The Effect of ZrO2 as Different Components of Ni-Based Catalysts for CO2 Reforming of Methane and Combined Steam and CO2 Reforming of Methane on Catalytic Performance with Coke Formation
Abstract
:1. Introduction
2. Results and Discussion
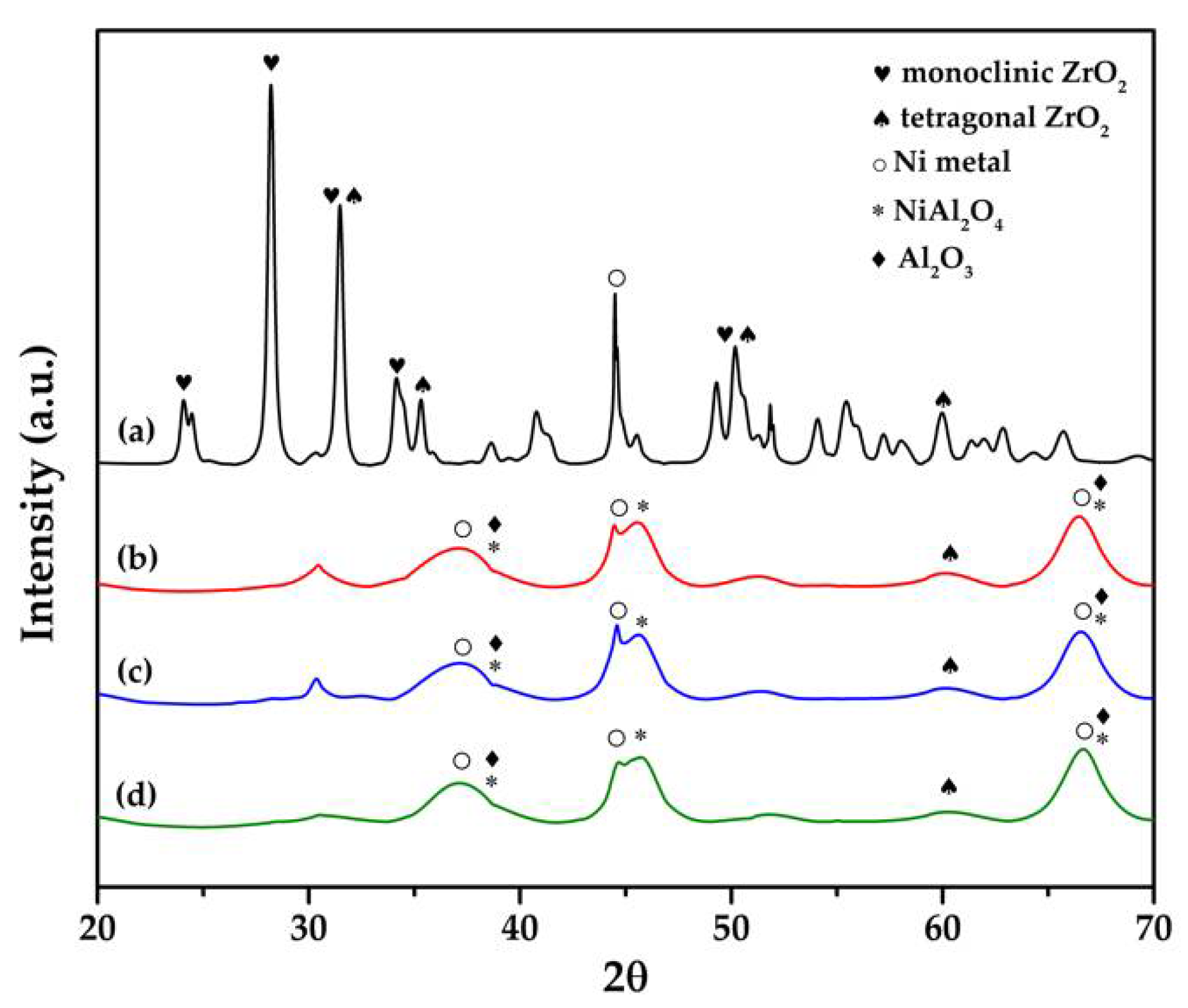
2.1. XRD Analysis and H2-Temperature Programmed Desorption
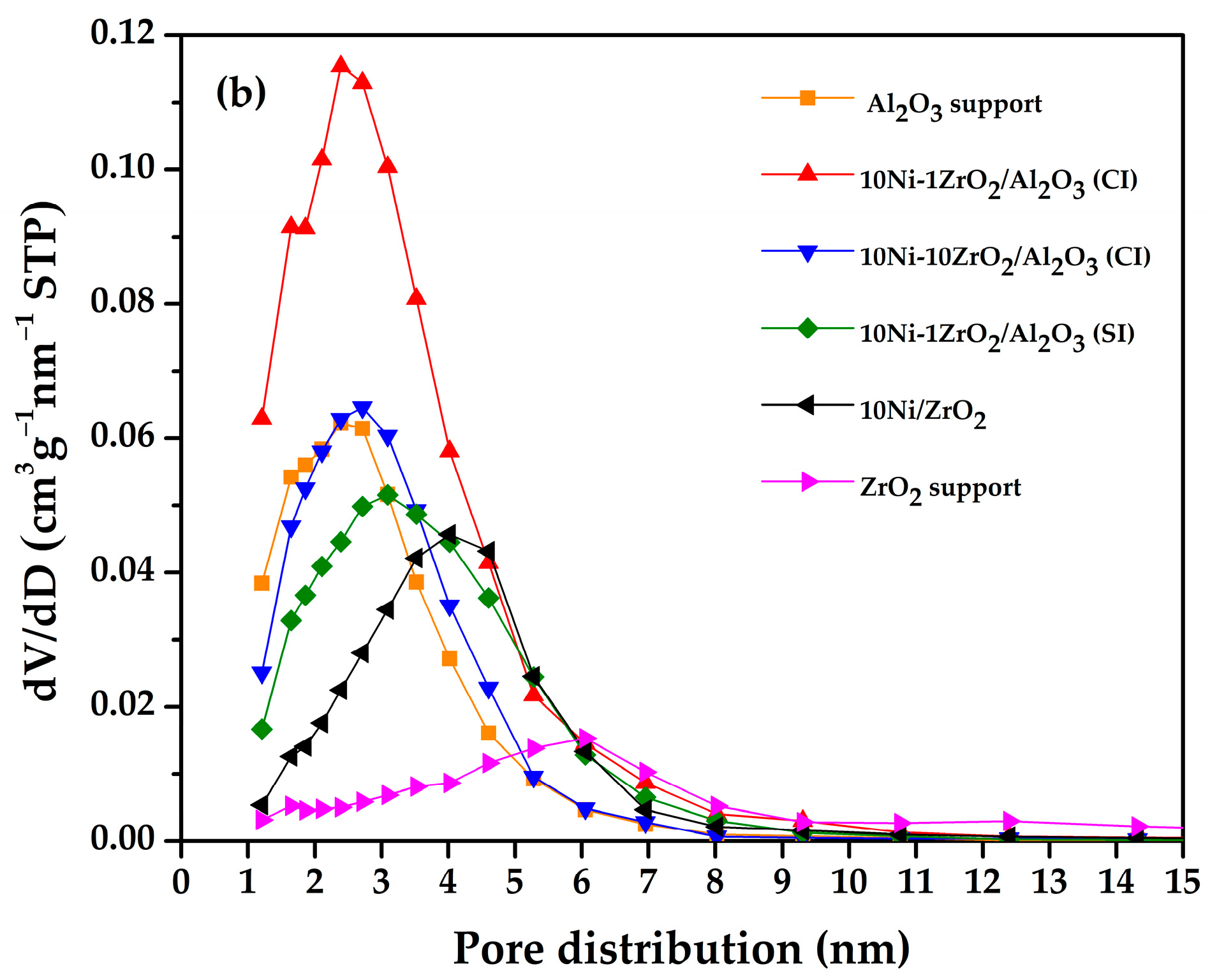
2.2. N2 Adsorption-Desorption
2.3. H2-Temperature Programmed Reduction
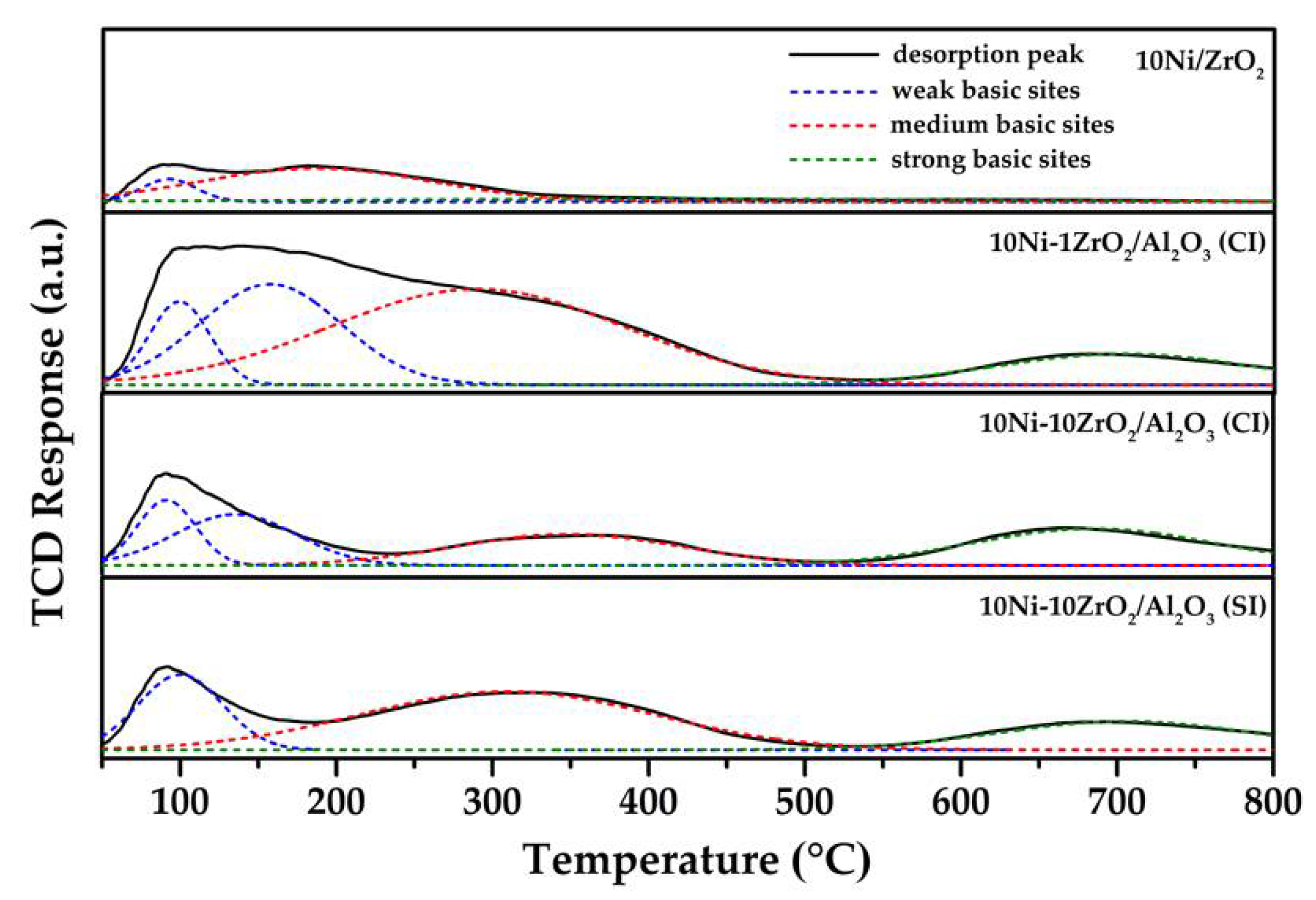
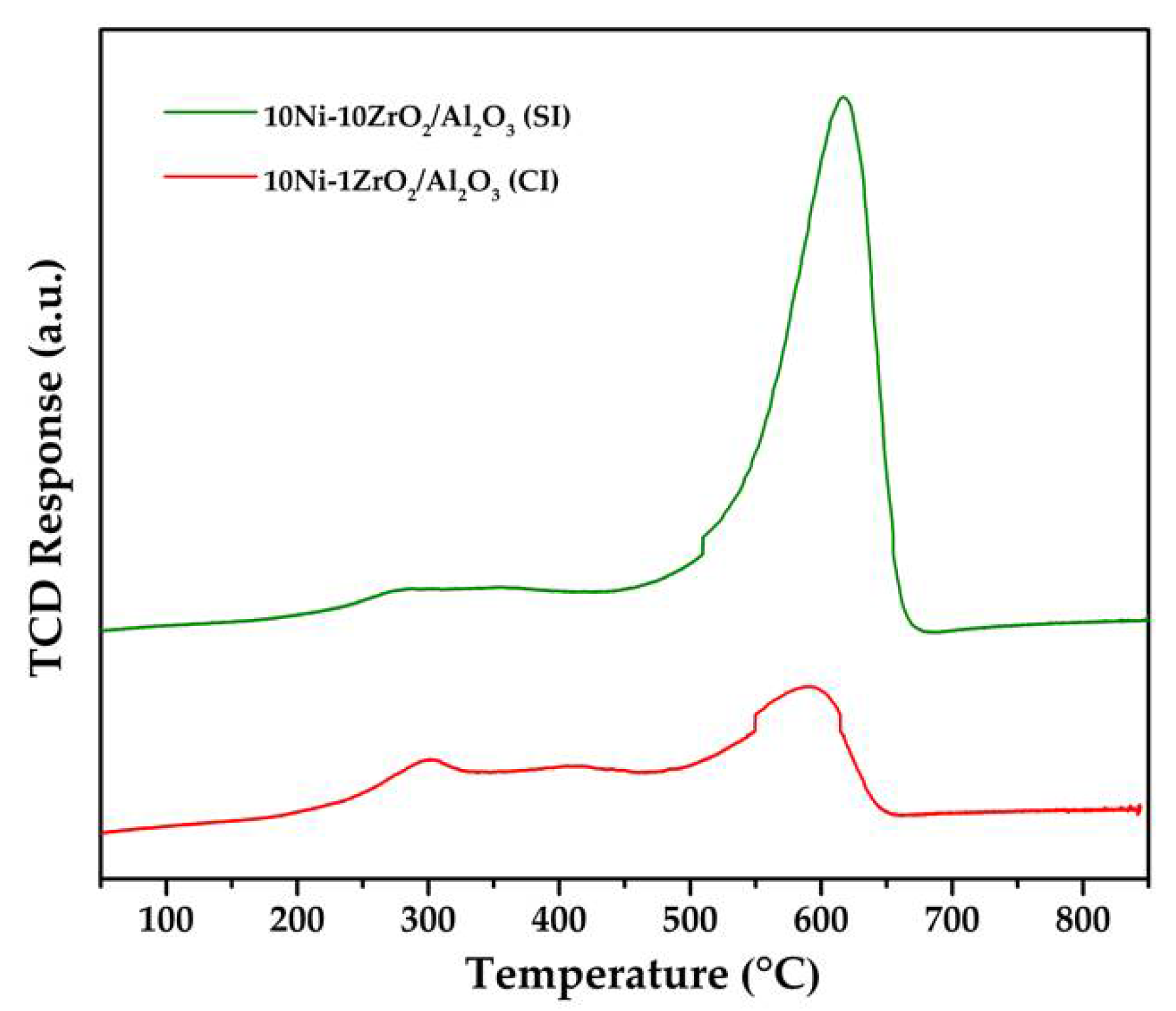
2.4. CO2-Temperature Programmed Desorption
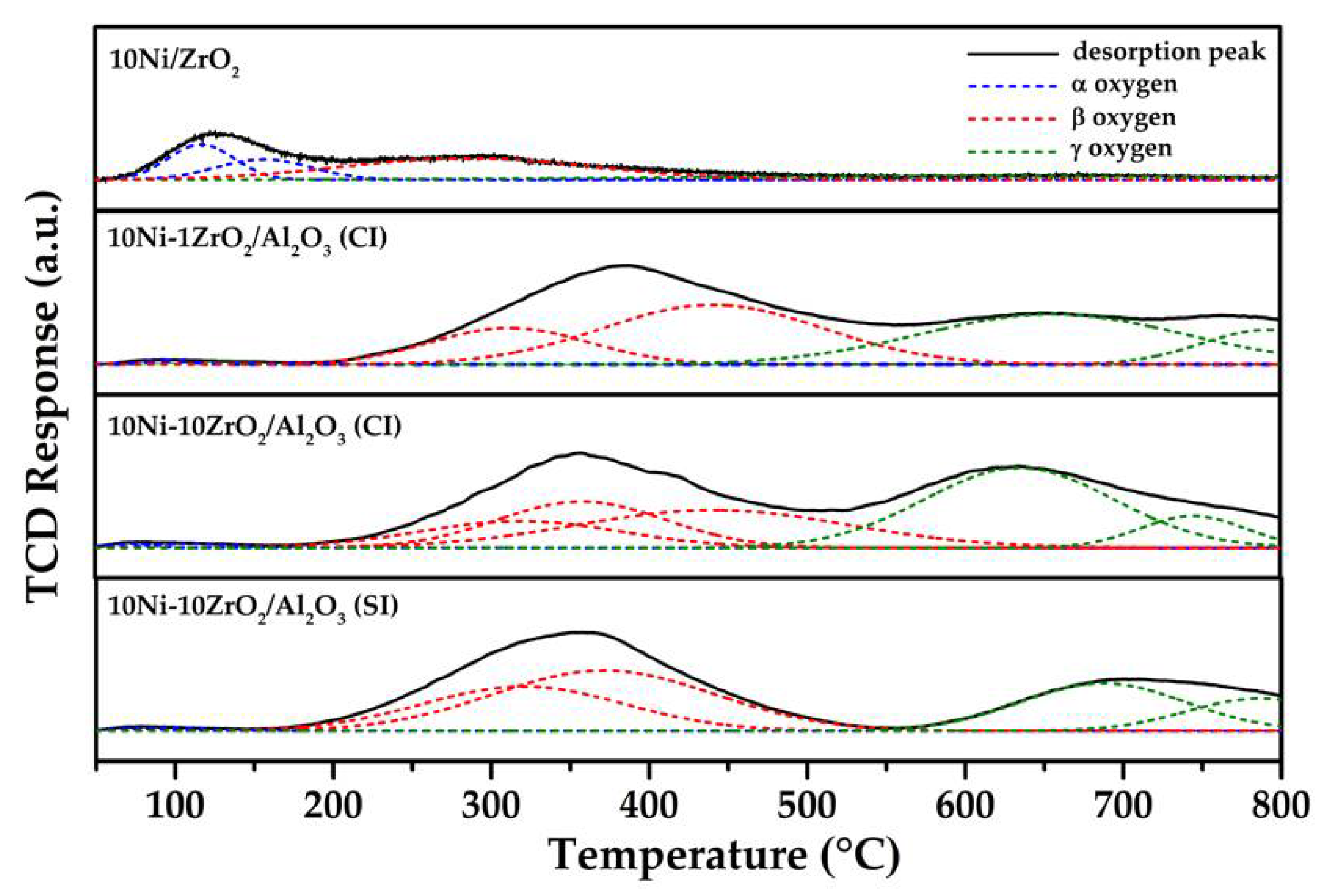
2.5. O2-Temperature Programmed Desorption
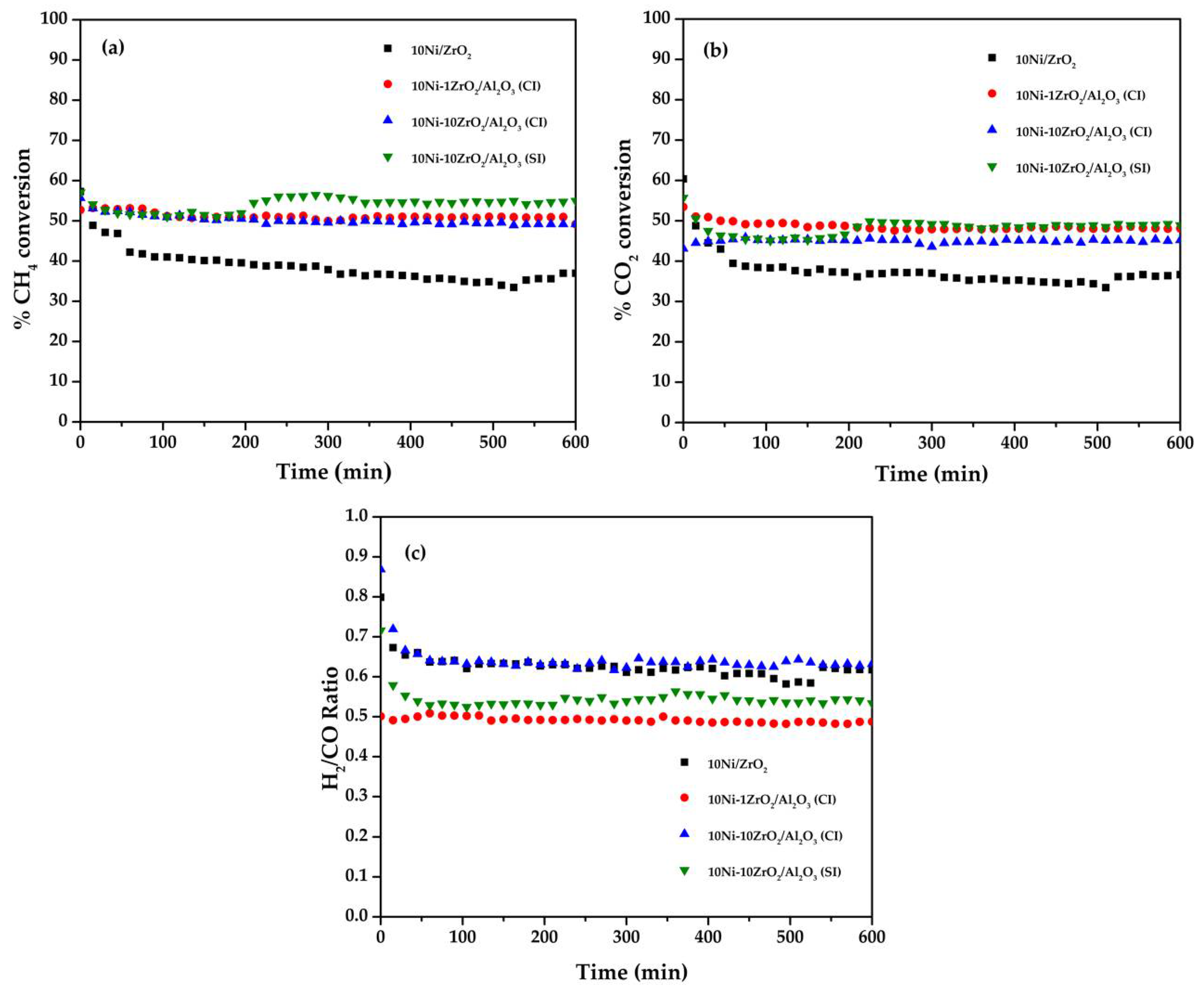
2.6. Catalytic Activity
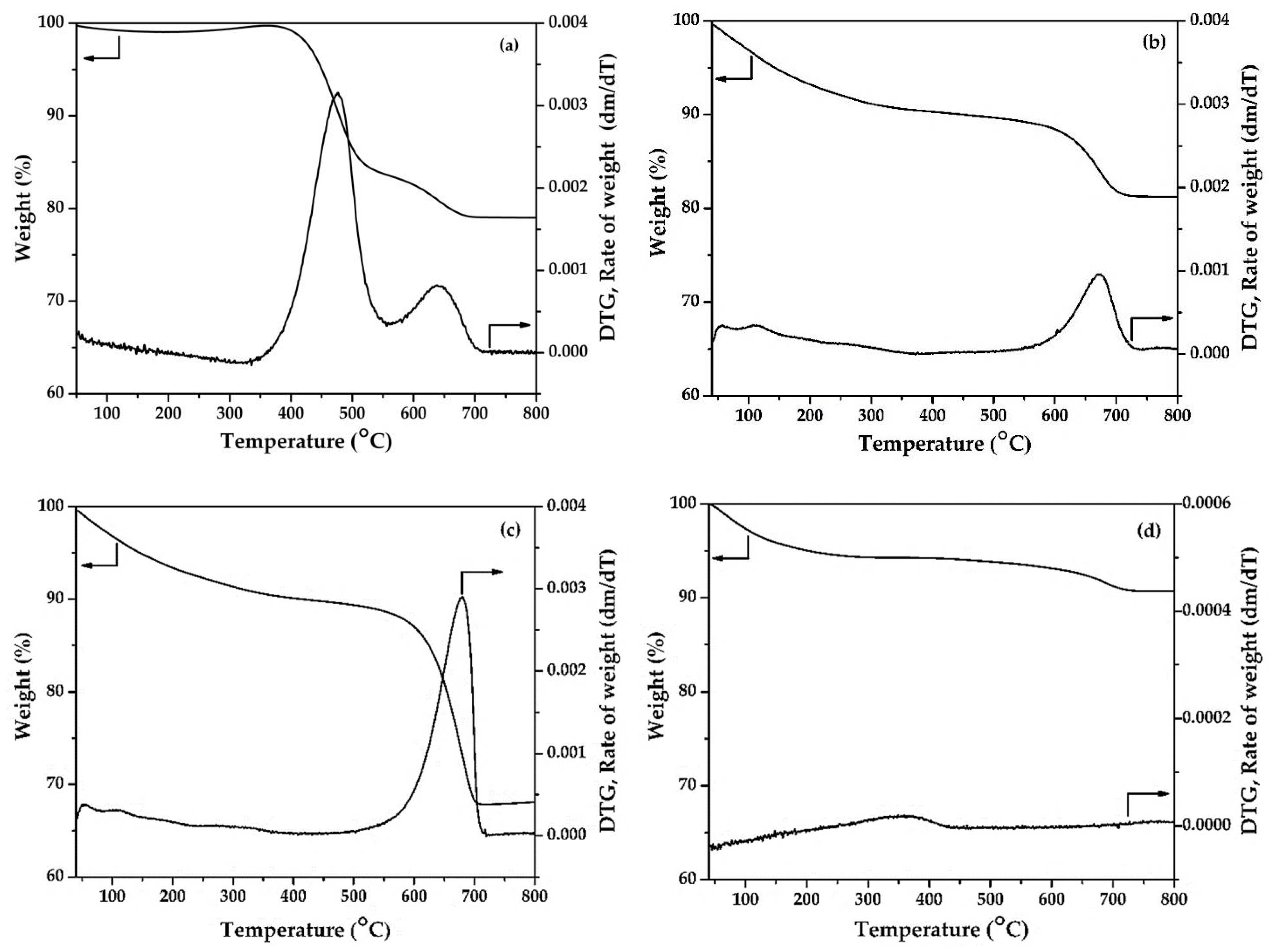
2.7. Coke Deposition
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalysts Characterization
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shamskar, F.R.; Rezaei, M.; Meshkani, F. The influence of Ni loading on the activity and coke formation of ultrasound-assisted co-precipitated Ni–Al2O3 nanocatalyst in dry reforming of methane. Int. J. Hydrogen Energy 2017, 42, 4155–4164. [Google Scholar] [CrossRef] [Green Version]
- Şener, A.N.; Günay, M.E.; Leba, A.; Yıldırım, R. Statistical review of dry reforming of methane literature using decision tree and artificial neural network analysis. Catal. Today 2018, 299, 289–302. [Google Scholar] [CrossRef]
- Kong, W.; Fu, Y.; Guan, C.; Pan, B.; Yuan, C.; Li, S.; Cai, F.; Zhang, J.; Sun, Y. Facile Synthesis of Highly Coking-Resistant and Active Nickel-Based Catalyst for Low-Temperature CO2 Reforming of Methane. Energy Technol. 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Tathod, A.P.; Hayek, N.; Shpasser, D.; Simakov, D.S.A.; Gazit, O.M. Mediating interaction strength between nickel and zirconia using a mixed oxide nanosheets interlayer for methane dry reforming. Appl. Catal. B Environ. 2019, 249, 106–115. