On the Use of Dual Cell Density Monoliths
Abstract
:1. Introduction
2. Computational Model
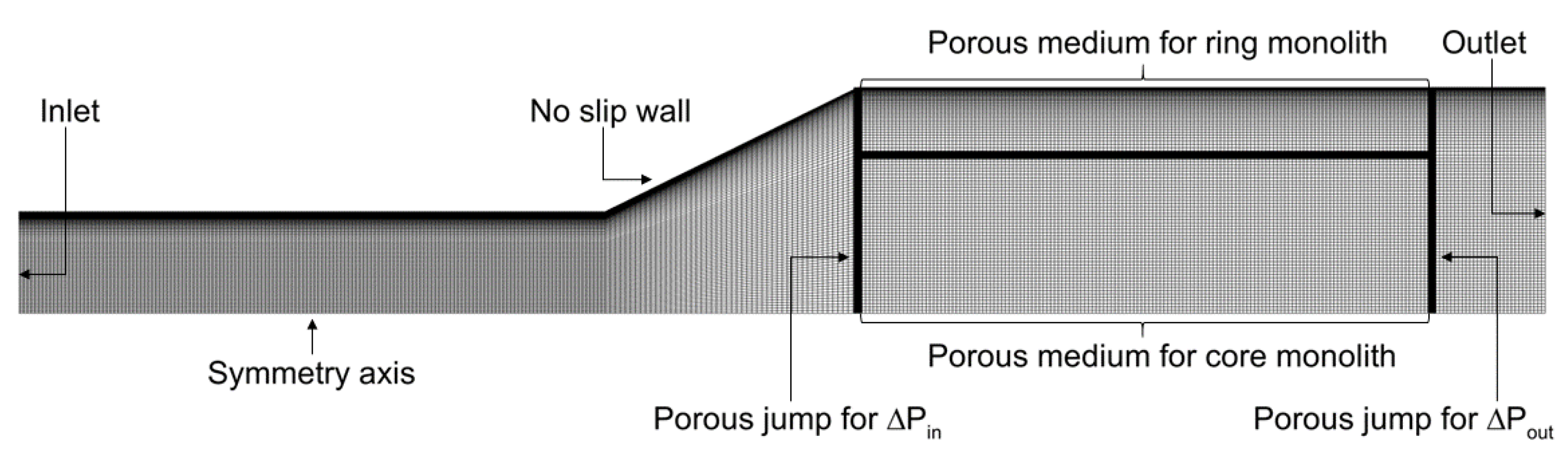
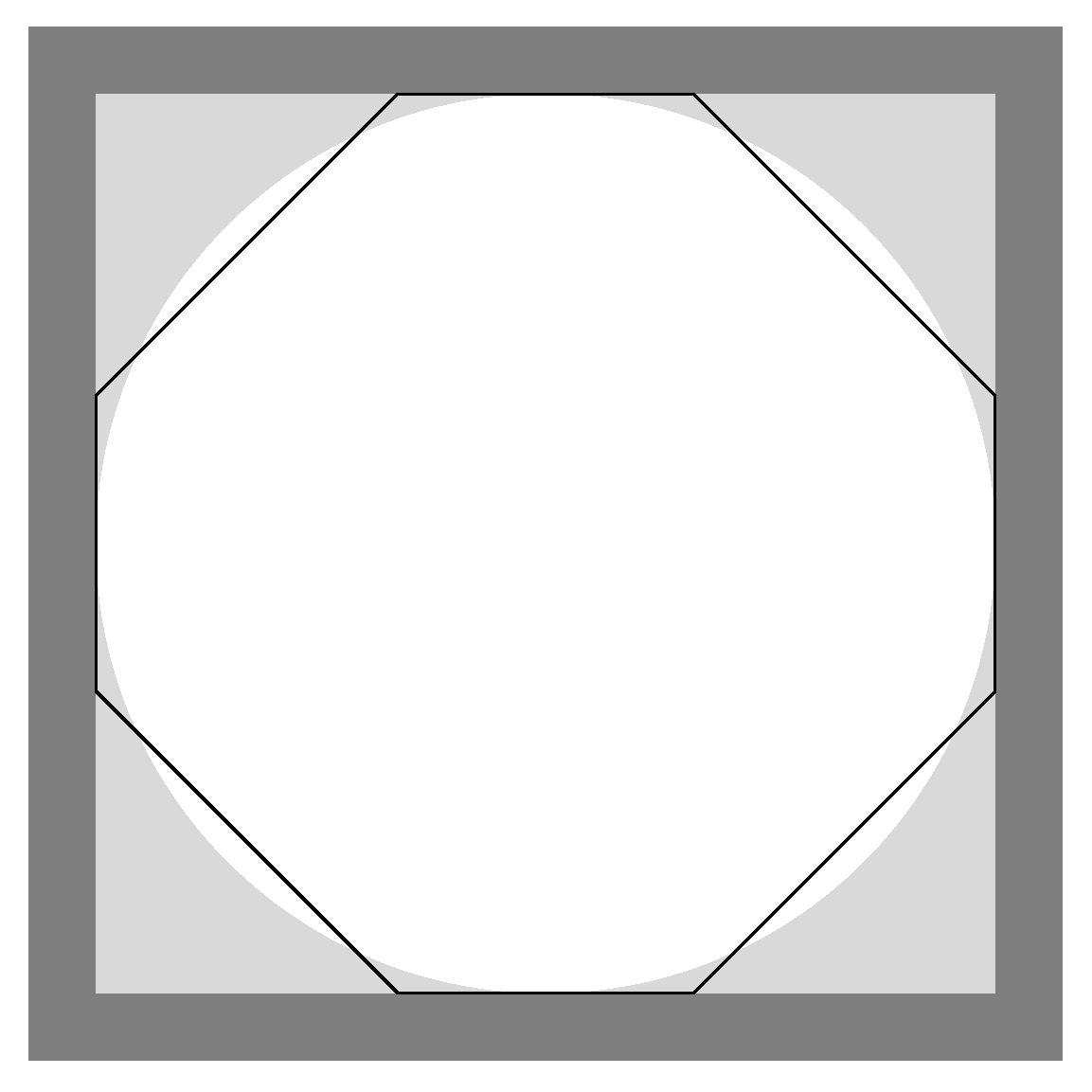
2.1. Description of the Domain
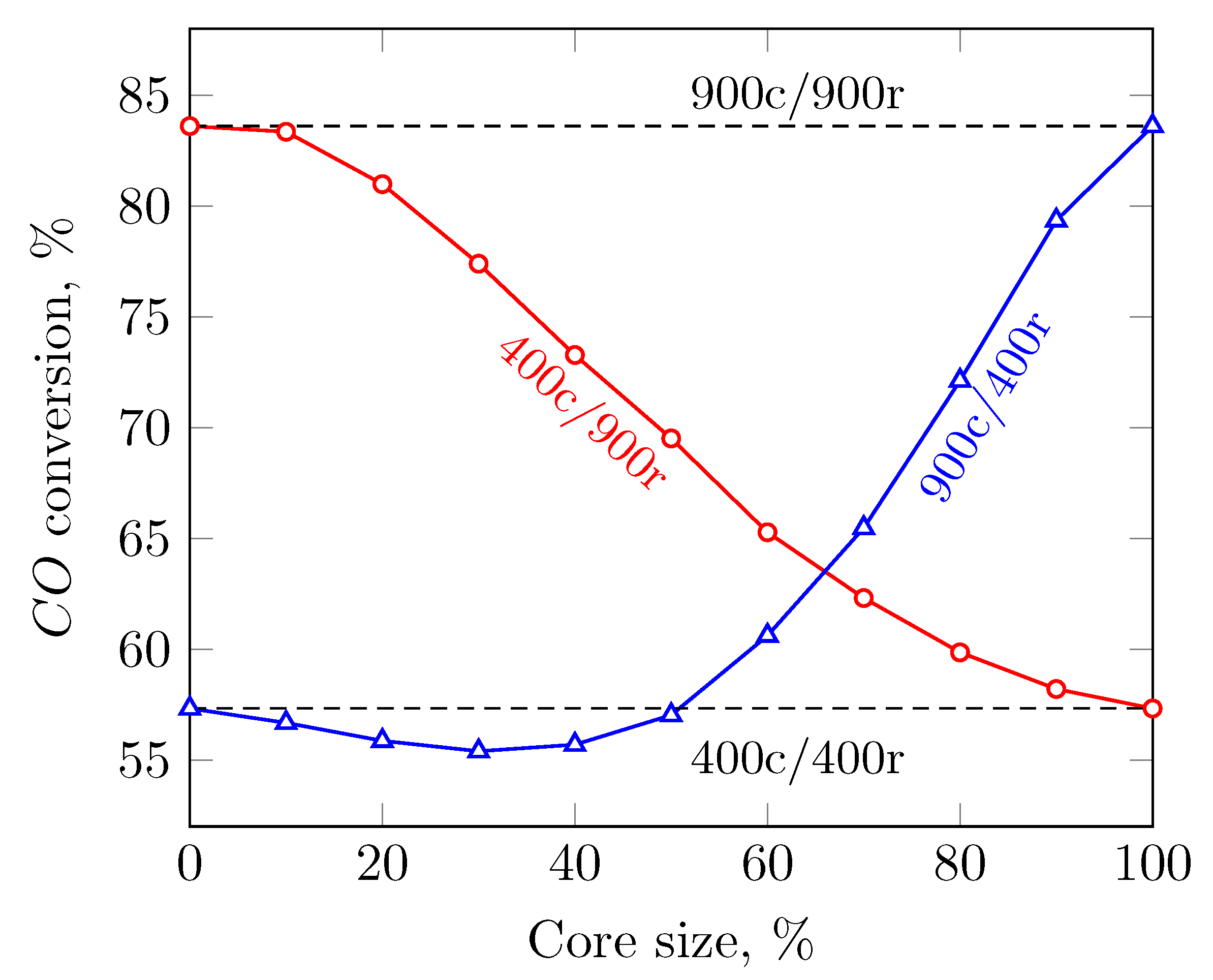
- i.
