Passivation of Co/Al2O3 Catalyst by Atomic Layer Deposition to Reduce Deactivation in the Fischer–Tropsch Synthesis
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Tests
2.2.1. Catalytic Performance at Regular Conditions (XCO ≈ 50%)
2.2.2. Catalytic Performance at Accelerated Deactivation Conditions (XCO ≈ 60%)
3. Materials and Methods
3.1. ALD Passivation Procedure
3.2. Sample Characterization
3.3. Catalytic Tests
3.4. Evaluation of Catalytic Performance
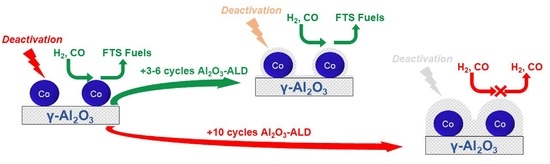
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panzone, C.; Philippe, R.; Chappaz, A.; Fongarland, P.; Bengaouer, A. Power-to-Liquid catalytic CO2 valorization into fuels and chemicals: Focus on the Fischer-Tropsch route. J. CO2 Util. 2020, 38, 314–347. [Google Scholar] [CrossRef]
- Pishahang, M.; Larring, Y.; Van Dijk, E.; Van Berkel, F.; Dahl, P.I.; Cobden, P.; McCann, M.; Bakken, E. Regenerative Copper-Alumina H2S Sorbent for Hot Gas Cleaning through Chemical Swing Adsorption. Ind. Eng. Chem. Res. 2016, 55, 1024–1032. [Google Scholar] [CrossRef]
- Tsakoumis, N.E.; Rønning, M.; Borg, Ø.; Rytter, E.; Holmen, A. Deactivation of cobalt based Fischer-Tropsch catalysts: A review. Catal. Today 2010, 154, 162–182. [Google Scholar] [CrossRef]
- Moodley, D.J.; van de Loosdrecht, J.; Saib, A.M.; Overett, M.J.; Datye, A.K.; Niemantsverdriet, J.W. Carbon deposition as a deactivation mechanism of cobalt-based Fischer–Tropsch synthesis catalysts under realistic conditions. Appl. Catal. A Gen. 2009, 354, 102–110. [Google Scholar] [CrossRef]
- Chen, W.; Kimpel, T.F.; Song, Y.; Chiang, F.K.; Zijlstra, B.; Pestman, R.; Wang, P.; Hensen, E.J.M. Influence of Carbon Deposits on the Cobalt-Catalyzed Fischer-Tropsch Reaction: Evidence of a Two-Site Reaction Model. ACS Catal. 2018, 8, 1580–1590. [Google Scholar] [CrossRef]
- Vázquez, F.V.; Koponen, J.; Ruuskanen, V.; Bajamundi, C.; Kosonen, A.; Simell, P.; Ahola, J.; Frilund, C.; Elfving, J.; Reinikainen, M.; et al. Power-to-X technology using renewable electricity and carbon dioxide from ambient air: SOLETAIR proof-of-concept and improved process concept. J. CO2 Util. 2018, 28, 235–246. [Google Scholar] [CrossRef]
- Rahmati, M.; Safdari, M.-S.; Fletcher, T.H.; Argyle, M.D.; Bartholomew, C.H. Chemical and Thermal Sintering of Supported Metals with Emphasis on Cobalt Catalysts During Fischer–Tropsch Synthesis. Chem. Rev. 2020, 120, 4455–4533. [Google Scholar] [CrossRef]
- van de Loosdrecht, J.; Balzhinimaev, B.; Dalmon, J.-A.; Niemantsverdriet, J.W.; Tsybulya, S.V.; Saib, A.M.; van Berge, P.J.; Visagie, J.L. Cobalt Fischer-Tropsch synthesis: Deactivation by oxidation? Catal. Today 2007, 123, 293–302. [Google Scholar] [CrossRef]
- Wolf, M.; Fischer, N.; Claeys, M. Water-induced deactivation of cobalt-based Fischer–Tropsch catalysts. Nat. Catal. 2020, 3, 962–965. [Google Scholar] [CrossRef]
