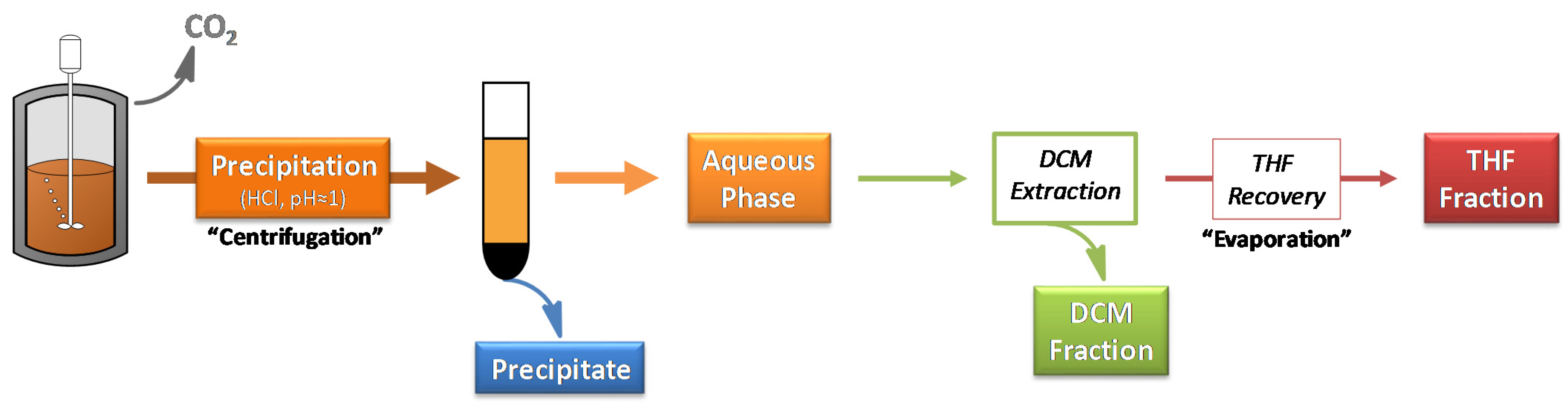
3. Materials and Methods
3.1. General Information
3.2. Lignin
3.3. Catalysts
3.4. Analytical Methods
3.5. Lignin Oxidative Depolymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, H.; Decina, S.; Crestini, C. Oxidative upgrade of lignin—Recent routes reviewed. Eur. Polym. J. 2013, 49, 1151–1173. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Guo, M.; Zhang, X. Recent advances in oxidative valorization of lignin. Catal. Today 2018, 302, 50–60. [Google Scholar] [CrossRef]
- Liu, X.; Bouxin, F.P.; Fan, J.; Budarin, V.L.; Hu, C.; Clark, J.H. Recent Advances in the Catalytic Depolymerization of Lignin towards Phenolic Chemicals: A Review. ChemSusChem 2020, 13, 4296–4317. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Wittmann, C. A field of dreams: Lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol. Adv. 2019, 37, 107360. [Google Scholar] [CrossRef] [PubMed]
- Joffres, B.; Laurenti, D.; Charon, N.; Daudin, A.; Quignard, A.; Geantet, C. Thermochemical Conversion of Lignin for Fuels and Chemicals: A Review. Oil Gas Sci. Technol.—Rev. IFP Energies Nouv. 2013, 68, 753–763. [Google Scholar] [CrossRef]
- Chatel, G.; Rogers, R.D. Review: Oxidation of Lignin Using Ionic Liquids—An Innovative Strategy to Produce Renewable Chemicals. ACS Sus. Chem. Eng. 2014, 2, 322–339. [Google Scholar] [CrossRef]
- Ha, J.-M.; Hwang, K.-R.; Kim, Y.-M.; Jae, J.; Kim, K.H.; Lee, H.W.; Kim, J.-Y.; Park, Y.-K. Recent progress in the thermal and catalytic conversion of lignin. Renew. Sustain. Energy Rev. 2019, 111, 422–441. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Deuss, P.J.; Barta, K. From models to lignin: Transition metal catalysis for selective bond cleavage reactions. Coord. Chem. Rev. 2016, 306, 510–532. [Google Scholar] [CrossRef]
- Hanson, S.K.; Baker, R.T. Knocking on Wood: Base Metal Complexes as Catalysts for Selective Oxidation of Lignin Models and Extracts. Acc. Chem. Res. 2015, 48, 2037–2048. [Google Scholar] [CrossRef]
- Almada, C.C.; Kazachenko, A.; Fongarland, P.; Da Silva Perez, D.; Kuznetsov, B.N.; Djakovitch, L. Oxidative depolymerization of lignins for producing aromatics: Variation of botanical origin and extraction methods. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Schutyser, W.; Kruger, J.S.; Robinson, A.M.; Katahira, R.; Brandner, D.G.; Cleveland, N.S.; Mittal, A.; Peterson, D.J.; Meilan, R.; Román-Leshkov, Y.; et al. Revisiting alkaline aerobic lignin oxidation. Green Chem. 2018, 20, 3828–3844. [Google Scholar] [CrossRef]
