Tandem Reactions Based on the Cyclization of Carbon Dioxide and Propargylic Alcohols: Derivative Applications of α-Alkylidene Carbonates
Abstract
:1. Introduction
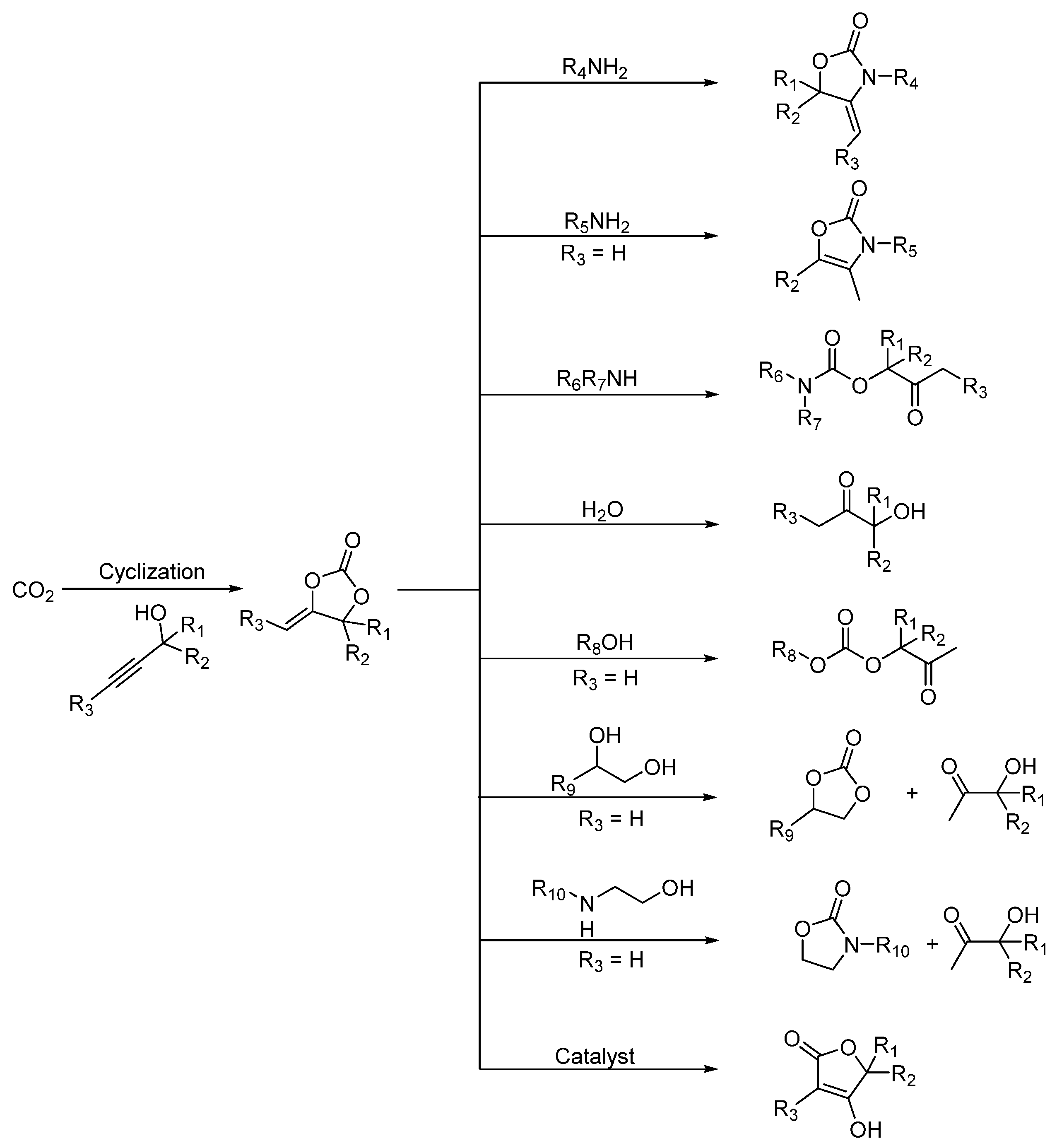
