Catalytic Combustion of Toluene over Highly Dispersed Cu-CeOx Derived from Cu-Ce-MOF by EDTA Grafting Method
Abstract
:1. Introduction
2. Results and Discussion
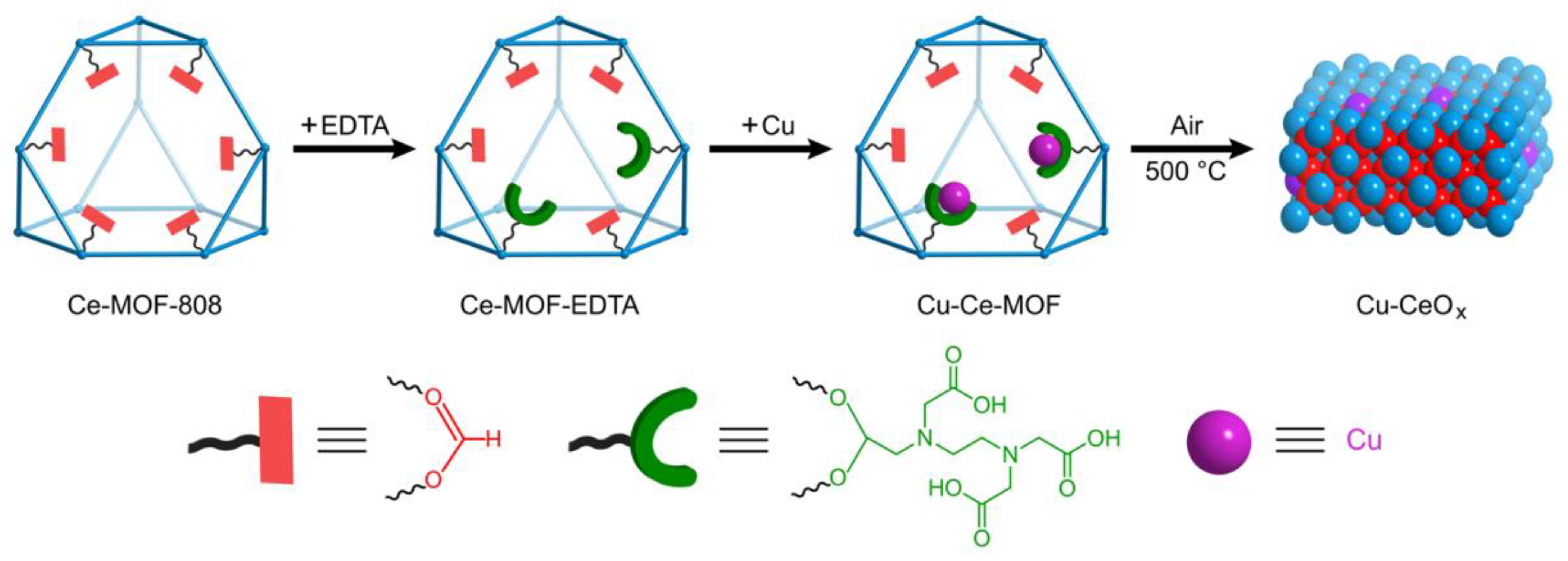
2.1. Preparation and Characterizations of Cu-CeOx-MOF-n
2.1.1. Preparation of Cu-CeOx-MOF-n
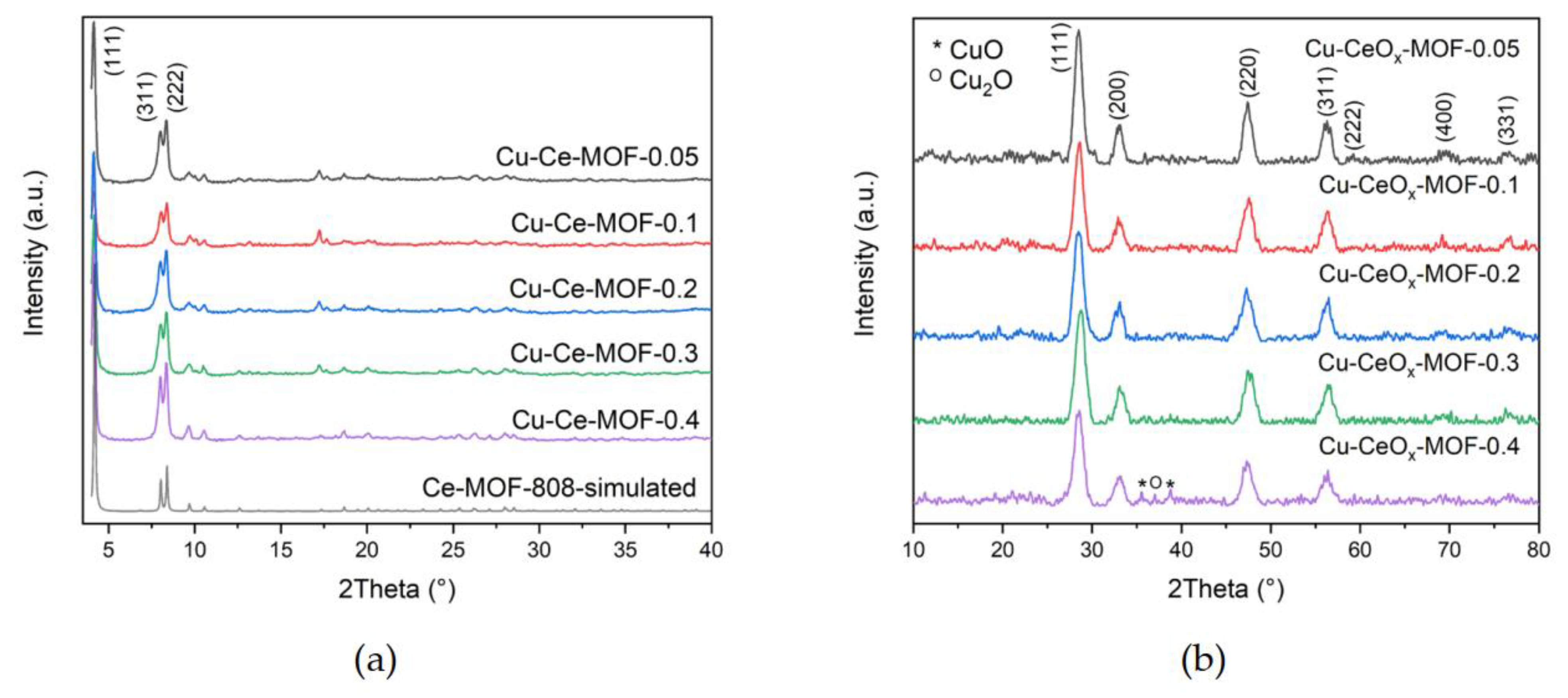
2.1.2. X-ray Diffraction (XRD)
2.1.3. N2 Adsorption and Desorption Measurements and Thermogravimetry (TG)
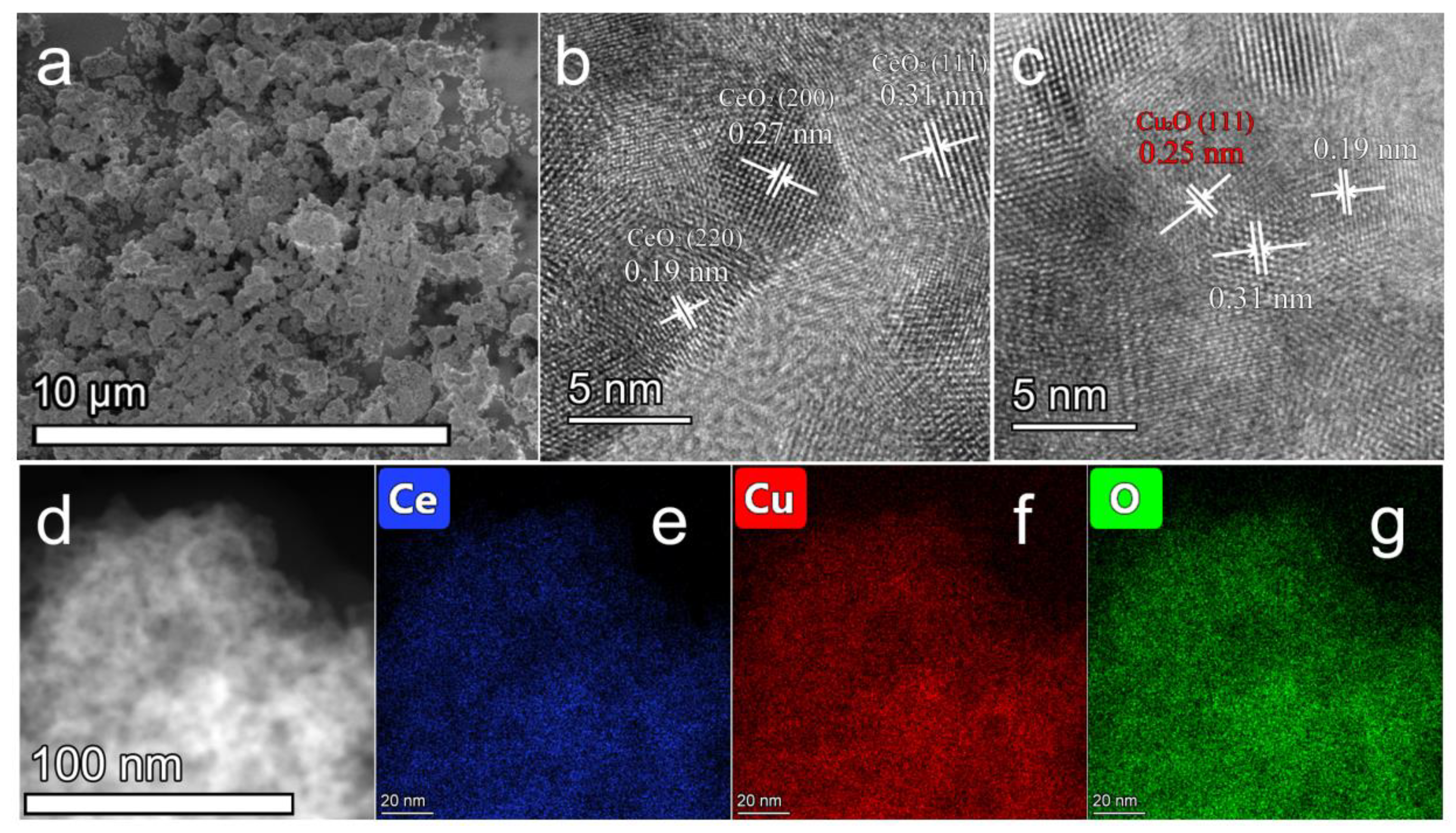
2.1.4. Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES), Field Emission Scanning Electron Microscope (FESEM) and High-Resolution Transmission Electron Microscope (HRTEM) Analyses
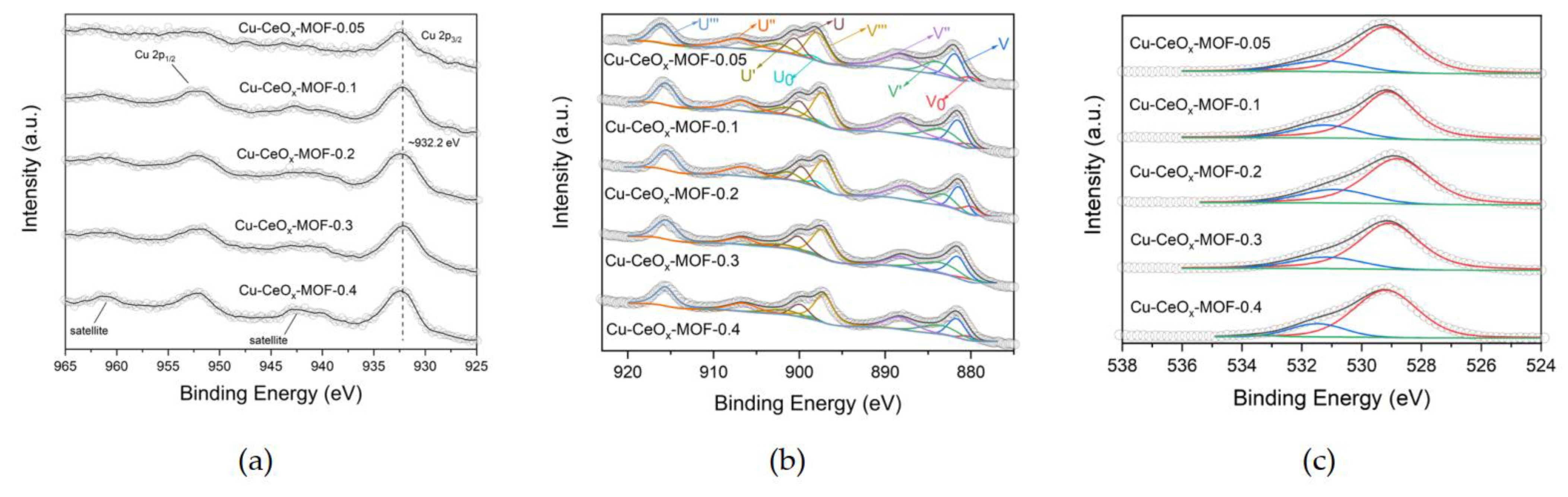
2.1.5. Raman Spectroscopy and X-ray Photoelectron Spectroscopy (XPS)
2.1.6. Hydrogen Temperature Programmed Reduction (H2-TPR)
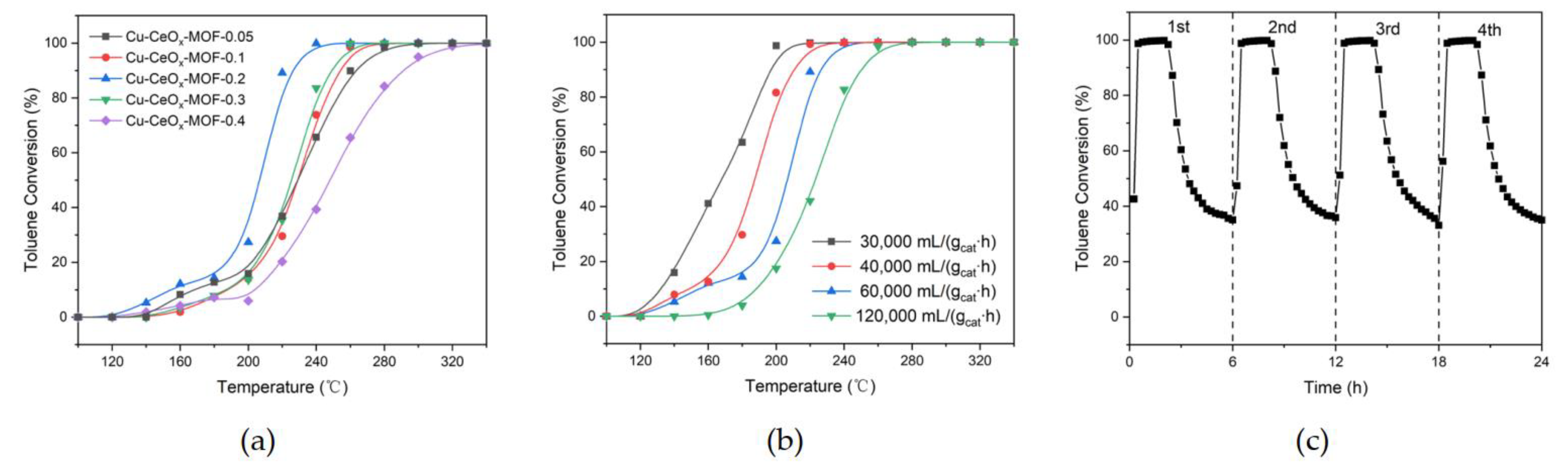
2.2. Catalytic Performance
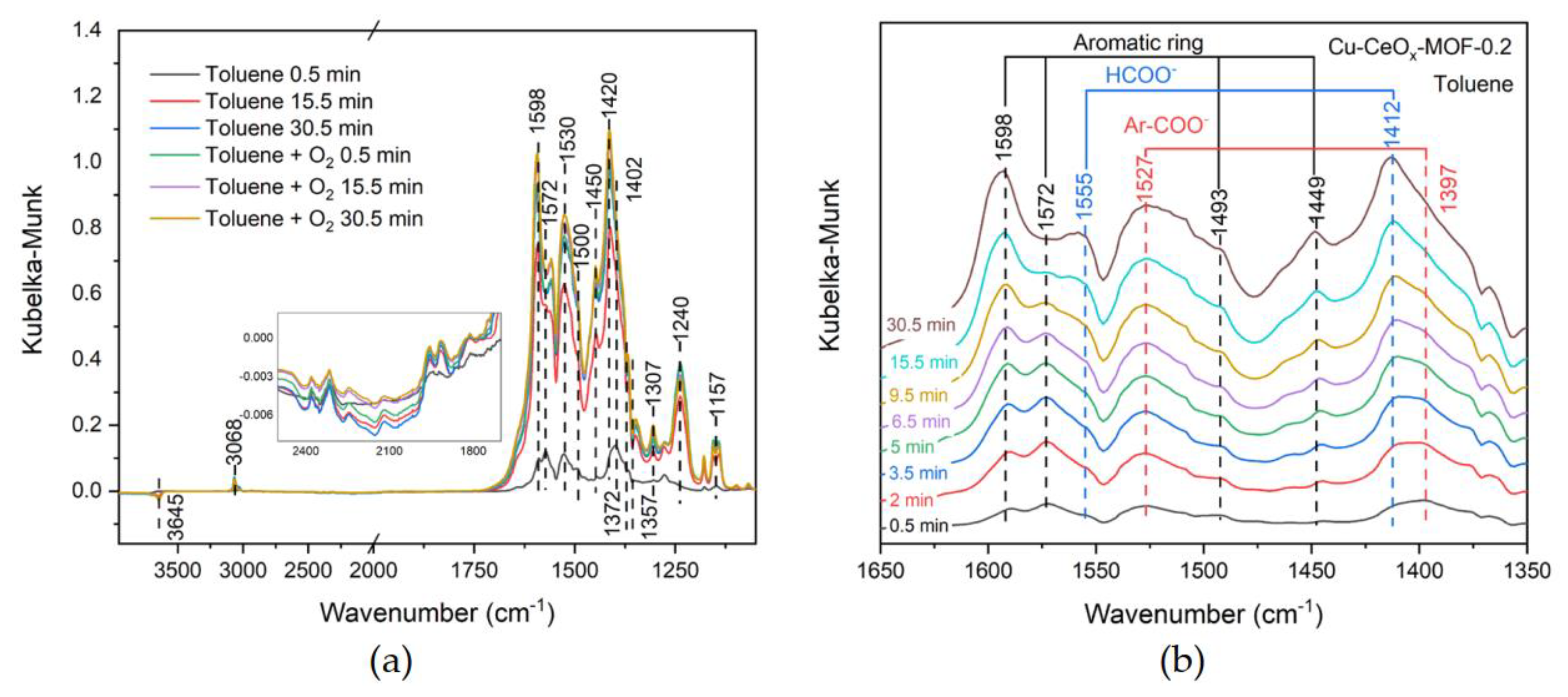
2.3. In Situ DRIFTS Study of Toluene Catalytic Combustion
3. Experimental Section
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Synthesis of Ce-MOF-808
3.2.2. Synthesis of Cu-Ce-MOF-n
3.2.3. Synthesis of Cu-CeOx-MOF-n
3.3. Catalyst Characterization
3.4. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Jiang, M. Characteristics, source apportionment and contribution of VOCs to ozone formation in Wuhan, Central China. Atmos. Environ. 2018, 192, 55–71. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Qu, Y.; Wang, J.; An, J.; Zhang, Y.; Zhang, F. Formation mechanism of continuous extreme haze episodes in the megacity Beijing, China, in January 2013. Atmos. Res. 2015, 155, 192–203. [Google Scholar] [CrossRef]
- Kinney, P.L. Interactions of Climate Change, Air Pollution, and Human Health. Curr. Environ. Health Rep. 2018, 5, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Sarigiannis, D.A.; Karakitsios, S.P.; Gotti, A.; Liakos, I.L.; Katsoyiannis, A. Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environ. Int. 2011, 37, 743–765. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Tang, C.; Ren, H.; Zhang, Q.; Xue, M.; Xiong, J.; Wang, D.; Yu, Q.; He, Z.; et al. Few-layered mesoporous graphene for high-performance toluene adsorption and regeneration. Environ. Sci. Nano 2019, 6, 3113–3122. [Google Scholar] [CrossRef]
