Agarose vs. Methacrylate as Material Supports for Enzyme Immobilization and Continuous Processing
Abstract
:1. Introduction
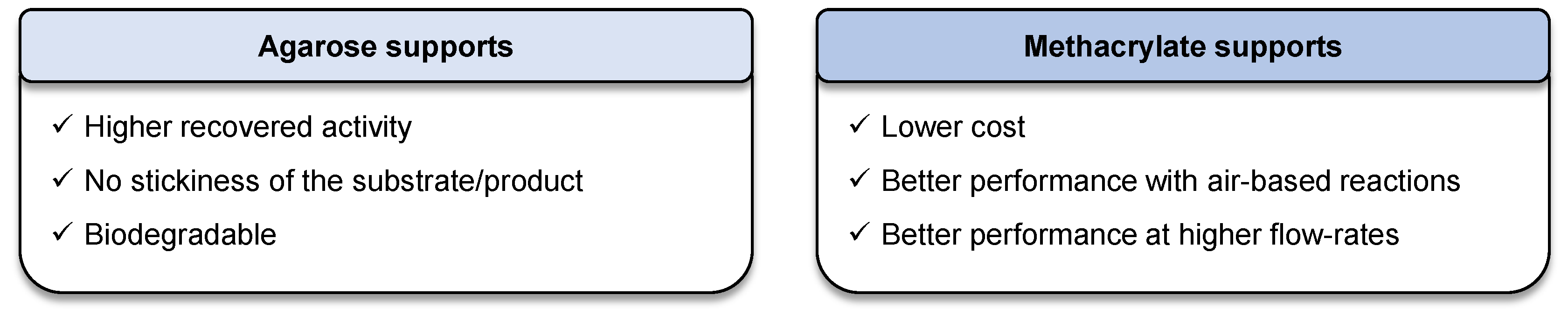
2. Results and Discussion
2.1. Comparison of Enzyme Activity upon Immobilization
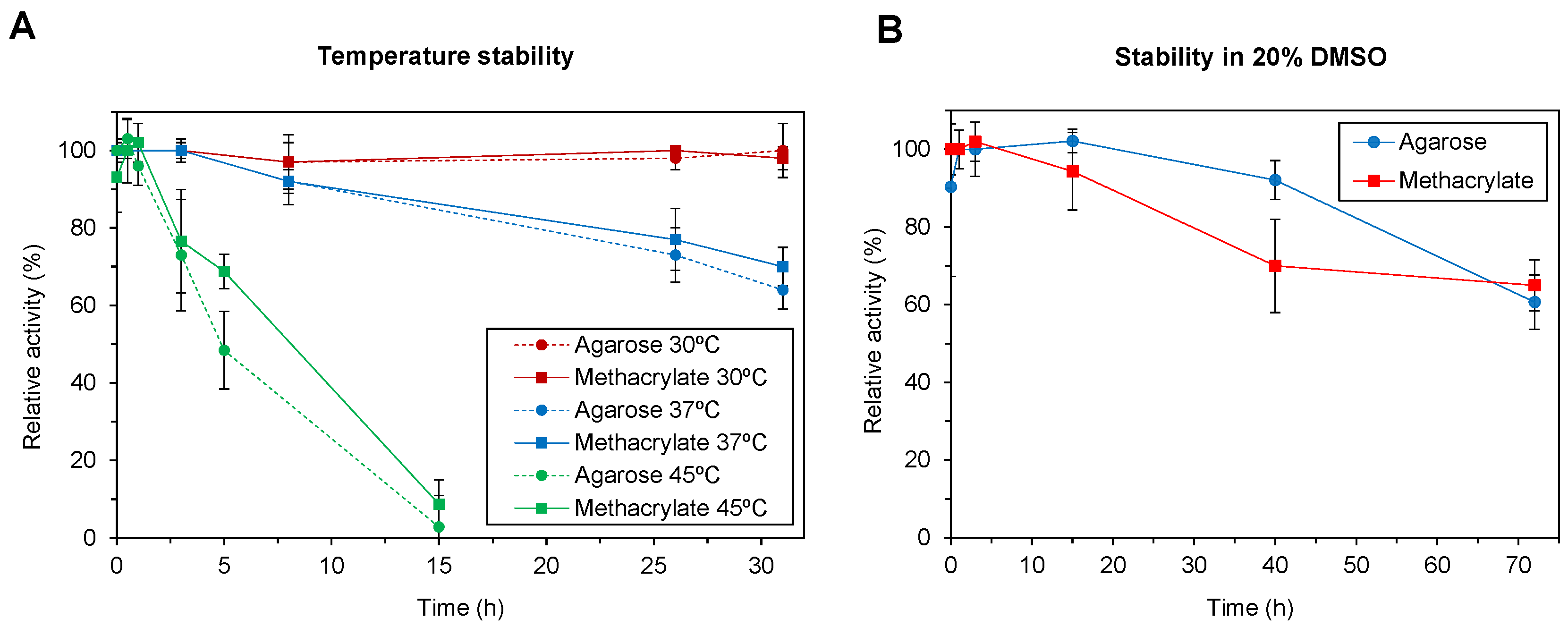
2.2. Stablity of the Immobilized Enzymes in Non-Natural Conditions
2.3. Stickiness of the Susbtrate/Product to the More Hydrophobic Support
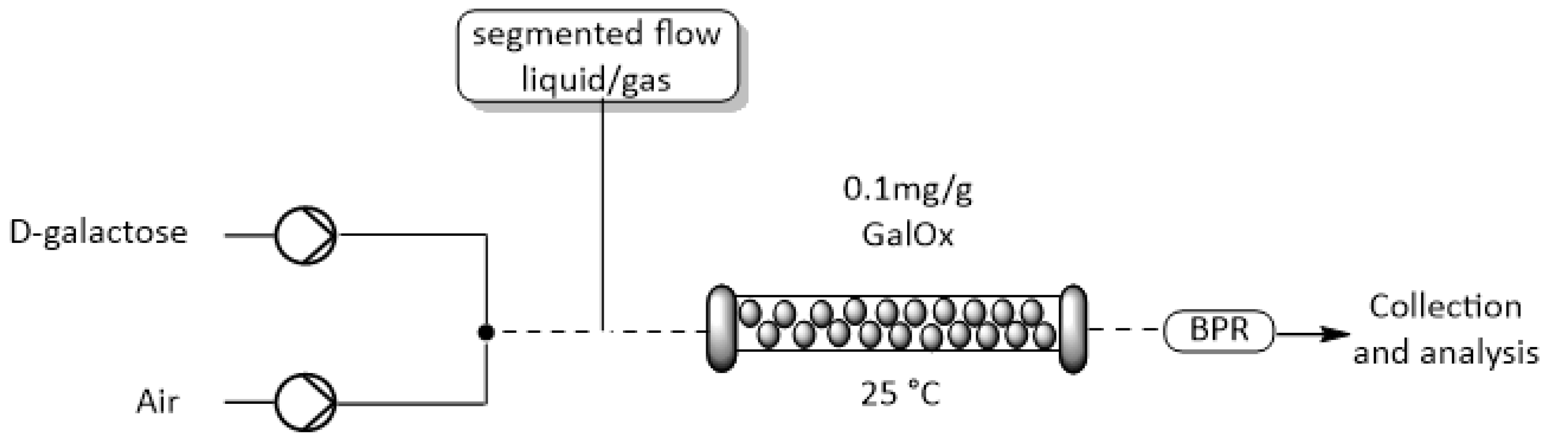
2.4. Integration of O2-Dependent Reactions into Flow Reactors
3. Materials and Methods
3.1. Materials
3.2. Protein Expression and Purification
3.3. Enzymatic Activity Assays
3.4. Enzyme Immobilization on Epoxy/Co2+-Supports
3.5. Enzyme Immobilization on Glyoxyl Supports
3.6. Enzyme Stability Test in DMSO
3.7. Enzyme Stability Test at 45 °C
3.8. Continuous Flow Reactions
3.9. HPLC Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagy, L.; Magyar, M.; Szabo, T.; Hajdu, K.; Giotta, L.; Dorogi, M.; Milano, F. Photosynthetic machineries in nano-systems. Curr. Protein Pept. Sci. 2014, 15, 363–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guisan, J.M.; López-Gallego, F.; Bolivar, J.M.; Rocha-Martín, J.; Fernandez-Lorente, G. The Science of Enzyme Immobilization. In Methods in Molecular Biology; Humana Press Inc.: New York, NY, USA, 2020; Volume 2100, pp. 1–26. [Google Scholar]
- Bommarius, A.S.; Paye, M.F. Stabilizing biocatalysts. Chem. Soc. Rev. 2013, 42, 6534–6565. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, E.A.; Dinu, Z.C. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Winkler, C.K.; Schrittwieser, J.H.; Kroutil, W. Power of Biocatalysis for Organic Synthesis. ACS Cent. Sci. 2021, 7, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Guisán, J.M.; Bolivar, J.M.; López-Gallego, F.; Rocha-Martín, J. Immobilization of Enzymes and Cells; Humana Press Inc.: New York, NY, USA, 2020; Volume 2100. [Google Scholar]
- Cao, L.; van Langen, L.; Sheldon, R.A. Immobilised enzymes: Carrier-bound or carrier-free? Curr. Opin. Biotechnol. 2003, 14, 387–394. [Google Scholar] [CrossRef]
- Romero-Fernández, M.; Paradisi, F. Protein immobilization technology for flow biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 1–8. [Google Scholar] [CrossRef]
