Phosphoric Acid Modification of Hβ Zeolite for Guaiacol Hydrodeoxygenation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.1.1. Physiochemical Property of P-Modified Hβ Zeolites
2.1.2. Acid Property of P-Modified Hβ Zeolites
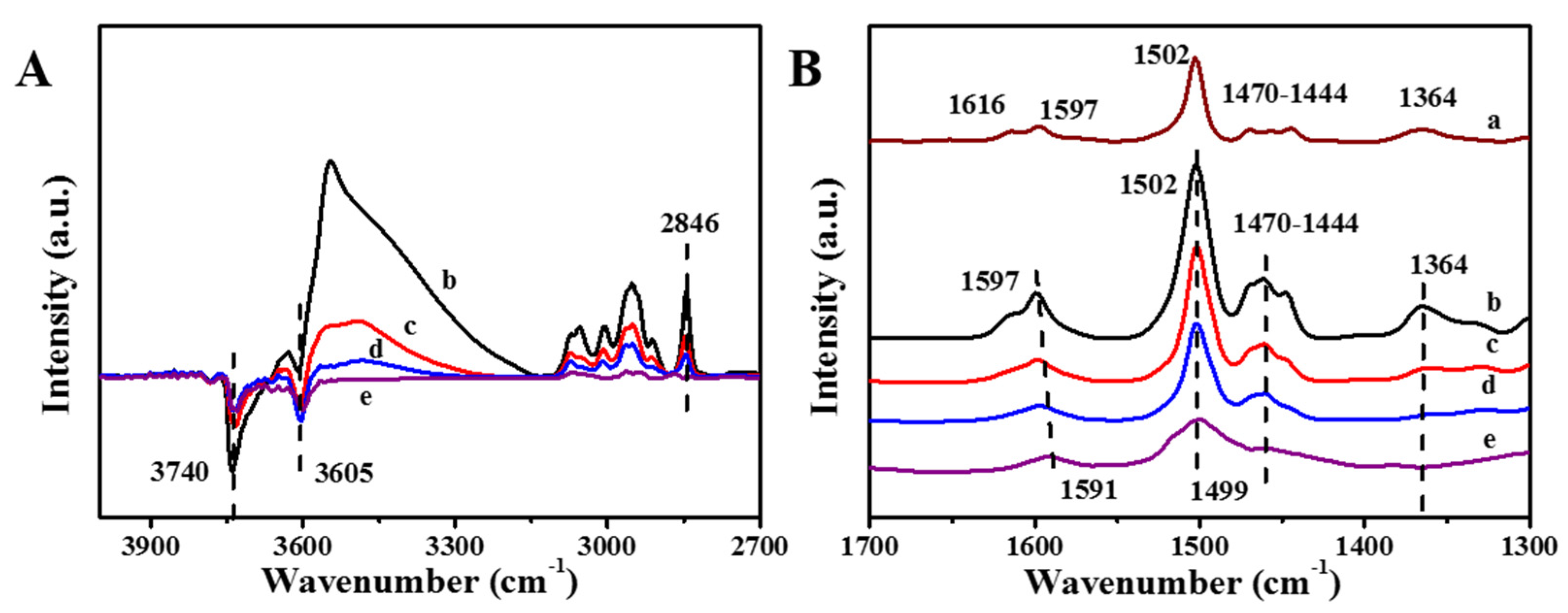
2.1.3. FT–IR Study of Guaiacol Adsorbed on P-Modified Hβ
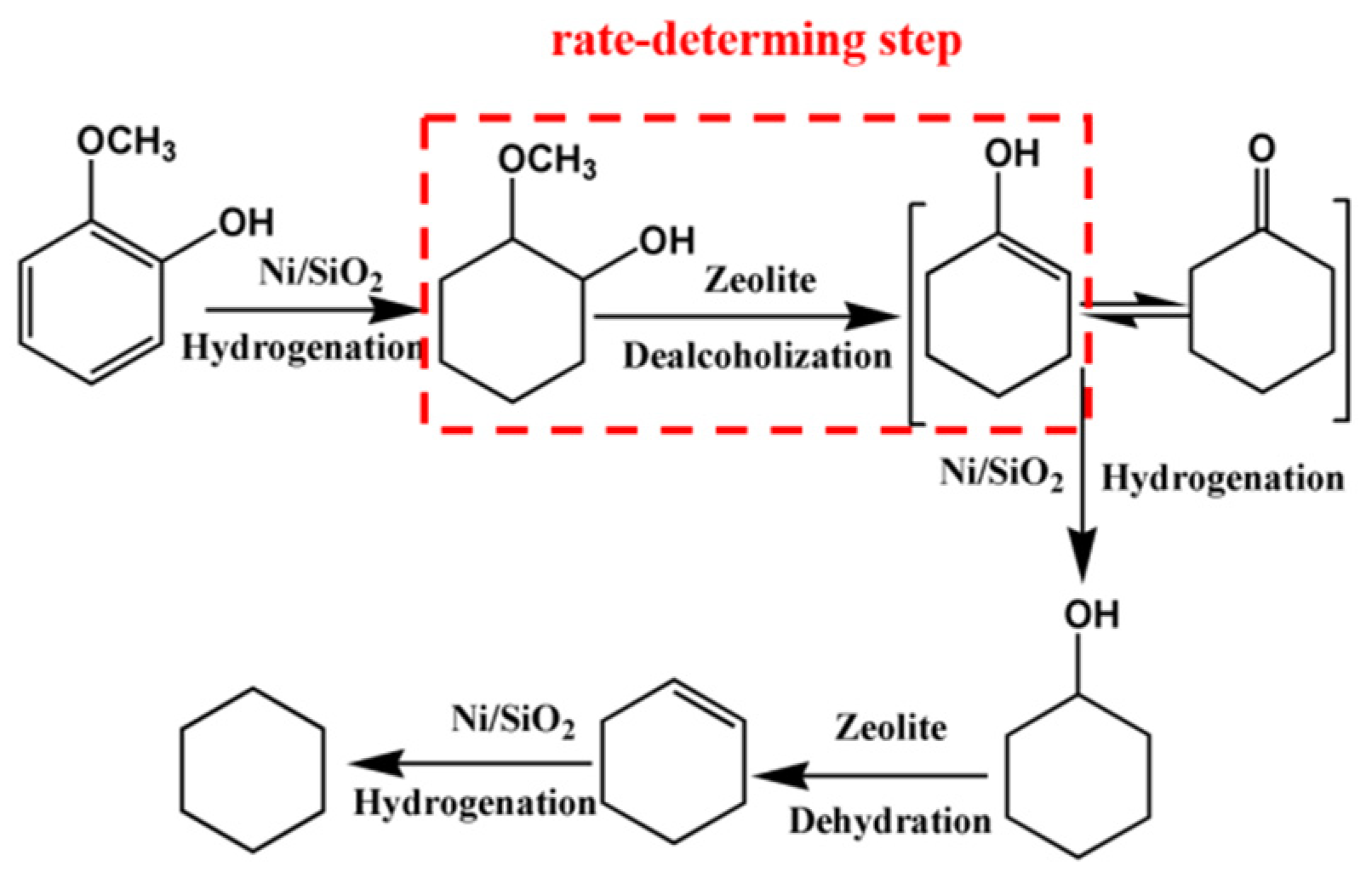
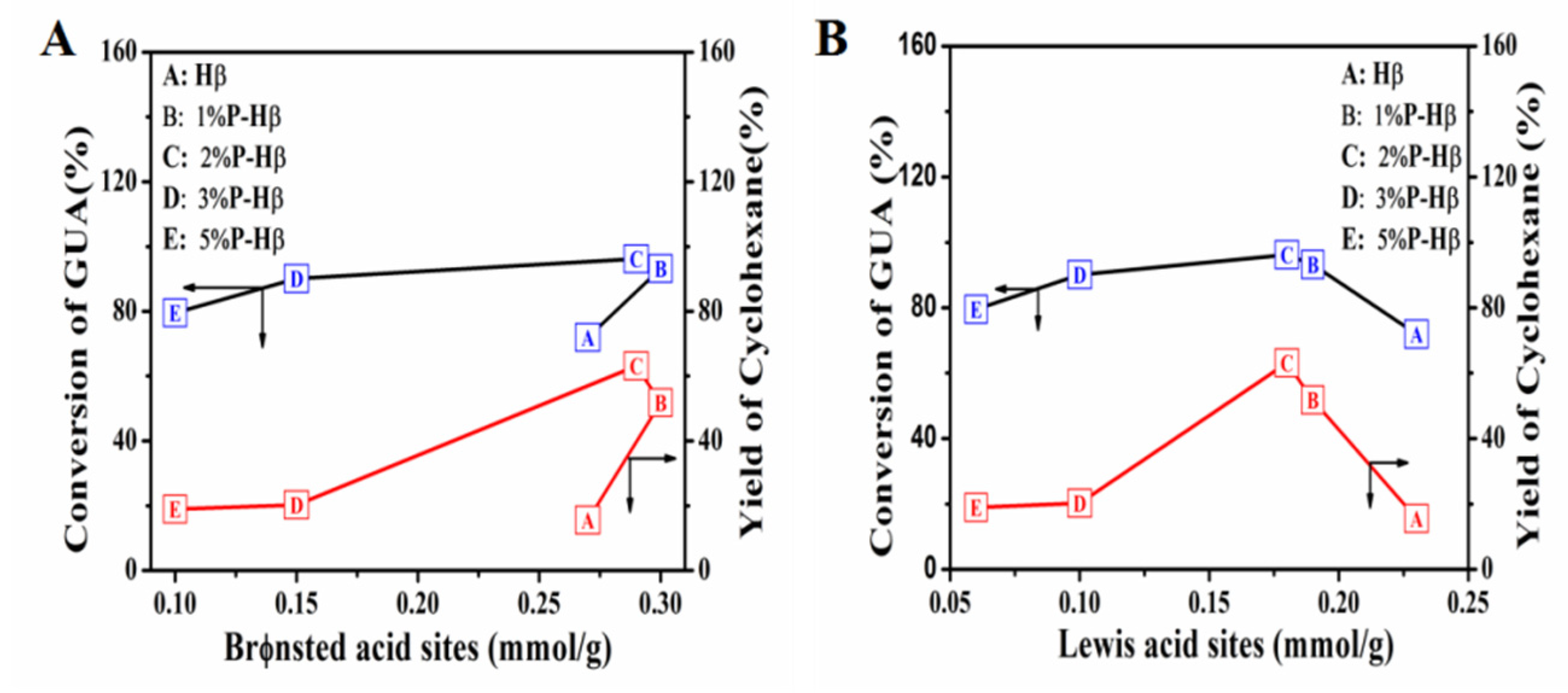
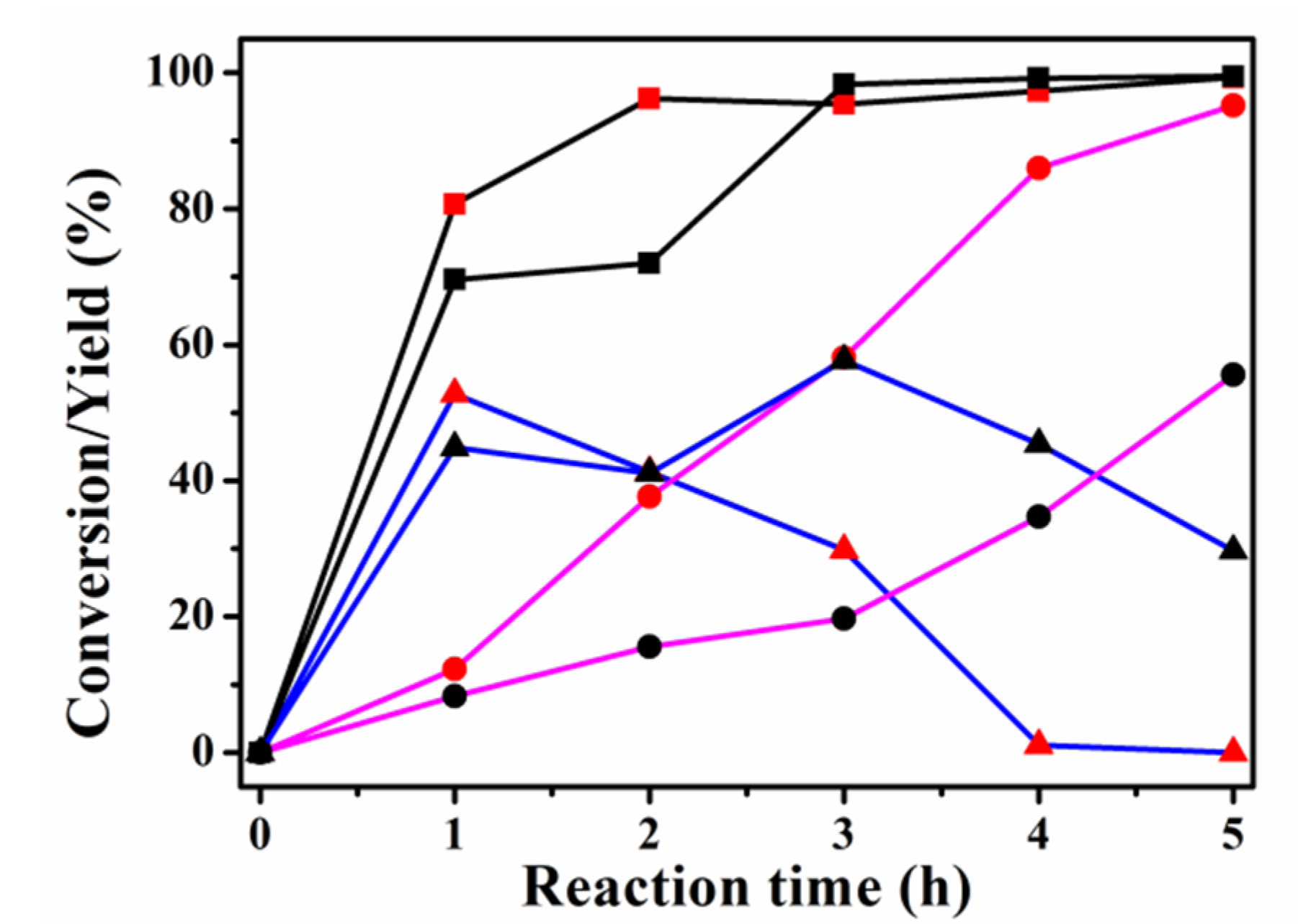
2.2. Effect of Acid Property on the Catalytic Performance of Guaiacol HDO
2.3. Effect of Phosphorus Loading on Guaiacol HDO over P-Modified Zeolite
3. Materials and Methods
3.1. Material
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. FTIR Study of Phenolic Compounds (Phenol, Anisole and Guaiacol) Adsorption on P-Modified Hβ Zeolites
3.5. Catalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Beckham, G.T.; Johnson, C.W.; Karp, E.M.; Salvachúa, D.; Vardon, D.R. Opportunities and challenges in biological lignin valorization. Curr. Opin. Biotech. 2016, 42, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Tuck, C.O.; Perez, E.; Horvath, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; De Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Arai, M.; Wu, Q.; Zhang, C.; Zhao, F. Hydrodeoxygenation of lignin-derived phenolics—a review on the active sites of supported metal catalysts. Green Chem. 2020, 22, 8140–8168. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Sun, Y.; Jiang, E.; Fan, X.; Tu, R.; Wang, J. Gas-phase hydrodeoxygenation of guaiacol over Ni-based HUSY zeolite catalysts under atmospheric H2 pressure. Renew. Energ. 2020, 152, 1380–1390. [Google Scholar] [CrossRef]
