Abstract
Pt/γ-Al2O3 catalysts coated on honeycomb-shaped stainless STS-444 steel substrates with a γ-Al2O3 intermediate layer were prepared using a conventional washcoating method. The intermediate layer was formed on the substrate surface through oxidation using pack cementation. The monolithic catalysts with the intermediate layer were fabricated for potential applications to pre-turbocharger catalysts, which suffer from severe conditions such as vibrations of the engine and high flow rates of exhaust gas. Adhesive strength tests and simultaneous oxidation reactions of CO and C3H6 were carried out for the Pt/γ-Al2O3 monolithic catalysts with and without the intermediate layer. The catalysts with an intermediate layer showed much stronger adhesion than the catalysts without an intermediate layer. Thus, the formation of a γ-Al2O3 intermediate layer by surface oxidation through pack cementation facilitated a significant enhancement of the catalyst adhesion strength without catalytic performance degradation.
1. Introduction
Hazardous gas pollutants, such as carbon monoxide (CO), hydrocarbons (HCs), and nitrogen oxides (NOx), emitted from gasoline and diesel engines have caused severe environmental problems [1]. Several types of exhaust gas after treatment technology to reduce the pollution have been developed, including three-way catalysts, diesel oxidation catalysts, diesel particulate filters, and selective catalytic reduction catalysts [2]. One efficient method to reduce the CO and HCs emissions is to place a small catalyst upstream of the engine’s turbocharger as a pre-turbocharger catalyst, PTC [2,3]. CO and HCs could be more effectively reduced by an oxidizing reaction and additional heat from the oxidation on the PTC, especially during cold start.
