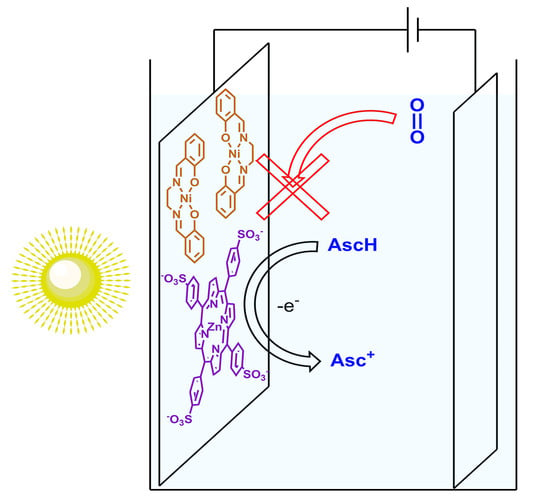
Inversion of the Photogalvanic Effect of Conductive Polymers by Porphyrin Dopants
Abstract
1. Introduction
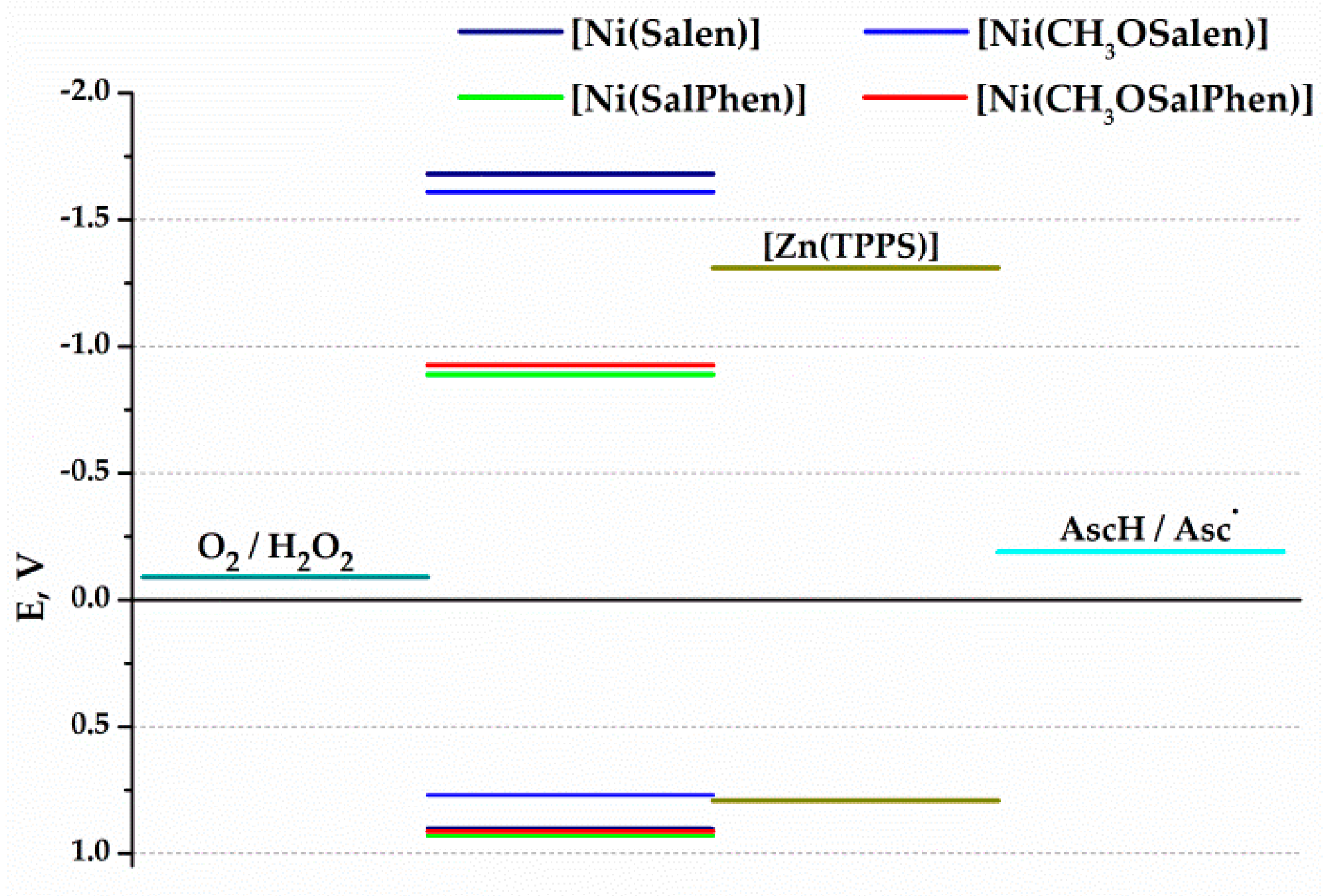
2. Results and Discussion
Photoelectrochemical Performance
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, H.; Lin, L.; Ji, M.; Zhang, S.; Yang, M.; Fu, Q. Progress on the morphological control of conductive network in conductive polymer composites and the use as electroactive multifunctional materials. Prog. Polym. Sci. 2014, 39, 627–655. [Google Scholar] [CrossRef]
- Luo, R.; Li, H.; Du, B.; Zhou, S.; Zhu, Y. A simple strategy for high stretchable, flexible and conductive polymer films based on PEDOT:PSS-PDMS blends. Org. Electron. 2020, 76, 105451. [Google Scholar] [CrossRef]
- Dou, J.; Tang, L.; Mou, L.; Zhang, R.; Jiang, X. Stretchable conductive adhesives for connection of electronics in wearable devices based on metal-polymer conductors and carbon nanotubes. Compos. Sci. Technol. 2020, 197, 108237. [Google Scholar] [CrossRef]
- Pankow, R.M.; Thompson, B.C. The development of conjugated polymers as the cornerstone of organic electronics. Polymer 2020, 207, 122874. [Google Scholar] [CrossRef]
- Ostroverkhova, O. Organic Optoelectronic Materials: Mechanisms and Applications. Chem. Rev. 2016, 116, 13279–13412. [Google Scholar] [CrossRef]
- Jamdegni, M.; Kaur, A. Highly efficient dark to transparent electrochromic electrode with charge storing ability based on polyaniline and functionalized nickel oxide composite linked through a binding agent. Electrochim. Acta 2020, 331, 135359. [Google Scholar] [CrossRef]
- Díaz-Sánchez, J.; Roquero, P.; Hernández-Alcántara, J.M.; Rosas-Aburto, A.; Vázquez-Torres, H.; Gimeno, M. Complementary electrochromic devices of PANI and PEDOT using the enzymatic poly(gallic acid). Sol. Energy Mater. Sol. Cells 2019, 200, 109973. [Google Scholar] [CrossRef]
- Tian, Y.; Dou, S.; Zhang, X.; Zhang, L.; Wang, L.; Zhao, J.; Zhao, X.; Li, Y. Synthesis of ordered bowl-like polyaniline film with enhanced electrochromic performances. Synth. Met. 2017, 232, 111–116. [Google Scholar] [CrossRef]
- Mjejri, I.; Rougier, A. PEDOT:PSS/Fe2O3 as hybrid composite film for tuning color in electrochromism. Mater. Today Proc. 2020, 33, 2470–2473. [Google Scholar] [CrossRef]
- Lv, D.; Chen, W.; Shen, W.; Peng, M.; Zhang, X.; Wang, R.; Xu, L.; Xu, W.; Song, W.; Tan, R. Enhanced flexible room temperature ammonia sensor based on PEDOT: PSS thin film with FeCl3 additives prepared by inkjet printing. Sens. Actuators B Chem. 2019, 298, 126890. [Google Scholar] [CrossRef]
