Effect of Pd and Ir as Promoters in the Activity of Ni/CeZrO2 Catalyst for the Reverse Water-Gas Shift Reaction
Abstract
:1. Introduction
2. Results and Discussion
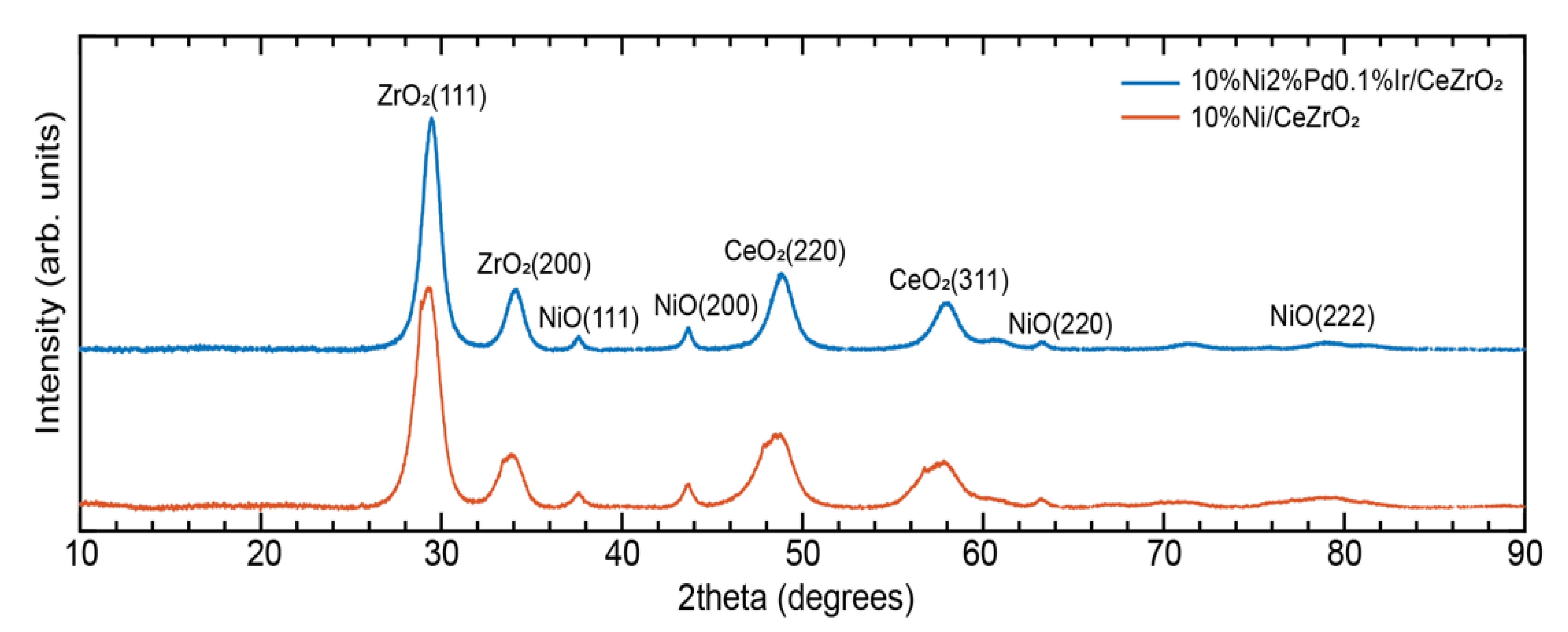
2.1. XRD Analysis
2.2. BET-BJH Measurements
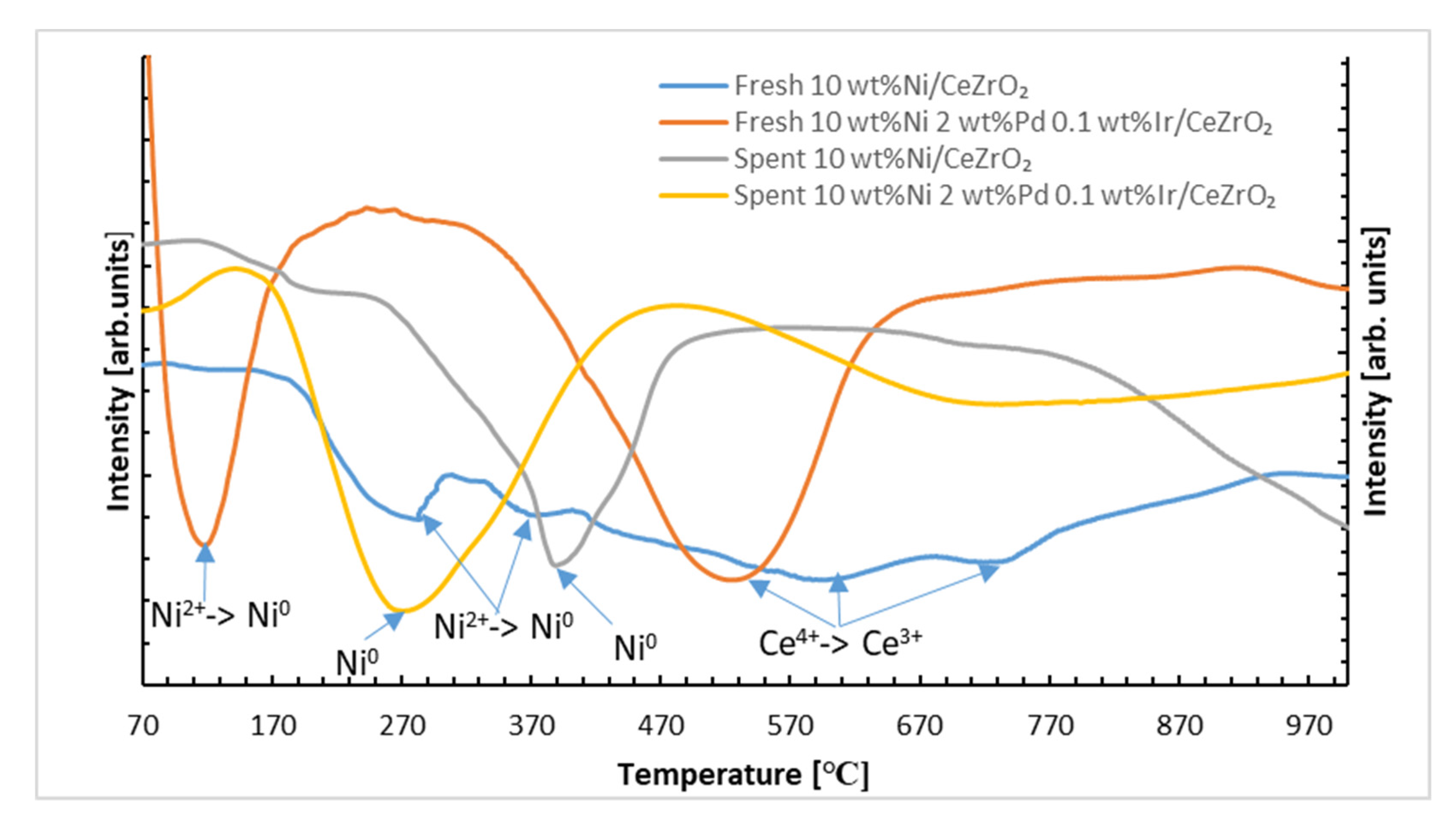
2.3. H2-TPR
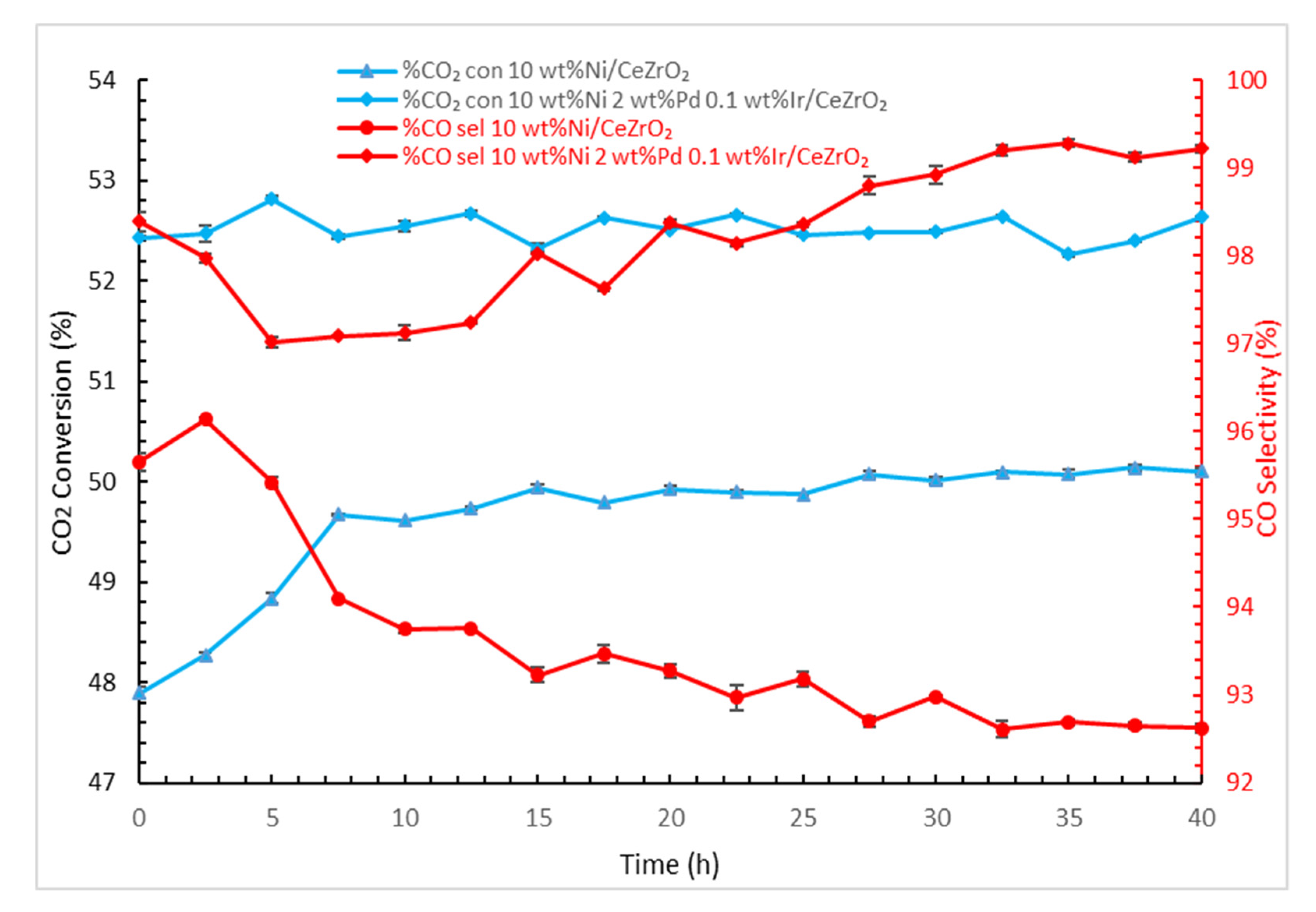
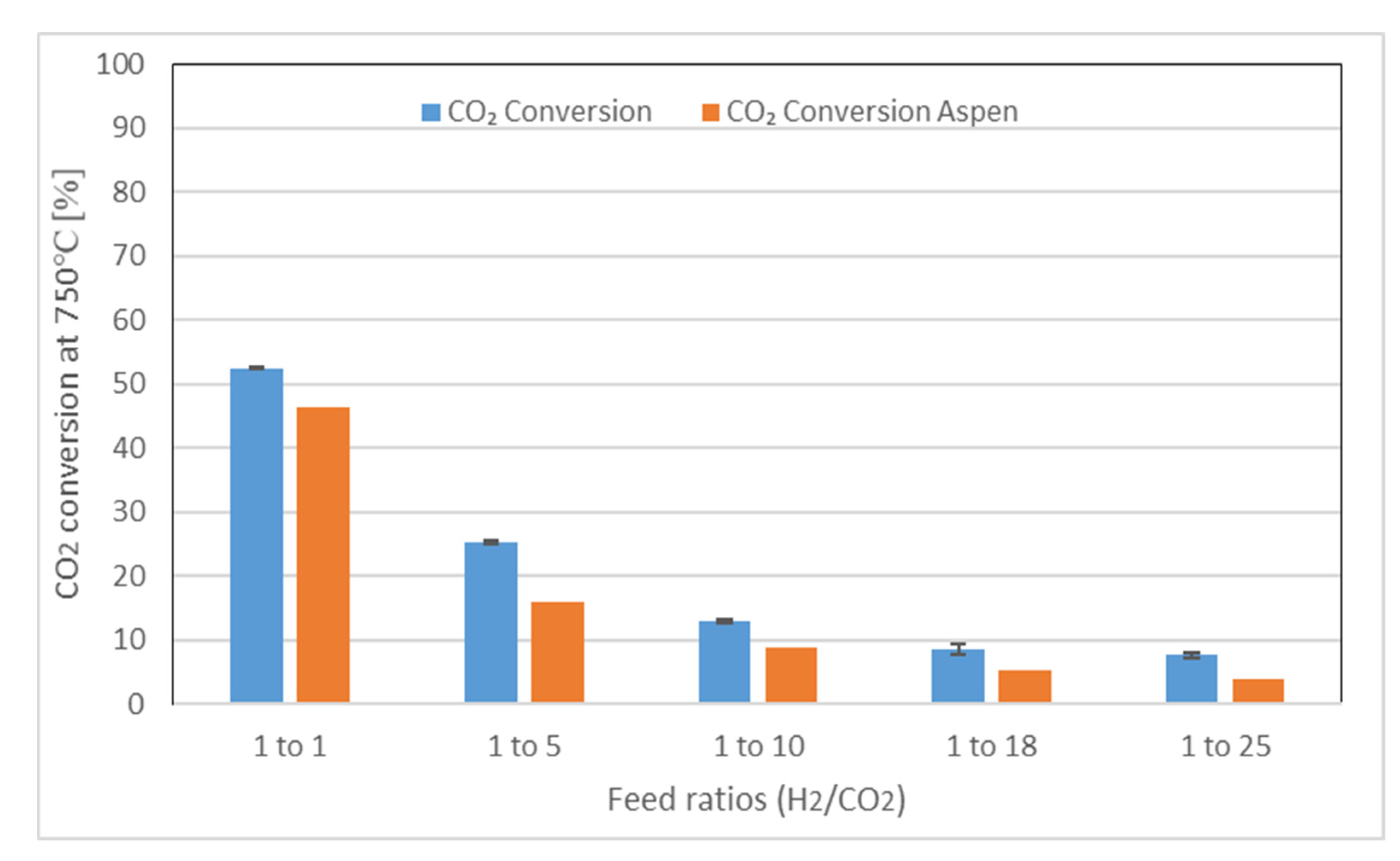
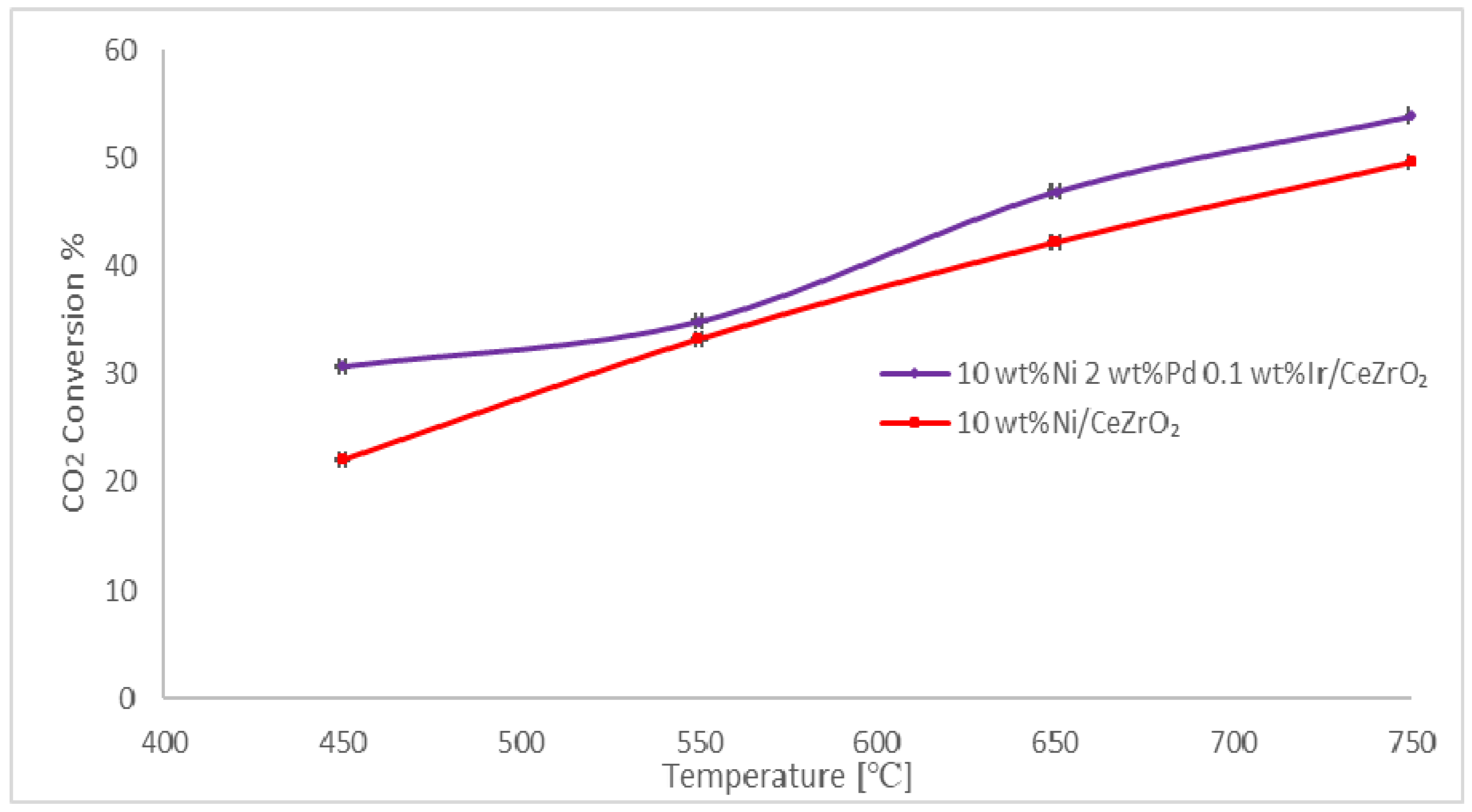
2.4. Catalyst Activity
3. Materials and Methods
3.1. Catalyst Preparation
3.2. RWGS Reaction
3.3. Characterization Techniques
3.3.1. X-ray Diffraction (XRD)
3.3.2. BET Surface Areas and Pore Distributions
3.3.3. H2-Temperature Program Reduction (H2-TPR)
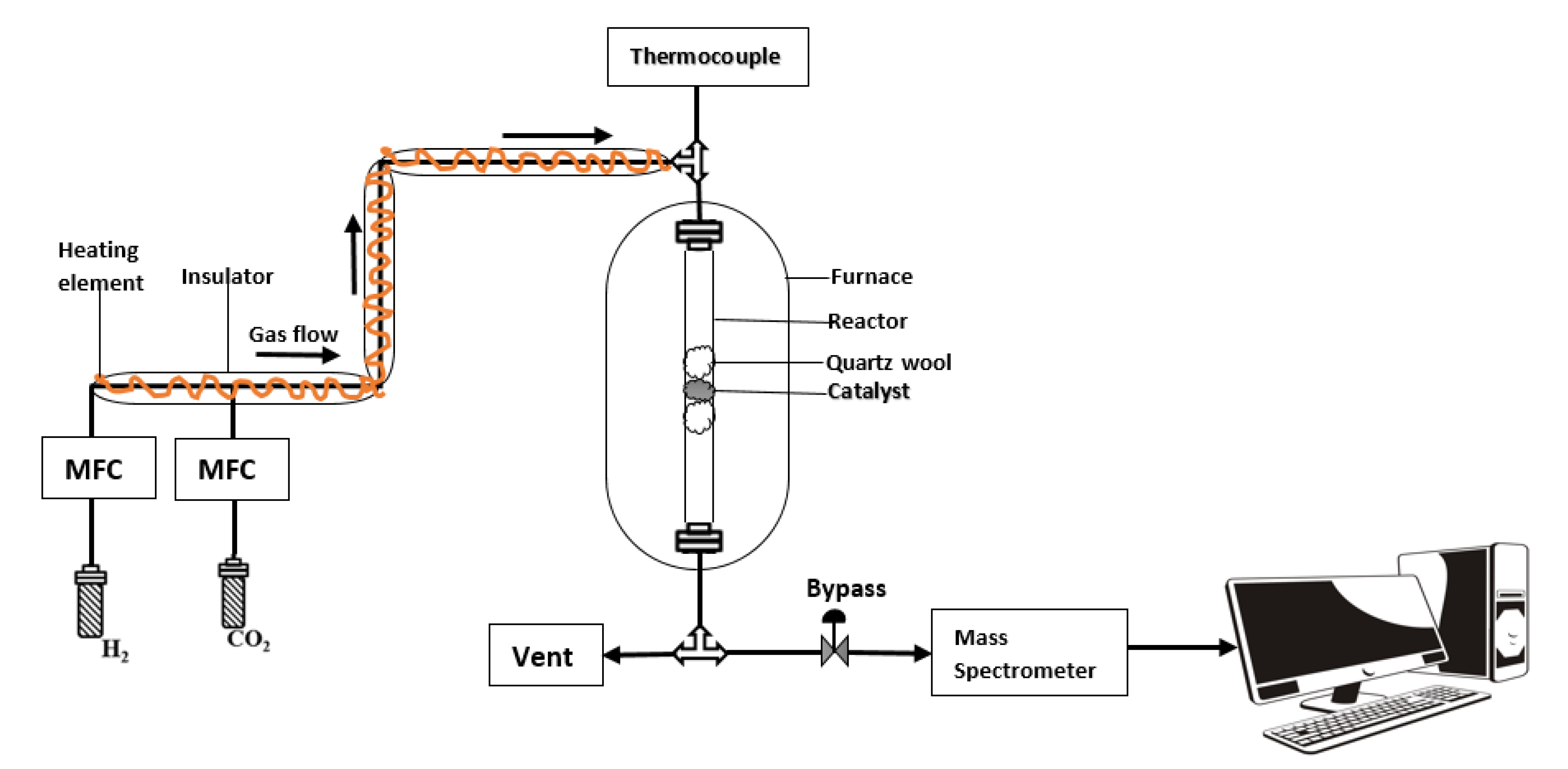
3.4. Reaction Procedure and Product Analysis
3.5. Thermodynamic Calculation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oshima, K.; Shinagawa, T.; Nogami, Y.; Manabe, R.; Ogo, S.; Sekine, Y. Low temperature catalytic reverse water gas shift reaction assisted by an electric field. Catal. Today 2014, 232, 27–32. [Google Scholar] [CrossRef]
