Abstract
Catalysts are usually used in the thermal conversion of waste tires to enhance the efficiency of the process and the quality of pyrolytic products. Recently, it has already been proved that Ni/Fe bimetallic catalysts had an effective catalytic effect on the thermal decomposition of organic solid wastes. Herein, we employed a TG-IR-GC/MS system to investigate the kinetics and product analysis of waste tire catalytic pyrolysis using Ni/Fe bimetallic ZSM-5 as catalysts. Iso-conversional methods and master-plot methods were employed to estimate the activation energies and reaction model of waste tire catalytic pyrolysis. The results illustrated that the ZSM-5 loading with 7 wt.% Ni and 3 wt.% Fe had the best catalytic effect on decreasing the activation energies with a reduction of 13%. The determination of kinetic models showed that both non-catalyzed and catalyzed pyrolysis were fitted to a Fn model while the addition of a catalyst reduced the reaction order to varying degrees. Both FTIR and GC/MS results suggested that the metallic Ni-based catalyst had strong ability to transform alkenes into aromatic hydrocarbons. Ni/Fe bimetallic catalysts showed almost the same catalytic performance as the Ni metallic catalyst, which could reduce the cost of the catalyst. Thus, this study could deepen the understanding and provide a basic guideline of Ni/Fe bimetallic catalysts on the catalytic pyrolysis process of waste tires.
1. Introduction
The transportation industry and the automobile manufacturing industry have made great progress in the last few decades. However, the tire consumption and the stream of waste dumped into the environment have been increased, which is a growing concern worldwide. According to statistics, in 2017, China produced about 340 million waste tires, weighing more than 13 million tons. In 2018, the annual output of waste tires increased to 14.58 million tons, and it is still growing at a rate of 5% to 8% each year. Hence, it is necessary to find out an effective route to reduce the stock of worn tires and convert them to high-value resources without causing any harm to the environment.
Compared with traditional incineration technique, waste tire pyrolysis is considered as a promising technology for the production of high value-added products such as syngas, fuel oil, and char. Thereinto, pyrolytic oil with high value chemicals such as benzene, toluene, xylenes, styrene, and d-limonene are of great interest to waste tire pyrolysis. For example, d-limonene is widely used in the production of industrial solvents, resins, and adhesives [1]. Benzene derivatives (ethylbenzene, cumene, etc.), which were originated from benzene, could be used to produce plastics, resins, fibers, surfactants, dyestuffs, and pharmaceuticals [2]. To improve the conversion efficiency, catalysts have been widely used in the process of waste tire pyrolysis. Catalytic pyrolysis can not only reduce the activation energy of the pyrolysis reaction, but also improve the quality of pyrolysis products, so as to improve the economic benefits of waste tire pyrolysis. Zeolites such as ZSM5 [3], USY [4], HY, SBA [5], and MCM [6] were widely used in thermal conversion of waste tire, biomass and electronic waste. Due to its suitable pore structure and acidity, zeolites can increase the gas yield and promote the yield of aromatic hydrocarbons, especially monocyclic aromatic hydrocarbons. Ding et al. [7] employed a Pyrolyze-Gas Chromatograph/Mass Spectrum (Py-GCMS) reactor to investigate the catalytic influence of HZSM5 and HY on the waste tire pyrolysis. The results showed that the presence of HY led to a dramatic decrease of alkenes and an enormous increase of aromatics, which indicated that HY had an excellent performance on converting alkenes to aromatics in the catalytic pyrolysis of waste tires. Meanwhile, compared with HY, HZSM5 showed a weaker performance on promoting the production of aromatics due to its poor ring-opening ability to d-limonene and its isomers while HZ had the superior selectivity to BTXE (benzene, toluene, xylene, and ethylbenzene). Wang et al. [4] explored the effect of SiO2/Al2O3 molar ratio of USY zeolites on the catalytic pyrolysis and found that the USY catalyst with the SiO2/Al2O3 molar ratio of 5.3 performed well in the formation of aromatic hydrocarbons. Moreover, a phenomenon was discovered that the USY catalyst with a low SiO2/Al2O3 ratio was more beneficial to the production of toluene and xylenes. Metal modification is often an effective method to improve the catalytic performance of catalysts. Transition metals such as Ni [8,9], Fe [10], Cu [11] and Zn [12] were loaded on the support as active ingredients, generally.
Ni is a very active metal which can catalyze the hydrogenation/dehydrogenation reactions [13] and is widely used in the stream reforming of biomass pyrolysis-tar. A previous study [14] has already proved that the catalytic pyrolysis of biomass with the presence of Ni showed well deoxygenation activity and high H2 production. Namchot et al. [15] used Ni/HZSM-5 and HZSM-5 as the catalysts to investigate the catalytic pyrolysis of waste tire in a bench-scale reactor. The increase of gasoline production indicated that the introduction of Ni greatly improved the cracking activity of catalyst. Moreover, they also found that Ni doping strongly enhanced the selectivity of ethylbenzene, toluene, cumene in pyrolytic oil. Meanwhile, Fe-based catalysts have attracted more attention due to the reason that it is an environment-friendly catalyst with low toxicity. Muenpol et al. [16] investigated the effect of Fe doping on the yield and quality of pyrolytic oil. The results illustrated that the addition of Fe enhanced the gas yield, which indicated that Fe enhanced the cracking ability. Compared with HZSM-5, Fe/HZSM-5 led to a sharp increase of the petrochemical contents in oils such as benzene, xylene, and ethylbenzene and a decrease of poly- and polar-aromatic hydrocarbons. As observed above, the Fe-based catalyst showed as high activity as the Ni-based catalyst.
Recently, bimetallic catalysts such as Ni-Mn [17] and Fe-Ru [18] were widely employed in the catalytic pyrolysis of solid wastes. Ni-Fe-based catalysts have been employed in biomass gasification to increase the conversion efficiency [19]. The results showed that Ni-Fe RHC (rice husk char) can increase the tar conversion efficiency up to 92.3%, which was almost the same as that of Ni RHA (rice husk ash). The introduction of Fe reduced the content of Ni, which resulted in a lower expense of catalyst synthesis. Moreover, according to the catalytic evaluations in Zhang et al.’s study [20], the activity of the Ni surface was passivated by Fe, which led to a reduction in the coke-tolerant property. The characterization of catalysts suggested that compared with single metal, the alloying of Fe with Ni enhanced the thermal stability and inhibited the thermal sintering. Thus, using Ni-Fe based catalysts in the thermal conversion of solid waste is a promising and economical strategy.
Thermogravimetric analysis (TGA) is an effective method to analyze the pyrolysis characteristics of waste tires. Based on the data obtained from the thermo-gravimetric analyzer, iso-conversional method [21] together with master-plot method [22] can estimate the kinetic triplets (activation energy, frequency factor, and reaction model). Kinetics is vital for the understanding of reaction mechanism, design of pyrolysis reactor and optimization of the pyrolysis process. Even though there have been many kinetic studies on waste pyrolysis, the effect of the catalyst on the pyrolysis kinetics and the pyrolysis products is still to be investigated, especially bimetallic catalysts. Therefore, in this study, wet impregnation method was employed to synthesize Ni/Fe bimetallic ZSM-5 catalysts with different loadings of Ni and Fe. The pyrolysis characteristics and product analysis of waste tire catalytic pyrolysis with five synthesized catalysts were investigated by employing TG-IR-GC/MS, which can analysis the properties of pyrolytic volatiles online. Moreover, iso-conversional method and master-plot method were employed to obtain the kinetic parameters and analyze the process of waste tire catalytic pyrolysis.
2. Results and Discussion
2.1. Characterization of the Catalyst
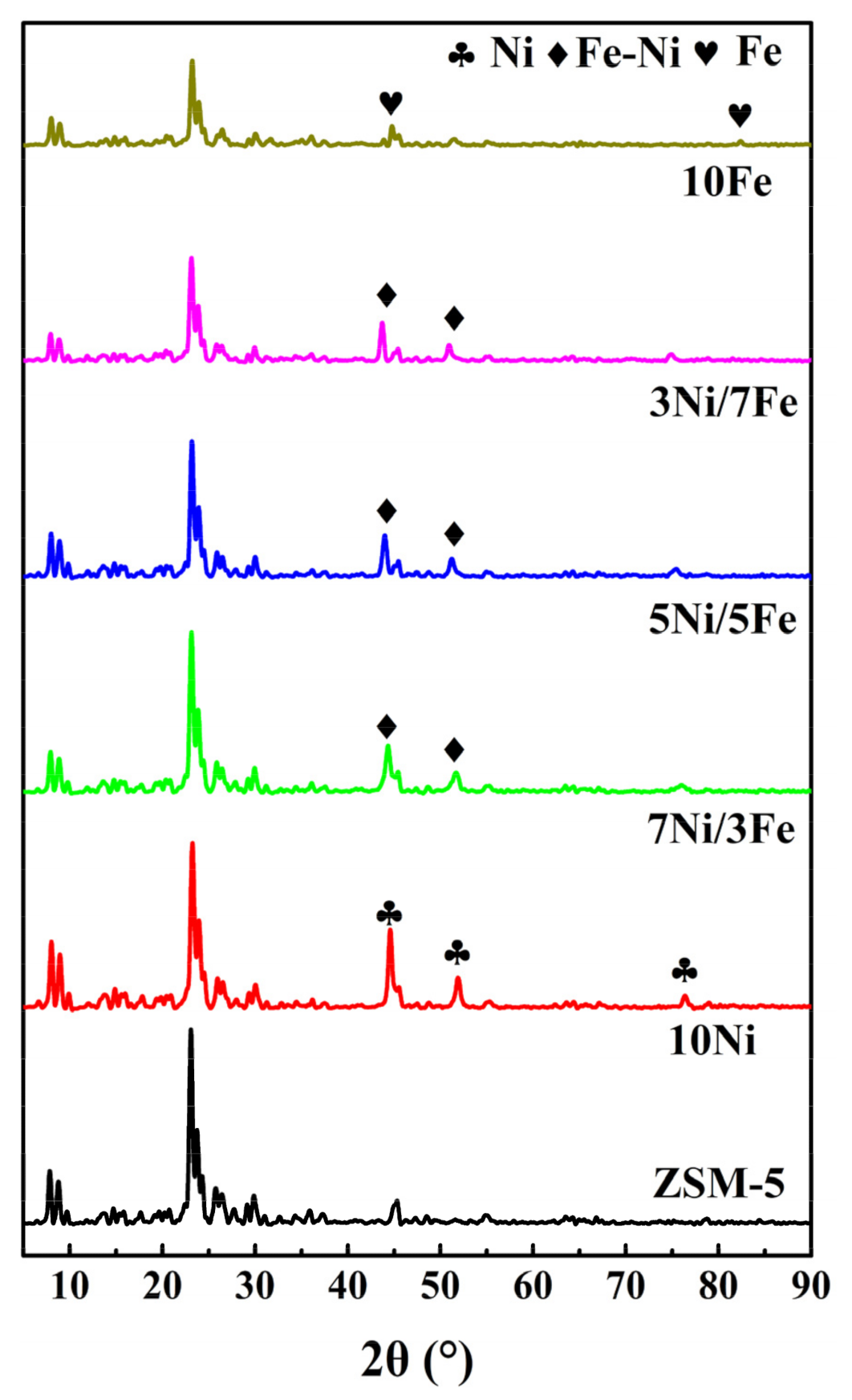
Figure 1 illustrated the XRD patterns of ZSM-5 and modified ZSM-5. All modified XRD patterns of catalysts displayed a ZSM-5 structure, which can be identified by diffraction peaks (101), (200), (332), and (051) at 2θ range of 7~10° and 23~25° [23]. This phenomenon indicated that after the modification by metal Fe and Ni, the modified catalysts remained good crystal structures of the parent ZSM-5 [24]. Diffraction peaks at 44.48°, 51.83°, and 76.35° in the pattern of Ni-ZSM-5, which were assigned to Ni(111), Ni(200), and Ni(220) respectively, indicated the presence of metal Ni particles [25]. The diffraction peaks observed at 44.67°, 65.02°, and 82.33° were assigned to the Fe crystal phase [25]. The patterns of Ni/Fe-ZSM-5 showed that with the increase of metal Fe, the diffraction peak between 44 and 45° was gradually shifted towards the smaller angle. According to Chen et al.’s study [26], the shift could be caused by the formation of Ni-Fe solid alloy. This peak shift indicated that there was interaction between Fe and Ni particles. Based on the previous literature [20], compared with the Ni-based catalysts, the formation of Ni-Fe solid alloy reduced the cost of catalyst preparation, which also provided an excellent catalytic effect. Besides, both thermal stability and coke-tolerant property were enhanced. Furthermore, the introduction of metal led to the decrease of the peak intensity because the X ray was adsorbed by Ni and Fe particles and the incorporation of metal decreased the degree of crystallinity of the ZSM-5 [27].
