Hierarchical Graphitic Carbon-Encapsulating Cobalt Nanoparticles for Catalytic Hydrogenation of 2,4-Dinitrophenol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Preparation
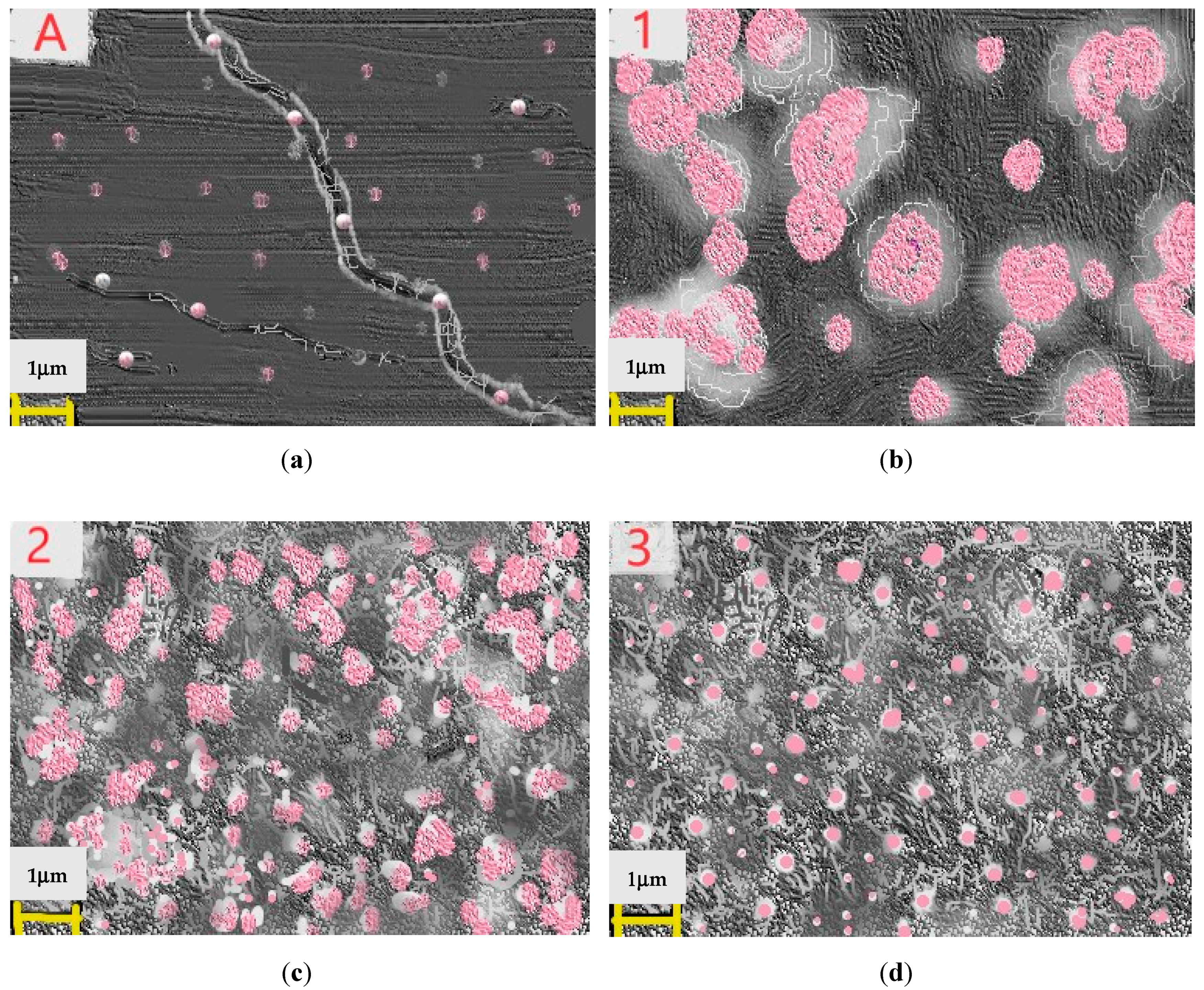
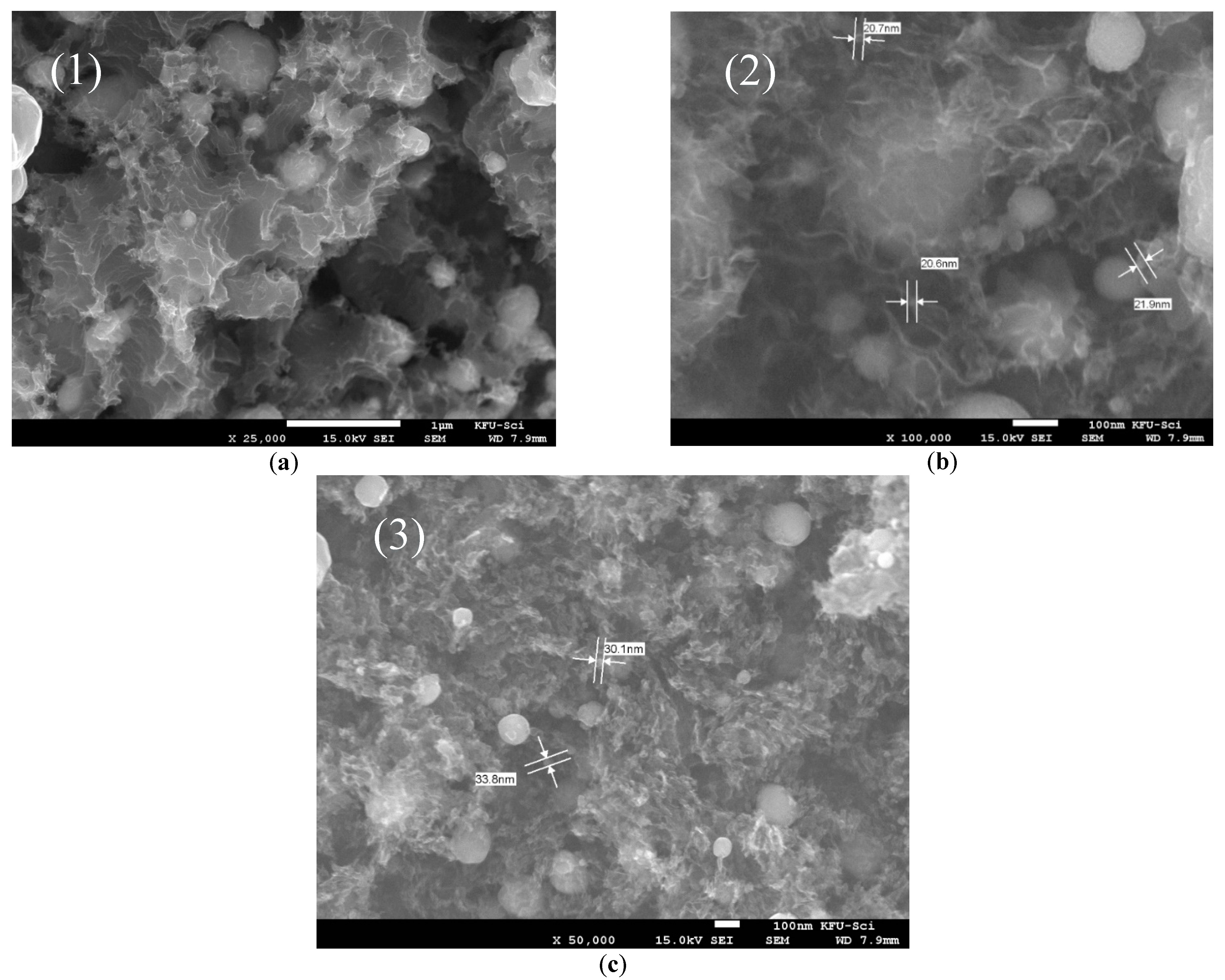
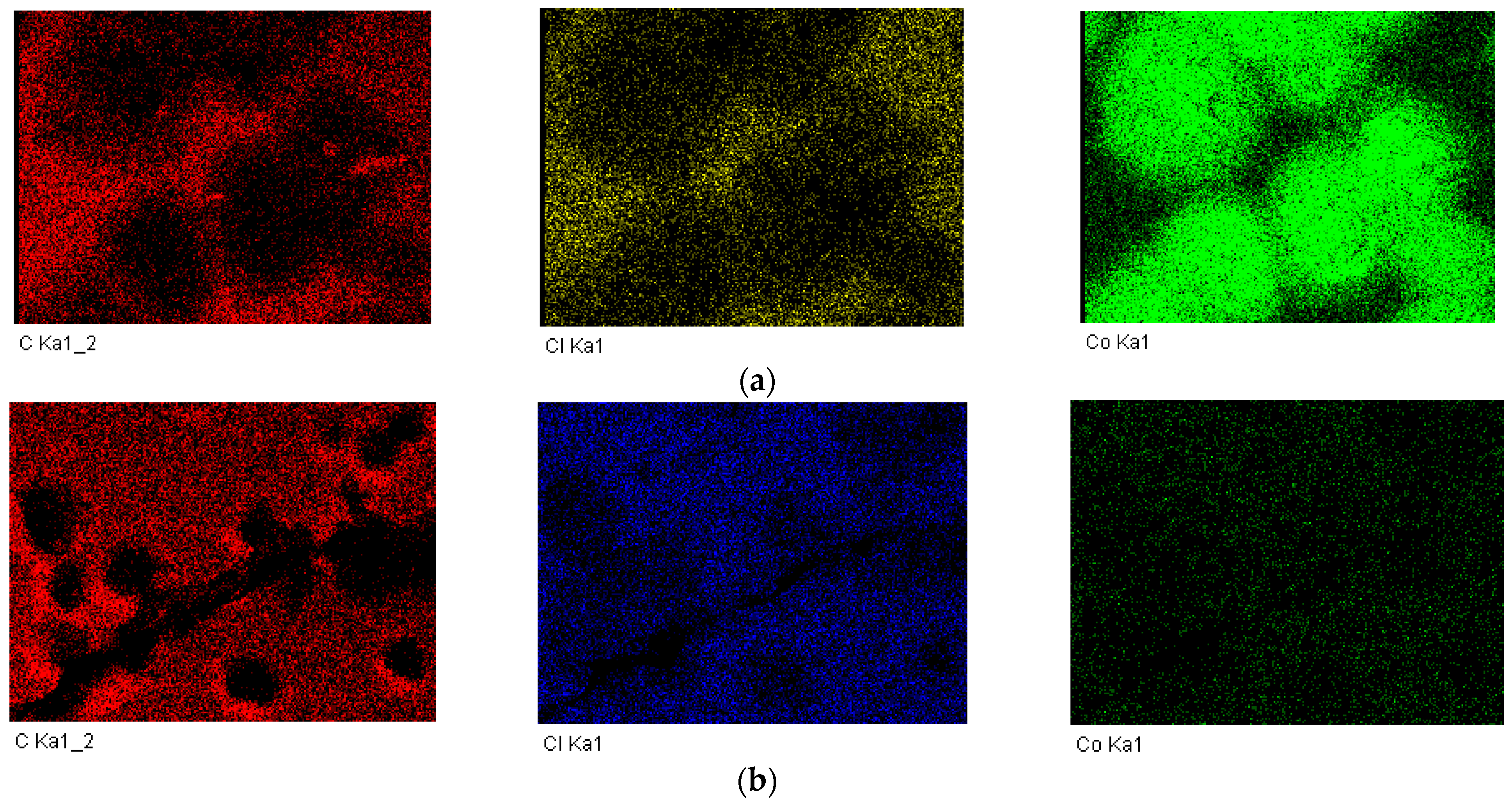
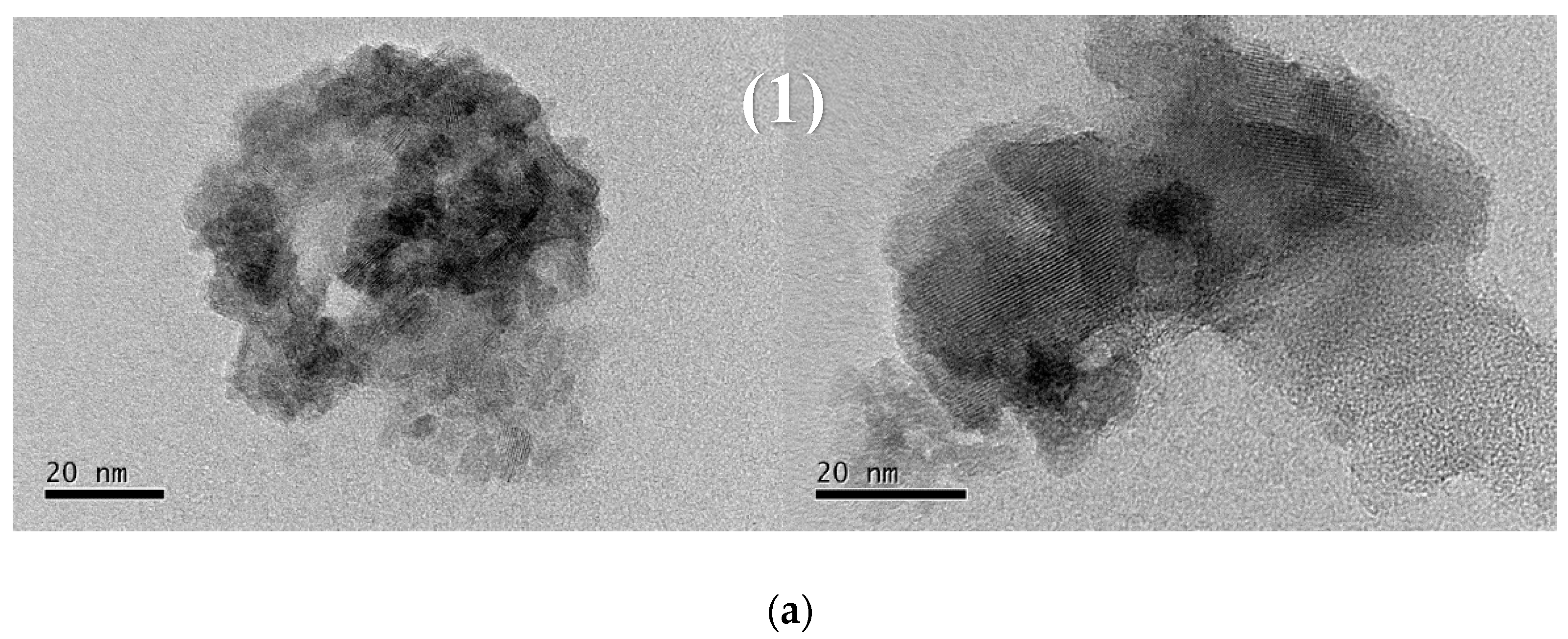
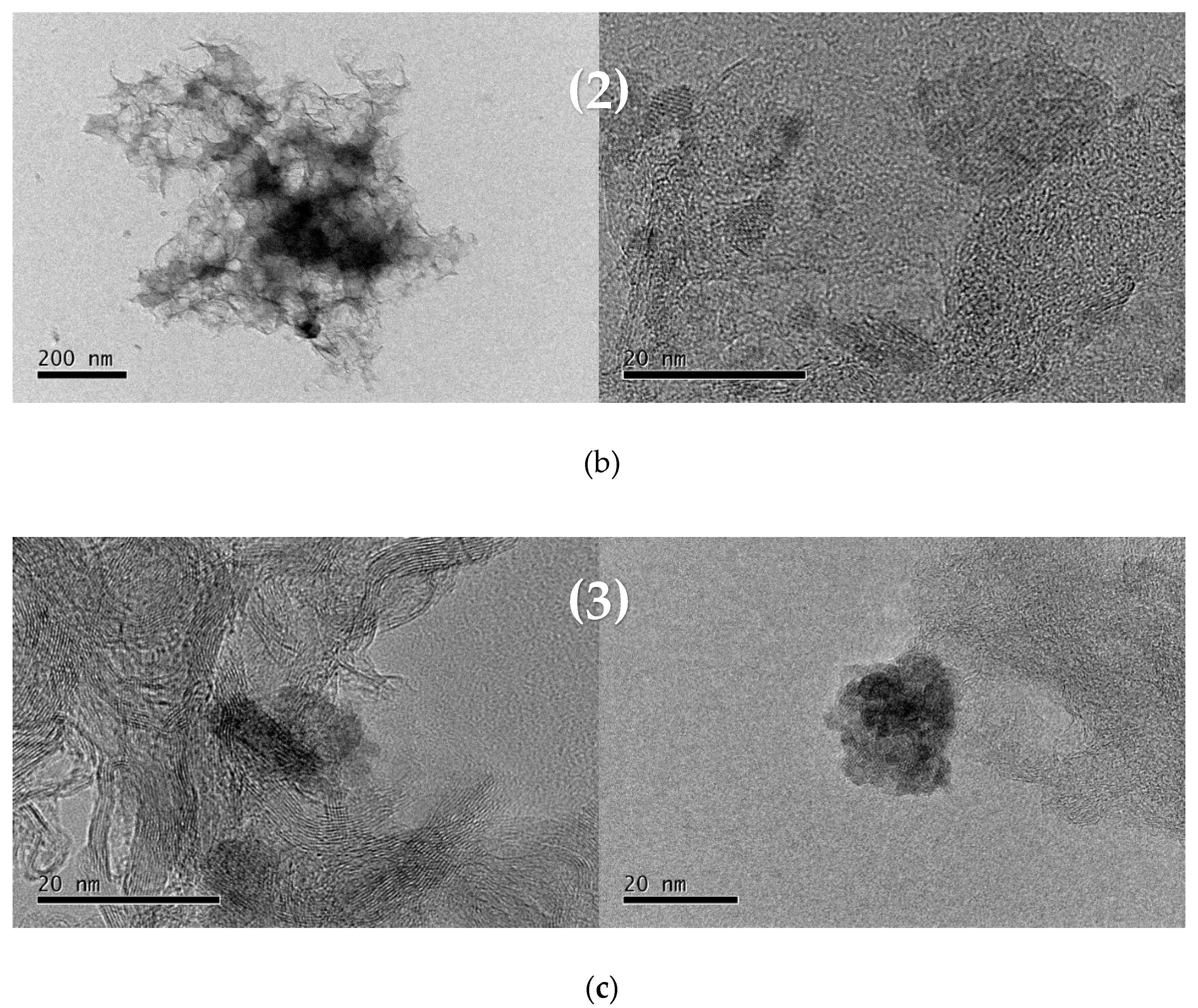
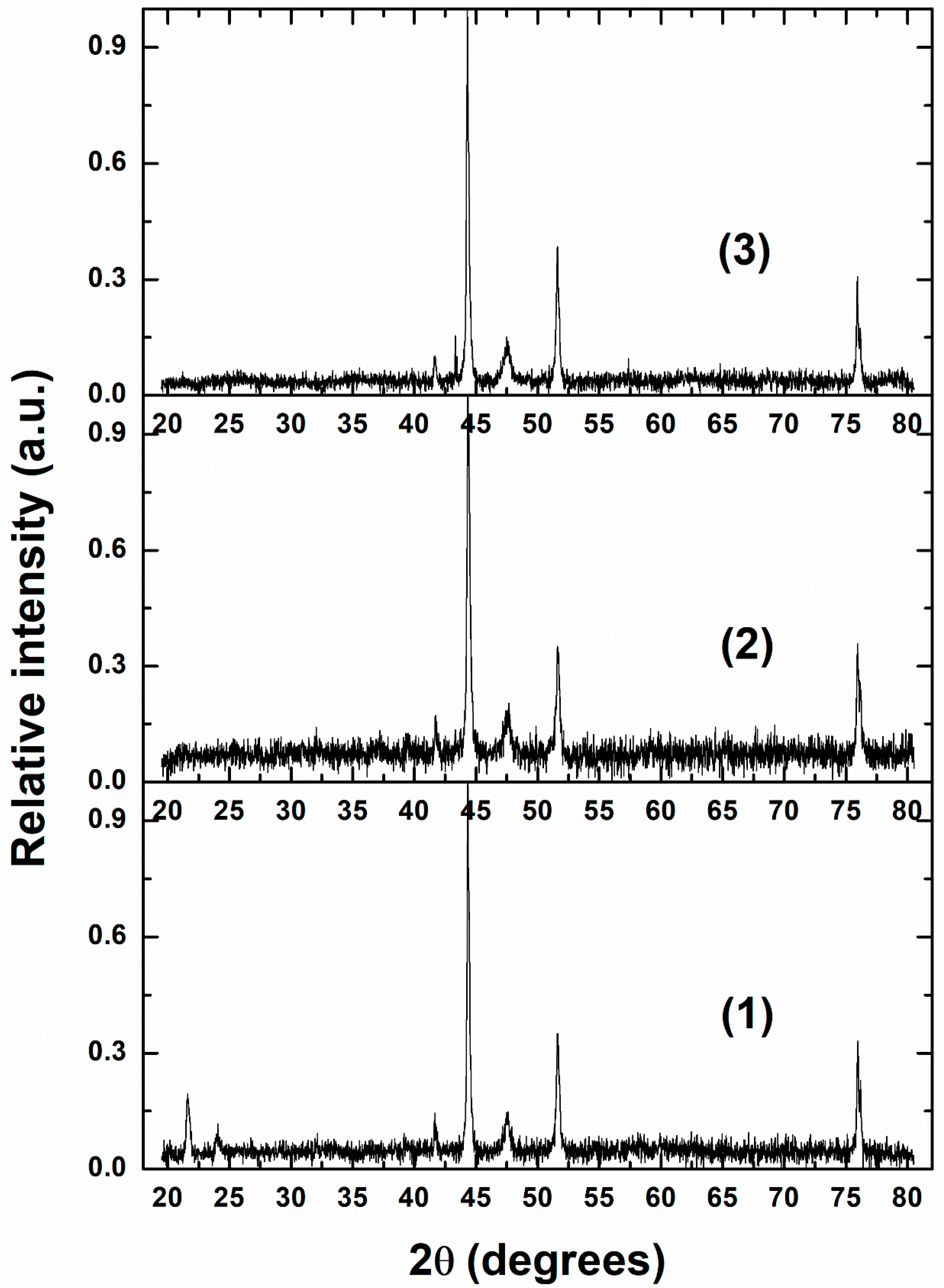
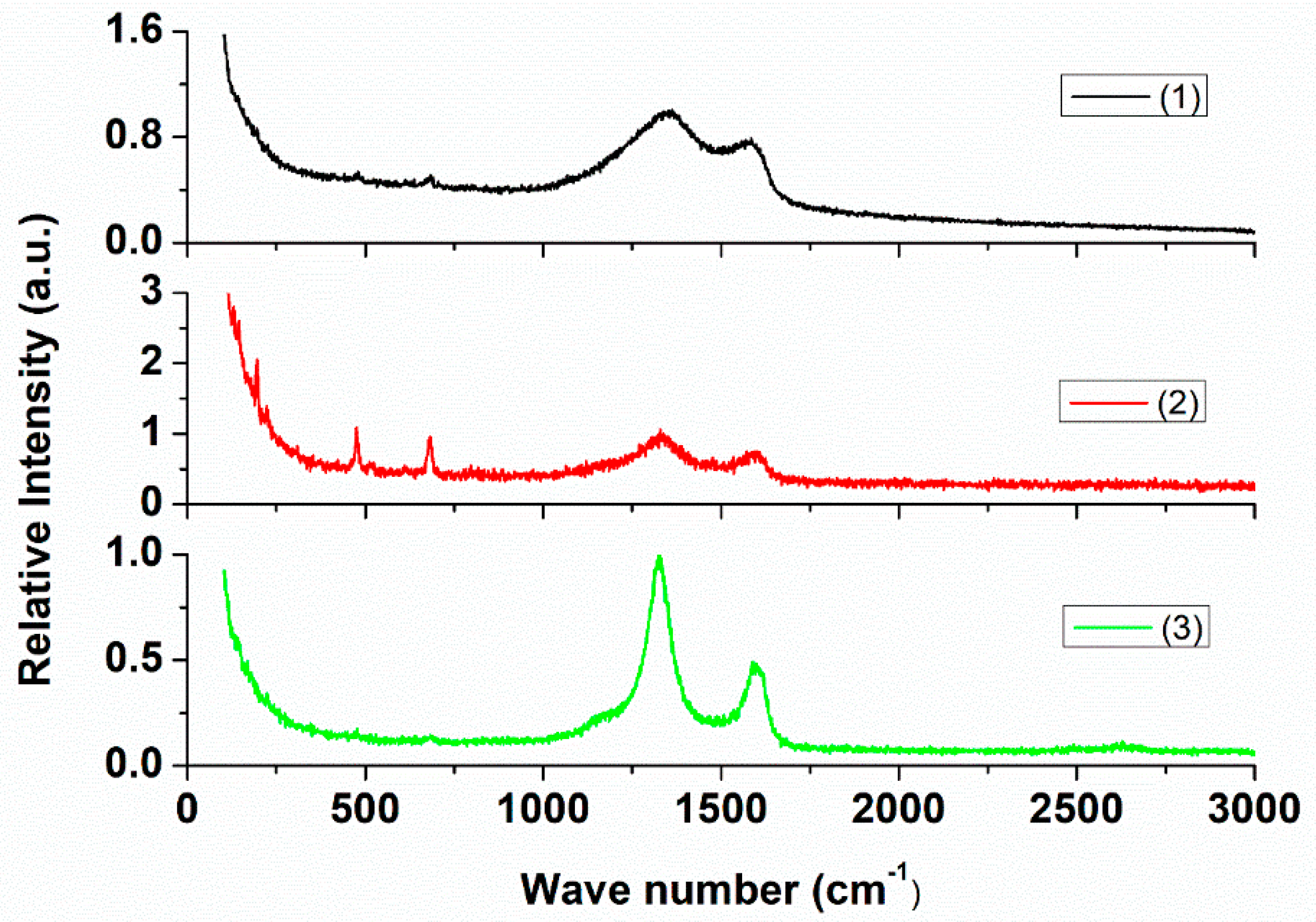
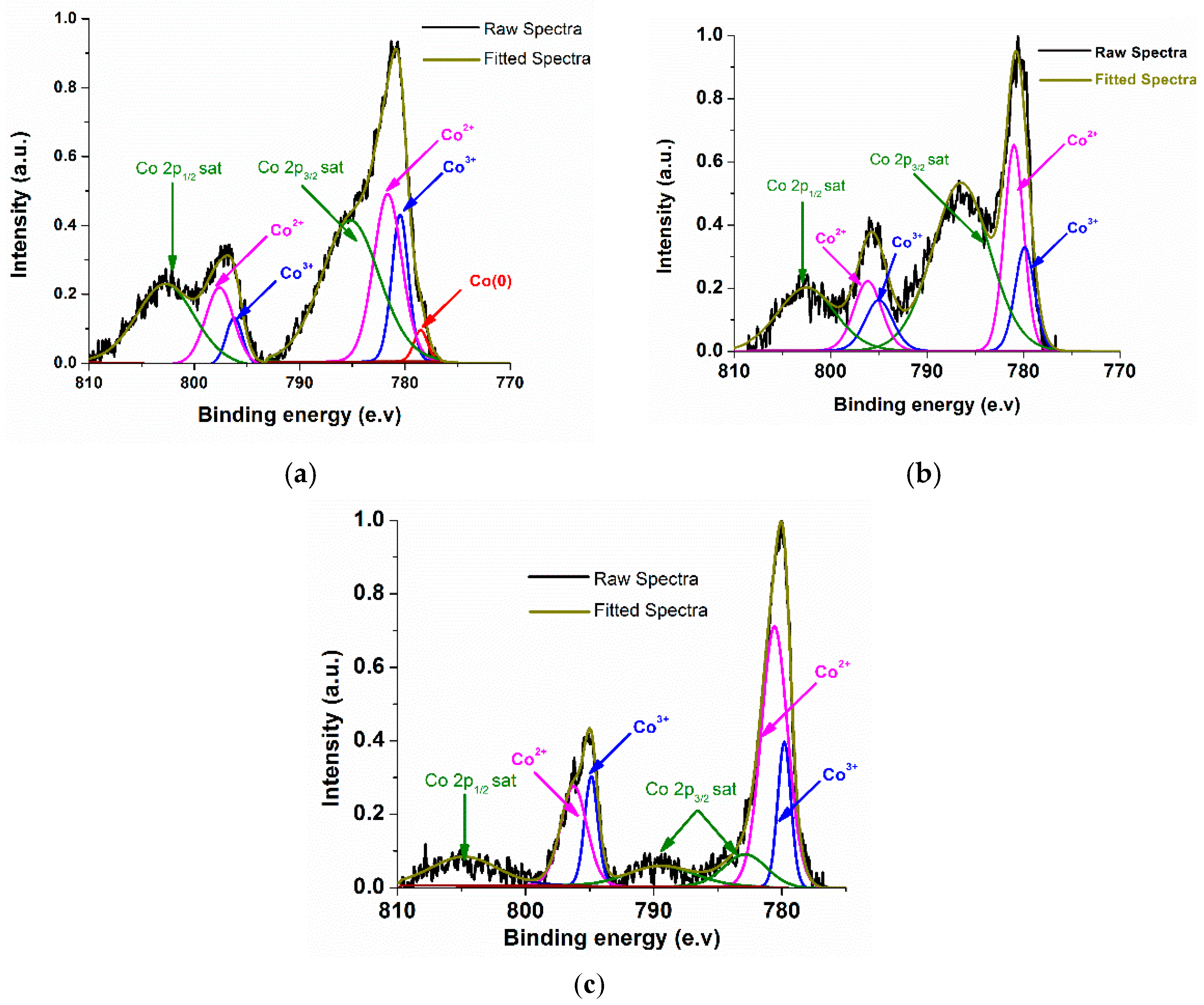
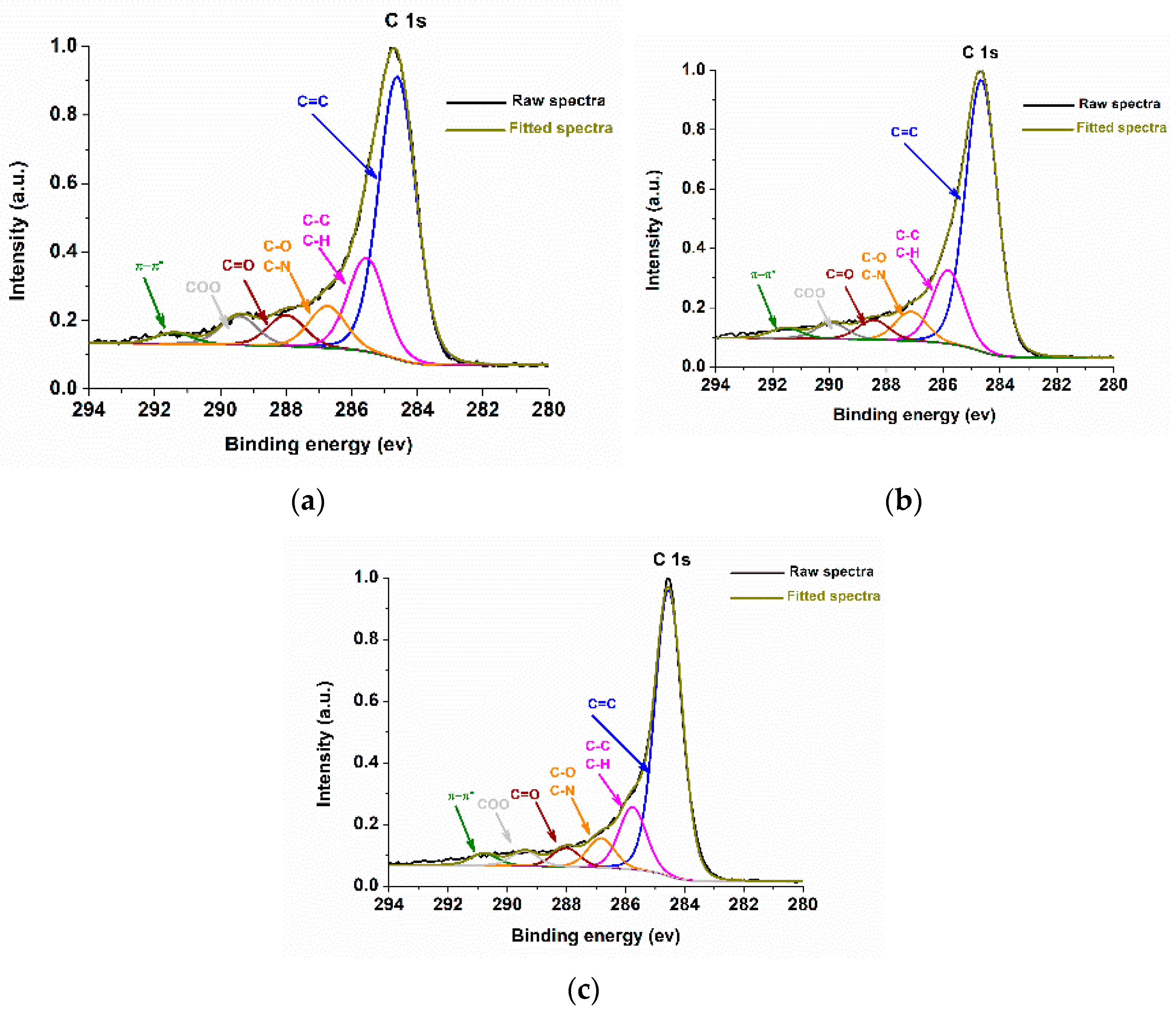
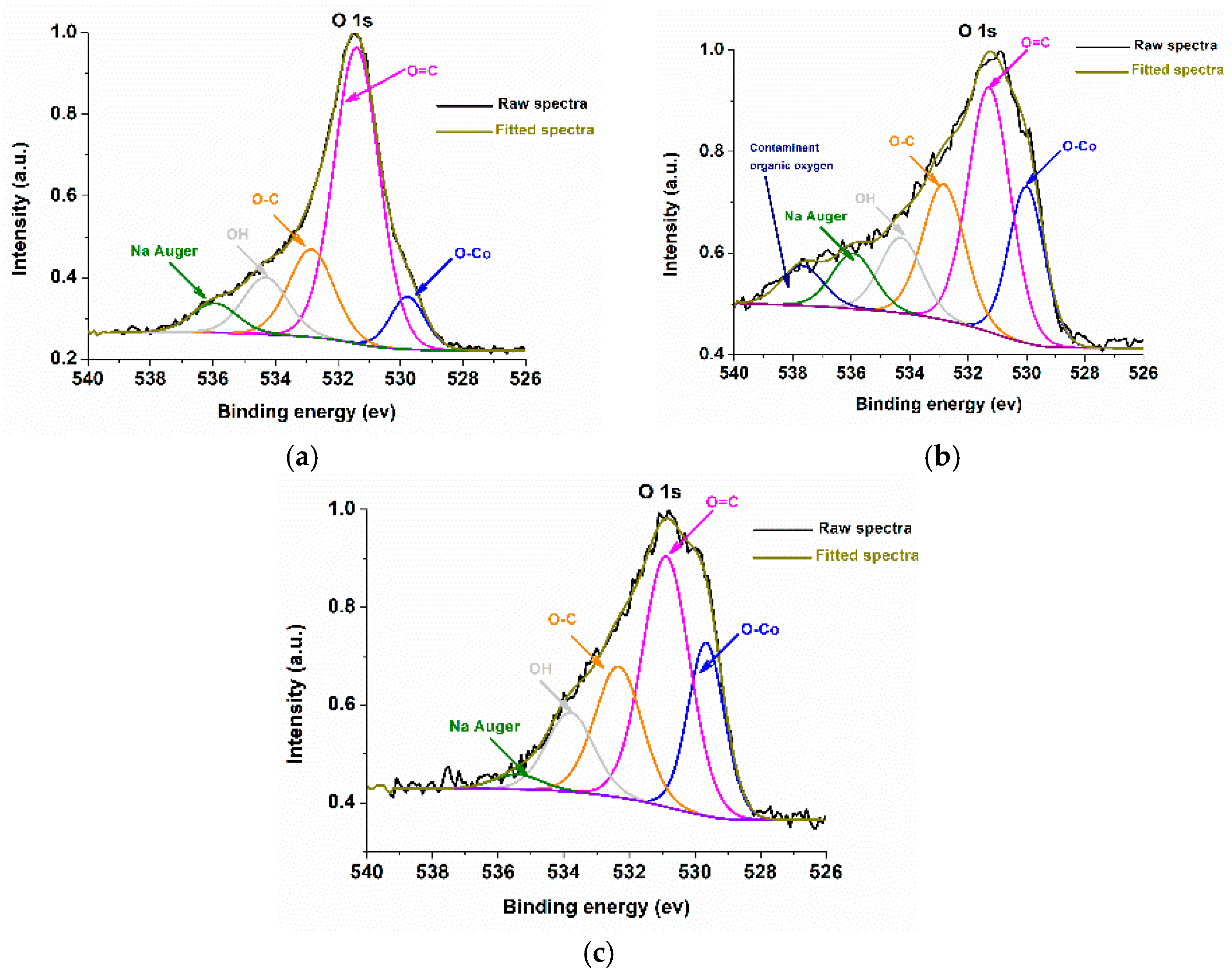
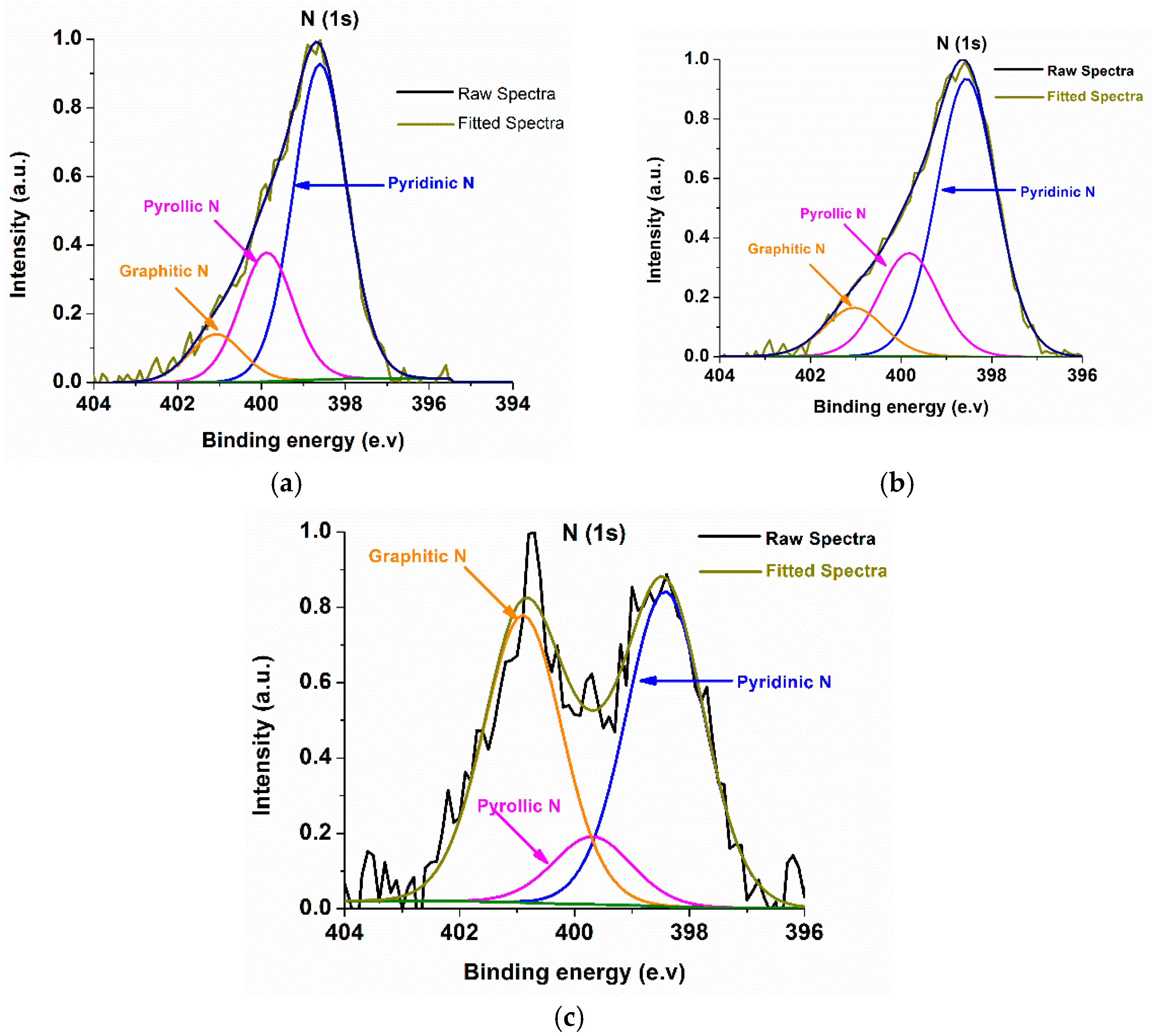
2.2. Characterizations of Nanomaterials (1), (2), and (3)
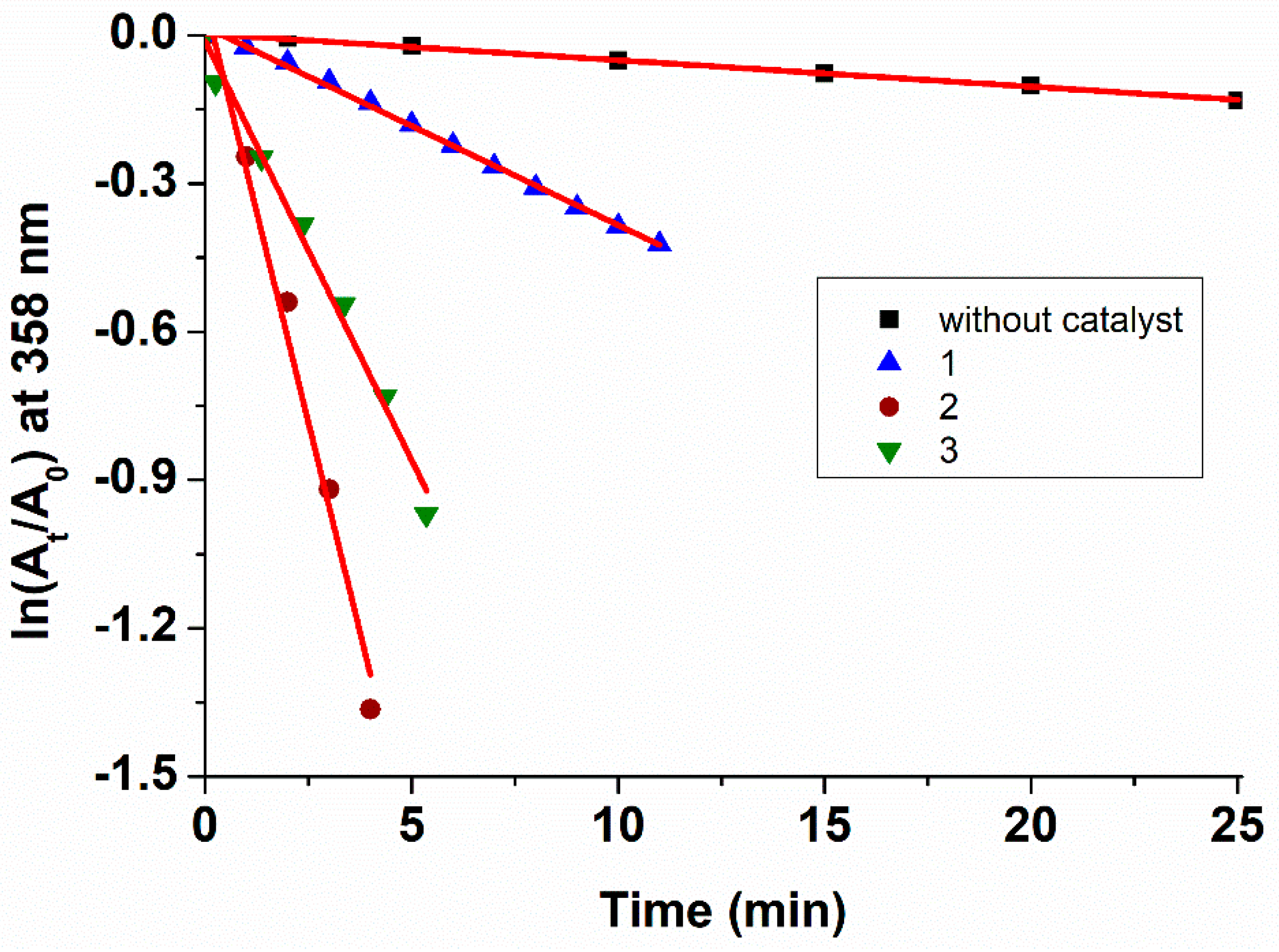
2.3. Kinetics
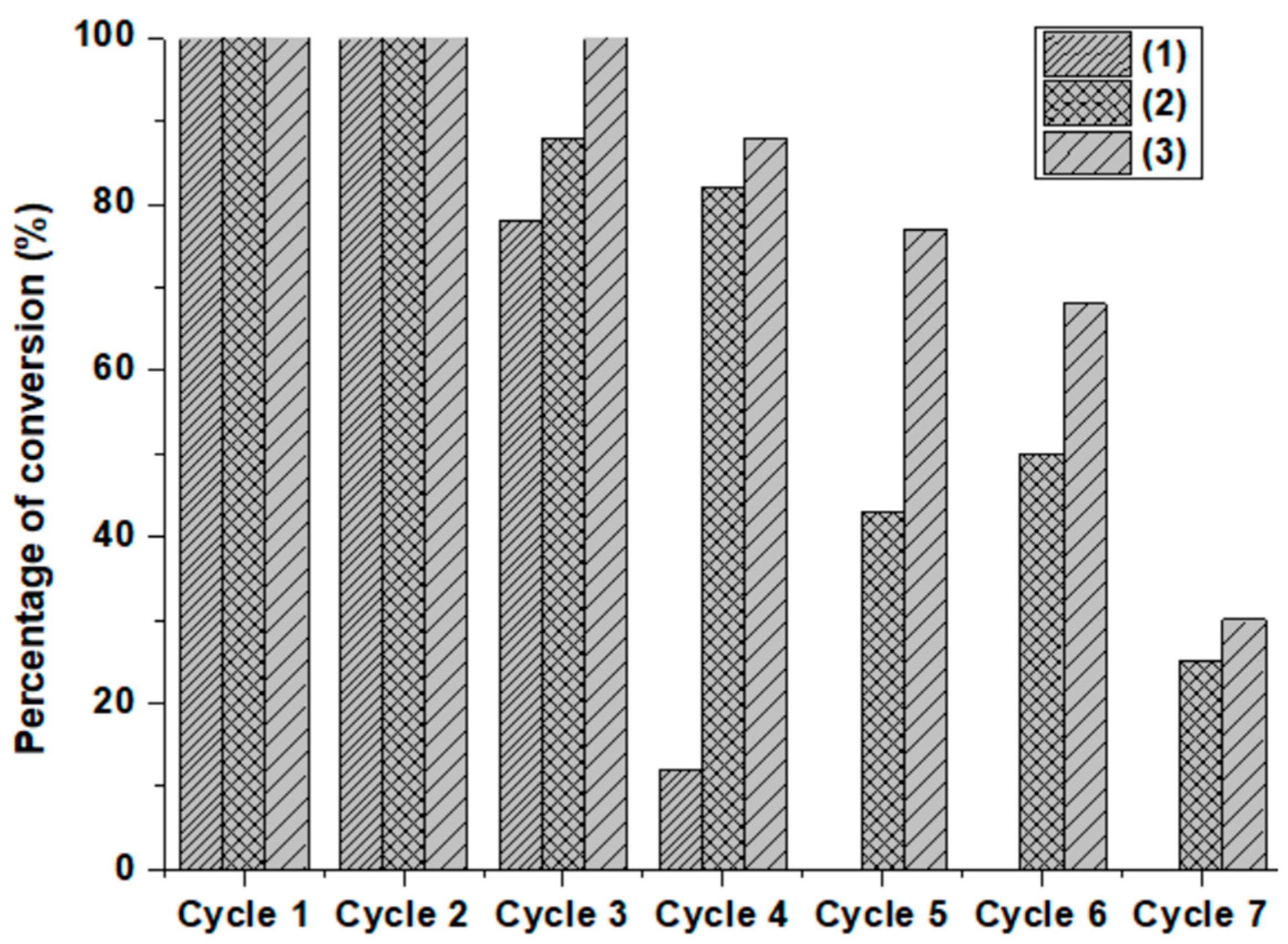
2.4. Deactivation and Regeneration of the Presented Composites
2.5. Effect of Adsorption of DNP on the Catalytic Activity of Nanocatalysts
3. Experimental Section
3.1. Preparation of Cobalt–Carbon Nanoparticles Catalyst Co@HGC (1), (2), and (3)
3.2. Characterization of Co@HGC
3.3. Kinetic Studies
3.4. Successive Reduction of 2,4-DNP
3.5. Reduction of 2,4-DNP Reduction under Sonication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, L.; Wu, J.; Chen, C.; Zhang, C.; Wan, L.; Wang, J.; Kong, Q.; Lv, C.; Li, K.; Sun, G. A novel asymmetric supercapacitor with an activated carbon cathode and a reduced graphene oxide-cobalt oxide nanocomposite anode. J. Power Sources 2013, 242, 148–156. [Google Scholar] [CrossRef]
- Mei, J.; Liao, T.; Ayoko, G.A.; Bell, J.; Sun, Z. Cobalt oxide-based nanoarchitectures for electrochemical energy applications. Prog. Mater. Sci. 2019, 103, 596–677. [Google Scholar] [CrossRef]
- Lin, X.; Nie, Z.; Zhang, L.; Mei, S.; Chen, Y.; Zhang, B.; Zhuc, R.; Liu, Z. Nitrogen-doped carbon nanotubes encapsulate cobalt nanoparticles as efficient catalysts for aerobic and solvent-free selective oxidation of hydrocarbons. Green Chem. 2017, 19, 2164–2173. [Google Scholar] [CrossRef]