- Gutiérrez-Rubio, S.; Berenguer, A.; Přech, J.; Opanasenko, M.; Ochoa-Hernández, C.; Pizarro, P.; Čejka, J.; Serrano, D.P.; Coronado, J.M.; Moreno, I. Guaiacol hydrodeoxygenation over Ni2P supported on 2D-zeolites. Catal. Today 2020, 345, 48–58. [Google Scholar] [CrossRef]
- Zhao, C.; Kou, Y.; Lemonidou, A.A.; Li, X.; Lercher, J.A. Hydrodeoxygenation of bio-derived phenols to hydrocarbons using RANEY Ni and Nafion/SiO2 catalysts. Chem. Commun. 2010, 46, 412–414. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Lercher, J.A. Selective Hydrodeoxygenation of Lignin-Derived Phenolic Monomers and Dimers to Cycloalkanes on Pd/C and HZSM-5 Catalysts. ChemCatChem 2012, 4, 64–68. [Google Scholar] [CrossRef]
- Hong, D.Y.; Miller, S.J.; Agrawal, P.K.; Jones, C.W. Hydrodeoxygenation and coupling of aqueous phenolics over bifunctional zeolite-supported metal catalysts. Chem. Commun. 2010, 46, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Park, R.S.; Kim, H.; Park, S.H.; Jung, S.C.; Jeon, J.K.; Kim, S.C.; Park, Y.K. Hydrodeoxygenation of guaiacol over Pt loaded zeolitic materials. J. Ind. Eng. Chem. 2016, 37, 18–21. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, S.; Wang, S.; He, Y.; Liu, Y.; Wang, J.; Fan, W.; Lv, Y. Low temperature hydrodeoxygenation of guaiacol into cyclohexane over Ni/SiO2 catalyst combined with Hβ zeolite. RSC Adv. 2019, 9, 3868–3876. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, H.; Yu, M.J.; Ko, C.H.; Jeon, J.-K.; Jae, J.; Park, S.H.; Jung, S.-C.; Park, Y.-K. Catalytic Hydrodeoxygenation of Bio-oil Model Compounds over Pt/HY Catalyst. SCI. Rep. 2016, 6, 28765–28772. [Google Scholar] [CrossRef]