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Hamid, M.Y.S. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Niu, J.; Liland, S.E.; Yang, J.; Rout, K.R.; Ran, J.; Chen, D. Effect of oxide additives on the hydrotalcite derived Ni catalysts for CO2 reforming of methane. Chem. Eng. J. 2019, 377, 119763. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Czaun, M.; Mathew, T.; May, R.B.; Prakash, G.K.S. Single Step Bi-reforming and Oxidative Bi-reforming of Methane (Natural Gas) with Steam and Carbon Dioxide to Metgas (CO-2H2) for Methanol Synthesis: Self-Sufficient Effective and Exclusive Oxygenation of Methane to Methanol with Oxygen. J. Am. Chem. Soc. 2015, 137, 8720–8729. [Google Scholar] [CrossRef] [PubMed]
- Stroud, T. Chemical CO2 recycling via dry and bi reforming of methane using Ni-Sn/Al2O3 and Ni-Sn/CeO2-Al2O3 catalysts. Appl. Catal. B Environ. 2017, 224, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Tillmann, L.; Schulwitz, J.; Van Veen, A.; Muhler, M. Dry Reforming of Methane at High Pressure in a Fixed-Bed Reactor with Axial Temperature Profile Determination. Catal. Lett. 2018, 148, 2256–2262. [Google Scholar] [CrossRef] [Green Version]
- Arbag, H.; Tasdemir, H.M.; Yagizatli, Y.; Kucuker, M.; Yasyerli, S. Effect of Preparation Technique on the Performance of Ni and Ce Incorporated Modified Alumina Catalysts in CO2 Reforming of Methane. Catal. Lett. 2020, 150, 3256–3268. [Google Scholar] [CrossRef]
- Jang, W.J. Combined steam and carbon dioxide reforming of methane and side reactions: Thermodynamic equilibrium analysis and experimental application. Appl. Energy. 2016, 173, 80–91. [Google Scholar] [CrossRef]
- Whang, H.S.; Lim, J.; Choi, M.S.; Lee, J.; Lee, H. Heterogeneous catalysts for catalytic CO2 conversion into value-added chemicals. BMC Chem. Eng. 2019, 1, 9. [Google Scholar] [CrossRef]
