Synergistic Effect of Alkali Na and K Promoter on Fe-Co-Cu-Al Catalysts for CO2 Hydrogenation to Light Hydrocarbons
Abstract
1. Introduction
2. Results and Discussion
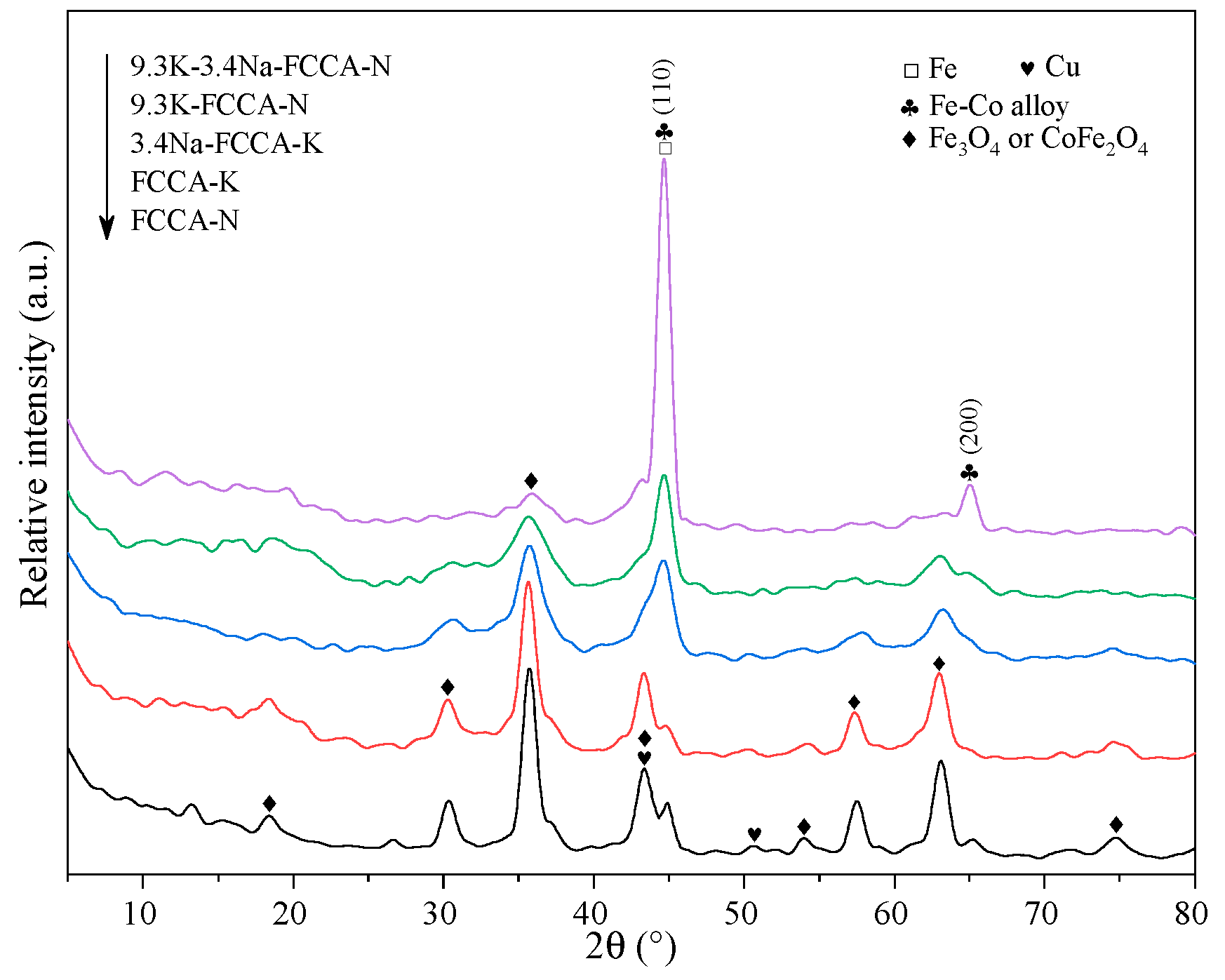
2.1. Textual and Structural Properties
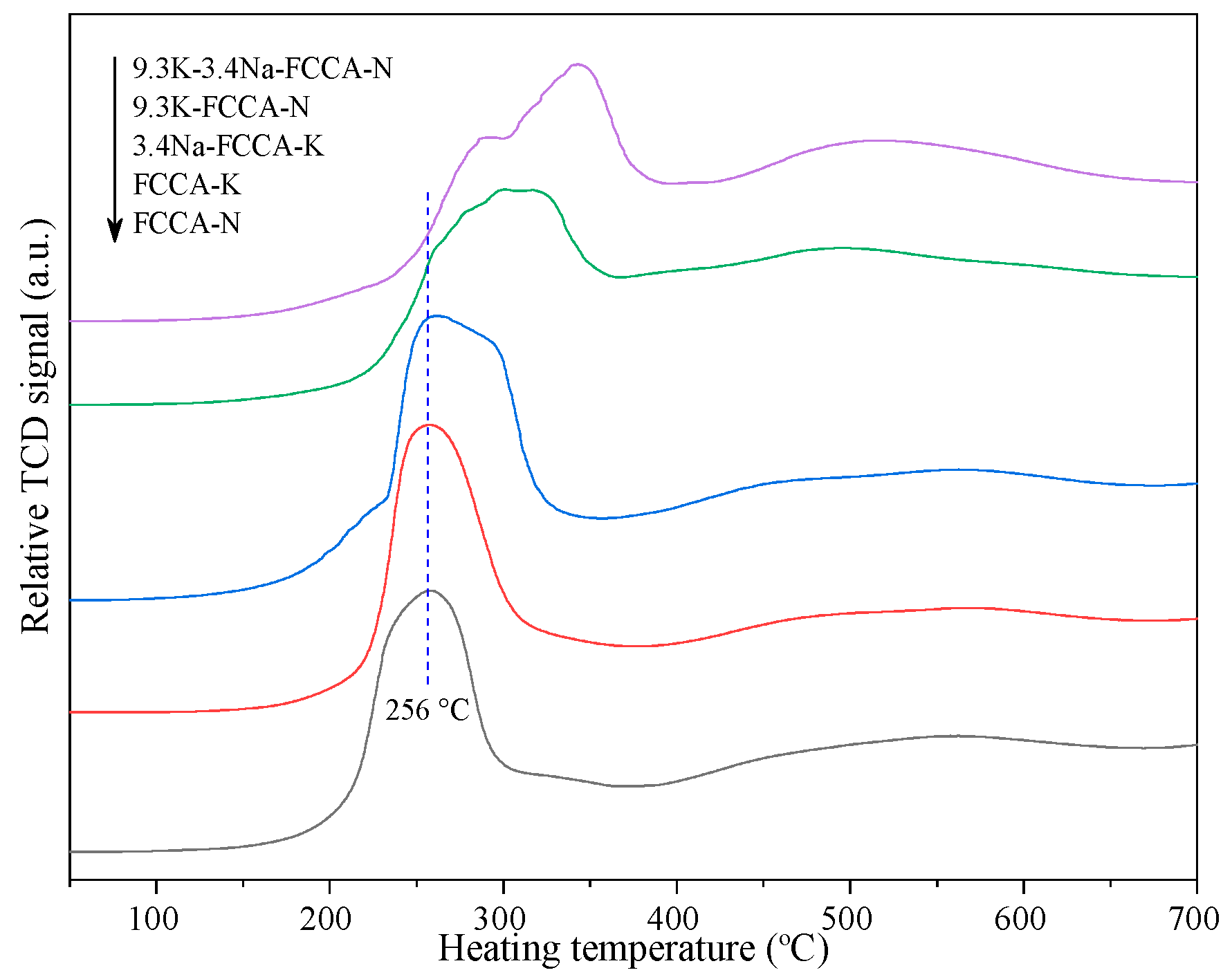
2.2. H2-TPR
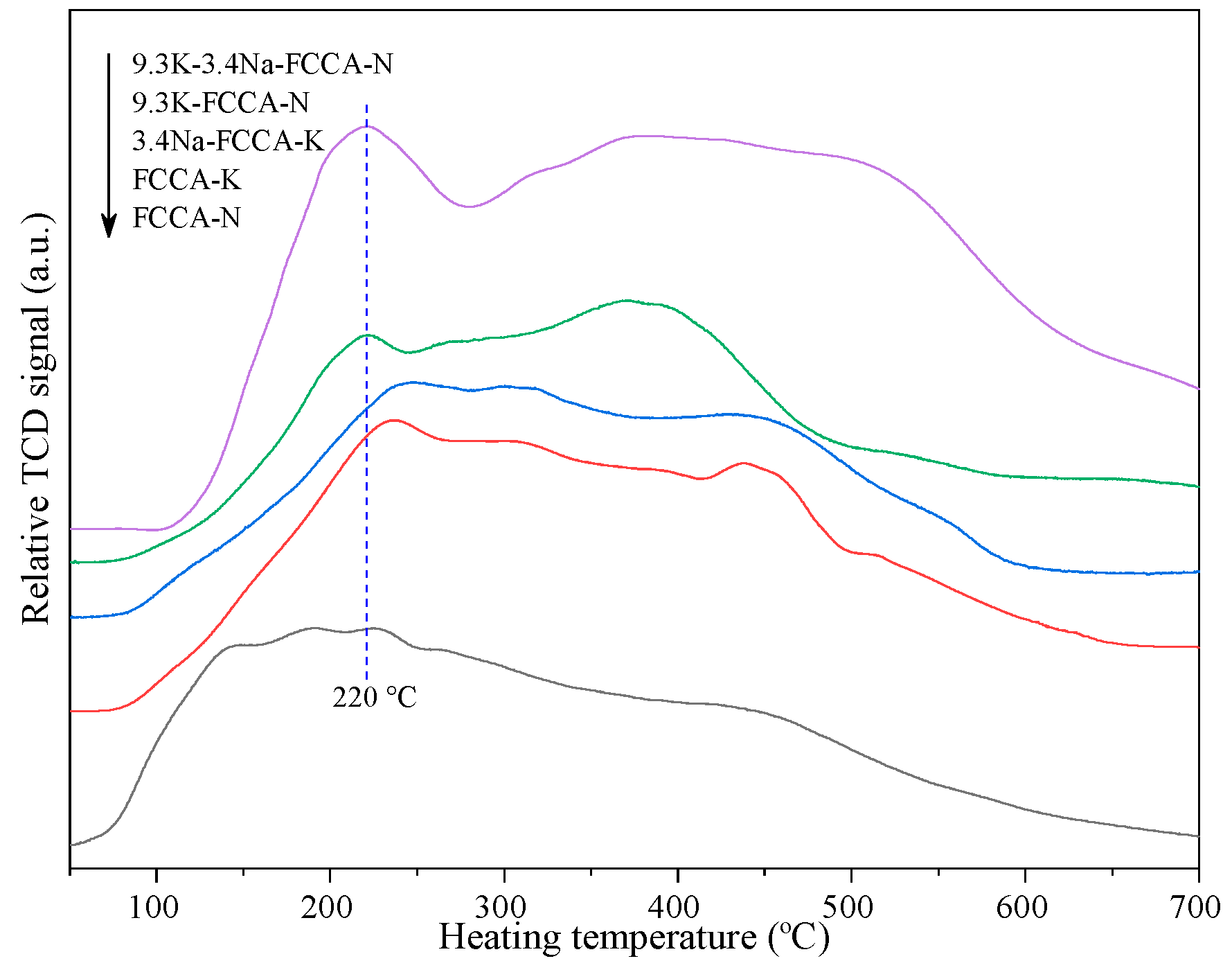
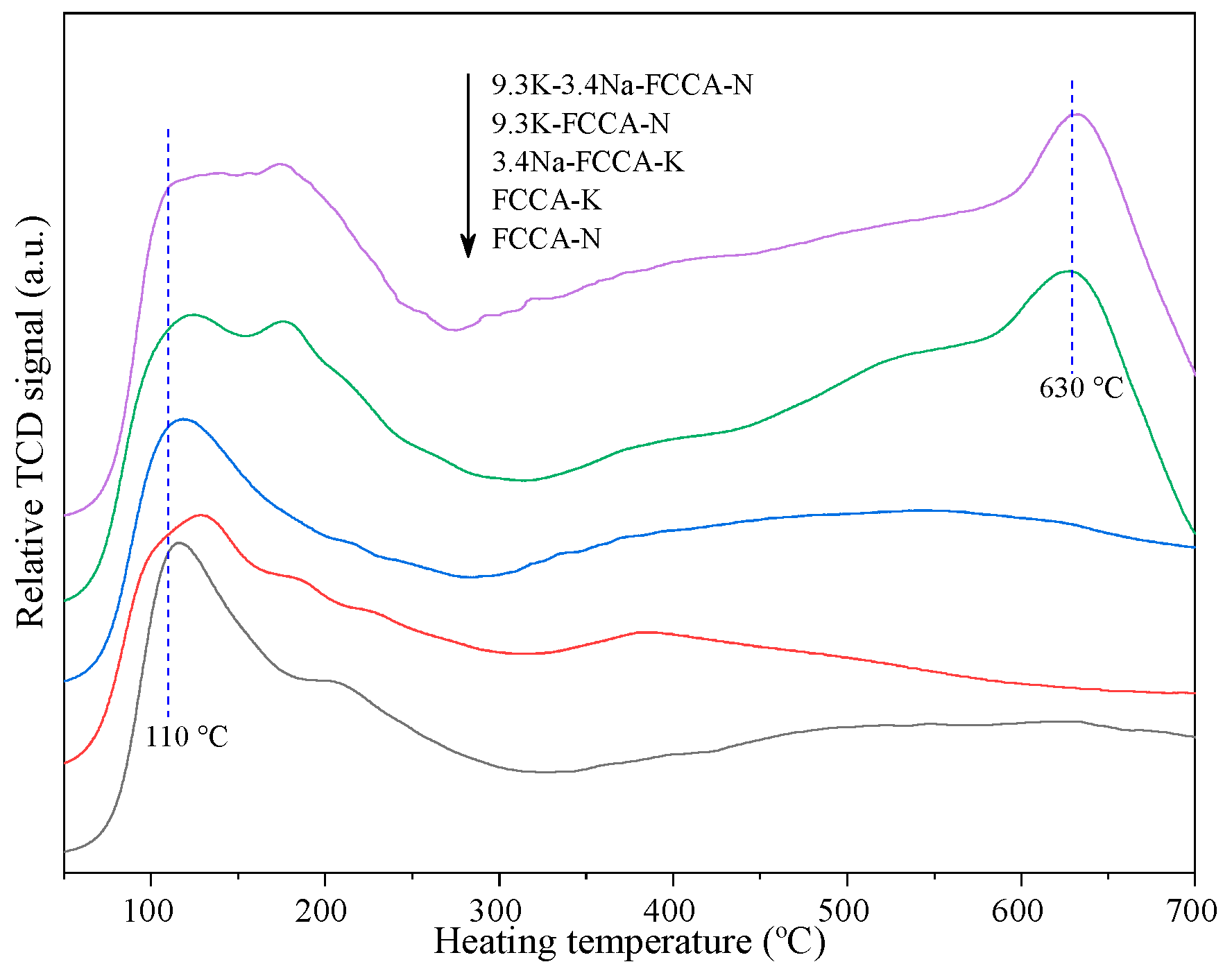
2.3. H2- and CO2-TPD
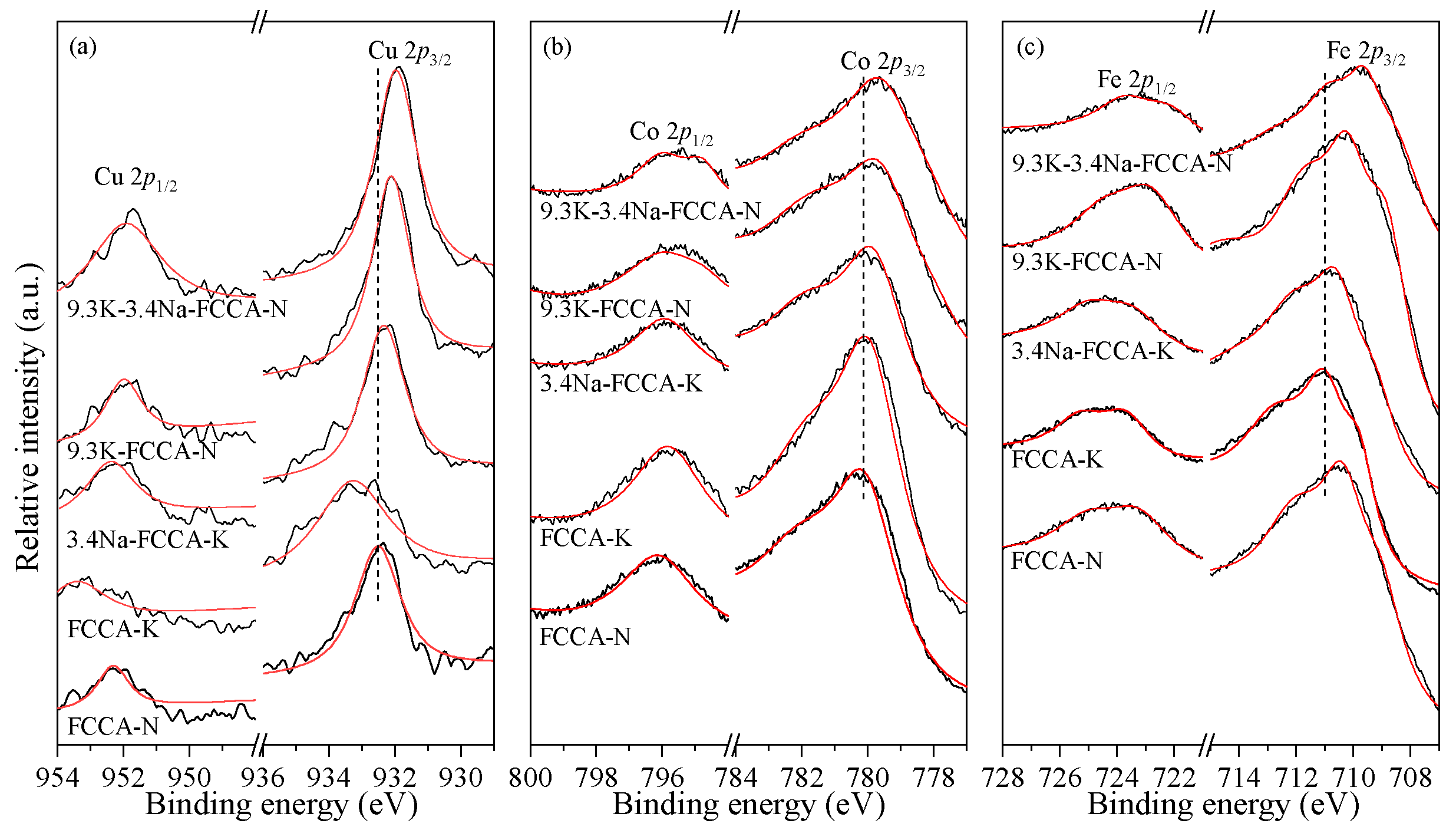
2.4. XPS
2.5. Catalytic Performances
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballantyne, A.P.; Alden, C.B.; Miller, J.B.; Tans, P.P.; White, J.W.C. Increase in observed net carbon dioxide uptake by land and oceans during the past 50 years. Nature 2012, 488, 70–72. [Google Scholar] [CrossRef]
- Wang, S.; Li, G.; Fang, C. Urbanization, economic growth, energy consumption, and CO2 emissions: Empirical evidence from countries with different income levels. Renew. Sustain. Energy Rev. 2018, 81, 2144–2159. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Wang, W.; Gong, J. Methanation of carbon dioxide: An overview. Front. Chem. Sci. Eng. 2011, 5, 2–10. [Google Scholar] [CrossRef]