- Chen, C.S.; Cheng, W.H.; Lin, S.S. Study of iron-promoted Cu/SiO2 catalyst on high temperature reverse water gas shift reaction. Appl. Catal. A Gen. 2004, 257, 97–106. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, S.W.; Yoon, H.S.; Kim, Y.D. Reverse water gas shift reaction catalyzed by Fe nanoparticles with high catalytic activity and stability. J. Ind. Eng. Chem. 2015, 23, 67–71. [Google Scholar] [CrossRef]
- Maitlis, P.M.; de Klerk, A. Greener Fischer-Tropsch Processes for Fuels and Feedstocks; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kim, D.H.; Park, J.L.; Park, E.J.; Kim, Y.D.; Uhm, S. Dopant Effect of Barium Zirconate-Based Perovskite-Type Catalysts for the Intermediate-Temperature Reverse Water Gas Shift Reaction. ACS Catal. 2014, 4, 3117–3122. [Google Scholar] [CrossRef]
- Hu, B.; Guild, C.; Suib, S.L. Thermal, electrochemical, and photochemical conversion of CO2 to fuels and value-added products. J. CO2 Util. 2013, 1, 18–27. [Google Scholar] [CrossRef]
- Luhui, W.A.; Hui, L.I.; Yuan, L.I.; Ying, C.H.; Shuqing, Y.A. Influence of preparation method on performance of Ni-CeO2 catalysts for reverse water-gas shift reaction. J. Rare Earths 2013, 31, 559–564. [Google Scholar]
