Dry Reforming of Methane Using Ni Catalyst Supported on ZrO2: The Effect of Different Sources of Zirconia
Abstract
:1. Introduction
2. Results and Discussions
2.1. Characterization
2.2. Results of Activity
3. Experimental
3.1. Materials
3.2. Catalyst Formation
3.3. Characterization
3.4. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Fatesh, A.S.; Kumar, R.; Kasim, S.O.; Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Alrasheed, R.; Bagabas, A.; Chaudhary, M.L.; Frusteri, F.; et al. The effect of modifier identity on the performance of Ni-based catalyst supported on γ-Al2O3 in dry reforming of methane. Catal. Today 2020, 348, 236–242. [Google Scholar] [CrossRef]
- Agus, E.; Zhang, L.; Sedlak, D.L. A framework for identifying characteristic odor compounds in municipal wastewater effluent. Water Res. 2012, 46, 5970–5980. [Google Scholar] [CrossRef]
- Gomes, R.; Costa, D.; Junior, R.; Santos, M.; Rodella, C.; Fréty, R.; Beretta, A.; Brandão, S. Dry Reforming of Methane over NiLa-Based Catalysts: Influence of Synthesis Method and Ba Addition on Catalytic Properties and Stability. Catalysts 2019, 9, 313. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Yang, B.; Miao, S.; Liu, W.; Xie, J.; Lee, S.; Pellin, M.J.; Xiao, D.; Su, D.; Ma, D. Lattice Strained Ni-Co alloy as a High-Performance Catalyst for Catalytic Dry Reforming of Methane. ACS Catal. 2019, 9, 2693–2700. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Rui, N.; Li, X.; Lin, L.; Betancourt, L.E.; Su, D.; Xu, W.; Cen, J.; Attenkofer, K.; et al. Highly Active Ceria-Supported Ru Catalyst for the Dry Reforming of Methane: In Situ Identification of Ruδ+-Ce3+ Interactions for Enhanced Conversion. ACS Catal. 2019, 9, 3349–3359. [Google Scholar] [CrossRef]
