SuFEx-Click Approach for the Synthesis of Soluble Polymer-Bound MacMillan Catalysts for the Asymmetric Diels–Alder Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design of Monomeric and Polymeric MacMillan Catalysts
2.2. Synthesis of Class I Polymeric MacMillan Catalysts
2.3. Synthesis of Class II Polymeric MacMillan Catalysts
2.4. Synthesis of Class III Polymeric MacMillan Catalysts
2.5. Optimization of Reaction Conditions for the Asymmetric Diels–Alder Reaction
2.6. Asymmetric Diels–Alder Reaction with Class I–III Organocatalysts
2.7. Substrate Scope of Asymmetric Diels–Alder Reaction with Chiral Polysulfate Organocatalyst 7a
2.8. Comparisons of Soluble Polymeric MacMillan Catalysts for the Asymmetric Diels–Alder Reaction
2.9. Recycling Test of Polysulfate Organocatalysts in Asymmetric Diels–Alder Reaction
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of Class I Polymeric MacMillan Catalysts
3.3. Synthesis of Class II Polymeric MacMillan Catalysts
3.4. Synthesis of Class III Polymeric MacMillan Catalysts
3.5. General Procedure for the Asymmetric Diels–Alder Reaction and Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cozzi, F. Immobilization of Organic Catalysts: When, Why, and How. Adv. Synth. Catal. 2006, 348, 1367–1390. [Google Scholar] [CrossRef]
- Benaglia, M. Recoverable and recyclable chiral organic catalysts. New J. Chem. 2006, 30, 1525–1533. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F.; Noto, R. Supported proline and proline-derivatives as recyclable organocatalysts. Chem. Soc. Rev. 2008, 37, 1666–1688. [Google Scholar] [CrossRef]
- Kristensen, T.E.; Hansen, T. Polymer-Supported Chiral Organocatalysts: Synthetic Strategies for the Road Towards Affordable Polymeric Immobilization. Eur. J. Org. Chem. 2010, 3179–3204. [Google Scholar] [CrossRef]
- Itsuno, S.; Parveza, M.M.; Haraguchi, N. Polymeric chiral organocatalysts. Polym. Chem. 2011, 2, 1942–1949. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, S.; Cheng, J.-P. Non-covalent immobilization of asymmetric organocatalysts. Catal. Sci. Technol. 2011, 1, 507–516. [Google Scholar] [CrossRef]
- Itsuno, S.; Hassan, M.M. Polymer-immobilized chiral catalysts. RSC Adv. 2014, 4, 52023–52043. [Google Scholar] [CrossRef]
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels−Alder Reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- Jen, W.S.; Wiener, J.J.M.; MacMillan, D.W.C. New Strategies for Organic Catalysis: The First Enantioselective Organocatalytic 1,3-Dipolar Cycloaddition. J. Am. Chem. Soc. 2000, 122, 9874–9875. [Google Scholar] [CrossRef] [Green Version]
- Pares, N.A.; MacMillan, D.W.C. New Strategies in Organic Catalysis: The First Enantioselective Organocatalytic Friedel−Crafts Alkylation. J. Am. Chem. Soc. 2001, 123, 4370–4371. [Google Scholar] [CrossRef] [Green Version]
- Austin, J.F.; MacMillan, D.W.C. Enantioselective Organocatalytic Indole Alkylations. Design of a New and Highly Effective Chiral Amine for Iminium Catalysis. J. Am. Chem. Soc. 2002, 124, 1172–1173. [Google Scholar] [CrossRef] [Green Version]