- 400c/400r
- ii.
- 400c/900r
- iii.
- 900c/900r
- iv.
- 900c/400r
2.2. Flow Model
2.3. Treatment of the Turbulence
2.4. Species Transport and Reactions Model
2.5. Heat Transfer Model
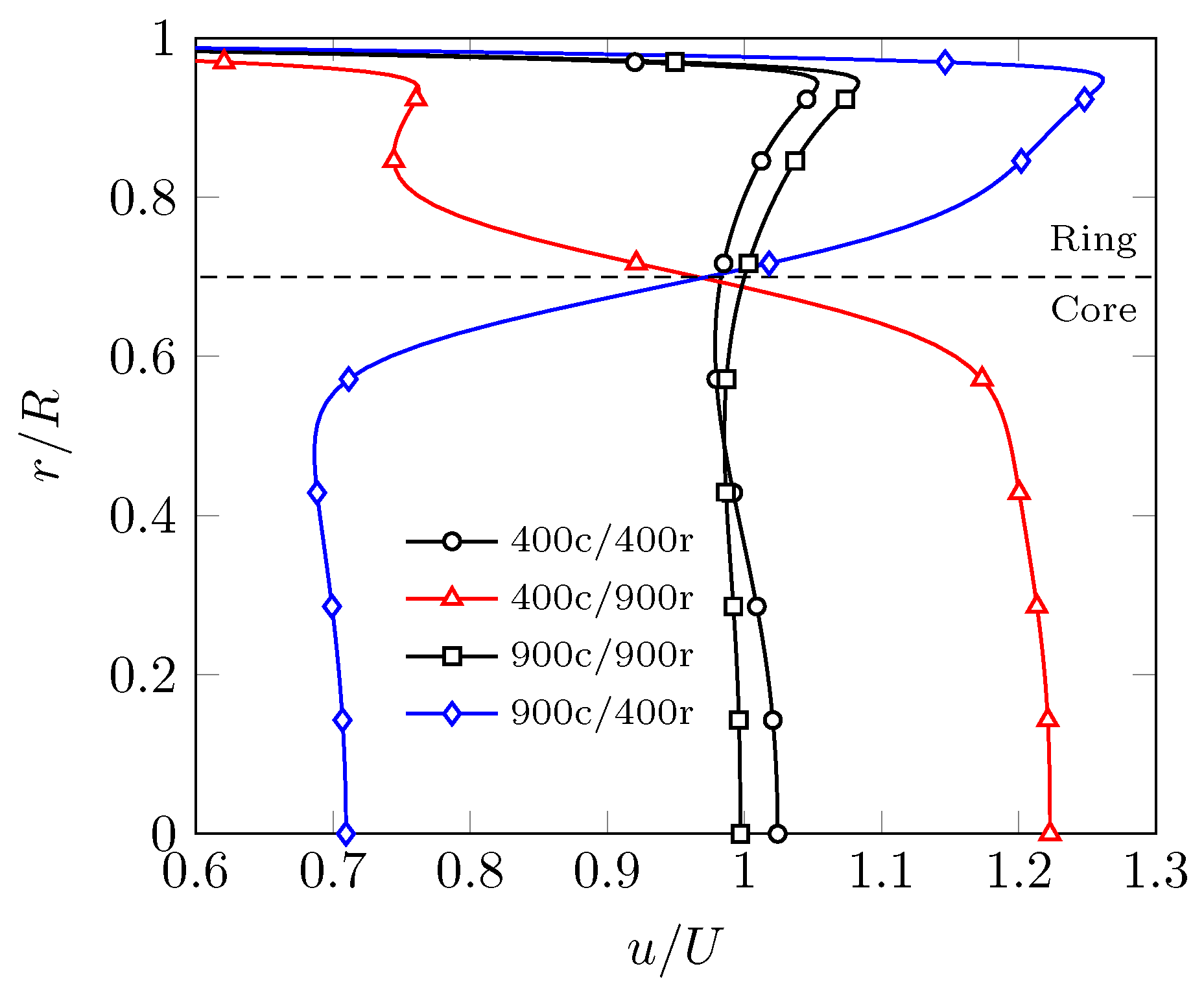
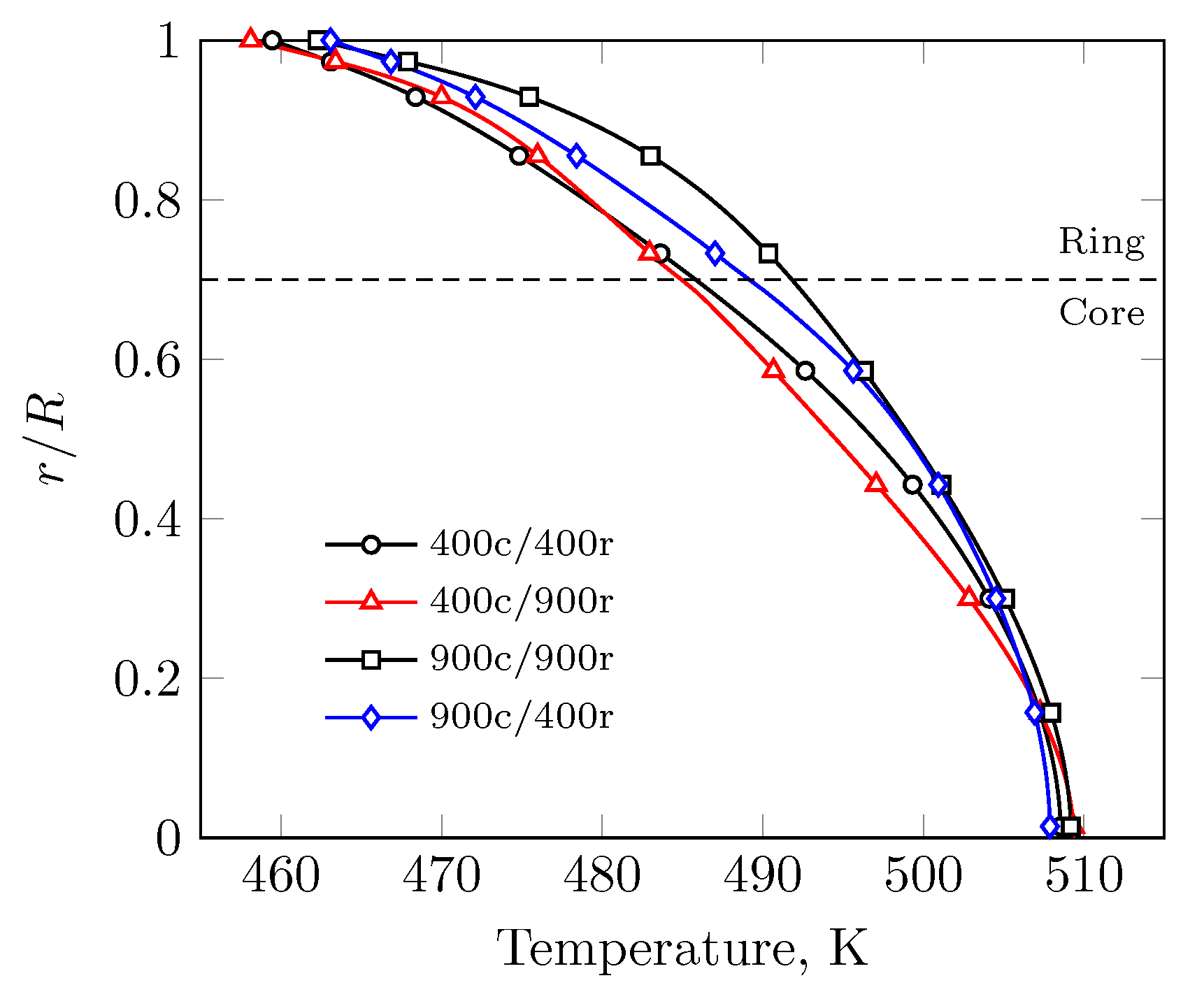
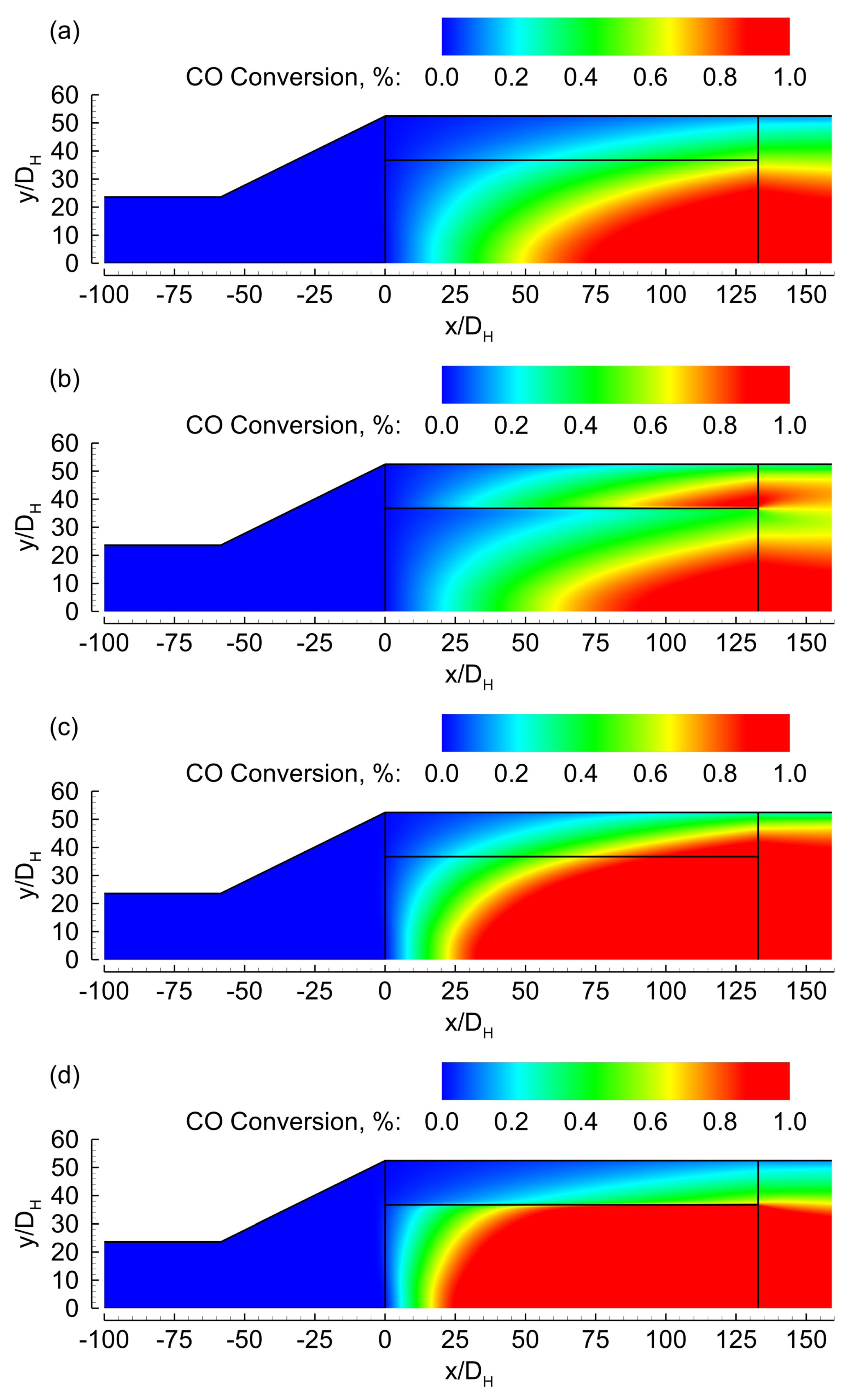
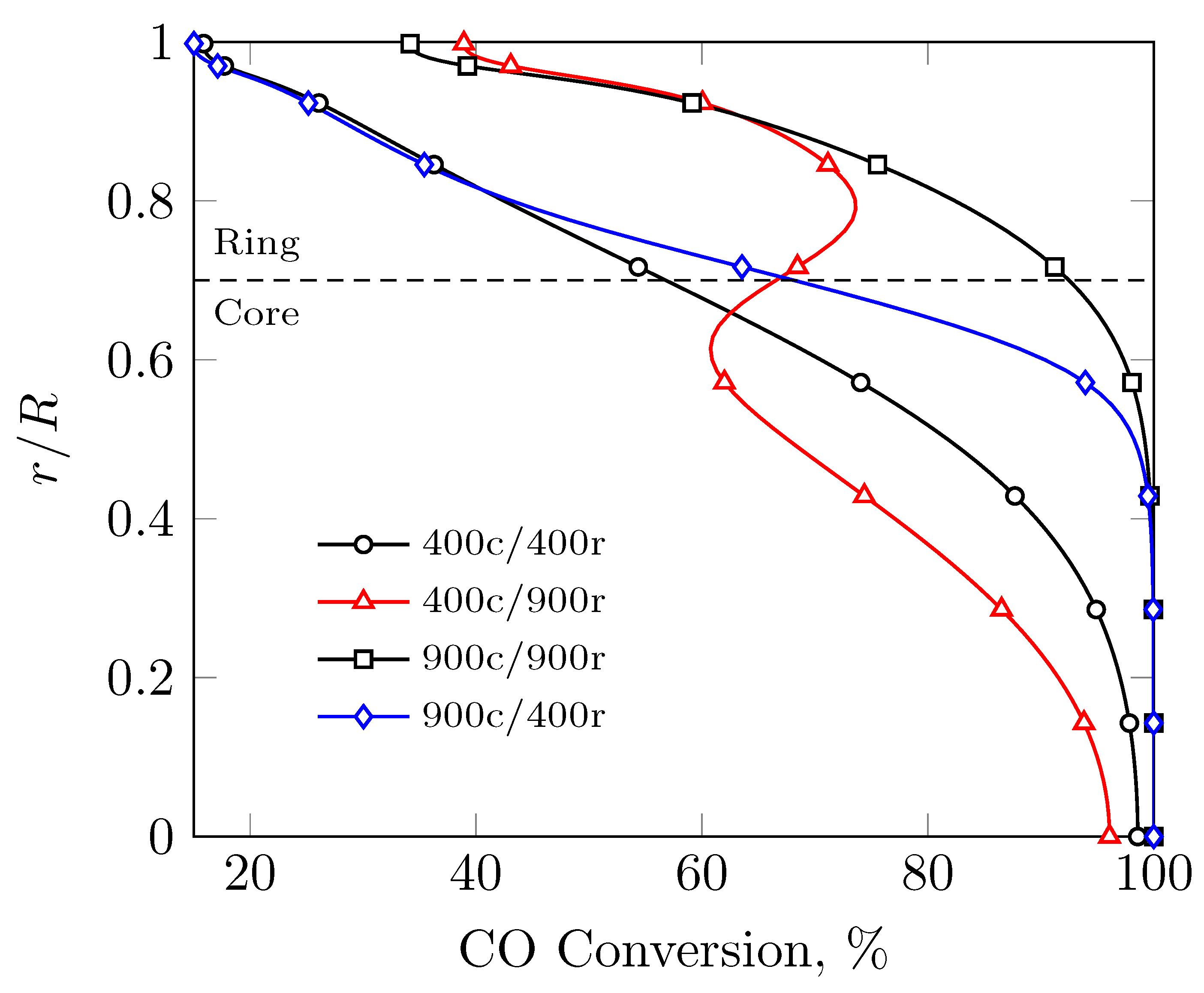
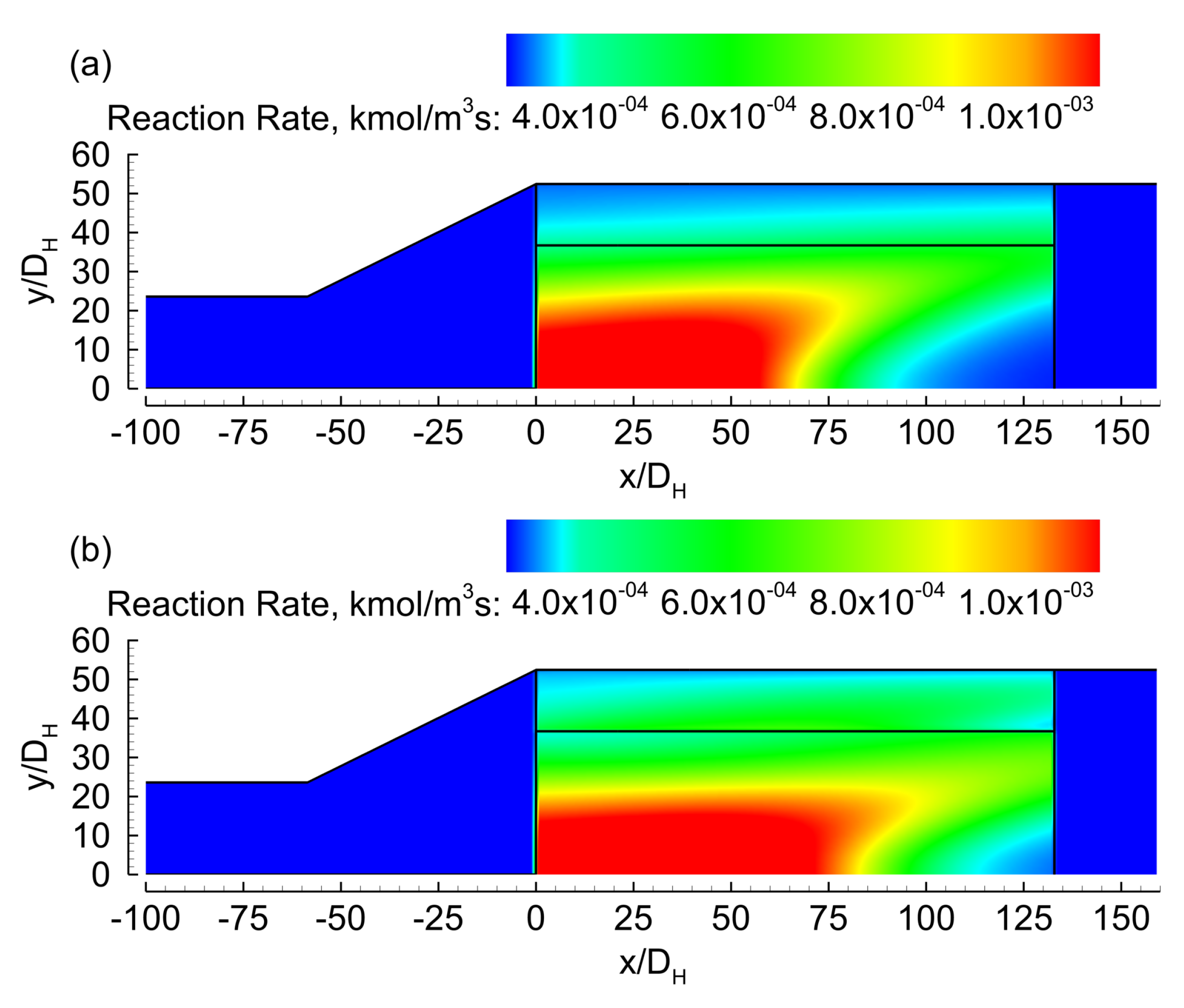
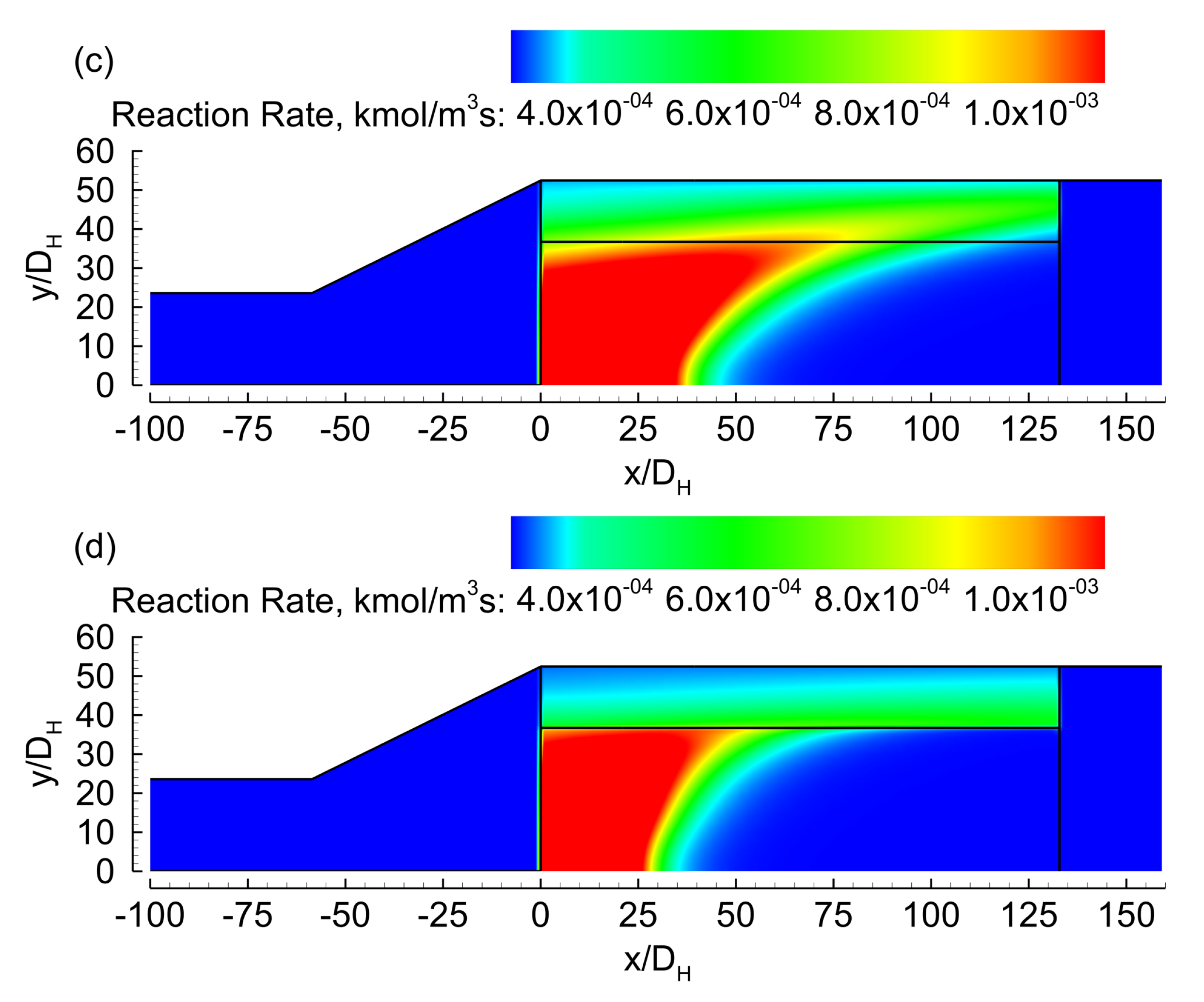
3. Results
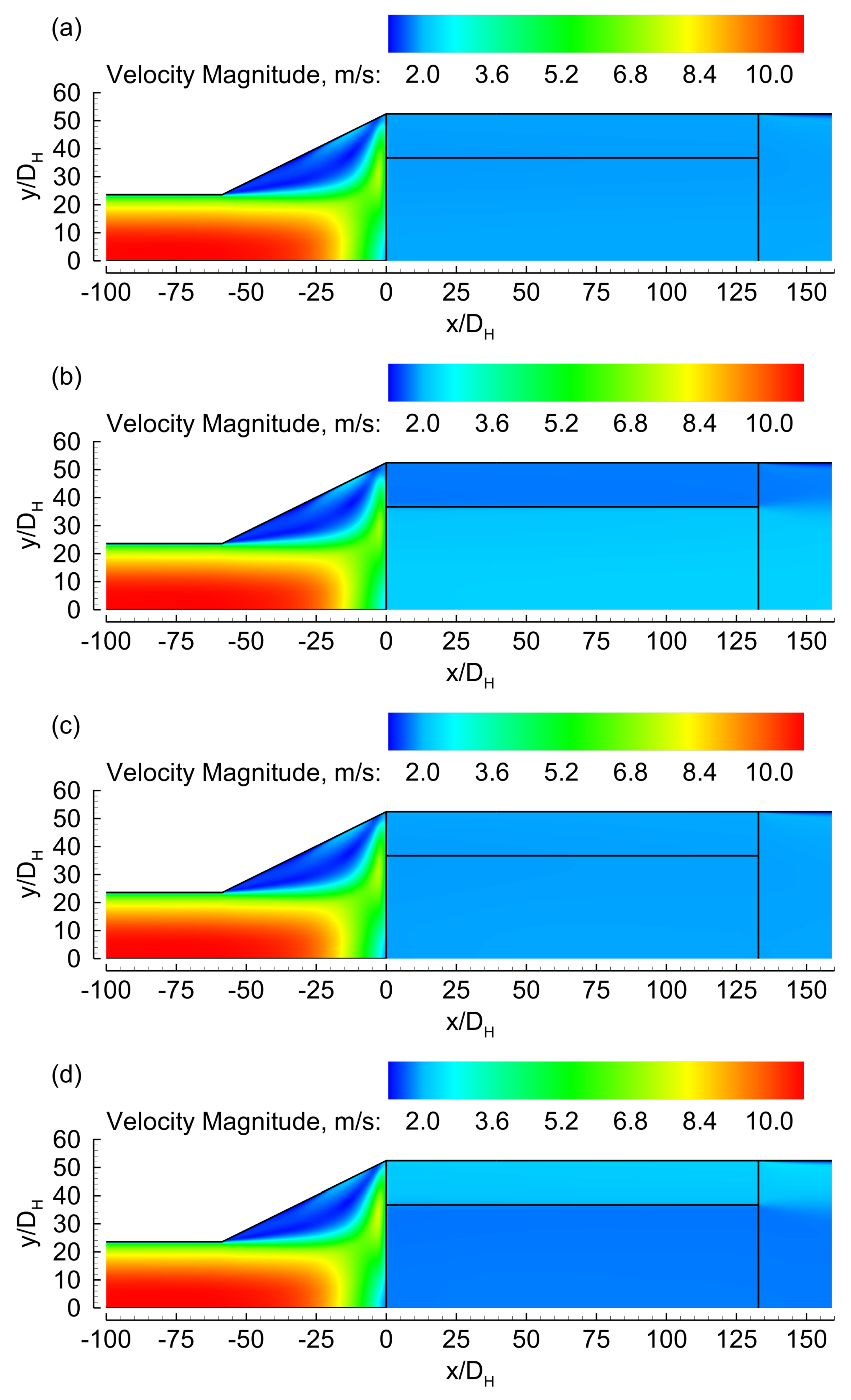
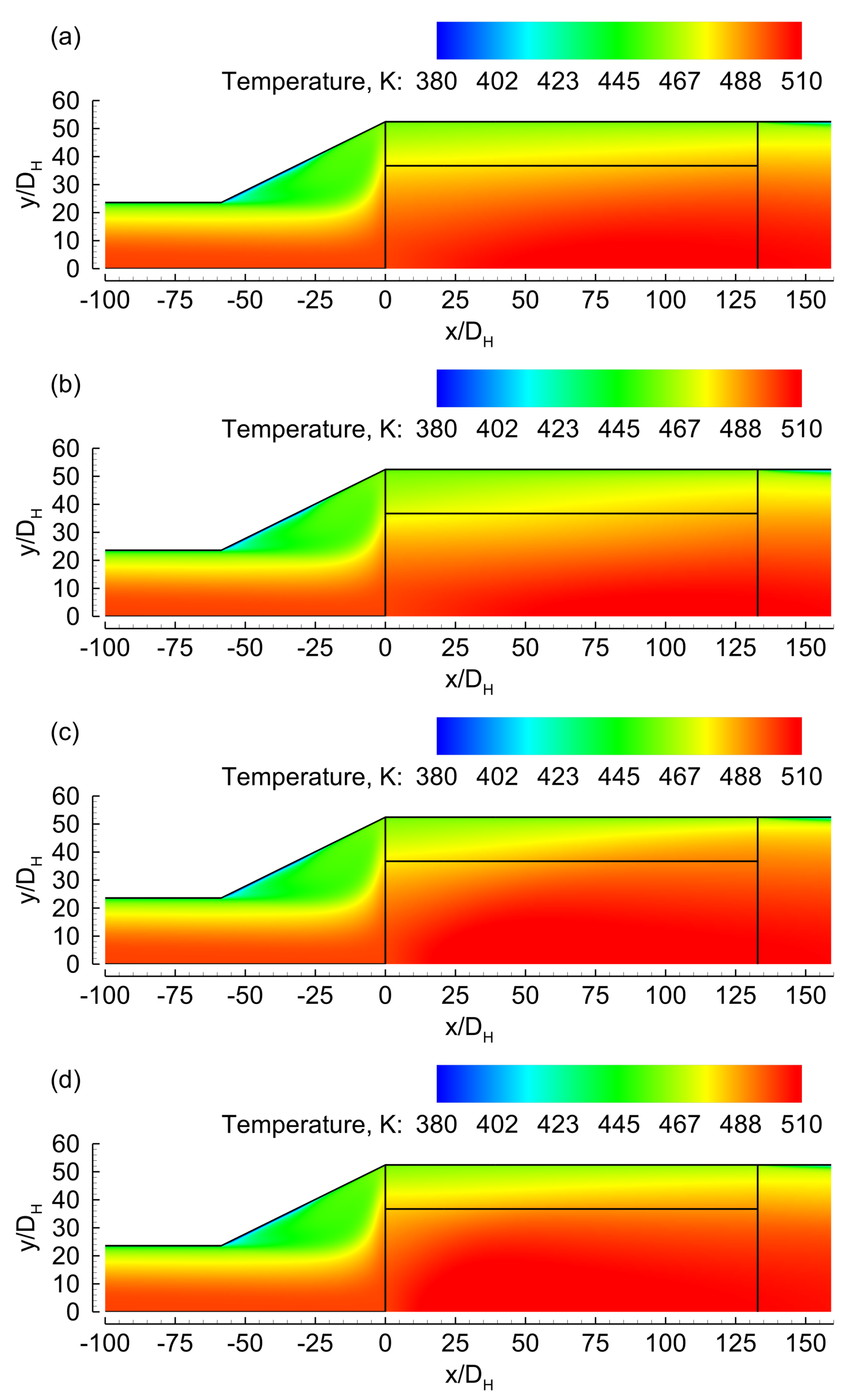
3.1. Evenly Distributed Dual Cell Density
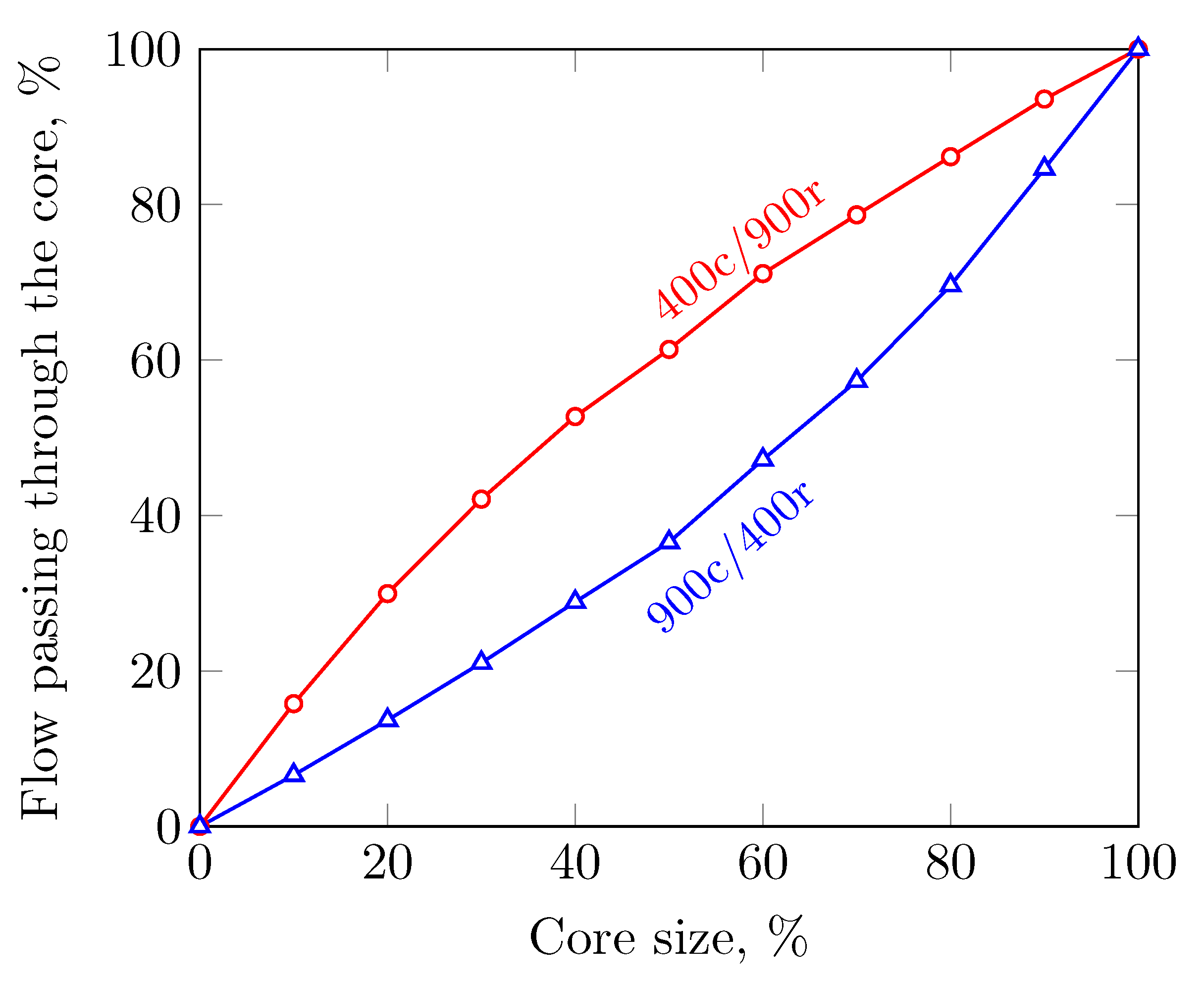
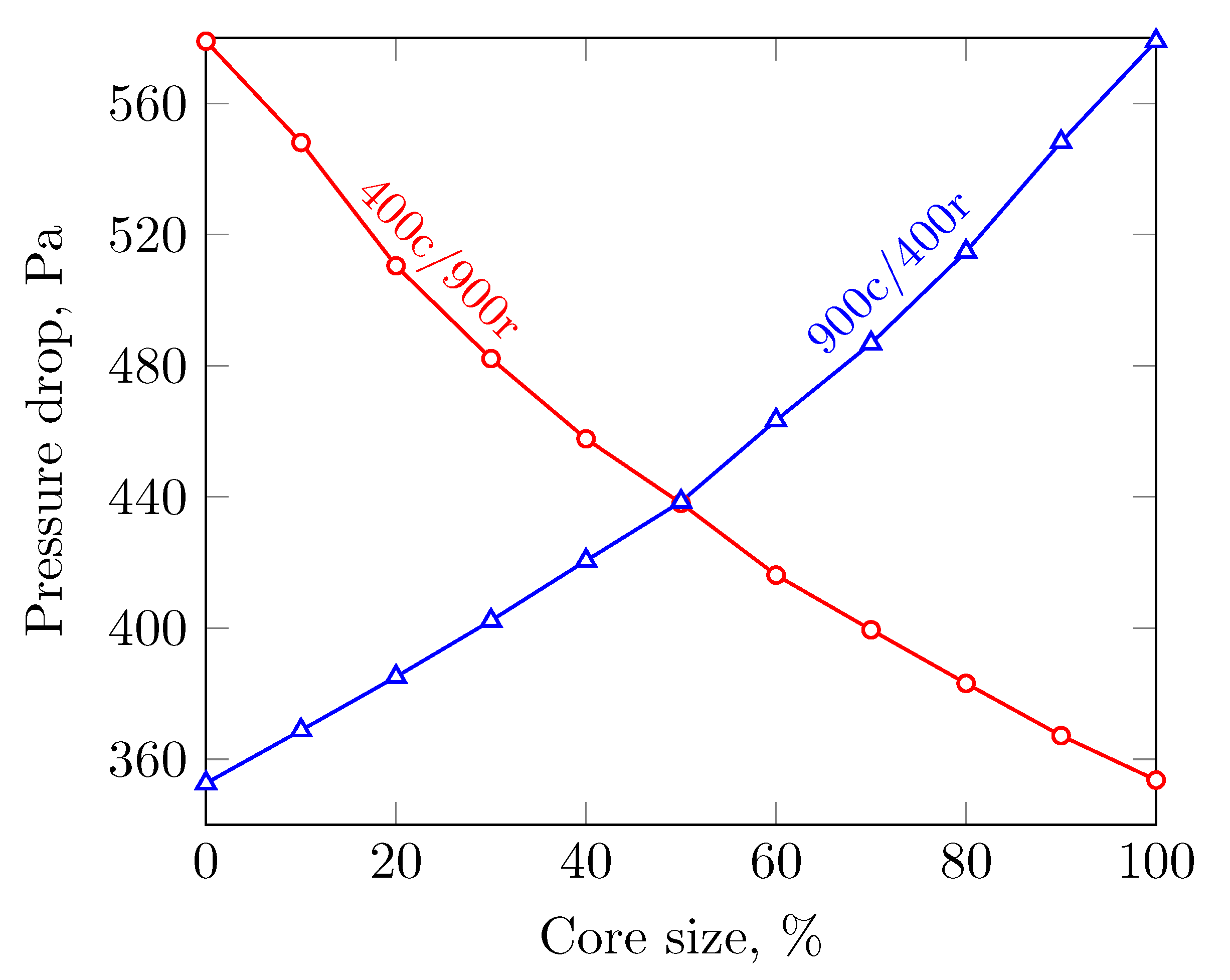
3.2. Sensitivity to the Cell Density Distribution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Specific heat capacity, J/kg-K | |
| Channel hydraulic diameter, m | |
| Characteristic pore diameter, m | |
| Gz | Graetz number |
| Thermal conductivity of porous cordierite, W/mol-K | |
| Thermal conductivity vector, W/mol-K | |
| Pseudo-first-order reaction rate constant, 1/s | |
| Washcoat characteristic length, m | |
| Monolith wall thickness, m | |
| p | Pressure, Pa |
| Pr | Prandlt number |
| R | Ideal gas constant, J/mol-K |
| Re | Reynolds number |