Metallic substrates have many advantages, such as higher structural rigidity, higher mechanical durability, and lower back pressure, so they are more appropriate as PTC than ceramic substrates [4,5,6]. The adhesive strength of the catalyst to the metallic substrates on PTC, however, might not be strong enough to be durable under very fast flow of the exhaust gas and vibration between engine and turbocharger, resulting in catalyst detachment problems. In particular, the coating layer of metal oxide-based catalysts on metallic substrates can be easily detached because the bonding property between metallic and ceramic materials is fundamentally poor [7,8]. To solve the problem, an intermediate layer with strong adhesion to both the metallic substrate and catalyst layer is adapted to reduce detachment of the catalyst on PTC. There have been several reports on the effect of intermediate layers, such as Al2O3 [9,10], mordenite framework inverted zeolite [11], carbon pencil [12], TiO2 [13], and gel coating [14], to enhance adhesion.
A pack cementation method was employed to form an Al2O3 intermediate layer suitable for the alumina-based catalyst layer. The gas diffusion method is a very simple and cost-effective process using STS-444 substrates and embedded powders, which undergo sublimation during heating under argon atmosphere. Thus, pack cementation has low equipment dependency and confers high bond strength to the coated layer [15]. When aluminum powder as a precursor was selected, the substrate surface was aluminized due to thermal reactions between the sublimated aluminum gas and substrates [16]. It was reported that an Fe2Al5 layer is formed on a stainless steel substrate when the substrate is coated with aluminum. Furthermore, the coated Fe2Al5 layer can act as a precursor for the Al2O3 formation when the coated substrate is oxidized at a high temperature in air [16].
In this study, we developed Pt/γ-Al2O3 catalyst with the γ-Al2O3 intermediate layer with strong adhesion through the oxidation of Fe2Al5 on the STS-444 substrate. The microstructure of the Pt/γ-Al2O3 catalyst coating layer was characterized through field emission-scanning electron microscopy (FE-SEM), energy-dispersive X-ray spectroscopy (EDS), and X-ray diffraction (XRD) analyses. The adhesion strengths of