Steam Reforming of Chloroform-Ethyl Acetate Mixture to Syngas over Ni-Cu Based Catalysts
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalytic Performance
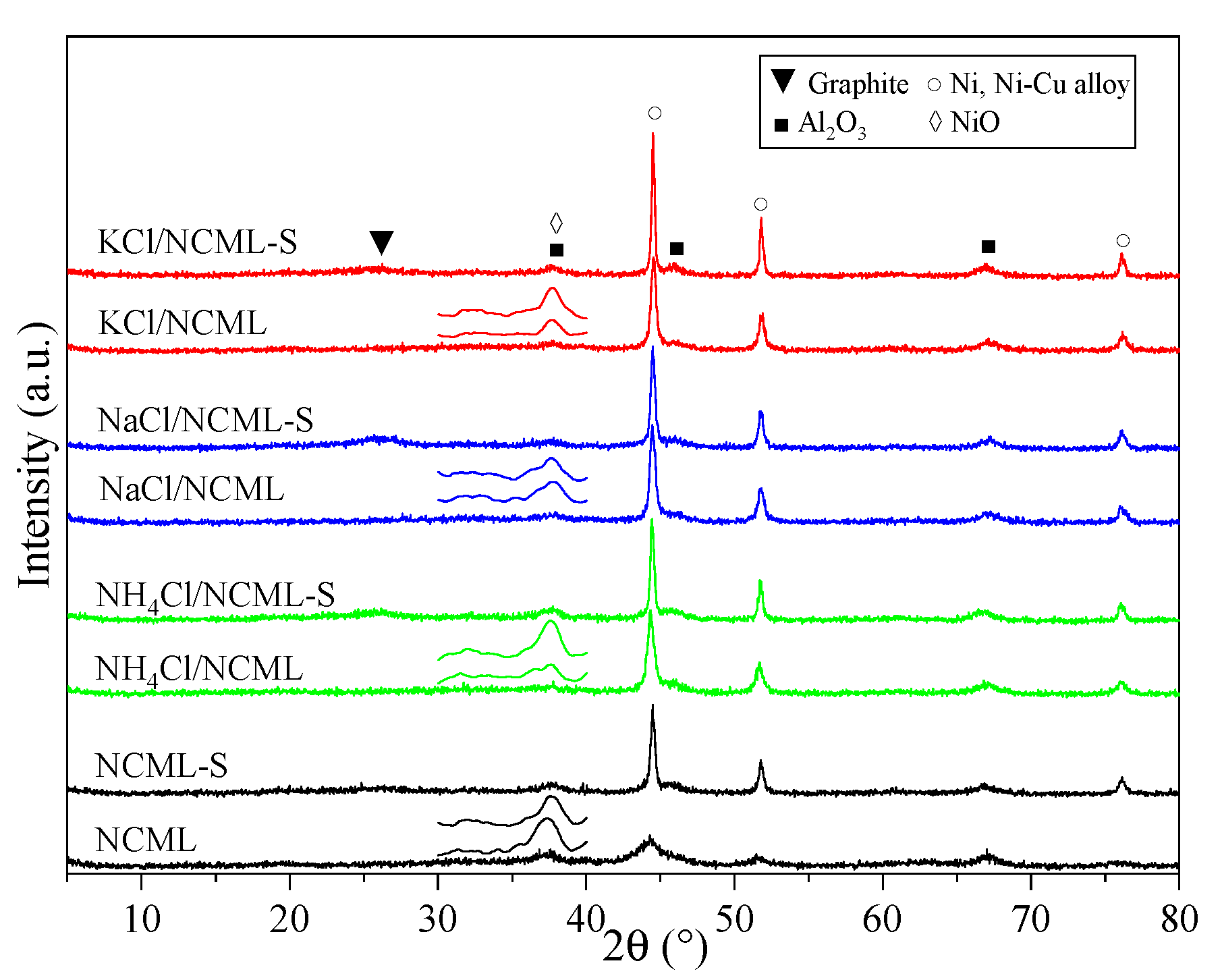
2.2. Textural and Structure Properties
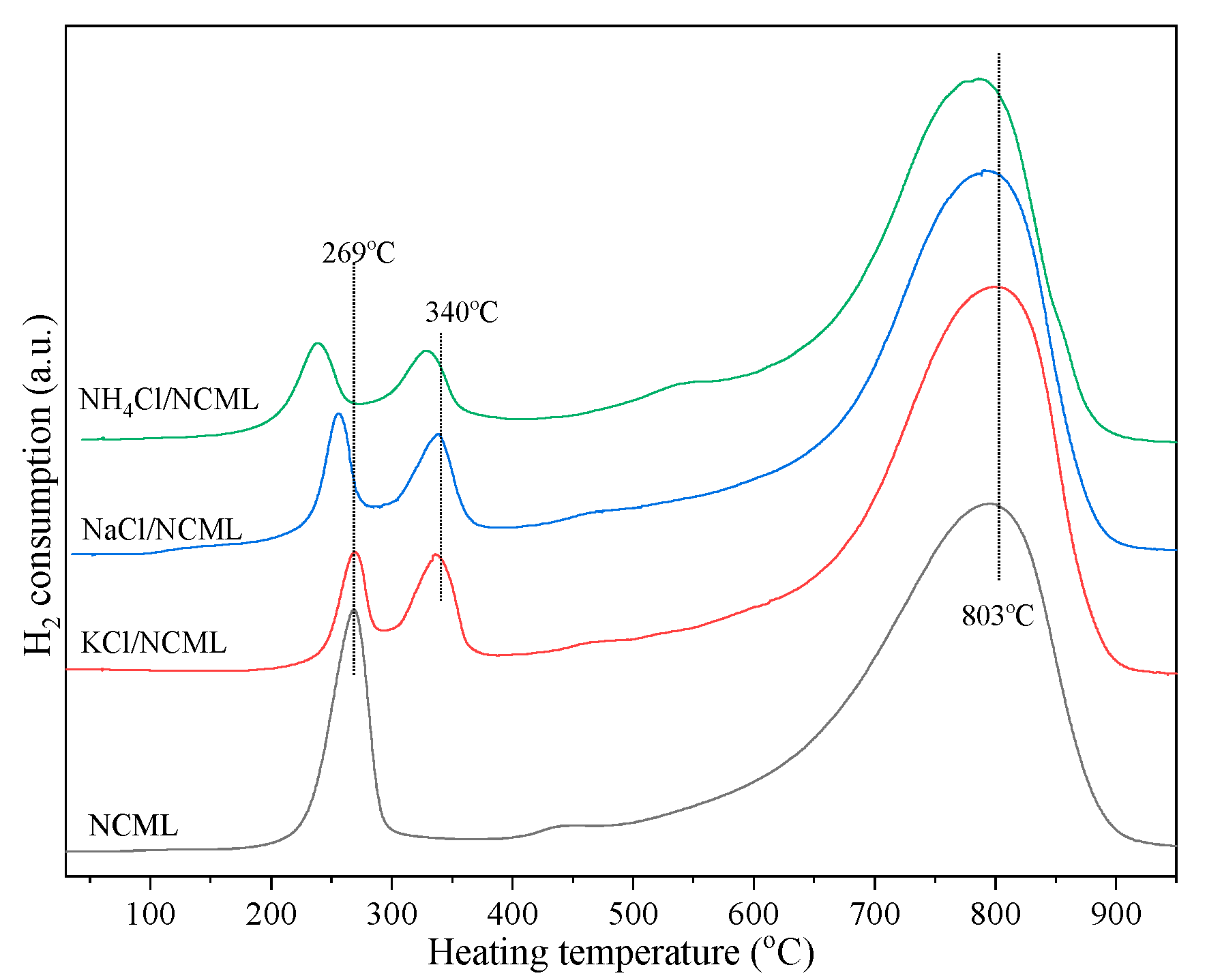
2.3. Reductive Property
2.4. Acid-Base Property
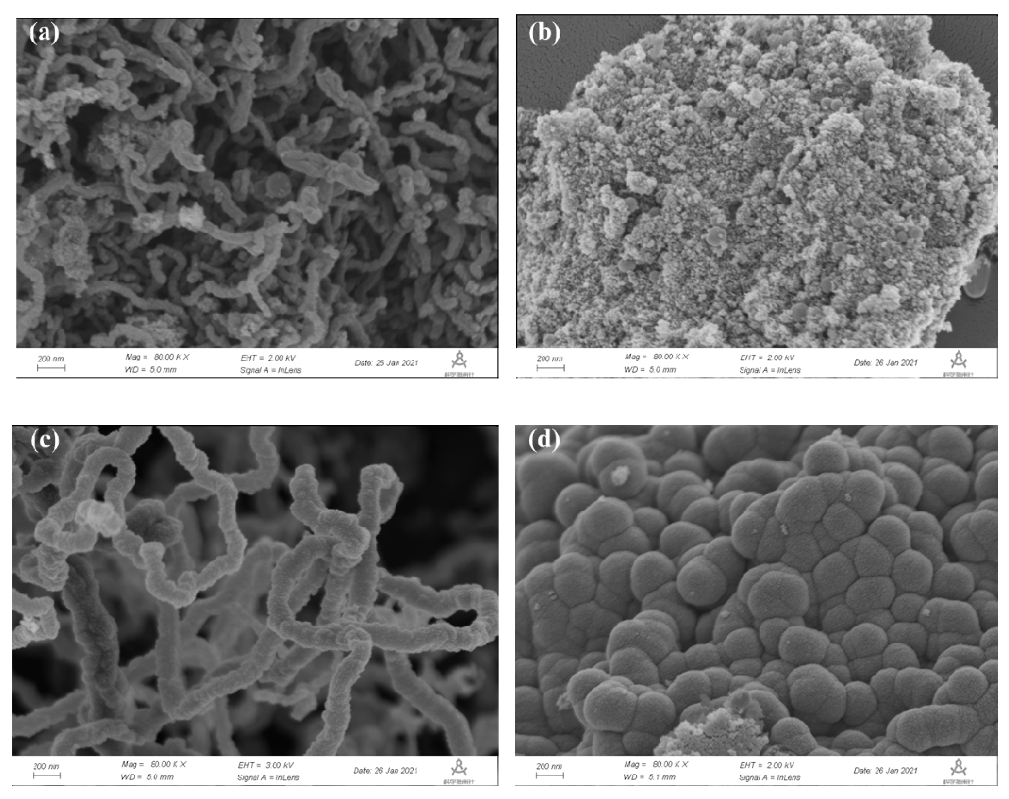
2.5. SEM Results
2.6. Effect of Reforming Parameters
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalytic Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, G.J.; Li, J.B.; Zeng, G.M. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef]
- Wang, R.K.; Ye, X.M.; Zhao, Z.H.; Yin, Q.Q.; Qi, Z.F.; Wang, Z.Y. Simultaneous disposal and utilization of coal chemical wastewater in coal and petroleum coke slurry preparation: Slurrying performance and mechanism. Fuel 2018, 215, 312–319. [Google Scholar] [CrossRef]
- Wang, S.N.; Liu, J.Z.; Pisupatil, S.V.; Li, D.D.; Wang, Z.H.; Cheng, J. Dispersion mechanism of coal water slurry prepared by mixing various high-concentration organic waste liquids. Fuel 2020, 287. [Google Scholar] [CrossRef]
- Wang, H.L.; Fu, P.B.; Li, J.P.; Huang, Y.; Zhao, Y.; Jiang, L.; Fang, X.C.; Yang, T.; Huang, Z.H.; Huang, C. Separation-and-recovery technology for organic waste liquid with a high concentration of inorganic particles. Engineering 2018, 4, 405–415. [Google Scholar] [CrossRef]
- Sun, W.L.; Qu, Y.Z.; Yu, Q.; Ni, J.R. Adsorption of organic pollutants from coking and papermaking wastewaters by bottom ash. J. Hazard. Mater. 2008, 154, 595–601. [Google Scholar] [CrossRef]
- Molina, R.; Martínez, F.; Melero, J.A.; Bremner, D.H.; Chakinala, A.G. Mineralization of phenol by a heterogeneous ultrasound/Fe-SBA-15/H2O2 process: Multivariate study by factorial design of experiments. Appl. Catal. B 2006, 66, 198–207. [Google Scholar] [CrossRef]
- Xing, Z.P.; Sun, D.Z.; Yu, X.J.; Zou, J.L.; Zhou, W. Treatment of antibiotic fermentation-based pharmaceutical wastewater using anaerobic and aerobic moving bed biofilm reactors combined with ozone/hydrogen peroxide process. Environ. Prog. Sustain. Energy 2013, 33, 170–177. [Google Scholar] [CrossRef]
