Experimental Study on Sulfur Deactivation and Regeneration of Ni-Based Catalyst in Dry Reforming of Biogas
Abstract
:1. Introduction
2. Thermodynamic Background
2.1. Dry Reforming of Biogas
2.2. Sulfur Chemisorption on Nickel
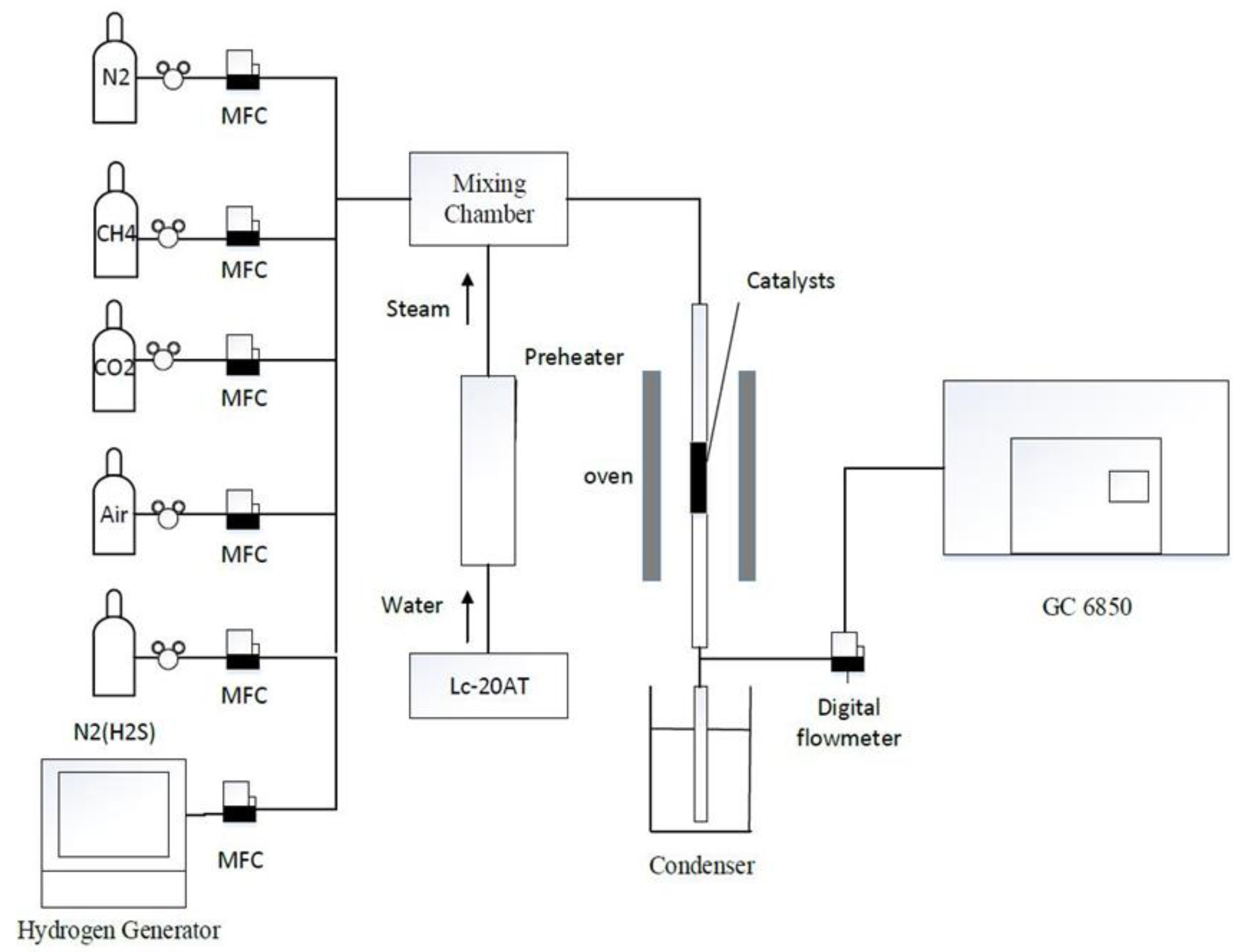
3. Experimental
4. Results and Discussion
4.1. Experimental Parameters
4.2. DRM without H2S
4.3. DRM with H2S
4.4. Bi-Reforming of Methane with H2S
4.5. Catalyst Regeneration
5. Conclusions
- (1)
- The catalyst poison depends on both the reaction temperature and time. The H2S coverage onto the catalyst surface decreases with the increased reaction temperature.