- Vinaches, P.; de Mello, M.S.; Meneau, F.; Costa, I.C.M.; Morgado, E.; Pergher, S. Effect of mother liquor addition on (P)MCM-22 synthesis. Microporous Mesoporous Mater. 2020, 306, 110370. [Google Scholar] [CrossRef]
- Faroldi, B.; Múnera, J.; Falivene, J.M.; Ramos, I.R.; García, Á.G.; Fernández, L.T.; Carrazán, S.G.; Cornaglia, L. Well-dispersed Rh nanoparticles with high activity for the dry reforming of methane. Int. J. Hydrog. Energy 2017, 42, 16127–16138. [Google Scholar] [CrossRef]
- Egawa, C. Methane dry reforming reaction on Ru(001)surfaces. J. Catal. 2018, 358, 35–42. [Google Scholar] [CrossRef]
- Jin, B.; Li, S.; Liang, X. Enhanced activity and stability of MgO-promoted Ni/Al2O3 catalyst for dry reforming of methane: Role of MgO. Fuel 2021, 284, 119082. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I.V. Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. Int. J. Hydrog. Energy 2017, 42, 13724–13740. [Google Scholar] [CrossRef]
- Taherian, Z.; Yousefpour, M.; Tajally, M.; Khoshandam, B. Catalytic performance of Samaria-promoted Ni and Co/SBA-15 catalysts for dry reforming of methane. Int. J. Hydrog. Energy 2017, 42, 24811–24822. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Al-Fatesh, A.S.; Khan, W.U.; Kasim, S.O.; Abasaeed, A.E.; Fakeeha, A.H.; Bonura, G.; Frusteri, F. Enhanced coke suppression by using phosphate-zirconia supported nickel catalysts under dry methane reforming conditions. Int. J. Hydrog. Energy 2019, 44, 27784–27794. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.; Trimm, D.L. Mechanisms of carbon formation on nickel-containing catalysts. J. Catal. 1977, 48, 155–165. [Google Scholar] [CrossRef]