- Benítez-Mateos, A.I.; Huber, C.; Nidetzky, B.; Bolivar, J.M.; López-Gallego, F. Design of the Enzyme–Carrier Interface to Overcome the O2 and NADH Mass Transfer Limitations of an Immobilized Flavin Oxidase. ACS Appl. Mater. Interfaces 2020, 12, 56027–56038. [Google Scholar] [CrossRef]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Roura Padrosa, D.; Benítez-Mateos, A.I.; Calvey, L.; Paradisi, F. Cell-free biocatalytic syntheses of l-pipecolic acid: A dual strategy approach and process intensification in flow. Green Chem. 2020, 22, 5310–5316. [Google Scholar] [CrossRef]
- Planchestainer, M.; Contente, M.L.; Cassidy, J.; Molinari, F.; Tamborini, L.; Paradisi, F. Continuous flow biocatalysis: Production and in-line purification of amines by immobilised transaminase from Halomonas elongata. Green Chem. 2017, 19, 372–375. [Google Scholar] [CrossRef]
- Roura Padrosa, D.; Nissar, Z.; Paradisi, F. Efficient Amino Donor Recycling in Amination Reactions: Development of a New Alanine Dehydrogenase in Continuous Flow and Dialysis Membrane Reactors. Catalysts 2021, 11, 520. [Google Scholar] [CrossRef]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for stabilization of enzymes in organic solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Pedroche, J.; del Mar Yust, M.; Mateo, C.; Fernández-Lafuente, R.; Girón-Calle, J.; Alaiz, M.; Vioque, J.; Guisán, J.M.; Millán, F. Effect of the support and experimental conditions in the intensity of the multipoint covalent attachment of proteins on glyoxyl-agarose supports: Correlation between enzyme-support linkages and thermal stability. Enzym. Microb. Technol. 2007, 40, 1160–1166. [Google Scholar] [CrossRef]
- Benítez-Mateos, A.I.; Contente, M.L.; Velasco-Lozano, S.; Paradisi, F.; López-Gallego, F. Self-Sufficient Flow-Biocatalysis by Coimmobilization of Pyridoxal 5′-Phosphate and ω-Transaminases onto Porous Carriers. ACS Sustain. Chem. Eng. 2018, 6, 13151–13159. [Google Scholar] [CrossRef]
- Contente, M.L.; Paradisi, F. Self-sustaining closed-loop multienzyme-mediated conversion of amines into alcohols in continuous reactions. Nat. Catal. 2018, 1, 452–459. [Google Scholar] [CrossRef]