- (2)
- Due to the stronger chemisorption of sulfur onto the catalyst as compared to O2 or H2O, catalyst deactivation cannot be regenerated by the bi-reforming of methane in which DRM is combined with POM, COM, or SRM.
- (3)
- The catalyst cannot be regenerated for the poison that occurs at low temperatures.
- (4)
- The poisoned catalyst can be effectively regenerated using a high-temperature oxidation process. A higher reaction time is required for the catalyst regenerated by the high-temperature steam process.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadhwani, N.; Adhikari, S.; Eden, M. Biomass Gasification Using Carbon Dioxide: Effect of Temperature, CO2/C Ratio, and the Study of Reactions Influencing the Process. Ind. Eng. Chem. Res. 2016, 55, 2883–2891. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, S.-C. Biogas partial oxidation in a heat recirculation reactor for syngas production and CO2 utilization. Appl. Energy 2018, 217, 113–125. [Google Scholar] [CrossRef]
- Hernández, B.; Martín, M. Optimal Process Operation for Biogas Reforming to Methanol: Effects of Dry Reforming and Biogas Composition. Ind. Eng. Chem. Res. 2016, 55, 6677–6685. [Google Scholar] [CrossRef]
- Kang, J.Y.; Kang, D.W.; Kim, T.S.; Hur, K.B. Comparative economic analysis of gas turbine-based power generation and combined heat and power systems using biogas fuel. Energy 2014, 67, 309–318. [Google Scholar] [CrossRef]
- Tamoor, M.; Tahir, M.S.; Sagir, M.; Iqbal, S.; Nawaz, T. Design of 3 kW integrated power generation system from solar and biogas. Int. J. Hydrogen Energy 2020, 45, 12711–12720. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A review of recent developments in hydrogen production via biogas dry reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Zhao, X.; Joseph, B.; Kuhn, J.; Ozcan, S. Biogas Reforming to Syngas: A Review. iScience 2020, 23, 101082. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, J.; Moon, D.H.; Kim, K.-H.; Kwon, E.E. Upgrading biogas into syngas through dry reforming. Renew. Sustain. Energy Rev. 2021, 143, 110949. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, M.-R.; Lu, J.-J.; Chao, Y.; Leu, T.-S. Thermodynamic analysis of hydrogen production from methane via autothermal reforming and partial oxidation followed by water gas shift reaction. Int. J. Hydrogen Energy 2010, 35, 11787–11797. [Google Scholar] [CrossRef]
- Cañete, B.; Gigola, C.E.; Brignole, N.B. Synthesis Gas Processes for Methanol Production via CH4Reforming with CO2, H2O, and O2. Ind. Eng. Chem. Res. 2014, 53, 7103–7112. [Google Scholar] [CrossRef]
- James, O.O.; Mesubi, A.M.; Ako, T.C.; Maity, S. Increasing carbon utilization in Fischer–Tropsch synthesis using H2-deficient or CO2-rich syngas feeds. Fuel Process. Technol. 2010, 91, 136–144. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Chen, M.; Liang, D.; Yang, Z.; Cheng, W.; Tang, Z.; Wang, J.; Zhang, H. Recent advances during CH4 dry reforming for syngas production: A mini review. Int. J. Hydrogen Energy 2021, 46, 5852–5874. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145. [Google Scholar] [CrossRef] [Green Version]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Chein, R.; Yang, Z. H2S effect on dry reforming of biogas for syngas production. Int. J. Energy Res. 2019, 43, 3330–3345. [Google Scholar] [CrossRef]
- Jablonski, W.S.; Villano, S.M.; Dean, A.M. A comparison of H2S, SO2, and COS poisoning on Ni/YSZ and Ni/K2O-CaAl2O4 during methane steam and dry reforming. Appl. Catal. A Gen. 2015, 502, 399–409. [Google Scholar] [CrossRef] [Green Version]
