Photo-Oxidation of Ammonia to Molecular Nitrogen in Water under UV, Vis and Sunlight Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials Characterisation
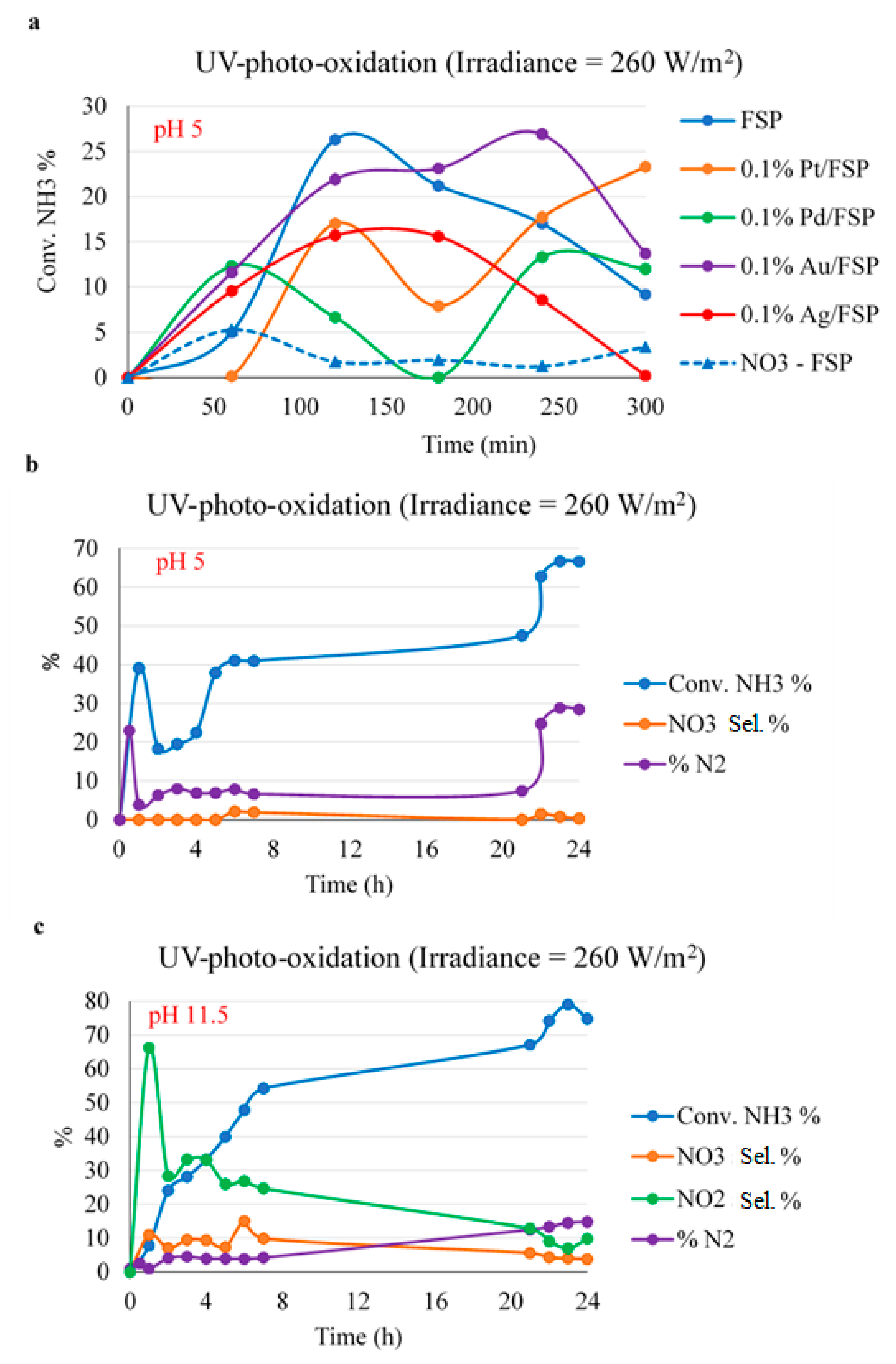