- Degnan, T.F.; Chitnis, G.K.; Schipper, P.H. History of ZSM-5 fluid catalytic cracking additive development at Mobil. Micropor. Mesopor. Mat. 2000, 35-36, 245–252. [Google Scholar] [CrossRef]
- Yi, D.; Meng, X.; Liu, N.; Shi, L. Catalytic performance of a phosphorus-modified H-IM-5@meso-SiO2 composite in the alkylation of toluene with methanol. New J. Chem. 2019, 43, 11758–11770. [Google Scholar] [CrossRef]
- Yi, D.; Meng, X.; Xu, X.; Liu, N.; Shi, L. Catalytic Performance of Modified ZSM-5 Designed with Selectively Passivated External Surface Acidity by Phosphorus. Ind. Eng. Chem. Res. 2019, 58, 10154–10163. [Google Scholar] [CrossRef]
- Van Der Bij, H.E.; Meirer, F.; Kalirai, S.; Wang, J.; Weckhuysen, B.M. Hexane Cracking over Steamed Phosphated Zeolite H-ZSM-5: Promotional Effect on Catalyst Performance and Stability. Chem. Eur. J. 2014, 20, 16922–16932. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, L.-L.; Li, G.-D.; Shang, Y.-S.; Zhao, X.-M.; Ma, T.; Zhang, L.-M.; Zhai, Y.-L.; Gong, Y.-J.; Xu, J.; et al. ZSM-5 extrudates modified with phosphorus as a super effective MTP catalyst: Impact of the acidity on binder. Fuel Process Technol. 2017, 168, 105–115. [Google Scholar] [CrossRef]
- Ma, T.; Ding, J.; Shao, R.; Yun, Z. Catalytic conversion of glycerol to acrolein over MCM-41 by the grafting of phosphorus species. Can. J. Chem. Eng. 2016, 94, 924–930. [Google Scholar] [CrossRef]