- Song, T.; Ren, P.; Duan, Y.; Wang, Z.; Chen, X.; Yang, Y. Cobalt Nanocomposites on N-Doped Hierarchical Porous Carbon for Highly Selective Formation of Anilines and Imines from Nitroarenes. Green Chem. 2018, 20, 4629–4637. [Google Scholar] [CrossRef]
- Zhu, W.; Ren, J.; Gu, X.; Azmat, M.U.; Lu, G.; Wang, Y. Synthesis of hermetically-sealed graphite-encapsulated metallic cobalt (alloy) core/shell nanostructures. Carbon 2011, 49, 1462–1472. [Google Scholar] [CrossRef]
- Hammud, H.H.; el Hamaoui, B.; Noubani, N.H.; Feng, X.; Wu, Z.S.; Müllen, K.; Ayub, K. Carbon-Cobalt Nanostructures as an Efficient Adsorbent of Malachite Green. Nanosci. Nanotechnol. Asia 2018, 8, 263–280. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; de Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Nishide, D.; Kataura, H.; Suzuki, S.; Okubo, S.; Achiba, Y. Growth of single-wall carbon nanotubes from ethanol vapor on cobalt particles produced by pulsed laser vaporization. Chem. Phys. Lett. 2004, 392, 309–313. [Google Scholar] [CrossRef]
- Terrado, E.; Redrado, M.; Munoz, E.; Maser, W.K.; Benito, A.M.; Martinez, M.T. Carbon nanotube growth on cobalt-sprayed substrates by thermal CVD. Mat. Sci. Eng. C 2006, 26, 1185–1188. [Google Scholar] [CrossRef]
- Hamaoui, B.E.; Zhi, L.; Wu, J.; Li, J.; Lucas, N.T.; Tomović, Z.; Kolb, U.; Müllen, K. Solid-State Pyrolysis of Polyphenylene–Metal Complexes: A Facile Approach Toward Carbon Nanoparticles. Adv. Funct. Mater. 2007, 17, 1179–1187. [Google Scholar] [CrossRef]
- Hamaoui, B.E.; Zhi, L.; Wu, J.; Kolb, U.; Müllen, K. Uniform Carbon and Carbon/Cobalt Nanostructures by Solid-State Thermolysis of Polyphenylene Dendrimer/Cobalt Complexes. Adv. Mater. 2005, 17, 2957–2960. [Google Scholar] [CrossRef]