- Kumar, N.; Shojaee, M.; Spivey, J.J. Catalytic bi-reforming of methane: From greenhouse gases to syngas. Curr. Opin. Chem. Eng. 2015, 9, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Batebi, D.; Abedini, R.; Mosayebi, A. Combined steam and CO2 reforming of methane (CSCRM) over Ni–Pd/Al2O3 catalyst for syngas formation. Int. J. Hydrogen Energy 2020, 45, 14293–14310. [Google Scholar] [CrossRef]
- Sangsong, S.; Ratana, T.; Tungkamani, S.; Sornchamni, T.; Phongaksorn, M.; Croiset, E. The Demonstration of the Superiority of the Dual Ni-Based Catalytic System for the Adjustment of the H2/CO Ratio in Syngas for Green Fuel Technologies. Catalysts 2020, 10, 1056. [Google Scholar] [CrossRef]
- Zhao, Z.; Ren, P.; Li, W.; Miao, B. Effect of mineralizers for preparing ZrO2 support on the supported Ni catalyst for steam-CO2 bi-reforming of methane. Int. J. Hydrogen Energy 2017, 42, 6598–6609. [Google Scholar] [CrossRef]
- Chawl, S.K.; George, M.; Patel, F.; Patel, S. Production of Synthesis Gas by Carbon Dioxide Reforming of Methane over Nickel based and Perovskite Catalysts. Procedia Eng. 2013, 51, 461–466. [Google Scholar] [CrossRef]
- Charisiou, N.; Papageridis, K.; Siakavelas, G.; Sebastian, V.; Hinder, S.; Baker, M.; Polychronopoulou, K.; Goula, M. The influence of SiO2 doping on the Ni/ZrO2 supported catalyst for hydrogen production through the glycerol steam reforming reaction. Catal. Today 2019, 319, 206–219. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liu, C.; Zhang, X.; Wang, W.; Wang, B.; Ma, X. Effect of ZrO2 on Catalyst Structure and Catalytic Sulfur-Resistant Methanation Performance of MoO3/ZrO2–Al2O3 Catalysts. Kinet. Catal. 2018, 59, 481–488. [Google Scholar] [CrossRef]
- Cai, M.; Wen, J.; Chu, W.; Cheng, X.; Li, Z. Methanation of carbon dioxide on Ni/ZrO2-Al2O3 catalysts: Effects of ZrO2 promoter and preparation method of novel ZrO2-Al2O3 carrier. J. Nat. Gas Chem. 2011, 20, 318–324. [Google Scholar] [CrossRef]
- Guo, C.; Wu, Y.; Qin, H.; Zhang, J. CO methanation over ZrO2/Al2O3 supported Ni catalysts: A comprehensive study. Fuel Process. Technol. 2014, 124, 61–69. [Google Scholar] [CrossRef]