Pt/γ-Al2O3 catalysts on the STS-444 substrate were compared with and without an intermediate layer. CO and C3H6 oxidation reaction tests were performed using Pt/γ-Al2O3 catalysts on STS-444 substrates before and after adhesion tests to evaluate their catalytic activities and the effects of the intermediate layer. The reactions were tested under very high gas hourly space velocity (GHSV) conditions mimicking the PTC circumstances.
2. Results and Discussion
2.1. Characterization of γ-Al2O3 Intermediate Layer and Pt/γ-Al2O3 Catalyst
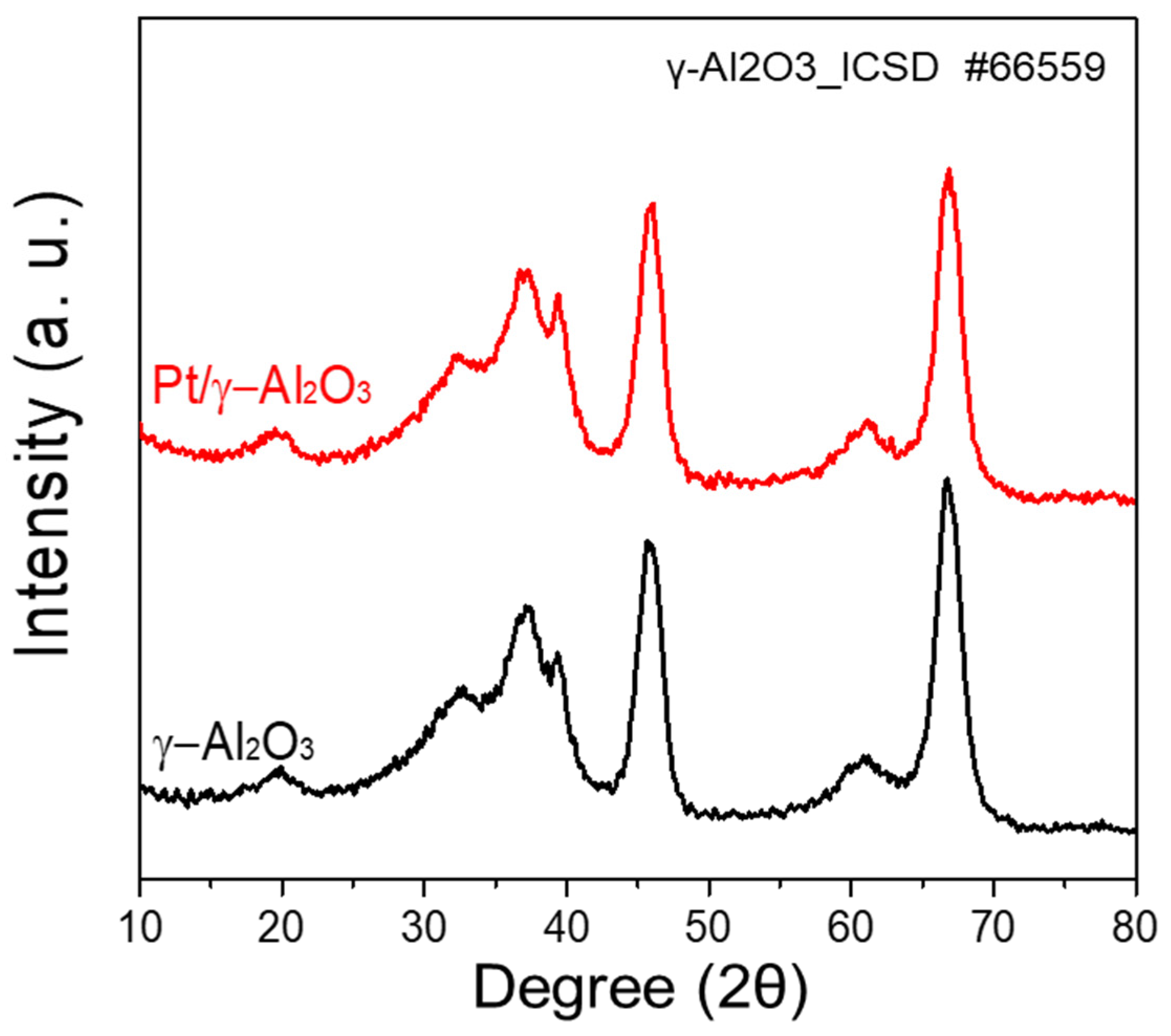
The XRD patterns of the γ-Al2O3 and Pt/γ-Al2O3 powders are shown in Figure 1. γ-Al2O3 showed diffraction peaks located at 2θ = 19.7°, 32.5°, 37.1°, 39.4°, 45.9°, 61.1°, and 66.8°, which correspond to the (111), (220), (311), (222), (400), (511), and (440) lattice planes of γ-Al2O3 (ICSD-66559), respectively [17,18]. However, no significant difference was observed in the XRD patterns of γ-Al2O3 and Pt/γ-Al2O3. In the XRD pattern of Pt/γ-Al2O3, the Pt peak could not be confirmed because the Pt content was as low as 2 wt% in the mixture, and nano-sized Pt particles were well dispersed on γ-Al2O3 [19,20]. However, the Pt content of 1.93 wt% was confirmed through inductively coupled plasma-optical emission spectrometry (ICP-OES) of Pt/γ-Al2O3. These results show that γ-Al2O3 and Pt are homogeneously dispersed.
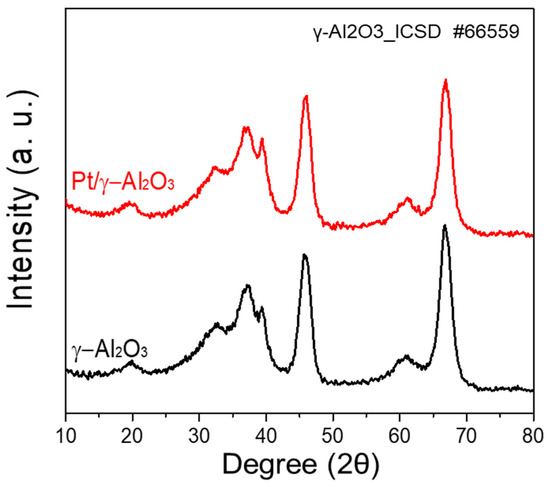
Figure 1.
XRD patterns of γ-Al2O3 and Pt/γ-Al2O3 powders.
2.2. Morphology of γ-Al2O3 Intermediate Layer on the STS-444 Substrate
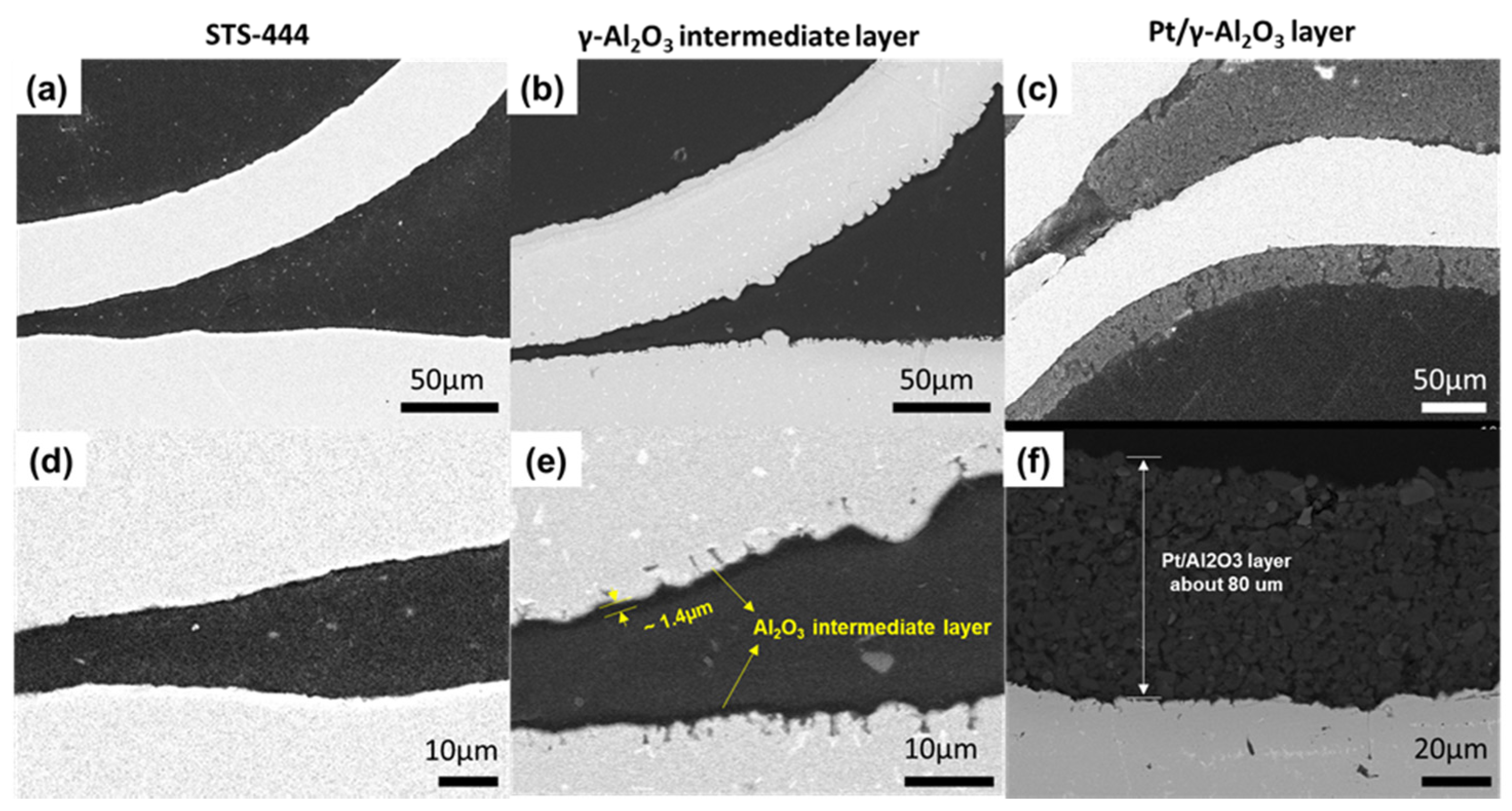
Cross-section images of the STS-444 substrate, γ-Al2O3 intermediate layer, and Pt/γ-Al2O3 catalyst was observed using FE-SEM and EDS analyses. Figure 2a,d show smooth surfaces without any cracks on the STS-444 substrate. When the γ-Al2O3 intermediate layer was coated onto the STS-444 surface, the substrate surface becomes rough, as shown in Figure 2b. The thickness of the γ-Al2O3 intermediate layer was around 1.4 μm (Figure 2e). Figure 2c,f shows 80 μm thick Pt/γ-Al2O3 catalyst coating adhered well to the intermediate layer.