- Lee, Y.H.; Von Gunten, U. Oxidative transformation of micropollutants during municipal wastewater treatment: Comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrateVI, and ozone) and non-selective oxidants (hydroxyl radical). Water Res. 2010, 44, 555–566. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Wang, N.N.; Zheng, T.; Zhang, G.S.; Wang, P. A review on fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Zhu, Q.S.; Guo, S.H.; Guo, C.M.; Dai, D.; Jiao, X.K.; Ma, T.Q.; Chen, J.F. Stability of Fe-C micro-electrolysis and biological process in treating ultra-high concentration organic wastewater. Chem. Eng. J. 2014, 255, 535–540. [Google Scholar] [CrossRef]
- El-Bahy, Z.M.; Ismail, A.A.; Mohamed, R.M. Enhancement of titania by doping rare earth for photodegradation of organic dye (direct blue). J. Hazard. Mater. 2009, 166, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, Z.D.; Chen, X.; Wang, H.; Dai, S.; Pan, X.Q.; Ren, Z.J.; Gu, J. Spontaneous solar syngas production from CO2 driven by energetically favorable wastewater microbial anodes. Joule 2020, 4, 2149–2161. [Google Scholar] [CrossRef]
- Kumar, G.; Eswari, A.P.; Kavitha, S.; Kumar, M.D.; Kannah, R.Y.; How, L.C.; Muthukaruppan, G.; Banu, J.R. Thermochemical conversion routes of hydrogen production from organic biomass: Processes, challenges and limitations. Biomass Conv. Bioref. 2020, 2, 1–26. [Google Scholar] [CrossRef]
- Nabgan, B.; Abdullah, T.A.T.; Tahir, M.; Nabgan, W.; Triwahyono, S.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Moghadamian, K. Pellet size dependent steam reforming of polyethylene terephthalate waste for hydrogen production over Ni/La promoted Al2O3 catalyst. Int. J. Hydrogen Energy 2017, 42, 21571–21585. [Google Scholar] [CrossRef]
- Shafiei, M.; Richardson, J.T. Dechlorination of chlorinated hydrocarbons by catalytic steam reforming. Appl. Catal. B 2004, 54, 251–259. [Google Scholar] [CrossRef]
