Studies of Nickel/Samarium-Doped Ceria for Catalytic Partial Oxidation of Methane and Effect of Oxygen Vacancy
Abstract
:1. Introduction
2. Results
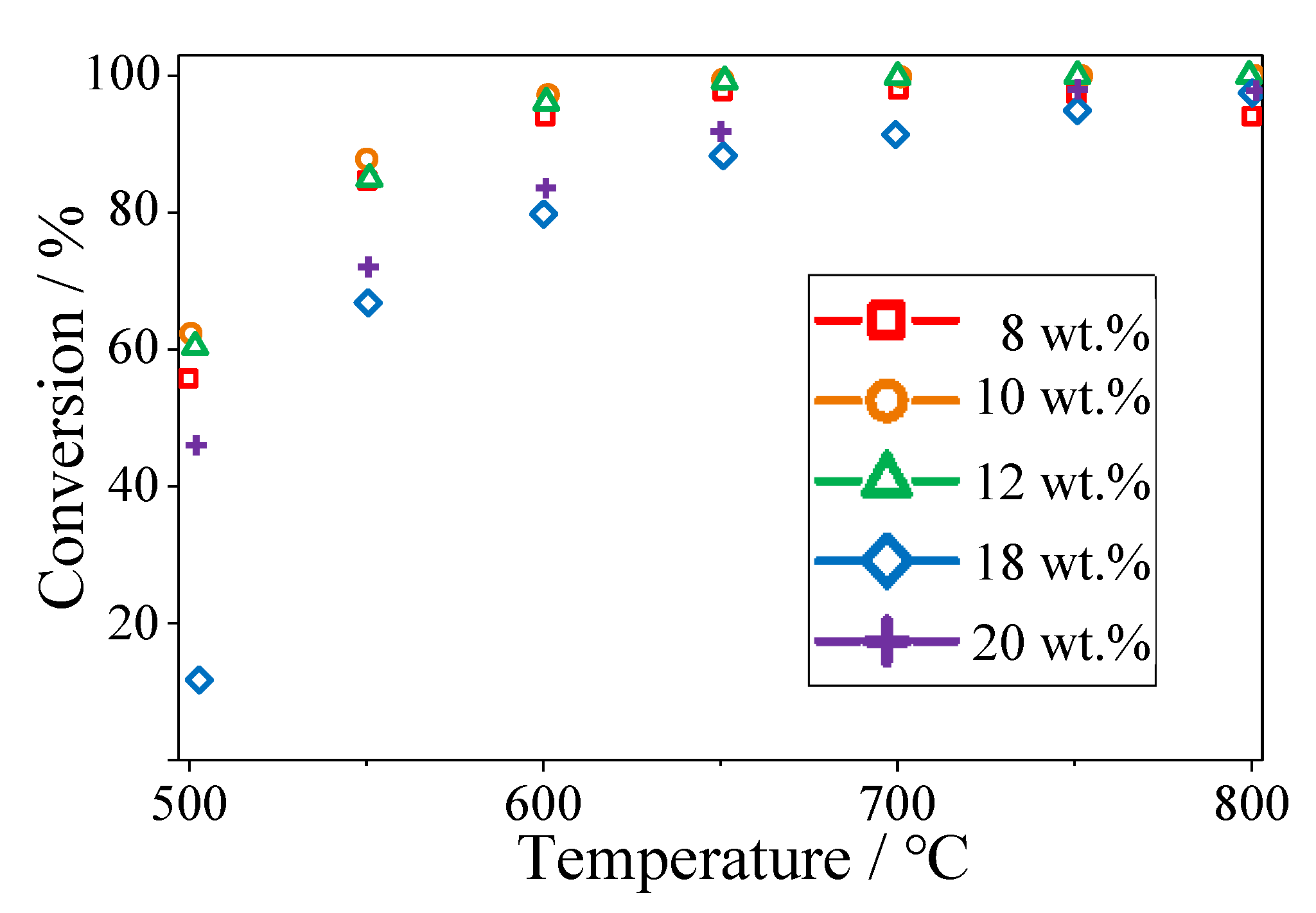
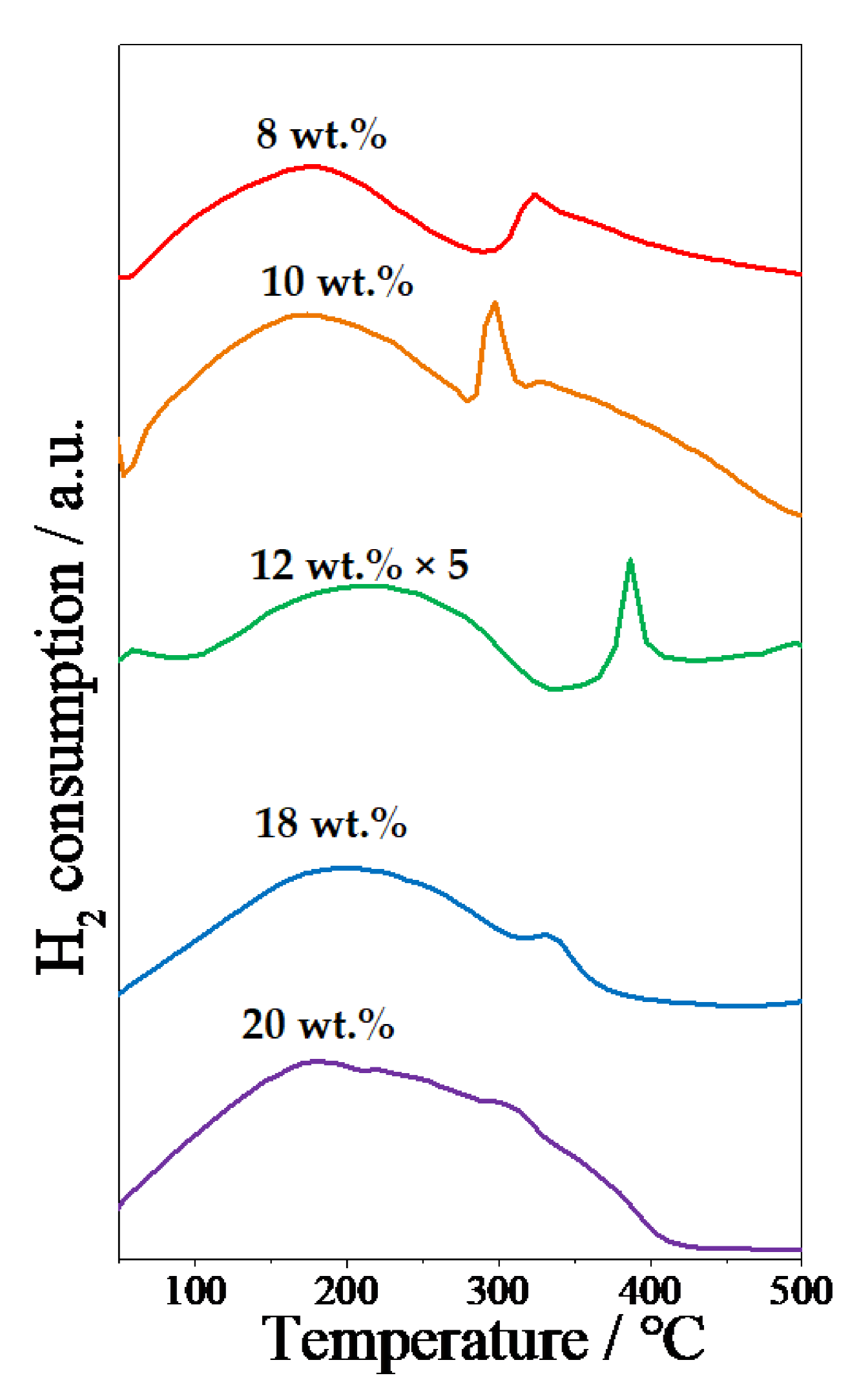
2.1. Effect of Ni Content on POM Activity
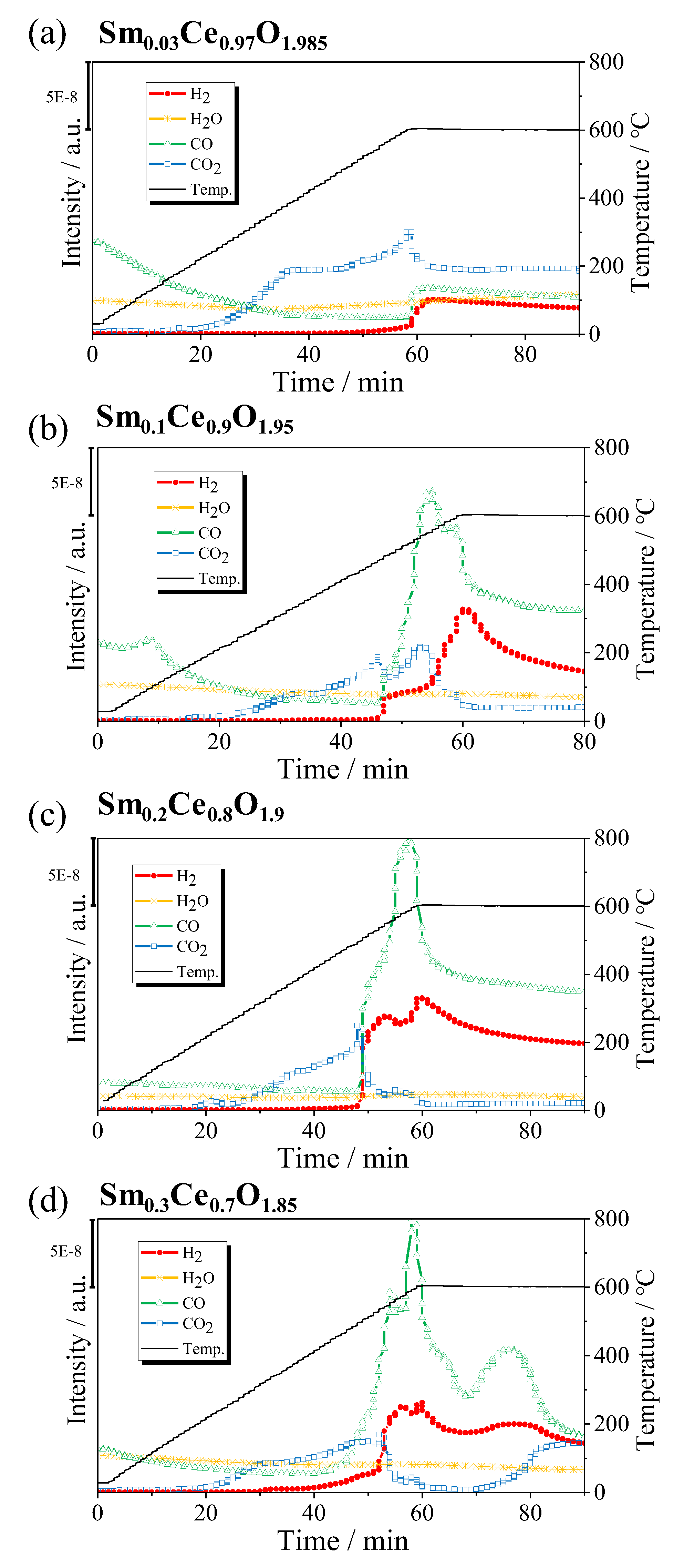
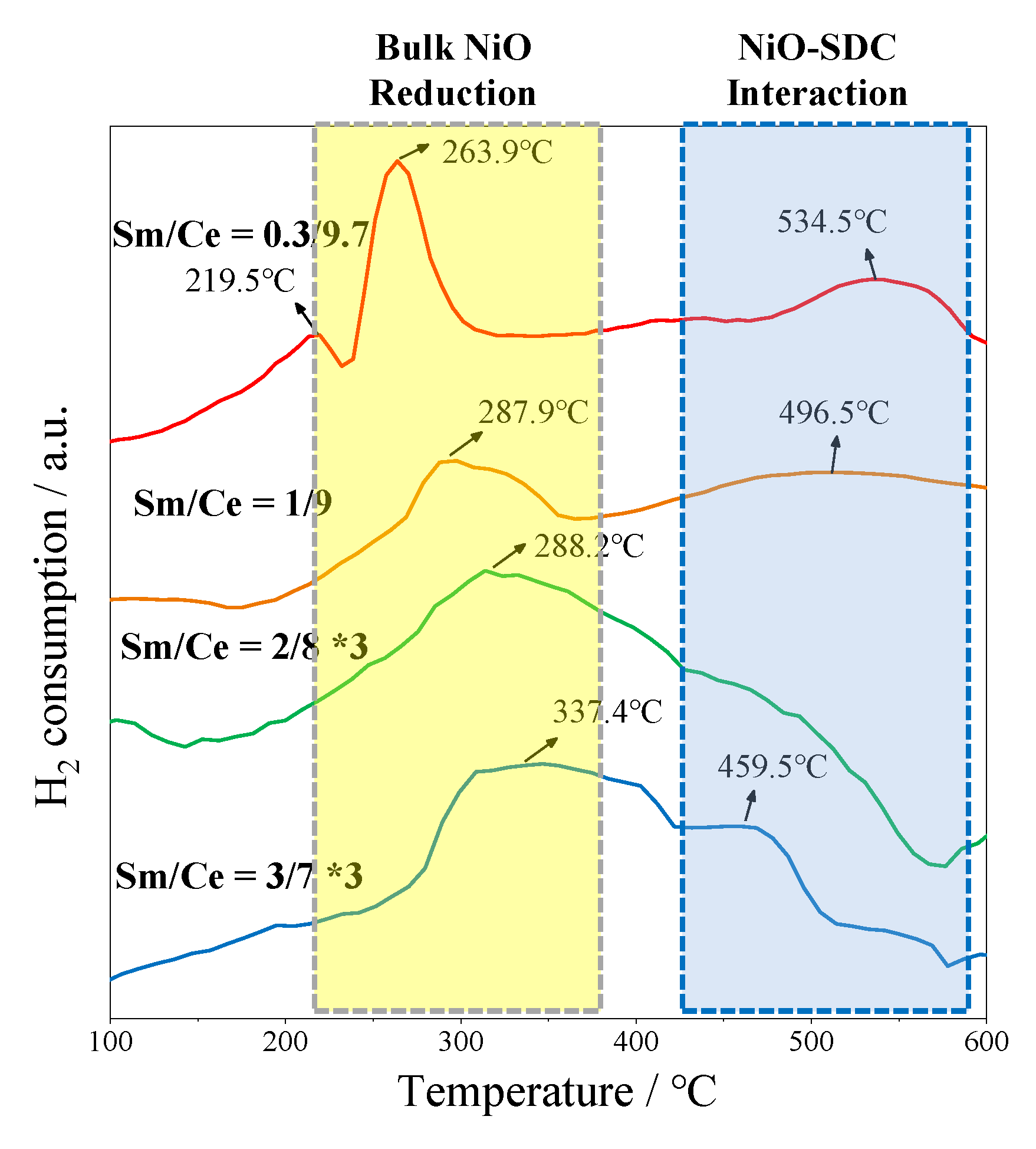
2.2. Effect of Sm/Ce Ratio on POM Activity
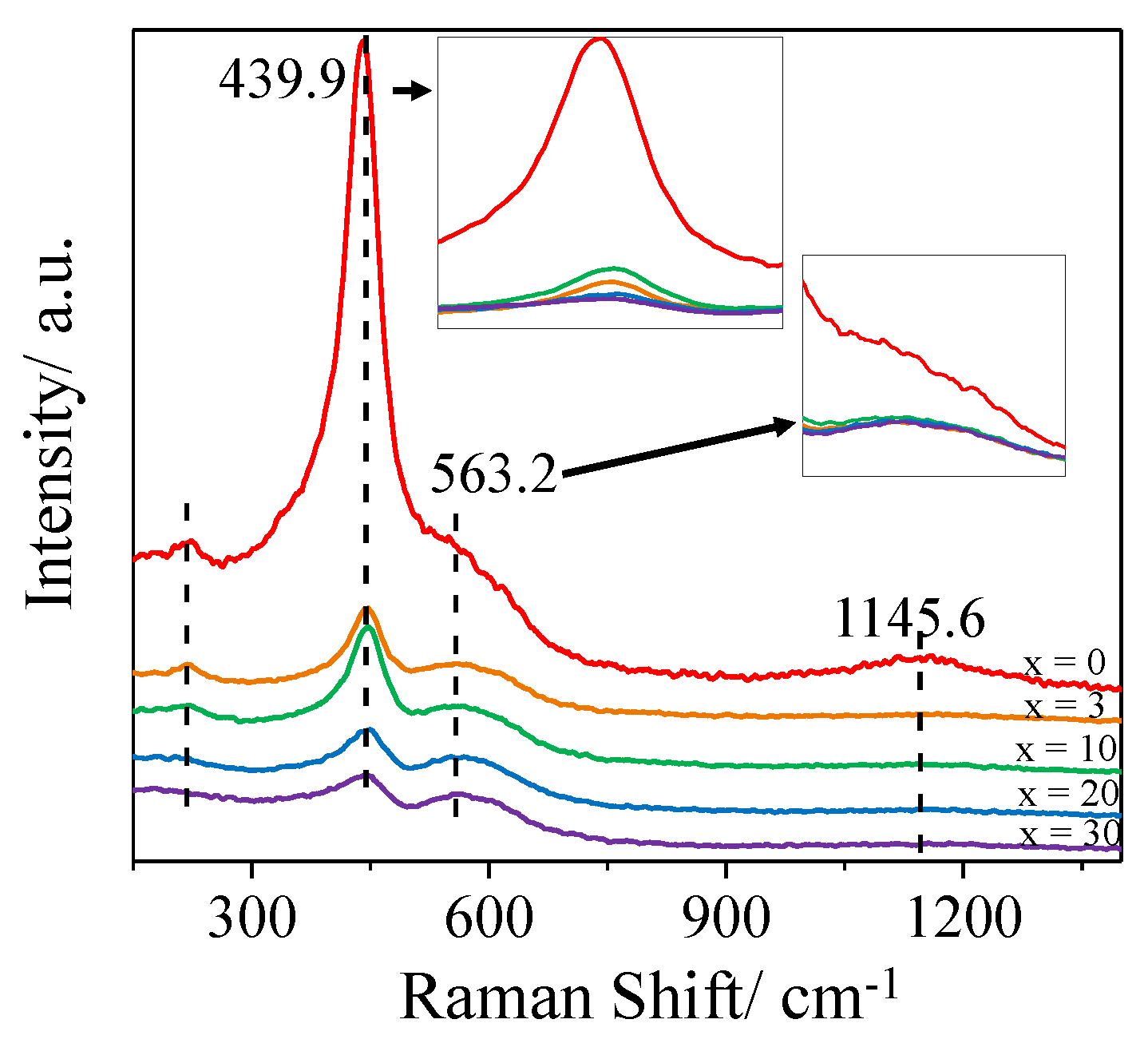
2.3. Crystalline Structure and Morphology
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Measurement of Catalytic Activity
4.3. Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bharadwaj, S.; Schmidt, L. Catalytic partial oxidation of natural gas to syngas. Fuel Process. Technol. 1995, 42, 109–127. [Google Scholar] [CrossRef]
- Enger, B.C.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A Gen. 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Pansanga, K.; Lohitharn, N.; Chien, A.C.; Lotero, E.; Panpranot, J.; Praserthdam, P.; Goodwin, J.G., Jr. Copper-modified alumina as a support for iron Fischer–Tropsch synthesis catalysts. Appl. Catal. A Gen. 2007, 332, 130–137. [Google Scholar] [CrossRef]