2. Three-Component Reactions of Propargylic Alcohols, CO2 and Amines
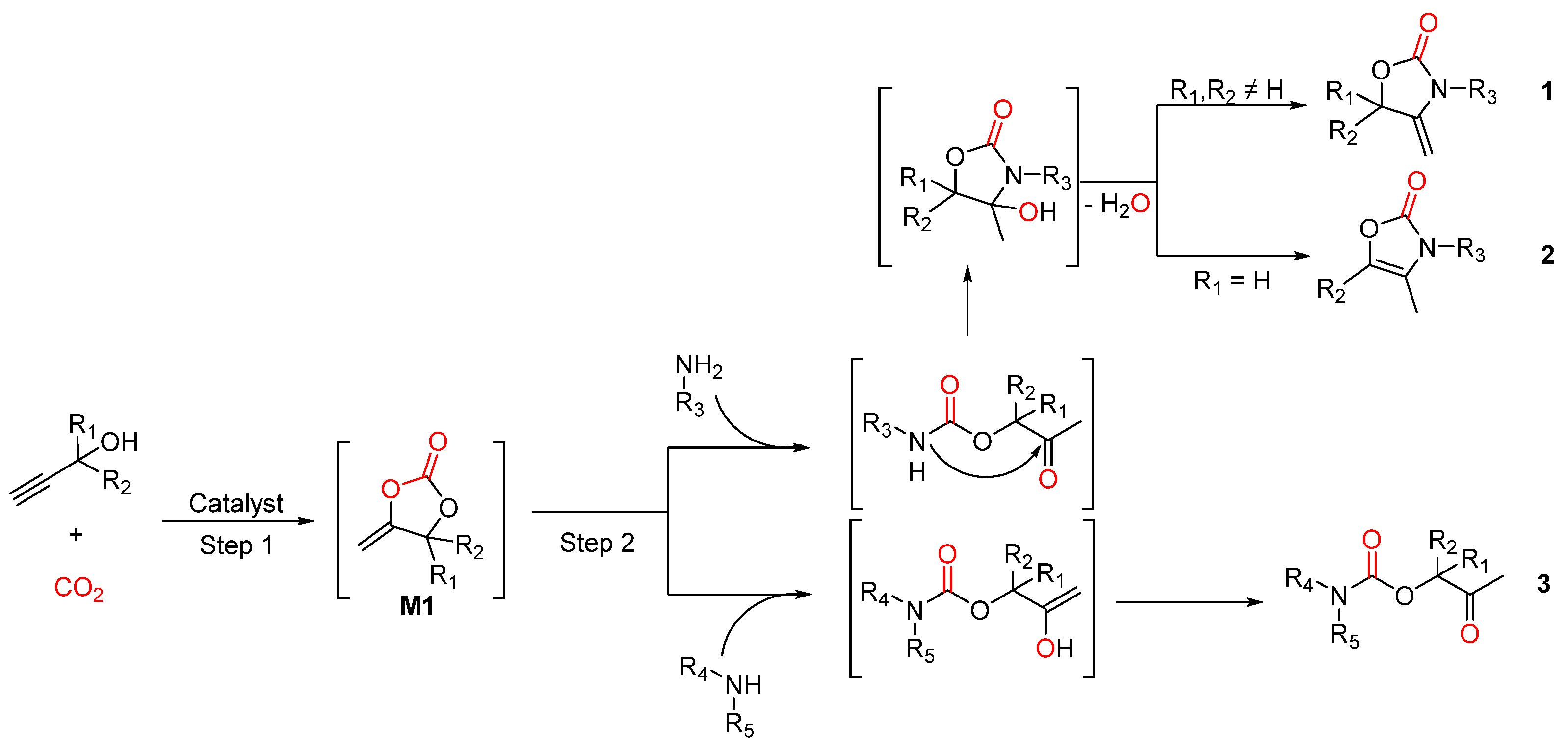
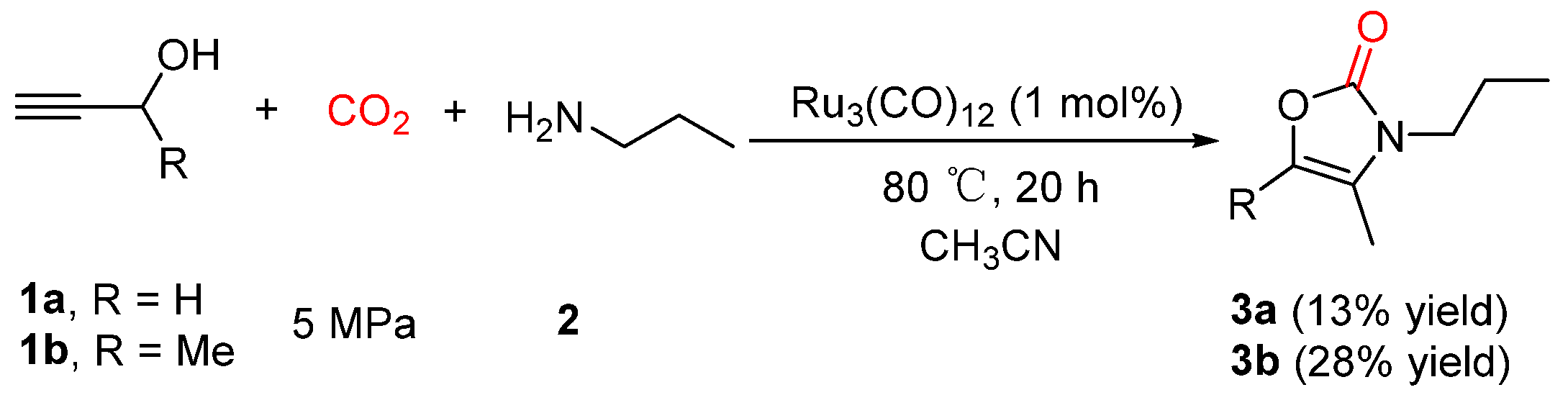
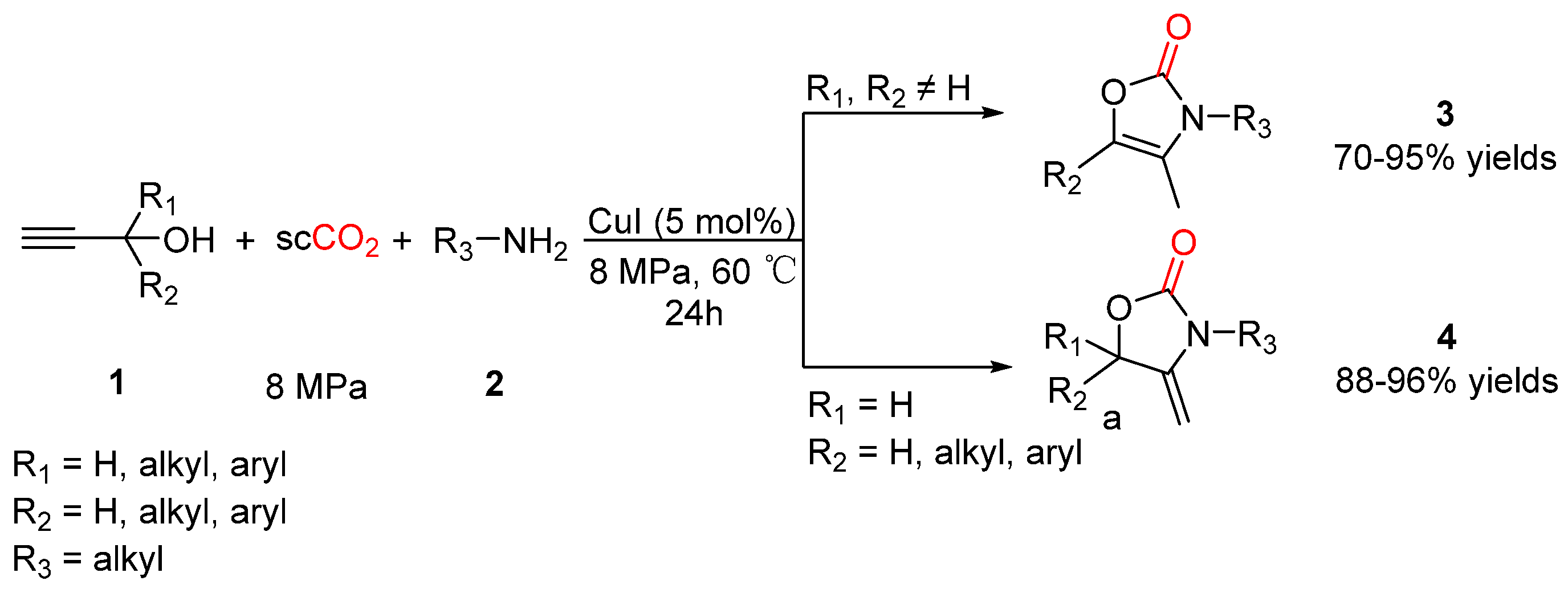
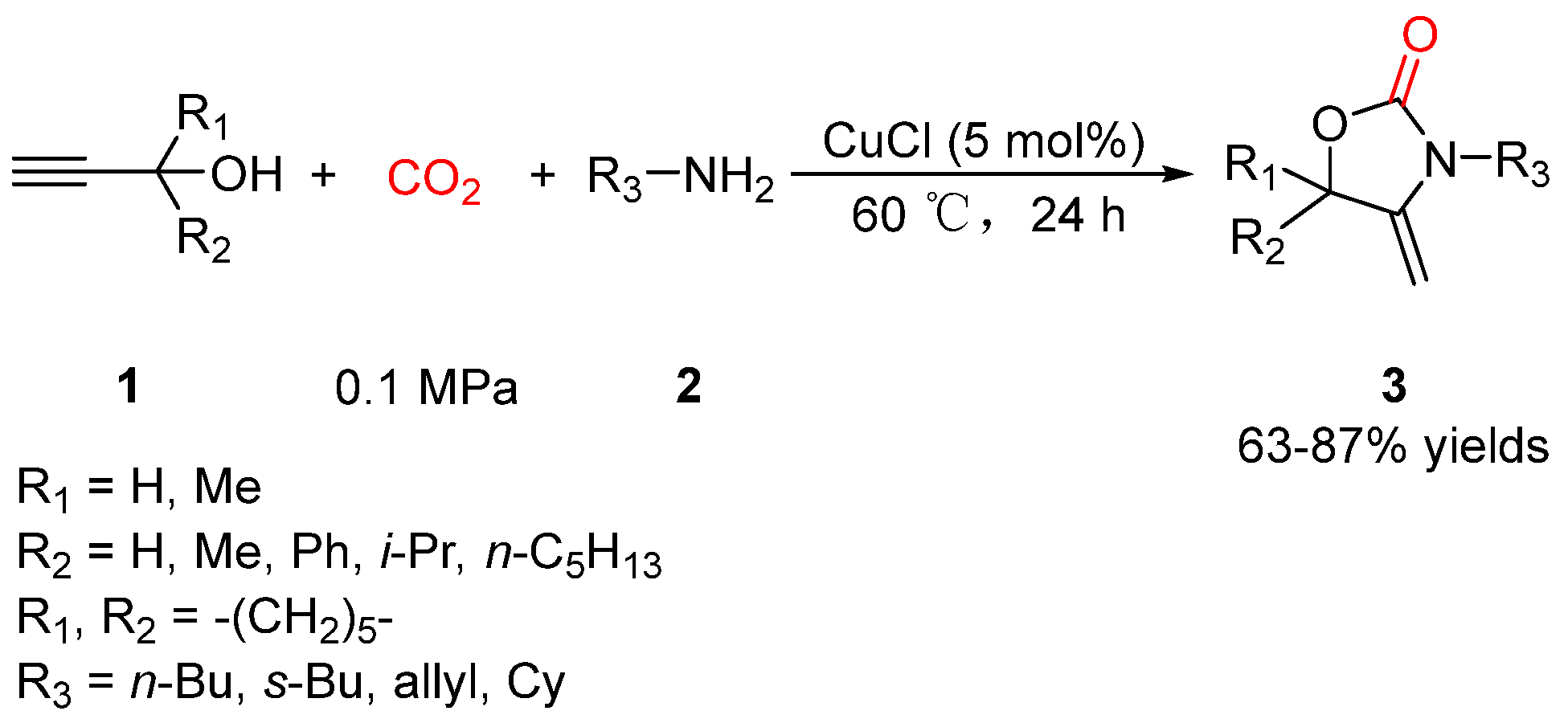