- Malakar, S.; Saha, P.D.; Baskaran, D.; Rajamanickam, R. Comparative study of biofiltration process for treatment of VOCs emission from petroleum refinery wastewater—A review. Environ. Technol. Innov. 2017, 8, 441–461. [Google Scholar] [CrossRef]
- Palma, V.; Cortese, M.; Renda, S.; Ruocco, C.; Martino, M.; Meloni, E. A Review about the Recent Advances in Selected NonThermal Plasma Assisted Solid-Gas Phase Chemical Processes. Nanomaterials 2020, 10, 1596. [Google Scholar] [CrossRef]
- Zhong, J.; Zeng, Y.; Chen, D.; Mo, S.; Zhang, M.; Fu, M.; Wu, J.; Su, Z.; Chen, P.; Ye, D. Toluene oxidation over Co3+-rich spinel Co3O4: Evaluation of chemical and by-product species identified by in situ DRIFTS combined with PTR-TOF-MS. J. Hazard. Mater. 2020, 386, 121957. [Google Scholar] [CrossRef]
- Zeng, Y.; Haw, K.-G.; Wang, Z.; Wang, Y.; Zhang, S.; Hongmanorom, P.; Zhong, Q.; Kawi, S. Double redox process to synthesize CuO–CeO2 catalysts with strong Cu–Ce interaction for efficient toluene oxidation. J. Hazard. Mater. 2021, 404, 124088. [Google Scholar] [CrossRef]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B Environ. 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Xu, W.; Xiao, K.; Lai, S.; Liang, J.; Jiang, X.; Liu, Z.; Li, F.; Zhang, Y.; Wu, X.; Zhou, X. Designing a dumbbell-brush-type Co3O4 for efficient catalytic toluene oxidation. Catal. Commun. 2020, 140, 106005. [Google Scholar] [CrossRef]
- Li, Y.; Han, W.; Wang, R.; Weng, L.-T.; Serrano-Lotina, A.; Banares, M.A.; Wang, Q.; Yeung, K.L. Performance of an aliovalent-substituted CoCeOx catalyst from bimetallic MOF for VOC oxidation in air. Appl. Catal. B Environ. 2020, 275, 119121. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; An, X.; Shi, J.; Shangguan, W.; Hao, X.; Xu, G.; Tang, B.; Abudula, A.; Guan, G. Generation of abundant defects in Mn-Co mixed oxides by a facile agar-gel method for highly efficient catalysis of total toluene oxidation. Appl. Catal. B Environ. 2021, 282, 119560. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Liu, J.; Zhao, J.; Quan, Y.; Li, T.; Pei, Y.; Li, X.; Ren, J. Catalytic combustion of toluene over CeO2-CoO(x)composite aerogels. New J. Chem. 2020, 44, 11557–11565. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, D.; Wu, G.; Fang, N.; Song, X.; Li, J.; Chen, J.; Liu, Y.; Guo, J.; Chu, Y. Enhanced Catalytic Combustion Performance of Toluene over a Novel Co–CeOx Monolith Catalyst. Energy Fuels 2021, 35, 6190–6201. [Google Scholar] [CrossRef]