- Martins, T.S.; Bott-Neto, J.L.; Raymundo-Pereira, P.A.; Ticianelli, E.A.; Machado, S.A.S. An electrochemical furosemide sensor based on pencil graphite surface modified with polymer film Ni-salen and Ni(OH)2/C nanoparticles. Sens. Actuators B Chem. 2018, 276, 378–387. [Google Scholar] [CrossRef]
- Pereira, C.F.; Olean-Oliveira, A.; David-Parra, D.N.; Teixeira, M.F.S. A chemiresistor sensor based on a cobalt(salen) metallopolymer for dissolved molecular oxygen. Talanta 2018, 190, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, A.I.; Chavan, H.S.; Kim, H.; Im, H. Mesoporous Ni-PANI composite electrode for electrochromic energy storage applications. Sol. Energy Mater. Sol. Cells 2019, 201, 110121. [Google Scholar] [CrossRef]
- Alekseeva, E.V.; Chepurnaya, I.A.; Malev, V.V.; Timonov, A.M.; Levin, O.V. Polymeric nickel complexes with salen-type ligands for modification of supercapacitor electrodes: Impedance studies of charge transfer and storage properties. Electrochim. Acta 2017, 225, 378–391. [Google Scholar] [CrossRef]
- Vereshchagin, A.A.; Lukyanov, D.A.; Kulikov, I.R.; Panjwani, N.A.; Alekseeva, E.A.; Behrends, J.; Levin, O.V. The Fast and the Capacious: A [Ni(Salen)]-TEMPO Redox-Conducting Polymer for Organic Batteries. Batter. Supercaps 2021, 4, 336–346. [Google Scholar] [CrossRef]
- Eliseeva, S.N.; Alekseeva, E.V.; Vereshchagin, A.A.; Volkov, A.I.; Vlasov, P.S.; Konev, A.S.; Levin, O.V. Nickel-Salen Type Polymers as Cathode Materials for Rechargeable Lithium Batteries. Macromol. Chem. Phys. 2017, 218, 1700361. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Zhu, H.; Wang, X. Hierarchical bead chain ZnFe2O4-PEDOT composites with enhanced Li-ion storage properties as anode materials for lithium-ion batteries. Appl. Surf. Sci. 2020, 529, 147078. [Google Scholar] [CrossRef]
- Oka, K.; Strietzel, C.; Emanuelsson, R.; Nishide, H.; Oyaizu, K.; Strømme, M.; Sjödin, M. Characterization of PEDOT-Quinone conducting redox polymers in water-in-salt electrolytes for safe and high-energy Li-ion batteries. Electrochem. Commun. 2019, 105, 106489. [Google Scholar] [CrossRef]
- He, Y.; Aïch, B.R.; Lu, J.; Alem, S.; Lang, S.; Movileanu, R.; Baribeau, J.-M.; Tao, Y. A diketopyrrolopyrrole conjugated polymer based on 4,4ʹ-difluoro-2,2ʹ-bithiophene for organic thin-film transistors and organic photovoltaics. Thin Solid Films 2020, 711, 138300. [Google Scholar] [CrossRef]