- Boldrin, P.; Ruiz-Trejo, E.; Mermelstein, J.; Menéndez, J.M.B.; Reina, T.R.; Brandon, N.P. Strategies for Carbon and Sulfur Tolerant Solid Oxide Fuel Cell Materials, Incorporating Lessons from Heterogeneous Catalysis. Chem. Rev. 2016, 116, 13633–13684. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Lin, Y.; Pillai, M.; Kim, I.; Barnett, S.A. High-rate electrochemical partial oxidation of methane in solid oxide fuel cells. J. Power Sources 2006, 161, 460–465. [Google Scholar] [CrossRef]
- Wang, W.; Su, C.; Wu, Y.; Ran, R.; Shao, Z. Progress in solid oxide fuel cells with nickel-based anodes operating on methane and related fuels. Chem. Rev. 2013, 113, 8104–8151. [Google Scholar] [CrossRef]
- Xu, S.; Wang, X. Highly active and coking resistant Ni/CeO2-ZrO2 catalyst for partial oxidation of methane. Fuel 2005, 84, 563–567. [Google Scholar] [CrossRef]
- Kim, D.K.; Stöwe, K.; Müller, F.; Maier, W.F. Mechanistic study of the unusual catalytic properties of a new Ni—Ce mixed oxide for the CO2 reforming of methane. J. Catal. 2007, 247, 101–111. [Google Scholar] [CrossRef]