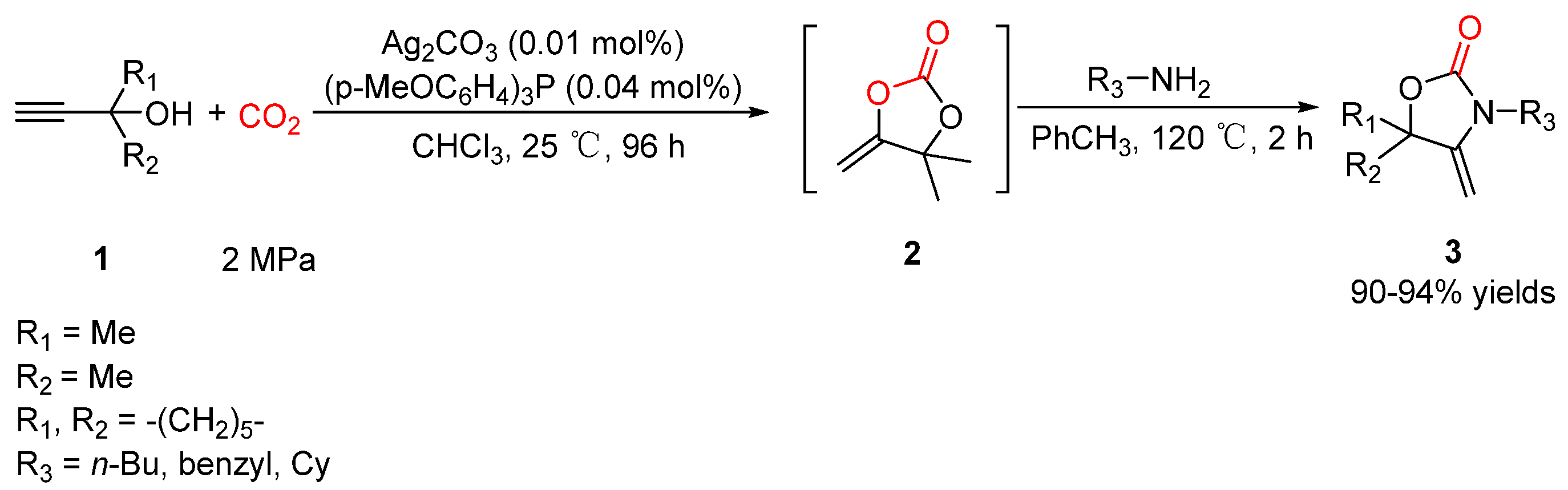
2.1. Oxazolidinones as Products
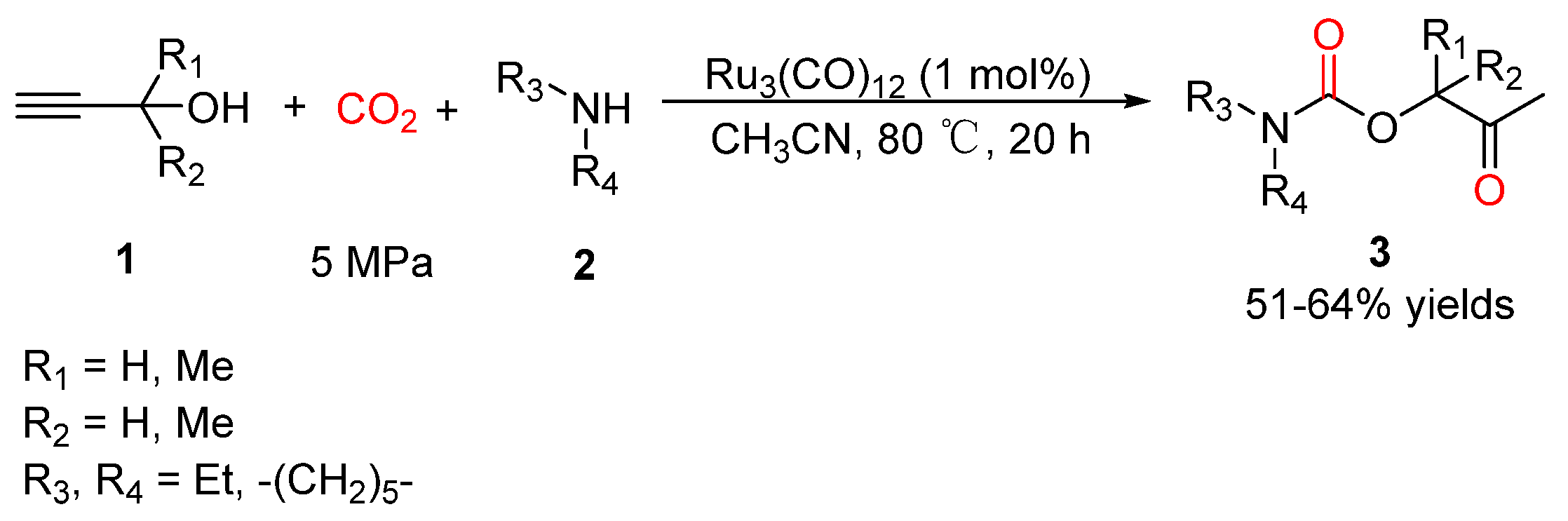
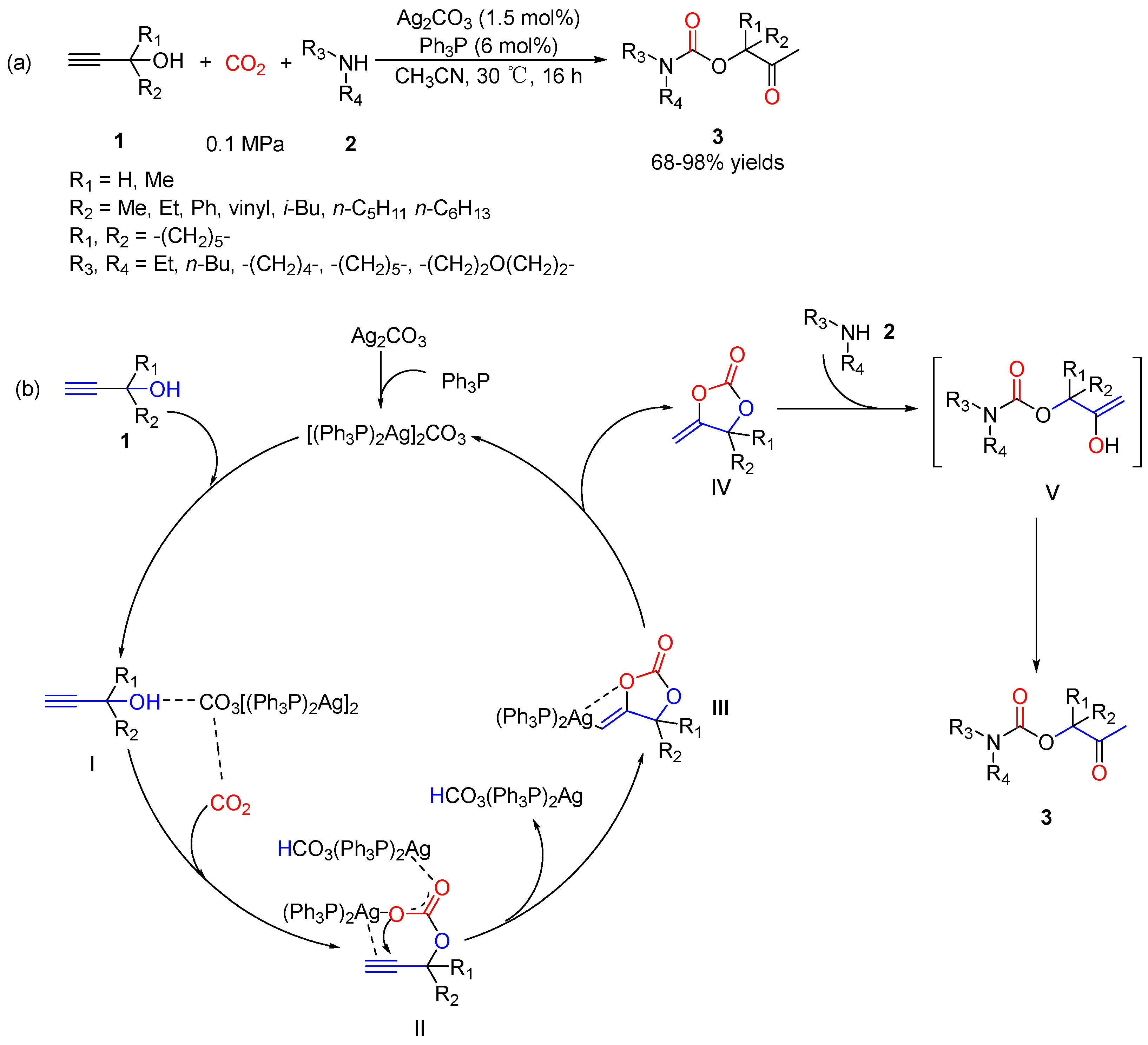
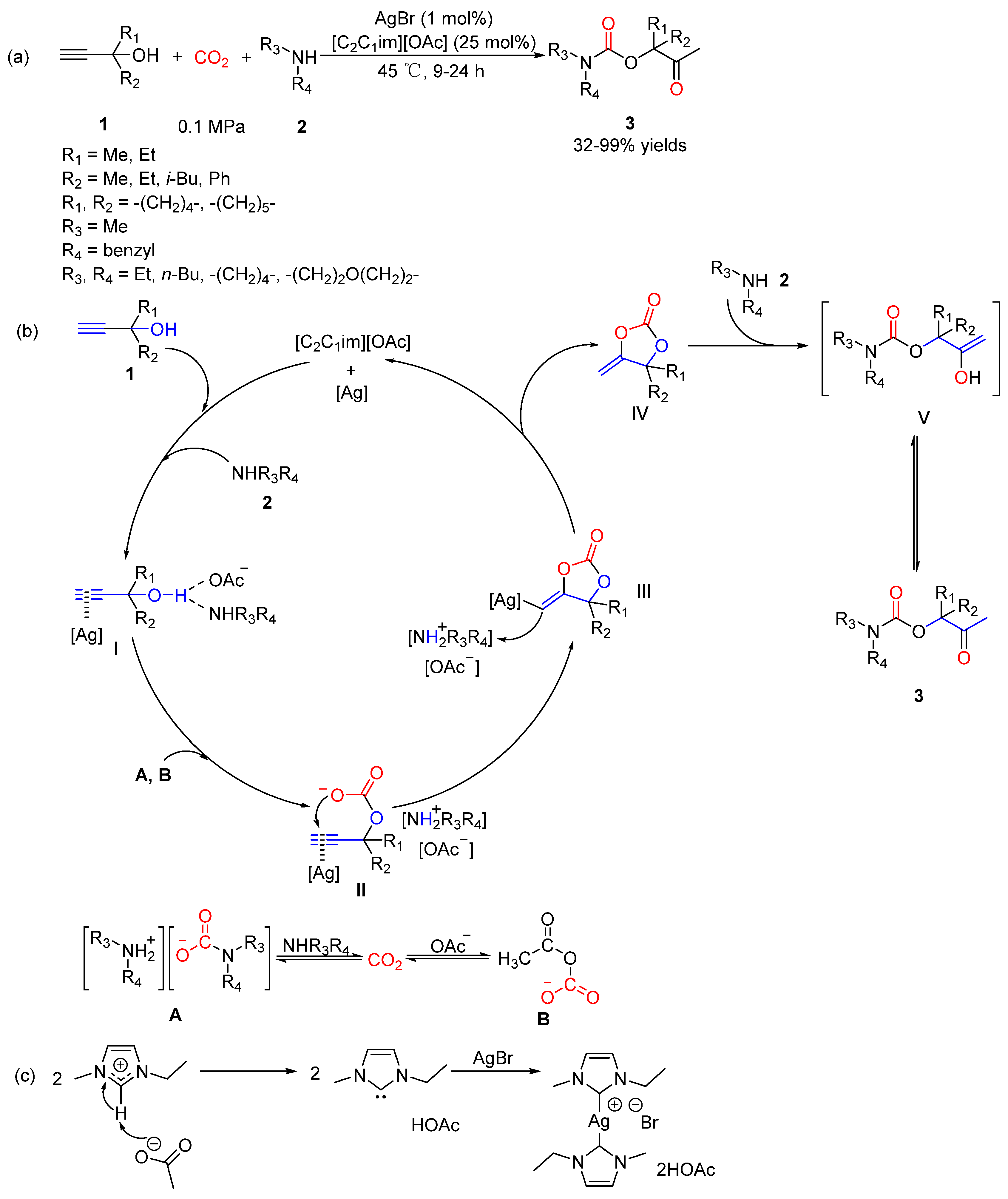