- Kattel, S.; Ramírez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-X.; Xu, C.-H.; Liu, Y.-L.; Li, J.-J.; Hu, X.-D.; Liu, J.; Liu, J.-Y.; Xu, Q. Improved methanol synthesis from CO2 hydrogenation over CuZnAlZr catalysts with precursor pre-activation by formaldehyde. J. Catal. 2019, 379, 147–153. [Google Scholar] [CrossRef]
- Ren, H.; Xu, C.-H.; Zhao, H.-Y.; Wang, Y.-X.; Liu, J.; Liu, J.-Y. Methanol synthesis from CO2 hydrogenation over Cu/γ-Al2O3 catalysts modified by ZnO, ZrO2 and MgO. J. Ind. Eng. Chem. 2015, 28, 261–267. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Qu, Y.; Liu, H.; Tang, C.; Miao, S.; Feng, Z.; An, H.; Li, C. Highly Selective Conversion of Carbon Dioxide to Lower Olefins. ACS Catal. 2017, 7, 8544–8548. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Ge, Q.; Tsubaki, N. Recent advances in direct catalytic hydrogenation of carbon dioxide to valuable C2+ hydrocarbons. J. Mater. Chem. A 2018, 6, 23244–23262. [Google Scholar] [CrossRef]
- Zhong, L.; Yu, F.; An, Y.; Zhao, Y.; Sun, Y.; Li, Z.; Lin, T.; Lin, Y.; Qi, X.; Dai, Y.; et al. Cobalt carbide nanoprisms for direct production of lower olefins from syngas. Nature 2016, 538, 84–87. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, M.; Liu, B.; Xu, Y.; Liu, X. Insights into the influence of support and potassium or sulfur promoter on iron-based Fischer–Tropsch synthesis: Understanding the control of catalytic activity, selectivity to lower olefins, and catalyst deactivation. Catal. Sci. Technol. 