- Chien, A.C.; Lin, E.Y.; Lai, A.D. Aid of a metallic functional layer on Ni/YSZ anode for Direct Methane Fuel Cell. Int. J. Hydrog. Energy 2020, 45, 23526–23532. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, Z.; Liu, J.; Barnett, S. Direct operation of solid oxide fuel cells with methane fuel. Solid State Ion. 2005, 176, 1827–1835. [Google Scholar] [CrossRef]
- Ideris, A.; Croiset, E.; Pritzker, M.; Amin, A. Direct-methane solid oxide fuel cell (SOFC) with Ni-SDC anode-supported cell. Int. J. Hydrog. Energy 2017, 42, 23118–23129. [Google Scholar] [CrossRef]
- Rismanchian, A.; Mirzababaei, J.; Chuang, S.S. Electroless plated Cu–Ni anode catalyst for natural gas solid oxide fuel cells. Catal. Today 2015, 245, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Stevens, J.A.R.W.; Chuang, S.S.C. In situ IR Study of Transient CO2 Reforming of CH4 over Rh/Al2O3. J. Phys. Chem. B 2004, 108, 696–703. [Google Scholar] [CrossRef]
- Chien, A.C.; Liao, B.Y. Oscillating syngas production on NiO/YSZ catalyst from methane oxidation. RSC Adv. 2020, 10, 26693–26698. [Google Scholar] [CrossRef]
- Chien, A.C.; Ye, N.J. Effect of preparation method and particle size of Ni/SDC catalyst on methane oxidation. Catal. Commun. 2021, 154, 106312. [Google Scholar] [CrossRef]