- Contente, M.L.; Dall’Oglio, F.; Tamborini, L.; Molinari, F.; Paradisi, F. Highly Efficient Oxidation of Amines to Aldehydes with Flow-based Biocatalysis. ChemCatChem 2017, 9, 3843–3848. [Google Scholar] [CrossRef]
- Roura Padrosa, D.; Alaux, R.; Smith, P.; Dreveny, I.; López-Gallego, F.; Paradisi, F. Enhancing PLP-Binding Capacity of Class-III ω-Transaminase by Single Residue Substitution. Front. Bioeng. Biotechnol. 2019, 7, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegarty, E.; Paradisi, F. Implementation of biocatalysis in continuous flow for the synthesis of small cyclic amines. Chimia 2020, 74, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Wang, Z.W.; Yang, L.C.; Dong, J.L.; Chi, C.Q.; Sui, D.N.; Wang, Y.Z.; Ren, J.G.; Hung, M.Y.; Jiang, Y.Y. A novel efficient route for preparation of chiral β-hydroxycarboxylic acid: Asymmetric hydration of unsaturated carboxylic acids catalyzed by heterobimetallic complex wool-palladium-cobalt. J. Mol. Catal. A Chem. 2007, 264, 60–65. [Google Scholar] [CrossRef]
- Contente, M.L.; Pinto, A.; Molinari, F.; Paradisi, F. Biocatalytic N-Acylation of Amines in Water Using an Acyltransferase from Mycobacterium smegmatis. Adv. Synth. Catal. 2018, 360, 4814–4819. [Google Scholar] [CrossRef] [Green Version]
- Delgado, L.; Parker, M.; Fisk, I.; Paradisi, F. Performance of the extremophilic enzyme BglA in the hydrolysis of two aroma glucosides in a range of model and real wines and juices. Food Chem. 2020, 323, 126825. [Google Scholar] [CrossRef]
- Heckmann, C.M.; Gourlay, L.J.; Dominguez, B.; Paradisi, F. An (R)-Selective Transaminase From Thermomyces stellatus: Stabilizing the Tetrameric Form. Front. Bioeng. Biotechnol. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Cerioli, L.; Planchestainer, M.; Cassidy, J.; Tessaro, D.; Paradisi, F. Characterization of a novel amine transaminase from Halomonas elongata. J. Mol. Catal. B Enzym. 2015, 120, 141–150. [Google Scholar] [CrossRef]
- Mateo, C.; Fernández-Lorente, G.; Cortés, E.; Garcia, J.L.; Fernández-Lafuente, R.; Guisan, J.M. One-step purification, covalent immobilization, and additional stabilization of poly-His-tagged proteins using novel heterofunctional chelate-epoxy supports. Biotechnol. Bioeng. 2001, 76, 269–276. [Google Scholar] [CrossRef]