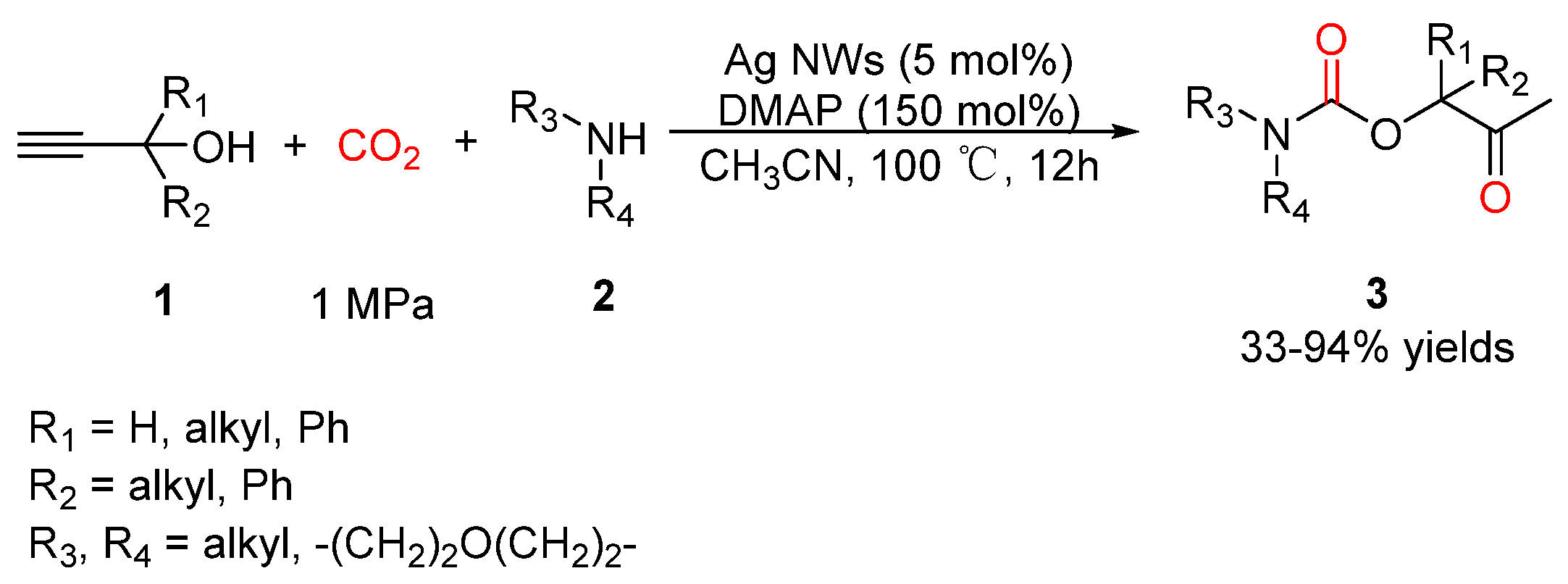
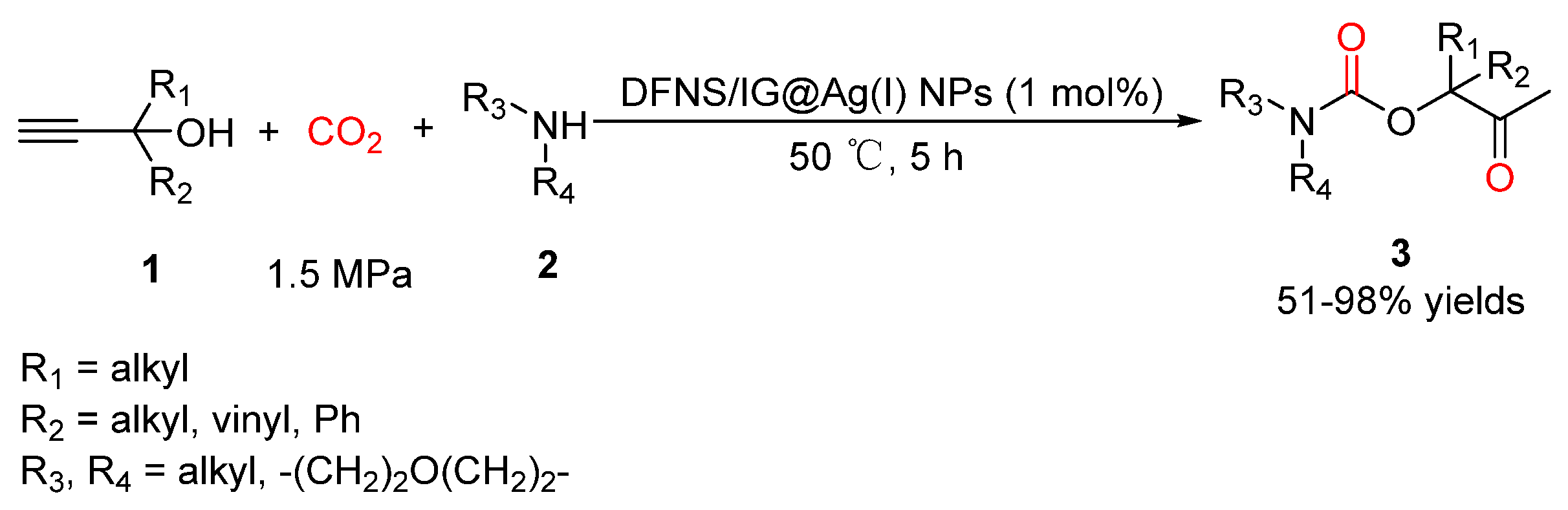
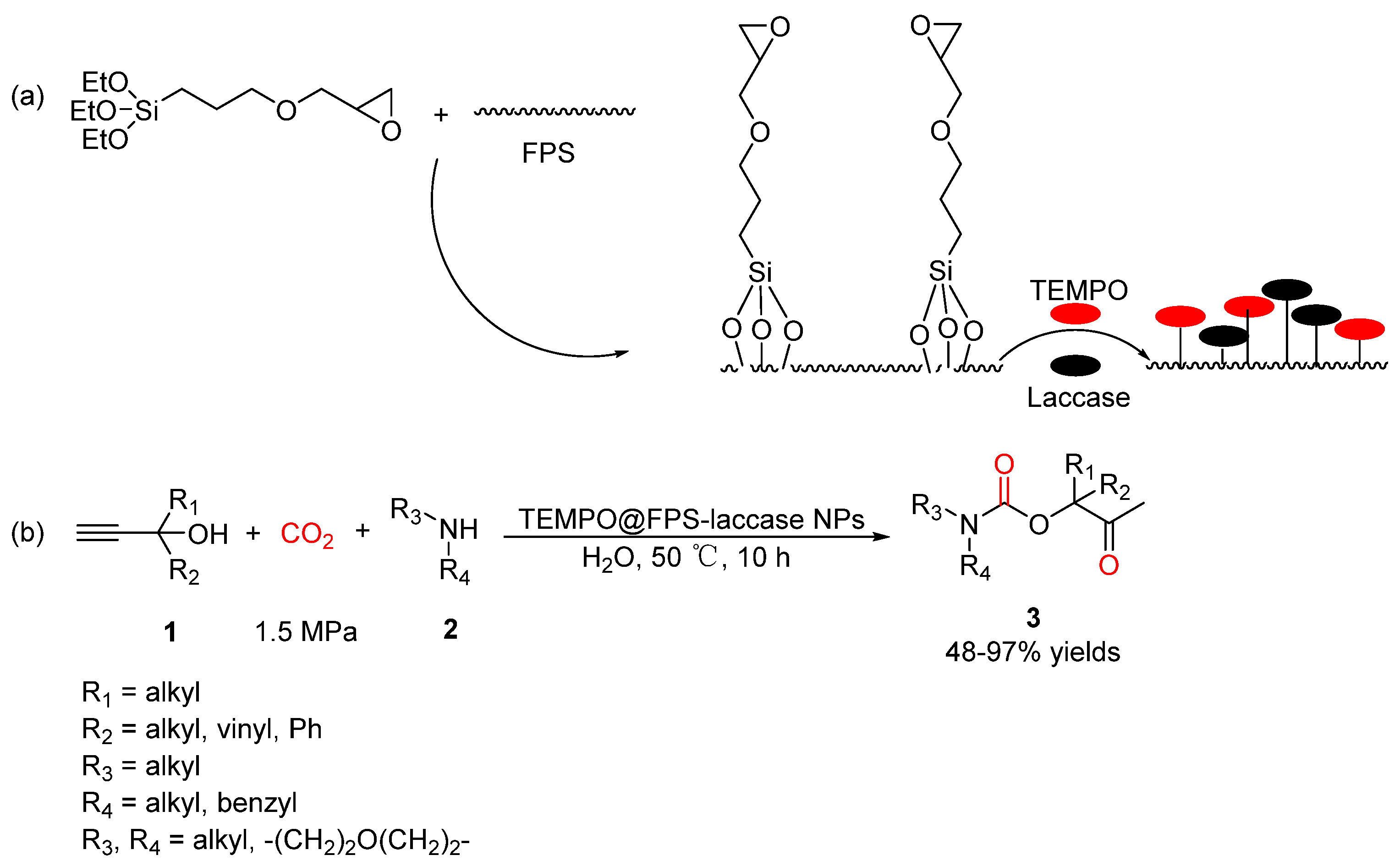
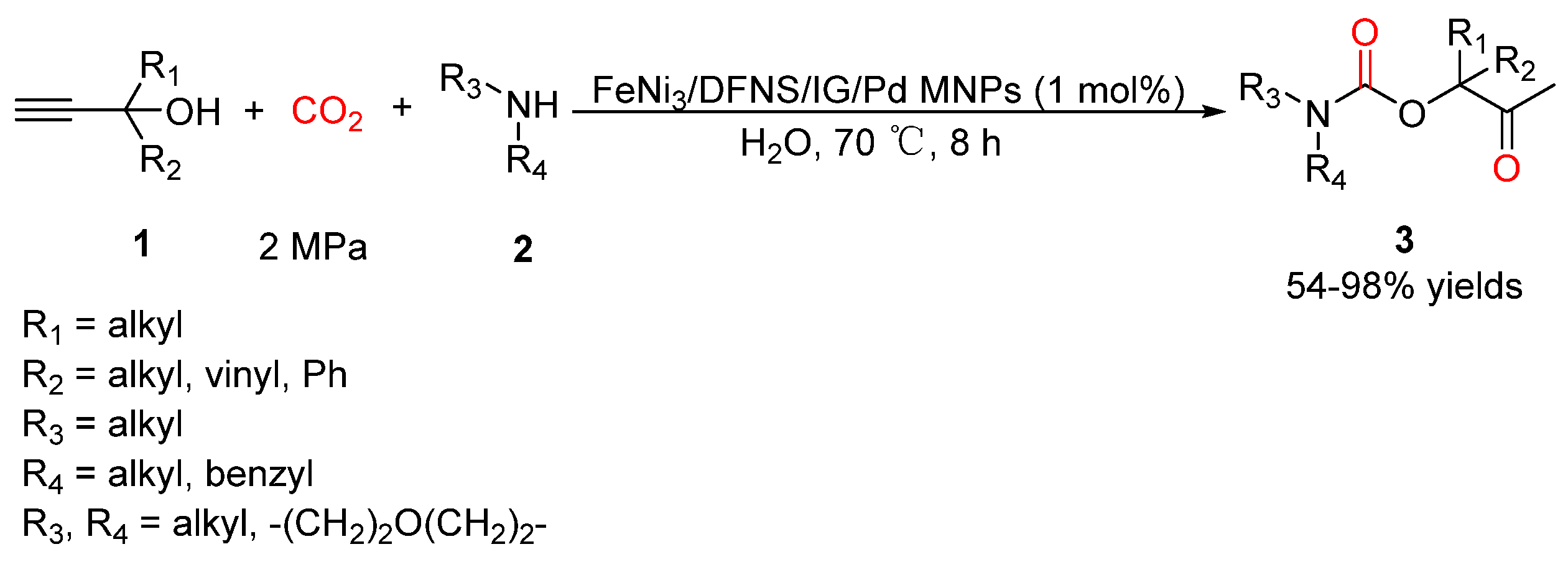
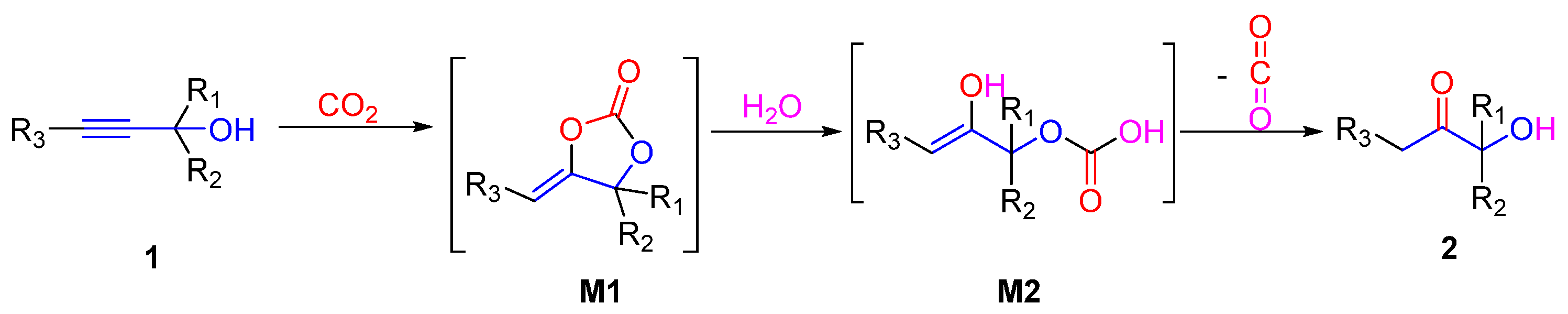