- Rytter, E.; Holmen, A. Deactivation and Regeneration of Commercial Type Fischer-Tropsch Co-Catalysts—A Mini-Review. Catalysts 2015, 5, 478–499. [Google Scholar] [CrossRef]
- Yang, J.; Fang, X.; Xu, Y.; Liu, X. Investigation of the deactivation behavior of Co catalysts in Fischer–Tropsch synthesis using encapsulated Co nanoparticles with controlled SiO2 shell layer thickness. Catal. Sci. Technol. 2020, 10, 1182–1192. [Google Scholar] [CrossRef]
- Phaahlamohlaka, T.N.; Dlamini, M.W.; Mogodi, M.W.; Kumi, D.O.; Jewell, L.L.; Billing, D.G.; Coville, N.J. A sinter resistant Co Fischer-Tropsch catalyst promoted with Ru and supported on titania encapsulated by mesoporous silica. Appl. Catal. A Gen. 2018, 552, 129–137. [Google Scholar] [CrossRef]
- Singh, J.A.; Yang, N.; Bent, S.F. Nanoengineering Heterogeneous Catalysts by Atomic Layer Deposition. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Elam, J.; Stair, P. Synthesis and stabilization of supported metal catalysts by atomic layer deposition. Acc. Chem. Res. 2013, 46. [Google Scholar] [CrossRef] [PubMed]
- Gorey, T.J.; Dai, Y.; Anderson, S.L.; Lee, S.; Lee, S.; Seifert, S.; Winans, R.E. Selective growth of Al2O3 on size-selected platinum clusters by atomic layer deposition. Surf. Sci. 2020, 691, 121485. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Ko, W.C.; Kang, S.; Kim, K.M.; Jeong, Y.K. Surface passivation of highly stable TiO2/V2O5 photocatalyst by atomic layer deposited-Al2O3. Appl. Surf. Sci. 2020, 507, 145128. [Google Scholar] [CrossRef]
- Baktash, E.; Littlewood, P.; Schomäcker, R.; Thomas, A.; Stair, P.C. Alumina coated nickel nanoparticles as a highly active catalyst for dry reforming of methane. Appl. Catal. B Environ. 2015, 179, 122–127. [Google Scholar] [CrossRef]
- Feng, H.; Lu, J.; Stair, P.C.; Elam, J.W. Alumina over-coating on Pd nanoparticle catalysts by atomic layer deposition: Enhanced stability and reactivity. Catal. Lett. 2011, 141, 512–517. [Google Scholar] [CrossRef]
- Lee, J.; Jackson, D.H.K.; Li, T.; Winans, R.E.; Dumesic, J.A.; Kuech, T.F.; Huber, G.W. Enhanced stability of cobalt catalysts by atomic layer deposition for aqueous-phase reactions. Energy Environ. Sci. 2014, 7, 1657–1660. [Google Scholar] [CrossRef]
- Moodley, D.J.; Saib, A.M.; Van De Loosdrecht, J.; Welker-Nieuwoudt, C.A.; Sigwebela, B.H.; Niemantsverdriet, J.W. The impact of cobalt aluminate formation on the deactivation of cobalt-based Fischer-Tropsch synthesis catalysts. Catal. Today 2011, 171, 192–200. [Google Scholar] [CrossRef]
- Taheri Najafabadi, A.; Khodadadi, A.A.; Parnian, M.J.; Mortazavi, Y. Atomic layer deposited Co/γ-Al2O3 catalyst with enhanced cobalt dispersion and Fischer-Tropsch synthesis activity and selectivity. Appl. Catal. A Gen. 2016, 511, 31–46. [Google Scholar] [CrossRef]