- Saeed, M.A.; Kim, S.H.; Lee, S.Y.; Shim, J.W. High indoor performance of flexible organic photovoltaics using polymer electrodes. Thin Solid Films 2020, 704, 138006. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Li, J.; Qin, M.; Wu, X.; Lv, Z.; Hsu, Y.-J.; Lu, X.; Wu, Y.; Fang, G. Constructing highly efficient all-inorganic perovskite solar cells with efficiency exceeding 17% by using dopant-free polymeric electron-donor materials. Nano Energy 2020, 75, 104933. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Liu, T.; Zhang, W.; Li, Y.; Cai, B.; He, L.; Guo, Y.; Yang, X.; Xu, B.; et al. A crosslinked polymer as dopant-free hole-transport material for efficient n-i-p type perovskite solar cells. J. Energy Chem. 2021, 55, 211–218. [Google Scholar] [CrossRef]
- Han, Q.; Liu, S.; Liu, Y.; Jin, J.; Li, D.; Cheng, S.; Xiong, Y. Flexible counter electrodes with a composite carbon/metal nanowire/polymer structure for use in dye-sensitized solar cells. Sol. Energy 2020, 208, 469–479. [Google Scholar] [CrossRef]
- Seema, H.; Zafar, Z.; Samreen, A. Evaluation of solution processable polymer reduced graphene oxide transparent films as counter electrodes for dye-sensitized solar cells. Arab. J. Chem. 2020, 13, 4978–4986. [Google Scholar] [CrossRef]
- Giri, D.; Raut, S.K.; Patra, S.K. Diketopyrrolopyrrole/perylene-diimide and thiophene based D-π-A low bandgap polymer sensitizers for application in dye sensitized solar cells. Dye. Pigment. 2020, 174, 108032. [Google Scholar] [CrossRef]
- Mansha, M.; Younas, M.; Gondal, M.A.; Ullah, N. 1,5-Naphthyridine-based conjugated polymers as co-sensitizers for dye-sensitized solar cells. Sol. Energy 2019, 194, 682–687. [Google Scholar] [CrossRef]
- Semenikhin, O.; Ovsyannikova, E.; Ehrenburg, M.; Alpatova, N.; Kazarinov, V. Electrochemical and photoelectrochemical behaviour of polythiophenes in non-aqueous solutions. J. Electroanal. Chem. 2000, 494, 1–11. [Google Scholar] [CrossRef]
- Glenis, S.; Tourillon, G.; Garnier, F. Photoelectrochemical properties of thin films of polythiophene and derivatives: Doping level and structure effects. Thin Solid Films 1984, 122, 9–17. [Google Scholar] [CrossRef]
- Semenikhin, O.A.; Ovsyannikova, E.V.; Alpatova, N.M.; Rotenberg, Z.A.; Kazarinov, V.E. Electrochemical and photoelectrochemical behaviour of poly-3-methylthiophene and polybithiophene in non-aqueous solutions. J. Electroanal. Chem. 1999, 463, 190–199. [Google Scholar] [CrossRef]
- Smirnova, E.A.; Besedina, M.A.; Karushev, M.P.; Vasil’ev, V.V.; Timonov, A.M. Photogalvanic and photovoltaic effects in systems based on metal complexes of Schiff bases. Russ. J. Phys. Chem. A 2016, 90, 1088–1094. [Google Scholar] [CrossRef]