- Fang, X.; Peng, C.; Peng, H.; Liu, W.; Xu, X.; Wang, X.; Li, C.; Zhou, W. Methane Dry Reforming over Coke-Resistant Mesoporous Ni-Al2O3 Catalysts Prepared by Evaporation-Induced Self-Assembly Method. ChemCatChem 2015, 7, 3753–3762. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Rabelo-Neto, R.C.; Epron, F.; Bion, N.; Toniolo, F.S.; Noronha, F.B. Embedded Ni nanoparticles in CeZrO2 as stable catalyst for dry reforming of methane. Appl. Catal. B Environ. 2020, 268, 118387. [Google Scholar] [CrossRef]
- Pu, J.; Luo, Y.; Wang, N.; Bao, H.; Wang, X.; Qian, E.W. Ceria-promoted Ni@Al2O3 core-shell catalyst for steam reforming of acetic acid with enhanced activity and coke resistance. Int. J. Hydrog. Energy 2018, 43, 3142–3153. [Google Scholar] [CrossRef]
- Van Beurden, P. On the Catalytic Aspects of Steam-Methane Reforming. A Literature Survey; ECN: Petten, The Netherlands, 2004. [Google Scholar]
- Pines, H.; Haag, W.O. Alumina: Catalyst and Support. I. Alumina, its Intrinsic Acidity and Catalytic Activity. J. Am. Chem. Soc. 1960, 82, 2471–2483. [Google Scholar] [CrossRef]
- Lahousse, C.; Aboulayt, A.; Maugé, F.; Bachelier, J.; Lavalley, J.C. Acidic and basic properties of zirconia-alumina and zirconia-titania mixed oxides. J. Mol. Catal. 1993, 84, 283–297. [Google Scholar] [CrossRef]
- Faria, E.C.; Neto, R.C.R.; Colman, R.C.; Noronha, F.B. Hydrogen production through CO2 reforming of methane over Ni/CeZrO2/Al2O3 catalysts. Catal. Today 2014, 228, 138–144. [Google Scholar] [CrossRef]