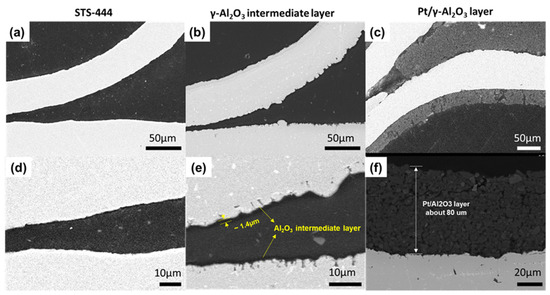
Figure 2.
SEM images of the bare STS-444 substrate (a,d), γ-Al2O3 intermediate layer (b,e), and Pt/Al2O3 catalyst layer (c,f).
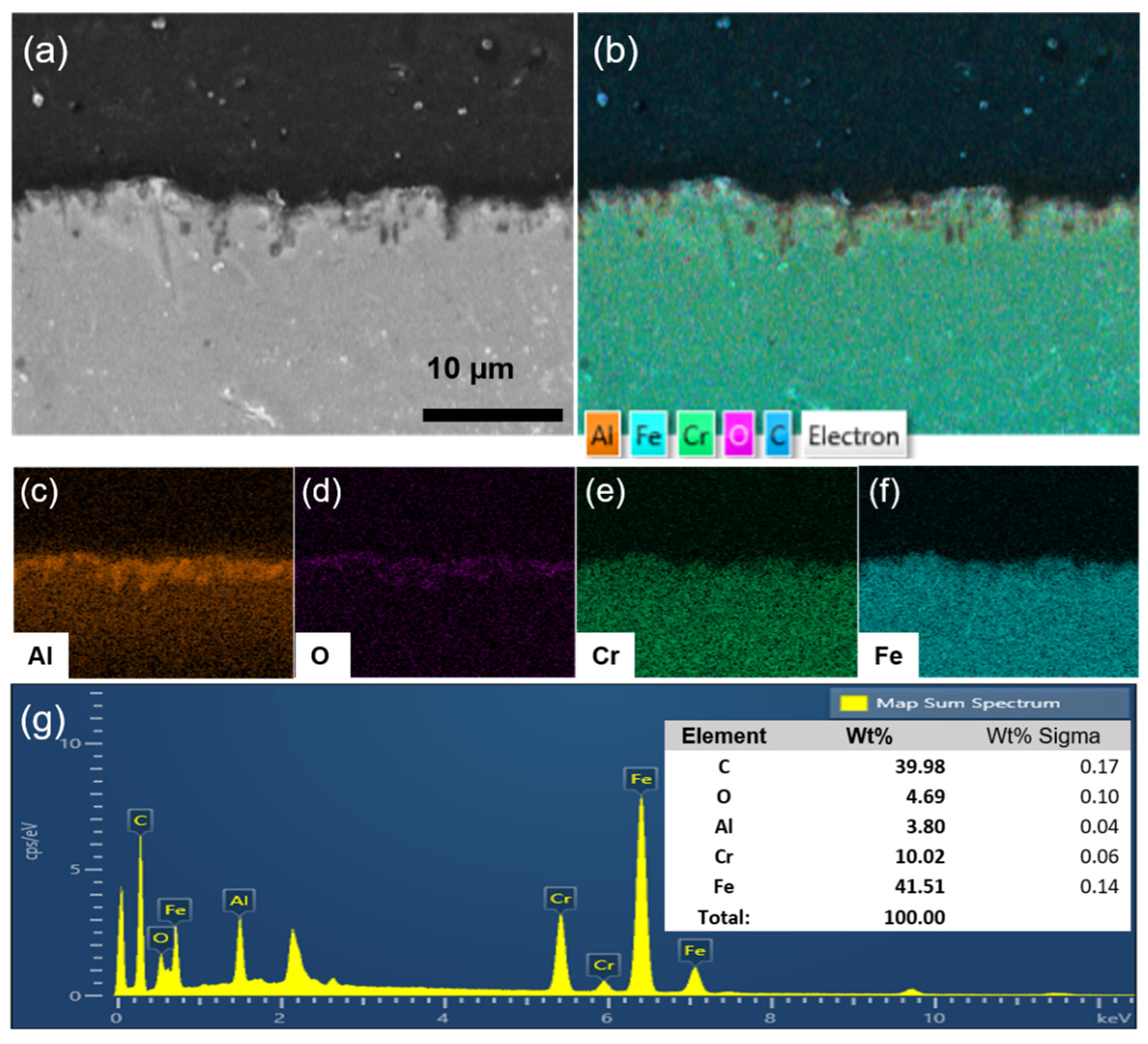
To investigate the elemental composition of the γ-Al2O3 intermediate layer on the STS-444 substrate, elemental mapping was conducted; the mapping profiles are shown in Figure 3a–f. Cr and Fe were uniformly found on the STS-444 surface, as shown in Figure 3e,f. In addition, Al and O show a high density on the surface and a low density on the substrate, demonstrating the formation of the γ-Al2O3 intermediate layer on the STS-444 substrate (Figure 3c,d). Therefore, the mapping result of γ-Al2O3/STS-444 indicates that the γ-Al2O3 intermediate layer was successfully formed on the STS-444 substrate. The EDS spectra shown in Figure 3g confirm the elemental composition of γ-Al2O3/STS-444, wherein the contents of C, O, Al, Cr, and Fe were 39.98, 4.69, 3.80, 10.02, and 41.51 wt%, respectively.
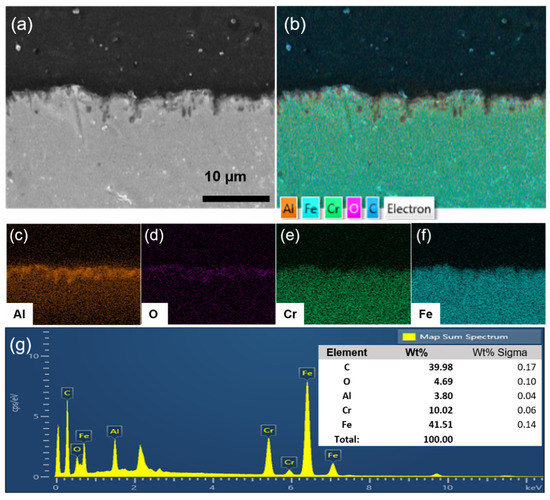
Figure 3.
SEM images of the bare γ-Al2O3 intermediate layer on the STS-444 substrate (a). Elemental mapping profiles of γ-Al2O3 intermediate layer bearing STS-444 substrate (b) and each element (c–f). (g) EDS spectra of the γ-Al2O3 intermediate layer on the STS-444 substrate.
2.3. Adhesion Strength of Washcoated Pt/γ-Al2O3 Catalyst Layer
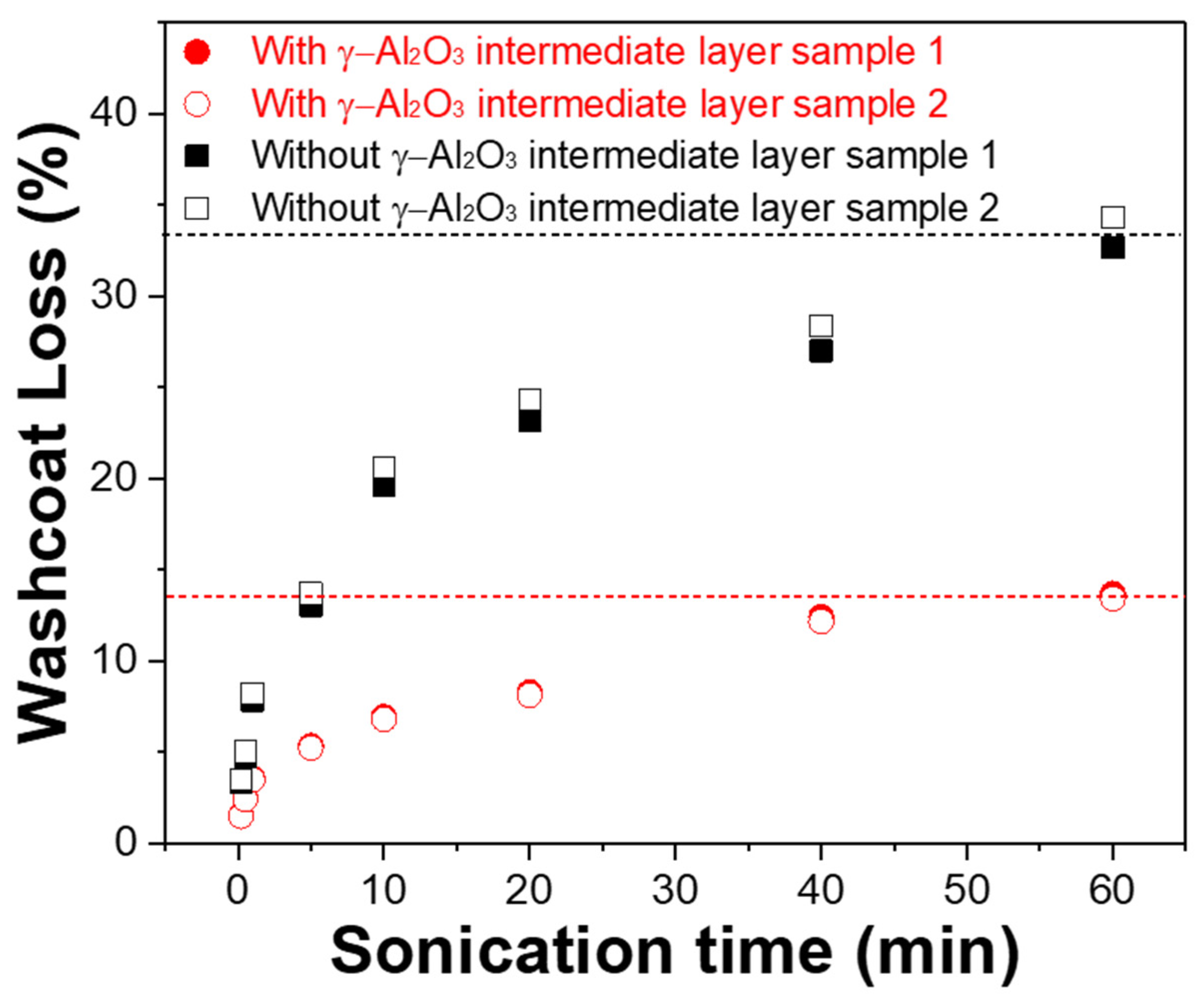
Figure 4 shows the weight loss of the Pt/γ-Al2O3 catalyst coating layer on STS-444 with and without the γ-Al2O3 intermediate layer during the adhesion tests to evaluate the effects of the intermediate layer on the adhesive strength of the catalyst layers coated on metallic substrates. The adhesion strength of the γ-Al2O3/STS-444 sample was much higher than that of the conventional STS-444 monolithic sample. After the 60-min adhesion test, only 13% of the catalyst coating layer was lost for the sample with the γ-Al2O3 intermediate