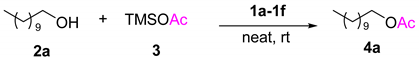
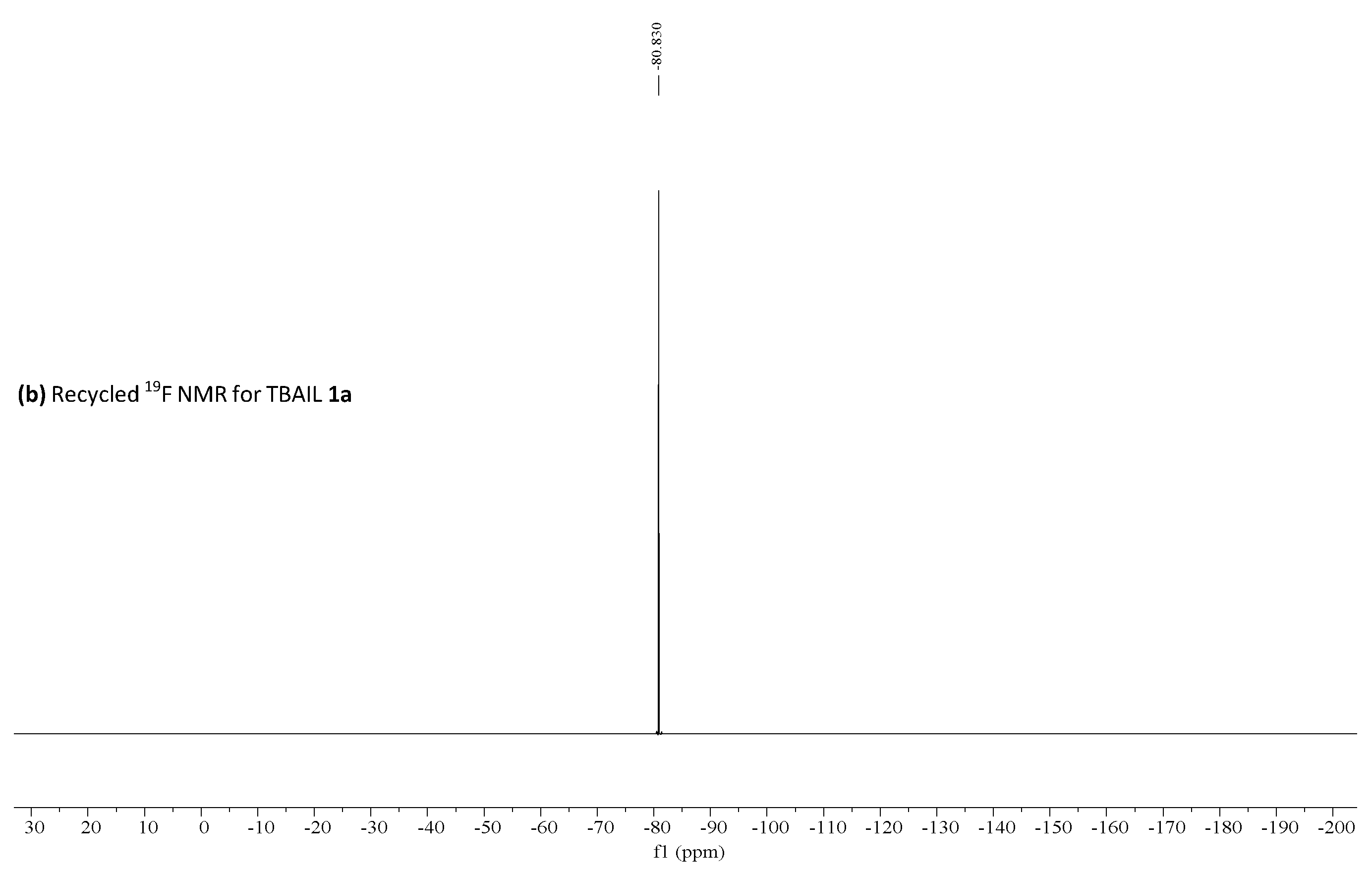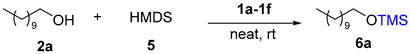Abstract
This report discloses a mild and efficient O-acetylation using easily accessible TMSOAc as a novel acetyl reagent and O-trimethylsilylation using HMDS for various alcohols catalyzed by tunable Brønsted acidic ionic liquids (TBAILs). Imidazolium-based TBAILs were prepared by a two-step atom-economic reaction and acidities measured by using UV-visible spectroscopy. Both protections for alcohols were accomplished at room temperature with good to excellent yields, while the products and TBAILs were separated by simple work-up for O-silylation and column chromatography for O-acetylation. Notably, with the simple post-process, TBAILs catalyst in this solvent free method easily recovered and recycled several times without significant degradation.
1. Introduction
The development of protections for synthesizing natural and unnatural products containing multiple functional groups is a foundational part of organic chemistry that is still being used today. Therefore, functional group protection is a fundamental reaction very commonly used in organic chemistry to perform selective reactions [1]. Among the other functional groups, alcoholic and phenolic moieties are the most common functional groups. One of the most fundamental and common transformations in organic chemistry is the acetylation of hydroxyl groups [2], which is achieved through acid halides and corresponding anhydrides [3,4]. The poor hydroxyl nucleophilicity, especially for phenols, demands the catalysts to activate anhydrides, such as protic acids, Lewis acids, and pyridine derivatives [5]. Recently, metal triflates and other metal salts [5,6,7,8,9] and ionic liquids [10,11,12,13] have also been discovered to be potential alternative catalysts. However, these catalysts have gripped grave disadvantages for acetylation in terms of its cost, longer reaction times, and toxicity. Hence, improvement of clean, cost-effective, versatile, and an eco-friendly procedure is continually being sought.
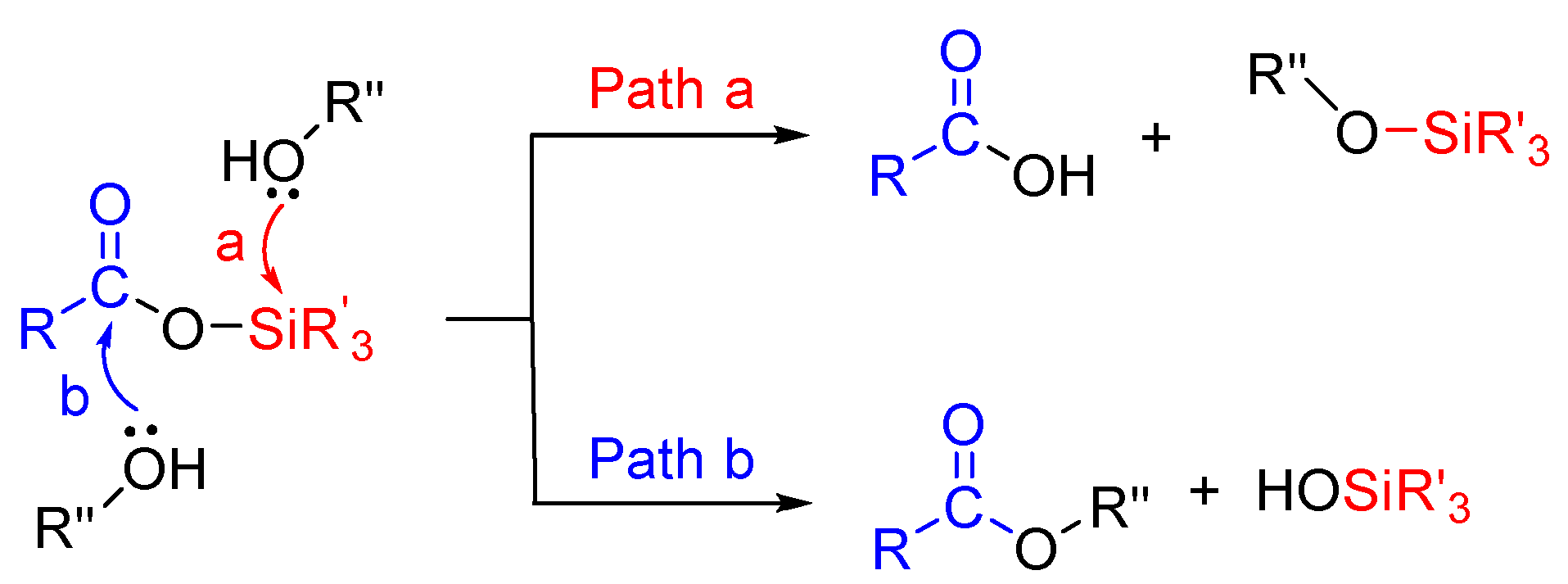
The use of silyl-based reagents has gained much more attention nowadays. Silyl carboxylates (acyloxysilane) were first studied by Yur’ev and Belyakova in 1960 [14]. Previous reports suggested that the reaction of nucleophiles with silyl carboxylates followed two possible pathways, where alcohol attacks silicon or the carbonyl group in Scheme 1 [14,15,16,17,18].
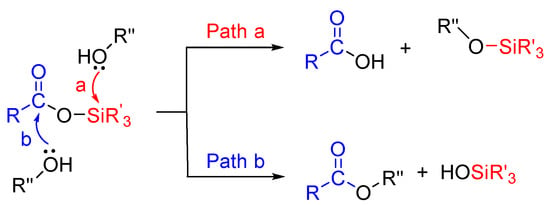
Scheme 1.
Possible pathways for O-nucleophile attack (O-acetylation) [14,15,16,17,18].
In 1970, Olah and co-workers reported that an iodotrimethylsilane mediated transesterification reaction would proceed through acyloxysilane as an intermediate state at a strictly neutral condition [19]. This method, acyloxysilane (trimethylsilyl acetate) with alcohols efficiently catalyzed by tunable Brønsted acidic ionic liquids (TBAILs), for the formation of a challenging acetylated product, was observed. To the best of our knowledge, TBAILs have never been used with trimethylsilyl acetate (TMSOAc) for acetylation reaction. Usually, trimethylsilyl acetate has been used for the preparation of by-product free oligosiloxane [20], α-silyl carboxylic acid [21], and silyl ketene acetals [22]. Recently, our lab reported that trimethylsilyl acetate was used for the silylation of sugars in the presence of a base [23]. Due to ease of handling, room temperature stability, and commercial availability, these reagents have much interest in organic transformation.