- Brochu, M.P.; Brown, S.P.; MacMillan, D.W.C. Direct and Enantioselective Organocatalytic α-Chlorination of Aldehydes. J. Am. Chem. Soc. 2004, 126, 4108–4109. [Google Scholar] [CrossRef] [Green Version]
- Beeson, T.D.; MacMillan, D.W.C. Enantioselective Organocatalytic α-Fluorination of Aldehydes. J. Am. Chem. Soc. 2005, 127, 8826–8828. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, M.T.H.; List, B. Catalytic Asymmetric Intramolecular Michael Reaction of Aldehydes. Angew. Chem. Int. Ed. 2004, 43, 3958–3960. [Google Scholar] [CrossRef]
- Lee, S.; MacMillan, D.W.C. Enantioselective organocatalytic epoxidation using hypervalent iodine reagents. Tetrahedron 2006, 62, 11413–11424. [Google Scholar] [CrossRef]
- Bartók, M. Advances in Immobilized Organocatalysts for the Heterogeneous Asymmetric Direct Aldol Reactions. Catal. Rev. Sci. Eng. 2015, 57, 192–255. [Google Scholar] [CrossRef]
- Sater, M.A.E.; Jaber, N.; Schulz, E. Chiral Salen Complexes for Asymmetric Heterogeneous Catalysis: Recent Examples for Recycling and Cooperativity. ChemCatChem 2019, 11, 3662–3687. [Google Scholar] [CrossRef] [Green Version]
- Franconetti, A.; De Gonzalo, G. Recent Developments on Supported Hydrogen-bond Organocatalysts. ChemCatChem 2018, 10, 5554–5572. [Google Scholar] [CrossRef]
- De Oliveira, P.H.R.; Santos, B.M.D.S.; Leão, R.A.C.; Miranda, L.S.M.; San Gil, R.A.S.; De Souza, R.O.M.A.; Finelli, F.G. From Immobilization to Catalyst Use: A Complete Continuous-Flow Approach Towards the Use of Immobilized Organocatalysts. ChemCatChem 2019, 11, 5553–5561. [Google Scholar] [CrossRef]
- Deepa; Singh, S. Recent Development of Recoverable MacMillan Catalyst in Asymmetric Organic Transformations. Adv. Synth. Catal. 2021, 363, 629–656. [Google Scholar] [CrossRef]
- Selkälä, S.A.; Tois, J.; Pihko, P.M.; Koskinen, A.M.P. Asymmetric Organocatalytic Diels–Alder Reactions on Solid Support. Adv. Synth. Catal. 2002, 344, 941–945. [Google Scholar] [CrossRef]
- Benaglia, M.; Celentano, G.; Cinquini, M.; Puglisi, A.; Cozzi, F. Poly(ethylene glycol)-Supported Chiral Imidazolidin-4-one: An Efficient Organic Catalyst for the Enantioselective Diels–Alder Cycloaddition. Adv. Synth. Catal. 2002, 344, 149–152. [Google Scholar] [CrossRef]
- Haraguchi, N.; Takemura, Y.; Itsuno, S. Novel polymer-supported organocatalyst via ion exchange reaction: Facile immobilization of chiral imidazolidin-4-one and its application to Diels–Alder reaction. Tetrahedron Lett. 2010, 51, 1205–1208. [Google Scholar] [CrossRef]
- Haraguchi, N.; Kiyono, H.; Takemura, Y.; Itsuno, S. Design of main-chain polymers of chiral imidazolidinone for asymmetric organocatalysis application. Chem. Commun. 2012, 48, 4011–4013. [Google Scholar] [CrossRef]
- Itsuno, S.; Oonami, T.; Takenaka, N.; Haraguchi, N. Synthesis of Chiral Polyethers Containing Imidazolidinone Repeating Units and Application as Catalyst in Asymmetric Diels–Alder Reaction. Adv. Synth. Catal. 2015, 357, 3995–4002. [Google Scholar] [CrossRef]