2.2. Carbamates as Products
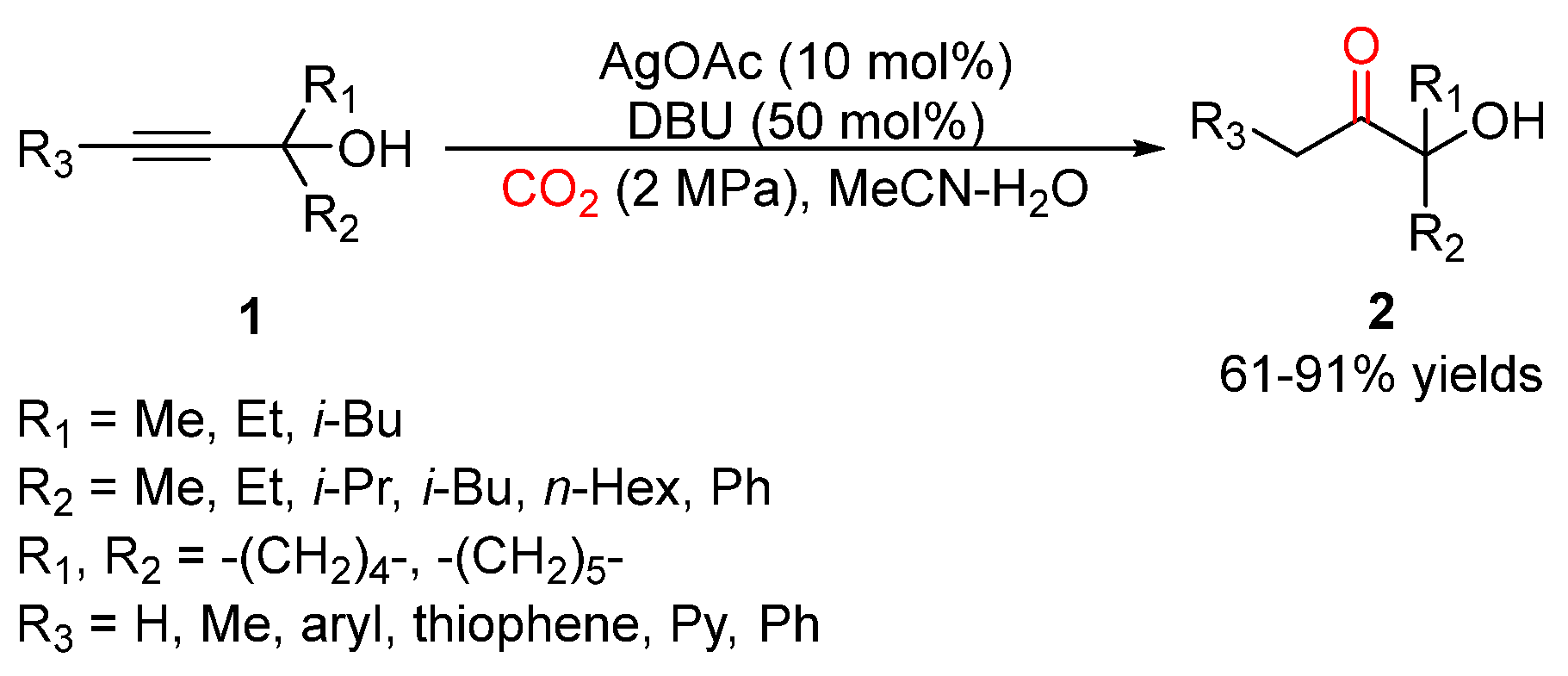
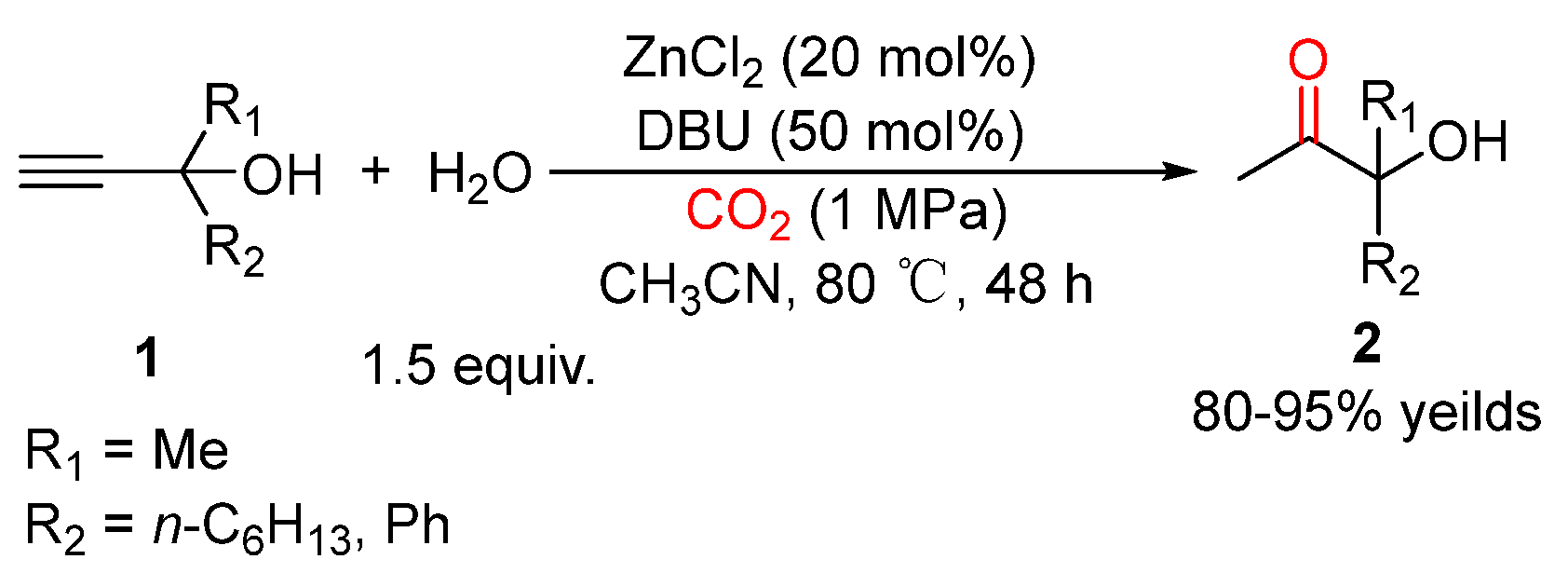
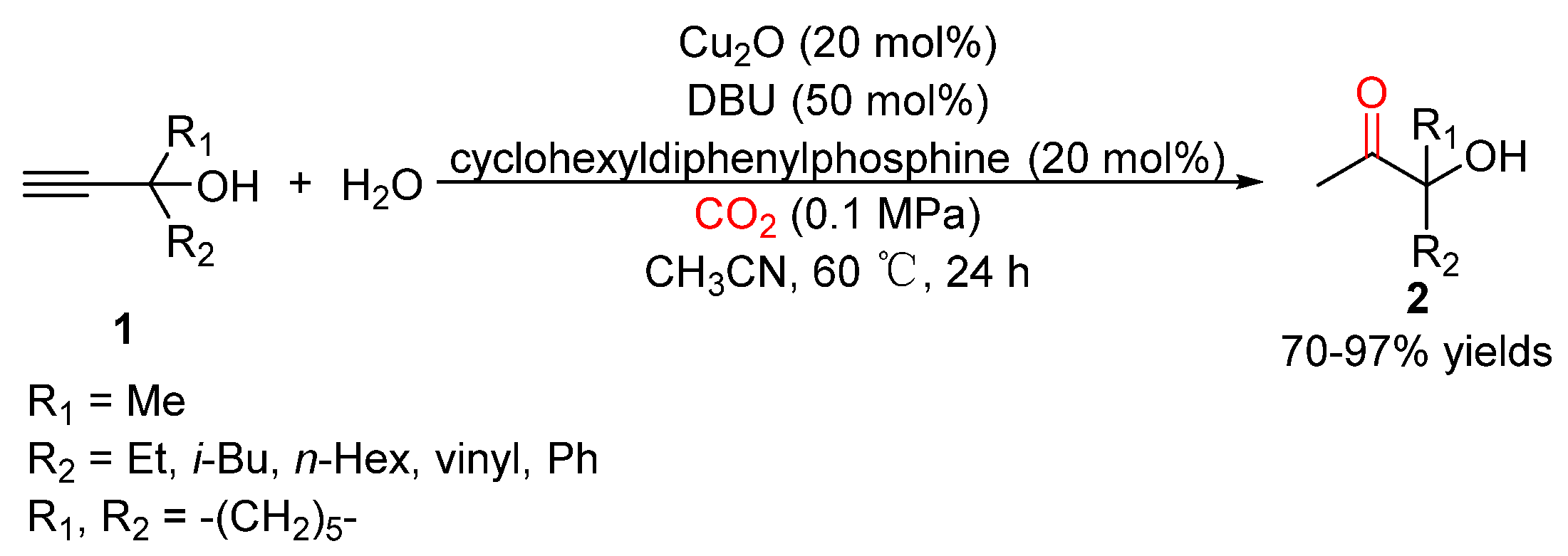
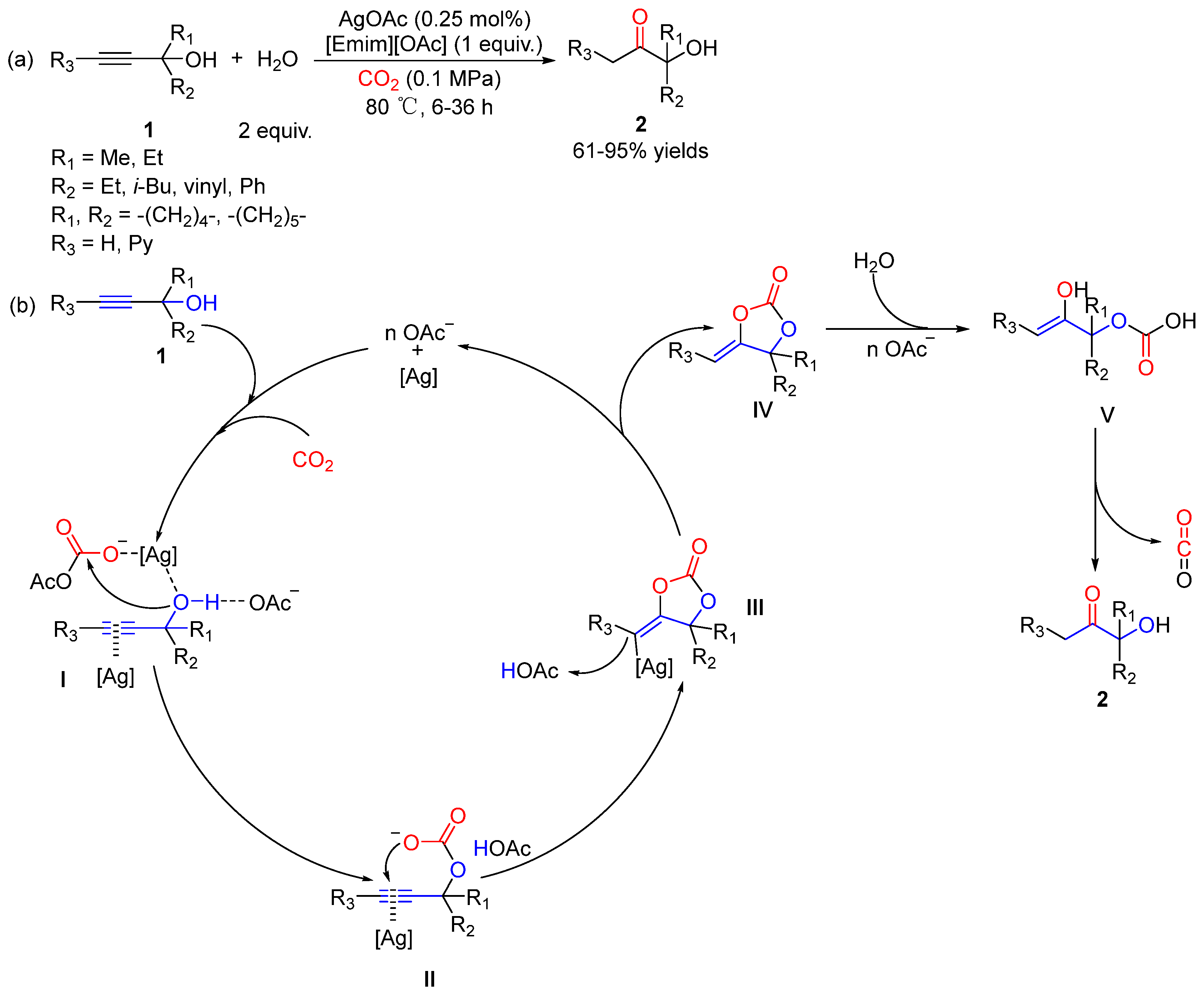
3. Three-Component Reactions of Propargylic Alcohols, CO2 and H2O