- Ito, M.; Tagawa, T.; Goto, S. Suppression of carbonaceous depositions on nickel catalyst for the carbon dioxide reforming of methane. Appl. Catal. A Gen. 1999, 177, 15–23. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q. Reforming of methane with carbon dioxide over Ni/Al2O3 catalysts: Effect of nickel precursor. Appl. Catal. A Gen. 1998, 169, 271–280. [Google Scholar] [CrossRef]
- Au, C.T.; Ng, C.F.; Liao, M.S. Methane dissociation and syngas formation on Ru, Os, Rh, Ir, Pd, Pt, Cu, Ag, and Au: A theoretical study. J. Catal. 1999, 185, 12–22. [Google Scholar] [CrossRef]
- Chang, J.S.; Park, S.E.; Chon, H. Catalytic activity and coke resistance in the carbon dioxide reforming of methane to synthesis gas over zeolite-supported Ni catalysts. Appl. Catal. A Gen. 1996, 145, 111–124. [Google Scholar] [CrossRef]
- Hou, Z.; Yokota, O.; Tanaka, T.; Yashima, T. Characterization of Ca-promoted Ni/A-Al2O3 catalyst for CH4 reforming with CO2. Appl. Catal. A Gen. 2003, 253, 381–387. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Roman-Martinez, M.C.; Illan-Gomez, M.J. Effect of potassium content in the activity of K-promoted Ni/Al2O3 catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2006, 301, 9–15. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Verykios, X.E. Carbon dioxide reforming of methane to synthesis gas over supported Ni catalysts. Catal. Today 1994, 21, 589–595. [Google Scholar] [CrossRef]
- Mihet, M.; Lazar, M.D. Methanation of CO2 on Ni/γ-Al2O3: Influence of Pt, Pd or Rh promotion. Catal. Today 2018, 306, 294–299. [Google Scholar] [CrossRef]
- Ertl, G.; Knözinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag Gmbh & Co. KGaA: Weinheim, Germany, 2008; Volume 1–5, pp. 1–2497. [Google Scholar]
- Vernoux, P.; Lizarraga, L.; Tsampas, M.N.; Sapountzi, F.M.; De Lucas-Consuegra, A.; Valverde, J.L.; Souentie, S.; Vayenas, C.G.; Tsiplakides, D.; Balomenou, S.; et al. Ionically conducting ceramics as active catalyst supports. Chem. Rev. 2013, 113, 8192–8260. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Koutsodontis, C.G. Non-Faradaic electrochemical activation of catalysis. J. Chem. Phys. 2008, 128, 182506. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.T.; Scates, R.; Twigg, M.V. X-ray diffraction study of nickel oxide reduction by hydrogen. Appl. Catal. A Gen. 2003, 246, 137–150. [Google Scholar] [CrossRef]
- Bastan, F.; Kazemeini, M.; Larimi, A.S. Aqueous-phase reforming of glycerol for production of alkanes over Ni/CexZr1-xO2 nano-catalyst: Effects of the support’s composition. Renew. Energy 2017, 108, 417–424. [Google Scholar] [CrossRef]
- Trovarelli, A.; Zamar, F.; Llorca, J.; De Leitenburg, C.; Dolcetti, G.; Kiss, J.T. Nanophase fluorite-structured CeO2–ZrO2Catalysts prepared by high-energy mechanical milling. J. Catal. 1997, 169, 490–502. [Google Scholar] [CrossRef]
- Roh, H.-S.; Potdar, H.; Jun, K.-W. Carbon dioxide reforming of methane over co-precipitated Ni–CeO2, Ni–ZrO2 and Ni–Ce–ZrO2 catalysts. Catal. Today 2004, 93, 39–44. [Google Scholar] [CrossRef]
- Sheikh, A.; Silva, E.L.; Moares, L.; Antonini, L.; Abellah, M.Y.; Malfatti, C. Pd-based catalysts for ethanol oxidation in alkaline electrolyte. Am. J. Min. Met. 2014, 2, 64–69. [Google Scholar]