- Haraguchi, N.; Nguyen, T.L.; Itsuno, S. Polyesters Containing Chiral Imidazolidinone Salts in Polymer Main Chain: Heterogeneous Organocatalysts for the Asymmetric Diels–Alder Reaction. ChemCatChem 2017, 9, 3786–3794. [Google Scholar] [CrossRef]
- Haraguchi, N.; Takenaka, N.; Najwa, A.; Takahara, Y.; Mun, M.K.; Itsuno, S. Synthesis of Main-Chain Ionic Polymers of Chiral Imidazolidinone Organocatalysts and Their Application to Asymmetric Diels–Alder Reactions. Adv. Synth. Catal. 2018, 360, 112–123. [Google Scholar] [CrossRef]
- Kristensen, T.E.; Vestli, K.; Jakobsen, M.G.; Hansen, F.K.; Hansen, T. A General Approach for Preparation of Polymer-Supported Chiral Organocatalysts via Acrylic Copolymerization. J. Org. Chem. 2010, 75, 1620–1629. [Google Scholar] [CrossRef]
- Guizzetti, S.; Benaglia, M.; Siegel, J.S. Poly(methylhydrosiloxane)-supported chiral imidazolinones: New versatile, highly efficient and recyclable organocatalysts for stereoselective Diels–Alder cycloaddition reactions. Chem. Commun. 2012, 48, 3188–3190. [Google Scholar] [CrossRef]
- Shen, Z.-L.; Cheong, H.-L.; Lai, Y.-C.; Loo, W.-Y.; Loh, T.-P. Application of recyclable ionic liquid-supported imidazolidinonecatalyst in enantioselective Diels–Alder reactions. Green Chem. 2012, 14, 2626–2627. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Kumar, P.; Singh, S. Synthesis of MacMillan catalyst modified with ionic liquid as a recoverable catalyst for asymmetric Diels–Alder reaction. RSC Adv. 2015, 5, 52636–52641. [Google Scholar] [CrossRef]
- Chu, Q.; Zhang, W.; Curran, D.P. A recyclable fluorous organocatalyst for Diels–Alder reactions. Tetrahedron Lett. 2006, 47, 9287–9290. [Google Scholar] [CrossRef] [Green Version]
- Riente, P.; Yadav, J.; Pericàs, M.A. A Click Strategy for the Immobilization of MacMillan Organocatalysts onto Polymers and Magnetic Nanoparticles. Org. Lett. 2012, 14, 3668–3671. [Google Scholar] [CrossRef]
- Moore, B.L.; Lu, A.; Longbottom, D.A.; O’Reilly, R.K. Immobilization of MacMillan catalyst via controlled radical polymerization: Catalytic activity and reuse. Polym. Chem. 2013, 4, 2304–2312. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.A.; Zhang, Y.; Shi, J.Y.; Wang, W. A Self-Supported Polymeric MacMillan Catalyst for Homogeneous Organocatalysis and Heterogeneous Recycling. Chem. Asian J. 2013, 8, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, A.; Benaglia, M.; Annunziata, R.; Chiroli, V.; Porta, R.; Gervasini, A. Chiral Hybrid Inorganic–Organic Materials: Synthesis, Characterization, and Application in Stereoselective Organocatalytic Cycloadditions. J. Org. Chem. 2013, 78, 11326–11334. [Google Scholar] [CrossRef]
- Pecchioli, T.; Muthyala, M.K.; Haag, R.; Christmann, M. Multivalent polyglycerol supported imidazolidin-4-one organocatalysts for enantioselective Friedel–Crafts alkylations. Beilstein J. Org. Chem. 2015, 11, 730–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-A.; Li, Y.-W.; Han, Y.-F.; Zhang, J.-P.; Wu, R.-T.; He, G.-F. The “bottom-up” construction of chiral porous organic polymers for heterogeneous asymmetric organocatalysis: MacMillan catalyst built-in nanoporous organic frameworks. Polym. Chem. 2017, 8, 5561–5569. [Google Scholar] [CrossRef]