- Stagg-Williams, S.M.; Noronha, F.B.; Fendley, G.; Resasco, D.E. CO2 Reforming of CH 4 over Pt/ZrO2 Catalysts Promoted with La and Ce Oxides. J. Catal. 2000, 194, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Maia, T.A.; Assaf, J.M.; Assaf, E.M. Performance of cobalt catalysts supported on CexZr1-xO2 (0 < x < 1) solid solutions in oxidative ethanol reforming. React. Kinet. Mech. Catal. 2013, 109, 181–197. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Al-Fatesh, A.S.; Arafat, Y.; Ibrahim, A.A.; Kasim, S.O.; Alharthi, A.; Fakeeha, A.H.; Abasaeed, A.E.; Bonura, G.; Frusteri, F. Catalytic behaviour of Ce-doped Ni systems supported on stabilized zirconia under dry reforming conditions. Catalysts 2019, 9, 473. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Zhou, Y.; Miao, S.; Li, Y.; Shen, W. Assembly of monoclinic ZrO2 nanorods: Formation mechanism and crystal phase control. CrystEngComm 2016, 18, 580–587. [Google Scholar] [CrossRef]
- El-Salamony, R.A.; El-Temtamy, S.A.; El Naggar, M.A.A.; Ghoneim, S.A.; El-Hafiz, D.R.A.; Ebiad, M.A.; Gendy, T.; Al-Sabagh, A.M. Valuation of Catalytic Activity of Nickel-Zirconia-Based Catalysts Using Lanthanum Co-Support for Dry Reforming of Methane; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Zafeiropoulos, G.; Nikolopoulos, N.; Kordouli, E.; Sygellou, L.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Developing nickel-zirconia Co-precipitated catalysts for production of green diesel. Catalysts 2019, 9, 210. [Google Scholar] [CrossRef] [Green Version]