| Sc | Schimdt number |
| External surface to volume ratio of the substrate, 1/m | |
| T | Temperature, K |
| u | Velocity Magnitude, m/s |
| Velocity vector, m/s | |
| Channel velocity, m/s | |
| Apparent permeability vector, m | |
| Washcoat porosity | |
| Effectiveness factor | |
| k | Turbulence kinetic energy, m/s |
| Turbulence specific dissipation rate, 1/s | |
| Substrate void fraction | |
| Thiele modulus | |
| Density, kg/m | |
| Washcoat tortuosity | |
| Molecular viscosity, Pa-s | |
| Turbulence viscosity, Pa-s |
References
- Gundlapally, S.R.; Balakotaiah, V. Analysis of the effect of substrate material on the steady-state and transient performance of monolith reactors. Chem. Eng. Sci. 2013, 92, 198–210. [Google Scholar] [CrossRef]
- Arab, S.; Commenge, J.M.; Portha, J.F.; Falk, L. Methanol synthesis from CO2 and H2 in multi-tubular fixed-bed reactor and multi-tubular reactor filled with monoliths. Chem. Eng. Res. Des. 2014, 92, 2598–2608. [Google Scholar] [CrossRef]
- Negri, M.; Wilhelm, M.; Hendrich, C.; Wingborg, N.; Gediminas, L.; Adelöw, L.; Maleix, C.; Chabernaud, P.; Brahmi, R.; Beauchet, R.; et al. New technologies for ammonium dinitramide based monopropellant thrusters–The project RHEFORM. Acta Astronaut. 2018, 143, 105–117. [Google Scholar] [CrossRef]