Silyl ether is considered as the most convenient extensively used hydroxyl protecting group [1], as a result of its augment stability under different conditions, soluble in non-polar solvents, and easy deprotection of silyl group gained increasing attention for protection. Moreover, many silylating methods are employed today in which trimethylsilylation is an extensively used method. Among different trimethylsilylating agents, chlorotrimethylsilane [24,25] and allyltrimethylsilane [26] have been commonly used to achieve trimethylsilylation. 1,1,1,3,3,3-Hexamethyldisilazane (HMDS) has been used frequently. by onsidering the propertiessuch as it does not form hydrogen halide as a byproduct, it has a simple work up, and can be handled in moisture. HMDS is a stable, and easily commercially available reagent producing only ammonia as a byproduct. Although, the steric hindrance of HMDS and poor silylating strength appeals to use catalysts and harsher reaction conditions [27,28,29]. Since then, a large number of catalysts have been reported, such as sulfonic acids [30], montmorillonite K-10 [31], ZnCl2 [32], metal chlorides [33], iodine [34], H3PW12O40 [35], TiO2–HClO4 [36], ZrO(OTf)2 [37], TiCl3(OTf) [38], alumina-supported heteropolyoxometalates [39], InBr3 [40], 1,3-disulfonic acid imidazolium hydrogen sulfate [41], ZrCl4 [42], {Al(OH)(BDC)} [43], CeO2 [44] TMSOTf [45], and CH3CN for per-O-trimethylsilylation [46], chlorozincate (II) ionic liquid [47], and silica-supported ionic liquid [48]. However, the existing reported methodologies have various limitations, such as expensive catalysts, reactions proceeding at high temperatures, and toxic metal catalysts. The principle of green chemistry stated that the development of a method should be constructed to reduce input energy by carrying reaction at ambient temperature and minimum use of reagents or catalysts. Thus, due to environmental concerns, an effective, cost-efficient, and green approach is needed.
Ionic liquids (ILs) because of their excellent properties and green solvent have gained widespread application in numerous fields. ILs are salts that are made up of the constituents of organic cations and organic or inorganic anions. Organic cations, for example, imidazolium, ammonium, phosphonium, and pyridinium ions, can be combined with a variety of anions, for example, BF4−, triflates, halides, (CF3SO2)2N−, or PF6− which give rise to various possible sequences. The physicochemical properties of ILs are perhaps tuned by switching the combinations of cation and anion [49]. In 2002, Cole and co-workers first reported the Brønsted-acidic ionic liquids (BAILs). Because of their excellent properties such as thermal stability, non-toxicity, high acidic character, ease of separation, and inertness towards air and water, they have gained considerable attention [50,51]. Since then, they have been utilized in a broad range of organic transformations. The use of green solvents results in a great decrease in the effects of global warming caused due to the use of conventional hazardous solvents. Since halide ions are generally non-favorable for the green chemistry [49]. Our previously synthesized ionic liquids were effectively utilized for applications in various organic transformations such as Friedel-Crafts acylation using acyl chloride and thioesterification [52], per-O-acetylation using acetic anhydride, and benzylidene ring-opening [53], and fatty acids esterification [54], based on our earlier experience in the area of ionic liquids and its utility. In this protocol, we aimed to provide an easy and eco-friendly method for the protection of alcohol. Thus, we reported TBAILs as our green catalysts for acetylation using TMSOAc as a novel reagent, and silylation using HMDS without metal and solvent.
2. Results and Discussion
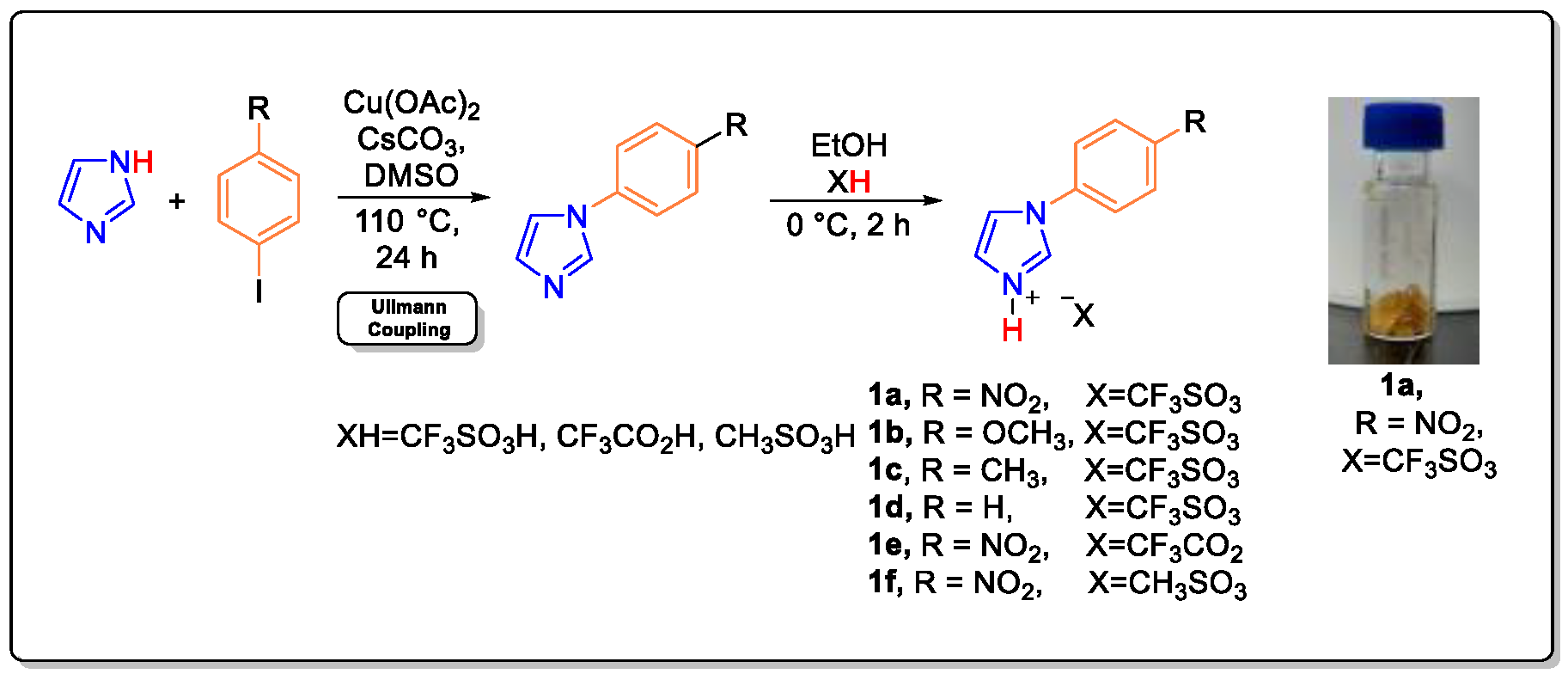
Tunable Brønsted acidic ionic liquids were prepared according to the reported method [52,53,54] by using a two-step atom-economic reaction in which aryl imidazole was synthesized by using Ullmann type coupling followed by treatment with an acid, as shown in Figure 1. Newly prepared TBAILs (1a–1f) are white and yellow solids in nature without any noticeable fumes or evident degree of vapor pressure. The Brønsted acidity functions were measured with UV-visible spectroscopy using crystal violet dye as an indicator [54]. The synthesized TBAILs are completely soluble in water and willingly soluble in organic polar solvents such as methanol, ethanol, and acetone. The solubility order for catalysts 1a–1f is as follows 1a > 1b > 1c > 1d > 1e > 1f [54]. With increasing interest toward a greener method for the protection of alcohol, efficient, safe, and cost-effective TBAILs were employed for the O-acetylation using TMSOAc, and O-silylation using HMDS were studied.
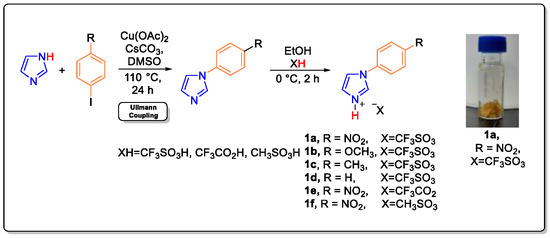
Figure 1.
Two steps synthesis of TBAILs 1a–1f reprinted with permission from ref. 54. Copyright 2021, Elsevier [54].
2.1. Acetylation of Alcohols Using TMSOAc
With the series of designed ionic liquid catalysts in hand, it is possible to study the condition for acetylation reaction by using TMSOAc as a reagent and the results are reported in Table 1. The initially step of our study was to define the best reaction conditions for the catalyst and reagent. These experiments were performed on undecanol 2a as a model substrate and TMSOAc 3 as a reagent to resolve the optimization condition. Firstly, we studied a series of experiments with various TBAILs, 1a–1f, where it was recommended that 1a was the finest catalyst for the acetylation providing the desired product 4a in excellent yield (95%, Table 1, entry 1). Ionic liquids 1b–1f showed less activity as compared to 1a, most expected due to weaker acidity [54] (Table 1, entries 2–6). Even low or high catalyst loadings (Table 1, entries 7 and 8) were not very influenced on reaction yields. We have observed that the reaction gives silylation intermediate, which was finally converted to an acetylated product. The next step was to study the amount of reagent used in the reaction for the conversion of the starting material. Lowering the reagent (Table 1, entry 9) further slightly lowers the reaction yield and increasing the reagent (Table 1, entry 10) does not affect the reaction yield.

Table 1.
Optimization of the acetylation conditions between undecanol 2a and TMSOAc 3 with 1a–1f. a
Ionic liquids 1b–1f showed less activity as compared to 1a, most expected due to weaker acidity [54] (Table 1, entries 2–6).