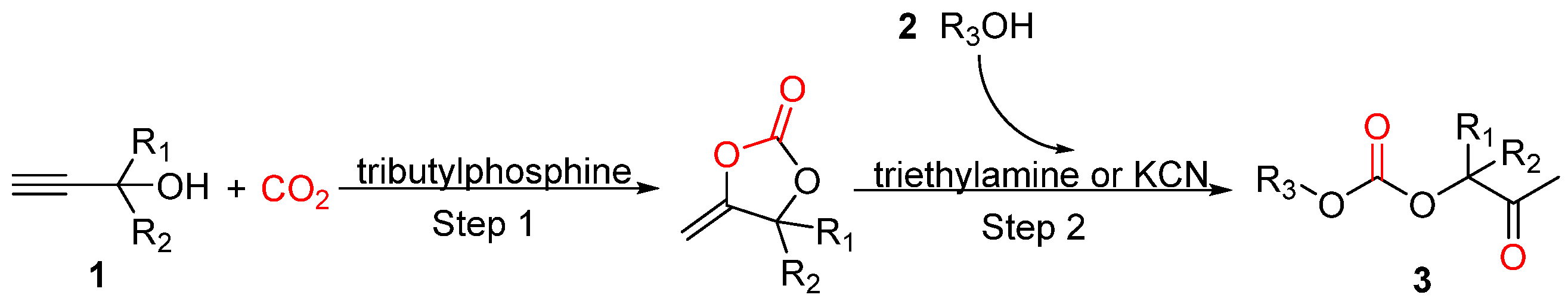
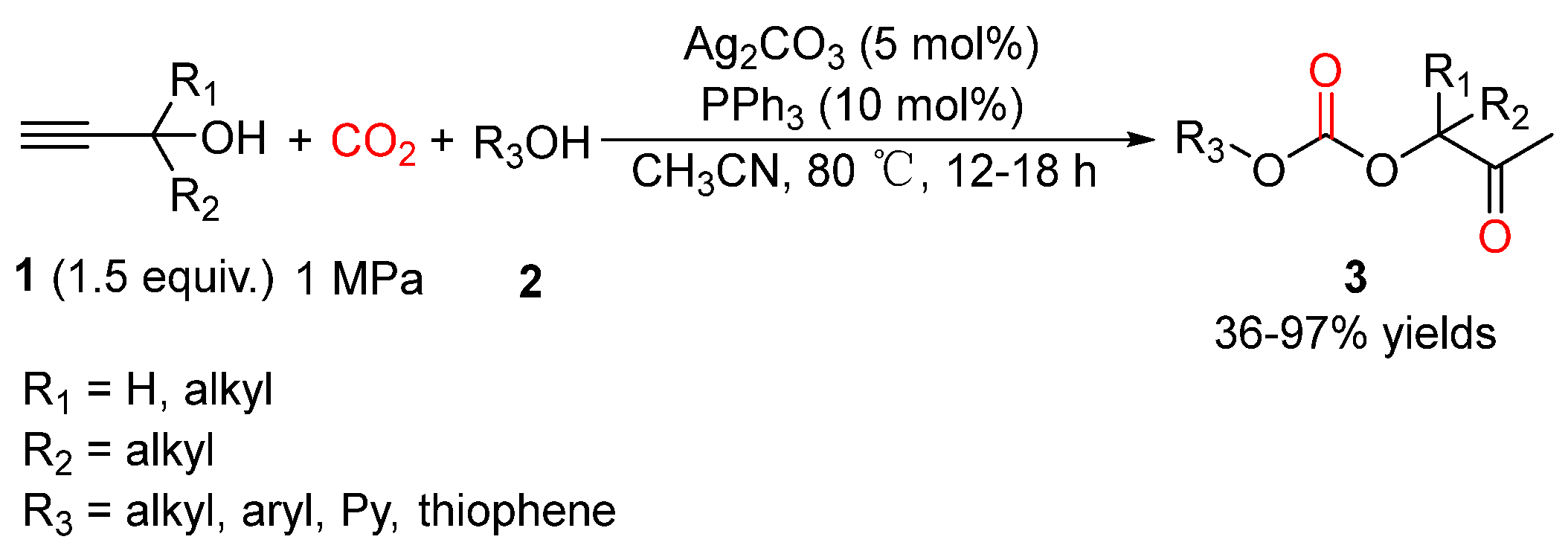
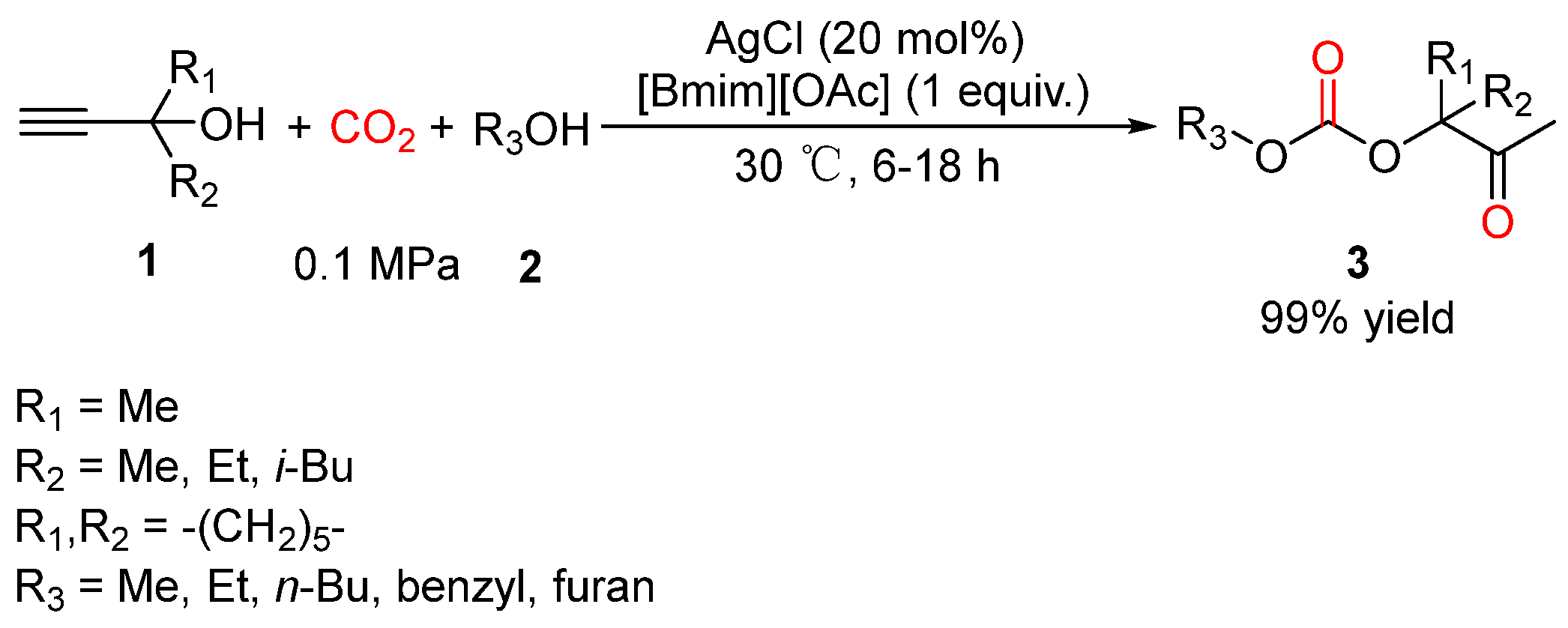
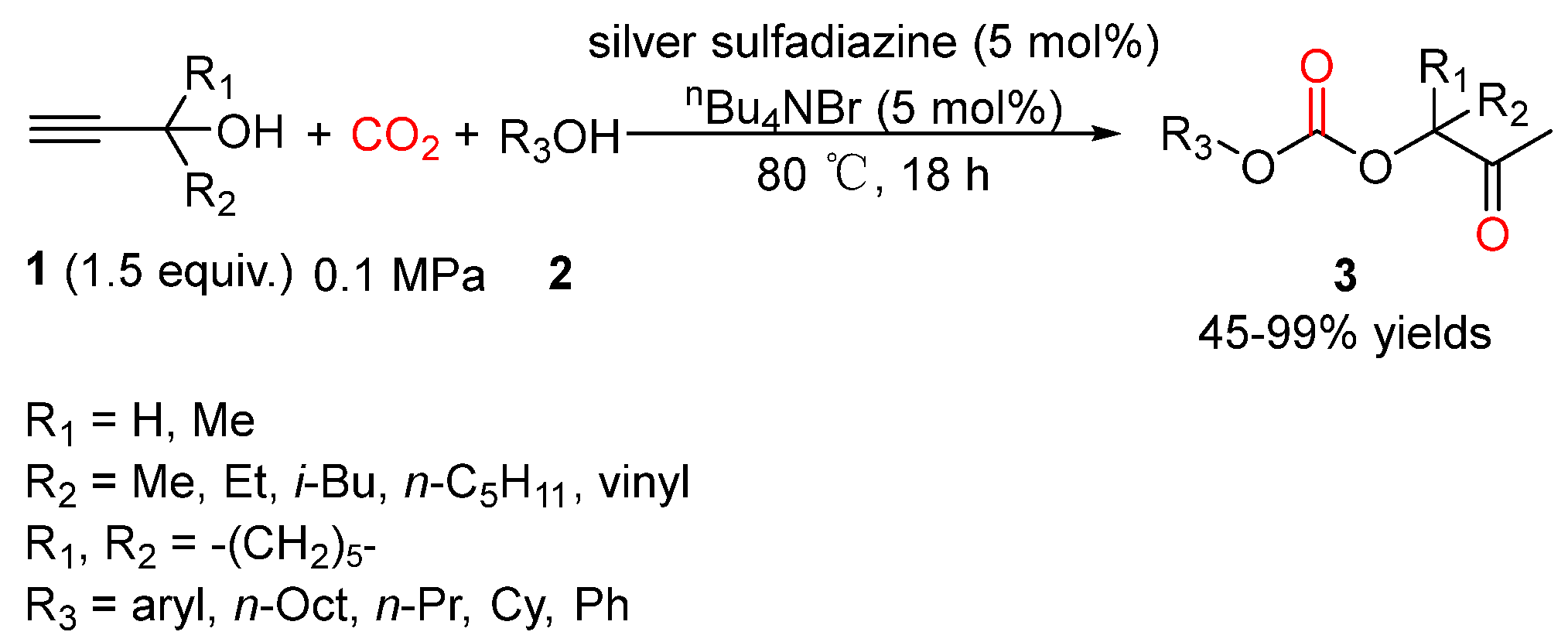