- De Lathouder, K.; Smeltink, M.; Straathof, A.; Paasman, M.; Van de Sandt, E.; Kapteijn, F.; Moulijn, J. Hydrogel coated monoliths for enzymatic hydrolysis of penicillin G. J. Ind. Microbiol. Biotechnol. 2008, 35, 815–824. [Google Scholar] [CrossRef] [Green Version]
- Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A. Shouldn’t catalysts shape up?: Structured reactors in general and gas–liquid monolith reactors in particular. Catal. Today 2006, 111, 111–118. [Google Scholar] [CrossRef]
- Cornejo, I.; Hayes, R.E. A Review of the Critical Aspects in the Multi-Scale Modelling of Structured Catalytic Reactors. Catalysts 2021, 11, 89. [Google Scholar] [CrossRef]
- Quintanilla, A.; Casas, J.; Miranzo, P.; Osendi, M.I.; Belmonte, M. 3D-Printed Fe-doped silicon carbide monolithic catalysts for wet peroxide oxidation processes. Appl. Catal. B Environ. 2018, 235, 246–255. [Google Scholar] [CrossRef]
- Hayes, R.E.; Kolaczkowski, S.T. Mass and heat transfer effects in catalytic monolith reactors. Chem. Eng. Sci. 1994, 49, 3587–3599. [Google Scholar] [CrossRef]