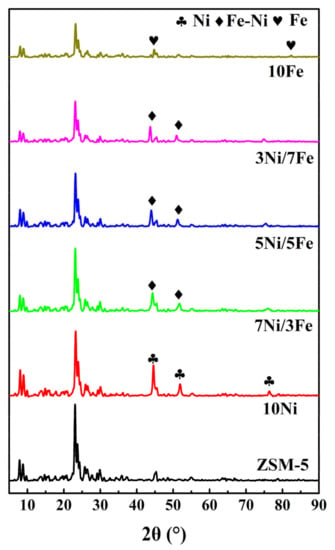
Figure 1.
XRD patterns of ZSM-5 and modified ZSM-5.
Figure S1 plotted the N2 adsorption-desorption isotherms of parent and modified ZSM-5. All catalysts presented type IV isotherm, which corresponds to the typical mesoporous structures. Table 1 summarized the texture properties of catalysts. The metal induced the decrease of the pore volume of ZSM-5. Moreover, after metal modification, with the increase of Fe loading and decrease of Ni loading, the pore volume of the catalyst decreased from 0.265 to 0.203 cm3/g, which indicated that Fe can enter the channel of ZSM-5 much easier than Ni. On the contrary, an opposite trend was discovered on the average pore size.

Table 1.
Texture properties of all samples.
The SEM images of catalysts were shown in Figure S2. After the metal modification, the surface of catalysts did not show obvious change which indicated the metal particles were not gathered on the surface of the support.
To investigate the thermal stability of the modified catalysts, all catalysts were heated from room temperature to 900 °C at the heating rate of 10 °C/min under oxygen-free atmosphere. As shown in Figure S3, all catalysts exhibited excellent thermal stability without showing any noticeable mass variation. The results suggested that in the thermal degradation process of the catalyst + WT mixture, the variation of thermogravimetric data should be all attributed to the thermal reaction of WT.
2.2. Thermal Characteristic Analysis
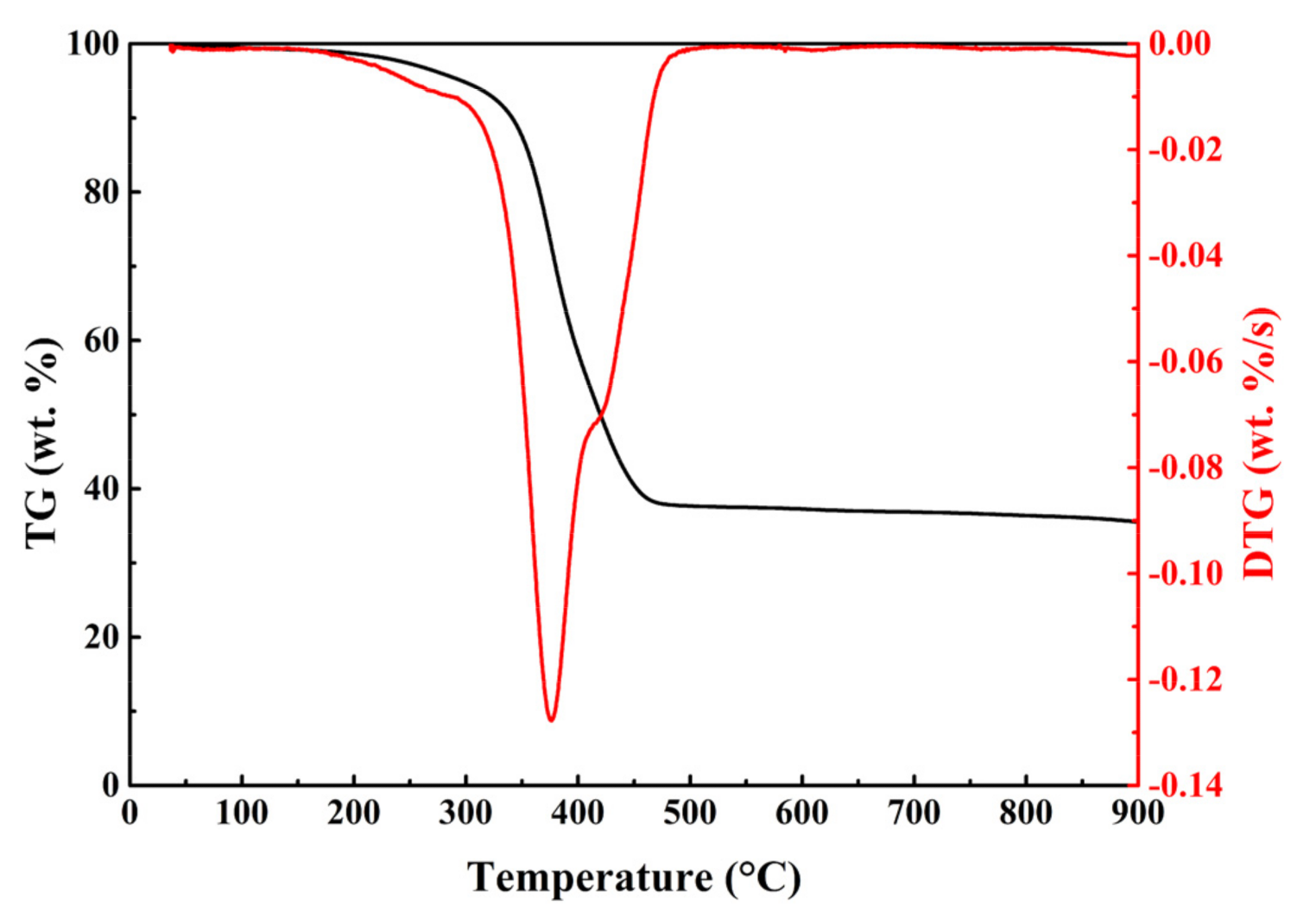
The TG and DTG curves of WT pyrolysis without catalysts were presented in Figure 2. The thermogravimetric analysis from the catalytic pyrolysis of WT with synthesized catalysts were presented in Figure S4. To compare the mass variation of WT, the mass of catalyst was subtracted from the TG data. Compared with the DTG data of WT pyrolysis without catalyst, adding catalysts to the WT pyrolysis did not significantly alter the shape of DTG curves. As shown in Figure 2, thermal degradation can be divided into three stages, namely low-boiling point oil and volatile additives release (stage I) [28], natural rubber (NR), styrene-butadiene rubber (SBR) and butyl rubber (BR) degradation (stage II) [29], and thermal degradation of refractory organic matter (stage III). Obviously, in stage II there were a sharp peak with a shoulder. Based on the previous study [29], it could be determined that the peak, which was located at the lower temperature range, corresponded to the thermal decomposition of NR while the shoulder corresponded to the degradation of BR and SBR. By contrast, it suggested that the waste tire used in this study was mainly composed of NR which indicated that the thermal decomposition of NR might be the major factor of determining the reaction rate in the whole pyrolysis process of waste tire. After adding the synthesized catalysts, the thermal decomposition process showed a slight change in stage I. At the temperature stage range 30~200 °C, all DTG curves of WT catalytic pyrolysis generated a new thermal degradation stage, which could not be observed in the DTG curve of WT pyrolysis without the catalyst. This phenomenon indicated that the addition of the modified catalysts reduced the degradation temperature of low-boiling point oil and volatile additives.
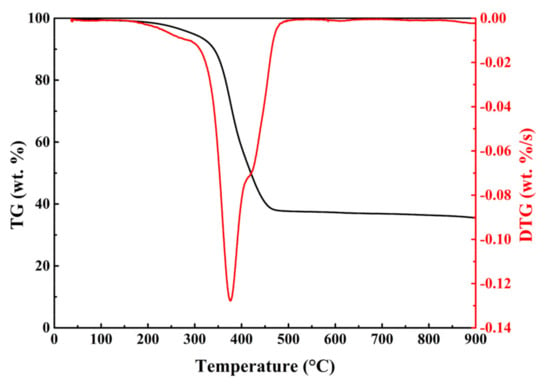
Figure 2.
TG and DTG curves of WT pyrolysis without catalysts at the heating rate of 10 °C/min.
Table 2 lists the pyrolysis characteristic parameters of WT with no catalysts and synthesized catalysts at 10 °C/min. WT pyrolysis without catalysts demonstrated the maximum weight loss rate (−0.127 wt.%/s) at 376.2 °C. By contrast, the maximum weight loss rate of WT catalytic pyrolysis increased slightly, and the corresponding temperature showed a minimal drop. For WT with 10Ni and 10Fe, the maximum weight loss rate was −0.137 wt.%/s at 373.9 °C and −0.137 wt.%/s at 373.5 °C. The maximum weight loss rates were slightly higher than that of WT pyrolysis with no catalysts and other three modified catalysts. The results indicated that single metal modification had better effect on the accelerating WT thermal decomposition than multi-metal modification. Moreover, the addition of Ni/Fe catalysts resulted in the decrease of residual mass fraction. This suggested that the catalysts with metal Ni and Fe can increase the pyrolysis potential of WT to generate more pyrolysis products

Table 2.
Pyrolysis characteristic parameters for WT catalytic pyrolysis at 10 °C/min.
Figure S5 presented the TG and DTG curves of WT pyrolysis with no catalyst and five synthesized catalysts at three different heating rates of 10, 20, and 30 °C/min. The similar trend of TG and DTG curves indicated that the reaction mechanism of waste tire pyrolysis was not affected by the change of heating rate. Note that due to the heat transfer limitation, the temperature corresponding to a peak is shifted toward the higher temperature region. Previous studies have also reported this trend [30,31].
2.3. Estimation of Activation Energy
Kissinger-Akahira-Sunose (KAS) method and Starink’s method were employed to determine the activation energy of WT thermal decomposition. The overall correlation coefficient R2 in Tables S1 and S2 was above 0.9, suggesting that the employment of KAS method and Starink’s method was suitable to model the process of WT catalytic pyrolysis. Table 3 and Table S3 list the values of activation energy obtained by using Starink’s method and KAS method, respectively. Due to the higher accuracy of Starink’s method than KAS method [32], the values of activation energy from Starink’s method were used in the following discussion. Figure 3 exhibited the activation energy with WT conversion from α = 0.2 to α = 0.8. The activation energies of all samples showed monotonically increasing trends. The case without the catalyst presented the highest activation energy from α = 0.2 to α = 0.65. In Table 5, the average activation energy of WT was 216.32 kJ/mol, which was higher than all other cases with synthesized catalysts. Moreover, our previous study had already demonstrated that parent ZSM-5 had little influence on the reduction of the energy barrier of pyrolysis reaction [33]. Thus, it could indicate that the metal, which was loaded on the ZSM-5, was the key to reducing the activation energy of WT pyrolysis.