Concerning the reaction rate and productivity, it was shown that 1a was the finest catalyst for acetylation reaction. Acetylation reactions were operated under different equivalents of catalyst 1a from 0.03 to 0.07 (Table 1, entries 7–8) and the different equivalents of TMSOAc 3 from 1.5 to 2.5 at room temperature (Table 1, entries 9–10). Taking into account its good reactivity, 1a was selected as the best TBAIL for acetylation of alcohols.
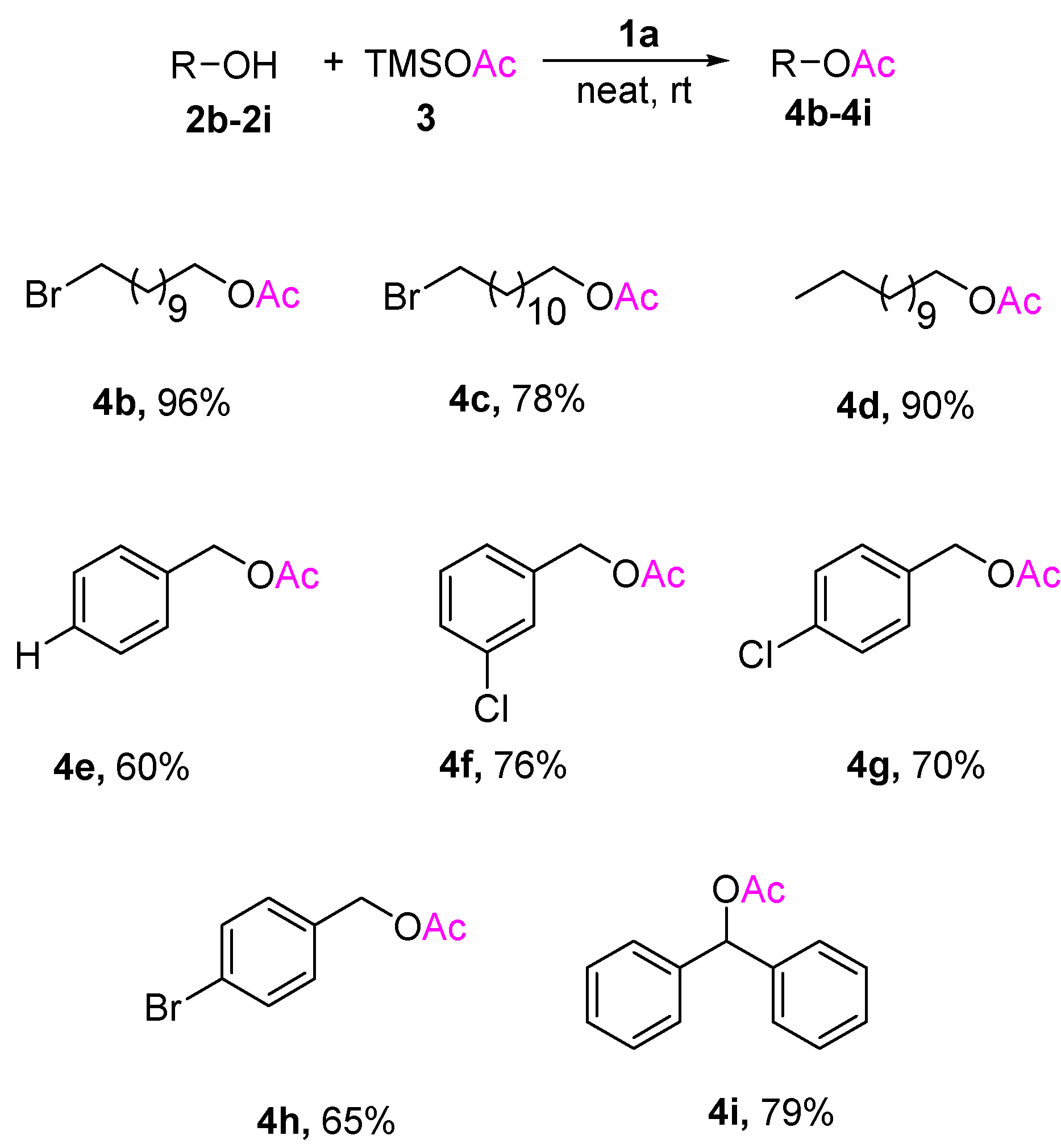
Various structurally diverse alcohols underwent acetylation using previously optimized reaction conditions to provide the corresponding acetates in moderate to excellent yields. The results are reported in Scheme 2. The bromoundecyl alcohol 2b in TMSOAc 3 was transformed to bromoundecyl acetate 4b in 96% excellent yield by using the 0.05 equivalent of ionic liquid 1a. Using the same reaction condition, other primary alcohols also resulted in good yields. Acetylation of hindered secondary alcohol 4i, which is often considered difficult, is also accomplished in reasonably good to moderate yields.
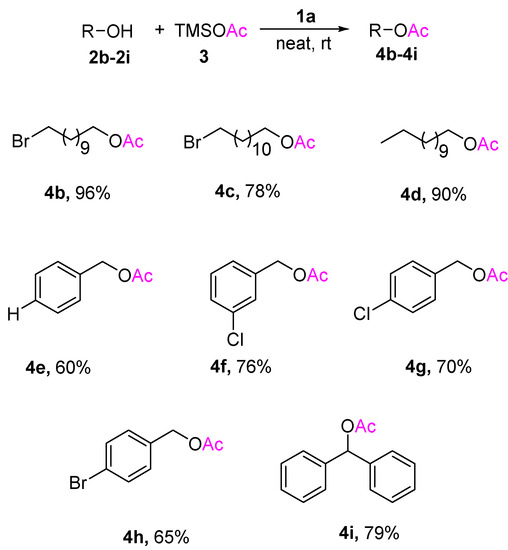
Scheme 2.
Ionic liquid 1a catalyzed acetylation of different alcohols using TMSOAc 3 (a,b). a Notes: All reactions were performed using 2b–2j (1 mmol), 1a (0.05 mmol), and TMSOAc 3 (2 mmol) for 4 h at room temperature. b Isolated yield. The reaction crudes were purified by column chromatography and identified by 1H-NMR.
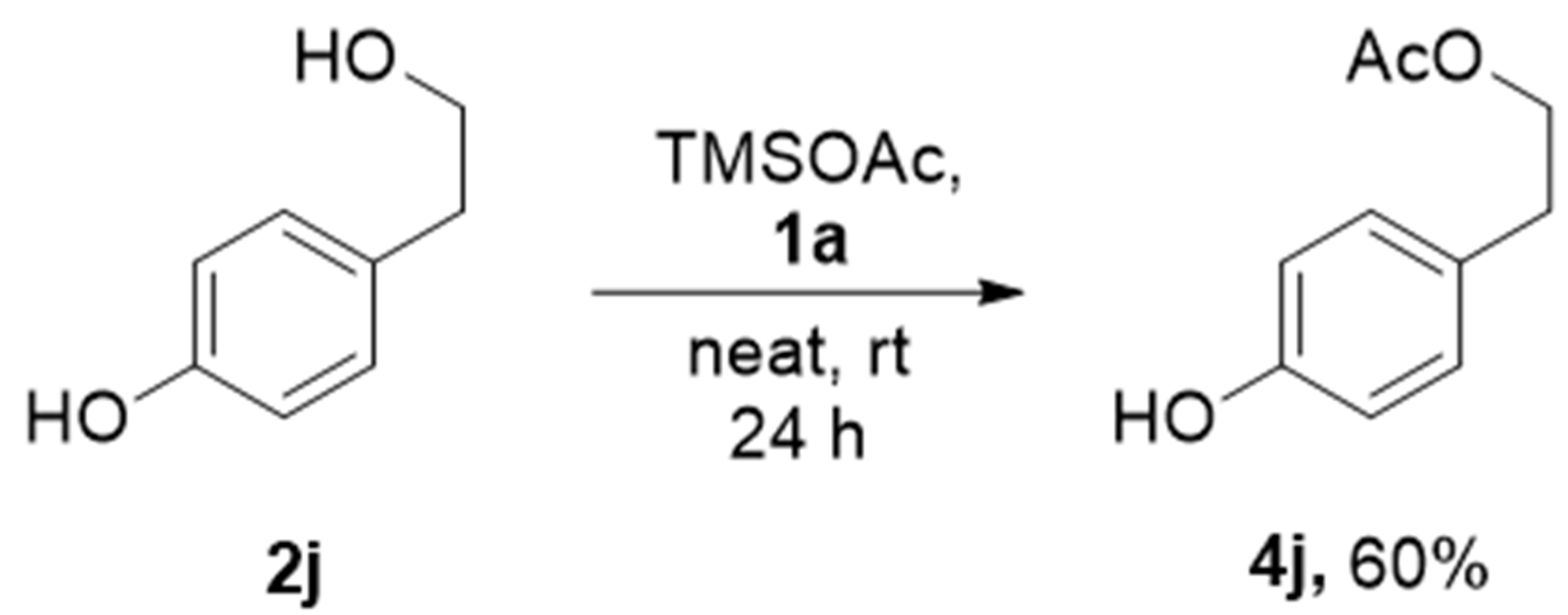
The reactions of sp2-hybridized carbon attached -OH, for example, o-cresol and p-cresol remained intact at standard reaction conditions. It is a good indication for obtaining the selective acetylation consisting of various alcohols. To further explore the universality of the acetylation reaction, a substrate containing sp2-hybridized carbon holding -OH and primary alcohol was examined using standard reaction conditions. We observed that only sp3-carbon holding -OH was selectively acetylated 2j with 60% yield after 24 h, as shown in Scheme 3. The poor hydroxyl nucleophilicity of sp2 carbon-holding hydroxy group, especially for phenols, did not proceed acetylation at ambient conditions. Thus, the good nucleophilic character of primary alcohols leads to acetylation predominantly over phenol. The acetylation of alcohols is a well-known process, but usually using either expensive metal salts or metal triflate, acid, or toxic base pyridine [4], with environmentally harmful solvents such as DCM or ACN [5,6,7,8,9]. Thus, there is space for improvement for a green method for acetylation. Table 2 shows the reported methods for acetylation in the presence of ionic liquids. However, some of these reported methods experience limitations in the reuse procedure and using acetic anhydride as the acylating agent cannot achieve selectivity for primary alcohol over phenolic -OH. Our present work using TMSOAc for acetylation showed some advantages in terms of selectivity and advantages such as room temperature reaction, solvent-free, and metal-free protocol.