2.2. Photo-Oxidation of Ammonium under UV Light
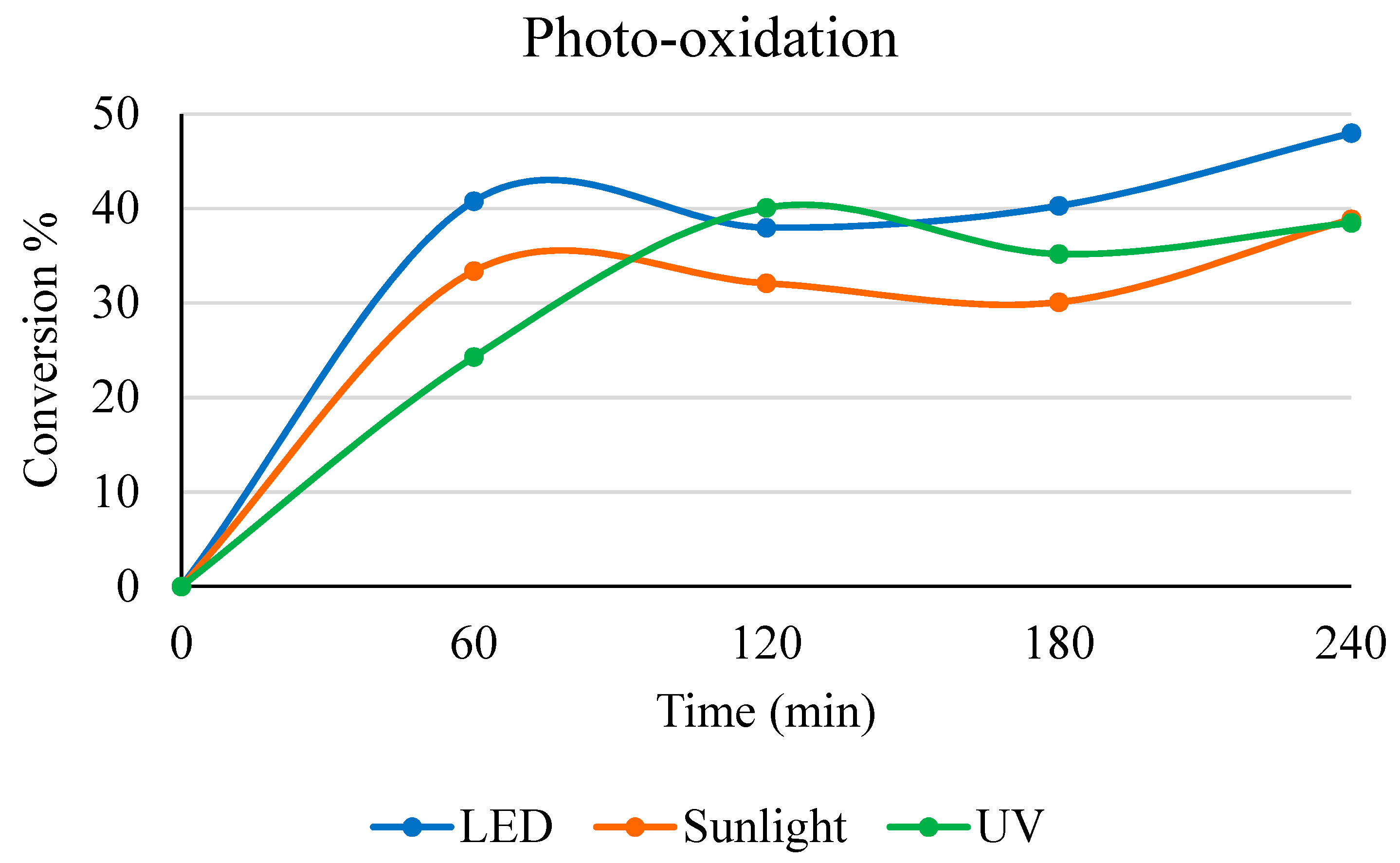
2.3. Comparison between Different Light Sources
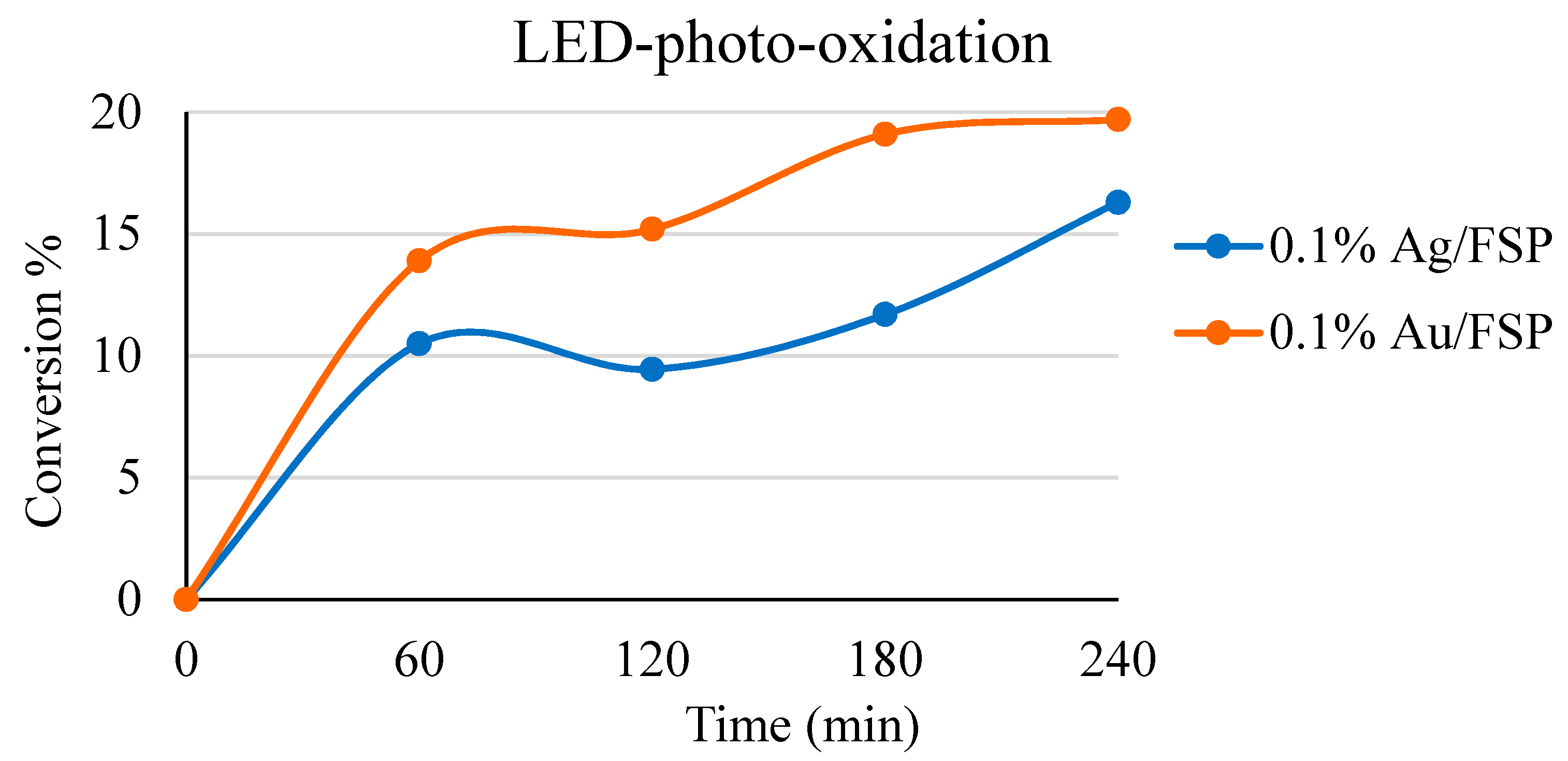
2.4. Optimisation of Reaction Conditions under LED White Light Irradiation