Table 3.
Activation energies of all samples with Starink’s method.
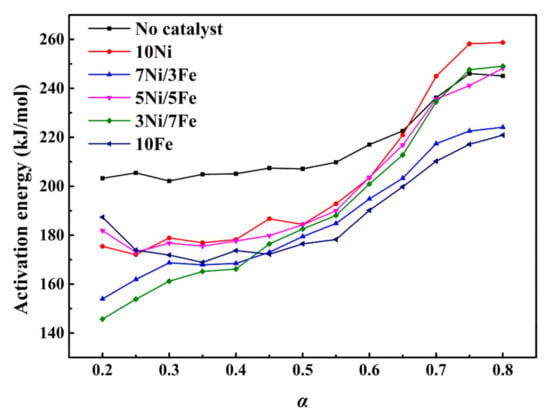
Figure 3.
Activation energy distributions of WT catalytic pyrolysis with no catalysts and different synthesized catalysts.
Table 4 lists the activation energies and methods presented in the open literatures. The addition of catalysts, besides zeolite, could reduce the activation energies of waste tire thermal composition in different degrees. Thereinto, metal nickel had the best catalytic effect on the reduction of activation energies, which agreed with the results in this study. The variations within the activation energies might be caused by the differences in tire components, kinetic methods, and catalyst types.

Table 4.
Summary of waste tire catalytic pyrolysis kinetic studies.
At α = 0.20–0.55, mainly corresponding to the thermal degradation of NR, the activation energy varied from 202.16 kJ/mol to 209.76 kJ/mol for WT pyrolysis without the catalyst. In this conversion stage, with the dual metal modified catalysts, the values of activation energy ranged from 153.97 kJ/mol to 184.79 kJ/mol for 7Ni/3Fe, from 145.69 kJ/mol to 188.03 kJ/mol for 3Ni/7Fe, which were much lower than that of the rest cases. This result indicated that Ni/Fe dual modified zeolite catalyst had a stronger catalytic effect on the thermal decomposition of NR than the single metal modified catalysts. The decrease of activation energy was due to the reason that Ni and Fe had an obvious catalytic effect on the C–C and C–H cleavage [37]. The improved catalytic effect of 7Ni/3Fe and 3Ni/7Fe might be caused by the synergistic catalysis between iron and nickel metals. At α = 0.55–0.80, an obvious increasing trend can be observed on the activation energy distribution of all samples, which could be explained by that SBR and BR had more complex chemical structures and higher thermal resistance than NR and the main reactant of thermal decomposition was gradually shifted from NR to SBR and BR. Moreover, comparing with WT pyrolysis without a catalyst, there was no reduction on the values of activation energy of WT thermal degradation with 10Ni, 5Ni/5Fe and 3Ni/7Fe, respectively. The catalytic effects of 7Ni/3Fe and 10Fe were also weakened. The monomer of SBR is a synthetic monomer of 1, 3-butadiene (CH2=CH–CH=CH2) and styrene (C6H5–CH=CH2), while NR is a polymer of cis-1, 4-polyisoprene (CH3–CH=CH–CH3). The benzene ring structure might be harder to rupture than C=C and C–C under the catalysis of iron and nickel metals. Hence, during the stage of BR and SBR degradation, the synthesized catalysts had almost no catalytic effect on the WT thermal decomposition.
2.4. Determination of Reaction Model
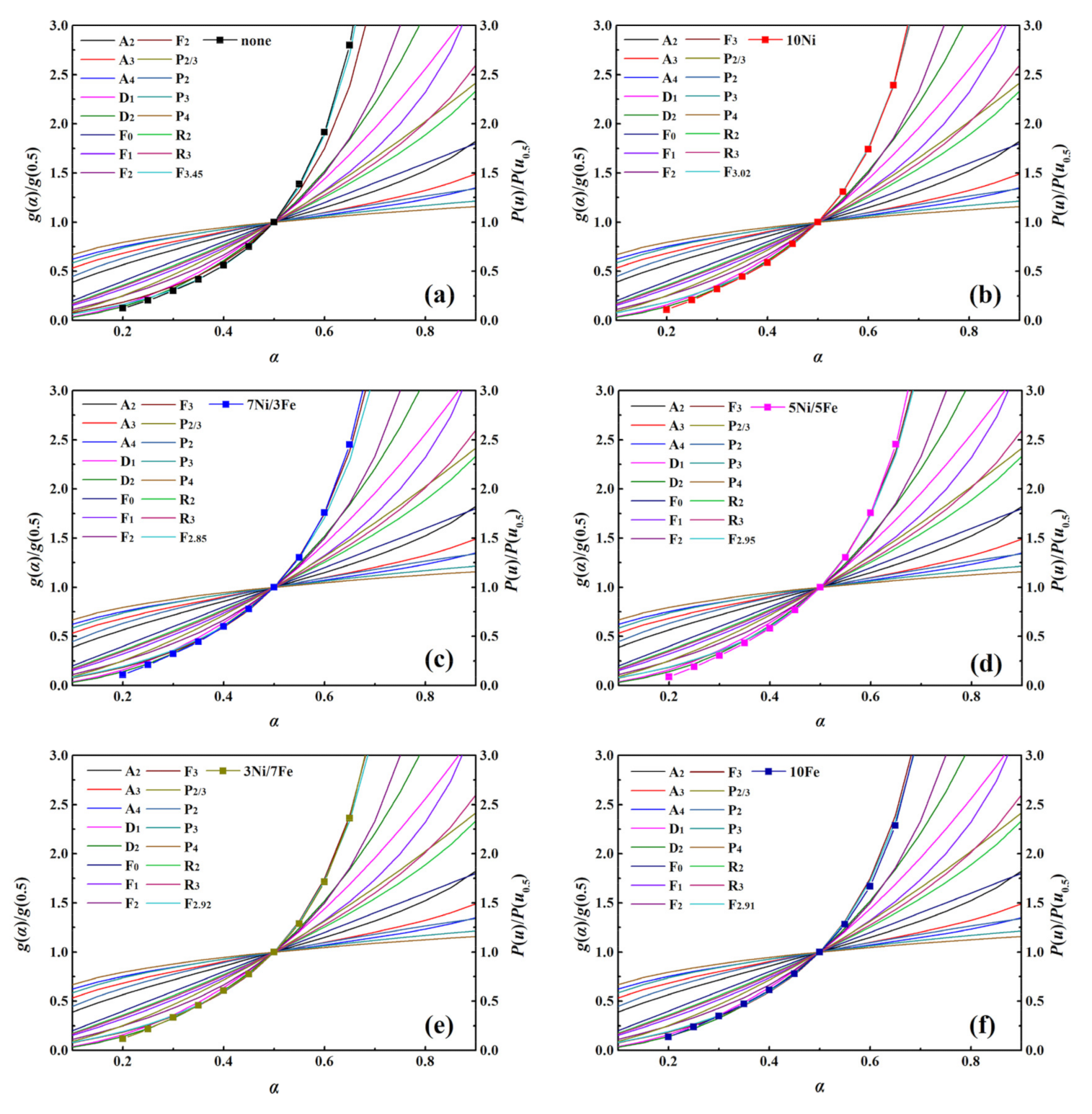
The reaction mechanism is an indispensable part which can give an insight into the behavior of the WT pyrolysis process. In this study, the reaction models of WT catalytic pyrolysis were determined by comparing the theoretical and experimental master-plots. Figure S6 showed the experimental master-plots of WT pyrolysis with no catalyst and five synthesized catalysts at three different heating rates. As shown in Figure S6, for all samples, it can be observed that the shape of the P(u)/P(u0.5) plots were similar, which illustrated that the heating rate had little effect on the catalytic pyrolysis processes of WT. The comparisons of theoretical master-plots g(α)/g(0.5) and experimental master-plots P(u)/P(u0.5) of WT catalytic pyrolysis with no catalyst and five synthesized catalysts at 20 °C/min were exhibited in Figure 4. Obviously, the experimental master-plot of WT without the catalyst was located between the theoretical master-plots of model F2 and F3, while those of WT with the modified catalysts were situated at the vicinity of the theoretical master-plots of model F3. Hence, the kinetic models of Fn, g(α) = [(1 − α)1−n − 1]/(n − 1), were employed to describe the thermal degradation of WT best.
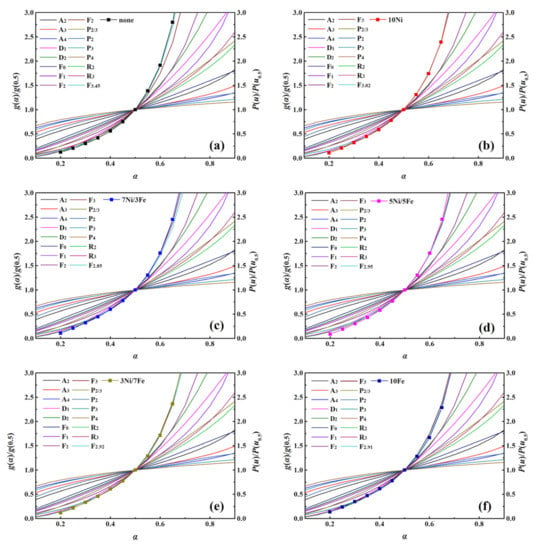
Figure 4.
g(α)/g(0.5) versus α for WT pyrolysis with no catalyst and five synthesized catalysts at the heating rate of 20 °C/min (a) No catalyst; (b) 10Ni; (c) 7Ni/3Fe; (d) 5Ni/5Fe; (e) 3Ni/7Fe; (f) 10Fe.
Combining the expression of the kinetic model Fn, Equation (1) can get:
where n is the reaction order. Figure S7 showed the plots of [(1 − α) (1−n) − 1]/(n − 1) versus EP(u)/βR. The most probable values of n of WT catalytic pyrolysis were the values which corresponded to the highest values of correlation coefficient R2 [38]. The value ranges of n were 2~3 for WT pyrolysis with five synthesized catalysts and 3~4 for WT pyrolysis without catalysts in increments of 0.01. As listed in Table 5, although the Ni/Fe-ZSM-5 catalysts were added into the thermal degradation process of WT, the pyrolysis was still based on the order-based reaction model, which indicated that the reaction rate of WT pyrolysis was mainly influenced by the concentration of reactants.

Table 5.
Kinetic triplets of WT catalytic pyrolysis at 10, 20, and 30 °C/min by master-plots method.
The pre-exponential values (A) obtained through Starink’s method were also listed in Table 5. Generally, A represented the collision degree of molecule in unit time [39]. It could be observed that the addition of Ni/Fe-ZSM-5 led to a reduction of pre-exponential values to some extent. A higher value of the per-exponential factor indicates a higher molecular collision, which requires more heat to be transferred [40]. Thus, the reduction of pre-exponential values indicated that the waste tire pyrolysis reaction needed less energy, which also can explain the reduction of activation energy after the addition of modified catalysts.