2017, 7, 1245–1265. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Zhang, J.; Gao, X.; Ma, Q.; Fan, S.; Zhao, T.-S. Highly selective conversion of CO2 to light olefins via Fischer-Tropsch synthesis over stable layered K–Fe–Ti catalysts. Appl. Catal. A 2019, 573, 32–40. [Google Scholar] [CrossRef]
- Leckel, D. Diesel Production from Fischer−Tropsch: The Past, the Present, and New Concepts. Energy Fuel. 2009, 23, 2342–2358. [Google Scholar] [CrossRef]
- Dorner, R.W.; Hardy, D.R.; Williams, F.W.; Willauer, H.D. Heterogeneous catalytic CO2 conversion to value-added hydrocarbons. Energy Environ. Sci. 2010, 3, 884–890. [Google Scholar] [CrossRef]
- Zhang, J.; Su, X.; Wang, X.; Ma, Q.; Fan, S.; Zhao, T.-S. Promotion effects of Ce added Fe–Zr–K on CO2 hydrogenation to light olefins. React. Kinet. Mech. Catal. 2018, 124, 575–585. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, X.; Wang, X.; Song, C. Fe–Cu Bimetallic Catalysts for Selective CO2 Hydrogenation to Olefin-Rich C2+ Hydrocarbons. Ind. Eng. Chem. Res. 2018, 57, 4535–4542. [Google Scholar] [CrossRef]