- Pereira, L.R.; Mondal, P.K.; Alves, M.S. Aromatic amines: Main sources, environmental impact and remediation. In Environmental Chemistry for a Sustainable World; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Berlin, Germany, 2013. [Google Scholar] [CrossRef]
- Raja, R.; Golovko, V.B.; Thomas, J.M.; Berenguer-Murcia, A.; Zhou, W.; Xiee, S.; Johnsona, B.F.G. Highly efficient catalysts for the hydrogenation of nitro-substituted aromatics. Chem. Commun. 2005, 15, 2026–2028. [Google Scholar] [CrossRef]
- Corma, A.; Gonález-Arellano, C.; Iglesias, M.; Sánchez, F. Gold complexes as catalysts: Chemoselective hydrogenation of nitroarenes. Appl. Catal. A 2009, 356, 99–102. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, P.; Huang, D.; Zeng, G.; Lai, C.; Qin, L.; Li, B.; He, J.; Yi, H.; Cheng, M.; et al. Au nanoparticles decorated on activated coke via a facile preparation for efficient catalytic reduction of nitrophenols and azo dyes. Appl. Surf. Sci. 2019, 473, 578–588. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Issaabadi, Z.; Sajadi, S.M. Green synthesis of the Ag/Al2O3 nanoparticles using Bryonia alba leaf extract and their catalytic application for the degradation of organic pollutants. J. Mater. Sci. Mater. Electron. 2019, 30, 3847–3859. [Google Scholar] [CrossRef]
- Das, R.; Sypu, V.S.; Paumo, H.K.; Bhaumik, M.; Maharaj, V.; Maity, A. Silver decorated magnetic nanocomposite (Fe3O4@PPy-MAA/Ag) as highly active catalyst towards reduction of 4-nitrophenol and toxic organic dyes. Appl. Catal. B Environ. 2019, 244, 546–558. [Google Scholar] [CrossRef]
- Makaudi, R.; Paumo, H.K.; Pone, B.K.; Katata-Seru, L. In Situ Stabilisation of Silver Nanoparticles at Chitosan-Functionalised Graphene Oxide for Reduction of 2,4-Dinitrophenol in Water. Polymers 2021, 13, 3800. [Google Scholar] [CrossRef]