2.5. Product Analysis
2.5.1. TG-FTIR
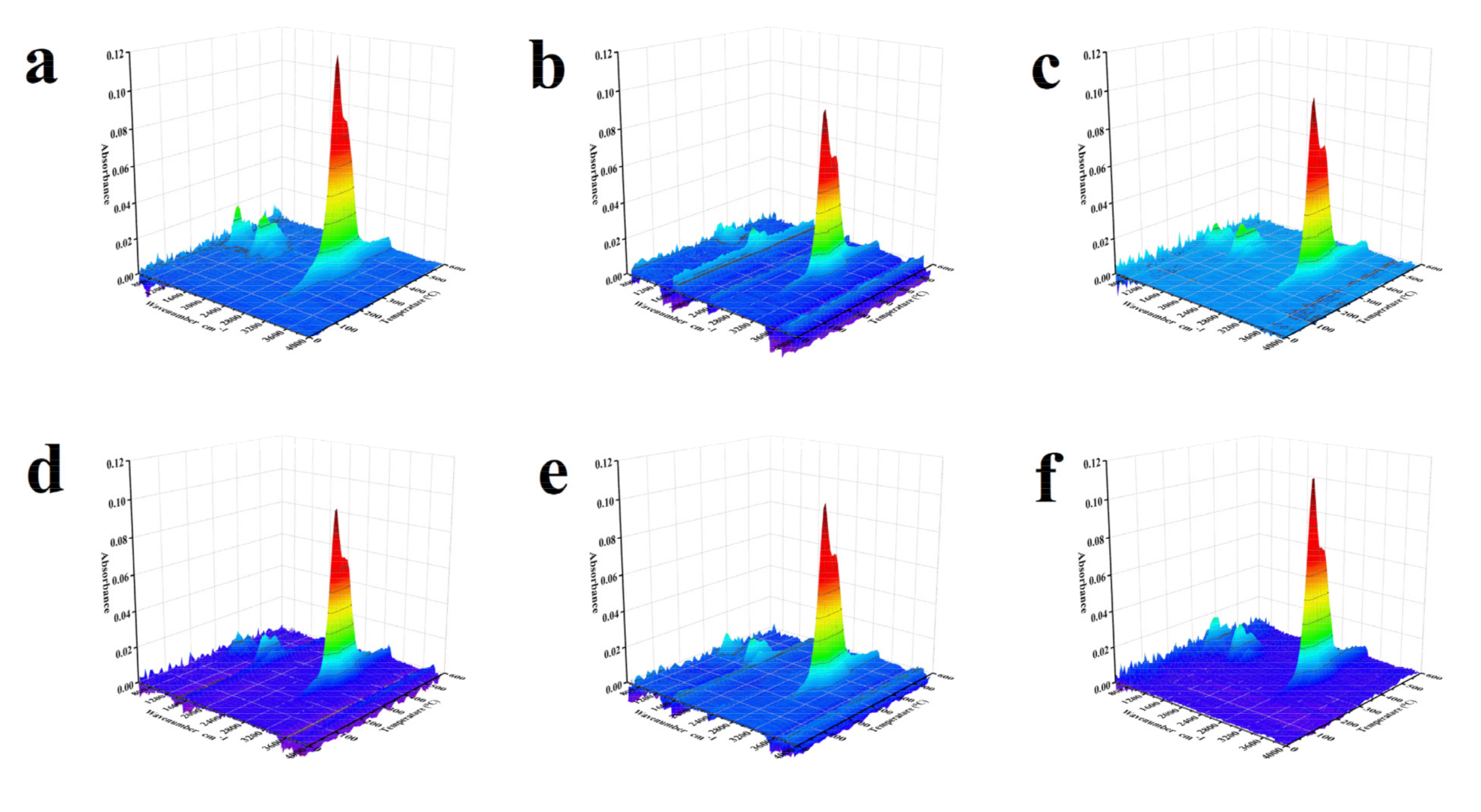
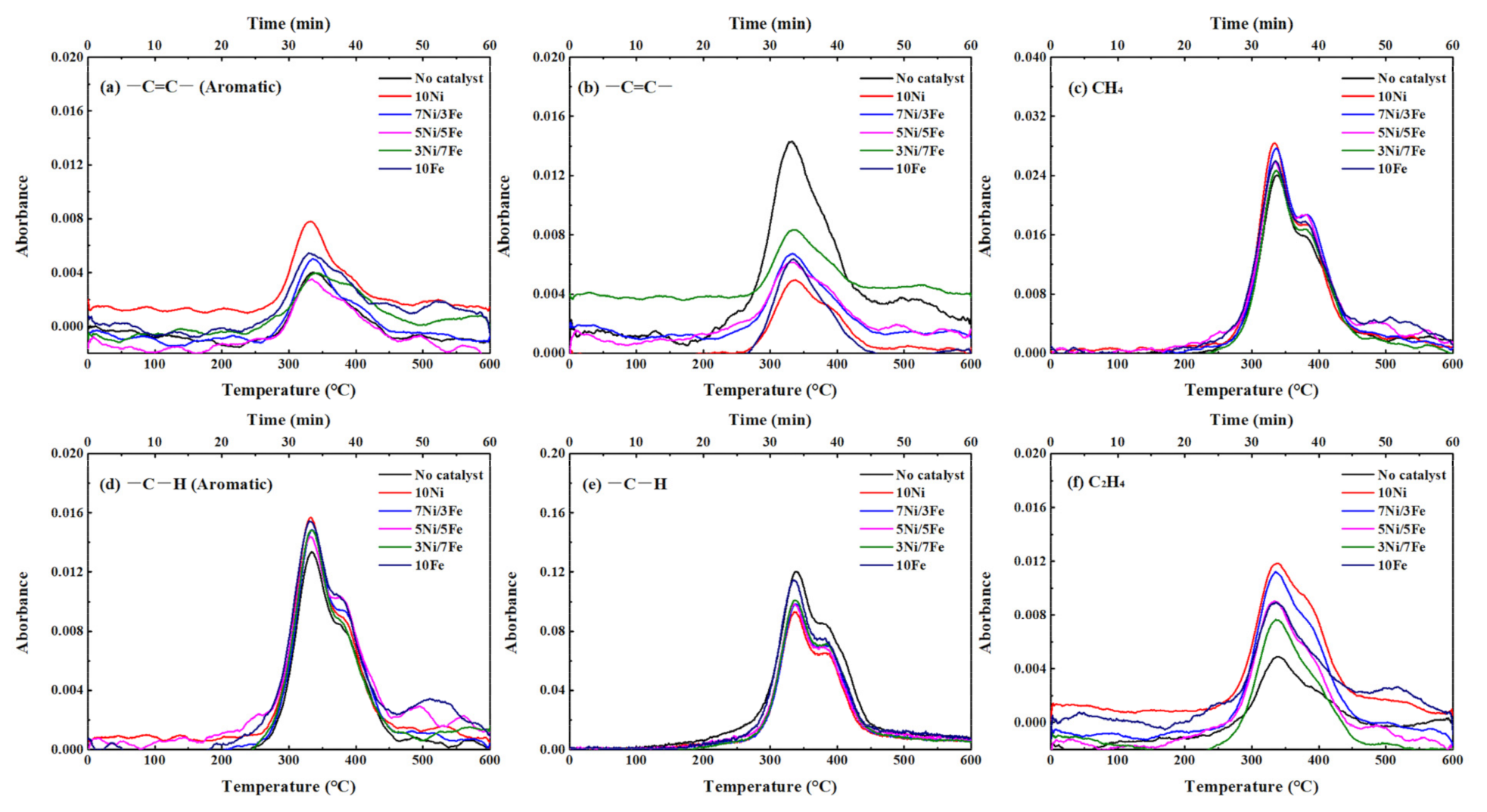
FTIR was used to detect the functional groups of volatiles from the thermal decomposition of waste tire in real time. According to the Lambert-Beer law [41], there is a linear relationship between the absorbance of the functional group and the concentration of the volatiles containing the functional group. Figure 5 presented the 3D FTIR spectra of the released volatiles from waste tire catalytic pyrolysis with no catalyst and five synthesized catalysts. Obviously, the functional group composition of each sample was almost the same and there were absorption peaks with wavenumber ranges of 800–1200, 1400–1700, and 2800–3200 cm−1 in all FTIR spectra. A summary of IR bonds identified by the FTIR absorption bands of typical functional groups is listed in Table 6. The wavenumber corresponding to functional groups and gas products shown in Figure 6 were 1520 cm−1 for –C=C– (Aromatic), 1650 cm−1 for –C=C– (aliphatic), 2887 cm−1 for –C–H (aliphatic), 3014 cm−1 for CH4, 3080 cm−1 for –C–H (Aromatic), and 950 cm−1 for C2H4, respectively.
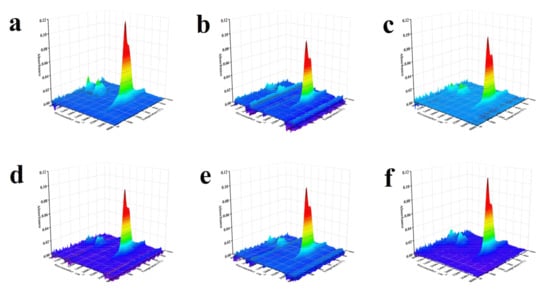
Figure 5.
3D FTIR spectra of the released volatiles from waste tire catalytic pyrolysis (a) No catalyst; (b) 10Ni; (c) 7Ni/3Fe; (d) 5Ni/5Fe; (e) 3Ni/7Fe; (f) 10Fe.

Table 6.
A summary of IR bonds identified by the FTIR absorption bands of the typical functional groups.
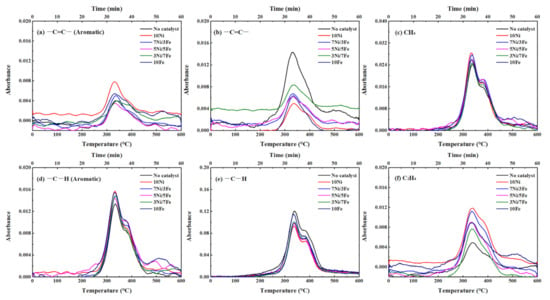
Figure 6.
Evolutions of the functional groups and gas products with temperature in waste tire catalytic pyrolysis (a) –C=C– (Aromatic); (b) –C=C–; (c) CH4; (d) –C–H (Aromatic); (e) –C–H; (f) C2H4.
As presented in Figure 6a, for WT pyrolysis without catalyst, an absorption peak of –C=C– in aromatic hydrocarbons during the WT pyrolysis process appeared at the temperature range of 250~500 °C. In the lower temperature range of 250~420 °C which mainly corresponds to the thermal decomposition of NR, the generation of –C=C– (aromatic) was attributed to the aromatization of cycloalkenes and olefins. With the increase of the temperature, the main reactant of thermal decomposition was shifted to BR and SBR. The evolution of –C=C– (aromatic) was related to the styrene, which was formed by the scission and dehydrogenation of SBR. At the same time, the evolution of –C–H (aromatic) was similar to that of –C=C– (aromatic), which was derived from the generation of aromatic hydrocarbons such as toluene, xylene, and cymene. With the addition of synthesized catalysts, the intensity of the absorption peaks of both –C=C– and –C–H in aromatic hydrocarbons increased obviously, which indicated that the Ni/Fe-ZSM-5 catalysts can enhance the yield of aromatic hydrocarbons. The order of catalytic effect on the formation of aromatic hydrocarbons was: 10Ni > 10Fe > 7Ni/3Fe ≈ 3Ni/7Fe > 5Ni/5Fe.