- Satthawong, R.; Koizumi, N.; Song, C.; Prasassarakich, P. Light olefin synthesis from CO2 hydrogenation over K-promoted Fe–Co bimetallic catalysts. Catal. Today 2015, 251, 34–40. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Hamdeh, H.H.; Jacobs, G.; Shafer, W.D.; Hopps, S.D.; Thomas, G.A.; Davis, B.H. Hydrogenation of Carbon Dioxide over K-Promoted FeCo Bimetallic Catalysts Prepared from Mixed Metal Oxalates. ChemCatChem 2017, 9, 1303–1312. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Su, X.; Fan, S.; Ma, Q.; Zhao, T. Selective formation of light olefins from CO2 hydrogenation over Fe–Zn–K catalysts. J. CO2 Util. 2015, 12, 95–100. [Google Scholar] [CrossRef]
- Visconti, C.G.; Martinelli, M.; Falbo, L.; Infantes-Molina, A.; Lietti, L.; Forzatti, P.; Iaquaniello, G.; Palo, E.; Picutti, B.; Brignoli, F. CO2 hydrogenation to lower olefins on a high surface area K-promoted bulk Fe-catalyst. Appl. Catal. B 2017, 200, 530–542. [Google Scholar] [CrossRef]
- Fischer, N.; Henkel, R.; Hettel, B.; Iglesias, M.; Schaub, G.; Claeys, M. Hydrocarbons via CO2 Hydrogenation Over Iron Catalysts: The Effect of Potassium on Structure and Performance. Catal. Lett. 2015, 146, 509–517. [Google Scholar] [CrossRef]