- Dejmkova, H.; Stoica, A.I.; Barek, J.; Zima, J. Voltammetric and amperometric determination of 2,4-dinitrophenol metabolites. Talanta 2011, 85, 2594–2598. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, L.; Zhang, Y.; Tang, H. Electrochemical sensoring of 2,4-dinitrophenol by using composites of graphene oxide with surface molecular imprinted polymer. Sens. Actuators B 2012, 171–172, 1151–1158. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Banerjee, D.; Arockiam, P.B.; Junge, H.; Junge, K.; Pohl, M.M.; Radnik, J.; Brückner, A.; Beller, M. Highly Selective Transfer Hydrogenation of Functionalysed Nitroarenes Using Cobalt-based Nanocatalysts. Green Chem. 2015, 17, 898–902. [Google Scholar] [CrossRef]
- Westerhaus, F.A.; Jagadeesh, R.V.; Wienho¨fer, G.; Pohl, M.; Radnik, J.; Surkus, A.; Rabeah, J.; Junge, K.; Junge, H.; Nielsen, M.; et al. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes. Nat. Chem. 2013, 5, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Yusran, Y.; Xu, D.; Fang, Q.; Zhang, D.; Qiu, S. MOF-derived Co@N-C nanocatalyst for catalytic reduction of 4-nitrophenol to 4-aminophenol. Microporous Mesoporous Mater. 2017, 241, 346–354. [Google Scholar] [CrossRef]
- Shultz, L.R.; McCullough, B.; Newsome, W.J.; Ali, H.; Shaw, T.E.; Davis, K.O.; Uribe-Romo, F.J.; Baudelet, M.; Jurca, T. A Combined Mechanochemical and Calcination, Route to Mixed Cobalt Oxides for the Selective Catalytic Reduction of Nitrophenols. Molecules 2020, 25, 89. [Google Scholar] [CrossRef] [Green Version]
- Naseem, K.; Begum, R.; Farooqi, Z.H.; Wu, W.; Irfan, A. Core-shell microgel stabilized silver nanoparticles for catalytic reduction of aryl nitro compounds. Appl. Organomet. Chem. 2020, 34, e5742. [Google Scholar] [CrossRef]
- Karakas, K.; Celebioglu, A.; Celebi, M.; Uyar, T.; Zahmakiran, M. Nickel nanoparticles decorated on electrospun polycaprolactone/chitosan nanofibers as flexible, highly active and reusable nanocatalyst in the reduction of nitrophenols under mildconditions. Appl. Catal. B Environ. 2017, 203, 549–562. [Google Scholar] [CrossRef]