- Kousi, K.; Kondarides, D.I.; Verykios, X.E.; Papadopoulou, C. Glycerol steam reforming over modified Ru/Al2O3 catalysts. Appl. Catal. A 2017, 542, 201–211. [Google Scholar] [CrossRef]
- Vita, A.; Cristiano, G.; Italiano, C.; Specchia, S.; Cipití, F.; Specchi, V. Methane oxy-steam reforming reaction: Performances of Ru/γ-Al2O3 catalysts loaded on structured cordierite monoliths. Int. J. Hydrogen. Energy 2014, 39, 18592–18603. [Google Scholar] [CrossRef]
- Shejale, A.D.; Yadav, G.D. Cu promoted Ni-Co/hydrotalcite catalyst for improved hydrogen production in comparison with several modified Ni-based catalysts via steam reforming of ethanol. Int. J. Hydrogen Energy 2017, 42, 11321–11332. [Google Scholar] [CrossRef]
- De Rogatis, L.; Montini, T.; Lorenzut, B.; Fornasiero, P. NixCuy/Al2O3 based catalysts for hydrogen production. Energy Environ. Sci. 2008, 1, 501–509. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Meloni, E.; Ricca, A. Oxidative reforming of ethanol over CeO2-SiO2 based catalysts in a fluidized bed reactor. Chem. Eng. Process. 2018, 124, 319–327. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Meloni, E.; Ricca, A. Influence of catalytic formulation and operative conditions on coke deposition over CeO2-SiO2 based catalysts for ethanol reforming. Energies 2017, 10, 1030. [Google Scholar] [CrossRef]
- Dou, B.L.; Song, Y.C.; Wang, C.; Chen, H.S.; Xu, Y.J. Hydrogen production from catalytic steam reforming of biodiesel byproduct glycerol: Issues and challenges. Renew. Sustain. Energy Rev. 2014, 30, 950–960. [Google Scholar] [CrossRef]
- Italiano, C.; Bizkarra, K.; Barrio, V.L.; Cambra, J.F.; Pino, L.; Vita, A. Renewable hydrogen production via steam reforming of simulated bio-oil over Ni-based catalysts. Int. J. Hydrogen Energy 2019, 44, 14671–14682. [Google Scholar] [CrossRef]
- Fu, P.; Zhang, A.; Luo, S.; Yi, W.; Hu, S.; Zhang, Y. Catalytic steam reforming of biomass-derived acetic acid over two supported Ni catalysts for hydrogen-rich syngas production. ACS Omega 2019, 4, 13585–13593. [Google Scholar] [CrossRef] [PubMed]