- Yan, L.; Ji, Y.; Wang, P.; Feng, C.; Han, L.; Li, H.; Yan, T.; Shi, L.; Zhang, D. Alkali and Phosphorus Resistant Zeolite-like Catalysts for NOx Reduction by NH3. Environ. Sci. Technol. 2020, 54, 9132–9141. [Google Scholar] [CrossRef]
- Huifang, C.H.S.B.P. Acidity and Catalytic Performance of ZSM5/Y Composite Zeolite for Heavy Oil Cracking. Chin. J. Catal. 2004, 25, 715–720. [Google Scholar]
- Blasco, T.; Corma, A.; Martinez-Triguero, J. Hydrothermal stabilization of ZSM-5 catalytic-cracking additives by phosphorus addition. J. Catal. 2006, 237, 267–277. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, S.; Wang, S.; Wang, J.; Fan, W.; Lv, Y. Ni nanoparticles entrapped in nickel phyllosilicate for selective hydrogenation of guaiacol to 2-methoxycyclohexanol. Appl. Catal. A-Gen. 2018, 568, 231–241. [Google Scholar] [CrossRef]
- Van Der Bij, H.E.; Aramburo, L.R.; Arstad, B.; Dynes, J.J.; Wang, J.; Weckhuysen, B.M. Phosphatation of Zeolite H-ZSM-5: A Combined Microscopy and Spectroscopy Study. ChemPhysChem 2014, 15, 283–292. [Google Scholar] [CrossRef]
- Zhao, G.; Teng, J.; Xie, Z.; Jin, W.; Yang, W.; Chen, Q.; Tang, Y. Effect of phosphorus on HZSM-5 catalyst for C-4-olefin cracking reactions to produce propylene. J. Catal. 2007, 248, 29–37. [Google Scholar] [CrossRef]
- Poncelet, G.; Dubru, M.L. An infrared study of the surface acidity of germanic near-faujasite zeolite by pyridine adsorption. J. Catal. 1978, 52, 321–331. [Google Scholar] [CrossRef]