- Shi-Jin, S.; Jian-Xin, W.; Ren-Jun, Z.; Jun-Rui, C. Study on Flow Characteristics of Automotive Catalytic Converters with Various Configurations; SAE Technical Paper; Report Number 2000-01-0208; SAE: Warrendale, PA, USA, 2000. [Google Scholar]
- Tsinoglou, D.N.; Koltsakis, G.C.; Missirlis, D.K.; Yakinthos, K.J. Transient modelling of flow distribution in automotive catalytic converters. Appl. Math. Model. 2004, 28, 775–794. [Google Scholar] [CrossRef]
- Martin, A.; Will, N.; Bordet, A.; Cornet, P.; Gondoin, C.; Mouton, X. Effect of flow distribution on emissions performance of catalytic converters. SAE Trans. 1998, 107, 384–390. [Google Scholar]
- Windmann, J.; Braun, J.; Zacke, P.; Tischer, S.; Deutschmann, O.; Warnatz, J. Impact of the inlet flow distribution on the light-off behavior of a 3-way catalytic converter. SAE Trans. 2003, 112, 713–723. [Google Scholar]
- Zygourakis, K. Transient operation of monolith catalytic converters: A two-dimensional reactor model and the effects of radially nonuniform flow distributions. Chem. Eng. Sci. 1989, 44, 2075–2086. [Google Scholar] [CrossRef]