- Landi, G.; Di Benedetto, A. Ni/CeO2 structured catalysts for solar reforming of spent solvents. Catalysts 2019, 9, 688. [Google Scholar] [CrossRef]
- Ramesh, S.; Yang, E.H.; Jung, J.S.; Moon, D.J. Copper decorated perovskite an efficient catalyst for low temperature hydrogen production by steam reforming of glycerol. Int. J. Hydrogen Energy 2015, 40, 11428–11435. [Google Scholar] [CrossRef]
- Liu, J.Y.; Su, W.N.; Rick, J.; Yang, S.C.; Cheng, J.H.; Pan, C.J.; Lee, J.F.; Hwang, B.J. Hierarchical copper-decorated nickel nanocatalysts supported on La2O3 for low-temperature steam reforming of ethanol. ChemSusChem 2014, 7, 570–576. [Google Scholar] [CrossRef]
- Toops, T.J.; Walters, A.B.; Vannice, M.A. The effect of CO2, H2O and SO2 on the kinetics of NO reduction by CH4 over La2O3. Appl. Catal. B 2002, 38, 183–199. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, H.H.; Gao, N.B. Development of activated biochar supported Ni catalyst for enhancing toluene steam reforming. Int. J. Energy Res. 2020, 44, 1–16. [Google Scholar] [CrossRef]
- Maluf, S.S.; Assaf, E.M. Ni catalysts with Mo promoter for methane steam reforming. Fuel 2009, 88, 1547–1553. [Google Scholar] [CrossRef]
- Saitoh, T.; Yamaguchi, M.; Hiraide, M. Surfactant-coated aluminum hydroxide for the rapid removal and biodegradation of hydrophobic organic pollutants in water. Water Res. 2011, 45, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Ortego, J.D., Jr.; Richardson, J.T.; Twigg, M.V. Catalytic steam reforming of chlorocarbons: Methyl chloride. Appl. Catal. B 1997, 12, 339–355. [Google Scholar] [CrossRef]
- Veksha, A.; Giannis, A.; Oh, W.D.; Chang, V.W.C.; Lisak, G.; Lim, T.T. Catalytic activities and resistance to HCl poisoning of Ni-based catalysts during steam reforming of naphthalene. Appl. Catal. A 2018, 557, 25–38. [Google Scholar] [CrossRef]
- Kiskinova, M.; Goodman, D.W. Modification of chemisorption properties by electronegative adatoms: H2 and CO on chlorided, sulfided, and phosphided Ni (100). Surf. Sci. 1981, 108, 64–76. [Google Scholar] [CrossRef]