layer, whereas 33% of the coating layer was detached for the sample without the intermediate layer. This result indicates that the γ-Al2O3 intermediate layer formed by surface oxidation through pack cementation drastically enhances the adhesion between the Pt/γ-Al2O3 catalyst coating layer and the metallic substrate.
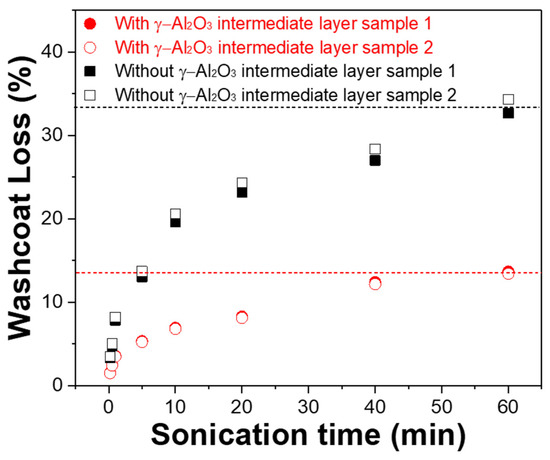
Figure 4.
Washcoat loss of Pt/γ-Al2O3 catalyst with and without the γ-Al2O3 intermediate layer on STS-444 substrate.
2.4. CO and C3H6 Oxidation over Pt/γ-Al2O3 Monolithic Catalysts with and without γ-Al2O3 Intermediate Layer
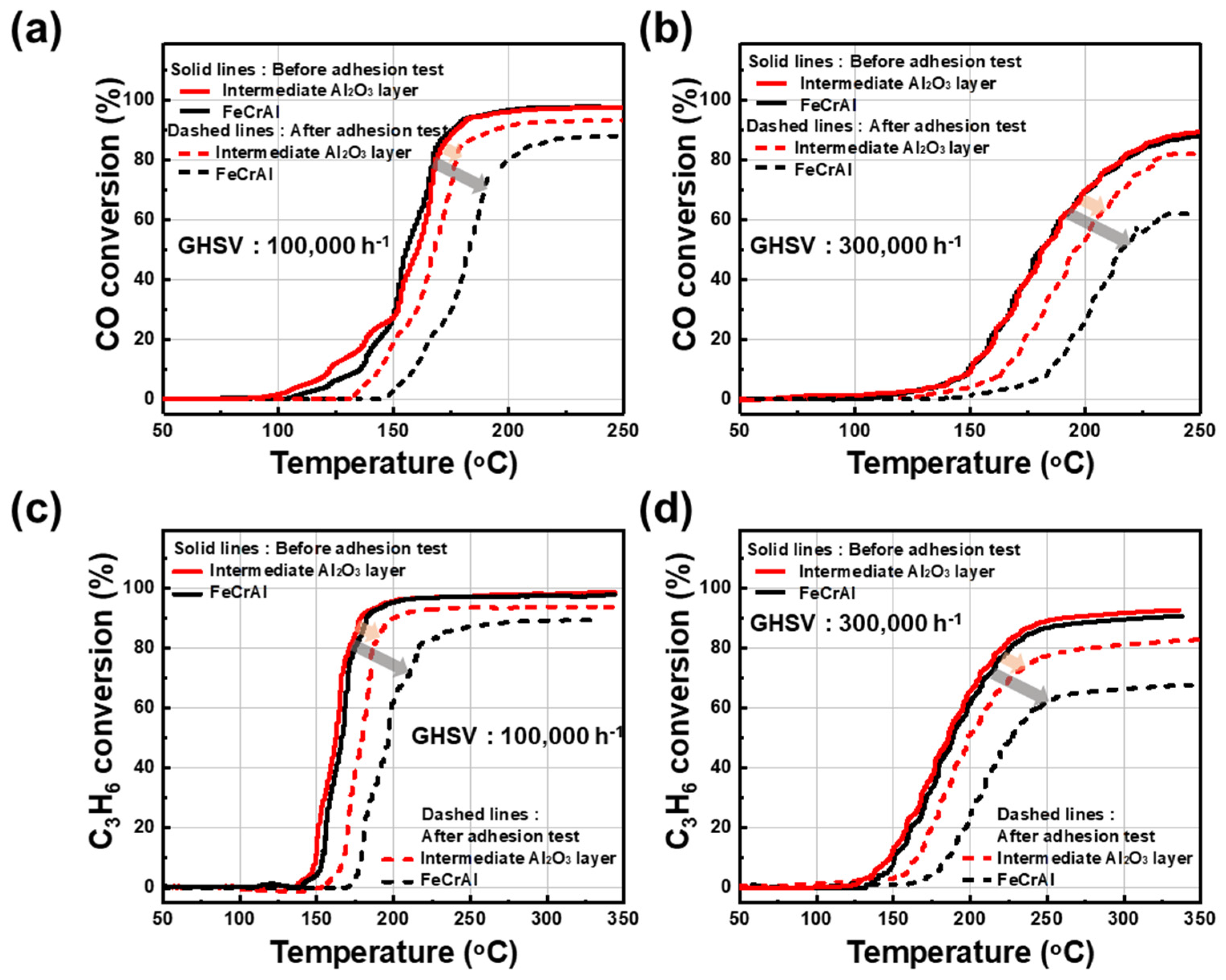
Figure 5 shows the light-off activities of Pt/γ-Al2O3 with and without an intermediate layer for CO and C3H6 oxidations under very high GHSV conditions of 100,000 and 300,000 h−1. The catalytic activities of Pt/γ-Al2O3 samples were compared before and after the adhesive strength tests. Figure 5a shows CO conversion over the catalysts with and without the intermediate layer under the GHSV of 100,000 h−1. First, both samples (STS-444 metallic substrate in the presence and absence of the γ-Al2O3 intermediate layer) showed a stable conversion graph with 80% conversion at approximately 160 °C and 90% conversion at around 170 °C. However, after the adhesion test, the intermediate layer sample showed a more stable conversion ratio than that without the intermediate layer. The conversion ratios of 80% and 90% were obtained at approximately 170 °C and 200 °C for the catalyst with the intermediate layer, respectively, but were 80% and 90% at 210 °C and 250 °C for the catalyst without the intermediate layer, respectively. As shown in Figure 5b, the CO conversion test was performed at 300,000 h−1 for both samples. Both catalyst samples presented similar CO conversion rates of 80% and 90% at 230 and 240 °C, respectively, before the adhesion test. In contrast, after the adhesion test, 80% conversion was observed for the catalyst with the intermediate layer, but the sample without an intermediate layer did not even