- Watanabe, M.; Sakai, T.; Oka, M.; Makinose, Y.; Miyazaki, H.; Iida, H. Non-Covalently Immobilized Chiral Imidazolidinone on Sulfated-Chitin: Reusable Heterogeneous Organocatalysts for Asymmetric Diels–Alder Reaction. Adv. Synth. Catal. 2020, 362, 255–260. [Google Scholar] [CrossRef]
- Ullah, M.W.; Haraguchi, N. Ionic, Core-Corona Polymer Microsphere-Immobilized MacMillan Catalyst for Asymmetric Diels–Alder Reaction. Catalysts 2019, 9, 960. [Google Scholar] [CrossRef] [Green Version]
- Gravert, D.J.; Janda, K.D. Organic Synthesis on Soluble Polymer Supports: Liquid-Phase Methodologies. Chem. Rev. 1997, 97, 489–510. [Google Scholar] [CrossRef]
- Toy, P.H.; Janda, K.D. Soluble Polymer-Supported Organic Synthesis. Acc. Chem. Res. 2000, 33, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, T.J.; Reed, N.N.; Janda, K.D. Soluble Polymers as Scaffolds for Recoverable Catalysts and Reagents. Chem. Rev. 2002, 102, 3325–3344. [Google Scholar] [CrossRef] [PubMed]
- Bergbreiter, D.E. Using Soluble Polymers To Recover Catalysts and Ligands. Chem. Rev. 2002, 102, 3345–3384. [Google Scholar] [CrossRef] [PubMed]
- Bergbreiter, D.E.; Tian, J.; Hongfa, C. Using Soluble Polymer Supports To Facilitate Homogeneous Catalysis. Chem. Rev. 2009, 109, 530–582. [Google Scholar] [CrossRef]
- Bergbreiter, D.E. Soluble Polymers as Tools in Catalysis. ACS Macro Lett. 2014, 3, 260–265. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Bergbreiter, D.E. Soluble polymer-supported organocatalysts. Pure Appl. Chem. 2013, 85, 493–509. [Google Scholar] [CrossRef]
- Dong, J.; Krasnova, L.; Finn, M.G.; Sharpless, K.B. Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angew. Chem. Int. Ed. 2014, 53, 9430–9448. [Google Scholar] [CrossRef]
- Dong, J.; Sharpless, K.B.; Kwisnek, L.; Oakdale, J.S.; Fokin, V.V. SuFEx-Based Synthesis of Polysulfates. Angew. Chem. Int. Ed. 2014, 53, 9466–9470. [Google Scholar] [CrossRef]
- Li, S.; Wu, P.; Moses, J.E.; Sharpless, K.B. Multidimensional SuFEx Click Chemistry: Sequential Sulfur(VI) Fluoride Exchange Connections of Diverse Modules Launched From An SOF4 Hub. Angew. Chem. Int. Ed. 2017, 56, 2903–2908. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Meng, G.; Zhan, X.; Yang, Q.; Ma, T.; Xu, L.; Sharpless, K.B.; Dong, J. A New Portal to SuFEx Click Chemistry: A Stable Fluorosulfuryl Imidazolium Salt Emerging as an “F−SO2+” Donor of Unprecedented Reactivity, Selectivity, and Scope. Angew. Chem. Int. Ed. 2018, 57, 2605–2610. [Google Scholar] [CrossRef]
- Gao, B.; Li, S.; Wu, P.; Moses, J.E.; Sharpless, K.B. SuFEx Chemistry of Thionyl Tetrafluoride (SOF4) with Organolithium Nucleophiles: Synthesis of Sulfonimidoyl Fluorides, Sulfoximines, Sulfonimidamides, and Sulfonimidates. Angew. Chem. Int. Ed. 2018, 57, 1939–1943. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, L.; Zheng, Q.; Zhou, F.; Klivansky, L.M.; Lu, J.; Liu, Y.; Dong, J.; Wu, P.; Sharpless, K.B. Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates. Nat. Chem. 2017, 9, 1083–1088. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, F.; Ren, G.; Zheng, Q.; Chen, H.; Gao, B.; Klivansky, L.; Liu, Y.; Wu, B.; Xu, Q.; et al. SuFEx-Based Polysulfonate Formation from Ethenesulfonyl Fluoride–Amine Adducts. Angew. Chem. Int. Ed. 2017, 56, 11203–11208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Jin, H.-S.; Chen, X.-M.; Lin, B.-P.; Yang, H. A sulfur(vi) fluoride exchange click chemistry approach towards main chain liquid crystal polymers bearing sulfate ester groups. Polym. Chem. 2019, 10, 3657–3664. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, F.; Gu, P.-Y.; Chen, D.; He, J.; Cappiello, J.R.; Wu, P.; Xu, Q.; Lu, J. Preparation of aryl polysulfonates via a highly efficient SuFEx click reaction, their controllable degradation and functionalized behavior. Polym. Chem. 2020, 11, 3120–3124. [Google Scholar] [CrossRef]
- Kulow, R.W.; Wu, J.W.; Kim, C.; Michaudel, Q. Synthesis of unsymmetrical sulfamides and polysulfamides via SuFEx click chemistry. Chem. Sci. 2020, 11, 7807–7812. [Google Scholar] [CrossRef] [PubMed]
- Yatvin, J.; Brooks, K.; Locklin, J. SuFEx on the Surface: A Flexible Platform for Postpolymerization Modification of Polymer Brushes. Angew. Chem. Int. Ed. 2015, 54, 13370–13373. [Google Scholar] [CrossRef] [PubMed]
- Durie, K.; Yatvin, J.; McNitt, C.D.; Reese, R.A.; Jung, C.; Popik, V.V.; Locklin, J. Multifunctional Surface Manipulation Using Orthogonal Click Chemistry. Langmuir 2016, 32, 6600–6605. [Google Scholar] [CrossRef] [PubMed]
- Gahtory, D.; Sen, R.; Pujari, S.; Li, S.; Zheng, Q.; Moses, J.E.; Sharpless, K.B.; Zuilhof, H. Quantitative and Orthogonal Formation and Reactivity of SuFEx Platforms. Chem. Eur. J. 2018, 24, 10550–10556. [Google Scholar] [CrossRef] [Green Version]
- Randall, J.D.; Eyckens, D.J.; Stojcevski, F.; Francis, P.S.; Doeven, E.H.; Barlow, A.J.; Barrow, A.S.; Arnold, C.L.; Moses, J.E.; Henderson, L.C. Modification of Carbon Fibre Surfaces by Sulfur-Fluoride Exchange Click Chemistry. ChemPhysChem 2018, 19, 3176–3181. [Google Scholar] [CrossRef]
- Liu, W.; Dong, Y.; Zhang, S.; Wu, Z.; Chen, H. A rapid one-step surface functionalization of polyvinyl chloride by combining click sulfur(vi)-fluoride exchange with benzophenone photochemistry. Chem. Commun. 2019, 55, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Durie, K.; Razavi, M.J.; Wang, X.; Locklin, J. Nanoscale Surface Creasing Induced by Post-polymerization Modification. ACS Nano 2015, 9, 10961–10969. [Google Scholar] [CrossRef]
- Li, S.; Beringer, L.T.; Chen, S.; Averick, S. Combination of AGET ATRP and SuFEx for post-polymerization chain-end modifications. Polymer 2015, 78, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Oakdale, J.S.; Kwisnek, L.; Fokin, V.V. Selective and Orthogonal Post-Polymerization Modification using Sulfur(VI) Fluoride Exchange (SuFEx) and Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC) Reactions. Macromolecules 2016, 49, 4473–4479. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Y.; Lu, X.; Wu, Z.; Chen, H. Combining Click Sulfur(VI)-Fluoride Exchange with Photoiniferters: A Facile, Fast, and Efficient Strategy for Postpolymerization Modification. Macromol. Rapid Commun. 2018, 39, 1700523. [Google Scholar] [CrossRef]