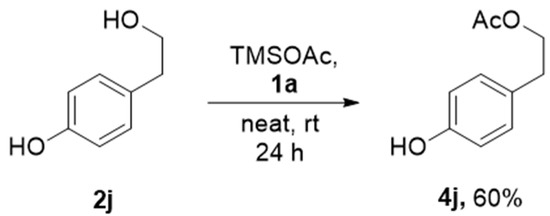
Scheme 3.
Selective acetylation of primary alcohol over phenolic -OH.

Table 2.
Comparison table of catalyst TBAIL 1a with previously reported ionic liquids for acetylation.
2.2. Silylation of Alcohol Using HMDS
Previously synthesized TBAILs were also tested for the silylation of alcohols. Undecanol 2a (1.0 equiv.) was selected as the model substrate and HMDS 5 (1.0 equiv.) was selected as the reagent; the results are reported in Table 3. Initially, our aim was to study the best reaction conditions for catalyst selection and equivalents. This reaction proceeded without a catalyst but remained unfinished even after 96 h (Table 3, entry 1). Subsequently, the same reaction was performed using 1a as the catalyst (0.05 eq.) and stirred at room temperature for 1 h, to afford product 6a with an excellent yield of 98% (Table 3, entry 2). Inspired by these results, various TBAILs 1b–1f were examined. Among all these TBAILs 1a–1f with the same amount of loading (5 mol%), 1a was given excellent yield at room temperature for 1 h.

Table 3.
Optimization for ionic liquid catalyzed O-silylation a.
As expected, the nature of the ionic liquid 1a played a pivotal role in the reaction, because ionic liquids 1b–1f resulted in relatively lower yields (Table 3, entries 3–7). Lowering the concentration of 1a to 0.03 equivalent led to a lower yield (93%, Table 3, entry 8), while increasing it to a 0.07 equivalent also reduced the reaction yield (92%, Table 3, entry 9). Finally, with the optimized reaction condition in hand, the scope of the method was explored. Various aliphatic and aromatic alcohol substrates were studied to evaluate the generality and effectiveness of the developed protocol (Scheme 4). Table 3 shows the silylation of long-chain 2a isolated with a 98% yield in 1 h.
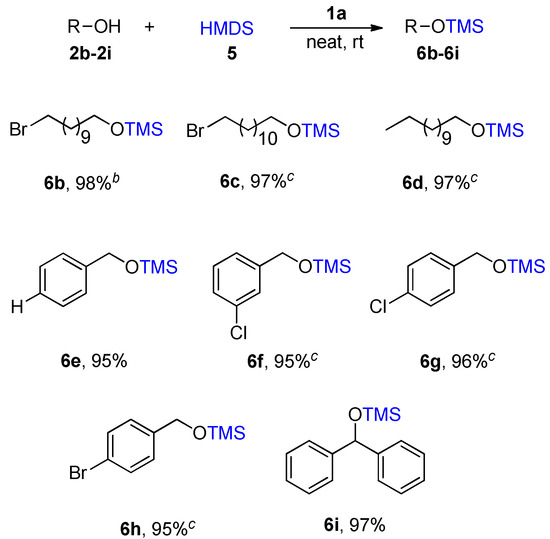
Scheme 4.
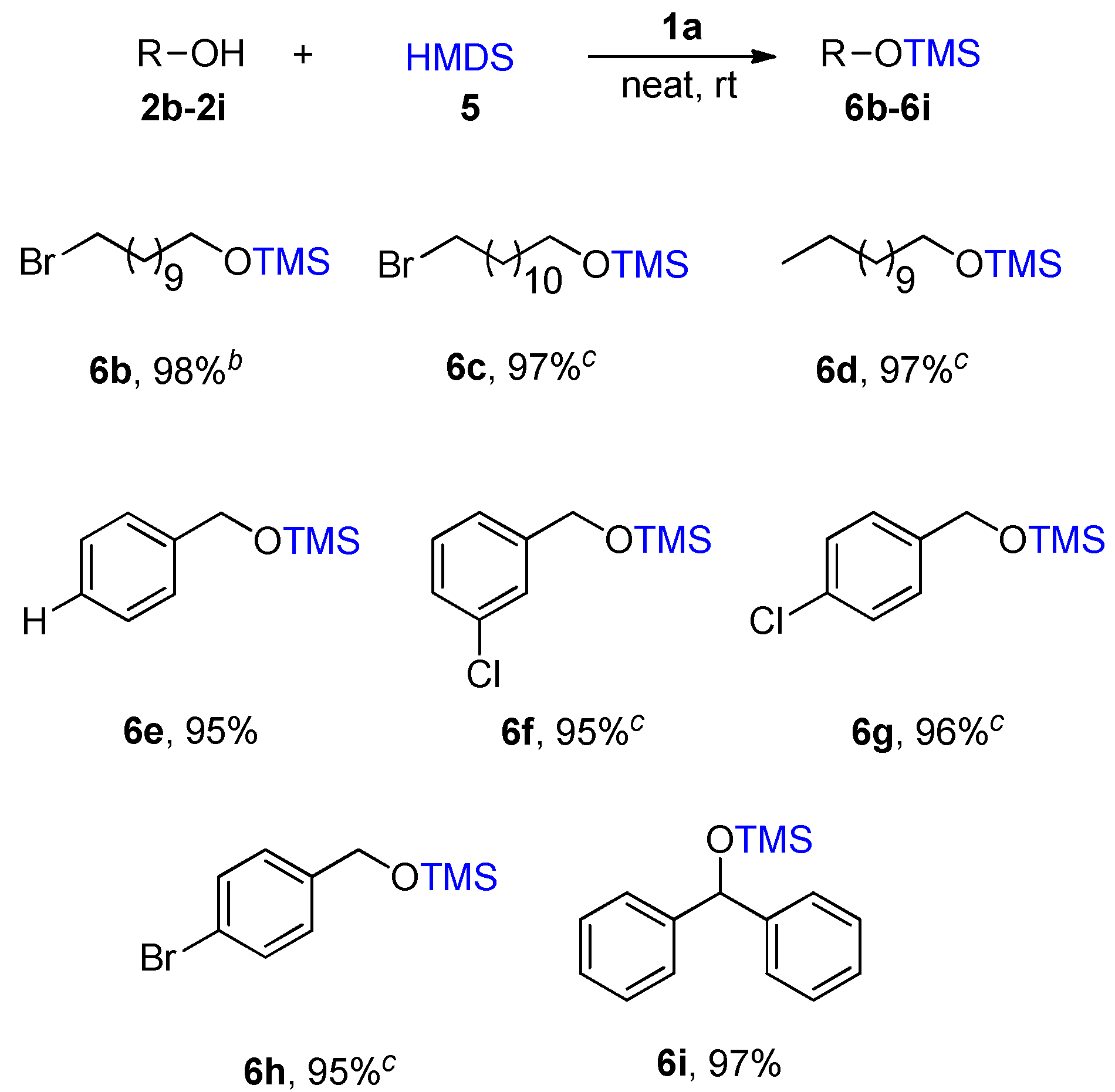
Ionic liquid 1a catalyzed silylation of different alcohols using HMDS 5 a. a Notes: All reactions were performed using 2b–2i (1 mmol), HMDS 5 (1 mmol), 1a (0.05 mmol) at room temperature. b The reaction was completed in 2 h, c The reaction was completed in 1.5 h. The products were extracted and identified by 1H-NMR.
Under the same condition, Bromo-substituted long-chain alcohols 2b was consumed in 2 h with a very good yield of 97%. Compounds 2c and 2d were formed the desired products 6c and 6d in high yield. The reaction proceeded very smoothly with benzyl alcohol derivatives containing a halogen group afforded the corresponding products with 6e in 95%, 6f in 95%, and 6g in 96% and 6h in 95% yields, respectively. Similarly, the silylation reaction of secondary alcohols such as hindered benzhydrol delivered the corresponding silylating product 6i in 97% yield.
The comparison of present catalyst TBAIL 1a with earlier reports for silylation with HMDS reveals that the present work exhibits some utility, as shown in Table 4. The salient features of TBAIL 1a as a catalyst for O-silylation are short reaction times, reaction proceeds at room temperature, two-step atom economy for the preparation of the catalyst, ease for separation of a catalyst, and product from the reaction mixture. In addition, the catalyst recovered easily without loss in its activity.

Table 4.
Comparison table of catalyst TBAIL 1a with previously reported ionic liquids for silylation.