- Guilera, J.; Del Valle, J.; Alarcón, A.; Díaz, J.A.; Andreu, T. Metal-oxide promoted Ni/Al2O3 as CO2 methanation micro-size catalysts. J. CO2 Util. 2019, 30, 11–17. [Google Scholar] [CrossRef]
- Che, M.; Bennett, C.O. The Influence of Particle Size on the Catalytic Properties of Supported Metals. Adv. Catal. 1989, 36, 55–172. [Google Scholar] [CrossRef]
- Prieto, G.; Zečević, J.; Friedrich, H.; De Jong, K.P.; De Jongh, P.E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 2012, 12, 34–39. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef] [Green Version]
- Rad, S.J.H.; Haghighi, M.; Eslami, A.A.; Rahmani, F.; Rahemi, N. Sol-gel vs. impregnation preparation of MgO and CeO2 doped Ni/Al2O3 nanocatalysts used in dry reforming of methane: Effect of process conditions, synthesis method and support composition. Int. J. Hydrog. Energy 2016, 41, 5335–5350. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Yan, Z.F. Nanocrystalline zirconia as support for nickel catalyst in methane reforming with CO2. Energy Fuels 2006, 20, 923–929. [Google Scholar] [CrossRef]
- Dantas, S.C.; Escritori, J.C.; Soares, R.R.; Hori, C.E. Effect of different promoters on Ni/CeZrO2 catalyst for autothermal reforming and partial oxidation of methane. Chem. Eng. J. 2010, 156, 380–387. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, M.; Patel, J.; Bordoloi, A. A study of the synergy between support surface properties and catalyst deactivation for CO2 reforming over supported Ni nanoparticles. Appl. Catal. A Gen. 2017, 545, 113–126. [Google Scholar] [CrossRef]
- Poole, C.F. Stavros Kromidas (Ed): The HPLC–MS Handbook for Practitioners. Chromatographia 2018, 81, 829–830. [Google Scholar] [CrossRef]