- Park, S.; Song, H.; Ko, N.; Kim, C.; Kim, K.; Lee, E. SuFEx in Metal–Organic Frameworks: Versatile Postsynthetic Modification Tool. ACS Appl. Mater. Interfaces 2018, 10, 33785–33789. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-y.; Liu, X.-y.; Zhao, G. Synthesis of Dendrimer-Supported Prolinols and Their Application in Enantioselective Reduction of Ketones. Synlett 2006, 1150–1154. [Google Scholar] [CrossRef]
- Zeitler, K.; Mager, I. An Efficient and Versatile Approach for the Immobilization of Carbene Precursors via Copper-Catalyzed [3+2]-Cycloaddition and their Catalytic Application. Adv. Synth. Catal. 2007, 349, 1851–1857. [Google Scholar] [CrossRef]
- Chung, C.W.Y.; Toy, P.H. Multipolymer Reaction System for Selective Aerobic Alcohol Oxidation: Simultaneous Use of Multiple Different Polymer-Supported Ligands. J. Comb. Chem. 2007, 9, 115–120. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Zheng, C.-W.; Zhao, G.; Cao, W.-G. Highly enantioselective tandem cyclopropanation/Wittig reaction of α,β-unsaturated aldehydes with arsonium ylides catalyzed by recyclable dendritic catalyst. Tetrahedron Asymmetry 2008, 19, 701–708. [Google Scholar] [CrossRef]
- Zhao, W.-X.; Liu, N.; Li, G.-W.; Chen, D.-L.; Zhang, A.-A.; Wang, M.-C.; Liu, L. Synthesis of dendrimer-supported ferrocenylmethyl aziridino alcohol ligands and their application in asymmetric catalysis. Green Chem. 2015, 17, 2924–2930. [Google Scholar] [CrossRef]
- Zhu, C.; Mu, A.U.; Wang, C.; Ji, X.; Fang, L. Synthesis and Solution Processing of a Rigid Polymer Enabled by Active Manipulation of Intramolecular Hydrogen Bonds. ACS Macro Lett. 2018, 7, 801–806. [Google Scholar] [CrossRef]
- Barclay, G.G.; Hawker, C.J.; Ito, H.; Orellana, A.; Malenfant, P.R.L.; Sinta, R.F. The “Living” Free Radical Synthesis of Poly(4-hydroxystyrene): Physical Properties and Dissolution Behavior. Macromolecules 1998, 31, 1024–1031. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.; Patil, P.; Kim, J.Y.; Kim, T.-H. Synthesis of succinylated poly(4-hydroxystyrene) and its application for negative-tone photoresist. J. Appl. Polym. Sci. 2007, 103, 3560–3566. [Google Scholar] [CrossRef]
- Brazier, J.B.; Jones, K.M.; Platts, J.A.; Tomkinson, N.C.O. On the Roles of Protic Solvents in Imidazolidinone-Catalyzed Transformations. Angew. Chem. Int. Ed. 2011, 50, 1613–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer-Gall, T.; Lee, J.-W.; Opwis, K.; List, B.; Gutmann, J.S. Textile Catalysts—An unconventional approach towards heterogeneous catalysis. ChemCatChem 2016, 8, 1428–1436. [Google Scholar] [CrossRef]
- Landini, D.; Molinari, H.; Pensa, M.; Rampoldi, A. Convenient Procedures for the Preparation of Lipophilic Quaternary Onium Fluorides, Hydrogendifluorides and Dihydrogentrifluorides via Ion Exchange in Two-Phase Systems. Synthesis 1988, 953–955. [Google Scholar] [CrossRef]


| Entry | Reactant | Product | Yield (%) b | Mw (Da) | Mn (Da) | PDI |