- Couté, N.; Richardson, J.T. Catalytic steam reforming of chlorocarbons: Polychlorinated biphenyls (PCBs). Appl. Catal. B 2000, 26, 265–273. [Google Scholar] [CrossRef]
- Couté, N.; Richardson, J.T. Steam reforming of chlorocarbons: Chlorinated aromatics. Appl. Catal. B 2000, 26, 217–226. [Google Scholar] [CrossRef]
- Cesteros, Y.; Salagre, P.; Medina, F.; Sueiras, J.E. Effect of the alumina phase and its modification on Ni/Al2O3 catalysts for the hydrodechlorination of 1,2,4-trichlorobenzene. Appl. Catal. B 1999, 22, 135–147. [Google Scholar] [CrossRef]
- Srinivas, S.T.; Prasad, P.S.S.; Madhavendra, S.S.; Rao, P.K. Hydrodechlorination of chlorobenzene over MgO modified Pt/A12O3 catalysts. Stud. Surf. Sci. Catal. 1998, 113, 835–839. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Xu, C.H.; Liu, C.Q.; Xiao, H.W.; Chen, J.; Zhang, Y.X.; Lei, Y.C. Glycerol steam reforming over Ni/γ-Al2O3 catalysts modified by metal oxides. Korean J. Chem. Eng. 2013, 30, 587–592. [Google Scholar] [CrossRef]
- Dou, X.M.; Veksha, A.; Chan, W.P.; Oh, W.D.; Liang, Y.N.; Teoh, F.; Mohamed, D.K.B.; Giannis, A.; Lisak, G.; Lim, T.T. Poisoning effects of H2S and HCl on the naphthalene steam reforming and water-gas shift activities of Ni and Fe catalysts. Fuel 2019, 241, 1008–1018. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over nickel catalyst supported on mesoporous alumina prepared by a non-ionic surfactant-templating method. Int. J. Hydrogen Energy 2009, 34, 1809–1817. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, E.G.; Joo, O.S.; Jung, K.D. Stabilization of Ni/Al2O3catalyst by Cu addition for CO2 reforming of methane. Appl. Catal. A 2004, 269, 1–6. [Google Scholar] [CrossRef]
- Li, D.L.; Koike, M.; Chen, J.H.; Nakagawa, Y.; Tomishige, K. Preparation of Ni-Cu/Mg/Al catalysts from hydrotalcite-like compounds for hydrogen production by steam reforming of biomass tar. Int. J. Hydrogen Energy 2014, 39, 10959–10970. [Google Scholar] [CrossRef]
- Yu, Z.J.; Zhang, L.J.; Zhang, C.T.; Gao, G.G.; Ye, Z.M.; Zhang, S.; Liu, Q.; Hu, G.Z.; Hu, X. Steam reforming of acetic acid over nickel catalysts: Impacts of fourteen additives on the catalytic behaviors. J. Energy Inst. 2020, 93, 1000–1019. [Google Scholar] [CrossRef]