- Crestini, C.; Pro, P.; Neri, V.; Saladino, R. Methyltrioxorhenium: A new catalyst for the activation of hydrogen peroxide to the oxidation of lignin and lignin model compounds. Bioorganic Med. Chem. 2005, 13, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crops Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Abejón, R.; Pérez-Acebo, H.; Clavijo, L. Alternatives for Chemical and Biochemical Lignin Valorization: Hot Topics from a Bibliometric Analysis of the Research Published During the 2000–2016 Period. Processes 2018, 6, 98. [Google Scholar] [CrossRef]
- Zucca, P.; Rescigno, A.; Rinaldi, A.C.; Sanjust, E. Biomimetic metalloporphines and metalloporphyrins as potential tools for delignification: Molecular mechanisms and application perspectives. J. Mol. Catal. A Chem. 2014, 388–389, 2–34. [Google Scholar] [CrossRef]
- Gasser, C.A.; Hommes, G.; Schäffer, A.; Corvini, P.F.X. Multi-catalysis reactions: New prospects and challenges of biotechnology to valorize lignin. Appl. Microbiol. Biotechnol. 2012, 95, 1115–1134. [Google Scholar] [CrossRef]
- Crestini, C.; Saladino, R.; Tagliatesta, P.; Boschi, T. Biomimetic degradation of lignin and lignin model compounds by synthetic anionic and cationic water soluble manganese and iron porphyrins. Bioorganic Med. Chem. 1999, 7, 1897–1905. [Google Scholar] [CrossRef]
- Crestini, C.; Pastorini, A.; Tagliatesta, P. Metalloporphyrins immobilized on motmorillonite as biomimetic catalysts in the oxidation of lignin model compounds. J. Mol. Catal. A Chem. 2004, 208, 195–202. [Google Scholar] [CrossRef]
- Zhu, C.; Ding, W.; Shen, T.; Tang, C.; Sun, C.; Xu, S.; Chen, Y.; Wu, J.; Ying, H. Metallo-Deuteroporphyrin as a Biomimetic Catalyst for the Catalytic Oxidation of Lignin to Aromatics. ChemSusChem 2015, 8, 1768–1778. [Google Scholar] [CrossRef]
- Harms, R.G.; Markovits, I.I.E.; Drees, M.; Herrmann, H.M.; Cokoja, M.; Kühn, F.E. Cleavage of C-O Bonds in Lignin Model Compounds Catalyzed by Methyldioxorhenium in Homogeneous Phase. ChemSusChem 2014, 7, 429–434. [Google Scholar] [CrossRef]
- Voitl, T.; Rudolf von Rohr, P. Oxidation of Lignin Using Aqueous Polyoxometalates in the Presence of Alcohols. ChemSusChem 2008, 1, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Gupta, P.; Singh, B.; Bhaskar, T.; Natte, K.; Narani, A. Molybdenum-catalyzed oxidative depolymerization of alkali lignin: Selective production of Vanillin. Appl. Catal. A Gen. 2020, 598, 117567. [Google Scholar] [CrossRef]
- Zhou, X.-F.; Lu, X.-J. Co(salen) supported on graphene oxide for oxidation of lignin. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Walch, F.; Abdelaziz, O.Y.; Meier, S.; Bjelić, S.; Hulteberg, C.P.; Riisager, A. Oxidative depolymerization of Kraft lignin to high-value aromatics using a homogeneous vanadium–copper catalyst. Catal. Sci. Technol. 2021, 11, 1843–1853. [Google Scholar] [CrossRef]
- Bjørsvik, H.-R.; Minisci, F. Fine Chemicals from Lignosulfonates. 1. Synthesis of Vanillin by Oxidation of Lignosulfonates. Org. Proc. Res. Dev. 1999, 3, 330–340. [Google Scholar] [CrossRef]