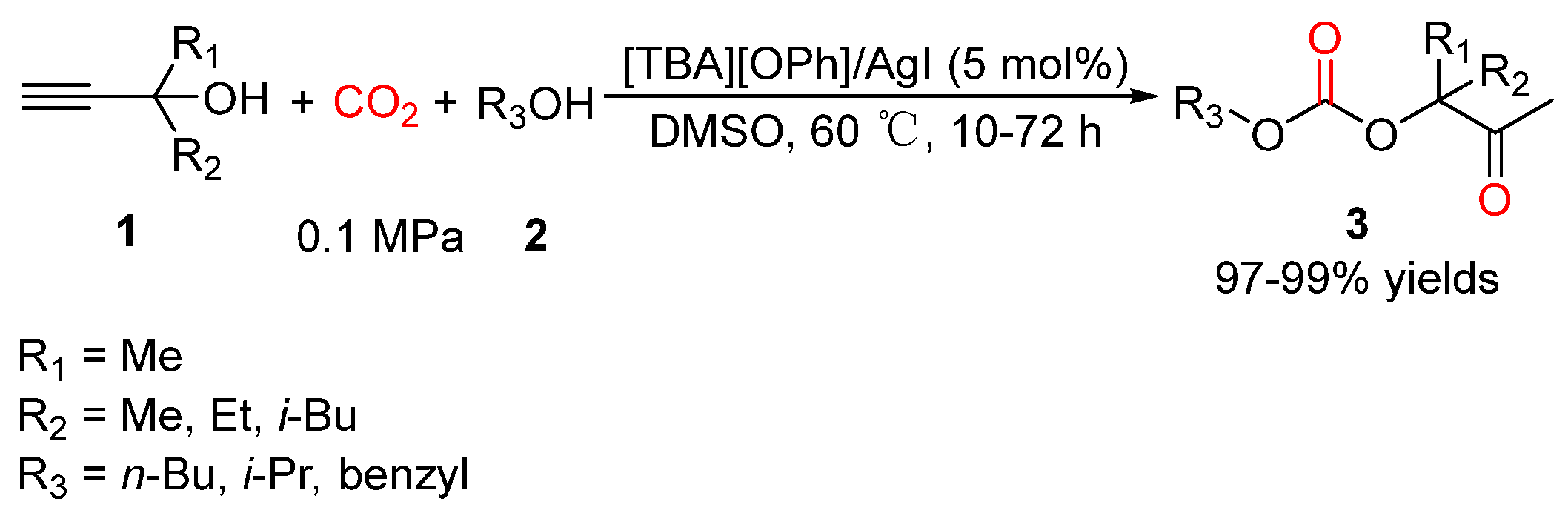
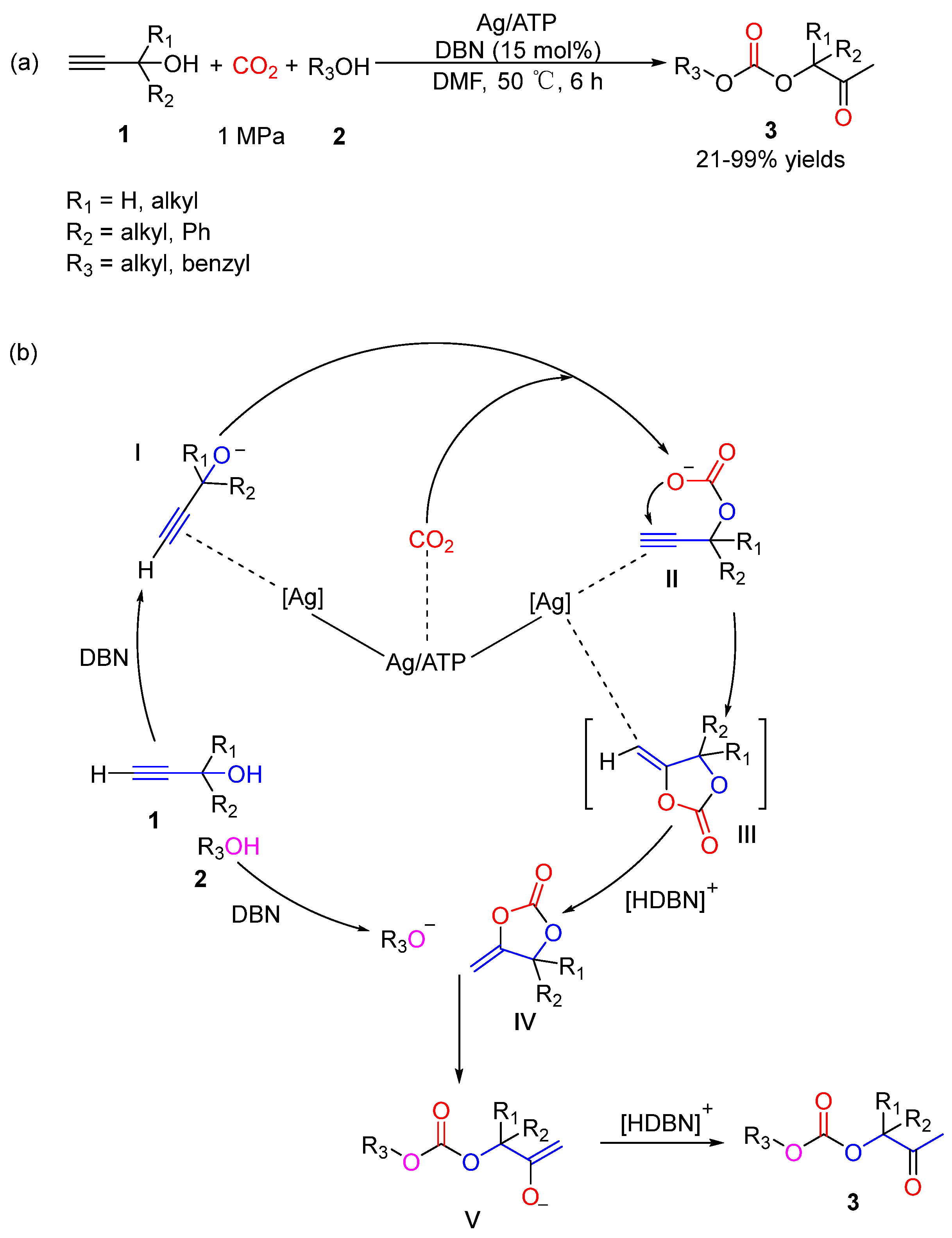
4. Three-Component Reactions of Propargylic Alcohols, CO2 and Monohydric Alcohols
5. Three-Component Reactions of Propargylic Alcohols, CO2 and Bi-Nucleophiles