- Rostrup-Nielsen, J.; Christiansen, L.J. Concepts in Syngas Manufacture; World Scientific: Singapore, 2011. [Google Scholar]
- Izquierdo, U.; García-García, I.; Gutierrez, Á.M.; Arraibi, J.R.; Barrio, V.L.; Cambra, J.F.; Arias, P.L. Catalyst Deactivation and Regeneration Processes in Biogas Tri-Reforming Process. The Effect of Hydrogen Sulfide Addition. Catalysts 2018, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Wachter, P.; Gaber, C.; Raic, J.; Demuth, M.; Hochenauer, C. Experimental investigation on H2S and SO2 sulphur poisoning and regeneration of a commercially available Ni-catalyst during methane tri-reforming. Int. J. Hydrogen Energy 2021, 46, 3437–3452. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q.; Millar, G.J. Carbon Dioxide Reforming of Methane To Produce Synthesis Gas over Metal-Supported Catalysts: State of the Art. Energy Fuels 1996, 10, 896–904. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, C.-Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Fidalgo, B.; Muradov, N.; Menéndez, J.A. Effect of H2S on carbon-catalyzed methane decomposition and CO2 reforming reactions. Int. J. Hydrogen Energy 2012, 37, 14187–14194. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. New aspects of syngas production and use. Catal. Today 2000, 63, 159–164. [Google Scholar] [CrossRef]
- Bartholomew, C.; Agrawal, P.; Katzer, J. Sulfur Poisoning of Metals. Adv. Catal. 1982, 31, 135–242. [Google Scholar]
- Chein, R.-Y.; Fung, W.-Y. Syngas production via dry reforming of methane over CeO2 modified Ni/Al2O3 catalysts. Int. J. Hydrogen Energy 2019, 44, 14303–14315. [Google Scholar] [CrossRef]
- Ashrafi, M.; Pfeifer, C.; Pröll, T.; Hofbauer, H. Experimental Study of Model Biogas Catalytic Steam Reforming: 2. Impact of Sulfur on the Deactivation and Regeneration of Ni-Based Catalysts. Energy Fuels 2008, 22, 4190–4195. [Google Scholar] [CrossRef]
- Yang, X. An experimental investigation on the deactivation and regeneration of a steam reforming catalyst. Renew. Energy 2017, 112, 17–24. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, J.; Yan, F.; Li, K.; Tian, S.; Gao, Y.; Zhou, H. Dry Reforming of Model Biogas on a Ni/SiO2 Catalyst: Overall Performance and Mechanisms of Sulfur Poisoning and Regeneration. ACS Sustain. Chem. Eng. 2017, 5, 10248–10257. [Google Scholar] [CrossRef]
- Kalai, D.Y.; Stangeland, K.; Tucho, W.M.; Jin, Y.; Yu, Z. Biogas reforming on hydrotalcite-derived Ni-Mg-Al catalysts: The effect of Ni loading and Ce promotion. J. CO2 Util. 2019, 33, 189–200. [Google Scholar] [CrossRef]
- Niu, J.; Liland, S.E.; Yang, J.; Rout, K.R.; Ran, J.; Chen, D. Effect of oxide additives on the hydrotalcite derived Ni catalysts for CO2 reforming of methane. Chem. Eng. J. 2019, 377, 119763. [Google Scholar] [CrossRef]
- Newnham, J.; Mantri, K.; Amin, M.H.; Tardio, J.; Bhargava, S.K. Highly stable and active Ni-mesoporous alumina catalysts for dry reforming of methane. Int. J. Hydrogen Energy 2012, 37, 1454–1464. [Google Scholar] [CrossRef]
- Appari, S.; Janardhanan, V.M.; Bauri, R.; Jayanti, S. Deactivation and regeneration of Ni catalyst during steam reforming of model biogas: An experimental investigation. Int. J. Hydrogen Energy 2014, 39, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Chattanathan, S.A.; Adhikari, S.; McVey, M.; Fasina, O. Hydrogen production from biogas reforming and the effect of H2S on CH4 conversion. Int. J. Hydrogen Energy 2014, 39, 19905–19911. [Google Scholar] [CrossRef]