- Lu, J.; Wang, J.; Zou, Q.; He, D.; Zhang, L.; Xu, Z.; He, S.; Luo, Y. Unravelling the Nature of the Active Species as well as the Doping Effect over Cu/Ce-Based Catalyst for Carbon Monoxide Preferential Oxidation. ACS Catal. 2019, 9, 2177–2195. [Google Scholar] [CrossRef]
- Delimaris, D.; Ioannides, T. VOC oxidation over CuO–CeO2 catalysts prepared by a combustion method. Appl. Catal. B Environ. 2009, 89, 295–302. [Google Scholar] [CrossRef]
- Zhou, G.; Lan, H.; Song, R.; Xie, H.; Du, Q. Effects of preparation method on CeCu oxide catalyst performance. RSC Adv. 2014, 4, 50840–50850. [Google Scholar] [CrossRef]
- Sun, W.; Li, X.; Sun, C.; Huang, Z.; Xu, H.; Shen, W. Insights into the Pyrolysis Processes of Ce-MOFs for Preparing Highly Active Catalysts of Toluene Combustion. Catalysts 2019, 9, 682. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, S.; Liu, Y.; Yang, Z.; Feng, X.; Lu, X.; Huo, F. Well-Dispersed and Size-Controlled Supported Metal Oxide Nanoparticles Derived from MOF Composites and Further Application in Catalysis. Small 2015, 11, 3130–3134. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Pachfule, P.; Banerjee, R.; Poddar, P. Metal and metal oxide nanoparticle synthesis from metal organic frameworks (MOFs): Finding the border of metal and metal oxides. Nanoscale 2012, 4, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-N.; Song, X.-Z.; Song, S.-Y.; Zhang, H.-J. Highly efficient heterogeneous catalytic materials derived from metal-organic framework supports/precursors. Coord. Chem. Rev. 2017, 337, 80–96. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M.; Guo, S.; Wang, Y.; Han, X.; Bai, Y. Efficient oxidation of o-xylene over CeO2 catalyst prepared from a Ce-MOF template: The promotion of K+ embedding substitution. Mol. Catal. 2017, 436, 120–127. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Yu, E.; Cai, S.; Jia, H.; Chen, J.; Liang, P. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, H.; Zhang, Y.; Kang, C.; Chen, S.; Song, L.; Liu, D.; Zhong, C. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 2018, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Huang, H.; Liu, P.; Song, X.; Mei, D.; Tang, Y.; Wang, X.; Zhong, C. Metal-organic framework encapsulated single-atom Pt catalysts for efficient photocatalytic hydrogen evolution. J. Catal. 2019, 375, 351–360. [Google Scholar] [CrossRef]
- Zhong, J.; Zeng, Y.; Zhang, M.; Feng, W.; Xiao, D.; Wu, J.; Chen, P.; Fu, M.; Ye, D. Toluene oxidation process and proper mechanism over Co3O4 nanotubes: Investigation through in-situ DRIFTS combined with PTR-TOF-MS and quasi in-situ XPS. Chem. Eng. J. 2020, 397, 125375. [Google Scholar] [CrossRef]
- Wang, Q.; Yeung, K.L.; Banares, M.A. Operando Raman-online FTIR investigation of ceria, vanadia/ceria and gold/ceria catalysts for toluene elimination. J. Catal. 2018, 364, 80–88. [Google Scholar] [CrossRef]
- Babazadeh, M.; Hosseinzadeh-Khanmiri, R.; Abolhasani, J.; Ghorbani-Kalhor, E.; Hassanpour, A. Solid phase extraction of heavy metal ions from agricultural samples with the aid of a novel functionalized magnetic metal-organic framework. RSC Adv. 2015, 5, 19884–19892. [Google Scholar] [CrossRef]
- Ghorbani-Kalhor, E. A metal-organic framework nanocomposite made from functionalized magnetite nanoparticles and HKUST-1 (MOF-199) for preconcentration of Cd(II), Pb(II), and Ni(II). Mikrochim. Acta 2016, 183, 2639–2647. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.-B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal–Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Lammert, M.; Glissmann, C.; Reinsch, H.; Stock, N. Synthesis and Characterization of New Ce(IV)-MOFs Exhibiting Various Framework Topologies. Cryst. Growth Des. 2017, 17, 1125–1131. [Google Scholar] [CrossRef]
- Schilling, C.; Hofmann, A.; Hess, C.; Ganduglia-Pirovano, M.V. Raman Spectra of Polycrystalline CeO2: A Density Functional Theory Study. J. Phys. Chem. C 2017, 121, 20834–20849. [Google Scholar] [CrossRef]
- Su, Z.; Yang, W.; Wang, C.; Xiong, S.; Cao, X.; Peng, Y.; Si, W.; Weng, Y.; Xue, M.; Li, J. Roles of Oxygen Vacancies in the Bulk and Surface of CeO2 for Toluene Catalytic Combustion. Environ. Sci. Technol. 2020, 54, 12684–12692. [Google Scholar] [CrossRef]