- Gerelbaatar, K.; Tsogoo, A.; Dashzeveg, R.; Tsedev, N.; Ganbold, E. Reduction of 2,4-Dinitrophenol to 2,4-Diaminophenol Using AuNPs and AgNPs as Catalyst. Solid State Phenom. 2018, 271, 76–84. [Google Scholar] [CrossRef]
- Alotaibi, N.; Hammud, H.H.; Karnati, R.K.; Hussain, S.G.; Mazher, J.; Prakasam, T. Cobalt–carbon/silica nanocomposites prepared by pyrolysis of a cobalt 2,2’-bipyridine terephthalate complex for remediation of cationic dyes. RSC Adv. 2020, 10, 17660–17672. [Google Scholar] [CrossRef]
- Hazell, A.; McGinley, J.; McKenzie, C.J. Dichlorobis(1,10-phenanthrolineN,N′)-cobalt(II)-Acetonitrile (1/1.5). Acta Cryst. 1997, C53, 723–725. [Google Scholar]
- Xu, Y.; Shan, W.; Liang, X.; Gao, X.; Li, W.; Li, H.; Qiu, X. Cobalt Nanoparticles Encapsulated in Nitrogen-Doped Carbon Shells: Efficient and Stable Catalyst for Nitrobenzene Reduction. Ind. Eng. Chem. Res. 2020, 59, 4367–4376. [Google Scholar] [CrossRef]
- Li, M.; Chen, S.; Jiang, Q.; Chen, Q.; Wang, X.; Yan, Y.; Liu, J.; Lv, C.; Ding, W.; Guo, X. Origin of the Activity of Co−N−C Catalysts for Chemoselective Hydrogenation of Nitroarenes. ACS Catal. 2021, 11, 3026–3039. [Google Scholar] [CrossRef]
- Yin, X.; Liu, Q.; Ding, Y.; Chen, K.; Cai, P.; Wen, Z. Hierarchical Carbon/Metal Nanostructure with a Combination of 0D Nanoparticles, 1D Nanofibers, and 2D Nanosheets: An Efficient Bifunctional Catalyst for Zinc-Air Batteries. ChemElectroChem 2021, 8, 1107–1116. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, P.; Li, M.; Wu, H.; Zhang, C.; Han, L.; Zhang, Y. Cobalt Nanoparticles Encapsulated in Nitrogen-Doped Carbon Nanotube as Bifunctional-Catalyst for Rechargeable Zn-Air Batteries. Front. Mater. 2019, 6, 85. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Li, Y.; Lan, X.; Ali, B.; Wang, T. Highly efficient hydrogenation of nitroarenes by N-doped carbon supported cobalt single-atom catalyst in ethanol/water mixed solvent. ACS Appl. Mater. Interfaces 2020, 12, 34021–34031. [Google Scholar] [CrossRef]