2.3. Reuse of TBAIL 1a for Silylation and Acetylation of Alcohols
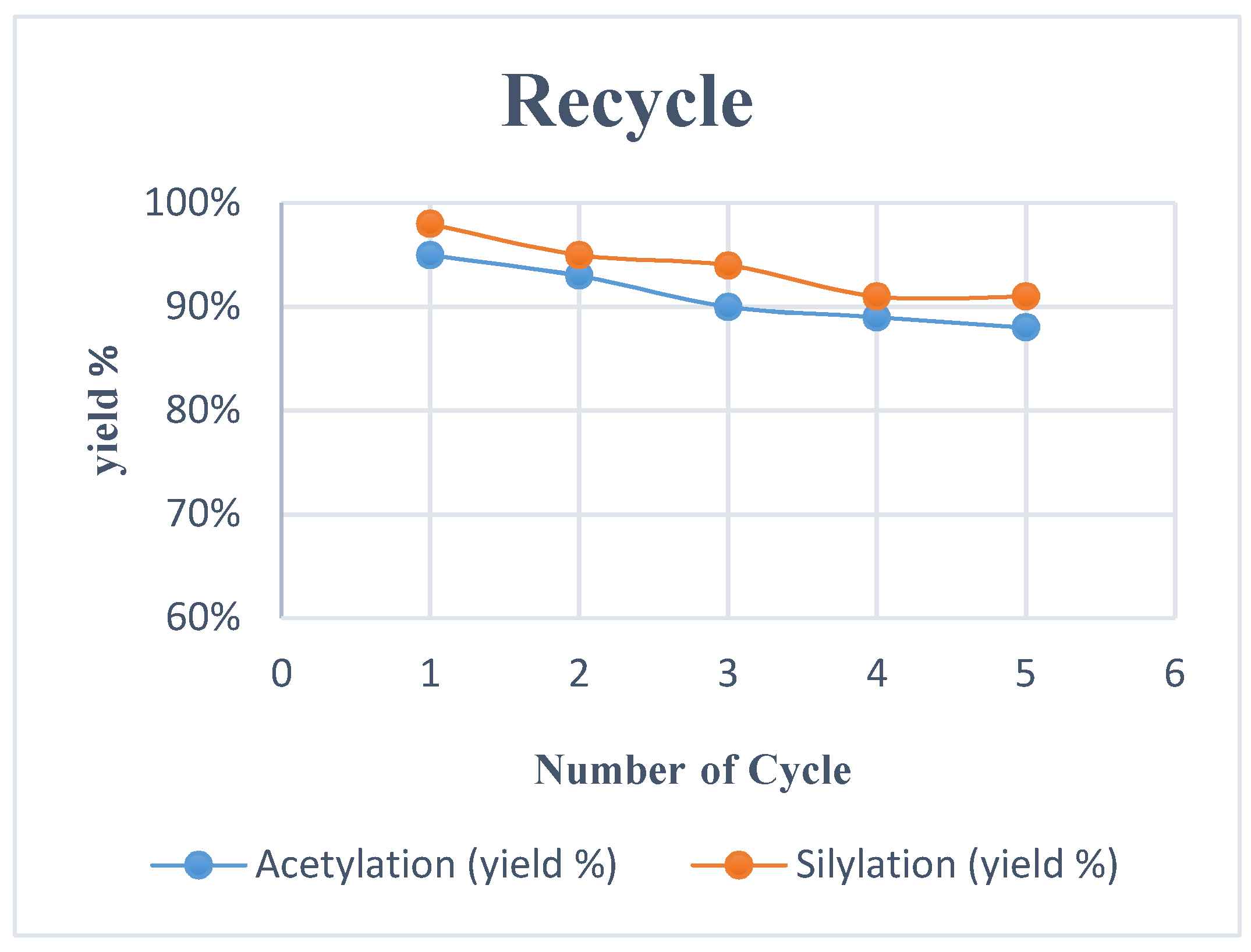
Ionic liquid 1a was recovered from the reaction mixture and reuse for acetylation of 2a using TMSOAc 3 without any considerable loss in reaction yield (run 1–95%, run 2–93%, run 3–90%, run 4–89%, run 5–88%), as shown in Figure 2. Notably, failure to cut out volatile from recovered IL can be a decrease in the yields of the consecutive reactions. TBAIL 1a could be reused several times with low cross-contamination.
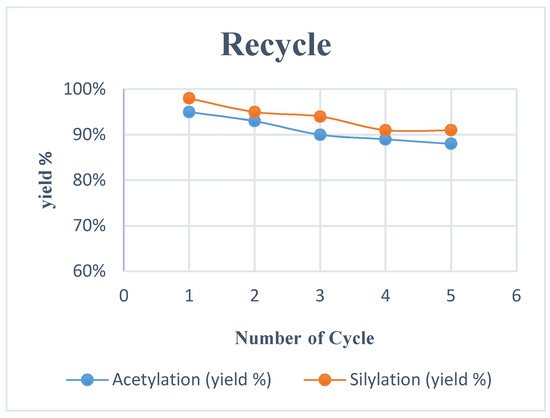
Figure 2.
Reuse of TBAIL 1a for acetylation and silylation.
The prospect of ionic liquid as a green solvent and catalyst on broad proportion applications, the recovery of the catalyst to help reduce cost and diminish their impact on the environment. The recycling of TBAIL 1a for silylation was tested between the reactions of 2a and 5, as shown in Figure 2.
After completion, the reaction mixture was added ether and then extracted with water. TBAIL 1a was recovered from the water layer and dried over the high vacuum without a significant loss in catalytic activity. Indeed, the reaction still afforded 91% after five cycles (Figure 2). Experimental data showed that 1a can be recycled and reused for several cycles.
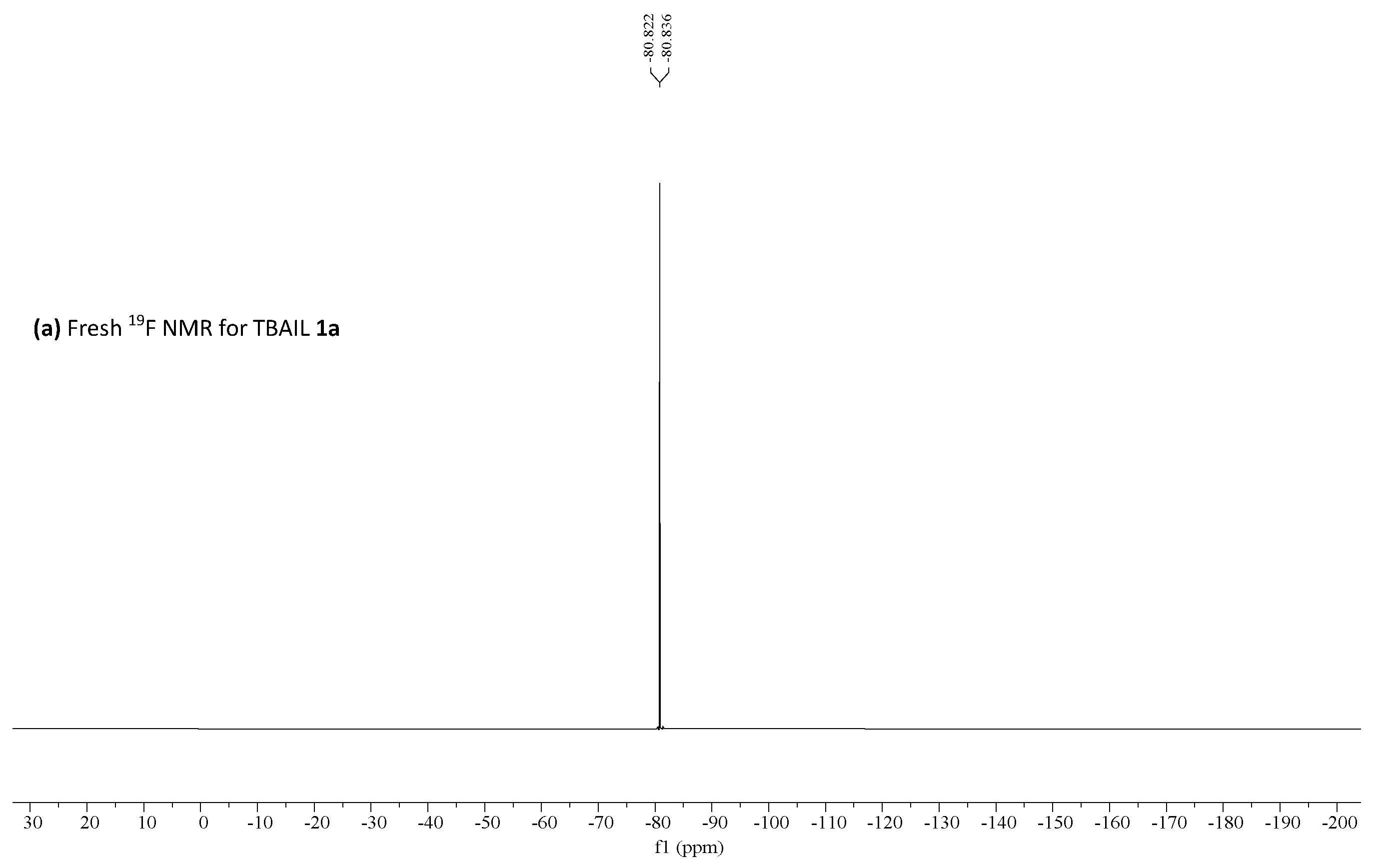
19F NMR was performed for fresh, recovered catalyst 1a and the obtained results are shown in Figure 3. These results suggested no significant changes in the catalyst and triflate anion remains in the aqueous phase—−80.083 ppm, as shown by 19F NMR [53].
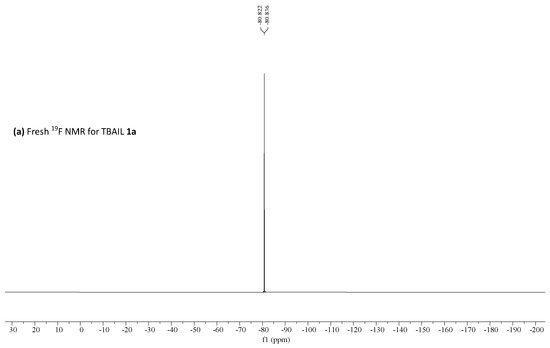
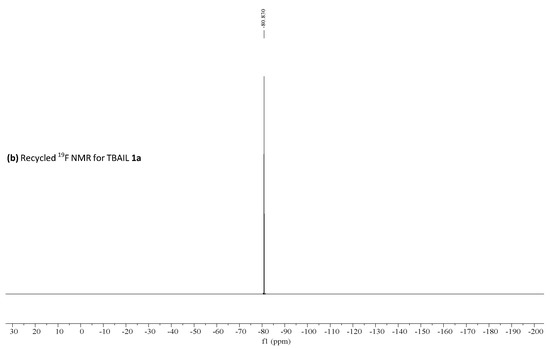
Figure 3.
(a) 19F HNMR for fresh TBAIL 1a, (b) 19F HNMR for recycled TBAIL 1a reprinted with permission from [53]. Copyright 2020, MDPI.