- Konev, A.S.; Kayumov, M.Y.; Karushev, M.P.; Novoselova, Y.V.; Lukyanov, D.A.; Alekseeva, E.V.; Levin, O.V. Polymeric Metal Salen-Type Complexes as Catalysts for Photoelectrocatalytic Hydrogen Peroxide Production. ChemElectroChem 2018, 5, 3138–3142. [Google Scholar] [CrossRef]
- Molino, P.J.; Garcia, L.; Stewart, E.M.; Lamaze, M.; Zhang, B.; Harris, A.R.; Winberg, P.; Wallace, G.G. PEDOT doped with algal, mammalian and synthetic dopants: Polymer properties, protein and cell interactions, and influence of electrical stimulation on neuronal cell differentiation. Biomater. Sci. 2018, 6, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Apraksin, R.V.; Volosatova, Y.A.; Volkov, A.I.; Vlasov, P.S.; Lukyanov, D.A.; Kulikov, I.R.; Eliseeva, S.N.; Levin, O.V. Electrochemical synthesis and characterization of poly [Ni(CH3Osalen)] with immobilized poly(styrenesulfonate) anion dopants. Electrochim. Acta 2021, 368, 137637. [Google Scholar] [CrossRef]
- Yan, W.; Jiang, D.; Liu, Q.; Kang, Q.; Zhou, F. Effects of doping methods and dopant sizes on the performance of solar cells constructed with anchor-guided photoelectrochemical polymerization of thiophene. Electrochim. Acta 2020, 330, 135250. [Google Scholar] [CrossRef]
- Mahmoodian, M.; Pourabbas, B.; Mohajerzadeh, S. Effect of anionic dopants on thickness, morphology and electrical properties of polypyrrole ultra-thin films prepared by in situ chemical polymerization. Thin Solid Films 2015, 583, 255–263. [Google Scholar] [CrossRef]
- Chen, J.; Wagner, P.; Tong, L.; Wallace, G.G.; Officer, D.L.; Swiegers, G.F. A Porphyrin-Doped Polymer Catalyzes Selective, Light-Assisted Water Oxidation in Seawater. Angew. Chem. Int. Ed. 2012, 51, 1907–1910. [Google Scholar] [CrossRef]
- Liu, H.; Blankenship, R.E. On the interface of light-harvesting antenna complexes and reaction centers in oxygenic photosynthesis. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 148079. [Google Scholar] [CrossRef]
- El-Khouly, M.E. Energy transfer between two light harvesting phthalocyanine derivatives as model for artificial photosynthetic antenna: Laser photolysis studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 508–513. [Google Scholar] [CrossRef]
- Arrigo, A.; Puntoriero, F.; La Ganga, G.; Campagna, S.; Burian, M.; Bernstorff, S.; Amenitsch, H. Aggregation-Induced Energy Transfer in a Decanuclear Os(II)/Ru(II) Polypyridine Light-Harvesting Antenna Dendrimer. Chem 2017, 3, 494–508. [Google Scholar] [CrossRef]
- Hambright, P.; Chock, P.B. Metal-porphyrin interactions. III. Dissociative-interchange mechanism for metal ion incorporation into porphyrin molecules. J. Am. Chem. Soc. 1974, 96, 3123–3131. [Google Scholar] [CrossRef]