|---|---|---|---|---|---|---|
| 1 | 2b + 4a | 5a | 96 | 38.8 k | 22.9 k | 1.70 |
| 2 | 2b + 4b | 5b | 97 | 37.4 k | 22.7 k | 1.64 |
| 3 | 2b + 4c | 5c | 92 | 21.2 k | 11.1 k | 1.91 |
| 4 | 2b + 4d | 5d | 96 | 35.0 k | 22.1 k | 1.58 |
| 5 | 2b + 4e | 5e | 91 | 15.6 k | 9.1 k | 1.72 |
| 6 | 2b + 4f | 5f | 94 | 30.8 k | 17.8 k | 1.73 |
| 7 | 2b + 4g | 5g | trace | n.d. | n.d. | n.d. |
| 8 | 2b + 2c | 5h | 91 | 7.1 k | 4.4 k | 1.64 |
| 9 | 2d | 5h | 98 | 31.6 k | 17.7 k | 1.78 |
| 10 | 2e | 5h | 92 c | 8.0 k | 4.9 k | 1.63 |

| Entry | Reactant | Product | Yield (%) b | Mw (Da) | Mn (Da) | PDI |
|---|---|---|---|---|---|---|
| 1 | 6b + 4a | 7a | 93 | 12.2 k | 6.3 k | 1.94 |
| 2 | 6b + 4b | 7b | 93 | 7.2 k | 4.5 k | 1.56 |
| 3 | 6b + 4c | 7c | 95 | 8.5 k | 4.4 k | 1.95 |
| 4 | 6b + 4d | 7d | 98 | 36.6 k | 8.8 k | 4.16 |
| 5 | 6b + 4e | 7e | 95 | 10.2 k | 5.6 k | 1.83 |
| 6 | 6b + 4f | 7f | 96 | 31.9 k | 7.2 k | 4.41 |
| 7 | 6b + 4g | 7g | 88 | 9.0 k | 6.0 k | 1.51 |
| 8 | 6b + 6c | 7h | 90 | 9.9 k | 4.5 k | 2.18 |
| 9 | 6d | 7h | n.r. | n.d. | n.d. | n.d. |
| 10 | 2b + 6c | 8 | 97 | 156 k | 22.3 k | 7.00 |

| Entry | Reactant | Product | Mw (Da) | Mn (Da) | PDI |
|---|---|---|---|---|---|
| 1 | 9b + 3c | 10a | 33.6 k | 21.7 k | 1.55 |
| 2 | 9c + 3b | 10b | 25.7 k | 19.2 k | 1.34 |
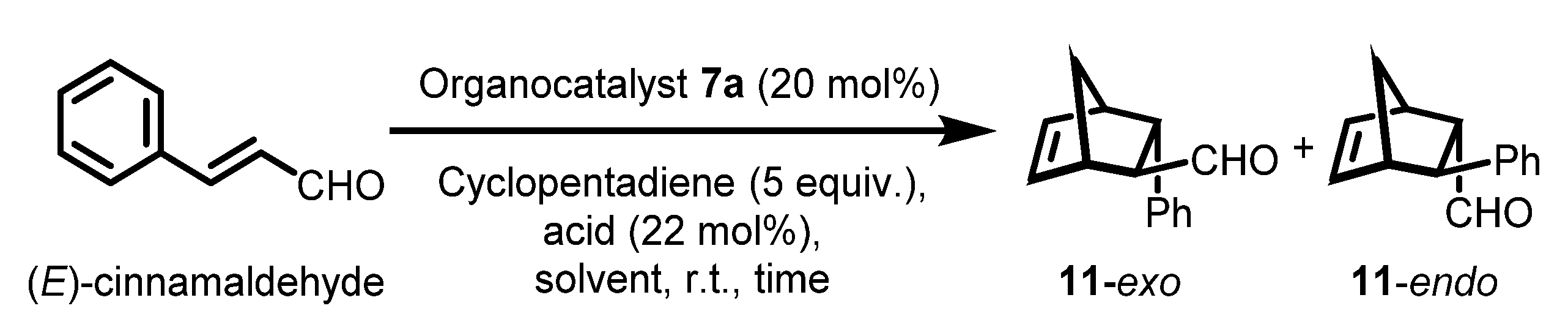
| Entry | Solvent | Acid | Time (h) | Yield (%) b | 11 c exo/endo | exo ee (%) d | endo ee (%) d |
|---|---|---|---|---|---|---|---|
| 1 | H2O | TFA | 24 | trace | n.d. | n.d. | n.d. |
| 2 | MeOH:H2O (95:5) | TFA | 24 | 57 | 46/53 | 63 | 59 |
| 3 | Toluene:H2O (95:5) | TFA | 24 | 46 | 57/43 | 73 | 64 |
| 4 | DCM:H2O (95:5) | TFA | 24 | 69 | 53/47 | 80 | 79 |
| 5 | ACN:H2O (95:5) | TFA | 24 | 79 | 60/40 | 89 | 91 |
| 6 | THF:H2O (95:5) | TFA | 24 | 88 | 54/46 | 91 | 94 |
| 7 | DMF:H2O (95:5) | TFA | 24 | 92 | 51/49 | 92 | 93 |
| 8 | DMF:H2O (95:5) | p-TsOH | 24 | 85 | 55/45 | 91 | 91 |
| 9 | DMF:H2O (95:5) | HCl | 24 | 92 | 56/44 | 90 | 89 |
| 10 | DMF:H2O (95:5) | HClO4 | 24 | 82 | 52/48 | 89 | 88 |
| 11 | DMF:H2O (95:5) | MsOH | 24 | 84 | 53/47 | 88 | 91 |
| 12 | DMF:H2O (95:5) | HBF4 | 24 | 79 | 56/44 | 91 | 89 |
| 13 e | DMF:H2O (95:5) | TFA | 24 | 62 | 53/47 | 92 | 92 |
| 14 f | DMF:H2O (95:5) | TFA | 48 | 80 | 51/49 | 94 | 94 |
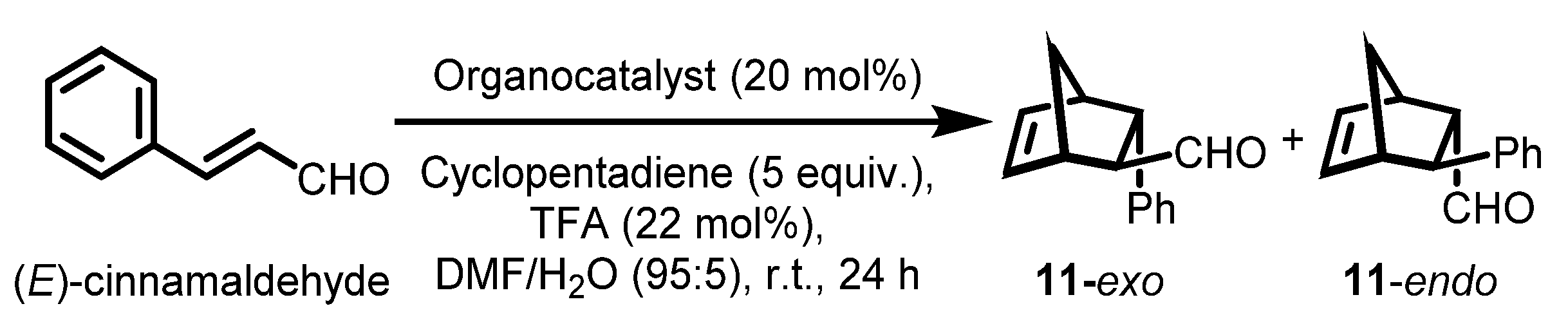
| Entry | Catalyst | Calculated Catalysts’ Loading (mmol/g) | Yield (%) b | 11 c exo/endo | exo ee (%) d | endo ee (%) d |
|---|---|---|---|---|---|---|
| Monomeric organocatalysts | ||||||
| 1 | 2a | 3.06 | 95 | 56/44 | 95 | 92 |
| 2 | 3a | 3.62 | 96 | 56/44 | 97 | 96 |
| 3 | 6a | 1.55 | 96 | 49/51 | 97 | 97 |
| Class I | ||||||
| 4 | 5a | 1.53 | 90 | 53/47 | 90 | 89 |
| 5 | 5b | 1.47 | 91 | 54/46 | 89 | 90 |
| 6 | 5c | 1.50 | 86 | 56/44 | 87 | 87 |
| 7 | 5d | 1.39 | 89 | 48/53 | 91 | 92 |
| 8 | 5e | 1.64 | 87 | 57/43 | 89 | 88 |
| 9 | 5f | 1.57 | 91 | 52/48 | 92 | 92 |
| 10 | 5he | 2.57 | 79 | 55/45 | 86 | 86 |
| 11 | 5hf | 2.57 | 73 | 56/44 | 85 | 86 |
| 12 | 5hg | 2.57 | 78 | 54/46 | 84 | 88 |
| Class II | ||||||