- Amoyal, M.; Vidruk-Nehemya, R.; Landau, M.V.; Herskowitz, M. Effect of potassium on the active phases of Fe catalysts for carbon dioxide conversion to liquid fuels through hydrogenation. J. Catal. 2017, 348, 29–39. [Google Scholar] [CrossRef]
- Numpilai, T.; Chanlek, N.; Poo-Arporn, Y.; Cheng, C.K.; Siri-Nguan, N.; Sornchamni, T.; Chareonpanich, M.; Kongkachuichay, P.; Yigit, N.; Rupprechter, G.; et al. Tuning Interactions of Surface-adsorbed Species over Fe−Co/K−Al2O3 Catalyst by Different K Contents: Selective CO2 Hydrogenation to Light Olefins. ChemCatChem 2020, 12, 3306–3320. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, P.; Ellis, P.; French, S.; Kelly, G.; Lok, C.M. Density Functional Theory Study of Iron and Cobalt Carbides for Fischer−Tropsch Synthesis. J. Phys. Chem. C 2010, 114, 1085–1093. [Google Scholar] [CrossRef]
- Liang, B.; Duan, H.; Sun, T.; Ma, J.; Liu, X.; Xu, J.; Su, X.; Huang, Y.; Zhang, T. Effect of Na Promoter on Fe-Based Catalyst for CO2 Hydrogenation to Alkenes. ACS Sustain. Chem. Eng. 2019, 7, 925–932. [Google Scholar] [CrossRef]
- Wei, J.; Sun, J.; Wen, Z.; Fang, C.; Ge, Q.; Xu, H. New insights into the effect of sodium on Fe3O4- based nanocatalysts for CO2 hydrogenation to light olefins. Catal. Sci. Technol. 2016, 6, 4786–4793. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Hamdeh, H.H.; Shafer, W.D.; Liu, F.; Hopps, S.D.; Thomas, G.A.; Davis, B.H. Hydrogenation of Carbon Dioxide over Co–Fe Bimetallic Catalysts. ACS Catal. 