- Shan, W.J.; Shen, W.J.; Li, C. Structural characteristics and redox behaviors of Ce1-xCuxOy solid solutions. Chem. Mater. 2003, 15, 4761–4767. [Google Scholar] [CrossRef]
- Yin, M.; Wu, C.K.; Lou, Y.B.; Burda, C.; Koberstein, J.T.; Zhu, Y.M.; O’Brien, S. Copper oxide nanocrystals. J. Am. Chem. Soc. 2005, 127, 9506–9511. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, K.; Xu, Y.; Wang, X.; Qian, Q.; Chen, Q. The role of Cu species in electrospun CuO-CeO2 nanofibers for total benzene oxidation. New J. Chem. 2015, 39, 1001–1005. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Song, F.; Zhang, S.; Zhong, Q. The effect of CuO loading on different method prepared CeO2 catalyst for toluene oxidation. Sci. Total Environ. 2020, 712, 135635. [Google Scholar] [CrossRef]
- Hocevar, S.; Krasovec, U.O.; Orel, B.; Arico, A.S.; Kim, H. CWO of phenol on two differently prepared CuO-CeO2 catalysts. Appl. Catal. B Environ. 2000, 28, 113–125. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Banares, M.A.; Weng, L.-T.; Gu, Q.; Price, J.; Han, W.; Yeung, K.L. A Novel Approach to High-Performance Aliovalent-Substituted Catalysts-2D Bimetallic MOF-Derived CeCuOx Microsheets. Small 2019, 15, 1903525. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.F.; Zhong, Y.J.; Yuan, X.X.; Zheng, X.M. TPR and TPD studies of CuO/CeO2 catalysts for low temperature CO oxidation. Appl. Catal. A Gen. 1997, 162, 121–131. [Google Scholar] [CrossRef]
- Sumrunronnasak, S.; Chanlek, N.; Pimpha, N. Improved CeCuOx catalysts for toluene oxidation prepared by aqueous cationic surfactant precipitation method. Mater. Chem. Phys. 2018, 216, 143–152. [Google Scholar] [CrossRef]
- Perez, A.; Molina, R.; Moreno, S. Enhanced VOC oxidation over Ce/CoMgAl mixed oxides using a reconstruction method with EDTA precursors. Appl. Catal. A Gen. 2014, 477, 109–116. [Google Scholar] [CrossRef]
- Lu, H.; Kong, X.; Huang, H.; Zhou, Y.; Chen, Y. Cu-Mn-Ce ternary mixed-oxide catalysts for catalytic combustion of toluene. J. Environ. Sci. 2015, 32, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Lan, H.; Gao, T.; Xie, H. Influence of Ce/Cu ratio on the performance of ordered mesoporous CeCu composite oxide catalysts. Chem. Eng. J. 2014, 246, 53–63. [Google Scholar] [CrossRef]