- Liu, Q.; Gu, F.; Gao, J.; Li, H.; Xu, G.; Su, F. Coking-resistant Ni-ZrO2/Al2O3 catalyst for CO methanation. J. Energy Chem. 2014, 23, 761–770. [Google Scholar] [CrossRef]
- Therdthianwong, S.; Siangchin, C.; Therdthianwong, A. Improvement of coke resistance of Ni/Al2O3 catalyst in CH4/CO2 reforming by ZrO2 addition. Fuel Process. Technol. 2008, 89, 160–168. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J.; Lindo, M. Effect of Ce and Zr Addition to Ni/SiO2 Catalysts for Hydrogen Production through Ethanol Steam Reforming. Catalysts 2015, 5, 58–76. [Google Scholar] [CrossRef] [Green Version]
- Barroso-Quiroga, M.M.; Castro-Luna, A.E. Catalytic activity and effect of modifiers on Ni-based catalysts for the dry reforming of methane. Int. J. Hydrogen Energy 2010, 35, 6052–6056. [Google Scholar] [CrossRef]
- Fakeeha, A. Hydrogen production by partial oxidation reforming of methane over ni catalysts supported on high and low surface area Alumina and Zirconia. Processes 2020, 8, 499. [Google Scholar] [CrossRef]
- Lou, Y.; Steib, M.; Zhang, Q.; Tiefenbacher, K.; Horváth, A.; Jentys, A.; Liu, Y.; Lercher, J.A. Design of stable Ni/ZrO2 catalysts for dry reforming of methane. J. Catal. 2017, 356, 147–156. [Google Scholar] [CrossRef]
- Therdthianwong, S.; Therdthianwong, A.; Siangchin, C.; Yongprapat, S. Synthesis gas production from dry reforming of methane over Ni/Al2O3 stabilized by ZrO2. Int. J. Hydrogen Energy 2008, 33, 991–999. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Cho, K.M.; Park, S.; Lee, S.H.; Lee, J.; Song, I.K. Effect of Al2O3-ZrO2 xerogel support on hydrogen production by steam reforming of LNG over Ni/Al2O3-ZrO2 catalyst. Korean J. Chem. Eng. 2008, 25, 41–45. [Google Scholar] [CrossRef]
- Roh, H.S.; Koo, K.Y.; Joshi, U.D.; Yoon, W.L. Combined H2O and CO2 reforming of methane over Ni-Ce-ZrO2 catalysts for gas to liquids (GTL). Catal. Lett. 2008, 125, 283–288. [Google Scholar] [CrossRef]