- Grabchenko, M.; Pantaleo, G.; Puleo, F.; Vodyankina, O.; Liotta, L. Ni/La2O3 catalysts for dry reforming of methane: Effect of La2O3 synthesis conditions on the structural properties and catalytic performances. Int. J. Hydrog. Energy 2021, 46, 7939–7953. [Google Scholar] [CrossRef]
- Devaiah, D.; Reddy, L.H.; Park, S.-E.; Reddy, B.M. Ceria–zirconia mixed oxides: Synthetic methods and applications. Catal. Rev. 2018, 60, 177–277. [Google Scholar] [CrossRef]
- Trovarelli, A.; Llorca, J. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Singha, R.K.; Shukla, A.; Yadav, A.; Konathala, L.S.; Bal, R. Effect of metal-support interaction on activity and stability of Ni-CeO2 catalyst for partial oxidation of methane. Appl. Catal. B Environ. 2017, 202, 473–488. [Google Scholar] [CrossRef]
- Lykhach, Y.; Kubát, J.; Neitzel, A.; Tsud, N.; Vorokhta, M.; Skála, T.; Dvořák, F.; Kosto, Y.; Prince, K.C.; Matolin, V.; et al. Charge transfer and spillover phenomena in ceria-supported iridium catalysts: A model study. J. Chem. Phys. 2019, 151, 204703. [Google Scholar] [CrossRef]
- Migani, A.; Vayssilov, G.N.; Bromley, S.T.; Illas, F.; Neyman, K.M. Greatly facilitated oxygen vacancy formation in ceria nanocrystallites. Chem. Commun. 2010, 46, 5936–5938. [Google Scholar] [CrossRef] [PubMed]