3. Materials and Methods
3.1. General Information
The reactions were conducted in glassware (sealed tube), under the nitrogen atmosphere. All reagents were purchased from Aldrich and Across and utilized without further purification unless otherwise mentioned. Silica Gel 60 was used for flash column chromatography. TLC was performed on pre-coated Aluminium plate of Silica Gel 60 F254. Detection was executed by spraying with a solution of Ce(NH4)2(NO3)6 (0.8 g), (NH4)6Mo7O24 (20.0 g) and 10% H2SO4 (400 mL) and subsequent heating on a hot plate. 1H, 13C NMR, DEPT, 1H-13C HSQC, NOESY, and 1H-1H COSY spectra were recorded using (Varian, Inc., Palo Alto, CA, USA) and (JEOL Ltd. Tokyo, Japan) 400 MHz instruments. Chemical shifts are in ppm from Me4Si generated from the CDCl3 lock signal at δ 7.26 ppm for 1HNMR and δ 77.0 ppm for 13C NMR. Mass spectra were analyzed on orbitrap instrument with an ESI source and FAB.
3.2. General Procedure for Preparation of TBAILs
Tunable Brønsted acidic ionic liquids (TBAILs) were prepared and characterized with different techniques such as 1HNMR, 13C NMR, Brønsted acidity using UV-visible spectroscopy and mass spectroscopy, as described in previously reported reference procedures [52,53,54].
3.3. General Procedure for O-Acetylation Reaction (A)
A sealed tube furnished with magnetic stirrer bar was charged with 2a–2i (1.0 equiv.), these were added trimethylsilyl acetate (2.0 equiv. for 2a–2i) and ionic liquids (0.05 equiv. for 2a–2i) at room temperature, and stirred for reaction time (4 h for 2a–2i, 24 h for 2j). After completion, the reaction mixture was concentrated. The crude products were purified by using column chromatography on silica gel to give the desired products 4a–4j. 1H NMR, and 13C NMR spectra are showed in Supplementary Materials.
Undecyl acetate (4a). Following the general procedure (A): as colorless liquid. (204 mg, 96%); Rf 0.46 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 4.05 (t, J = 6.8 Hz, 2H), 2.05 (s, 3H), 1.61 (quintet, J = 6.8 Hz, 2H), 1.38–1.20 (m, 16H), 0.88 (t, J = 6.8 Hz, 3H).; 13C NMR (100 MHz, CDCl3) δ 171.3, 64.7, 31.9, 29.58, 29.56, 29.5, 29.3, 29.2, 28.6, 25.9, 22.7, 21.0, 14.1. HRMS (ESI, M + H+) calcd for C13H27O2 215.2011, found 215.2015.
11-Bromoundecyl acetate (4b). Following the general procedure (A): as colorless liquid. (280 mg, 96%); Rf 0.55 (EtOAc/Hex = 1/4); 1H NMR (400 MHz, CDCl3) δ 4.04 (t, J = 6.8 Hz, 2H), 3.39 (t, J = 6.8 Hz, 2H), 2.03 (s, 3H), 1.84 (quintet, J = 7.2 Hz, 2H), 1.60 (quintet, J = 7.2 Hz, 2H), 1.40 (quintet, J = 7.2 Hz, 2H), 1.28 (m, 12H).; 13C NMR (100 MHz, CDCl3) δ 171.2, 64.6, 34.0, 32.7, 29.41, 29.36, 29.1, 28.7, 28.5, 28.1, 25.8, 21.0. HRMS (ESI, M+Na+) calcd for C13H25BrO2Na 315.0936, found 315.0928.
12-Bromododecyl acetate (4c). Following the general procedure (A): as colorless liquid. (240 mg, 78%); Rf 0.43 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 4.03 (t, J = 6.8 Hz, 2H), 3.39 (t, J = 6.8 Hz, 2H), 2.03 (s, 3H), 1.84 (quintet, J = 6.8 Hz, 2H), 1.60 (quintet, J = 7.2 Hz, 2H), 1.40 (quintet, J = 7.2 Hz, 2H), 1.27 (m, 14H).; 13C NMR (100 MHz, CDCl3) δ 171.1, 64.6, 34.0, 32.7, 29.4, 29.3, 29.1, 28.7, 28.5, 28.1, 25.8, 20.9. HRMS (ESI, M + Na+) calcd for C14H27BrO2Na 329.1092, found 329.1092.
Dodecyl acetate (4d). Following the general procedure (A): as colorless liquid. (205 mg, 90%); Rf 0.55 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 4.02 (t, J = 6.8 Hz, 3H), 2.02 (s, 3H), 1.59 (m, J = 6.4 Hz, 2H), 1.26 (m, 18H), 0.85 (t, J = 6.8 Hz, 3H).; 13C NMR (100 MHz, CDCl3) δ 171.2, 64.6, 31.9, 29.6, 29.5, 29.5, 29.3, 29.2, 28.5. HRMS (FAB, M + H+) calcd for C13H28O2 229.2169, found 229.2168.
Benzyl acetate (4e). Following the general procedure (A): as colorless liquid. (60 mg, 40%); Rf 0.42 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 7.41–7.29 (m, 5H), 5.11 (s, 2H), 2.11 (s, 3H).; 13C NMR (100 MHz, CDCl3) δ 170.7, 135.8, 128.4, 128.1, 66.1, 20.8. HRMS (ESI, M + Na+) calcd for C9H10O2Na 173.0579, found 173.0584.
3-Chlorobenzyl acetate (4f). Following the general procedure (A): as colorless liquid. (140 mg, 76%); Rf 0.46 (EtOAc/Hex = 1/2); 1H NMR (400 MHz, CDCl3) δ 7.35 (s, 1H), 7.29 (d, J = 2.8 Hz, 2H), 7.23 (d, J = 7.2 Hz, 1H), 2.11 (d, J = 7.2 Hz, 3H).; 13C NMR (100 MHz, CDCl3) δ 170.6, 137.7, 134.3, 129.8, 128.3, 126.1, 65.3, 20.9. HRMS (ESI, M+) calcd for C9H10ClO2Na 184.0288, found 185.0291.
4-Chlorobenzyl acetate (4g). Following the general procedure (A): as colorless liquid. (130 mg, 70%); Rf 0.48 (EtOAc/Hex = 1/2); 1H NMR (400 MHz, CDCl3) δ 7.40 (m, 4H), 5.04 (s, 2H), 2.08 (s, 3H).; 13C NMR (100 MHz, CDCl3) δ 170.7, 134.4, 134.1, 129.6, 128.7, 65.4, 20.9. HRMS (ESI, M + H+) calcd for C9H10ClO2Na 185.0369, found 185.0368.
4-Bromobenzyl acetate (4h). Following the general procedure (A): as colorless liquid. (150 mg, 65%); Rf 0.49 (EtOAc/Hex = 1/2); 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 6.4 Hz, 2H), 7.23 (d, J = 8.4 Hz, 2H), 5.04 (s, 2H) 2.09 (s, 2H).; 13C NMR (100 MHz, CDCl3) δ 170.7, 134.8, 131.6, 129.8, 122.2, 65.4, 20.9. The physical and spectral data were matched with those previously reported [55].
Benzhydryl acetate (4i). Following the general procedure (A): as colorless liquid. (180 mg, 79%); Rf 0.47 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 7.40 (m, 10H), 6.96 (s, 1H), 2.21 (s, 3H).; 13C NMR (100 MHz, CDCl3) δ 169.9, 142.10, 140.11, 128.4, 128.3, 127.8, 127.3, 127.1, 126.9, 79.8, 76.7, 21.2. HRMS (ESI, M + Na+) calcd for C15H14O2Na 249.0891, found 249.0885.
4-hydroxyphenethyl acetate (4j). Following the general procedure (A): as colorless liquid. (108 mg, 60%); Rf 0.42 (EtOAc/Hex = 1/3); 1H NMR (400 MHz, CDCl3) δ 7.05 (m, 2H), 6.79 (m, 2H), 4.25 (t, J = 6.8 Hz, 2H), 2.86 (t, J = 7.2 Hz, 2H), 2.05 (s, 3H).; 13C NMR (100 MHz, CDCl3) δ 171.9, 154.5, 129.8, 129.2, 115.3, 65.5, 34.0, 20.9. The physical and spectral data were matched with those previously reported [55].
3.4. General Procedure for O-Trimethylsilylation Reaction (B)
A sealed tube furnished with magnetic stirrer bar was charged with 2a–2i (1.0 equiv.), and added HMDS (1.0 equiv.) and ionic liquid (0.05 equiv.) at room temperature. Then, it was stirred (6a, 6e, and 6i for 1 h, 6c–6d and 6f–6h for 1.5 h, 6b for 2 h) at room temperature. After completion of the reaction, the reaction mixture was diluted with ether and extracted with water (20 × 3). The crude product was concentrated and put on under high vacuum to give the desired product 6a–6i.
Trimethyl(undecyloxy)silane (6a). Following general procedure (B): as colorless liquid. (238 mg, 98%); Rf 0.67 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 3.55 (t, J = 6.8 Hz, 2H), 1.51 (m, 2H), 1.24–1.26 (m, 16H), 0.86 (t, J = 7.2 Hz, 3H), 0.94 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 62.7, 32.7, 31.9, 29.6, 29.4, 29.3, 25.8, 22.6, 14.1, −0.4. HRMS (ESI+, M + Na+) calcd for C14H32O2SiNa 267.2120, found 267.2120.
[(11-Bromoundecyl)oxy]trimethylsilane (6b). Following general procedure (B): as colorless liquid. (316 mg, 98%); Rf 0.66 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 3.56 (t, J = 6.8 Hz, 2H), 3.40 (t, J = 6.8, 2H), 1.85 (quintet, J = 7.6 Hz, 2H), 1.53–1.50 (m, 2H), 1.43-1.41 (m, 2H), 1.27 (s, 12H), 0.11 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 62.7, 34.0, 32.8, 32.7, 29.5, 29.4, 28.7, 28.1, 25.8, −0.4. HRMS (ESI+, M + Na+) calcd for C14H31O1BrSiNa 345.1225, found 345.1210.