- Gürbüz, E.I.; Kunkes, E.L.; Dumesic, J.A. Integration of C-C coupling reactions of biomass-derived oxygenates to fuel-grade compounds. Appl. Catal. B Environ. 2010, 94, 134–141. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Ren, S.; Wang, W.; Shen, B. Study on the basic centers and active oxygen species of solid-base catalysts for oxidation of iso-mercaptans. Appl. Catal. A Gen. 2014, 473, 125–131. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Avdeeva, L.B.; Khassin, A.A.; Kustova, G.N.; Ushakov, V.A.; Moroz, E.M.; Shmakov, A.N.; Kriventsov, V.V.; Kochubey, D.I.; Pavlyukhin, Y.T.; et al. Coprecipitated iron-containing catalysts (Fe-Al2O3, Fe-Co-Al2O3, Fe-Ni-Al2O3) for methane decomposition at moderate temperatures I. Genesis of calcined and reduced catalysts. Appl. Catal. A Gen. 2004, 268, 127–138. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on Carbon Nanotubes and Graphene Raman. Spectroscopy 2010. [Google Scholar] [CrossRef]
- Conner, W.C.; Falconer, J.L. Spillover in Heterogeneous Catalysis. Chem. Rev. 1995, 95, 759–788. [Google Scholar] [CrossRef]
- Xu, Y.; Du, X.H.; Li, J.; Wang, P.; Zhu, J.; Ge, F.J.; Zhou, J.; Song, M.; Zhu, W.-Y. A comparison of Al2O3 and SiO2 supported Ni-based catalysts in their performance for the dry reforming of methane. J. Fuel Chem. Technol. 2019, 47, 199–208. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Khan, W.U.; Al-Fatesh, A.S.; Abasaeed, A.E. Stabilities of zeolite-supported Ni catalysts for dry reforming of methane. Chin. J. Catal. 2013, 34, 764–768. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Z.; Zhu, Y.A.; Liu, Z.; Sui, Z.; Zhu, K.; Zhou, X. Dry reforming of methane on Ni-Fe-MgO catalysts: Influence of Fe on carbon-resistant property and kinetics. Appl. Catal. B Environ. 2020, 264. [Google Scholar] [CrossRef]