reach 70% conversion up to 250 °C. The absence of an intermediate layer induces a very weak catalytic effect toward CO conversion under these conditions. Furthermore, Figure 5c shows the C3H6 conversion for both samples under the GHSV condition of 100,000 h−1. The conversion temperatures of the samples with and without the intermediate layer were similar at 165 °C (80%) and 175 °C (90%) before the adhesion test. However, the catalyst with the intermediate layer exhibited conversion temperatures of approximately 175 °C (80%) and 200 °C (90%) after the adhesive strength test, whereas the sample without an intermediate layer showed conversion temperatures of 200 °C (80%) and 250 °C (85%). Figure 5d shows the C3H6 conversion at 300,000 h−1 for both samples. Both presented the same C3H6 conversion temperature at 220 °C (80%) and 250 °C (90%) before the adhesion test. However, after the adhesion test, the conversion temperature of the sample with the intermediate layer was at 250 °C (80%). The samples without an intermediate layer did not even reach 70% conversion up to 350 °C.
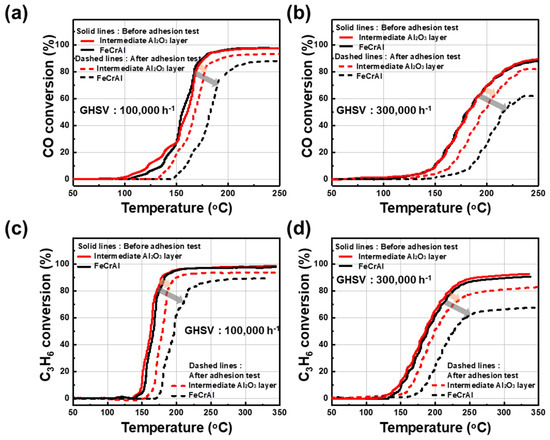
Figure 5.
CO (a,b) and C3H6 (c,d) oxidation performance of the catalysts with and without the γ-Al2O3 intermediate layer on the STS-444 substrate under two GHSV (100,000 and 300,000 h−1) conditions.
As a result of these tests, both the gas conversion performance of the Pt/γ-Al2O3 catalysts without the γ-Al2O3 intermediate layer was severely degraded after the adhesive strength test because a significant amount of the catalyst coating layer was detached from the STS-444 substrate. However, the Pt/γ-Al2O3 catalysts with the γ-Al2O3 intermediate layer showed excellent CO and C3H6 oxidation activity even after the adhesive strength test. Thus, the intermediate layer formation on the metallic substrate greatly enhances the adhesive strength and CO/C3H6 oxidation performance of the metallic monolithic catalysts, which, in turn, can considerably strengthen the long-term mechanical durability of the catalysts.