- Guisán, J.M. Aldehyde-agarose gels as activated supports for immobilization-stabilization of enzymes. Enzym. Microb. Technol. 1988, 10, 375–382. [Google Scholar] [CrossRef]

| Enzyme | Material Support | Recovered Activity 1 (%) | Results Source |
|---|---|---|---|
| Gs-Lys6DH | Agarose | 91 | Ref. [15] |
| Methacrylate | 63 | ||
| He-P5C | Agarose | 10 | Ref. [15] |
| Methacrylate | <5 | ||
| HeWT | Agarose | 60 | This work |
| Methacrylate | 30 | Ref. [16] | |
| He-AlaDH | Agarose | 42 | Ref. [17] |
| Methacrylate | 19 | ||
| TsRTA | Agarose | 95 | This work |
| Methacrylate | 67 | ||
| HRP | Agarose | 48 | This work |
| Methacrylate | 27 | ||
| Tt-NOX | Agarose | 55 | Ref. [12] |
| Methacrylate | 24 | ||
| MsAcT | Agarose | 78 | This work |
| Methacrylate | 35 | ||
| HOR | Agarose | 62 | This work |
| Methacrylate | 30 | ||
| GalOx | Agarose | 100 | This work |
| Methacrylate | 98 | ||
| KRED1-Pglu | Agarose | 35 | This work |
| Methacrylate | 16 |
| Material Support | Product | ||
|---|---|---|---|
| % | mg | ||
| Trapped on support 1 | Agarose | 4 | 0.2 |
| Methacrylate | 66 | 4.7 | |
| Released with toluene 2 | Agarose | 100 | 0.2 |
| Methacrylate | 67 | 3.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez-Mateos, A.I.; Contente, M.L. Agarose vs. Methacrylate as Material Supports for Enzyme Immobilization and Continuous Processing. Catalysts 2021, 11, 814. https://doi.org/10.3390/catal11070814
Benítez-Mateos AI, Contente ML. Agarose vs. Methacrylate as Material Supports for Enzyme Immobilization and Continuous Processing. Catalysts. 2021; 11(7):814. https://doi.org/10.3390/catal11070814
Chicago/Turabian StyleBenítez-Mateos, Ana I., and Martina L. Contente. 2021. "Agarose vs. Methacrylate as Material Supports for Enzyme Immobilization and Continuous Processing" Catalysts 11, no. 7: 814. https://doi.org/10.3390/catal11070814
APA StyleBenítez-Mateos, A. I., & Contente, M. L. (2021). Agarose vs. Methacrylate as Material Supports for Enzyme Immobilization and Continuous Processing. Catalysts, 11(7), 814. https://doi.org/10.3390/catal11070814