- Kang, R.; Wei, X.; Li, H.; Bin, F.; Zhao, R.; Hao, Q.; Dou, B. Sol-gel enhanced mesoporous Cu-Ce-Zr catalyst for toluene oxidation. Combust. Sci. Technol. 2018, 190, 878–892. [Google Scholar] [CrossRef] [Green Version]
- Mo, S.; Zhang, Q.; Li, J.; Sun, Y.; Ren, Q.; Zou, S.; Zhang, Q.; Lu, J.; Fu, M.; Mo, D.; et al. Highly efficient mesoporous MnO2 catalysts for the total toluene oxidation: Oxygen-Vacancy defect engineering and involved intermediates using in situ DRIFTS. Appl. Catal. B Environ. 2020, 264. [Google Scholar] [CrossRef]
- Li, J.; Lu, D.-F.; Zhang, Z.; Liu, Q.; Qi, Z.-M. Hierarchical mesoporous silica film modified near infrared SPR sensor with high sensitivities to small and large molecules. Sens. Actuators B Chem. 2014, 203, 690–696. [Google Scholar] [CrossRef]
- Zhang, Q.; Mo, S.; Li, J.; Sun, Y.; Zhang, M.; Chen, P.; Fu, M.; Wu, J.; Chen, L.; Ye, D. In situ DRIFT spectroscopy insights into the reaction mechanism of CO and toluene co-oxidation over Pt-based catalysts. Catal. Sci. Technol. 2019, 9, 4538–4551. [Google Scholar] [CrossRef]
- Ma, L.; Seo, C.Y.; Chen, X.; Sun, K.; Schwank, J.W. Indium-doped Co3O4 nanorods for catalytic oxidation of CO and C3H6 towards diesel exhaust. Appl. Catal. B Environ. 2018, 222, 44–58. [Google Scholar] [CrossRef]
- Abd El-Moemen, A.; Abdel-Mageed, A.M.; Bansmann, J.; Parlinska-Wojtan, M.; Behm, R.J.; Kučerová, G. Deactivation of Au/CeO2 catalysts during CO oxidation: Influence of pretreatment and reaction conditions. J. Catal. 2016, 341, 160–179. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Huang, H.; Zeng, K.; Wang, Z.; Jia, H.-p.; Li, X. Insights into the size and structural effects of zeolitic supports on gaseous toluene oxidation over MnOx/HZSM-5 catalysts. Appl. Surf. Sci. 2019, 486, 108–120. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, Q.; Sun, Y.; Zhang, M.; Li, J.; Ren, Q.; Fu, M.; Wu, J.; Chen, L.; Ye, D. Gaseous CO and toluene co-oxidation over monolithic core–shell Co3O4-based hetero-structured catalysts. J. Mater. Chem. A 2019, 7, 16197–16210. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Z.; Li, Y.; Leng, X.; Zhang, T.; Yuan, F.; Niu, X.; Zhu, Y. Synthesis of CeaMnOx hollow microsphere with hierarchical structure and its excellent catalytic performance for toluene combustion. Appl. Catal. B Environ. 2019, 245, 502–512. [Google Scholar] [CrossRef]