2016, 6, 913–927. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Han, S.J.; Min, J.E.; Park, H.-G.; Jun, K.-W.; Kim, S.K. Mechanistic insights into Cu and K promoted Fe-catalyzed production of liquid hydrocarbons via CO2 hydrogenation. J. CO2 Util. 2019, 34, 522–532. [Google Scholar] [CrossRef]
- Wan, H.; Wu, B.; Zhang, C.; Xiang, H.; Li, Y. Promotional effects of Cu and K on precipitated iron-based catalysts for Fischer–Tropsch synthesis. J. Mol. Catal. A 2008, 283, 33–42. [Google Scholar] [CrossRef]
- Cheng, K.; Virginie, M.; Ordomsky, V.V.; Cordier, C.; Chernavskii, P.A.; Ivantsov, M.I.; Paul, S.; Wang, Y.; Khodakov, A.Y. Pore size effects in high-temperature Fischer–Tropsch synthesis over supported iron catalysts. J. Catal. 2015, 328, 139–150. [Google Scholar] [CrossRef]
- Hou, Z.; Yashima, T. Supported Co catalysts for methane reforming with CO. React. Kinet. Catal. Lett. 2004, 81, 153–159. [Google Scholar] [CrossRef]
- Liu, B.; Geng, S.; Zheng, J.; Jia, X.; Jiang, F.; Liu, X. Unravelling the New Roles of Na and Mn Promoter in CO2 Hydrogenation over Fe3O4 -Based Catalysts for Enhanced Selectivity to Light α-Olefins. ChemCatChem 2018, 10, 4718–4732. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, A.; Jiang, X.; Liu, M.; Sun, Y.; Song, C.; Guo, X. Selective CO2 Hydrogenation to Hydrocarbons on Cu-Promoted Fe-Based Catalysts: Dependence on Cu–Fe Interaction. ACS Sustain. Chem. Eng. 2018, 6, 10182–10190. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Chen, J.; Ma, Q.; Fan, S.; Zhao, T.-S. Effect of preparation methods on the structure and catalytic performance of Fe–Zn/K catalysts for CO2 hydrogenation to light olefins. Chin. J. Chem. Eng. 2018, 26, 761–767. [Google Scholar] [CrossRef]
- Prasad, P.S.S.; Bae, J.W.; Jun, K.-W.; Lee, K.-W. Fischer–Tropsch Synthesis by Carbon Dioxide Hydrogenation on Fe-Based Catalysts. Catal. Surv. Asia 2008, 12, 170–183. [Google Scholar] [CrossRef]
| Catalysts | BET Specific Surface Area (m2·g−1) | BJH Pore Volume (mL·g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| FCCA-N | 134.1 | 0.42 | 4.1 |
| FCCA-K | 102.9 | 0.32 | 4.0 |
| 3.4Na-FCCA-K | 135.9 | 0.34 | 3.2 |
| 9.3K-FCCA-N | 105.4 | 0.36 | 4.2 |
| 9.3K-3.4Na-FCCA-N | 110.3 | 0.28 | 3.2 |
| Catalysts | CO2 Conversion (mol%) | Distribution of Products (mol%) | ||||
|---|---|---|---|---|---|---|
| CO | CH4 | C2+ | Olefin C2-C4 | Paraffin C2-C4 | ||
| FCCA-N | 35.02 | 2.56 | 26.13 | 71.31 | 20.98 | 44.50 |