- Eskelinen, P.; Fransila, S. Cobalt Catalyst Characterization and Modification by Atomic Layer Deposition for Fischer-Tropsch Synthesis; Aalto University: Espoo, Finland, 2019. [Google Scholar]
- Rouquerol, J.; Avnir, D.; Everett, D.H.; Fairbridge, C.; Haynes, M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Guidelines for the Characterization of Porous Solids. In Studies in Surface Science and Catalysis; Elsevier Science B.V.: Amsterdam, The Netherlands, 1994; Volume 87, pp. 1–9. [Google Scholar]
- Trépanier, M.; Tavasoli, A.; Dalai, A.K.; Abatzoglou, N. Co, Ru and K loadings effects on the activity and selectivity of carbon nanotubes supported cobalt catalyst in Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2009, 353, 193–202. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the Development of Novel Cobalt Fischer−Tropsch Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.; Ellis, P.R.; Humble, R.; Kelly, G.J.; McKenna, M.; West, J. Deactivation of alumina supported cobalt FT catalysts during testing in a Continuous-stirred tank reactor (CSTR). Appl. Catal. A Gen. 2018, 550, 28–37. [Google Scholar] [CrossRef]
- Lögdberg, S.; Yang, J.; Lualdi, M.; Walmsley, J.C.; Järås, S.; Boutonnet, M.; Blekkan, E.A.; Rytter, E.; Holmen, A. Further insights into methane and higher hydrocarbons formation over cobalt-based catalysts with γ-Al2O3, α-Al2O3 and TiO2 as support materials. J. Catal. 2017, 352, 515–531. [Google Scholar] [CrossRef]
- de la Peña O’Shea, V.A.; Homs, N.; Fierro, J.L.G.; Ramírez de la Piscina, P. Structural changes and activation treatment in a Co/SiO2 catalyst for Fischer–Tropsch synthesis. Catal. Today 2006, 114, 422–427. [Google Scholar] [CrossRef]
- Argyle, M.D.; Frost, T.S.; Bartholomew, C.H. Cobalt Fischer–Tropsch Catalyst Deactivation Modeled Using Generalized Power Law Expressions. Top. Catal. 2014, 57, 415–429. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Sintering kinetics of supported metals: New perspectives from a unifying GPLE treatment. Appl. Catal. A Gen. 1993, 107, 1–57. [Google Scholar] [CrossRef]
- Tavasoli, A.; Malek Abbaslou, R.M.; Dalai, A.K. Deactivation behavior of ruthenium promoted Co/γ-Al2O3 catalysts in Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2008, 346, 58–64. [Google Scholar] [CrossRef]








| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | TPR-Tmax (°C) a | Co Crystallite Size (nm) b | Extent of Co Reduction (%) c |
|---|---|---|---|---|---|
| γ-Al2O3 | 216 | 0.492 | - | - | - |
| REF | 163 | 0.320 | 268/481 | 12.4 | 61.4 |
| 3ALD-REF | 163 | 0.331 | 260/466 | 11.6 | 56.5 |
| 6ALD-REF | 161 | 0.322 | 281/514 | 11.1 | 65.4 |
| 10ALD-REF | 161 | 0.327 | 283/530 | 12.1 | 11.1 |
| Sample | Time (h) | XCO (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|---|
| CH4 | CO2 | C2–C4 a | C5+ | |||||