- Guczi, L.; Stefler, G.; Geszti, O.; Sajó, I.; Pászti, Z.; Tompos, A.; Schay, Z. Methane dry reforming with CO2: A study on surface carbon species. Appl. Catal. A Gen. 2010, 375, 236–246. [Google Scholar] [CrossRef]
- Asai, K.; Takane, K.; Nagayasu, Y.; Iwamoto, S.; Yagasaki, E.; Inoue, M. Decomposition of methane in the presence of carbon dioxide over Ni catalysts. Chem. Eng. Sci. 2008, 63, 5083–5088. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Al Fatesh, A.S.; Ibrahim, A.A.; Kurdi, A.N.; Abasaeed, A.E. Yttria Modified ZrO2 Supported Ni Catalysts for CO2 Reforming of Methane: The Role of Ce Promoter. ACS Omega 2021. [Google Scholar] [CrossRef]
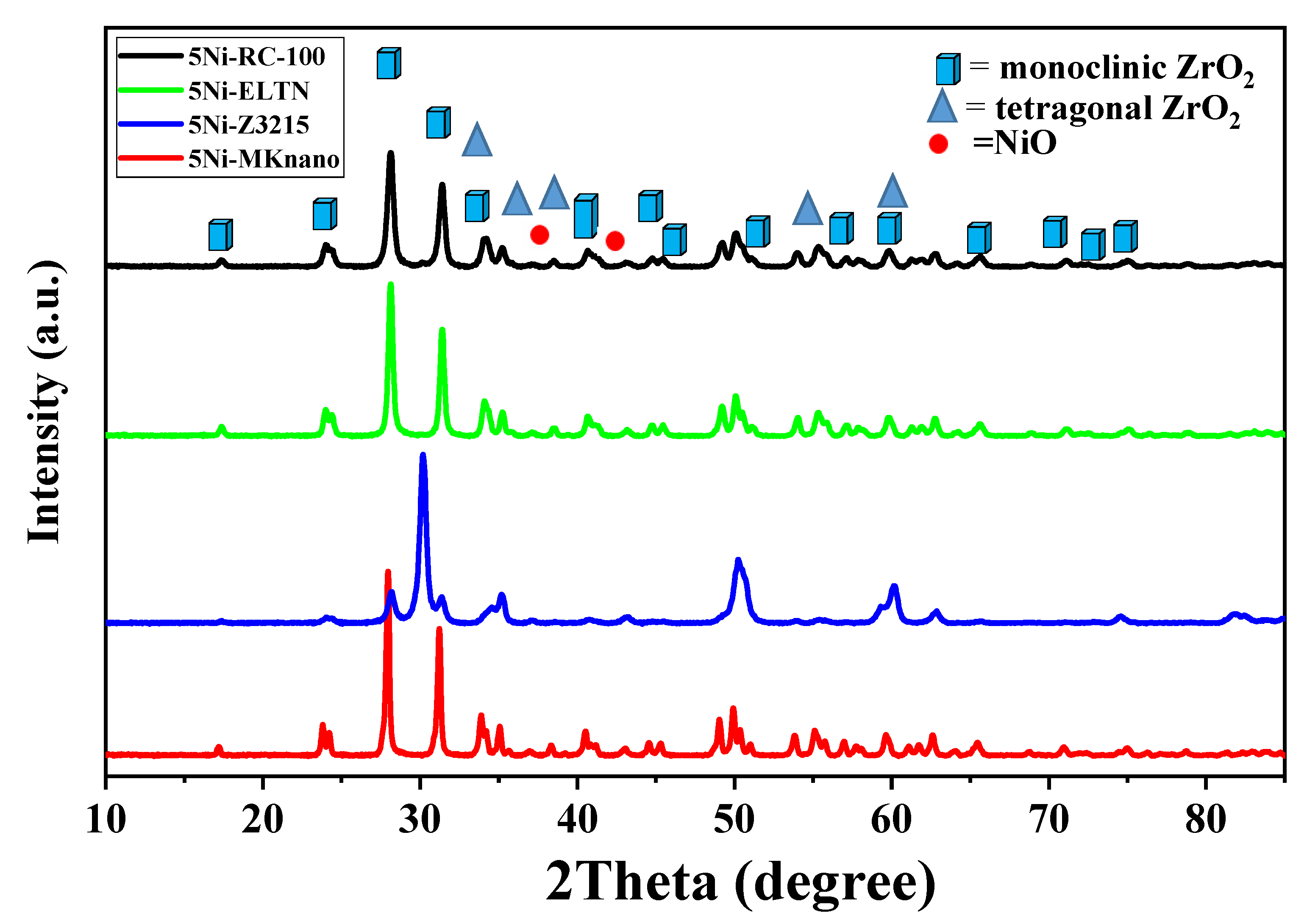
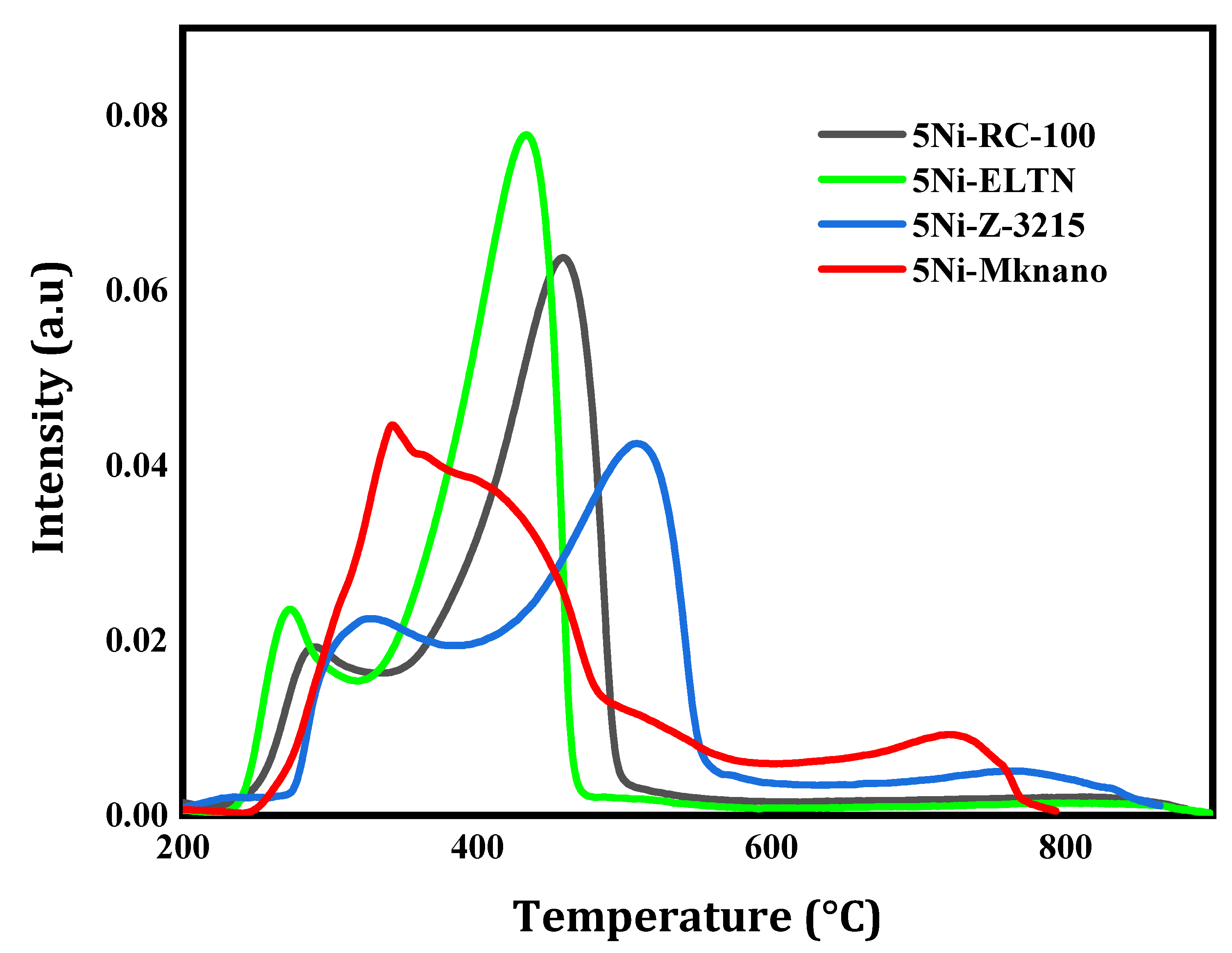
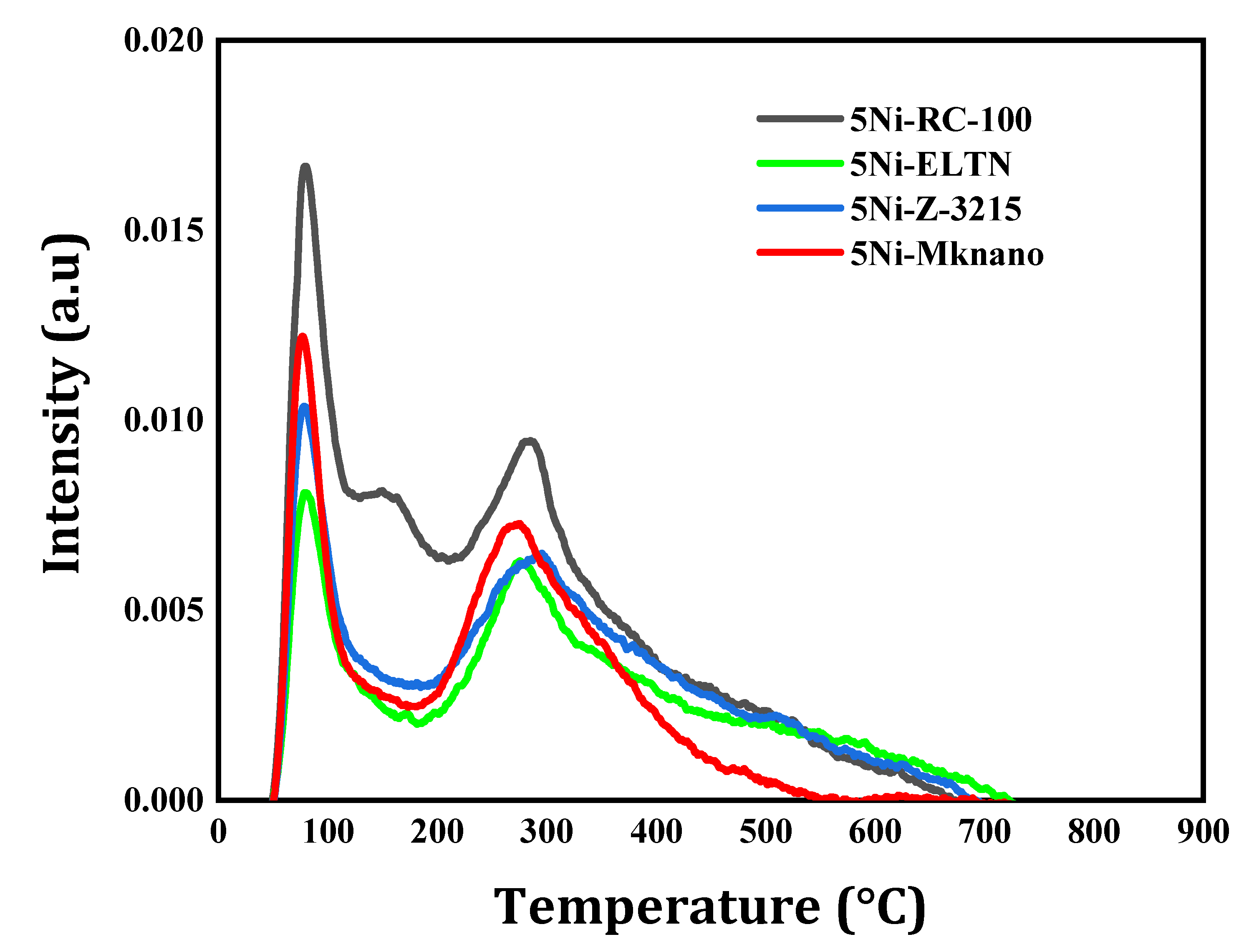
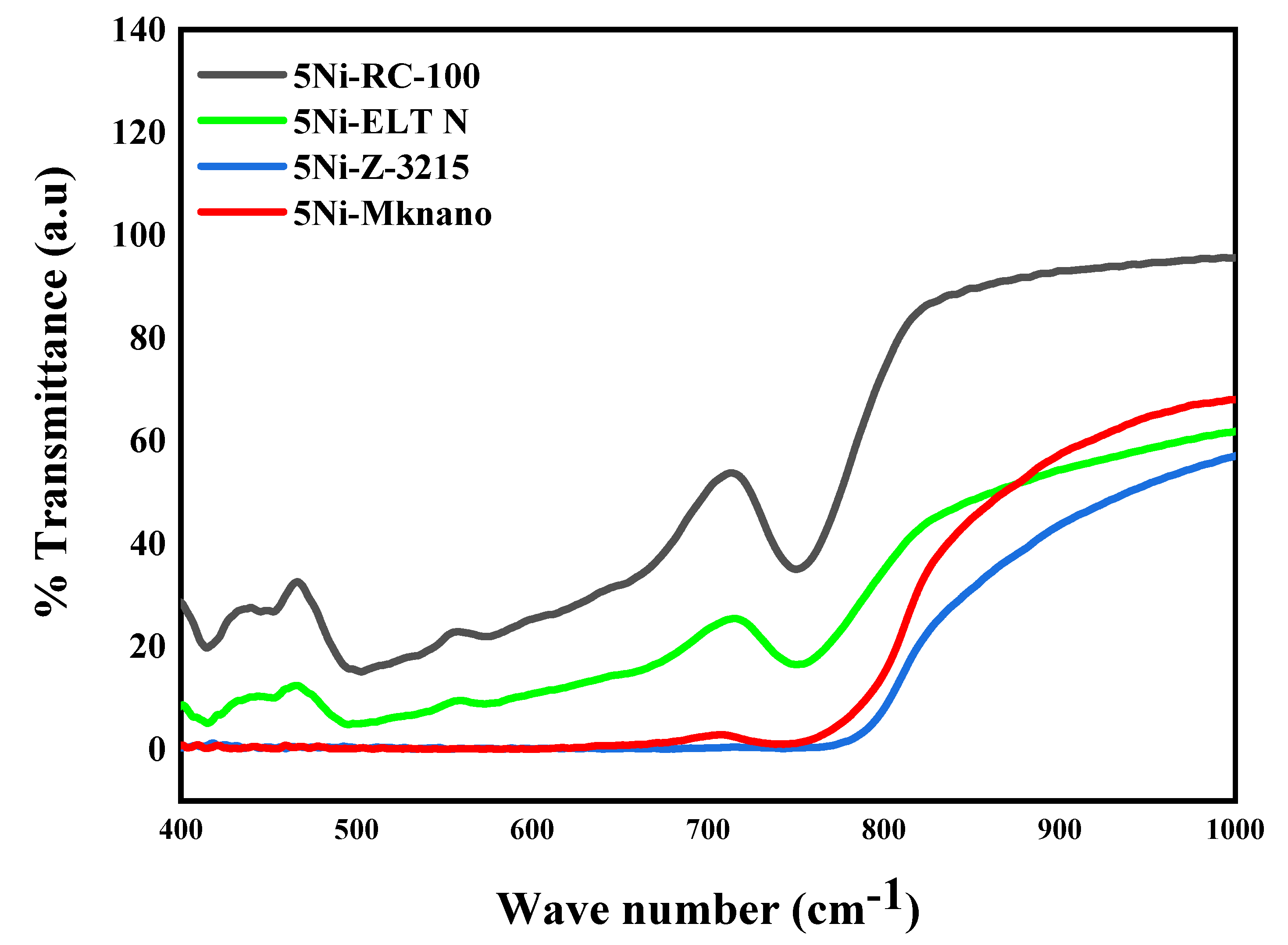



| Catalysts | SBET (m2 /g) | Vp (cm3 /g) | Dp (nm) |
|---|---|---|---|
| 5Ni-RC-100 | 17.7 | 0.12 | 31.7 |
| 5Ni-ELTN | 15.5 | 0.13 | 38.6 |
| 5Ni-Z-3215 | 9.8 | 0.05 | 24.8 |
| 5Ni-MKnano | 3.2 | 0.02 | 4.1 |
| Samples | Temperature (°C) | Quantity Consumed (µmol/g) | Exp. Total (µmol/g) | Reduction % | Dispersion % |
|---|---|---|---|---|---|
| 5Ni-RC-100 | 282.5 | 42.1 | 576.9 | 68 | 0.39 |
| 463.0 | 534.8 | ||||
| 5Ni-ELTN | 275.4 | 54.0 | 624.1 | 73 | 0.55 |
| 443.6 | 570.1 | ||||
| 5Ni-Z-3215 | 284.3 | 129.0 | 742 | 87 | 0.58 |
| 411.6 | 582.1 | ||||
| 752.5 | 30.9 | ||||
| 5Ni-MKnano | 327.8 | 305.4 | 805.6 | 94 | 0.14 |
| 404.9 | 450.0 | ||||