- Asanova, T.I.; Asanov, I.P.; Kim, M.G.; Gerasimov, E.Y.; Zadesenets, A.V.; Plyusnin, P.E.; Korenev, S.V. On formation mechanism of Pd–Ir bimetallic nanoparticles through thermal decomposition of [Pd (NH 3) 4][IrCl 6]. J. Nanopart. Res. 2013, 15, 1–15. [Google Scholar] [CrossRef]
- Masui, T.; Fujiwara, K.; Peng, Y.; Sakata, T.; Machida, K.I.; Mori, H.; Adachi, G.Y. Characterization and catalytic properties of CeO2–ZrO2 ultrafine particles prepared by the microemulsion method. J. Alloys Compd. 1998, 269, 116–122. [Google Scholar] [CrossRef]
- Montoya, J.A.; Romero-Pascual, E.; Gimon, C.; Del Angel, P.; Monzon, A. Methane reforming with CO2 over Ni/ZrO2-CeO2 catalysts prepared by sol-gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Dantas, S.C.; Escritori, J.C.; Soares, R.R.; Hori, C.E. Effect of different promoters on Ni/CeZrO2 catalyst for autothermal reforming and partial oxidation of methane. Chem. Eng. J. 2010, 156, 380–387. [Google Scholar] [CrossRef]