- Rainone, F.; Bulushev, D.A.; Kiwi-Minsker, L.; Renken, A. DRIFTS and transient-response study of vanadia/titania catalysts during toluene partial oxidation. Phys. Chem. Chem. Phys. 2003, 5, 4445–4449. [Google Scholar] [CrossRef] [Green Version]
- Mukai, D.; Murai, Y.; Higo, T.; Tochiya, S.; Hashimoto, T.; Sugiura, Y.; Sekine, Y. In situ IR study for elucidating reaction mechanism of toluene steam reforming over Ni/La0.7Sr0.3AlO3−δ catalyst. Appl. Catal. A Gen. 2013, 466, 190–197. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Z.; Chen, S.; Quan, X. The role of lattice oxygen on the activity and selectivity of the OMS-2 catalyst for the total oxidation of toluene. Chem. Eng. J. 2015, 270, 58–65. [Google Scholar] [CrossRef]
- Besselmann, S.; Löffler, E.; Muhler, M. On the role of monomeric vanadyl species in toluene adsorption and oxidation on V2O5/TiO2 catalysts: A Raman and in situ DRIFTS study. J. Mol. Catal. A Chem. 2000, 162, 401–411. [Google Scholar] [CrossRef]



| n | ICP-AES | XRD | N2 Adsorption and Desorption | Raman | XPS | H2-TPR | ||
|---|---|---|---|---|---|---|---|---|
| mCu/mCe | D (nm) | SSA (m2/g) | AD/AF2g (%) | Oα/(Oα+Oβ) (%) | Ce3+/(Ce3++Ce4+) (%) | TPeak 1 (°C) | TPeak 2 (°C) | |
| 0.05 | 0.053 | 8.0 | 54 | 8 | 18.7 | 21.3 | / | 178 |
| 0.1 | 0.105 | 6.9 | 67 | 9 | 19.3 | 21.4 | 144 | 177 |
| 0.2 | 0.122 | 6.6 | 57 | 13 | 20.7 | 22.4 | 145 | 174 |
| 0.3 | 0.147 | 6.9 | 61 | 9 | 18.5 | 21.5 | 163 | 191 |
| 0.4 | 0.225 | 6.6 | 57 | 8 | 17.5 | 21.0 | 178 | 211 |
| Composition | Preparation Method | Toluene Concentration (ppm) | WHSV (mL/gcat∙h) | T50 (°C) | T90 |
|---|---|---|---|---|---|
| (°C) | |||||
| Cu-CeOx-0.05 | MOF precursor via EDTA grafting method | 1000 | 60,000 | 224 | 263 |
| Cu-CeOx-0.1 | 1000 | 60,000 | 224 | 254 | |
| Cu-CeOx-0.2 | 1000 | 60,000 | 207 | 226 | |
| 1000 | 30,000 | 163 | 197 | ||
| 1000 | 40,000 | 182 | 211 | ||
| 1000 | 120,000 | 221 | 250 | ||
| Cu-CeOx-0.3 | 1000 | 60,000 | 222 | 249 | |
| Cu-CeOx-0.4 | 1000 | 60,000 | 244 | 291 | |
| Cu0.15Ce0.85 [20] | combustion method | 600 | 50,000 | 209 | 210 |