Figure 6b,e displayed the evolution of both –C=C– and –C–H in aliphatic hydrocarbons. At around 270 °C, there was an obvious change in the absorption of –C=C–, which was caused by the thermal decomposition of the main components in WT. As the pyrolysis temperature further increased, the absorption intensity of –C=C– appeared as a reduction, which was attributed to the aromatization of alkenes and the secondary decomposition of the intermediate such as isoprene and d-limonene. As for –C–H in aliphatic hydrocarbons, the generation mechanism was the cleavage of alkyl side chains and β bond scission of alkenes [42]. All Ni/Fe-ZSM-5 catalysts reduce the yield of those in aliphatic hydrocarbons, which indicated that metal modified catalysts might inhibit the formation or enhance the transition of aliphatic hydrocarbons to aromatic compounds. As observed in Figure 6b,e, the highest absorption intensity of –C=C– and –C–H (aliphatic) was obtained in no catalyst, while 10Ni yield the lowest absorption intensity. This phenomenon was opposite to the catalytic effect on the formation of aromatic hydrocarbons, which suggested that Ni/Fe-ZSM-5 favors the aromatization of alkenes.
As depicted in Figure 6c, the evolution process of CH4 and –C–H in both aromatic and aliphatic hydrocarbons featured a good similarity, which could speculate that the release of CH4 was related to the formation and transformation of –C–H. Obviously, there was one CH4 evolution peak with a shoulder in the temperature range of 250~375 °C and 375~500 °C. According to the Liu et al.’s study [43], the generation of CH4 during the thermal cracking process was caused by the combination of hydrogen donors and unstable functional groups and fragment such as –CH3 and –CH2–. In the temperature range of 250~375 °C, the source of methyl free radicals might be primarily the alkyl free radicals, which were located at the aliphatic hydrocarbons [42]. Afterwards, the methyl free radicals can capture the H free radicals, which were from the weak C–H in the aliphatic hydrocarbons to form methane. With the increase of pyrolysis temperature, the methyl free radicals were mainly originated from the cracking of alkyl chains located on the aromatic rings and cycloalkene rings [42,44]. As for C2H4, the formation mechanism was similar to CH4, which was mainly attributed to the β scission of the free alkyl radicals, while a certain amount of C2H4 was generated by the dissociation of C–H bonds in free ethyl radicals [44,45]. After adding the catalysts, the yields of CH4 and C2H4 increased significantly. Thereinto, 10Ni favored the formation of both CH4 and C2H4 the most. The positive effect on the formation of aromatic hydrocarbons and negative effect on the formation of aliphatic hydrocarbons implied that Ni was very active in hydrogenation/dehydrogenation reactions so that 10Ni could promote the transition of aliphatic hydrocarbons into aromatic hydrocarbons. However, 10Fe did not catalyze the pyrolysis process of WT as effective as 10Ni, which indicated that Fe had a weaker catalytic effect on the C–C and C–H cleavage than Ni. Moreover, three bimetallic catalysts could also obviously catalyze the thermal decomposition process of WT to generate CH4 and C2H4, while the catalytic effect of 7Ni/3Fe was almost the same as that of 10Ni. The similar catalytic effect might be caused by the synergy between nickel and iron.
2.5.2. TG-GCMS
TG-GCMS test was run during the pyrolysis to obtain a further understanding of composition and distribution of products. Figure 7 illustrated the chromatogram of pyrolytic products released from the catalytic pyrolysis process of waste tire. Many peaks were detected and identified by NIST MS library. The volatile products of WT catalytic pyrolysis were more diverse than those of WT non-catalytic pyrolysis. Moreover, all pyrolytic products could be mainly classified into two types: alkenes and aromatics.
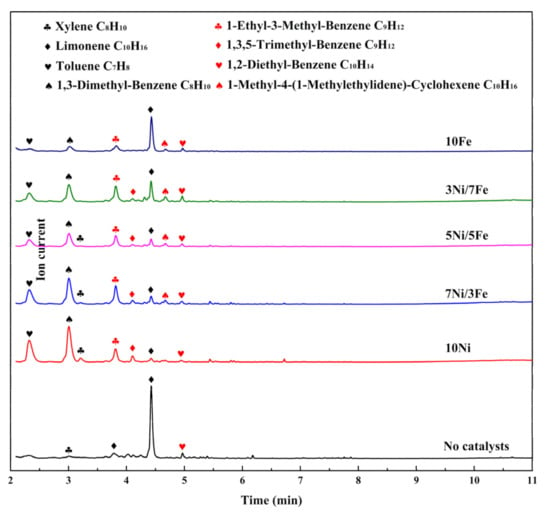
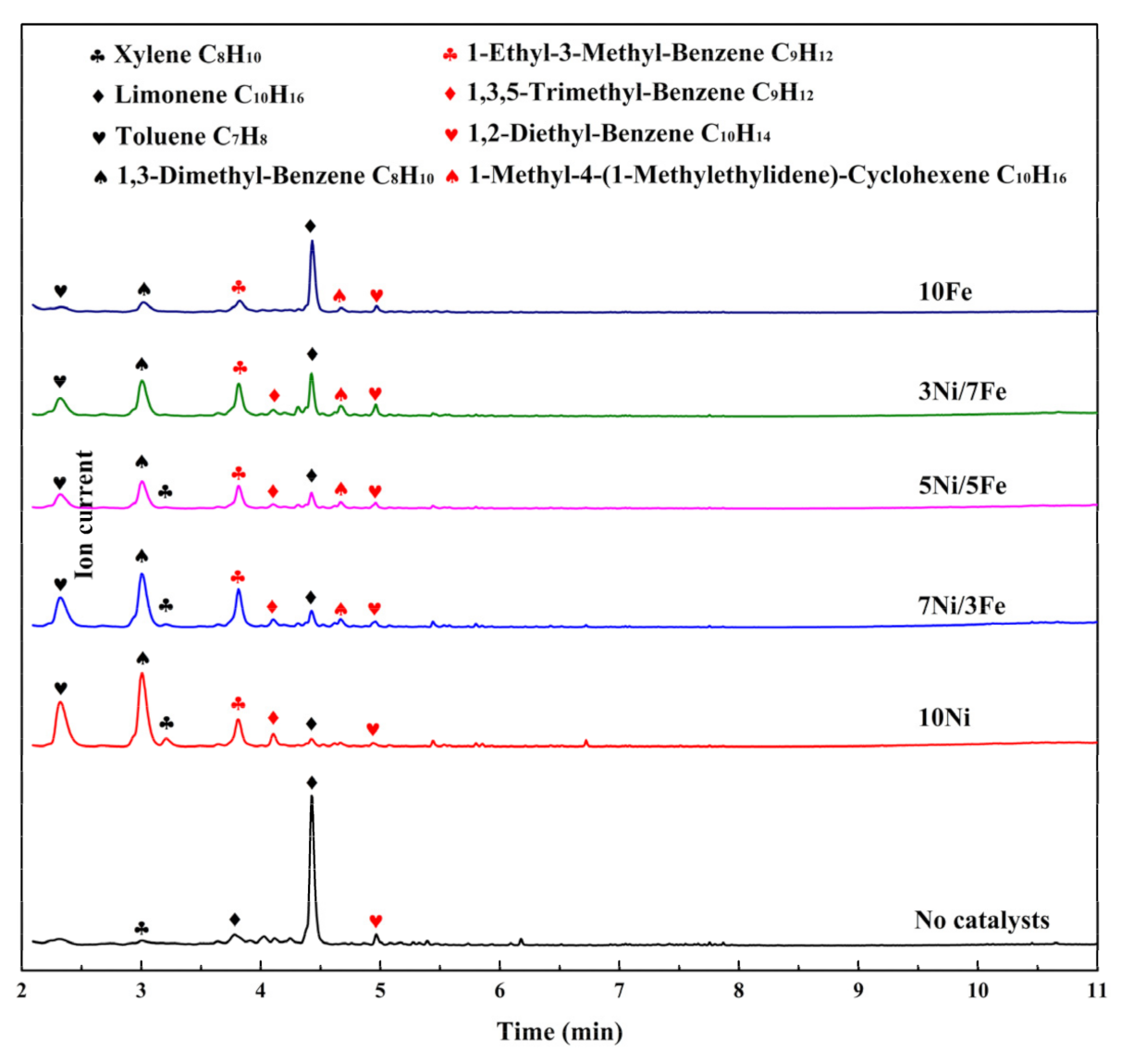
Figure 7.
Chromatogram of pyrolytic products released from the catalytic pyrolysis process of waste tire.
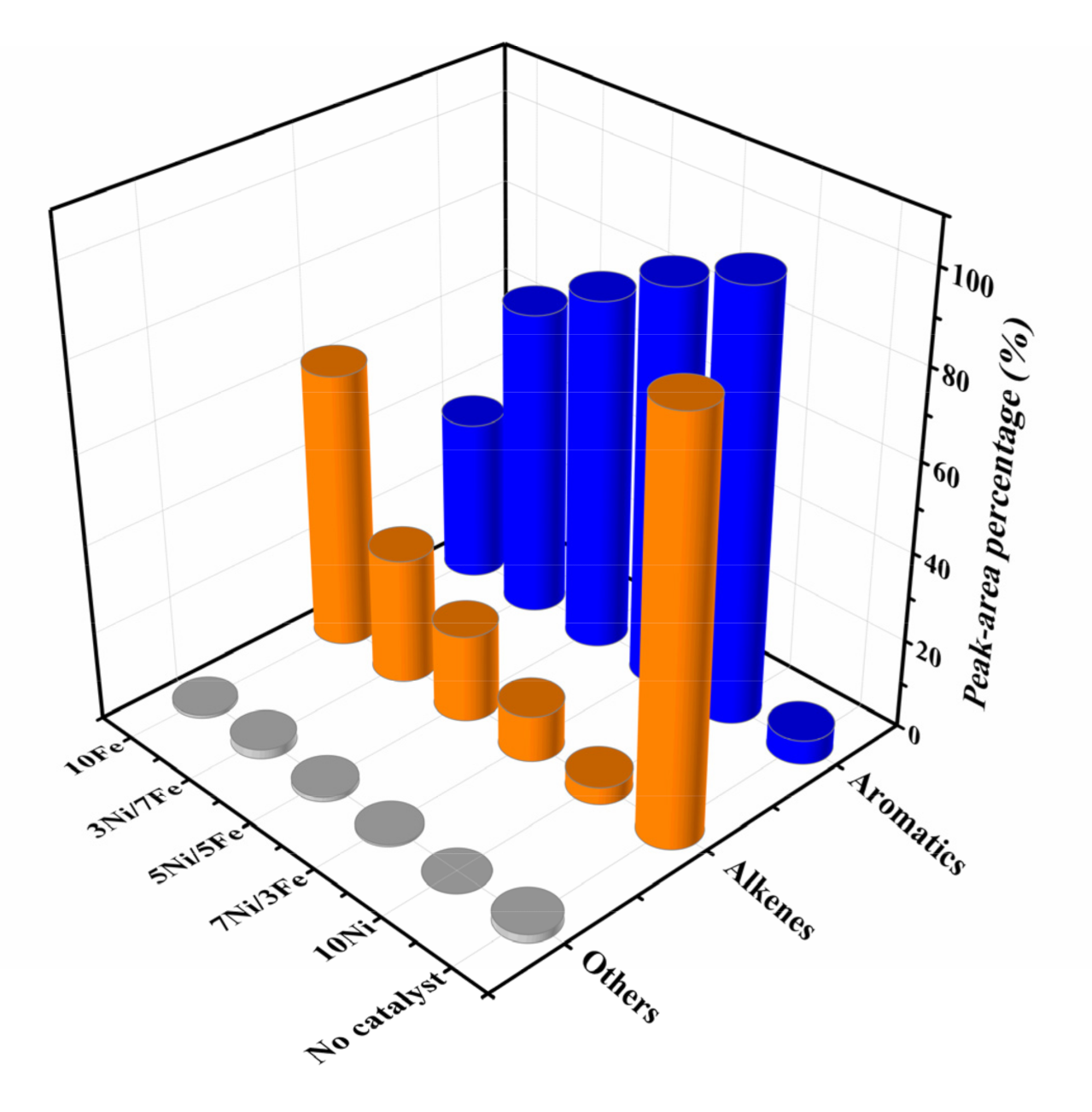
Figure 8 exhibited the product distribution of waste tire catalytic pyrolysis. Alkenes (92.56%) were dominant compositions in non-catalytic pyrolysis products while only 5.54% aromatic hydrocarbons were detected. Alkenes were mainly composed of d-limonene, which was formed from isoprene by the Diels-Alder reaction [46,47]. Compared to the uncatalyzed pyrolysis, the addition of Ni/Fe-ZSM-5 significantly changed the distribution of pyrolytic products. The catalysts greatly reduced the content of alkenes and significantly increased that of aromatic hydrocarbons, which indicated that Ni/Fe-ZSM-5 catalysts could decomposed d-limonene to form aromatic hydrocarbons [4]. Moreover, among all Ni/Fe modified ZSM-5 catalysts, 10Ni was more effective at producing aromatic hydrocarbons, with a concentration up to 96.06%. The best catalytic performance might be caused by higher surface area, larger pore volume and higher Ni loading, which enhanced the ability of C–C and C–H cleavage and hydrogen transfer [4]. With respect to the remaining catalysts, the concentrations of aromatic hydrocarbons were obtained in the following order: 7Ni/3Fe (88.98%) > 5Ni/5Fe (79.10%) > 3Ni/7Fe (69.25%) > 10Fe (36.74%). The increase of Ni loading led to the improvement of catalytic performance, which implied that the ability of Ni to reduce the activation energy of C–C and C–H cleavage was stronger than Fe. 7Ni/3Fe also showed excellent catalytic performance, which was close to 10Ni. It might be caused by the synergetic enhancement in catalysis of cracking after the alloying of Fe with Ni.