3. Materials and Methods
3.1. Formation of γ-Al2O3 Intermediate Layer on the STS-444 Substrate
The honeycomb-shaped stainless STS-444 steel substrates used in this study are cylindrical with a diameter of 2.54 cm and a height of 2.54 cm. To coat the substrate with aluminum, mixed powders of Al (coating material), AlCl3 (evaporator), and Al2O3 (anti-sintering material) were placed with the substrate in an alumina crucible. The alumina crucible and alumina lid were tightly bonded with a ceramic bond (Cerabond®, Cera-Chem, Chennai, India) for the thermal treatment. The tightly sealed crucibles were placed in a tube furnace under Ar atmosphere and maintained at 500 °C for 6 h. Further details of the process can be found in our previous report [16]. The oxidation treatments were carried out at 500 °C for 12 h to form an in situ γ-Al2O3 layer on top of the Fe2Al5 layer formed on the surface of the STS-444 substrate. The oxidation treatments can form a well adhered γ-Al2O3 layer on top of the aluminide-coated surface.
3.2. Preparation of Pt-Based Monolithic Catalysts Coated on γ-Al2O3/STS-444 Substrate
Pt/γ-Al2O3 catalyst was synthesized by incipient wetness impregnation. The γ-alumina (MI386, Solvay, Brussels, Belgium) support, which has a large surface area (~200 m2/g), and a platinum ethanolamine solution (16 wt% Pt, Strong Industry, Incheon, Korea) were mixed together in the deionized water solvent. The mixture was mixed at room temperature for 30 min using a vacuum evaporator and then mixed at 90 °C to evaporate the moisture. After drying for 2 h in a circulation dryer to remove residual moisture from the synthetic catalyst, the catalyst particle size was adjusted with a 300 μm sieve and calcined at 120 °C for 4 h and 500 °C for 4 h. A monolithic catalyst was prepared by coating the prepared Pt/γ-Al2O3 catalyst on a metallic STS-444 substrate as follows. In order to coat the catalyst on the manufactured metal monolithic structure, the synthesized catalyst and distilled water were mixed to prepare a slurry, followed by ball-milling for 24 h to uniformly produce an average particle size of 1–5 μm. Next, a commercial boehmite binder (Disperal P2, Sasol, Sandton, South Africa) was added to increase the adhesion strength, and washcoating was performed on the metal monolithic structure. It was coated with the desired catalyst whose weight was relative to that of the metal monolithic structure, followed by drying at 120 °C for 4 h and at 500 °C for 2 h.
3.3. Characterization of γ-Al2O3 Intermediate Layer and Pt/γ-Al2O3 Catalysts
The crystalline phases of the γ-Al2O3 and Pt/γ-Al2O3 powders were identified through XRD analysis (D-MAX 2500, Rigaku, Tokyo, Japan) with Cu-Kα radiation (40 kV, 100 mA). The thickness and components of the γ-Al2O3 and Pt/γ-Al2O3 layers were observed by scanning electron microscopy (JSM-6610LV, JEOL, Tokyo, Japan) at 15 kV coupled with EDS.
3.4. Adhesive Strength Test of Washcoated Pt/γ-Al2O3 Catalysts
The adhesive strength tests of the proposed catalyst layer were carried out using an ultrasonic vibration cleaner (SD-D400H, 40 kHz, 400 W). The monolithic catalysts with and without the γ-Al2O3 intermediate layer were placed in a cleaner filled with deionized water. The adhesive strength tests were performed for periods of 30–60 min. Tested samples were perfectly dried at 120 °C for 30 min prior to measuring the sample weight.
The weight loss of the catalyst coating layer was calculated by the following equation:
where W0, W1, and W2 mean the weights of the bare substrate and washcoated monolithic catalysts before and after the adhesive strength test, respectively [2].
3.5. Simultaneous CO and C3H6 Oxidation Reactions
The performance of the monolithic catalysts toward the oxidation of CO and C3H6 gases was investigated using an in-house lab bench reactor. A total of 10 and 30 L/min of feed gas was introduced to the catalysts for GHSV (volumetric gas flow rate)/(volume of monolithic catalyst) of 100,000 and 300,000 h−1, respectively. The reactant gas is composed of 500 ppm of CO, 500 ppm of C3H6, 5% of CO2 in a dry air balance. The reactor was heated from 50 to 350 °C at a ramping rate of 1.7 °C/min. The product gases after catalysis were analyzed using a gas chromatography (GC) equipped with a thermal conductivity detector (TCD) and flame ionization detector (FID) (Younglin, Anyang, Korea).
The conversions of CO and C3H6 were calculated by the following equations:
where Molinlet and Moloutlet represent the mole number of CO or C3H6 before and after catalytic reactions, respectively.
4. Conclusions
Monolithic Pt/γ-Al2O3 catalysts coated on honeycomb-shaped stainless STS-444 steel substrates with a γ-Al2O3 intermediate layer were successfully prepared for potential pre-turbocharger catalyst applications. The intermediate layer was formed through aluminum pack cementation and oxidation process and acted as a buffer layer to enhance the catalyst adhesion property between the substrate and catalyst coating layer. The adhesion of the Pt/γ-Al2O3 catalysts with the intermediate layer was much stronger than those without an intermediate layer. This is because the γ-Al2O3 intermediate layer was well adhered to both the substrate and catalyst layers. CO and C3H6 oxidation reaction results after the adhesion tests showed that the catalysts with the intermediate layer have better reaction performance than those without the layer under very harsh conditions at GHSV of 100,000 and 300,000 h−1. The CO and C3H6 oxidation performance of the conventional monolithic catalysts without the intermediate layer was degraded significantly after the adhesion test. The results show that the enhancement of catalyst adhesion by well-adhered intermediate layer formation could be a critical factor for catalytic applications, such as a small catalyst located near engines exposed to very high space velocity.
Author Contributions
Conceptualization, J.S.P. and J.-H.C.; formal analysis, S.-H.R., C.H.H. and G.K.; investigation, S.-H.R. and C.H.H.; writing—original draft preparation, S.-H.R.; writing—review and editing, S.-H.R., H.J., J.S.P. and J.-H.C.; supervision, S.I.A. and J.-H.C.; project administration, J.-H.C.; funding acquisition, J.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Korea Institute of Materials Science, grant number PNK7410 and National Research Foundation (NRF) of Korea, grant number 2020R1I1A3070554.