- Crestini, C.; Caponi, M.C.; Argyropoulos, D.S.; Saladino, R. Immobilized methyltrioxo rhenium (MTO)/H2O2 systems for the oxidation of lignin and lignin model compounds. Bioorganic Med. Chem. 2006, 14, 5292–5302. [Google Scholar] [CrossRef]
- Crestini, C.; Pastorini, A.; Tagliatesta, P. The Immobilized Porphyrin-Mediator System Mn(TMePyP)/clay/HBT (clay-PMS): A Lignin Peroxidase Biomimetic Catalyst in the Oxidation of Lignin and Lignin Model Compounds. Eur. J. Inorg. Chem. 2004, 2004, 4477–4483. [Google Scholar] [CrossRef]
- Kumar, A.; Jain, N.; Chauhan, S.M.S. Biomimetic Oxidation of Veratryl Alcohol with H2O2 Catalyzed by Iron(III) Porphyrins and Horseradish Peroxidase in Ionic Liquid. Synlett 2007, 2007, 0411–0414. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, S.; Liu, M.; Zhu, G.; Wang, M.; Du, W.; Gao, J.; Xu, J. Covalent triazine framework catalytic oxidative cleavage of lignin models and organosolv lignin. Green Chem. 2018, 20, 1270–1279. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Ojo, O.S.; Tran, F.; Westwood, N.J. Isolation of Functionalized Phenolic Monomers through Selective Oxidation and C–O Bond Cleavage of the β-O-4 Linkages in Lignin. Angew. Chem. Int. Ed. 2015, 54, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Lin, L.; Sun, Y.; Pang, C.; Zhuang, J.; Ouyang, P.; Li, Z.; Liu, S. Perovskite-type Oxide LaMnO3: An Efficient and Recyclable Heterogeneous Catalyst for the Wet Aerobic Oxidation of Lignin to Aromatic Aldehydes. Catal. Lett. 2008, 126, 106. [Google Scholar] [CrossRef]
- Deng, H.; Lin, L.; Liu, S. Catalysis of Cu-Doped Co-Based Perovskite-Type Oxide in Wet Oxidation of Lignin To Produce Aromatic Aldehydes. Energy Fuels 2010, 24, 4797–4802. [Google Scholar] [CrossRef]
- Deng, H.; Lin, L.; Sun, Y.; Pang, C.; Zhuang, J.; Ouyang, P.; Li, J.; Liu, S. Activity and Stability of Perovskite-Type Oxide LaCoO3 Catalyst in Lignin Catalytic Wet Oxidation to Aromatic Aldehydes Process. Energy Fuels 2009, 23, 19–24. [Google Scholar] [CrossRef]
- Yang, M.; Xu, A.; Du, H.; Sun, C.; Li, C. Removal of salicylic acid on perovskite-type oxide LaFeO3 catalyst in catalytic wet air oxidation process. J. Hazard. Mater. 2007, 139, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Royer, S.; Levasseur, B.; Alamdari, H.; Barbier, J.; Duprez, D.; Kaliaguine, S. Mechanism of stearic acid oxidation over nanocrystalline La1−xA′xBO3 (A′=Sr, Ce; B=Co, Mn): The role of oxygen mobility. Appl. Catal. B Environ. 2008, 80, 51–61. [Google Scholar] [CrossRef]
- Villar, J.C.; Caperos, A.; García-Ochoa, F. Oxidation of hardwood kraft-lignin to phenolic derivatives with oxygen as oxidant. Wood Sci.Technol. 2001, 35, 245–255. [Google Scholar] [CrossRef]