- Dou, X.; Veksha, A.; Chan, W.P.; Oh, W.-D.; Liang, Y.N.; Teoh, F.; Mohamed, D.K.B.; Giannis, A.; Lisak, G.; Lim, T.-T. Poisoning effects of H2S and HCl on the naphthalene steam reforming and water-gas shift activities of Ni and Fe catalysts. Fuel 2019, 241, 1008–1018. [Google Scholar] [CrossRef]
- Chein, R.; Chen, Y.; Yu, C.; Chung, J. Thermodynamic analysis of dry reforming of CH4 with CO2 at high pressures. J. Nat. Gas Sci. Eng. 2015, 26, 617–629. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Shojaee, M.; Spivey, J. Catalytic bi-reforming of methane: From greenhouse gases to syngas. Curr. Opin. Chem. Eng. 2015, 9, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Olah, G.A.; Goeppert, A.; Czaun, M.; Prakash, G.K.S. Bi-reforming of Methane from Any Source with Steam and Carbon Dioxide Exclusively to Metgas (CO–2H2) for Methanol and Hydrocarbon Synthesis. J. Am. Chem. Soc. 2013, 135, 648–650. [Google Scholar] [CrossRef]
- Pawar, V.; Appari, S.; Monder, D.S.; Janardhanan, V.M. Study of the Combined Deactivation Due to Sulfur Poisoning and Carbon Deposition during Biogas Dry Reforming on Supported Ni Catalyst. Ind. Eng. Chem. Res. 2017, 56, 8448–8455. [Google Scholar] [CrossRef]
- Sehested, J. Four challenges for nickel steam-reforming catalysts. Catal. Today 2006, 111, 103–110. [Google Scholar] [CrossRef]
- Li, L.; Howard, C.; King, D.L.; Gerber, M.; Dagle, R.; Stevens, D. Regeneration of Sulfur Deactivated Ni-Based Biomass Syngas Cleaning Catalysts. Ind. Eng. Chem. Res. 2010, 49, 10144–10148. [Google Scholar] [CrossRef]
- Lombard, C.; Ledoze, S.; Marencak, E.; Marquaire, P.; Lenoc, D.; Bertrand, G.; Lapicque, F. In situ regeneration of the Ni-based catalytic reformer of a 5kW PEMFC system. Int. J. Hydrogen Energy 2006, 31, 437–440. [Google Scholar] [CrossRef]








| Case | Molar Ratio | CH4 (sccm) | CO2 (sccm) | N2 (sccm) |
|---|---|---|---|---|
| 1 | CH4/CO2 = 1/0.5 | 15 | 7.5 | 27.5 |
| 2 | CH4/CO2 = 1/1 | 15 | 15 | 20 |
| 3 | CH4/CO2= 1/2 | 15 | 30 | 5 |
| Present Study | Ref [29] | Ref [30] | Ref [31] | |||||
|---|---|---|---|---|---|---|---|---|
| Temperature | XCH4 | XCO2 | XCH4 | XCO2 | XCH4 | XCO2 | XCH4 | XCO2 |
| 700 °C | 55% | 68% | 53% | 68% | 53% | 64% | 56% | 70% |
| 800 °C | 78% | 91% | 82% | 88% | 80% | 85% | 80% | 89% |
| 900 °C | 88% | 96% | N/A | N/A | N/A | N/A | 94% | 98% |
| Regeneration | Detail Conditions |
|---|---|
| High-temperature reaction | T = 700~900 °C. The catalyst was poisoned from 0 to 7 h, followed by a H2S-free DRM test for 8 h. |
| high-temperature steam | T = 800 °C. The catalyst was poisoned from 0 to 12 h, regenerated by 10 sccm steam from 12 to 14 h to form NiO, reduced NiO to Ni by 20 sccm H2 from 14 to 16 h, and DRM-tested using regenerated catalyst from 16 to 33 h. |
| High-temperature oxidation | T = 800 °C. The catalyst was poisoned from 0 to 12 h, regenerated by 10 sccm air from 12 to 14 h to form NiO, reduced NiO to Ni by 20 sccm H2 from 14 to 16 h, and DRM test using regenerated catalyst from 16 to 33 h. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chein, R.-Y.; Chen, Y.-C.; Chen, W.-H. Experimental Study on Sulfur Deactivation and Regeneration of Ni-Based Catalyst in Dry Reforming of Biogas. Catalysts 2021, 11, 777. https://doi.org/10.3390/catal11070777
Chein R-Y, Chen Y-C, Chen W-H. Experimental Study on Sulfur Deactivation and Regeneration of Ni-Based Catalyst in Dry Reforming of Biogas. Catalysts. 2021; 11(7):777. https://doi.org/10.3390/catal11070777
Chicago/Turabian StyleChein, Rei-Yu, Yen-Chung Chen, and Wei-Hsin Chen. 2021. "Experimental Study on Sulfur Deactivation and Regeneration of Ni-Based Catalyst in Dry Reforming of Biogas" Catalysts 11, no. 7: 777. https://doi.org/10.3390/catal11070777