- Vayssilov, G.N.; Lykhach, Y.; Migani, A.; Staudt, T.; Petrova, G.P.; Tsud, N.; Skála, T.; Bruix, A.; Illas, F.; Prince, K.C.; et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 2011, 10, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-J.; Huang, M.-C. Electrochemical promotion of bulk lattice-oxygen extraction for direct methane conversion to syngas in SOFCs with Ni-YSZ anodes. Chem. Eng. J. 2008, 138, 538–547. [Google Scholar] [CrossRef]
- La Parola, V.; Pantaleo, G.; Venezia, A.M. Effects of Synthesis on the Structural Properties and Methane Partial Oxidation Activity of Ni/CeO2 Catalyst. Catalysts 2018, 8, 220. [Google Scholar] [CrossRef] [Green Version]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Vivier, L.; Duprez, D. Ceria-Based Solid Catalysts for Organic Chemistry. ChemSusChem 2010, 3, 654–678. [Google Scholar] [CrossRef]
- Tao, R.; Xu, J.; Zhong, H.; Wen, W.; Pan, Q.; Liu, Y.; Chen, J. Finely Tuned Structure and Catalytic Performance of Cerium Oxides by a Continuous Samarium Doping from 0 to 100%. Inorg. Chem. 2019, 58, 13066–13076. [Google Scholar] [CrossRef]
- Presto, S.; Artini, C.; Pani, M.; Carnasciali, M.M.; Massardo, S.; Viviani, M. Ionic conductivity and local structural features in Ce1−xSmxO2−x/2. Phys. Chem. Chem. Phys. 2018, 20, 28338–28345. [Google Scholar] [CrossRef]