- Crestini, C.; Crucianelli, M.; Orlandi, M.; Saladino, R. Oxidative strategies in lignin chemistry: A new environmental friendly approach for the functionalisation of lignin and lignocellulosic fibers. Catal. Today 2010, 156, 8–22. [Google Scholar] [CrossRef]
- Luo, J.; Melissa, P.; Zhao, W.; Wang, Z.; Zhu, Y. Selective Lignin Oxidation towards Vanillin in Phenol Media. ChemistrySelect 2016, 1, 4596–4601. [Google Scholar] [CrossRef]
- Pearl, I.A.; Beyer, D.L. Oxidation of Alkali Lignin. In Lignin Structure and Reactions; American Chemical Society: Washington, DC, USA, 1966; Volume 59, pp. 145–156. [Google Scholar]
- Kumar, A.; Biswas, B.; Saini, K.; Kumar, A.; Kumar, J.; Krishna, B.B.; Bhaskar, T. Copper and manganese bimetallic catalysts for oxidation of prot lignin: Effects of metal oxide on product yield. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Hdidou, L.; Khallouk, K.; Solhy, A.; Manoun, B.; Oukarroum, A.; Barakat, A. Synthesis of CoFeO mixed oxides via an alginate gelation process as efficient heterogeneous catalysts for lignin depolymerization in water. Catal. Sci. Technol. 2018, 8, 5445–5453. [Google Scholar] [CrossRef]
- Sun, K.; Chen, S.; Zhang, J.; Lu, G.-P.; Cai, C. Cobalt Nanoparticles Embedded in N-Doped Porous Carbon Derived from Bimetallic Zeolitic Imidazolate Frameworks for One-Pot Selective Oxidative Depolymerization of Lignin. ChemCatChem 2019, 11, 1264–1271. [Google Scholar] [CrossRef]
- Song, Y.; Mobley, J.K.; Motagamwala, A.H.; Isaacs, M.; Dumesic, J.A.; Ralph, J.; Lee, A.F.; Wilson, K.; Crocker, M. Gold-catalyzed conversion of lignin to low molecular weight aromatics. Chem. Sci. 2018, 9, 8127–8133. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhang, H.; Wu, X.; Li, R.; Zhang, Q.; Wang, Y. Oxidative conversion of lignin and lignin model compounds catalyzed by CeO2-supported Pd nanoparticles. Green Chem. 2015, 17, 5009–5018. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sharypov, V.I.; Baryshnikov, S.V.; Miroshnikova, A.V.; Taran, O.P.; Yakovlev, V.A.; Lavrenov, A.V.; Djakovitch, L. Catalytic hydrogenolysis of native and organosolv lignins of aspen wood to liquid products in supercritical ethanol medium. Catal. Today 2020. [Google Scholar] [CrossRef]
- Sales, F.G.; Maranhão, L.C.A.; Lima Filho, N.M.; Abreu, C.A.M. Kinetic Evaluation and Modeling of Lignin Catalytic Wet Oxidation to Selective Production of Aromatic Aldehydes. Ind. Eng. Chem. Res. 2006, 45, 6627–6631. [Google Scholar] [CrossRef]
- Bjelić, A.; Grilc, M.; Huš, M.; Likozar, B. Hydrogenation and hydrodeoxygenation of aromatic lignin monomers over Cu/C, Ni/C, Pd/C, Pt/C, Rh/C and Ru/C catalysts: Mechanisms, reaction micro-kinetic modelling and quantitative structure-activity relationships. Chem. Eng. J. 2019, 359, 305–320. [Google Scholar] [CrossRef]