- Iglesias, I.; Forti, M.; Baronetti, G.; Mariño, F. Zr-enhanced stability of ceria based supports for methane steam reforming at severe reaction conditions. Int. J. Hydrogen Energy 2019, 44, 8121–8132. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Wachs, I.E. Catalysis by supported metal oxides. In Handbook of Surfaces and Interfaces of Materials; Academic Press: San Diego, CA, USA, 2001; Volume 1, pp. 613–648. [Google Scholar]
- Lanre, M.S.; Al-Fatesh, A.S.; Fakeeha, A.H.; Kasim, S.O.; Ibrahim, A.A.; Al-Awadi, A.S.; Al-Zahrani, A.A.; Abasaeed, A.E. Catalytic Performance of Lanthanum Promoted Ni/ZrO2 for Carbon Dioxide Reforming of Methane. Processes 2020, 8, 1502. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Arafat, Y.; Ibrahim, A.A.; Shaikh, H.; Atia, H.; Abasaeed, A.E.; Armbruster, U.; Al-Fatesh, A.S. Highly Selective Syngas/H2 Production via Partial Oxidation of CH4 Using (Ni, Co and Ni–Co)/Zr–Al2O3 Catalysts: Influence of Calcination Temperature. Processes 2019, 7, 141. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, S.P.; Patel, S. Dry Reforming of Methane over Supported Nickel Catalysts Promoted by Zerconia, Ceria and Magnesia. Int. J. Adv. Res. Eng. Technol. 2015, 6, 131–146. [Google Scholar]
- Santos, D.C.R.M.; Lisboa, J.S.; Passos, F.B.; Noronha, F.B. Characterization of steam-reforming catalysts. Braz. J. Chem. Eng. 2004, 21, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Sajjadi, S.M.; Haghighi, M.; Rahmani, F. Sol-gel synthesis and catalytic performance of Ni-Co/Al2O3-MgO-ZrO2 nanocatalyst with different ZrO2-loadings used in CH4/CO2 reforming for hydrogen production. Int. J. Oil Gas Coal Technol. 2014, 8, 304–324. [Google Scholar] [CrossRef]
- Zhou, L.; Li, L.; Wei, N.; Li, J.; Basset, J.-M. Effect of NiAl2O4 Formation on Ni/Al2O3 Stability during Dry Reforming of Methane. ChemCatChem 2015, 7, 2508–2516. [Google Scholar] [CrossRef] [Green Version]