- Campbell, B.; Finch, A.; Tancell, P.; Hitchings, A.; Marsh, M.; Lloyd-Thomas, D.; Gregory, D. Effect of Catalyst Inlet Cone Flow Mal-Distribution on Emissions Performance of a Close-Coupled Catalytic Converter; SAE Technical Paper; Report Number 2004-01-1489; SAE: Warrendale, PA, USA, 2004. [Google Scholar]
- Guojiang, W.; Song, T. CFD simulation of the effect of upstream flow distribution on the light-off performance of a catalytic converter. Energy Convers. Manag. 2005, 46, 2010–2031. [Google Scholar] [CrossRef]
- Heibel, A.; Spaid, M.A. A New Converter Concept Providing Improved Flow Distribution and Space Utilization; SAE Technical Paper; Report Number 1999-01-0768; SAE: Warrendale, PA, USA, 1999. [Google Scholar]
- Wollin, J.; Benjamin, S.F. A Study of the Flow Performance of Ceramic Contoured Substrates for Automotive Exhaust Catalyst Systems; SAE Technical Paper; Report Number 1999-01-3626; SAE: Warrendale, PA, USA, 1999. [Google Scholar]
- Hughes, K.W.; Schmitz, K.M.; Radke, D.A.; Zerafa, K.L.; Light, B. Impact of ultra thinwall catalyst substrates for TIER2 emission standards. SAE Trans. 2003, 112, 282–296. [Google Scholar]
- Gong, J.; Cai, L.; Peng, W.; Liu, J.; Liu, Y.; Cai, H.; Jiaqiang, E. Analysis to the Impact of Monolith Geometric Parameters on Emission Conversion Performance Based on an Improved Three-Way Catalytic Converter Simulation Model; SAE Technical Paper; Report Number 2006-32-0089; SAE: Warrendale, PA, USA, 2006. [Google Scholar]