| REF | 16 | 43.5 | 15.9 | 0.70 | 4.78 | 78.6 | 1 | 1 |
| 87 | 35.9 | 14.9 | 0.59 | 11.6 | 73.0 | 1 | 1 | |
| 3ALD-REF | 17 | 43.0 | 17.2 | 0.76 | 5.2 | 76.9 | 0.990 | 0.967 |
| 87 | 36.7 | 15.2 | 0.57 | 10.9 | 73.3 | 1.018 | 1.026 | |
| 6ALD-REF | 17 | 40.1 | 15.3 | 0.74 | 7.6 | 76.3 | 0.945 | 0.895 |
| 64 | 34.8 | 14.0 | 0.66 | 11.5 | 73.9 | 0.972 b | 0.981 | |
| 10ALD-REF | 15 | 28.1 | 32.1 | 0.78 | 9.9 | 57.2 | 0.706 | 0.470 |
| 62 | 30.2 | 21.2 | 0.62 | 12.2 | 66.0 | 0.860 b | 0.760 | |
| Deactivation Order | 0 | 1 | 2 | |||
|---|---|---|---|---|---|---|
| Sample | ||||||
| REF | 2.38 × 10−4 | 0.8021 | 2.63 × 10−4 | 0.8154 | 2.92 × 10−4 | 0.8279 |
| 3ALD-REF | 2.02 × 10−4 | 0.8496 | 2.20 × 10−4 | 0.8619 | 2.41 × 10−4 | 0.8735 |
| 6ALD-REF | 1.73 × 10−4 | 0.7384 | 1.90 × 10−4 | 0.7528 | 2.07 × 10−4 | 0.7665 |
| Sample | Time (h) | XCO (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|---|
| CH4 | CO2 | C2–C4 a | C5+ | |||||
| REF | 17 | 56.8 | 13.6 | 0.87 | 5.33 | 80.2 | 1 | 1 |
| 100 | 47.6 | 12.5 | 0.54 | 10.3 | 76.7 | 1 | 1 | |
| 200 | 44.5 | 12.6 | 0.53 | 10.7 | 76.2 | 1 | 1 | |
| 3ALD-REF | 17 | 56.7 | 14.9 | 0.96 | 5.64 | 78.5 | 1.014 | 0.977 |
| 100 | 47.7 | 12.4 | 0.56 | 10.0 | 77.0 | 1.011 | 1.001 | |
| 200 | 44.8 | 12.3 | 0.51 | 10.3 | 76.9 | 1.013 | 1.016 | |
| 6ALD-REF | 17 | 56.8 | 16.3 | 1.08 | 6.72 | 75.9 | 1.012 | 0.946 |
| 100 | 48.4 | 14.1 | 0.70 | 10.8 | 74.4 | 1.029 | 0.986 | |
| 200 | 45.7 | 13.4 | 0.58 | 10.7 | 75.3 | 1.034 | 1.015 | |
| Reference Sample | Number of ALD Cycles | Nomenclature |
|---|---|---|
| 16Co/γ-Al2O3 (Johnson Matthey) | 0 | REF |
| 3 | 3ALD-REF | |
| 6 | 6ALD-REF | |
| 10 | 10ALD-REF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-López, J.A.; Guilera, J.; Biset-Peiró, M.; Enache, D.; Kelly, G.; Andreu, T. Passivation of Co/Al2O3 Catalyst by Atomic Layer Deposition to Reduce Deactivation in the Fischer–Tropsch Synthesis. Catalysts 2021, 11, 732. https://doi.org/10.3390/catal11060732
Díaz-López JA, Guilera J, Biset-Peiró M, Enache D, Kelly G, Andreu T. Passivation of Co/Al2O3 Catalyst by Atomic Layer Deposition to Reduce Deactivation in the Fischer–Tropsch Synthesis. Catalysts. 2021; 11(6):732. https://doi.org/10.3390/catal11060732
Chicago/Turabian StyleDíaz-López, José Antonio, Jordi Guilera, Martí Biset-Peiró, Dan Enache, Gordon Kelly, and Teresa Andreu. 2021. "Passivation of Co/Al2O3 Catalyst by Atomic Layer Deposition to Reduce Deactivation in the Fischer–Tropsch Synthesis" Catalysts 11, no. 6: 732. https://doi.org/10.3390/catal11060732
APA StyleDíaz-López, J. A., Guilera, J., Biset-Peiró, M., Enache, D., Kelly, G., & Andreu, T. (2021). Passivation of Co/Al2O3 Catalyst by Atomic Layer Deposition to Reduce Deactivation in the Fischer–Tropsch Synthesis. Catalysts, 11(6), 732. https://doi.org/10.3390/catal11060732