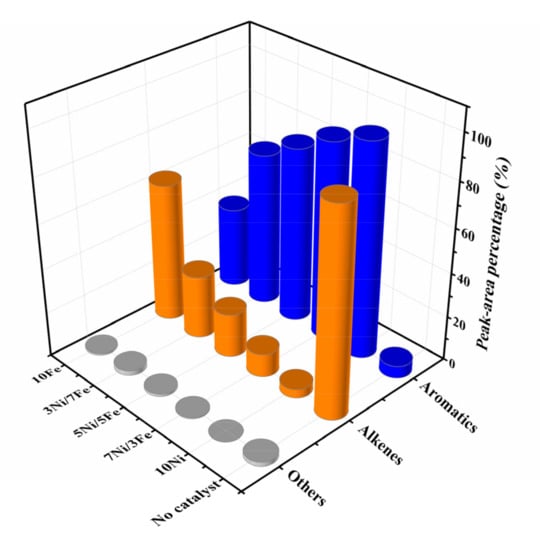
Figure 8.
Product distribution of waste tire catalytic pyrolysis.
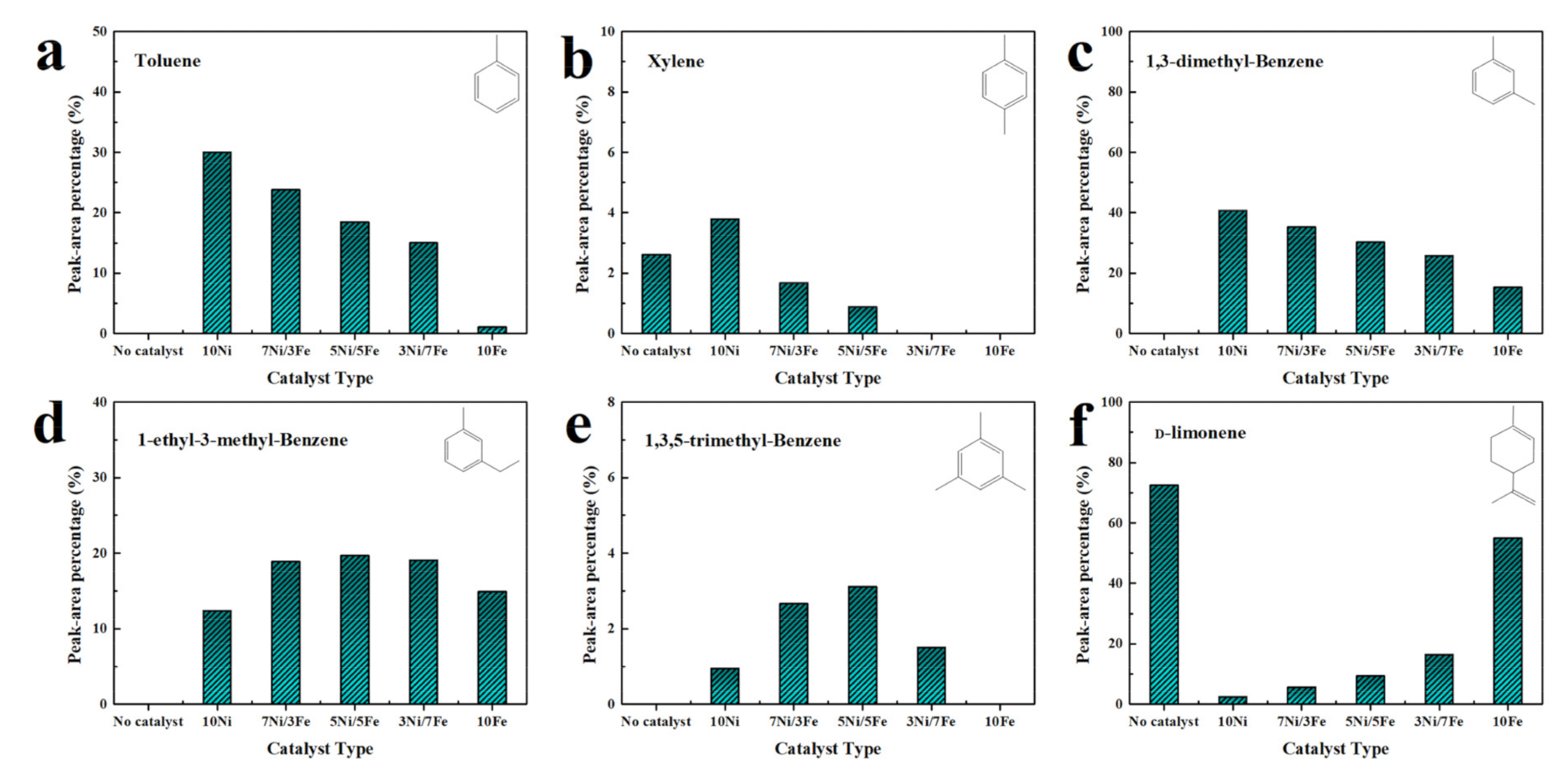
Figure 9 illustrated the main chemical compounds obtained from catalytic pyrolysis of waste tire over different catalysts. As illustrated, with the addition of catalysts, the yields of toluene, xylene and 1,3-dimethyl-benzene were all improved. 10Ni favored the formation of toluene, xylene, and 1,3-dimethyl-benzene with the highest relative selectivity of 30.00%, 3.79% and 40.72%, respectively. Compared to 10Ni, 10Fe presented a weaker catalytic performance. It was worth noting that the bimetallic catalysts had much stronger catalytic effect on the yield of toluene, xylene, and 1,3-dimethyl-benzene than 10Fe. Besides, 5Ni/5Fe catalyzed the formation of 1-ethyl-3-methyl-benzene and 1,3,5-trimethyl-benzene with the highest relative selectivity of 19.69% and 3.14%, which was 1.59 and 3.31 times higher than those in the case of 10Ni. A previous study [48] proved that metal favored the dealkylation of alkylbenzenes with multiple branched chains to generate xylenes, toluene, and benzene. Due to this reason, 10Ni showed the best performance in the dealkylation of alkylbenzenes. The massive generation of 1-ethyl-3-methyl-benzene and 1,3,5-trimethyl-benzene by 5Ni/5Fe might be due to the reason that the activity of Ni sites was passivated by Fe, inhibiting the dealkylation of them.
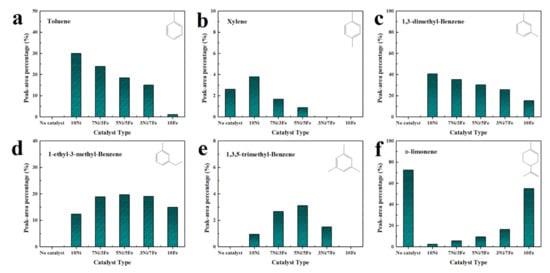
Figure 9.
Main chemical compounds obtained from catalytic pyrolysis of waste tire over different catalysts (a) Toluene; (b) Xylene; (c) 1,3-dimethyl-benzene; (d) 1-ethyl-3-methyl-benzene; (e) 1,3,5-trimethyl-benzene; (f) d-limonene.
3. Materials and Methods
3.1. Materials and Catalysts
Waste tires (WT) supplied from a garage in Dalian, Liaoning Province, China were used as experiment feedstocks in the study. Before the pyrolysis experiment, the samples were crushed and sieved into powder with a particle size of 0.15–0.20 mm. The ultimate and proximate analyses of WT were carried out by a Vario EL elemental analyzer (Element, Germany) and a SDTGA5000A Industrial Analyzer (Sundy Co., Changsha, Hunan, China). The results were shown in Table 7. ZSM-5 (SiO2/Al2O3 = 25) was purchased from Catalyst Plant of Nankai University (Tianjin, China). Conventional wet impregnation method was used to load metal catalysts onto ZSM-5. Before modification, ZSM-5 was activated in a muffle furnace at 550 °C for 3 h with a heating rate of 5 °C/min. Active metal precursors were prepared by mixing Ni (NO3)2⋅6H2O (Tianjin Kermel co., Tianjin, China) and Fe(NO3)3⋅9H2O (Tianjin Kermel co., Tianjin, China) into 10 mL of deionized water. Then, the ZSM-5 powder was added to the solution and the mixture was continuously stirred for 12 h to ensure that the adsorption of metal precursor in the ZSM-5 was in equilibrium. The moisture was removed in an oven at 105 °C. The solids were then calcined at 600 °C for 4 h with a heating rate of 5 °C/min. Finally, the catalyst activation was implemented in H2/N2 (5 vol.% H2) mixture gas at 700 °C for 1 h. The obtained catalysts were denoted as xNi/yFe, where x and y (wt.%) represent the metal Ni and Fe loading.

Table 7.
Proximate and ultimate analysis of waste tire.
N2 physisorption, X-ray diffraction (XRD), scan electron microscope (SEM), and thermo-gravimetric analyzer (TGA) were carried out to analyze textual property, crystal phases, microscopic morphology and thermal degradation characteristics of the catalysts.