| FCCA-K | 36.70 | 2.68 | 32.15 | 65.17 | 16.29 | 33.33 |
| 3.4Na-FCCA-K | 47.52 | 4.77 | 10.22 | 85.01 | 28.35 | 40.00 |
| 9.3K-FCCA-N | 46.22 | 2.89 | 17.00 | 80.11 | 25.51 | 33.67 |
| 9.3K-3.4Na-FCCA-N | 52.87 | 2.29 | 8.01 | 89.70 | 32.14 | 39.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Xu, C.; Zhang, X.; Wu, Q.; Liu, J. Synergistic Effect of Alkali Na and K Promoter on Fe-Co-Cu-Al Catalysts for CO2 Hydrogenation to Light Hydrocarbons. Catalysts 2021, 11, 735. https://doi.org/10.3390/catal11060735
Zheng Y, Xu C, Zhang X, Wu Q, Liu J. Synergistic Effect of Alkali Na and K Promoter on Fe-Co-Cu-Al Catalysts for CO2 Hydrogenation to Light Hydrocarbons. Catalysts. 2021; 11(6):735. https://doi.org/10.3390/catal11060735
Chicago/Turabian StyleZheng, Yuhao, Chenghua Xu, Xia Zhang, Qiong Wu, and Jie Liu. 2021. "Synergistic Effect of Alkali Na and K Promoter on Fe-Co-Cu-Al Catalysts for CO2 Hydrogenation to Light Hydrocarbons" Catalysts 11, no. 6: 735. https://doi.org/10.3390/catal11060735
APA StyleZheng, Y., Xu, C., Zhang, X., Wu, Q., & Liu, J. (2021). Synergistic Effect of Alkali Na and K Promoter on Fe-Co-Cu-Al Catalysts for CO2 Hydrogenation to Light Hydrocarbons. Catalysts, 11(6), 735. https://doi.org/10.3390/catal11060735