- Soni, S.; Kumar, S.; Dalela, B.; Kumar, S.; Alvi, P.; Dalela, S. Defects and oxygen vacancies tailored structural and optical properties in CeO2 nanoparticles doped with Sm3+ cation. J. Alloy. Compd. 2018, 752, 520–531. [Google Scholar] [CrossRef]
- Coles-Aldridge, A.V.; Baker, R.T. Oxygen ion conductivity in ceria-based electrolytes co-doped with samarium and gadolinium. Solid State Ion. 2020, 347, 115255. [Google Scholar] [CrossRef]
- Kumar, S.A.; Kuppusami, P.; Amirthapandian, S.; Fu, Y.-P. Effect of Sm co-doping on structural, mechanical and electrical properties of Gd doped ceria solid electrolytes for intermediate temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2020, 45, 29690–29704. [Google Scholar] [CrossRef]
- Jung, G.-B.; Huang, T.-J.; Chang, C.-L. Effect of temperature and dopant concentration on the conductivity of samaria-doped ceria electrolyte. J. Solid State Electrochem. 2001, 6, 225–230. [Google Scholar] [CrossRef]
- Pantaleo, G.; La Parola, V.; Deganello, F.; Singha, R.; Bal, R.; Venezia, A. Ni/CeO2 catalysts for methane partial oxidation: Synthesis driven structural and catalytic effects. Appl. Catal. B Environ. 2016, 189, 233–241. [Google Scholar] [CrossRef]