- Fadic, A.; Nien, T.W.; Mmbaga, J.; Hayes, R.E.; Votsmeier, M. A case study in multi-scale model reduction: The effect of cell density on catalytic converter performance. Can. J. Chem. Eng. 2014, 92, 1607–1617. [Google Scholar] [CrossRef]
- Kim, J.Y.; Son, S. Improving Flow Efficiency of a Catalytic Converter Using the Concept of Radially Variable Cell Density-PART I; SAE Technical Paper; Report Number 1999-01-0769; SAE: Warrendale, PA, USA, 1999. [Google Scholar]
- Hayes, R.E.; Fadic, A.; Mmbaga, J.; Najafi, A. CFD modelling of the automotive catalytic converter. Catal. Today 2012, 188, 94–105. [Google Scholar] [CrossRef]
- Clarkson, R.J. A Theoretical and Experimental Study of Automotive Catalytic Converters. Ph.D. Thesis, Coventry University, Coventry, UK, 1997. [Google Scholar]
- Menter, F. Improved Two-Equation K-ω Turbulence Models for Aerodynamic Flows; NASA Technical Memorandum; ID 19930013620; NASA: Washington, DC, USA, 1992. [Google Scholar]
- ANSYS Fluent User’s Guide Release 2020R2; ANSYS Inc.: Canonsburg, PA, USA, 2020.