- Rauta, P.R.; Manivasakan, P.; Rajendran, V.; Sahu, B.B.; Panda, B.K.; Mohapatra, P. Phase transformation of ZrO2 nanoparticles produced from zircon. Phase Transit. 2012, 85, 13–26. [Google Scholar] [CrossRef]
- Xu, Y.; Du, X.-H.; Li, J.; Wang, P.; Zhu, J.; Ge, F.-J.; Zhou, J.; Song, M.; Zhu, W.-Y. A comparison of Al2O3 and SiO2 supported Ni-based catalysts in their performance for the dry reforming of methane. J. Fuel Chem. Technol. 2019, 47, 199–208. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Zhang, X.; Wang, Z.; Wang, X.; Peng, H. Design of Ni-ZrO2@SiO2 catalyst with ultra-high sintering and coking resistance for dry reforming of methane to prepare syngas. J. CO2 Util. 2018, 27, 297–307. [Google Scholar] [CrossRef]
- Kauppi, E.I.; Honkala, K.; Krause, A.O.I.; Kanervo, J.M.; Lefferts, L. ZrO2 Acting as a Redox Catalyst. Top. Catal. 2016, 59, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Santamaria, L.; Arregi, A.; Alvarez, J.; Artetxe, M.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Performance of a Ni/ZrO2 catalyst in the steam reforming of the volatiles derived from biomass pyrolysis. J. Anal. Appl. Pyrolysis 2018, 136, 222–231. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Wu, Y.; Pan, J.; Zhang, Q.; Tan, Y.; Han, Y. Insight into the effects of the oxygen species over Ni/ZrO2 catalyst surface on methane reforming with carbon dioxide. Appl. Catal. B Environ. 2019, 244, 427–437. [Google Scholar] [CrossRef]
- Wang, X.; Bai, X.; Guo, Y.; Liu, Q.; Ji, S.; Wang, Z.J. A nanoscale Ni/ ZrO2 catalyst coated with Al2O3 for carbon dioxide reforming of methane. J. Chem. Technol. Biotechnol. 2021, 96, 474–480. [Google Scholar] [CrossRef]
- Sangsong, S.; Ratana, T.; Tungkamani, S.; Sornchamni, T.; Phongaksorn, M. Effect of CeO2 loading of the Ce-Al mixed oxide on ultrahigh temperature water-gas shift performance over Ce-Al mixed oxide supported Ni catalysts. Fuel 2019, 252, 488–495. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Nabgan, W.; Nabgan, B.; Abdullah, T.A.T.; Ngadi, N.; Jalil, A.A.; Hassan, N.S.; Izan, S.M.; Luing, W.S.; Abdullah, S.N.; Majeed, F.S.A. Conversion of polyethylene terephthalate plastic waste and phenol steam reforming to hydrogen and valuable liquid fuel: Synthesis effect of Ni–Co/ZrO2 nanostructured catalysts. Int. J. Hydrogen Energy 2020, 45, 6302–6317. [Google Scholar] [CrossRef]
- Vrijburg, W.L.; Van Helden, J.W.A.; Parastaev, A.; Groeneveld, E.; Pidko, E.A.; Hensen, E.J.M. Ceria–zirconia encapsulated Ni nanoparticles for CO2 methanation. Catal. Sci. Technol. 2019, 9, 5001–5010. [Google Scholar] [CrossRef] [Green Version]
- Dharmasaroja, N.; Ratana, T.; Tungkamani, S.; Sornchamni, T.; Simakov, S.A.; Phongaksorn, M. Catalysts and the Performance of the Ni/Al2O3-MgO Catalyst for the Combined Steam and CO2 Reforming of Methane Using Ultra-Low Steam to Carbon Ratio. Catalysts 2020, 10, 1450. [Google Scholar] [CrossRef]



| Samples | N2 Adsorption-Desorption | H2-TPD | ||
|---|---|---|---|---|
| Surface Area (m2·g−1) | Total Pore Volume (cm3·g−1) | Ni Dispersion (%) | Ni Particle Size (nm) | |
| Al2O3 support | 165 | 0.20 | - | - |
| ZrO2 support | 95 | 0.21 | - | - |
| 10Ni/ZrO2 | 75 | 0.16 | 3.0 | 21.5 |
| 10Ni-1ZrO2/Al2O3 (CI) | 153 | 0.21 | 8.1 | 7.8 |
| 10Ni-10ZrO2/Al2O3 (CI) | 134 | 0.20 | 7.1 | 8.9 |
| 10Ni-10ZrO2/Al2O3 (SI) | 112 | 0.20 | 10.0 | 6.3 |
| Samples | CO2-TPD Deconvolution (mmol·g−1) | O2-TPD Deconvolution (mmol·g−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Weak | Medium | Strong | Total Basicity | α Oxygen | β Oxygen | γ Oxygen | Total O2 Desorption | |
| 10Ni/ZrO2 | 0.058 | 0.042 | 0.007 | 0.107 | 0.151 | 0.020 | 0.005 | 0.176 |
| 10Ni-1ZrO2/Al2O3 (CI) | 0.191 | 0.225 | 0.034 | 0.450 | 0.009 | 0.260 | 0.028 | 0.297 |
| 10Ni-10ZrO2/Al2O3 (CI) | 0.081 | 0.046 | 0.047 | 0.174 | 0.008 | 0.215 | 0.121 | 0.344 |
| 10Ni-10ZrO2/Al2O3 (SI) | 0.069 | 0.112 | 0.030 | 0.211 | 0.017 | 0.316 | 0.096 | 0.429 |