2.5. Light Source Comparison
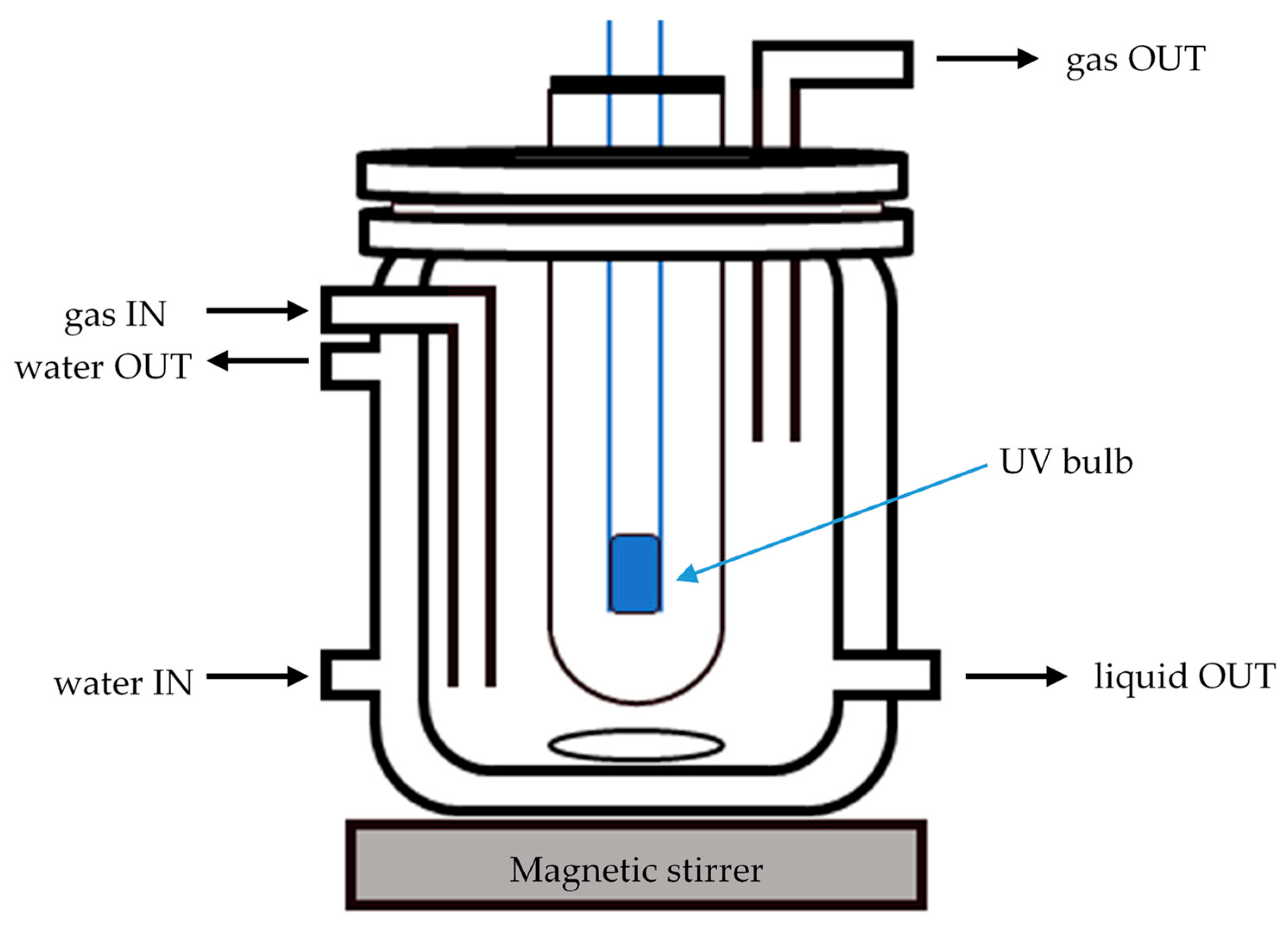
3. Experimental
3.1. Materials Preparation
3.2. Materials Characterisation
3.3. Instrumentation Setup and Procedures
3.4. Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roser, M.; Ritchie, H.; Ortiz-Ospina, E. World Population Growth. 2020. Available online: https://ourworldindata.org/world-population-growth (accessed on 15 May 2021).