- Sun, X.; Olivos-Suarez, A.I.; Osadchii, D.; Romero, M.J.V.; Kapteijn, F.; Gascon, J. Single Cobalt Sites in Mesoporous N-Doped Carbon Matrix for Selective Catalytic Hydrogenation of Nitroarenes. J. Catal. 2018, 357, 20–28. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Arellano-Jiménez, M.J.; Ye, G.; Kim, N.D.; Samuel, E.L.G.; Peng, Z.; Zhu, Z.; Qin, F.; Bao, J.; et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 8668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Lau, S.P.; Huang, L.; Tay, B.K. Carbon nanotubes grown on cobalt-containing amorphous carbon composite films. Diam. Relat. Mater. 2006, 15, 171–175. [Google Scholar] [CrossRef]
- Xie, S.; Liu, Y.; Deng, J.; Yang, J.; Zhao, X.; Han, Z.; Zhang, K.; Dai, H. Insights into the active sites of ordered mesoporous cobalt oxide catalysts for the total oxidation of o-xylene. J. Catal. 2017, 352, 282–292. [Google Scholar] [CrossRef]
- Andrieux, J.; Swierczynski, D.; Laversenne, L.; Garron, A.; Bennici, S.; Goutaudier, C.; Miele, P.; Auroux, A.; Bonnetot, B. A multifactor study of catalyzed hydrolysis of solid NaBH4 on cobalt nanoparticles: Thermodynamics and kinetics. Int. J. Hydrog. Energy 2009, 34, 938–951. [Google Scholar] [CrossRef]
- Lee, D.; XunXia, Q.; Yun, J.M.; HoKim, K. High-performance cobalt carbonate hydroxide nano-dot/NiCo(CO3)(OH)2 electrode for asymmetric supercapacitors. Appl. Surf. Sci. 2018, 433, 16–26. [Google Scholar] [CrossRef]
- Shi, R.; Chen, G.; Ma, W.; Zhang, D.; Qiu, G.; Liu, X. Shape-controlled synthesis and characterization of cobalt oxides hollow spheres and octahedra. Dalton Trans. 2012, 41, 5981. [Google Scholar] [CrossRef]
- Tang, C.; Surkus, A.-E.; Chen, F.; Pohl, M.-M.; Agostini, G.; Schneider, M.; Junge, H.; Beller, M. A Stable Nanocobalt Catalyst with Highly Dispersed CoNx Active Sites for the Selective Dehydrogenation of Formic Acid. Angew. Chem. Int. Ed. 2017, 56, 16616–16620. [Google Scholar] [CrossRef]