- Bjelić, A.; Grilc, M.; Likozar, B. Bifunctional metallic-acidic mechanisms of hydrodeoxygenation of eugenol as lignin model compound over supported Cu, Ni, Pd, Pt, Rh and Ru catalyst materials. Chem. Eng. J. 2020, 394, 124914. [Google Scholar] [CrossRef]
- Figueirêdo, M.B.; Venderbosch, R.H.; Deuss, P.J.; Heeres, H.J. A Two-Step Approach for the Conversion of Technical Lignins to Biofuels. Adv. Sustain. Syst. 2020, 4, 1900147. [Google Scholar] [CrossRef]
- Ma, R.; Guo, M.; Zhang, X. Selective Conversion of Biorefinery Lignin into Dicarboxylic Acids. ChemSusChem 2014, 7, 412–415. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Li, F.; Cao, X.; Sun, R. Production of vanillin from lignin: The relationship between β-O-4 linkages and vanillin yield. Ind. Crops Prod. 2018, 116, 116–121. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, J.; Liao, Y.; Lv, W.; Ma, L.; Wang, C. Degradation of Vanillin During Lignin Valorization Under Alkaline Oxidation. Top. Curr. Chem. 2018, 376, 29. [Google Scholar] [CrossRef]
- Casimiro, F.M.; Costa, C.A.E.; Botelho, C.M.; Barreiro, M.F.; Rodrigues, A.E. Kinetics of Oxidative Degradation of Lignin-Based Phenolic Compounds in Batch Reactor. Ind. Eng. Chem. Res. 2019, 58, 16442–16449. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Petukhov, D.V.; Selyutin, G.E. New Mechanism for the Catalytic Oxidation of Lignin to Vanillin. Kinet. Catal. 2004, 45, 569–577. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Fomova, N.A.; Kuznetsov, B.N.; Ivanchenko, N.M.; Kudryashev, A.V. On the mechanism of vanillin formation in the catalytic oxidation of lignin with oxygen. React. Kinet. Catal. Lett. 1995, 55, 161–170. [Google Scholar] [CrossRef]
- Hita, I.; Deuss, P.J.; Bonura, G.; Frusteri, F.; Heeres, H.J. Biobased chemicals from the catalytic depolymerization of Kraft lignin using supported noble metal-based catalysts. Fuel Process. Technol. 2018, 179, 143–153. [Google Scholar] [CrossRef]
- Ait Rass, H.; Essayem, N.; Besson, M. Selective Aerobic Oxidation of 5-HMF into 2,5-Furandicarboxylic Acid with Pt Catalysts Supported on TiO2- and ZrO2-Based Supports. ChemSusChem 2015, 8, 1206–1217. [Google Scholar] [CrossRef]
- Granata, A.; Argyropoulos, D.S. 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane, a Reagent for the Accurate Determination of the Uncondensed and Condensed Phenolic Moieties in Lignins. J. Agric. Food. Chem. 1995, 43, 1538–1544. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Popescu, M.-C.; Singurel, G.; Vasile, C.; Argyropoulos, D.S.; Willfor, S. Spectral Characterization of Eucalyptus Wood. Appl. Spectrosc. 2007, 61, 1168–1177. [Google Scholar] [CrossRef] [PubMed]


| Kinetic Constant (h−1) | ||||||
|---|---|---|---|---|---|---|
| Kraft | EOL-Ab | EOL-As | ||||
| 0.26 | 1.72 | 0.50 | 1.77 | 2.17 | 4.41 | |