- Galloway, J.N.; Cowling, E.B. Reactive nitrogen and the world: 200 Years of change. Ambio R. Swed. Acad. Sci. 2002, 31, 64–71. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operation space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.K. The Use of Nutrients in Crop Plants; Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Goebes, M.D.; Strader, R.; Davidson, C. An ammonia emission inventory for fertilizer application in the United States. Atmos. Environ. 2003, 37, 2539–2550. [Google Scholar] [CrossRef]
- Shepherd, M.F.; Barzetti, S.; Hastie, D.R. The production of atmospheric NOx and N2O from a fertilized agricultural soil. Atmos. Environ. Part A Gen. Top. 1991, 25, 1961–1969. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Whithall, D.; Aber, J.D.; Boyer, E.; Castro, M.; Cronon, C.; Goodale, C.L.; Groffman, P.M.; Hopkinson, C.; Lambert, K.F.; et al. Nitrogen Pollution: Source and Consequences in the U. S. Northeast. Environment 2003, 45, 22. [Google Scholar]
- Chislock, M.; Doster, E. Eutrophication: Causes, Consequences, and Controls in Aquatic Ecosystems. Nat. Educ. Knowl. 2013, 4, 10. [Google Scholar]
- Camargo, J.A.; Alonso, A.; Salamanca, A. Nitrate toxicity to aquatic animals: A review with new data for freshwater invertebrates. Chemosphere 2005, 58, 1255–1267. [Google Scholar] [CrossRef]
- Herschy, R.W. Water Quality for Drinking: WHO Guidelines; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar] [CrossRef]
- Volkmer, B.G.; Ernst, B.; Simon, J.; Kuefer, R.; Bartsch, G.; Bach, D.; Gschwend, J.E. Influence of nitrate levels in drinking water on urological malignancies: A community-based cohort study. BJU Int. 2005, 95, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Pyatt, F.B. Potential effects on human health of an ammonia rich atmospheric environment in an archaeologically important cave in southeast Asia. Occup. Environ. Med. 2003, 60, 986–988. [Google Scholar] [CrossRef] [Green Version]
- Mohseni-Bandpi, A.; Elliott, D.J.; Zazouli, M.A. Biological nitrate removal processes from drinking water supply—A review. J. Environ. Health Sci. Eng. 2013, 11, 1–11. [Google Scholar] [CrossRef]
- John, G.T.; Crittenden, C.; Trussell, R.R.; Hand, D.W.; Howe, K.J. MWH’s Water Treatment: Principles and Design, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Nur, T.; Shim, W.G.; Loganathan, P.; Vigneswaran, S.; Kandasamy, J. Nitrate removal using Purolite A520E ion exchange resin: Batch and fixed-bed column adsorption modelling. Int. J. Environ. Sci. Technol. 2015, 12, 1311–1320. [Google Scholar] [CrossRef]
- Ferro, S.; Australia, E. Removal of nitrates from highly-contaminated industrial wastewater Sustainable Environmental Technology for Chemical and Allied industries (SETCA) View project Innovative Technologies for the Remediation of Soils and Waters View project. La Chim. E L’Industria 2012, 94, 100–110. [Google Scholar]
- Ren, H.T.; Liang, Y.; Han, X.; Liu, Y.; Wu, S.H.; Bai, H.; Jia, S.Y. Photocatalytic oxidation of aqueous ammonia by Ag2O/TiO2 (P25): New insights into selectivity and contributions of different oxidative species. Appl. Surf. Sci. 2020, 504, 144433. [Google Scholar] [CrossRef]