- Ramesh, B.; Borade, A. Probing Acid Sites in Zeolites by X-ray Photoelectron Spectroscopy Using Pyridine as a Probe Molecule. Stud. Surf. Sci. Catal. 1994, 84, 661–668. [Google Scholar]
- Liu, M.; Yin, Y.; Guo, X.; Song, C. Oxalic Acid Modification of β Zeolite for Dehydration of 2-(4′-Ethylbenzoyl) Benzoic Acid. Ind. Eng. Chem. Res. 2017, 56, 8850–8856. [Google Scholar] [CrossRef]
- Derewinski, M.; Sarv, P.; Sun, X.; Mueller, S.; Van Veen, A.C.; Lercher, J.A. Reversibility of the Modification of HZSM-5 with Phosphate Anions. J. Phys. Chem. C 2014, 118, 6122–6131. [Google Scholar] [CrossRef]
- Damodaran, K.; Cabral de Menezes, S.M.; Lam, Y.L.; Trebosc, J.; Amoureux, J.-P.; Pruski, M. Modification of H-ZSM-5 zeolites with phosphorus. 2. Interaction between phosphorus and aluminum studied by solid-state NMR spectroscopy. Micropor. Mesopor. Mat. 2006, 95, 296–305. [Google Scholar] [CrossRef]
- Caeiro, G.; Magnoux, P.; Lopes, J.M.; Ribeiro, F.R.; Menezes, S.M.C.; Costa, A.F.; Cerqueira, H.S. Stabilization effect of phosphorus on steamed H-MFI zeolites. Appl. Catal. A Gen. 2006, 314, 160–171. [Google Scholar] [CrossRef]
- Kiricsi, I.; Flego, C.; Pazzuconi, G.; Parker, W.O., Jr.; Millini, R.; Perego, C.; Bellussi, G. Progress toward Understanding Zeolite.beta. Acidity: An IR and 27Al NMR Spectroscopic Study. J. Phys. Chem. 1994, 98, 4627–4634. [Google Scholar] [CrossRef]
- Popov, A.; Kondratieva, E.; Goupil, J.M.; Mariey, L.; Bazin, P.; Gilson, J.-P.; Travert, A.; Mauge, F. Bio-oils Hydrodeoxygenation: Adsorption of Phenolic Molecules on Oxidic Catalyst Supports. J. Phys. Chem. C. 2010, 114, 15661–15670. [Google Scholar] [CrossRef]