- Nguyen-Huy, C.; Lee, J.; Seo, J.H.; Yang, E.; Lee, J.; Choi, K.; Lee, H.; Kim, J.H.; Lee, M.S.; Joo, S.H.; et al. Structure-Dependent Catalytic Properties of Mesoporous Cobalt Oxides in Furfural Hydrogenation. Appl. Catal. A Gen. 2019, 583, 117125. [Google Scholar] [CrossRef]
- Lesiak, B.; Kövér, L.; Tóth, J.; Zemek, J.; Jiricek, P.; Kromka, A.; Rangama, N. C sp2/sp3 hybridisations in carbon nanomaterials—XPS and (X)AES study. Appl. Surf. Sci. 2018, 452, 223–231. [Google Scholar] [CrossRef]
- Benkoula, S.; Sublemontier, O.; Patanen, M.; Nicolas, C.; Sirotti, F.; Naitabdi, A.; Gaie-Levrel, F.; Antonsson, E.; Aureau, D.; Ouf, F.; et al. Catalin Miron Water adsorption on TiO2 surfaces probed by soft X-ray spectroscopies: Bulk materials vs. isolated nanoparticles. Sci. Rep. 2015, 5, 10588. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Chen, J.; Zhao, J.; Zheng, J.; Song, C.; Lia, L.; Zhu, Z. Synthesis of highly nitrogen-doped hollow carbon nanoparticles and their excellent electrocatalytic properties in dye-sensitized solar cell. J. Mater. Chem. 2010, 20, 10829–10834. [Google Scholar] [CrossRef]
- Sudhakar, P.; Soni, H. Catalytic reduction of Nitrophenols using silver nanoparticles-supported activated carbon derived from agro-waste. J. Environ. Chem. Eng. 2018, 6, 28–36. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Naseem, K.; Begum, R.; Ijaz, A. Catalytic reduction of 2-nitroaniline in aqueous medium using silver nanoparticles functionalized polymer microgels. J. Inorg. Organomet. Polym. Mater. 2015, 25, 1554–1568. [Google Scholar] [CrossRef]
- Oar-Arteta, L.; Wezendonk, T.; Sun, X.; Kapteijn, F.; Gascon, J. Metal organic frameworks as precursors for the manufacture of advanced catalytic materials. Mater. Chem. Front. 2017, 1, 1709. [Google Scholar] [CrossRef] [Green Version]
- Mondal, A.; Mondal, A.; Adhikary, B.; Mukherjee, D.K. Cobalt nanoparticles as reusable catalysts for reduction of 4-nitrophenol under mild conditions. Bull. Mater. Sci. 2017, 40, 321–328. [Google Scholar] [CrossRef]