- Peters, M.K.; Herges, R. Insertion of Ni(I) into Porphyrins at Room Temperature: Preparation of Ni(II)porphyrins, and Ni(II)chlorins and Observation of Hydroporphyrin Intermediates. Inorg. Chem. 2018, 57, 3177–3182. [Google Scholar] [CrossRef] [PubMed]
- Mergen, Ö.B.; Arda, E. Determination of Optical Band Gap Energies of CS/MWCNT Bio-nanocomposites by Tauc and ASF Methods. Synth. Met. 2020, 269, 116539. [Google Scholar] [CrossRef]
- Neumann-Spallart, M.; Kalyanasundaram, K. On the One and Two-Electron Oxidations of Water-Soluble Zinc Porphyrins in Aqueous Media. Z. Nat. B 1981, 36, 596–600. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Breith, E.; Lübbe, E.; Tsumaki, T. Tricyclische orthokondensierte Nebenvalenzringe. Justus Liebig’s Ann. Chem. 1933, 503, 84–130. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.W.; Lee, C.Y. Fullerene–porphyrin–ferrocene triad self-assembled monolayers (SAMs) for photovoltaic applications. J. Organomet. Chem. 2014, 761, 20–27. [Google Scholar] [CrossRef]





| LE [a], µA cm−2 Mlx−1 | |||||
|---|---|---|---|---|---|
| Ni-L | λ, nm | O2 Reduction | Asc− Oxidation | O2 Reduction | Asc− Oxidation |
| Ni-L [b] | Ni-L [b] | Ni-L:P [c] | Ni-L:P [c] | ||
| poly[Ni(Salen)] | 470 | −130.4 | −2.1 | −18.0 | 7.9 |
| 420 | −174.7 | −5.6 | −17.4 | 21.1 | |
| poly [NiCH3O(Salen)] | 470 | −98.2 | −4.0 | −2.6 | 3.2 |
| 420 | −53.7 | −4.5 | −9.3 | 5.7 | |
| poly [Ni(SalPhen)] | 470 | −38.8 | −1.5 | −2.1 | 33.5 |
| 420 | −120.1 | −5.3 | −2.5 | 68.3 | |
| poly [Ni(CH3OSalPhen)] | 470 | −20.5 | −0.8 | −3.4 | 9.2 |
| 420 | −40.7 | −6.7 | −6.2 | 20.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrov, A.A.; Lukyanov, D.A.; Kopytko, O.A.; Novoselova, J.V.; Alekseeva, E.V.; Levin, O.V. Inversion of the Photogalvanic Effect of Conductive Polymers by Porphyrin Dopants. Catalysts 2021, 11, 729. https://doi.org/10.3390/catal11060729
Petrov AA, Lukyanov DA, Kopytko OA, Novoselova JV, Alekseeva EV, Levin OV. Inversion of the Photogalvanic Effect of Conductive Polymers by Porphyrin Dopants. Catalysts. 2021; 11(6):729. https://doi.org/10.3390/catal11060729
Chicago/Turabian StylePetrov, Alexey A., Daniil A. Lukyanov, Oleg A. Kopytko, Julia V. Novoselova, Elena V. Alekseeva, and Oleg V. Levin. 2021. "Inversion of the Photogalvanic Effect of Conductive Polymers by Porphyrin Dopants" Catalysts 11, no. 6: 729. https://doi.org/10.3390/catal11060729
APA StylePetrov, A. A., Lukyanov, D. A., Kopytko, O. A., Novoselova, J. V., Alekseeva, E. V., & Levin, O. V. (2021). Inversion of the Photogalvanic Effect of Conductive Polymers by Porphyrin Dopants. Catalysts, 11(6), 729. https://doi.org/10.3390/catal11060729