- Shafaghat, H.; Rezaei, P.S.; Daud, W.M.A.W. Catalytic hydrogenation of phenol, cresol and guaiacol over physically mixed catalysts of Pd/C and zeolite solid acids. RSC Adv. 2015, 5, 33990–33998. [Google Scholar] [CrossRef]
- Hellinger, M.; Carvalho, H.W.P.; Baier, S.; Wang, D.; Kleist, W.; Grunwaldt, J.-D. Catalytic hydrodeoxygenation of guaiacol over platinum supported on metal oxides and zeolites. Appl. Catal. A-Gen. 2015, 490, 181–192. [Google Scholar] [CrossRef]
- Qiu, S.B.; Xu, Y.; Weng, Y.J.; Ma, L.L.; Wang, T.J. Efficient Hydrogenolysis of Guaiacol over Highly Dispersed Ni/MCM-41 Catalyst Combined with HZSM-5. Catalysts 2016, 6, 134. [Google Scholar] [CrossRef] [Green Version]
- Madeira, F.F.; Ben Tayeb, K.; Pinard, L.; Vezin, H.; Maury, S.; Cadran, N. Ethanol transformation into hydrocarbons on ZSM-5 zeolites: Influence of Si/Al ratio on catalytic performances and deactivation rate. Study of the radical species role. Appl. Catal. A-Gen. 2012, 443, 171–180. [Google Scholar] [CrossRef]
- Han, J.; Cho, J.; Kim, J.-C.; Ryoo, R. Confinement of Supported Metal Catalysts at High Loading in the Mesopore Network of Hierarchical Zeolites, with Access via the Microporous Windows. ACS Catal. 2018, 8, 876–879. [Google Scholar] [CrossRef]
- Na, K.; Choi, M.; Ryoo, R. Cyclic diquaternary ammoniums for nanocrystalline BEA, MTW and MFI zeolites with intercrystalline mesoporosity. J. Mater. Chem. 2009, 19, 6713. [Google Scholar] [CrossRef]






| Catalyst | P Loading a | SBET(m2·g−1) b | Pore Volume(m3·g−1) | ||||
|---|---|---|---|---|---|---|---|