- Delmas, J.; Laversenne, L.; Rougeaux, I.; Capron, P.; Garron, A.; Bennici, S.; Swierczynski, D.; Auroux, A. Improved hydrogen storage capacity through hydrolysis of solid NaBH4 catalyzed with cobalt boride. Int. J. Hydrog. Energy 2011, 36, 2145–2153. [Google Scholar] [CrossRef]
- Simagina, V.I.; Komova, O.V.; Ozerova, A.M.; Netskina, O.V.; Odegova, G.V.; Kellerman, D.G.; Bulavchenko, O.A.; Ishchenko, A.V. Cobalt oxide catalyst for hydrolysis of sodium borohydride and ammonia borane. Appl. Catal. A Gen. 2011, 394, 86–92. [Google Scholar] [CrossRef]
- Alotaibi, N.; Hammud, H.H.; al Otaibi, N.; Hussain, S.G. Thirumurugan Prakasam Novel cobalt–carbon@silica adsorbent. Sci. Rep. 2020, 10, 18652. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammud, H.H.; Traboulsi, H.; Karnati, R.K.; Hussain, S.G.; Bakir, E.M. Hierarchical Graphitic Carbon-Encapsulating Cobalt Nanoparticles for Catalytic Hydrogenation of 2,4-Dinitrophenol. Catalysts 2022, 12, 39. https://doi.org/10.3390/catal12010039
Hammud HH, Traboulsi H, Karnati RK, Hussain SG, Bakir EM. Hierarchical Graphitic Carbon-Encapsulating Cobalt Nanoparticles for Catalytic Hydrogenation of 2,4-Dinitrophenol. Catalysts. 2022; 12(1):39. https://doi.org/10.3390/catal12010039
Chicago/Turabian StyleHammud, Hassan H., Hassan Traboulsi, Ranjith Kumar Karnati, Syed Ghazanfar Hussain, and Esam M. Bakir. 2022. "Hierarchical Graphitic Carbon-Encapsulating Cobalt Nanoparticles for Catalytic Hydrogenation of 2,4-Dinitrophenol" Catalysts 12, no. 1: 39. https://doi.org/10.3390/catal12010039
APA StyleHammud, H. H., Traboulsi, H., Karnati, R. K., Hussain, S. G., & Bakir, E. M. (2022). Hierarchical Graphitic Carbon-Encapsulating Cobalt Nanoparticles for Catalytic Hydrogenation of 2,4-Dinitrophenol. Catalysts, 12(1), 39. https://doi.org/10.3390/catal12010039