- Abd El-Hafiz, D.R.; Ebiad, M.A.; El-salamony, R.A. Hydrogen selectivity and carbon behavior during gasoline steam reforming over nano-Al2O3 catalysts. Mater. Renew. Sustain. Energy 2014, 3, 34–46. [Google Scholar] [CrossRef][Green Version]
- Lorenzut, B.; Montini, T.; De Rogatis, L.; Canton, P.; Benedetti, A.; Fornasiero, P. Hydrogen production through alcohol steam reforming on Cu/ZnO-based catalysts. Appl. Catal. B 2011, 101, 397–408. [Google Scholar] [CrossRef]
- Wang, X.T.; Su, X.X.; Zhang, Q.W.; Hu, H.P. Effect of additives on Ni-based catalysts for hydrogen-enriched production from steam reforming of biomass. Energy Technol. 2020, 8, 2000136. [Google Scholar] [CrossRef]
- Ochoa, A.; Bilbao, J.; Gayubo, A.G.; Castano, P. Coke formation and deactivation during catalytic reforming of biomass and waste pyrolysis products: A review. Renew. Sustain. Energy Rev. 2020, 119, 109600. [Google Scholar] [CrossRef]



| Catalysts | Conversion of Oil (%) | Capacity of Gas Production (m3·k) | Catalytic Life (min) | Carbon Deposit (mg ·h−1) | Gaseous Products Distribution (mol%) | |||
|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | CH4 | |||||
| NCML | 99.46 | 4.07 | 248 | 43.7 | 68.25 | 16.36 | 14.27 | 1.12 |
| NH4Cl/NCML | 98.83 | 4.52 | 272 | 49.6 | 70.07 | 13.16 | 15.16 | 1.61 |
| NaCl/NCML | 98.30 | 4.55 | 291 | 42.4 | 69.27 | 12.15 | 15.21 | 3.37 |
| KCl/NCML | 97.83 | 4.35 | 408 | 40.7 | 69.83 | 12.85 | 14.77 | 2.55 |
| KCl/NCML @ | 97.97 | 4.27 | 638 | 38.0 | 69.68 | 13.15 | 14.06 | 3.11 |
| Catalysts | N2 Adsorption–Desorption | Acidic Sites (mmol·) a | Cell Size of Nickel b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SBET (m2·g−1) | VBJH (mL·g−1) | Dpore (nm) | AW | AM | AT | dNi,F (nm) | dNi,S (nm) | ∆dNi (nm) | |
| NCML | 87.91 | 0.40 | 6.25 | 0.038 | 0.173 | 0.211 | 8.10 | 22.89 | 14.78 |
| NH4Cl/NCML | 92.83 | 0.51 | 6.83 | 0.106 | 0.112 | 0.218 | 14.61 | 23.23 | 8.63 |