- Pollema, C.H.; Milosavljevic’, E.B.; Hendrix, J.L.; Solujic’, L.; Nelson, J.H. Photocatalytic oxidation of aqueous ammonia (ammonium ion) to nitrite or nitrate at TiO2 particles. Mon. Chem. Chem. Mon. 1992, 123, 333–339. [Google Scholar] [CrossRef]
- Bravo, A.; Garcia, J.; Domenech, X.; Peral, J. Some aspects of the photocatalytic oxidation ofammoniumion by titanium dioxide. J. Chem. Res. 1993, 9, 376–377. [Google Scholar]
- Huang, L.; Li, L.; Dong, W.; Liu, Y.; Hou, H. Removal of ammonia by OH radical in aqueous phase. Environ. Sci. Technol. 2008, 42, 8070–8075. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Edwards, J.G.; Davies, J.A. Photooxidation of aqueous ammonia with titania-based heterogeneous catalysts. Sol. Energy 1994, 52, 459–466. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Sá, J.; Agüera, C.A.; Gross, S.; Anderson, J.A. Photocatalytic nitrate reduction over metal modified TiO2. Appl. Catal. B Environ. 2009, 85, 192–200. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase Characterization of TiO2 Powder by XRD and TEM. Nat. Sci. 2008, 42, 357–361. [Google Scholar]
- Bahadori, E.; Rapf, M.; Di Michele, A.; Rossetti, I. Photochemical vs. photocatalytic azo-dye removal in a pilot free-surface reactor: Is the catalyst effective? Sep. Purif. Technol. 2020, 237, 116320. [Google Scholar] [CrossRef]
- Rossetti, I.; Compagnoni, M.; Ramis, G.; Freyria, F.; Armandi, M.; Bonelli, B. Development of unconventional photocatalytic reactors and processes for the abatement of harmful N-containing pollutants. Chem. Eng. Trans. 2017, 57, 1663. [Google Scholar] [CrossRef]
- Bahadori, E.; Tripodi, A.; Ramis, G.; Rossetti, I. Semi-Batch Photocatalytic Reduction of Nitrates: Role of Process Conditions and Co-Catalysts. ChemCatChem 2019, 11, 4642–4652. [Google Scholar] [CrossRef]
- Bahadori, E.; Conte, F.; Tripodi, A.; Ramis, G.; Rossetti, I. Photocatalytic selective oxidation of ammonia in a semi-batch reactor: Unravelling the effect of reaction conditions and metal co-catalysts. Catalysts 2021, 11, 209. [Google Scholar] [CrossRef]
- Zeng, M. Influence of TiO2 surface properties on water pollution treatment and photocatalytic activity. Bull. Korean Chem. Soc. 2013, 34, 953–956. [Google Scholar] [CrossRef] [Green Version]
- Nemoto, J.; Gokan, N.; Ueno, H.; Kaneko, M. Photodecomposition of ammonia to dinitrogen and dihydrogen on platinized TiO2 nanoparticules in an aqueous solution. J. Photochem. Photobiol. A Chem. 2007, 185, 295–300. [Google Scholar] [CrossRef]
- Shibuya, S.; Aoki, S.; Sekine, Y.; Mikami, I. Influence of oxygen addition on photocatalytic oxidation of aqueous ammonia over platinum-loaded TiO2. Appl. Catal. B Environ 2013, 138--139, 294–298. [Google Scholar] [CrossRef]
- Bahadori, E.; Compagnoni, M.; Tripodi, A.; Freyria, F.; Armandi, M.; Bonelli, B.; Ramis, G.; Rossetti, I. Photoreduction of nitrates from waste and drinking water. Mater. Today Proc. 2018, 5, 17404–17413. [Google Scholar] [CrossRef]
- Atchard, C.G.H. A new sensitive chemical actinometer—II. Potassium ferrioxalate as a standard chemical actinometer. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1956, 235, 518–536. [Google Scholar] [CrossRef]
- P25—EVONIK. Available online: https://corporate.evonik.com/en/products/search-products/pages/product-details.aspx?productId=43469 (accessed on 15 May 2021).