- Larsen, J.H.; Chorkendorff, I. From fundamental studies of reactivity on single crystals to the design of catalysts. Surf. Sci. Rep. 1999, 35, 163–222. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-Based Catalysts for CO2 Methanation: A Review. Nanomaterials 2021, 11, 28. [Google Scholar] [CrossRef]
- Blomberg, S.; Johansson, N.; Kokkonen, E.; Rissler, J.; Kollberg, L.; Preger, C.; Franzén, S.M.; Messing, M.E.; Hulteberg, C. Bimetallic Nanoparticles as a Model System for an Industrial NiMo Catalyst. Materials 2019, 12, 3727. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P. The Determination of Pore Volume and Area Distributions in Porous Substances. 1. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar]
- Li, Y.; Zhang, H.; Zhang, L.; Zhang, H. Bimetallic NiPd/SBA-15 alloy as an effective catalyst for selective hydrogenation of CO2 to methane. Int. J. Hydrog. Energy 2019, 44, 13354–13363. [Google Scholar] [CrossRef]
| Catalyst | BET | Surface Area (m2/g) | |
|---|---|---|---|
| Pore Volume (cm3/gcat) | Pore Size (Å) | ||
| Fresh 10 wt%Ni 2 wt%Pd 0.1 wt%Ir/CeZrO2 | 0.19 | 78 | 95 |
| Fresh 10 wt% Ni/CeZrO2 | 0.20 | 78 | 100 |