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Pressure correction for automotive catalytic converters: A multi-zone permeability approach. Chem. Eng. Res. Des. 2019, 147, 232–243. [Google Scholar] [CrossRef]
- Mesquida, I.M.V.; Cornejo, I.; Nikrityuk, P.; Greiner, R.; Votsmeier, M.; Hayes, R.E. Towards a fully predictive multi-scale pressure drop model for a wall-flow filter. Chem. Eng. Res. Des. 2020, 164, 261–280. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. The influence of channel geometry on the pressure drop in automotive catalytic converters: Model development and validation. Chem. Eng. Sci. 2020, 212, 115317. [Google Scholar] [CrossRef]
- Benjamin, S.F.; Haimad, N.; Roberts, C.A.; Wollin, J. Modelling the flow distribution through automotive catalytic converters. Proc. Inst. Mech. Eng. Part J. Mech. Eng. Sci. 2001, 215, 379–383. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Turbulence decay inside the channels of an automotive catalytic converter monolith. Emiss. Control Sci. Technol. 2017, 3, 302–309. [Google Scholar] [CrossRef]
- Cornejo, I.; Hayes, R.E.; Nikrityuk, P. A new approach for the modeling of turbulent flows in automotive catalytic converters. Chem. Eng. Res. Des. 2018, 140, 308–319. [Google Scholar] [CrossRef]
- Kalitzin, G.; Medic, G.; Iaccarino, G.; Durbin, P. Near-wall behavior of RANS turbulence models and implications for wall functions. J. Comput. Phys. 2005, 204, 265–291. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Lange, C.; Hayes, R.E. Influence of upstream turbulence on the pressure drop inside a monolith. Chem. Eng. Process.-Process. Intensif. 2020, 147, 107735. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Turbulence generation after a monolith in automotive catalytic converters. Chem. Eng. Sci. 2018, 187, 107–116. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Effect of substrate geometry and flow condition on the turbulence generation after a monolith. Can. J. Chem. Eng. 2020, 98, 947–956. [Google Scholar] [CrossRef]
- Voltz, S.E.; Morgan, C.R.; Liederman, D.; Jacob, S.M. Kinetic study of carbon monoxide and propylene oxidation on platinum catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1973, 12, 294–301. [Google Scholar] [CrossRef]
- Hayes, R.; Kolaczkowskib, S.; Li, P.; Awdry, S. Evaluating the effective diffusivity of methane in the washcoat of a honeycomb monolith. Appl. Catal. Environ. 2000, 25, 93–104. [Google Scholar] [CrossRef]
- Lommerts, B.; Graaf, G.; Beenackers, A. Mathematical modeling of internal mass transport limitations in methanol synthesis. Chem. Eng. Sci. 2000, 55, 5589–5598. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Heat and mass transfer inside of a monolith honeycomb: From channel to full size reactor scale. Catal. Today 2020, in press. [Google Scholar] [CrossRef]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. Improved Nu number correlations for gas flow in monolith reactors using temperature-dependent fluid properties. Int. J. Therm. Sci. 2020, 155, 106419. [Google Scholar] [CrossRef]
- Cornejo, I.; Cornejo, G.; Nikrityuk, P.; Hayes, R.E. Entry length convective heat transfer in a monolith: The effect of upstream turbulence. Int. J. Therm. Sci. 2019, 138, 235–246. [Google Scholar] [CrossRef]
- Hayes, R.E.; Rojas, A.; Mmbaga, J. The effective thermal conductivity of monolith honeycomb structures. Catal. Today 2009, 147, S113–S119. [Google Scholar] [CrossRef]
- Weltens, H.; Bressler, H.; Terres, F.; Neumaier, H.; Rammoser, D. Optimisation of Catalytic Converter Gas Flow Distribution by CFD Prediction; SAE Technical Paper; Report Number 930780; SAE: Warrendale, PA, USA, 1993. [Google Scholar]
- Salasc, S.; Barrieu, E.; Leroy, V. Impact of Manifold Design on Flow Distribution of a Close-Coupled Catalytic Converter; SAE Technical Paper; 2005-01-1626; SAE: Warrendale, PA, USA, 2005. [Google Scholar]
- Dammalapati, S.; Aghalayam, P.; Kaisare, N. Modeling the Effects of the Inlet Manifold Design on the Performance of a Diesel Oxidation Catalytic Converter. Ind. Eng. Chem. Res. 2021, 60, 3860–3870. [Google Scholar]
- Portha, J.F.; Parkhomenko, K.; Kobl, K.; Roger, A.C.; Arab, S.; Commenge, J.M.; Falk, L. Kinetics of methanol synthesis from carbon dioxide hydrogenation over copper–zinc oxide catalysts. Ind. Eng. Chem. Res. 2017, 56, 13133–13145. [Google Scholar] [CrossRef]
- Salamon, E.; Cornejo, I.; Mmbaga, J.P.; Kołodziej, A.; Lojewska, J.; Hayes, R.E. Investigations of a three channel autogenous reactor for lean methane combustion. Chem. Eng. Process.-Process. Intensif. 2020, 153, 107956. [Google Scholar] [CrossRef]
| 400/6.5 | 900/2.5 | |
|---|---|---|
| , m | 17933 | 28607 |
| 0.637 | 0.736 | |
| , m/m | 0.513 | 0.481 |
| , m | 55 | 37 |
| , mm | 1.144 | 0.819 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornejo, I.; Garreton, G.; Hayes, R.E. On the Use of Dual Cell Density Monoliths. Catalysts 2021, 11, 1075. https://doi.org/10.3390/catal11091075
Cornejo I, Garreton G, Hayes RE. On the Use of Dual Cell Density Monoliths. Catalysts. 2021; 11(9):1075. https://doi.org/10.3390/catal11091075
Chicago/Turabian StyleCornejo, Ivan, Gonzalo Garreton, and Robert E. Hayes. 2021. "On the Use of Dual Cell Density Monoliths" Catalysts 11, no. 9: 1075. https://doi.org/10.3390/catal11091075
APA StyleCornejo, I., Garreton, G., & Hayes, R. E. (2021). On the Use of Dual Cell Density Monoliths. Catalysts, 11(9), 1075. https://doi.org/10.3390/catal11091075