| 0.41 | 0.69 | 0.38 | 1.60 | 0.54 | 1.18 | |
| 1.87 | 1.87 | 1.01 | 2.59 | 6.39 | 7.04 | |
| 0 | 0.002 | 0.016 | 0.004 | 0 | 0 | |
| 2.54 | 4.29 | 1.89 | 5.96 | 9.10 | 12.63 | |
| 0.63 | 0.41 | 0.45 | 0.83 | 0.34 | 1.55 | |
| 0.51 | 0.11 | 0.86 | 0.21 | 1.26 | 0.98 | |
| / | 1.2 | 3.9 | 0.5 | 3.9 | 0.3 | 1.6 |
| 0.12 | 0 | 0.24 | 0 | 0.26 | 0 | |
| 0.05 | 0.002 | 0.10 | 0.004 | 0.14 | 0.26 | |
| 0.4 | 1.3 | 0.9 | 1.3 | 0.4 | 0.8 | |
| / | 1.2 | 3.9 | 0.5 | 3.9 | 0.3 | 1.6 |
| Kinetic Constant (·102·h−1) | |||||||
|---|---|---|---|---|---|---|---|
| Kraft | EOL-Ab | EOL-As | |||||
| Vanillin (V) | 0.02 | 0.05 | 0.00 | 0.20 | 0.19 | 0.005 | |
| 9.6 | 17.0 | 13.4 | 39.1 | 17.4 | 31.0 | ||
| 17.4 | 23.3 | 22.2 | 52.2 | 32.0 | 70.9 | ||
| Acetovanillone (Ac) | 0.09 | 0.06 | 0.07 | 0.07 | 0.03 | 0.03 | |
| 1.88 | 3.38 | 1.54 | 4.69 | 1.54 | 3.33 | ||
| 77.7 | 69.4 | 75.0 | 76.8 | 27.3 | 57.8 | ||
| Vanillic acid (VA) | 0.68 | 1.04 | 0.00 | 1.10 | 0.00 | 0.07 | |
| 31.8 | 18.0 | 32.6 | 19.3 | 7.3 | 6.5 | ||
| 1201 | 1218 | 887 | 898 | 67.3 | 61.9 | ||
| Syringaldehyde (S) | - | - | - | - | 1.15 | 1.16 | |
| - | - | - | - | 36.6 | 69.7 | ||
| - | - | - | - | 184 | 198 | ||
| Acetosyringone (AS) | - | - | - | - | 0.00 | 0.13 | |
| - | - | - | - | 5.0 | 7.3 | ||
| - | - | - | - | 103 | 112 | ||
| Kraft | EOL-Ab | EOL-As | ||||
|---|---|---|---|---|---|---|
| Vanillin | 0.55 | 0.73 | 0.60 | 0.75 | 0.55 | 0.44 |
| Acetovanillone | 0.03 | 0.05 | 0.02 | 0.06 | 0.06 | 0.06 |
| Vanillic Acid | 0.03 | 0.02 | 0.04 | 0.02 | 0.11 | 0.11 |
| Syringaldehyde | - | - | - | - | 0.21 | 0.36 |
| Acetosyringone | - | - | - | - | 0.05 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral Almada, C.; Kazachenko, A.; Fongarland, P.; Da Silva Perez, D.; Kuznetsov, B.N.; Djakovitch, L. Supported-Metal Catalysts in Upgrading Lignin to Aromatics by Oxidative Depolymerization. Catalysts 2021, 11, 467. https://doi.org/10.3390/catal11040467
Cabral Almada C, Kazachenko A, Fongarland P, Da Silva Perez D, Kuznetsov BN, Djakovitch L. Supported-Metal Catalysts in Upgrading Lignin to Aromatics by Oxidative Depolymerization. Catalysts. 2021; 11(4):467. https://doi.org/10.3390/catal11040467
Chicago/Turabian StyleCabral Almada, Cédric, Aleksandr Kazachenko, Pascal Fongarland, Denilson Da Silva Perez, Boris N. Kuznetsov, and Laurent Djakovitch. 2021. "Supported-Metal Catalysts in Upgrading Lignin to Aromatics by Oxidative Depolymerization" Catalysts 11, no. 4: 467. https://doi.org/10.3390/catal11040467
APA StyleCabral Almada, C., Kazachenko, A., Fongarland, P., Da Silva Perez, D., Kuznetsov, B. N., & Djakovitch, L. (2021). Supported-Metal Catalysts in Upgrading Lignin to Aromatics by Oxidative Depolymerization. Catalysts, 11(4), 467. https://doi.org/10.3390/catal11040467