| CuO-CeO2 [47] | calcination | 500 | 50,000 | 211 | 231 |
| Ce0.8Cu0.2O [45] | surfactant precipitation method | 5000 | 9000 | >250 | >250 |
| CeCu-HT [48] | hard-template method | 10,000 | 66,000 | 221 | 223 |
| Ce0.4Cu0.6 [9] | double redox method | 500 | 50,000 h−1 | 228 | 245 |
| CuCeZr4 [49] | sol-gel method | 1500 | 24,000 h−1 | 183 | 219 |
| Wavenumber/cm−1 | Assignment | Corresponding Species |
|---|---|---|
| 1158, 1098, 1069 | C–O stretching vibration | benzyl alcohol [50] |
| 1180 | antisymmetric Ar–C stretching vibration | aromatic ring (fingerprint region) [51] |
| 1242 | C–O stretching vibration (phenolics) | phenol [52] |
| 1397, 1527; 1412, 1555 | symmetric C–O stretching vibration and antisymmetric C–O stretching vibration (carboxylate) | carboxylate [53,54] |
| 1593, 1574, 1498, 1440 | C=C skeleton vibration | aromatic ring [55,56] |
| 1647 | C=O stretching vibration (aromatic aldehydes) | benzaldehyde [57] |
| 1960, 1917, 1813, 1740, 1307 | symmetric and antisymmetric C=O stretching vibration (cyclic anhydrides) | maleic anhydride [30,58] |
| 2320 | antisymmetric CO2 stretching vibration | CO2 [59] |
| 3066 | Csp2–H stretching vibration | aromatic ring or benzyl [60,61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Huang, Y.; Li, X.; Huang, Z.; Xu, H.; Shen, W. Catalytic Combustion of Toluene over Highly Dispersed Cu-CeOx Derived from Cu-Ce-MOF by EDTA Grafting Method. Catalysts 2021, 11, 519. https://doi.org/10.3390/catal11040519
Sun W, Huang Y, Li X, Huang Z, Xu H, Shen W. Catalytic Combustion of Toluene over Highly Dispersed Cu-CeOx Derived from Cu-Ce-MOF by EDTA Grafting Method. Catalysts. 2021; 11(4):519. https://doi.org/10.3390/catal11040519
Chicago/Turabian StyleSun, Wenjie, Yijia Huang, Xiaomin Li, Zhen Huang, Hualong Xu, and Wei Shen. 2021. "Catalytic Combustion of Toluene over Highly Dispersed Cu-CeOx Derived from Cu-Ce-MOF by EDTA Grafting Method" Catalysts 11, no. 4: 519. https://doi.org/10.3390/catal11040519
APA StyleSun, W., Huang, Y., Li, X., Huang, Z., Xu, H., & Shen, W. (2021). Catalytic Combustion of Toluene over Highly Dispersed Cu-CeOx Derived from Cu-Ce-MOF by EDTA Grafting Method. Catalysts, 11(4), 519. https://doi.org/10.3390/catal11040519