Data Availability Statement
The data that support the plots within this paper and other findings of this study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank the XRD, SEM, and EDS measurement services provided by the National Research Facilities & Equipment center in Korea Institute of Materials Science.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bowker, M. Automotive catalysis studied by surface science. Chem. Soc. Rev. 2008, 37, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Hwang, J.; Kim, G.; Choi, J.-J.; Ahn, C.-W.; Kim, J.-W.; Hahn, B.-D.; Yoon, W.-H.; Min, Y. Catalyst adhesion enhancement by porous TiO2 layer formed on anodized titanium honeycomb substrate. Ceram. Int. 2021, 47, 7241–7247. [Google Scholar] [CrossRef]
- Carberry, B.; Grasi, G.; Guerin, S.; Jayat, F.; Konieczny, R. Pre-Turbocharger Catalyst–Fast Catalyst Light-Off Evaluation; 2005-01-2142; SAE: Warrendale, PA, USA, 2005. [Google Scholar]
- Hwang, J.; Ha, H.-J.; Ryu, J.; Choi, J.-J.; Ahn, C.-W.; Kim, J.-W.; Hahn, B.-D.; Yoon, W.-H.; Lee, H.; Choi, J.-H. Enhancement of washcoat adhesion for SCR catalysts to convert nitrogen oxide using powder spray coating of TiO2 on metallic honeycomb substrate. Catal. Commun. 2017, 94, 1–4. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Liang, B.; Li, Y. Effect of acid treatment on the high-temperature surface oxidation behavior of FeCrAlloy foil used for methane combustion catalyst support. Ind. Eng. Chem. Res. 2009, 48, 5117–5122. [Google Scholar] [CrossRef]
- Tomašíc, V.; Jovíc, F. State-of-the-art in the monolithic catalysts/reactors. Appl. Catal. A 2006, 311, 112–121. [Google Scholar] [CrossRef]
- Johnson, B.R.; Canfield, N.L.; Tran, D.N.; Dagle, R.A.; Li, X.S.; Holladay, J.D.; Wang, Y. Engineered SMR catalysts based on hydrothermally stable, porous, ceramic supports for microchannel reactors. Catal. Today 2007, 120, 54–62. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, J.; Weng, D.; Wu, X. A method to form well-adhered γ-Al2O3 layers on FeCrAl metallic supports. Surf. Coat. Technol. 2003, 167, 97–105. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.N.W.; Sazali, N.; Ismail, A.F. Effect of intermediate layer on gas separation performance of disk supported carbon membrane. Sep. Sci. Technol. 2017, 52, 2137–2149. [Google Scholar] [CrossRef]
- Lee, P.-S.; Kim, D.; Nam, S.-E.; Bhave, R.R. Carbon molecular sieve membranes on porous composite tubular supports for high performance gas separations. Micropor. Mesopor. Mater. 2016, 224, 332–338. [Google Scholar] [CrossRef]
- Wey, M.-Y.; Tseng, H.-H.; Chiang, C.-K. Effect of MFI zeolite intermediate layers on gas separation performance of carbon molecular sieve (CMS) membranes. J. Membr. Sci. 2013, 446, 220–229. [Google Scholar] [CrossRef]
- Wang, C.; Ling, L.; Huang, Y.; Yao, Y.; Song, Q. Decoration of porous ceramic substrate with pencil for enhanced gas separation performance of carbon membrane. Carbon 2015, 84, 151–159. [Google Scholar] [CrossRef]
- Tseng, H.-H.; Wang, C.-T.; Zhuang, G.-L.; Uchytil, P.; Reznickova, J.; Setnickova, K. Enhanced H2/CH4 and H2/CO2 separation by carbon molecular sieve membrane coated on titania modified alumina support: Effects of TiO2 intermediate layer preparation variables on interfacial adhesion. J. Membr. Sci. 2016, 510, 391–404. [Google Scholar] [CrossRef]
- Wang, C.; Hu, X.; Yu, J.; Wei, L.; Huang, Y. Intermediate gel coating on macroporous Al2O3 substrate for fabrication of thin carbon membranes. Ceram. Int. 2014, 40, 10367–10373. [Google Scholar] [CrossRef]
- Choi, K.; Yang, W.; Baik, K.H.; Kim, Y.; Lee, S.; Lee, S.; Park, J.S. Growth kinetics and isothermal oxidation behavior of a Si pack cementation-coated Mo-Si-B alloy. Appl. Surf. Sci. 2019, 489, 668–676. [Google Scholar] [CrossRef]
- Yang, W.C.; Lee, S.; Chung, C.-H.; Sakidja, R.; Park, J.S. Ablation stability of in situ Al2O3 layer in aluminized AISI 4130 steels under high-temperature plasma flame environments. J. Mater. Eng. Perform. 2021, 30, 7488–7493. [Google Scholar] [CrossRef]
- Ladd, D.M.; Volosin, A.; Seo, D.-K. Preparation of highly porous γ-alumina via combustion of bio renewableoil. J. Mater. Chem. 2010, 20, 5923–5929. [Google Scholar] [CrossRef]
- Samain, L.; Jaworski, A.; Edén, M.; Ladd, D.M.; Seo, D.-K.; Javier Garcia-Garcia, F.J.; Häussermann, U. Structural analysis of highly porous γ-Al2O3. J. Solid State Chem. 2014, 217, 1–8. [Google Scholar] [CrossRef]
- Bai, Q.; Li, D.; Hailian, L.H.; Sui, X.N.; Liu, M. Solvent-free selective hydrogenation of o-chloronitrobenzene to o-chloroaniline over alumina supported Pt nanoparticles. Prog. Nat. Sci. 2015, 25, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.-L.; Zhang, W.-Q.; Xia, C.-G.; Xiong, X.-M.; Mu, X.-Y.; Hu, B. Low temperature ruthenium catalyst for ammonia synthesis supported on BaCeO3 nanocrystals. Catal. Commun. 2010, 11, 867–870. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).