[(12-bromododecyl)oxy]trimethylsilane (6c). Following general procedure (B): as colorless liquid. (326 mg, 97%); Rf 0.66 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 3.55 (t, J = 6.4 Hz, 2H), 3.58 (t, J = 6.8, 2H), 1.83 (quintet, J = 7.2 Hz, 2H), 1.51–1.48 (m, 2H), 1.40 (dd, J = 1.2, 6 Hz, 2H), 1.26 (s, 14H), 0.09 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 62.6, 33.9, 32.7, 32.6, 29.6, 29.5, 39.4, 29.3, 28.7, 28.1, 25.7, −0.5. HRMS (ESI+, M+) calcd for C15H33O1Br1Si 336.1484, found 336.1484.
(Dodecyloxy)trimethylsilane (6d). Following general procedure (B): as colorless liquid. (258 mg, 97%); Rf 0.69 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 3.56 (t, J = 6.8 Hz, 2H), 1.52 (quintet, J = 6.8 Hz, 2H), 1.27–1.25 (m, 18H), 0.88 (t, J = 7.2 Hz, 3H), 0.11 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 62.7, 32.7, 31.9, 29.6, 29.4, 29.3, 25.8, 22.6, 14.1, −0.5. The physical and spectral data were matched with those previously reported [47].
(Benzyloxy)trimethylsilane (6e). Following general procedure (B): as colorless liquid. (171 mg, 95%); Rf 0.67 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 7.44 (m, 4H), 7.36–7.34 (m, 1H), 4.81 (s, 2H), 0.28 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 140.8, 128.1, 126.3, 64.5, −0.48. The physical and spectral data were matched with those previously reported [47].
[(3-Chlorobenzyl)oxy]trimethylsilane (6f). Following general procedure (B): as colorless liquid. (204 mg, 95%); Rf 0.71 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 7.32 (br, 2H), 7.24–7.21 (m, 2H), 7.20–7.16 (m,1H), 4.65 (s, 2H), 0.16 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 143.0, 134.1, 129.4, 127.1, 126.4, 124.3, 63.8, −0.4. The physical and spectral data were matched with those previously reported [47].
[(4-Chlorobenzyl)oxy]trimethylsilane (6g). Following general procedure (B): as colorless liquid. (206 mg, 96%); Rf 0.74 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 7.29–7.27 (m, 2H), 7.24–7.22 (m, 2H), 4.64 (s, 2H), 0.13 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 139.4, 132.6, 128.3, 127.7, 63.8, −0.5. The physical and spectral data were matched with those previously reported [47].
[(4-Bromobenzyl)oxy]trimethylsilane (6h). Following general procedure (B): as colorless liquid. (246 mg, 95%); Rf 0.73 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 7.46–7.43 (m, 2H), 7.20–7.18 (m, 2H), 4.63 (s, 2H), 0.15 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 140.0, 131.3, 128.1, 120.7, 63.9, −0.4. The physical and spectral data were matched with those previously reported [47].
(Diphenylmethoxy)trimethylsilane (6i). Following general procedure (B): as colorless liquid. (249 mg, 97%); Rf 0.70 (EtOAc/Hex = 1/9); 1H NMR (400 MHz, CDCl3) δ 7.38–7.35 (m, 4H), 7.34–7.29 (m, 4H), 7.25–7.21(m, 2h), 5.79 (s, 1H), 0.11 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 139.4, 132.6, 128.3, 127.7, 63.8, −0.5. The physical and spectral data were matched with those previously reported [47].
4. Conclusions
A mild and efficient method has been established for acetylation using novel TMSOAc as an acetyl donor and silylation using HMDS in the presence of a recyclable tunable Brønsted acidic ionic liquid. Our investigation suggested that TBAIL catalyzed acetylation using TMSOAc proceeds through silylation intermediate. Both transformations were proceeded at room temperature and provided mild to excellent yields. The use of TBAIL for diverse alcohols presents a simple alternative to costly and toxic catalysts. This method may be more useful in the future for extensive study of acetylation and silylation of different alcohols, and for the synthesis of natural products and small molecules TBAIL was recycled and reused at least 5 times, which provided another eco-friendly method in the field of alcohol protection.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11070825/s1, 1H NMR, 13C NMR of all related compounds.
Author Contributions
S.-Y.L. and Y.-J.L. designed the research. H.-R.W. analyzed the Mass data. M.T., A.R.P., M.L., and W.L. prepared the compounds. A.R.P. wrote the manuscript with the help of S.R.J. and S.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology in Taiwan (MOST 108-2113-M-005-021 and MOST 110-2113-M-005-017) and National Chung Hsing University.
Data Availability Statement
Data is contained within article or Supplementary Materials.
Acknowledgments
The authors thank the Ministry of Science and Technology in Taiwan (MOST 108-2113-M-005-021 and MOST 110-2113-M-005-017) and National Chung Hsing University for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wuts, P.G.M.; Greene, T.W. Greene’s Protective Groups in Organic Synthesis, 4th ed.; Wiley: Hoboken, NJ, USA, 2007; pp. 1–16. [Google Scholar]
- Sartori, G.; Ballini, R.; Bigi, F.; Bosica, G.; Maggi, R.; Righi, P. Protection (and deprotection) of functional groups in organic synthesis by heterogeneous catalysis. Chem. Rev. 2004, 104, 199–250. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.W.; Wuts, M.G.P. Protective Groups in Organic Synthesis, 3rd ed.; Wiley: New York, NY, USA, 1999; pp. 1–52. [Google Scholar]
- Larock, C.R. Comprehensive Organic Transformations, 3rd ed.; VCH Publishers Inc.: New York, NY, USA, 1989. [Google Scholar]
- Ishihara, K.; Kubota, M.; Kurihara, H.; Yamamoto, H. Scandium trifluromethanesulfonate as an extrely active acylation catalyst. J. Am. Chem. Soc. 1995, 117, 4413–4414. [Google Scholar] [CrossRef]
- Ishihara, K.; Kubota, M.; Kurihara, H.; Yamamoto, H. Scandium trifluoromethanesulfonate as an extremely active Lewis acid catalyst in acylation of alcohols with acid anhydrides and mixed anhydrides. J. Org. Chem. 1996, 61, 4560–4567. [Google Scholar] [CrossRef]
- Procopiou, P.A.; Baugh, S.P.D.; Flack, S.S.; Inglis, G.G.A. An extremely powerful acylation reaction of alcohols with acid anhydrides catalyzed by trimethylsilyl trifluoromethanesulfonate. J. Org. Chem. 1998, 63, 2342–2347. [Google Scholar] [CrossRef]
- Procopiou, P.A.; Baugh, S.P.D.; Flack, S.S.; Inglis, G.G.A. An extremely fast and efficient acylation reaction of alcohols with acid anhydrides in the presence of trimethylsilyl trifluoromethanesulfonate as catalyst. Chem. Commun. 1996, 23, 2625–2626. [Google Scholar] [CrossRef]
- Carrigan, M.D.; Freiberg, D.; Smith, R.C.; Zerth, H.M.; Mohan, R.S. A simple and practical method for large-Scale acetylation of alcohols and diols using bismuth triflate. Synthesis 2001, 14, 2091–2094. [Google Scholar] [CrossRef]
- Chaubey, S.A.; Mishra, R. Synthesis of task-specifc imidazolium ionic liquid as an efcient catalyst in acetylation of alcohols, phenols, and amines. Chem. Pap. 2020, 74, 3259–3268. [Google Scholar] [CrossRef]
- Lee, S.G.; Park, J.H. Metallic Lewis acids-catalyzed acetylation of alcohols with acetic anhydride and acetic acid in ionic liquids: Study on reactivity and reusability of the catalysts. J. Mol. Catal. A Chem. 2003, 194, 49–52. [Google Scholar] [CrossRef]
- Qian, L.; Ji, C.; Chen, X.Z. Lewis basic ionic liquid as an efficient and facile catalyst for acetylation of alcohols, phenols, and amines under solvent-free conditions. Mon. Chem. Chem. Mon. 2013, 144, 369–374. [Google Scholar]
- Liu, Y.; Liu, L.; Lu, Y.; Cai, Y.Q. An imidazolium tosylate salt as efficient and recyclable catalyst for acetylation in an ionic liquid. Mon. Chem. Chem. Mon. 2008, 139, 633–638. [Google Scholar] [CrossRef]
- Yurev, Y.K.; Belyakova, Z.V. Acyloxysilanes. Russ. Chem. Rev. 1960, 29, 383–394. [Google Scholar] [CrossRef]
- Gilman, H.; Smart, G.N.R. Steric hindrances in highly-substituted organosilicon compounds. III. The preparation and properties of some triaryl silyl ethers. J. Org. Chem. 1954, 19, 441–450. [Google Scholar] [CrossRef]
- Sommer, L.H.; Parker, G.A.; Frye, C.L. Stereochemistry of asymmetric silicon. III. carboxylate and tosylate leaving groups. J. Am. Chem. Soc. 1964, 86, 3280–3283. [Google Scholar] [CrossRef]
- Gornowicz, A.G.; West, R. Lithiation of trimethylsilyl compounds. J. Am. Chem. Soc. 1968, 90, 4478–4479. [Google Scholar] [CrossRef]
- Anderson, H.H.; Fischer, H. Methylsilicon esters. Esters in an organosilicon “Conversion series”. J. Org. Chem. 1954, 19, 1296–1299. [Google Scholar] [CrossRef]
- Olah, G.A.; Narang, C.S.; Salem, F.G.; Gupta, B.G.B. Synthetic methods and reactions. Iodotrimethylsilane mediated mild and neutral transesterification of esters. Synthesis 1981, 2, 142–143. [Google Scholar] [CrossRef]
- Matsumoto, K.; Sajna, K.V.; Satoh, Y.; Sato, K.; Shimada, S. By-product free siloxane bond formation and one pot oligosiloxane synthesis. Angew. Chem. Int. Ed. 2017, 129, 3216–3219. [Google Scholar] [CrossRef]