- Chiarello, G.L.; Rossetti, I.; Forni, L. Flame-spray pyrolysis preparation of perovskites for methane catalytic combustion. J. Catal. 2005, 236, 251–261. [Google Scholar] [CrossRef]
- Compagnoni, M.; Lasso, J.; Di Michele, A.I.; Rossetti, I. Flame-pyrolysis-prepared catalysts for the steam reforming of ethanol. Catal. Sci. Technol. 2016, 6, 6247–6256. [Google Scholar] [CrossRef]
- Bahadori, E.; Tripodi, A.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G.; Dimitratos, N.; Wang, D.; Rossetti, I. High pressure CO2 photoreduction using Au/TiO2: Unravelling the effect of co-catalysts and of titania polymorphs. Catal. Sci. Technol. 2019, 9, 2253–2265. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Morales, A.E.; Mora, E.S.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. Física 2007, 53, 18–22. [Google Scholar]
- Verdouw, H.; Van Echteld, C.J.A.; Dekkers, E.M.J. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 1978, 12, 399–402. [Google Scholar] [CrossRef]
- Aillet, T.; Loubiere, K.; Dechy-Cabaret, O.; Prat, L. Accurate measurement of the photon flux received inside two continuous flow microphotoreactors by actinometry. Int. J. Chem. React. Eng. 2014, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]

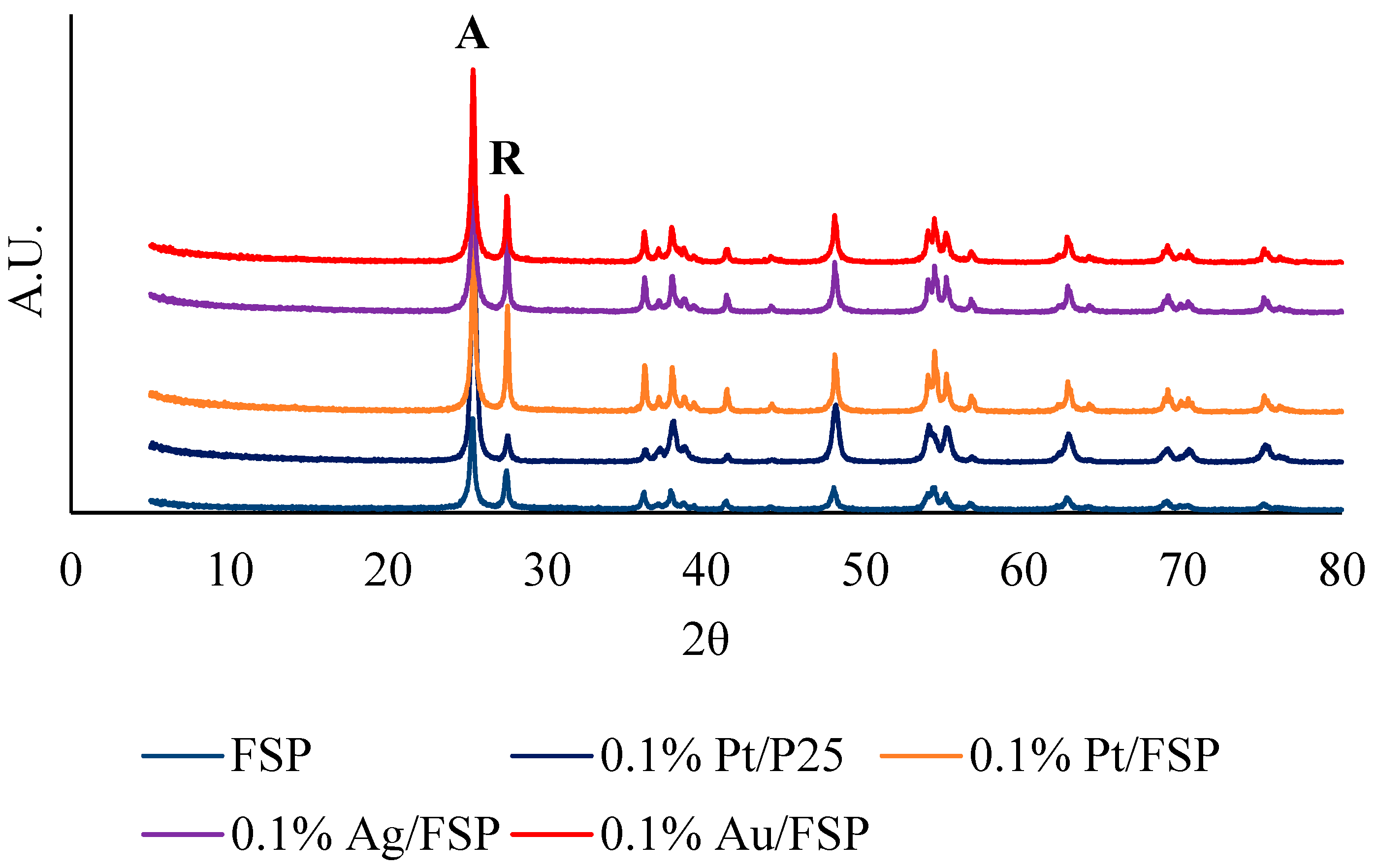




| Sample | P25 | 0.1% Pt/P25 | FSP | 0.1% Ag/FSP | 0.1% Pd/FSP | 0.1% Pt/FSP |
|---|---|---|---|---|---|---|
| Phase % | A(78) + R(22) | A(87) + R(13) | A(65) + R(35) | A(70) + R(30) | A(53) + R(47) | A(64) + R(36) |
| BET Surface Area (m2/g) | 45 | 55 | 67 | 72 | 57 | 60 |
| Crystallite Size (nm) | 15 | 21 | 23 | 30 | 18 | 26 |
| Total Pore Volume (cm3/g) | 0.11 | 0.32 | 0.14 | 0.36 | 0.21 | 0.25 |