- Ding, G.; Gan, T.; Yu, J.; Li, P.; Yao, X.; Hou, N.; Fan, L.; Zhao, Y.; Li, Y. Carbon-resistant Ni1-xCox-Ce0.8Sm0.2O1.9 anode for solid oxide fuel cells fed with methanol. Catal. Today 2017, 298, 250–257. [Google Scholar] [CrossRef]
- Swatsitang, E.; Phokha, S.; Hunpratub, S.; Maensiri, S. Characterization of Sm-doped CeO2 nanoparticles and their magnetic properties. Phys. B Condens. Matter 2016, 485, 14–20. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Zedan, A.F.; Katsiotis, M.; Baker, M.; Alkhoori, A.; AlQaradawi, S.Y.; Hinder, S.; Alhassan, S. Rapid microwave assisted sol-gel synthesis of CeO2 and CexSm1-xO2 nanoparticle catalysts for CO oxidation. Mol. Catal. 2017, 428, 41–55. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Ge, L.; Zhang, J.; Zhuang, L.; Liu, S.; Tan, X.; Wang, S.; Zhu, Z. Partial oxidation of methane to syngas in catalytic membrane reactor: Role of catalyst oxygen vacancies. Chem. Eng. J. 2020, 392, 123739. [Google Scholar] [CrossRef]