| Catalyst | Preparation Method | Reactor Type | Operating Temperature | % Conversion | Ref. | |
|---|---|---|---|---|---|---|
| CH4 | CO2 | |||||
| 2.5%Ni nanosheet/ZrO2 | Wet impregnation | Fixed-bed quartz reactor | 800 °C | 46.0 | 60.0 | [4] |
| 10%Ni/m-ZrO2-Al2O3 | Evaporation-induced self-assembly | Fixed-bed quartz reactor | 700 °C | 60.2 | 78.0 | [45] |
| 15%Ni-7%ZrO2/Al2O3 | Sequential impregnation | Fixed-bed reactor | 700 °C | 42.5 | n/a | [28] |
| 3%Ni/ZrO2 treated with N2 | Deposition-precipitation | Fixed-bed quartz microreactor | 700 °C | 62.5 | 63.0 | [44] |
| 10%Ni-10%ZrO2/Al2O3 | Sequential impregnation | Fixed-bed reactor | 620 °C | 54.3 | 49.8 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumarasingha, W.; Supasitmongkol, S.; Phongaksorn, M. The Effect of ZrO2 as Different Components of Ni-Based Catalysts for CO2 Reforming of Methane and Combined Steam and CO2 Reforming of Methane on Catalytic Performance with Coke Formation. Catalysts 2021, 11, 984. https://doi.org/10.3390/catal11080984
Sumarasingha W, Supasitmongkol S, Phongaksorn M. The Effect of ZrO2 as Different Components of Ni-Based Catalysts for CO2 Reforming of Methane and Combined Steam and CO2 Reforming of Methane on Catalytic Performance with Coke Formation. Catalysts. 2021; 11(8):984. https://doi.org/10.3390/catal11080984
Chicago/Turabian StyleSumarasingha, Wassachol, Somsak Supasitmongkol, and Monrudee Phongaksorn. 2021. "The Effect of ZrO2 as Different Components of Ni-Based Catalysts for CO2 Reforming of Methane and Combined Steam and CO2 Reforming of Methane on Catalytic Performance with Coke Formation" Catalysts 11, no. 8: 984. https://doi.org/10.3390/catal11080984
APA StyleSumarasingha, W., Supasitmongkol, S., & Phongaksorn, M. (2021). The Effect of ZrO2 as Different Components of Ni-Based Catalysts for CO2 Reforming of Methane and Combined Steam and CO2 Reforming of Methane on Catalytic Performance with Coke Formation. Catalysts, 11(8), 984. https://doi.org/10.3390/catal11080984