| t-plot Micropore Volume (cm3/g) | 0.012 | 0.0036 | 0.02 | 0.00044 | 0.0003 | 0.0037 |
| BJH Adsorption Pore Width (nm) | 22 | 23 | 20 | 17 | 15 | 19 |
| Band Gap (eV) | 3.41 | 3.12 | 3.31 | 3.24 | 3.13 | 3.11 |
| Precursor | Metal Loading (%mol) | Titania Source | Reduction T (°C) | Colour |
|---|---|---|---|---|
| AgNO3 (pur. > 99%) | 0.1 | FSP | 150 | Gry-Brown |
| AuCl3 (pur. > 99%) | 0.1 | FSP | 700 | Light Purple |
| Pd(NO3)2·2H2O | 0.1 | FSP | 300 | Grey |
| Pt(acac)2 (pur. > 97%) | 0.1 | FSP | 700 | Grey |
| Pt(acac)2 (pur. > 97%) | 0.1 | P25 | 700 | Grey |
| Cu(ac)2 (pur. 98%) + Pt(acac)2 (pur > 97%) | 0.1 Cu + 0.1 Pt | P25 | 700 | White |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conte, F.; Pellegatta, V.; Tripodi, A.; Ramis, G.; Rossetti, I. Photo-Oxidation of Ammonia to Molecular Nitrogen in Water under UV, Vis and Sunlight Irradiation. Catalysts 2021, 11, 975. https://doi.org/10.3390/catal11080975
Conte F, Pellegatta V, Tripodi A, Ramis G, Rossetti I. Photo-Oxidation of Ammonia to Molecular Nitrogen in Water under UV, Vis and Sunlight Irradiation. Catalysts. 2021; 11(8):975. https://doi.org/10.3390/catal11080975
Chicago/Turabian StyleConte, Francesco, Veronica Pellegatta, Antonio Tripodi, Gianguido Ramis, and Ilenia Rossetti. 2021. "Photo-Oxidation of Ammonia to Molecular Nitrogen in Water under UV, Vis and Sunlight Irradiation" Catalysts 11, no. 8: 975. https://doi.org/10.3390/catal11080975
APA StyleConte, F., Pellegatta, V., Tripodi, A., Ramis, G., & Rossetti, I. (2021). Photo-Oxidation of Ammonia to Molecular Nitrogen in Water under UV, Vis and Sunlight Irradiation. Catalysts, 11(8), 975. https://doi.org/10.3390/catal11080975