| 714.8 | 50.2 |
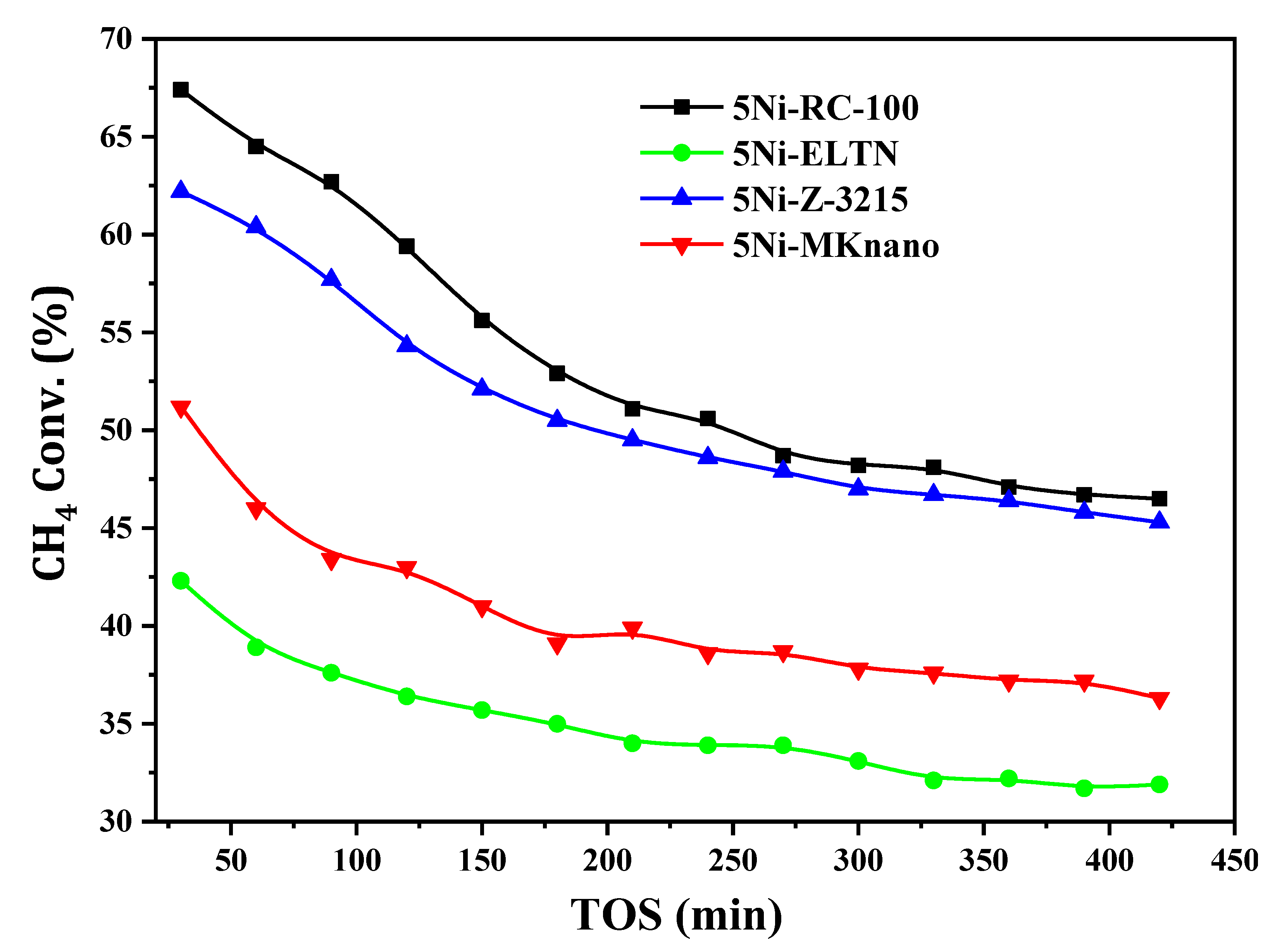
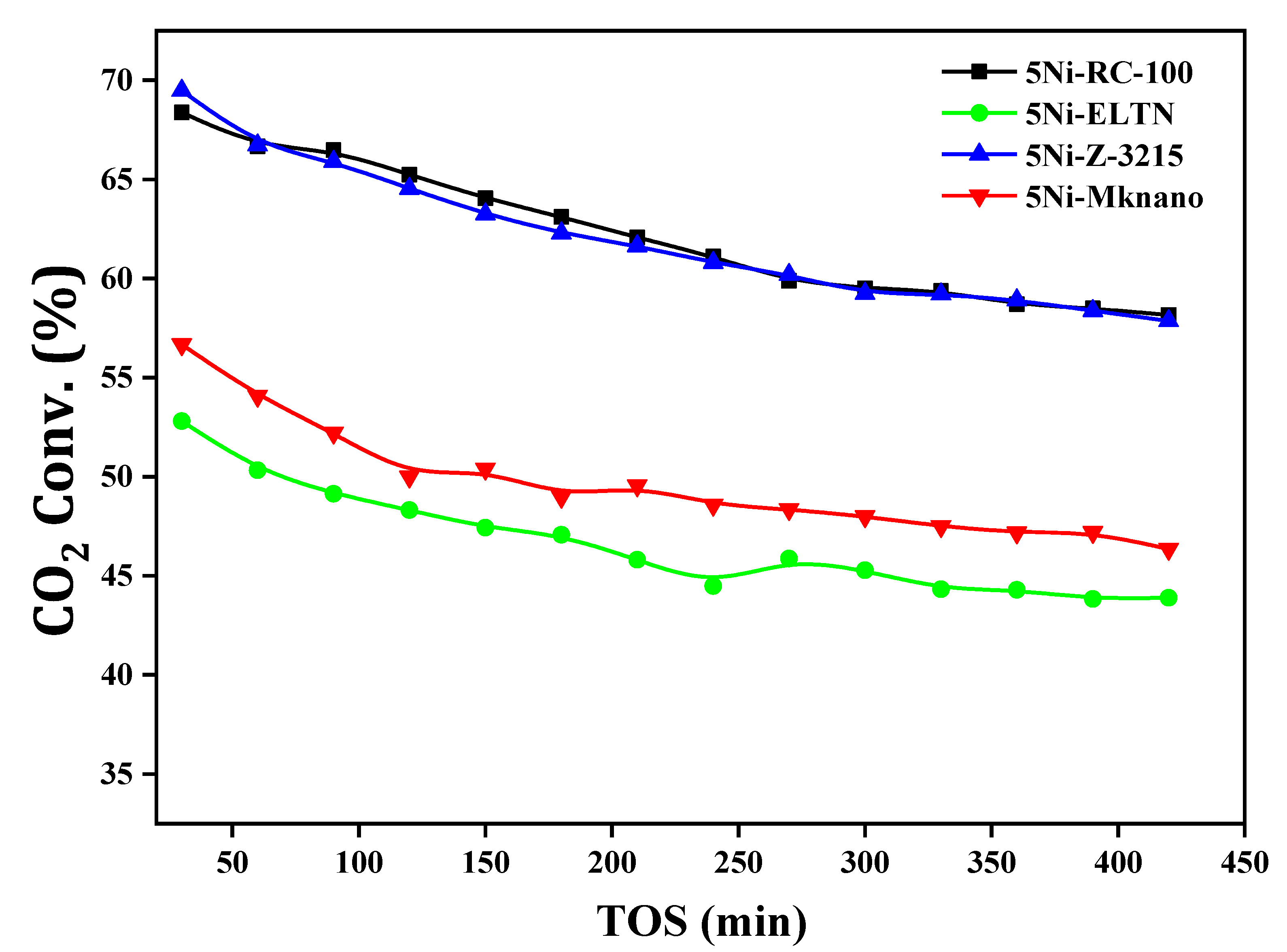
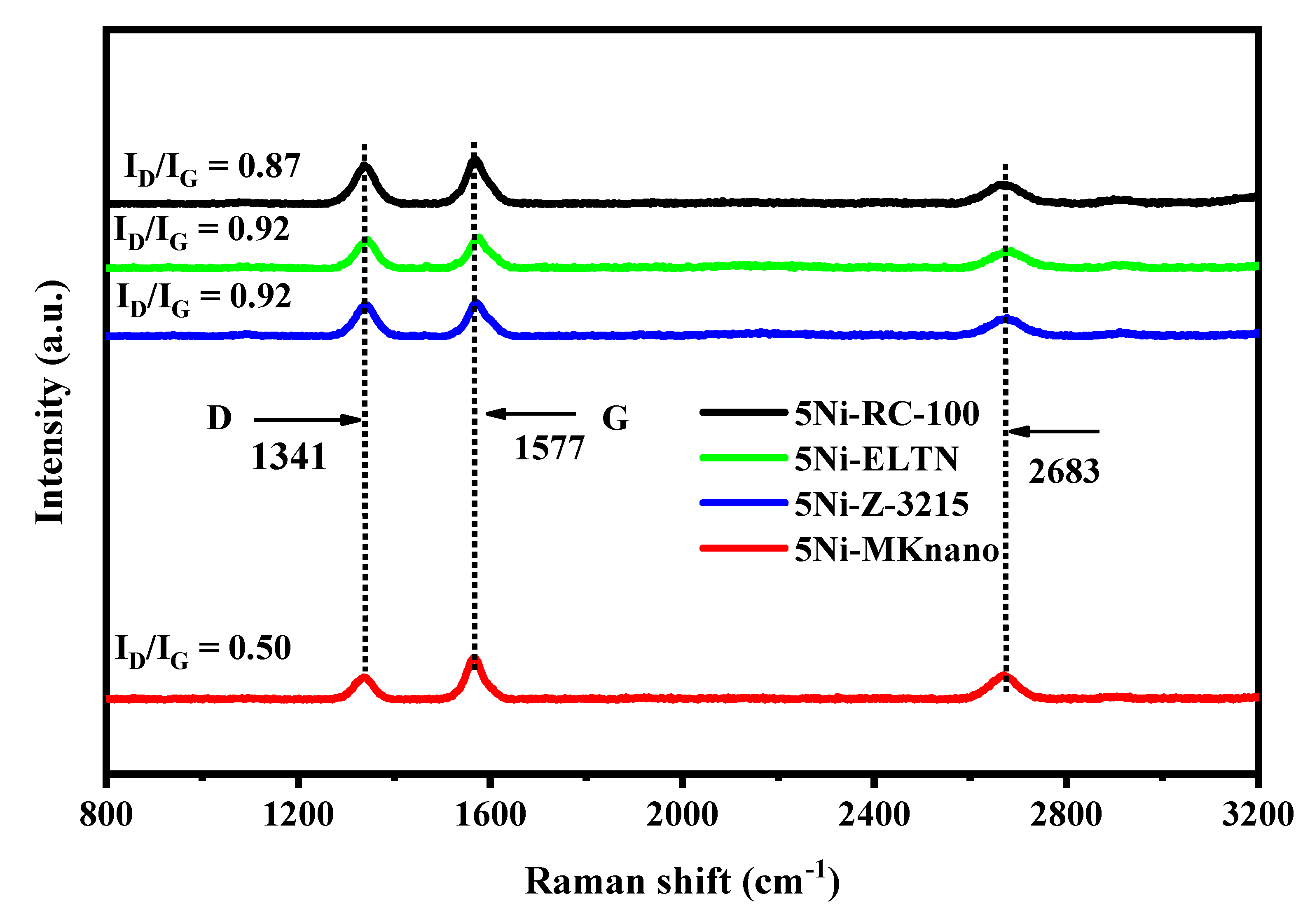
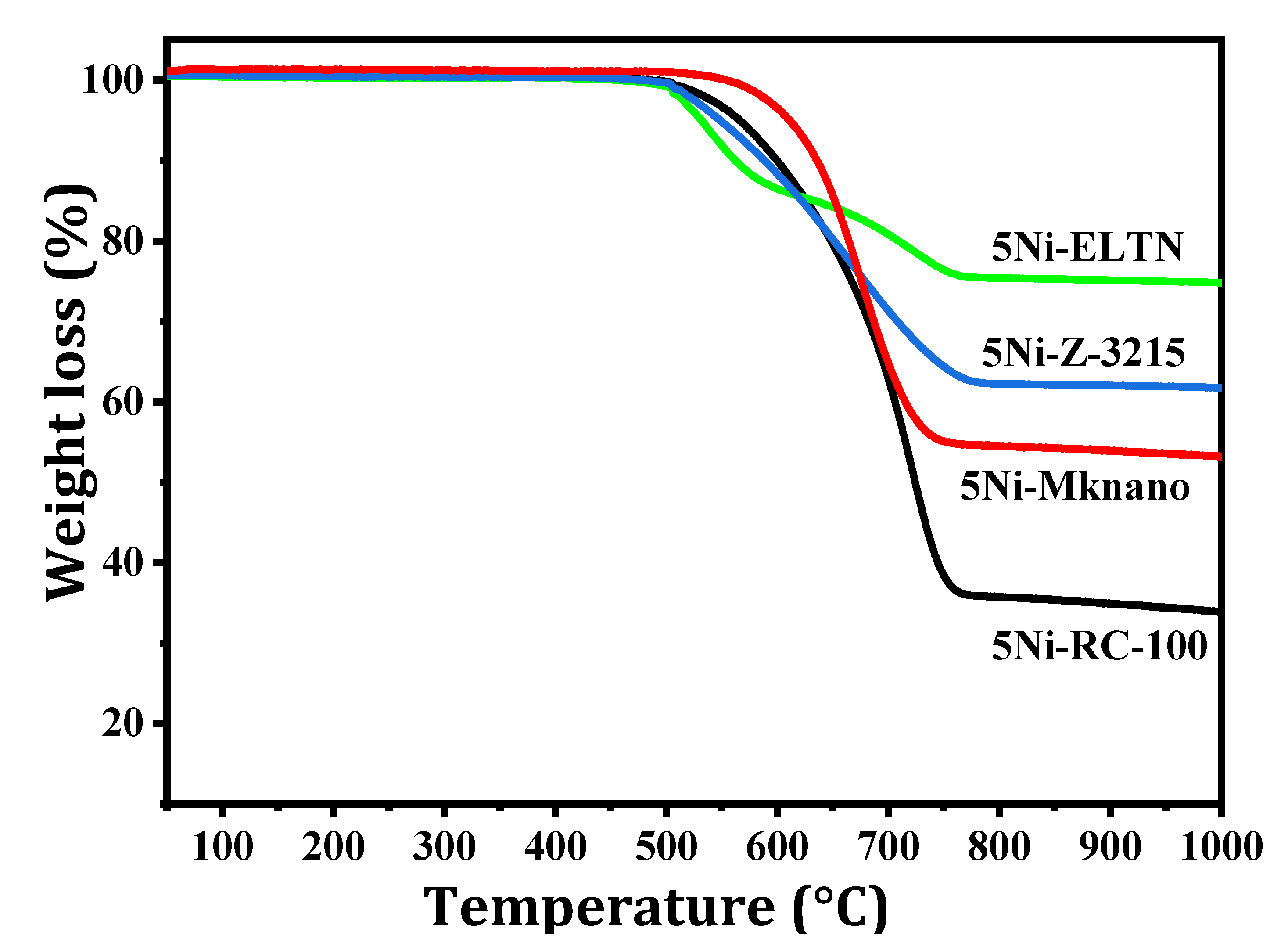
| Samples | Initial CH4 Conv. (%) | Final CH4 Conv. (%) | Initial CO2 Conv. (%) | Final CO2 Conv. (%) | DF (%) | Coke %wt. Loss |
|---|---|---|---|---|---|---|
| 5Ni-RC-100 | 67.4 | 46.5 | 68.4 | 58.2 | 31 | 66.3 |
| 5Ni-ELTN | 42.3 | 31.9 | 52.3 | 43.9 | 27 | 25.2 |
| 5Ni-Z-3215 | 62.2 | 45.3 | 69.5 | 58.4 | 24 | 38.3 |
| 5Ni-MKnano | 51.2 | 36.3 | 56.7 | 46.4 | 29 | 46.9 |
| Reference | Sample | CH4:CO2 | %CH4 Conv. | WHSV | T °C |
|---|---|---|---|---|---|
| [9] | Ni-Al2O3 | 1:1 | 36 | 6000 | 800 |
| [42] | Ni-SiO2 | 1:1 | 50 | 48,000 | 800 |
| [43] | Ni-H-ZSM-5 | 1:1 | 76 | 3600 | 700 |
| [44] | Ni-MgO | 1:1 | 70 | 86,000 | 760 |
| This work | Ni-ZrO2 (RC-100) | 1:1 | 54 | 42,000 | 700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Al-Fatesh, A.S. Dry Reforming of Methane Using Ni Catalyst Supported on ZrO2: The Effect of Different Sources of Zirconia. Catalysts 2021, 11, 827. https://doi.org/10.3390/catal11070827
Ibrahim AA, Fakeeha AH, Abasaeed AE, Al-Fatesh AS. Dry Reforming of Methane Using Ni Catalyst Supported on ZrO2: The Effect of Different Sources of Zirconia. Catalysts. 2021; 11(7):827. https://doi.org/10.3390/catal11070827
Chicago/Turabian StyleIbrahim, Ahmed Aidid, Anis Hamza Fakeeha, Ahmed Elhag Abasaeed, and Ahmed Sadeq Al-Fatesh. 2021. "Dry Reforming of Methane Using Ni Catalyst Supported on ZrO2: The Effect of Different Sources of Zirconia" Catalysts 11, no. 7: 827. https://doi.org/10.3390/catal11070827