- Rakovshik, A.; Shtelman, A.V.; Becker, J.Y. Synthesis of α-germyl and α-silylcarboxylic acids and selected electrochemical oxidations. J. Organomet. Chem. 2012, 706–709, 13–19. [Google Scholar] [CrossRef]
- Bellassoued, M.; Mouelhi, S.; Fromentin, P.; Gonzalez, A. Two-carbon homologation of ketones via sily ketene acetals: Synthesis of α, β-unsaturated acids and a trimethylsilyl δ-ketoacids. J. Organomet. Chem. 2005, 690, 2172–2179. [Google Scholar] [CrossRef]
- Chen, J.S.; Ke, Y.F.; Lin, H.Y.; Lin, W.; Yen, W.C.; Wud, H.R.; Luo, S.Y. Development of a novel method for trimethylsilylation of saccharides. Synthesis 2021, 53, 2000–2006. [Google Scholar] [CrossRef]
- Langer, S.H.; Connell, S.; Wender, J. Preparation and properties of trimethylsilyl ethers and related compounds. J. Org. Chem. 1958, 23, 50–58. [Google Scholar] [CrossRef]
- Gauuret, P.; El-Ghamarli, S.; Legrand, A.; Couirier, D.; Rigo, B. On the silylation of diarylcarbinols. Synth. Commun. 1996, 26, 707–713. [Google Scholar] [CrossRef]
- Morita, T.; Okamoto, Y.; Sakurai, H. Use of allyl silanes as a new type of silylating agent for alcohols and carboxylic acids. Tetrahedron Lett. 1980, 21, 835–838. [Google Scholar] [CrossRef]
- Azizi, N.; Yousefi, R.; Saidi, M.R. Efficient and practical protocol for silylation of hydroxylgroups using reusable lithium perchlorate dispreadin silica gel under neutral condition. J. Organometal. Chem. 2006, 691, 817–820. [Google Scholar] [CrossRef]
- Zadehahmadi, F.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Baltork, I.M.; Kardanpour, R. Highly efficient protection of alcohols and phenols catalysed by tin porphyrin supported on MIL-101. Appl. Organometal. Chem. 2015, 29, 209–215. [Google Scholar] [CrossRef]
- Gharaati, S.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Baltork, I.M. Highly efficient and selective trimethylsilylation of alcohols and phenols with hexamethyldisilazane catalyzed by polystyrene-bound tin (IV) porphyrin. Polyhedron 2012, 35, 87–95. [Google Scholar] [CrossRef]
- Boerner, D.; Koerner, G.; Spieker, F.; Wiemann, M. Verfahren zur Silylierung. German Patent DE2757936, 21 September 1978. [Google Scholar]
- Zhang, Z.H.; Li, T.S.; Yang, F.; Fu, C.G. Montmorillonite clay catalysis XI: Protection and deprotection of hydroxyl group by formation and cleavage of trimethylsilyl ethers catalysed by montmorillonite K-10. Synth. Commun. 1998, 28, 3105–3114. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Karimi, B. Zinc chloride catalyzed silylation of alcohols and phenols by hexamethyldisilazane. A highly chemoselective reaction. Synth. Commun. 1993, 23, 1633–1641. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Sardarian, A.R.; Khayat, Z.; Karimi, B.; Tangestaninejad, S. Nitrogen ligand complexes of metal chlorides as effective catalysts for the highly regioand chemoselective silylation of hydroxyl groups with hexamethyldisilazane (HMDS) at room temperature. Synth. Commun. 1997, 27, 2709–2719. [Google Scholar] [CrossRef]
- Karimi, B.; Golshani, B. Mild and highly efficient method for the silylation of alcohols using hexamethyldisilazane catalyzed by iodine under nearly neutral reaction conditions. J. Org. Chem. 2000, 65, 7228–7230. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, H.; Iranpoor, N.; Amani, K.; Nowrouzi, F. Tungstophosphoric acid (H3PW12O40) as a heterogeneous inorganic catalyst. Activation of hexamethyldisilazane (HMDS) by tungstophosphoric acid for efficient and selective solvent-free O-silylation reactions. J. Chem. Soc. Perkin Trans. 2002, 1, 2601–2604. [Google Scholar] [CrossRef]
- Shirini, F.; Atghia, S.V.; Jirdehi, M.G. Nanocrystalline TiO2-HClO4 as a new, efficient and recyclable catalyst for the chemoselective trimethylsilylation of alcohols, phenols and deprotection of silyl ethers. Catal. Commun. 2012, 18, 5–10. [Google Scholar] [CrossRef]
- Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Baltork, M.I.; Chahardahcheric, S.; Tavakoli, Z. Rapid and highly efficient trimethylsilylation of alcohols and phenols with hexamethyldisilazane (HMDS) catalyzed by reusable zirconyl triflate, [ZrO(OTf)2]. Organomet. Chem. 2008, 693, 2041–2046. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Farahi, S. Highly regio- and chemoselective silylation of diethyla-hydroxyphosphonates, alcohols and phenols in the presence of solid TiCl3(OTf) as a catalyst with hexamethyldisilazane (HMDS) at room temperature in the absence of solvent. Catal. Commun. 2009, 10, 1547–1550. [Google Scholar] [CrossRef]
- Villabrille, P.; Romanelli, G.; Quaranta, N.; Vázquez, P. An efficient catalytic route for the preparation of silyl ethers usingalumina-supported heteropolyoxometalates. Appl. Catal. B 2010, 96, 379–386. [Google Scholar] [CrossRef]
- Sridhar, M.; Raveendra, J.; Ramanaiah, B.C.; Narsaiah, C. An efficient synthesis of silyl ethers of primary alcohols, secondary alcohols, phenols and oximes with a hydrosilane using InBr3 as a catalyst. Tetrahedron Lett. 2011, 52, 5980–5982. [Google Scholar] [CrossRef]
- Shirini, F.; Khaligh, G.N.; Dadamahaleh, S.A. Preparation, characterization and use of 1,3-disulfonic acid imidazolium hydrogen sulfate as an efficient, halogen-free and reusable ionic liquid catalyst for the trimethylsilyl protection of hydroxyl groups and deprotection of the obtained trimethylsilanes. J. Mol. Catal. A Chem. 2012, 365, 15–23. [Google Scholar]
- Shirini, F.; Mollarazi, E. Efficient trimethylsilylation of alcohols and phenols in the presence of ZrCl4 as a reusable catalyst. Catal. Commun. 2007, 8, 1393–1396. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Portillo, A.S.; Concepción, P.; Herance, R.J.; Navalón, S.; Alvaro, P.; Garcia, H. Room temperature silylation of alcohols catalyzed by metal organic frameworks. Catal. Sci. Technol. 2017, 7, 2445–2449. [Google Scholar] [CrossRef]
- Anbu, N.; Vijayan, C.; Dhakshinamoorthy, A. A simple and efficient room temperature silylation of diverse functional groups with hexamethyldisilazane using CeO2 nanoparticles as solid catalysts. Mol. Catal. 2019, 474, 110357. [Google Scholar] [CrossRef]
- Ko, Y.C.; Tsai, C.F.; Wang, C.C.; Dhurandhare, V.M.; Hu, L.P.; Su, T.Y.; Lico, L.S.; Zulueta, M.M.L.; Hung, S.C. Microwave-assisted one-pot synthesis of 1,6-anhydrosugars and orthogonally protected thioglycosides. J. Am. Chem. Soc. 2014, 136, 14425–14431. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.A.; Dhurandhare, V.M.; Chang, C.W.; Verma, V.P.; Mishra, G.P.; Ku, C.C.; Lin, C.C.; Wang, C.C. Chemoselective per-O-trimethylsilylation and homogeneous N-functionalisation of amino sugars. Chem. Commun. 2015, 51, 104. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Azizi, N.; Senejani, M.A. Chlorozincate (II) acidic ionic liquid: Efficient and biodegradable silylation catalyst. Appl. Organomet. Chem. 2017, 31, e3790. [Google Scholar] [CrossRef]
- Tajik, H.; Niknama, K.; Karimian, S. Silylation of alcohols and phenols by HMDS in the presence of ionic liquid and silica-supported ionic liquids. Iran. J. Catal. 2013, 3, 107–113. [Google Scholar]
- Ahrens, S.; Peritz, A.; Strassner, T. Tunable aryl alkyl ionic liquids (TAAILs): The next generation of ionic liquids. Angew. Chem. Int. Ed. 2009, 48, 7908–7910. [Google Scholar] [CrossRef]
- Cole, A.C.; Jensen, L.J.; Ntai, I.; Tran, K.L.T.; Weaver, K.J.; Forbes, D.C.; Davis, J.H. Novel Brønsted acidic ionic liquids and their use as dual solvent-catalysts. J. Am. Chem. Soc. 2002, 124, 5962–5963. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.; Lu, X.; Zhang, L.; Jiang, J.; Zhang, L. Reusable Brønsted acidic ionic liquid efficiently catalyzed N-formylation and N-acylation of amines. ACS Sustain. Chem. Eng. 2020, 8, 4353–4361. [Google Scholar] [CrossRef]
- Lin, Y.J.; Wu, Y.P.; Thul, M.; Hung, M.W.; Chou, S.H.; Chen, W.T.; Lin, W.; Lin, M.; Reddy, D.M.; Wu, H.R.; et al. Tunable aryl imidazolium recyclable ionic liquid with dual Brønsted-Lewis acid as green catalyst for Friedel-Crafts acylation and thioesterification. Molecules 2020, 25, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thul, M.; Wu, P.Y.; Lin, Y.J.; Du, S.L.; Wu, H.R.; Ho, W.Y.; Luo, S.Y. Ionic iquid catalyzed per-O-acetylation and benzylidene ring-opening reaction. Catalysts 2020, 10, 642. [Google Scholar] [CrossRef]
- Thul, M.; Pantawane, A.; Lin, W.; Lin, Y.J.; Po, P.F.; Tseng, S.A.; Wu, H.R.; Ho, W.Y.; Luo, S.Y. Tunable aryl imidazolium ionic liquids (TAIILs) as environmentally benign catalysts for the esterification of fatty acids to biodiesel fuel. Catal. Commun. 2021, 149, 106243. [Google Scholar] [CrossRef]
- Orita, A.; Mitsutome, A.; Otera, J. Distannoxane-catalyzed highly selective acylation of alcohols. J. Org. Chem. 1998, 63, 2420–2421. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).