| NaCl/NCML | 94.37 | 0.52 | 6.65 | 0.039 | 0.090 | 0.128 | 17.08 | 22.02 | 4.94 |
| KCl/NCML | 95.04 | 0.46 | 6.34 | 0.036 | 0.071 | 0.107 | 18.44 | 24.21 | 6.78 |
| Heating Rate during Catalyst Reduction (°C⋅min−1) | LHSV (h−1) | H2O/C Ratio | Conversion of Oil (%) | Capacity of Gas Production (m3 k) | Catalytic Life (min) | Gaseous Products Distribution (mol%) | |||
|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | CH4 | ||||||
| 2 | 3.30 | 2.13 | 96.24 | 3.95 | 460 | 66.38 | 12.89 | 14.94 | 5.79 |
| 5 | 3.30 | 2.13 | 97.37 | 4.26 | 450 | 67.34 | 13.32 | 14.88 | 4.46 |
| 8 | 3.30 | 2.13 | 97.83 | 4.35 | 408 | 69.83 | 12.85 | 14.78 | 2.55 |
| 10 | 3.30 | 2.13 | 98.16 | 4.27 | 412 | 69.56 | 12.53 | 14.89 | 3.02 |
| 5 | 6.66 | 2.13 | 83.64 | 3.04 | 190 | 66.19 | 16.77 | 11.14 | 5.90 |
| 5 | 4.40 | 2.13 | 90.20 | 4.03 | 280 | 66.84 | 15.62 | 12.04 | 5.51 |
| 5 | 3.77 | 2.13 | 94.96 | 4.11 | 430 | 68.10 | 15.06 | 12.52 | 4.31 |
| 5 | 3.30 | 4.21 | 97.96 | 4.55 | 1140 | 68.96 | 11.56 | 16.86 | 2.62 |
| 5 | 3.30 | 5.17 | 98.34 | 4.72 | 1620 | 69.07 | 11.36 | 16.97 | 2.60 |
| 5 | 3.30 | 8.11 | 98.64 | 5.48 | 3060 | 70.31 | 8.01 | 19.51 | 2.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Xu, C.; Zheng, Y.; Liu, J.; Deng, Z.; Liu, J. Steam Reforming of Chloroform-Ethyl Acetate Mixture to Syngas over Ni-Cu Based Catalysts. Catalysts 2021, 11, 826. https://doi.org/10.3390/catal11070826
Wu Q, Xu C, Zheng Y, Liu J, Deng Z, Liu J. Steam Reforming of Chloroform-Ethyl Acetate Mixture to Syngas over Ni-Cu Based Catalysts. Catalysts. 2021; 11(7):826. https://doi.org/10.3390/catal11070826
Chicago/Turabian StyleWu, Qiong, Chenghua Xu, Yuhao Zheng, Jie Liu, Zhiyong Deng, and Jianying Liu. 2021. "Steam Reforming of Chloroform-Ethyl Acetate Mixture to Syngas over Ni-Cu Based Catalysts" Catalysts 11, no. 7: 826. https://doi.org/10.3390/catal11070826
APA StyleWu, Q., Xu, C., Zheng, Y., Liu, J., Deng, Z., & Liu, J. (2021). Steam Reforming of Chloroform-Ethyl Acetate Mixture to Syngas over Ni-Cu Based Catalysts. Catalysts, 11(7), 826. https://doi.org/10.3390/catal11070826