| Total | External | Vtotal c | Vmeso d | Vmicro e | |||
| 1 | Hβ | 0 | 489 | 139 | 0.41 | 0.26 | 0.15 |
| 2 | 1%P-Hβ | 1.1 | 477 | 133 | 0.41 | 0.27 | 0.14 |
| 3 | 2%P-Hβ | 1.8 | 466 | 124 | 0.40 | 0.26 | 0.14 |
| 4 | 3%P-Hβ | 3.0 | 400 | 86 | 0.32 | 0.18 | 0.14 |
| 5 | 5%P-Hβ | 4.6 | 328 | 73 | 0.29 | 0.18 | 0.11 |
| Entry | Catalyst | Acid Site(mmol/g) a | B/L | Acid Site(mmol/g) b | |||
|---|---|---|---|---|---|---|---|
| B | L | Total | Weak | Strong | |||
| 1 | Hβ | 0.27 | 0.23 | 1.17 | 0.97 | 0.68 | 0.29 |
| 2 | 1%P-Hβ | 0.30 | 0.19 | 1.58 | 0.90 | 0.55 | 0.35 |
| 3 | 2%P-Hβ | 0.29 | 0.18 | 1.61 | 0.91 | 0.57 | 0.34 |
| 4 | 3%P-Hβ | 0.15 | 0.10 | 1.5 | 0.47 | 0.29 | 0.18 |
| 5 | 5%P-Hβ | 0.10 | 0.06 | 1.67 | 0.31 | 0.19 | 0.12 |
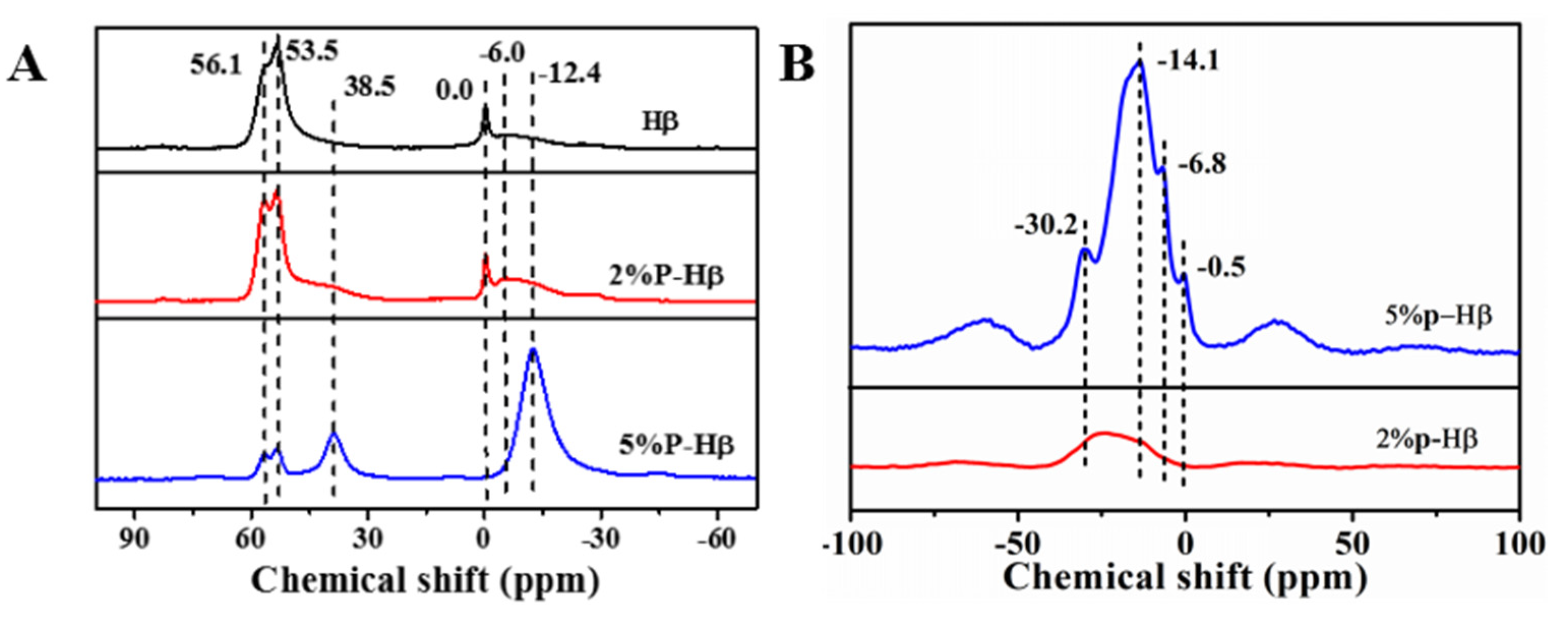
| Entry | Catalyst | Chemical Shift (ppm) | |||||
|---|---|---|---|---|---|---|---|
| 56.1 | 53.5 | 38.5 | 0.0 | −6.0 | −12.4 | ||
| 1 | Hβ | 15.3 | 54.3 | 0 | 5.9 | 24.5 | 0 |
| 2 | 2%P-Hβ | 14.4 | 39.5 | 12.6 | 0.3 | 9.2 | 23.9 |
| 3 | 5%P-Hβ | 3.7 | 6.4 | 17.8 | 0 | 0 | 72.1 |
| Entry | Substrate | Catalyst | Reaction Time | Conv. (%) | Main Product |
|---|---|---|---|---|---|
| 1 | GUA | Ni/SiO2 | 2 h | 81.8 | MCH |
| 2 | GUA | Ni/SiO2 + Hβ | 2 h | 72.0 | MCH, cyclohexane |
| 3 | GUA | Ni/SiO2 + Al2O3 | 5 h | >99 | MCH |
| 4 | GUA | Ni/SiO2 + SiO2 | 5 h | >99 | MCH (95.6) |
| 5 | MCH b | SiO2 | 5 h | - | - |
| 6 | MCH | Hβ | 1 h | >99 | cyclohexanone |
| 7 | MCH | Al2O3 | 5 h | - | - |
| Entry | Catalyst | Conv. (%) | Yield (%) | ||
|---|---|---|---|---|---|