- Zheng, Y.; He, S.; Ge, L.; Zhou, M.; Chen, H.; Guo, L. Effect of Sr on Sm-doped ceria electrolyte. Int. J. Hydrog. Energy 2011, 36, 5128–5135. [Google Scholar] [CrossRef]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E. Microwaves and Heterogeneous Catalysis: A Review on Selected Catalytic Processes. Catalysts 2020, 10, 246. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, A.C.; Ye, N.J.; Huang, C.-W.; Tseng, I.-H. Studies of Nickel/Samarium-Doped Ceria for Catalytic Partial Oxidation of Methane and Effect of Oxygen Vacancy. Catalysts 2021, 11, 731. https://doi.org/10.3390/catal11060731
Chien AC, Ye NJ, Huang C-W, Tseng I-H. Studies of Nickel/Samarium-Doped Ceria for Catalytic Partial Oxidation of Methane and Effect of Oxygen Vacancy. Catalysts. 2021; 11(6):731. https://doi.org/10.3390/catal11060731
Chicago/Turabian StyleChien, Andrew C., Nicole J. Ye, Chao-Wei Huang, and I-Hsiang Tseng. 2021. "Studies of Nickel/Samarium-Doped Ceria for Catalytic Partial Oxidation of Methane and Effect of Oxygen Vacancy" Catalysts 11, no. 6: 731. https://doi.org/10.3390/catal11060731
APA StyleChien, A. C., Ye, N. J., Huang, C.-W., & Tseng, I.-H. (2021). Studies of Nickel/Samarium-Doped Ceria for Catalytic Partial Oxidation of Methane and Effect of Oxygen Vacancy. Catalysts, 11(6), 731. https://doi.org/10.3390/catal11060731