| Spent 10 wt%Ni 2 wt%Pd 0.1 wt%Ir/CeZrO2 | 0.17 | 170 | 35 |
| Spent 10 wt% Ni/CeZrO2 | 0.20 | 159 | 41 |
| Feed H2/CO2 (v/v) | Volume of H2 (mL/min) | Volume of CO2(mL/min) |
|---|---|---|
| 1/1 | 25 | 25 |
| 1/5 | 20 | 100 |
| 1/10 | 17 | 170 |
| 1/18 | 12 | 216 |
| 1/25 | 12 | 300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajakaiye Jensen, L.I.; Blomberg, S.; Hulteberg, C. Effect of Pd and Ir as Promoters in the Activity of Ni/CeZrO2 Catalyst for the Reverse Water-Gas Shift Reaction. Catalysts 2021, 11, 1076. https://doi.org/10.3390/catal11091076
Ajakaiye Jensen LI, Blomberg S, Hulteberg C. Effect of Pd and Ir as Promoters in the Activity of Ni/CeZrO2 Catalyst for the Reverse Water-Gas Shift Reaction. Catalysts. 2021; 11(9):1076. https://doi.org/10.3390/catal11091076
Chicago/Turabian StyleAjakaiye Jensen, Lucy Idowu, Sara Blomberg, and Christian Hulteberg. 2021. "Effect of Pd and Ir as Promoters in the Activity of Ni/CeZrO2 Catalyst for the Reverse Water-Gas Shift Reaction" Catalysts 11, no. 9: 1076. https://doi.org/10.3390/catal11091076