| Cyclohexane | Cyclohexanol | MCH b | |||
| 1 | Ni/SiO2 + Hβ | 72.0 | 15.6 | 1.0 | 41.1 |
| 2 | Ni/SiO2 + 1%P-Hβ | 93.2 | 51.8 | 0.6 | 40.7 |
| 3 | Ni/SiO2 + 2%P-Hβ | 96.2 | 63.1 | 0.5 | 32.5 |
| 4 | Ni/SiO2 + 3%P-Hβ | 90.1 | 20.2 | 1.2 | 67.4 |
| 5 | Ni/SiO2 + 5%P-Hβ | 79.6 | 18.9 | 1.3 | 60.2 |
| Entry | Catalyst | T (°C) | PH2 (MPa) | Conv. (%) | YiledCYH a (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Pd/C + zeolite | 275 | 1.5 | 100 | 0.3 | [33] |
| 2 | Pt/zeolite | 250 | 4 | >90 | 45.3 | [10] |
| 3 | Pt/H-MFI-60 | 180 | 5 | 100 | 93 | [34] |
| 4 | Ni/MCM-41 + HZSM-5 | 240 | 5 | 100 | 84.1 | [35] |
| 5 | Ni/SiO2 + Hβ | 140 | 5 | >99 | 55.2 | This work |
| 6 | Ni/SiO2 + 2%P-Hβ | 140 | 5 | >99 | 95.2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lv, Y.; Zhu, S.; Wang, X.; Deng, C. Phosphoric Acid Modification of Hβ Zeolite for Guaiacol Hydrodeoxygenation. Catalysts 2021, 11, 962. https://doi.org/10.3390/catal11080962
Wang X, Lv Y, Zhu S, Wang X, Deng C. Phosphoric Acid Modification of Hβ Zeolite for Guaiacol Hydrodeoxygenation. Catalysts. 2021; 11(8):962. https://doi.org/10.3390/catal11080962
Chicago/Turabian StyleWang, Xun, Yongkang Lv, Shanhui Zhu, Xuefeng Wang, and Cunbao Deng. 2021. "Phosphoric Acid Modification of Hβ Zeolite for Guaiacol Hydrodeoxygenation" Catalysts 11, no. 8: 962. https://doi.org/10.3390/catal11080962
APA StyleWang, X., Lv, Y., Zhu, S., Wang, X., & Deng, C. (2021). Phosphoric Acid Modification of Hβ Zeolite for Guaiacol Hydrodeoxygenation. Catalysts, 11(8), 962. https://doi.org/10.3390/catal11080962