3.2. TG-IR-GC/MS Experiments
The waste tire catalytic pyrolysis experiments were carried out by a Thermo-gravimetric analyzer (STA449F5, Netzsch, Germany) coupled with Fourier Transform Infrared spectrometer (IS 20, Nicolet, Waltham, MA, USA) and Gas Chromatography/Mass Spectrometry (8890-5977B, Agilent, Santa Clara, CA, USA). The catalysts and feedstocks were mixed with 1:1 mass ratio in advance and about 15 mg sample was heated from room temperature to 900 °C in a nitrogen atmosphere with a flow rate of 200 mL/min. For kinetic analysis, multiple catalytic pyrolysis experiments were carried out under three heating rates of 10, 20, and 30 °C/min. All experiments were reduplicated three times to ensure the validity of the results. For the product analysis, during the catalytic pyrolysis process, the released volatiles were input to FTIR and GC/MS by a heated connection (200 °C). The scanning range of FTIR applied in this study was from 4000 to 400 cm−1 with a resolution of 4 cm−1. The GC/MS was run to determine the composition of pyrolytic volatiles at 373 °C. The temperature program of GC column oven was as follows: (i) 50 °C for 2 min; (ii) from 50 °C to 325 °C at the heating rate of 5 °C/min; (iii) 325 °C for 15 min.
3.3. Kinetic Analysis Method
The catalytic pyrolysis of WT could be described as follows:
thus, the thermogravimetric data could express the reaction process of WT thermal degradation as follows:
where mo, mt, and m∞ represent the initial sample mass, the mass at time t, and the final mass, respectively.
Waste tire → Organic volatiles + Chars,
The conversion rate for solid-state reaction could be described as [21]:
where f(α) and k(T) are reaction model (a function of conversion listed in Table 8 [49]) and reaction rate constant. Thereinto, the process, which was based on the A2~A4, was regarded as nucleation and nuclei growth. At the beginning of nucleation, the driving force of nucleation is proportional to the volume of the nucleus. However, due to the reason that the nuclei interfere and overlap with each other, the reaction rate decreased. The mechanism of model P2/3~P4 is similar to that of An. The difference was the negligence of interference between growing nuclei, which led to a monotonically increase of reaction rate. Model D1 and D2 were based the assumption that the formed products covered the surface of the reactants and hindered the mass transfer so that the reaction rate was limited by the mass transfer rate. As for model R2 and R3, it assumed that the reaction happened at the interface between reactants and products, which led to a contraction of the reactant core at a constant speed. Model Fn assume that the reaction rate is proportional to the concentration of the remaining reactants or a particular power of the concentration. Generally, Arrhenius equation can be employed to represent the reaction rate constant.
where T is the absolute temperature, A is the pre-exponential factor, E is the activation energy, and R is the gas constant.

Table 8.
Common solid reaction models.
Thus, Equation (4) could be transformed as:
For the non-isothermal process, the sample is heated at a constant heating rate, which could be expressed as:
Substituting Equation (7) into Equation (6) leads to Equation (8).
After the separation of variables (α and T), the integral of reaction model g(α) could be obtained:
Employing different approximations of the integration term on the right-hand side of Equation (9) could obtain different iso-conversional methods to determine the activation energy.
By introducing Murray and White’s integral approximation [50], the equation of KAS method was achieved:
The equation of Starink’s method, which used Starink’s approximation [32], is expressed as:
At a series of specified conversions, the values of activation energy could be determined from the slope of ln(β/T2) versus (−1/RT) for KAS and ln(β/T1.92) versus (1.0008/RT) for Starink’s method.
The integral master plot method was employed to identify the reaction model of WT catalytic thermal degradation. In this method, the integral form of reaction model can be expressed as:
where P(u) is the temperature integral and u = E/RT, because the temperature integral P(u) does not have any analytical solution. In this study, an empirical equation named Tang–Liu–Zhang–Wang–Wang approximation [51] was adopted to approximate the solution.
The method assumed that entire process of WT catalytic pyrolysis is a single-step reaction. Thus, the values of A and E can be treated as constants. Using α = 0.5 as an anchor point [22], Equation (11) can be expressed as:
where g(0.5) is the value of the integral of reaction model at α = 0.5. u0.5 = E/RT0.5 and T0.5 is the temperature at α = 0.5.
The ratio of Equations (11) and (13) yields the equation of the integral master plot method:
The plot of g(α)/g(0.5) versus α (g(α) from Table 2) can be attained as the theoretical master plots. The plot of g(α)/g(0.5) versus α can be calculated from thermogravimetric data as the experimental master plots. The best fit reaction model of WT catalytic pyrolysis could be identified by comparing the experimental plot to the theoretical plots.
4. Conclusions
In this study, pyrolysis behaviors, kinetics, and product analysis of waste tire catalytic pyrolysis with five Ni/Fe bimetallic ZSM-5 catalysts were investigated by TG-IR-GC/MS. Iso-conversional methods (KAS and Starink’s methods) and the master-plot method were employed to determine the activation energies and reaction model. The comparison of activation energies between non-catalyzed and catalyzed pyrolysis revealed that 7Ni/3Fe had the lowest average activation energy (186.19 kJ/mol), which indicated that Ni-rich Ni-Fe alloy catalysts had the best catalytic effect on the reduction of reaction barrier of waste tire pyrolysis. The results of the master-plot methods suggested that both non-catalyzed and catalyzed pyrolysis reactions were fitted to model Fn, suggesting that the reaction rate of waste tire thermal decomposition was dominated by the concentration of reactants, but the addition of the catalyst reduced the reaction order to varying degrees. FTIR analysis found that the absorption intensity of CH4, C2H4, –C=C– and –C–H in aromatic hydrocarbons increased with the addition of catalysts. Thereinto, the catalytic effect of 10Ni was the best among all modified ZSM-5 catalysts, demonstrating the strongest dehydrogenation ability to form aromatic hydrocarbons. Note that 7Ni/3Fe showed almost the same catalytic performance as 10Ni, which might be caused by the synergy between nickel and iron. GC/MS analysis illustrated that all modified catalysts could significantly reduce the concentration of alkenes (especially d-limonene) and enrich aromatics such as toluene, xylene, and 1,3-dimethyl-benzene, which could be widely employed in production of pesticides, dyestuffs, and surfactants and applied in the plastic industry to produce plasticizers, indicating that Ni and Fe favored the C–C and C–H cleavage. Besides, due to the highest selectivity of 1-ethyl-3-methyl-benzene and 1,3,5-trimethyl-benzene, 5Ni/5Fe had better catalytic effect on the formation of alkylbenzenes with multiple branched chains than 10Ni, which might be caused by the reason that Ni-Fe alloy inhibited the dealkylation reaction.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11091031/s1, Figure S1: N2 adsorption-desorption isotherms of parent and modified ZSM-5, Figure S2: The SEM images of parent and modified catalysts (a) ZSM-5; (b) 10Ni; (c) 7Ni/3Fe; (d) 5Ni/5Fe; (e) 3Ni/7Fe; (f) 10Fe, Figure S3: The TG curves of parent and modified catalysts heated from room temperature to 900 °C at the heating rate of 10 °C/min (a) 10Ni; (b) 7Ni/3Fe; (c) 5Ni/5Fe; (d) 3Ni/7Fe; (e) 10Fe, Figure S4: The TG and DTG curves of modified catalysts at the heating rate of 10 °C/min (a) 10Ni; (b) 7Ni/3Fe; (c) 5Ni/5Fe; (d) 3Ni/7Fe; (e) 10Fe, Figure S5: The TG and DTG curves of WT pyrolysis with no catalyst and five synthesized catalysts at three different heating rates of 10, 20 and 30 °C/min (a) No catalyst; (b) 10Ni; (c) 7Ni/3Fe; (d) 5Ni/5Fe; (e) 3Ni/7Fe; (f) 10Fe, Figure S6: P(u)/P(u0.5) versus α for WT pyrolysis with no catalyst and five synthesized catalysts at three different heating rates of 10, 20 and 30 °C/min (a) No catalyst; (b) 10Ni; (c) 7Ni/3Fe; (d) 5Ni/5Fe; (e) 3Ni/7Fe; (f) 10Fe, Figure S7: Plots of [(1 − α) (1 − n) − 1]/(n − 1) versus EP(u)/βR for WT pyrolysis with no catalyst and five synthesized catalysts (a) No catalyst; (b) 10Ni; (c) 7Ni/3Fe; (d) 5Ni/5Fe; (e) 3Ni/7Fe; (f) 10Fe, Table S1: Correlations of all samples with Starink’s method, Table S2: Correlations of all samples with KAS method, Table S3: Activation energies of all samples with KAS method.