| 13 | 7a | 1.03 | 92 | 51/49 | 92 | 93 |
| 14 | 7b | 1.00 | 93 | 54/46 | 92 | 92 |
| 15 | 7c | 1.02 | 89 | 51/49 | 90 | 91 |
| 16 | 7d | 0.96 | 92 | 53/47 | 89 | 92 |
| 17 | 7e | 1.08 | 88 | 60/40 | 89 | 90 |
| 18 | 7f | 1.05 | 90 | 54/46 | 91 | 91 |
| 19 | 7g | 1.08 | 88 | 53/47 | 93 | 93 |
| 20 | 7h | 1.41 | 93 | 50/50 | 86 | 85 |
| Class III | ||||||
| 21 | 10a | 2.18 | 64 | 52/48 | 61 | 67 |
| 22 | 10b | 2.18 | 69 | 51/49 | 66 | 69 |
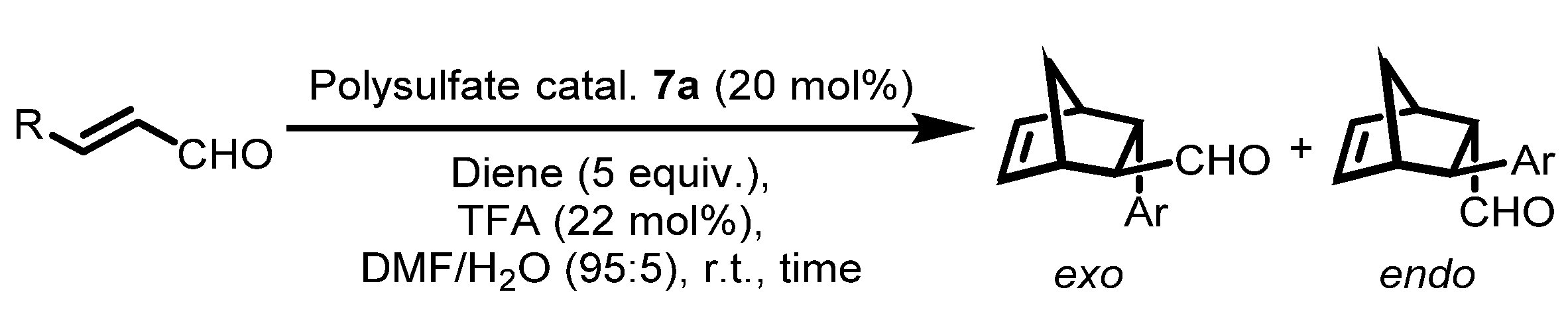
| Entry | Diene | R | Time (h) | Yield (%) b | 11 c exo/endo | exo ee (%) d | endo ee (%) d |
|---|---|---|---|---|---|---|---|
| 1 | Cyclopentadiene | p-F-C6H4 | 20 | 97 | 51/49 | 93 | 93 |
| 2 | Cyclopentadiene | p-Cl-C6H4 | 20 | 95 | 54/46 | 91 | 95 |
| 3 | Cyclopentadiene | p-Br-C6H4 | 24 | 96 | 51/49 | 90 | 92 |
| 4 | Cyclopentadiene | p-NO2-C6H4 | 18 | 90 | 53/47 | 95 | 94 |
| 5 | Cyclopentadiene | o-NO2-C6H4 | 18 | 93 | 42/58 | 52 | 82 |
| 6 | Cyclopentadiene | Me | 24 | 76 | 53/47 | 64 e | 81 e |
| 7 | 1,3-Diphenylisobenzofuran | Me | 16 | 85 | 90/10 | 84 | n.d. |
| Entry | Immobilization Strategy | Catalysts’ Loading (mol%) | Time (h) | Temperature (°C) | Yield (%) | TON | exo/endo | exo ee (%) | end oee (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 [22] a | PEG5000 monomethyl ether | 10 | 40 | 24 | 67 | 6.7 | 6/94 | 86 | 92 |
| 2 [29] | Poly(methyl hydrosiloxane) (PMHS) | 10 | 40 | 0 | 65 | 6.5 | 55/45 | 92 | 93 |
| 3 [34] | Polyacrylate copolymer | 5 | 6 | r.t. | 92 | 18.4 | 1/1.12 | 68 | 79 |
| 4 [35] | Chiral organosilica polymer (ChiOSP) | 20 | 36 | r.t. | 94 | 4.7 | 1.1/1 | 90 | 86 |
| 5 | Linear polysulfate 7a | 20 | 24 | r.t. | 92 | 4.6 | 51/49 | 92 | 93 |

| Entry | Catalyst | Cycle | Time (h) | Yield (%) b | 11 c exo/endo | exo ee (%) d | endo ee (%) d |
|---|---|---|---|---|---|---|---|
| 1 a | 5b | Fresh | 24 | 91 | 54/46 | 89 | 90 |
| 2 | 5b | 1st | 24 | 92 | 53/47 | 89 | 90 |
| 3 | 5b | 2nd | 24 | 87 | 54/46 | 89 | 89 |
| 4 | 5b | 3rd | 36 | 86 | 55/44 | 87 | 87 |
| 5 | 5b | 4th | 36 | 77 | 54/46 | 81 | 81 |
| 6 a | 7a | Fresh | 24 | 92 | 51/49 | 92 | 93 |
| 7 | 7a | 1st | 24 | 92 | 52/48 | 92 | 92 |
| 8 | 7a | 2nd | 24 | 88 | 52/48 | 89 | 90 |
| 9 | 7a | 3rd | 24 | 83 | 52/48 | 88 | 89 |
| 10 | 7a | 4th | 30 | 85 | 51/49 | 87 | 88 |
| 11 | 7a | 5th | 36 | 80 | 51/49 | 86 | 86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-S.; Li, L.; Kim, B.M. SuFEx-Click Approach for the Synthesis of Soluble Polymer-Bound MacMillan Catalysts for the Asymmetric Diels–Alder Reaction. Catalysts 2021, 11, 1044. https://doi.org/10.3390/catal11091044
Lee W-S, Li L, Kim BM. SuFEx-Click Approach for the Synthesis of Soluble Polymer-Bound MacMillan Catalysts for the Asymmetric Diels–Alder Reaction. Catalysts. 2021; 11(9):1044. https://doi.org/10.3390/catal11091044
Chicago/Turabian StyleLee, Woong-Sup, Linzi Li, and Byeong Moon Kim. 2021. "SuFEx-Click Approach for the Synthesis of Soluble Polymer-Bound MacMillan Catalysts for the Asymmetric Diels–Alder Reaction" Catalysts 11, no. 9: 1044. https://doi.org/10.3390/catal11091044