Author Contributions
Conceptualization, B.Q. and G.J.; methodology, B.Q. and Y.Z.; formal analysis, B.Q. and T.W.; investigation, B.Q.; resources, B.Q. and Z.W.; writing—original draft preparation, B.Q.; writing—review and editing, G.J. and Z.W.; supervision, A.L.; project administration, G.J. and A.L.; funding acquisition, G.J. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Fundamental Research Funds for the Central Universities, grant number: DUT20LAB304.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pakdel, H.; Roy, C.; Aubin, H.; Jean, G.; Coulombe, S. Formation of dl-limonene in used tire vacuum pyrolysis oils. Environ. Sci. Technol. 1991, 25, 1646–1649. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.T.; Brindle, A.J. Aromatic chemicals from the catalytic pyrolysis of scrap tyres. J. Anal. Appl. Pyrolysis 2003, 67, 143–164. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Wang, X.; Liu, P.; Li, J.; Liu, G.; Wang, K.; Li, M.; Zhong, Z.; Xu, J.; et al. Catalytic conversion of rubber wastes to produce aromatic hydrocarbons over USY zeolites: Effect of SiO2/Al2O3 mole ratio. Energy Convers. Manag. 2019, 197, 111857. [Google Scholar] [CrossRef]
- Zhao, M.; Church, T.L.; Harris, A.T. SBA-15 supported Ni-Co bimetallic catalysts for enhanced hydrogen production during cellulose decomposition. Appl. Catal. B Environ. 2011, 101, 522–530. [Google Scholar] [CrossRef]
- Yu, L.; Farinmade, A.; Ajumobi, O.; Su, Y.; John, V.T.; Valla, J.A. MCM-41/ZSM-5 composite particles for the catalytic fast pyrolysis of biomass. Appl. Catal. A Gen. 2020, 602, 117727. [Google Scholar] [CrossRef]
- Ding, K.; Zhong, Z.; Zhang, B.; Wang, J.; Min, A.; Ruan, R. Catalytic pyrolysis of waste tire to produce valuable aromatichydrocarbons: An analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2016, 122, 55–63. [Google Scholar] [CrossRef]
- Ji, G.; Xu, X.; Yang, H.; Zhao, X.; He, X.; Zhao, M. Enhanced Hydrogen Production from Sawdust Decomposition Using Hybrid-Functional Ni-CaO-Ca2SiO4 Materials. Env. Sci. Technol. 2017, 51, 11484–11492. [Google Scholar] [CrossRef] [PubMed]
- Kantarelis, E.; Yang, W.; Blasiak, W. Effect of zeolite to binder ratio on product yields and composition during catalytic steam pyrolysis of biomass over transition metal modified HZSM5. Fuel 2014, 122, 119–125. [Google Scholar] [CrossRef]
- Hong, Y.; Hensley, A.; McEwen, J.-S.; Wang, Y. Perspective on Catalytic Hydrodeoxygenation of Biomass Pyrolysis Oils: Essential Roles of Fe-Based Catalysts. Catal. Lett. 2016, 146, 1621–1633. [Google Scholar] [CrossRef]
- Widayatno, W.B.; Guan, G.; Rizkiana, J.; Yang, J.; Hao, X.; Tsutsumi, A.; Abudula, A. Upgrading of bio-oil from biomass pyrolysis over Cu-modified β-zeolite catalyst with high selectivity and stability. Appl. Catal. B Environ. 2016, 186, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Yu, J.; Chen, T.; Liu, X.; Sun, L. Study on catalytic pyrolysis mechanism of natural rubber (NR) over Zn-modified ZSM5 catalysts. J. Energy Inst. 2021, 94, 210–221. [Google Scholar] [CrossRef]
- Botas, J.A.; Serrano, D.P.; García, A.; de Vicente, J.; Ramos, R. Catalytic conversion of rapeseed oil into raw chemicals and fuels over Ni- and Mo-modified nanocrystalline ZSM-5 zeolite. Catal. Today 2012, 195, 59–70. [Google Scholar] [CrossRef]
- Kantarelis, E.; Yang, W.; Blasiak, W. Effects of Silica-Supported Nickel and Vanadium on Liquid Products of Catalytic Steam Pyrolysis of Biomass. Energy Fuels 2014, 28, 591–599. [Google Scholar] [CrossRef]
- Namchot, W.; Jitkarnka, S. Upgrading of waste tyre-derived oil from waste tyre pyrolysis over Ni catalyst supported on HZSM-5 zeolite. Chem. Eng. Trans. 2015, 45, 775–780. [Google Scholar] [CrossRef]
- Muenpol, S.; Jitkarnka, S. Effects of Fe supported on zeolites on structures of hydrocarbon compounds and petrochemicals in waste tire-derived pyrolysis oils. J. Anal. Appl. Pyrolysis 2016, 117, 147–156. [Google Scholar] [CrossRef]
- Wu, C.; Nahil, M.A.; Miskolczi, N.; Huang, J.; Williams, P.T. Production and application of carbon nanotubes, as a co-product of hydrogen from the pyrolysis-catalytic reforming of waste plastic. Process. Saf. Environ. Prot. 2016, 103, 107–114. [Google Scholar] [CrossRef]
- Popovska, N.; Danova, K.; Jipa, I.; Zenneck, U. Catalytic growth of carbon nanotubes on zeolite supported iron, ruthenium and iron/ruthenium nanoparticles by chemical vapor deposition in a fluidized bed reactor. Powder Technol. 2011, 207, 17–25. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, P.; Shao, Q.; Takahashi, F.; Yoshikawa, K. In situ catalytic conversion of tar using rice husk char/ash supported nickel–iron catalysts for biomass pyrolytic gasification combined with the mixing-simulation in fluidized-bed gasifier. Appl. Energy 2015, 160, 808–819. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Z.; Zhu, Y.-A.; Liu, Z.; Sui, Z.; Zhu, K.; Zhou, X. Dry reforming of methane on Ni-Fe-MgO catalysts: Influence of Fe on carbon-resistant property and kinetics. Appl. Catal. B Environ. 2020, 264, 118497. [Google Scholar] [CrossRef]
- Vyazovkina, S.; Burnhamb, A.K.; Criadoc, J.M.; Pérez-Maquedac, L.A.; Popescud, C.; Sbirrazzuolie, N. ICTAC Kinetics Committee recommendations for performing kineticcomputations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Pérez-Maqueda, L.A.; Ortega, A.; Criado, J.M. The use of master plots for discriminating the kinetic model of solid state reactions from a single constant-rate thermal analysis (CRTA) experiment. Thermochim. Acta 1996, 277, 165–173. [Google Scholar] [CrossRef]
- Chai, M.; Liu, R.; He, Y. Effects of SiO2/Al2O3 ratio and Fe loading rate of Fe-modified ZSM-5 on selection of aromatics and kinetics of corn stalk catalytic pyrolysis. Fuel Process. Technol. 2020, 206, 106458. [Google Scholar] [CrossRef]
- Al-Dughaither, A.S.; de Lasa, H. HZSM-5 Zeolites with Different SiO2/Al2O3 Ratios. Characterization and NH3 Desorption Kinetics. Ind. Eng. Chem. Res. 2014, 53, 15303–15316. [Google Scholar] [CrossRef]
- Ma, C.; Kamo, T. Enhanced debromination by Fe particles during the catalytic pyrolysis of non-metallic fractions of printed circuit boards over ZSM-5 and Ni/SiO2-Al2O3 catalyst. J. Anal. Appl. Pyrolysis 2019, 138, 170–177. [Google Scholar] [CrossRef]
- Chen, H.S.; Polk, D.E. Mechanical properties of Ni-Fe based alloy glasses. J. Non-Cryst. Solids 1974, 15, 174–178. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Chen, L.; Zhao, B.; Yang, S.; Xie, X. Comparision of catalytic fast pyrolysis of biomass to aromatic hydrocarbons over ZSM-5 and Fe/ZSM-5 catalysts. J. Anal. Appl. Pyrolysis 2016, 121, 342–346. [Google Scholar] [CrossRef]
- Leung, C.L.W. Kinetic Modeling of Scrap Tire Pyrolysis. Energy Fuels 1999, 13, 421–427. [Google Scholar] [CrossRef]
- Seidelt, S.; Müller-Hagedorn, M.; Bockhorn, H. Description of tire pyrolysis by thermal degradation behaviour of main components. J. Anal. Appl. Pyrolysis 2006, 75, 11–18. [Google Scholar] [CrossRef]
- Irmak Aslan, D.; Parthasarathy, P.; Goldfarb, J.L.; Ceylan, S. Pyrolysis reaction models of waste tires: Application of master-plots method for energy conversion via devolatilization. Waste Manag. 2017, 68, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhan, J.-H.; Lai, D.; Li, M.; Liu, X.; Xu, G. Primary understanding of non-isothermal pyrolysis behavior for oil shale kerogen using reactive molecular dynamics simulation. Int. J. Hydrogen Energy 2016, 41, 12093–12100. [Google Scholar] [CrossRef]
- Starink, M.J. The Determination of Activation Energy from Linear Heating Rate Experiments: A Comparison of the Accuracy of Isoconversion Methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Qu, B.; Li, A.; Qu, Y.; Wang, T.; Zhang, Y.; Wang, X.; Gao, Y.; Fu, W.; Ji, G. Kinetic analysis of waste tire pyrolysis with metal oxide and zeolitic catalysts. J. Anal. Appl. Pyrolysis 2020, 152, 104949. [Google Scholar] [CrossRef]
- Osorio-Vargas, P.; Lick, I.D.; Sobrevía, F.; Correa-Muriel, D.; Menares, T.; Manrique, R.; Casella, M.L.; Arteaga-Pérez, L.E. Thermal Behavior, Reaction Pathways and Kinetic Implications of Using a Ni/SiO2 Catalyst for Waste Tire Pyrolysis. Waste Biomass Valorization 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Alsurakji, I.H.; El-Qanni, A.; El-Hamouz, A.M.; Warad, I.; Odeh, Y. Thermogravimetric Kinetics Study of Scrap Tires Pyrolysis Using Silica Embedded With NiO and/or MgO Nanocatalysts. J. Energy Resour. Technol. 2021, 143, 092302. [Google Scholar] [CrossRef]
- Kordoghli, S.; Paraschiv, M.; Kuncser, R.; Tazerout, M.; Zagrouba, F. Catalysts’ influence on thermochemical decomposition of waste tires. Environ. Prog. Sustain. Energy 2017, 36, 1560–1567. [Google Scholar] [CrossRef]
- Yang, H.; Ji, G.; Clough, P.T.; Xu, X.; Zhao, M. Kinetics of catalytic biomass pyrolysis using Ni-based functional materials. Fuel Process. Technol. 2019, 195, 106145. [Google Scholar] [CrossRef]
- Chen, J.; Mu, L.; Jiang, B.; Yin, H.; Song, X.; Li, A. TG/DSC-FTIR and Py-GC investigation on pyrolysis characteristics of petrochemical wastewater sludge. Bioresour. Technol. 2015, 192, 1–10. [Google Scholar] [CrossRef]
- Fong, M.J.B.; Loy, A.C.M.; Chin, B.L.F.; Lam, M.K.; Yusup, S.; Jawad, Z.A. Catalytic pyrolysis of Chlorella vulgaris: Kinetic and thermodynamic analysis. Bioresour. Technol. 2019, 289, 121689. [Google Scholar] [CrossRef]
- Chong, C.T.; Mong, G.R.; Ng, J.-H.; Chong, W.W.F.; Ani, F.N.; Lam, S.S.; Ong, H.C. Pyrolysis characteristics and kinetic studies of horse manure using thermogravimetric analysis. Energy Convers. Manag. 2019, 180, 1260–1267. [Google Scholar] [CrossRef]
- Tian, B.; Qiao, Y.; Bai, L.; Feng, W.; Jiang, Y.; Tian, Y. Pyrolysis behavior and kinetics of the trapped small molecular phase in a lignite. Energy Convers. Manag. 2017, 140, 109–120. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Ming, X.; Jiang, Y.; Hao, J.; Qiao, Y.; Tian, Y. TG-FTIR and Py-GC/MS study on pyrolysis mechanism and products distribution of waste bicycle tire. Energy Convers. Manag. 2018, 175, 288–297. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.; Shen, J.; Zhang, H. Pyrolysis of superfine pulverized coal. Part 1. Mechanisms of methane formation. Energy Convers. Manag. 2014, 87, 1027–1038. [Google Scholar] [CrossRef]
- Hao, J.; Feng, W.; Qiao, Y.; Tian, Y.; Zhang, J.; Che, Y. Thermal cracking behaviors and products distribution of oil sand bitumen by TG-FTIR and Py-GC/TOF-MS. Energy Convers. Manag. 2017, 151, 227–239. [Google Scholar] [CrossRef]
- Zeng, M.; Yuan, W.; Wang, Y.; Zhou, W.; Zhang, L.; Qi, F.; Li, Y. Experimental and kinetic modeling study of pyrolysis and oxidation of n-decane. Combust. Flame 2014, 161, 1701–1715. [Google Scholar] [CrossRef]
- Danon, B.; van der Gryp, P.; Schwarz, C.E.; Görgens, J.F. A review of dipentene (dl-limonene) production from waste tire pyrolysis. J. Anal. Appl. Pyrolysis 2015, 112, 1–13. [Google Scholar] [CrossRef]
- Pakdel, H.; Pantea, D.M.; Roy, C. Production of dl-limonene by vacuum pyrolysis of used tires. J. Anal. Appl. Pyrolysis 2001, 57, 91–107. [Google Scholar] [CrossRef]
- Busca, G. Chapter 9—Metal Catalysts for Hydrogenations and Dehydrogenations. In Heterogeneous Catalytic Materials; Busca, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 297–343. [Google Scholar]
- Zhao, M.; Raheem, A.; Memon, Z.M.; Vuppaladadiyam, A.K.; Ji, G. Iso-conversional kinetics of low-lipid micro-algae gasification by air. J. Clean. Prod. 2019, 207, 618–629. [Google Scholar] [CrossRef]
- Murray, P.; White, J. Kinetic of the thermal dehydration of clays. Part IV. Interpretation of the differential thermal analysis of the clay minerals. Trans. Brot Ceram. Soc. 1955, 54, 204–238. [Google Scholar]
- Qu, Y.; Li, A.; Wang, D.; Zhang, L.; Ji, G. Kinetic study of the effect of in-situ mineral solids on pyrolysis process of oil sludge. Chem. Eng. J. 2019, 374, 338–346. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).