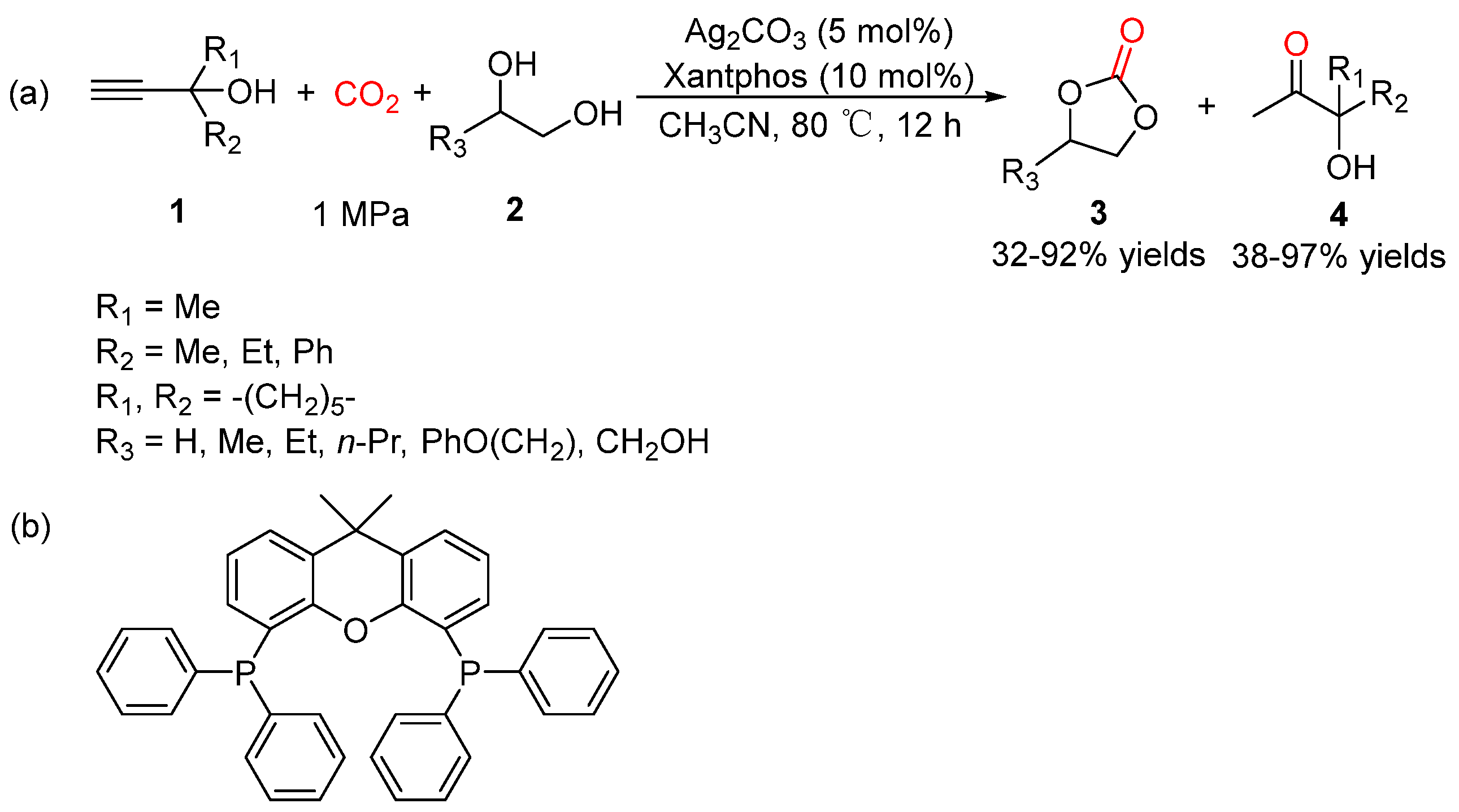
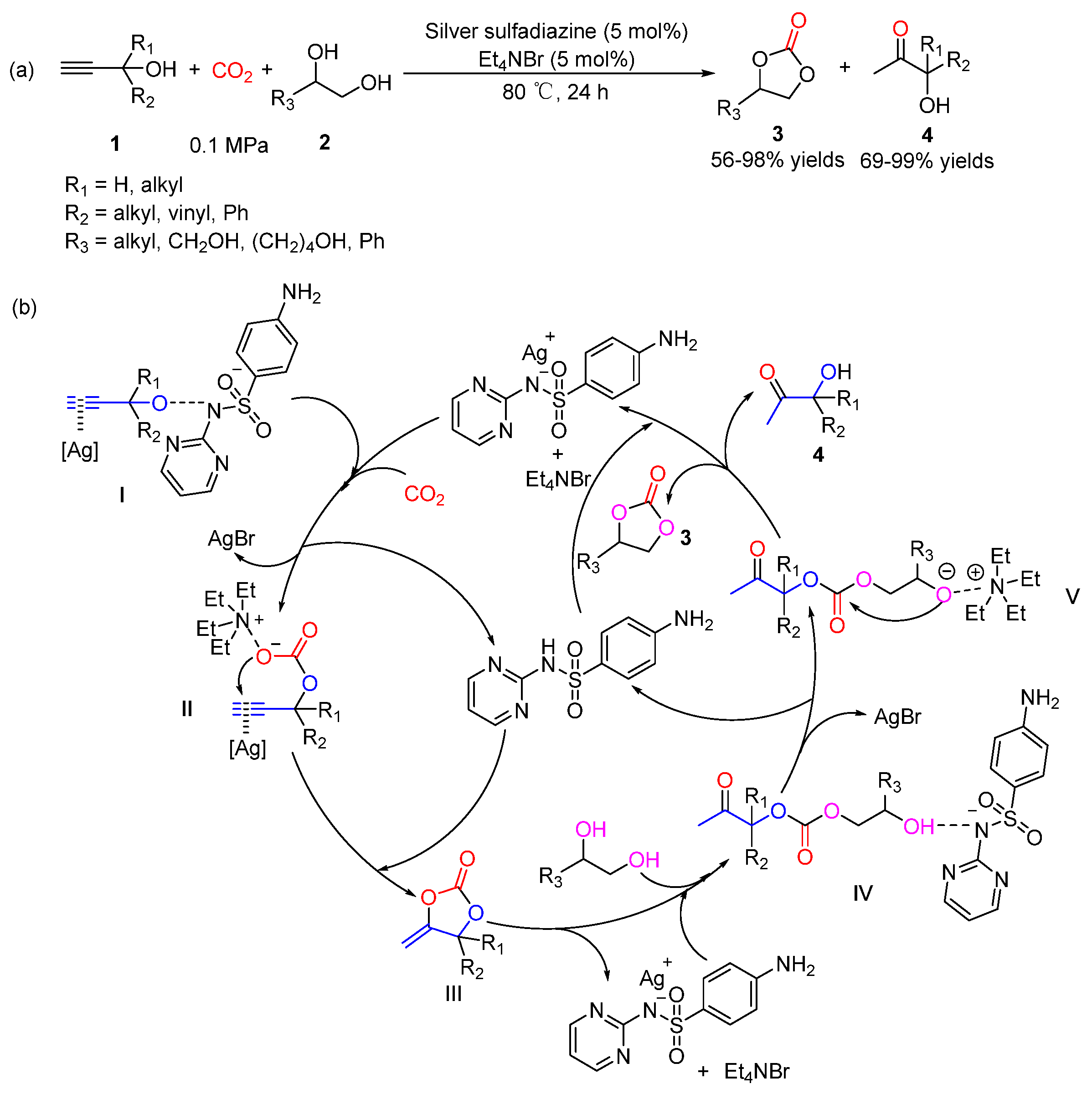
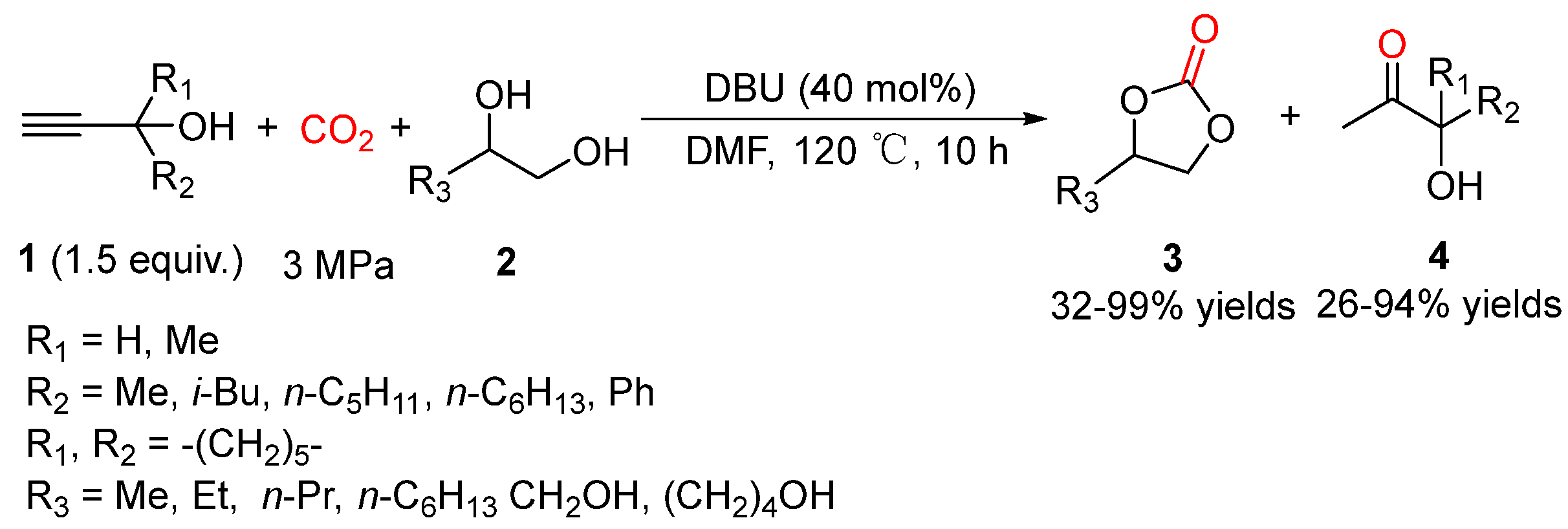
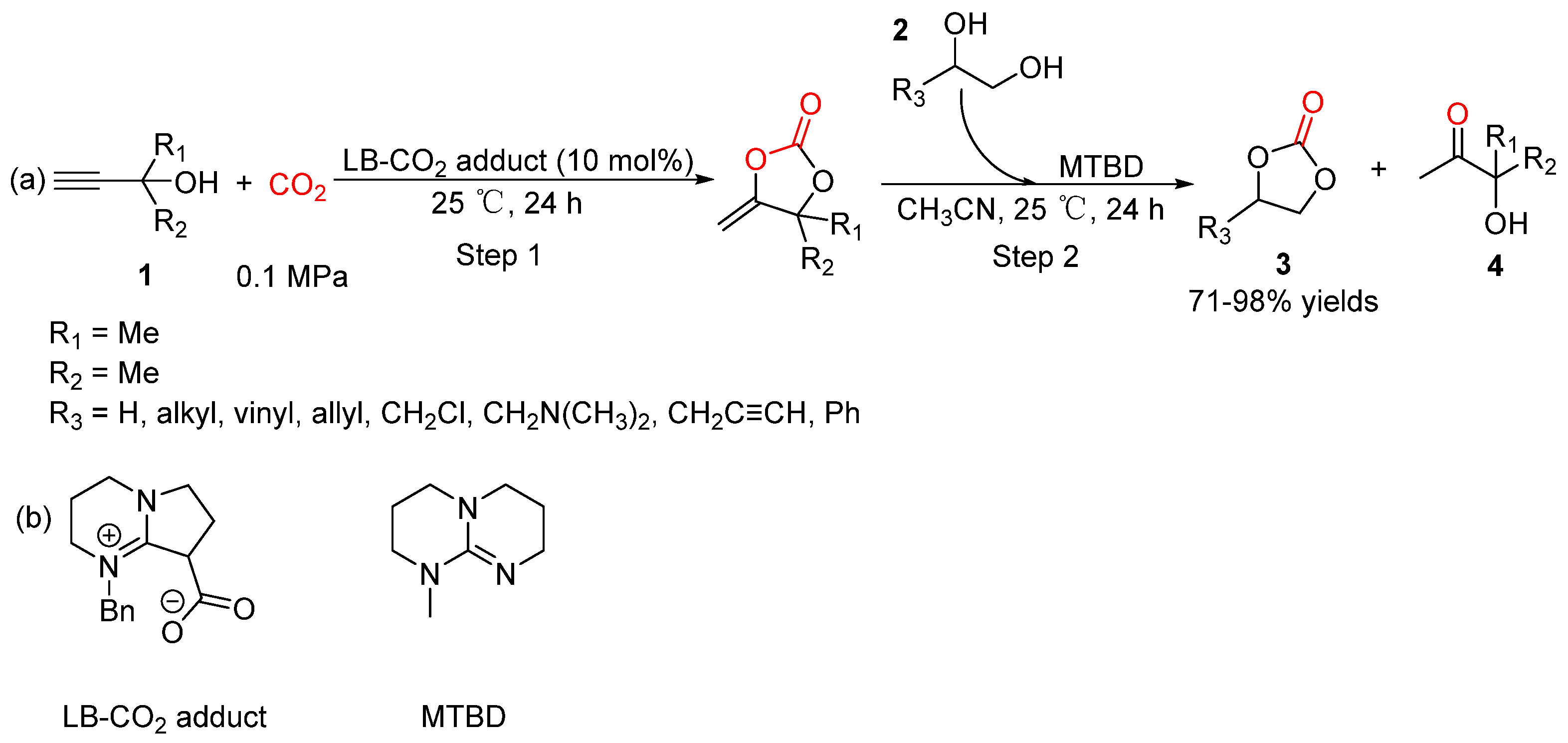
5.1. Vicinal Diols as Bi-Nucleophiles
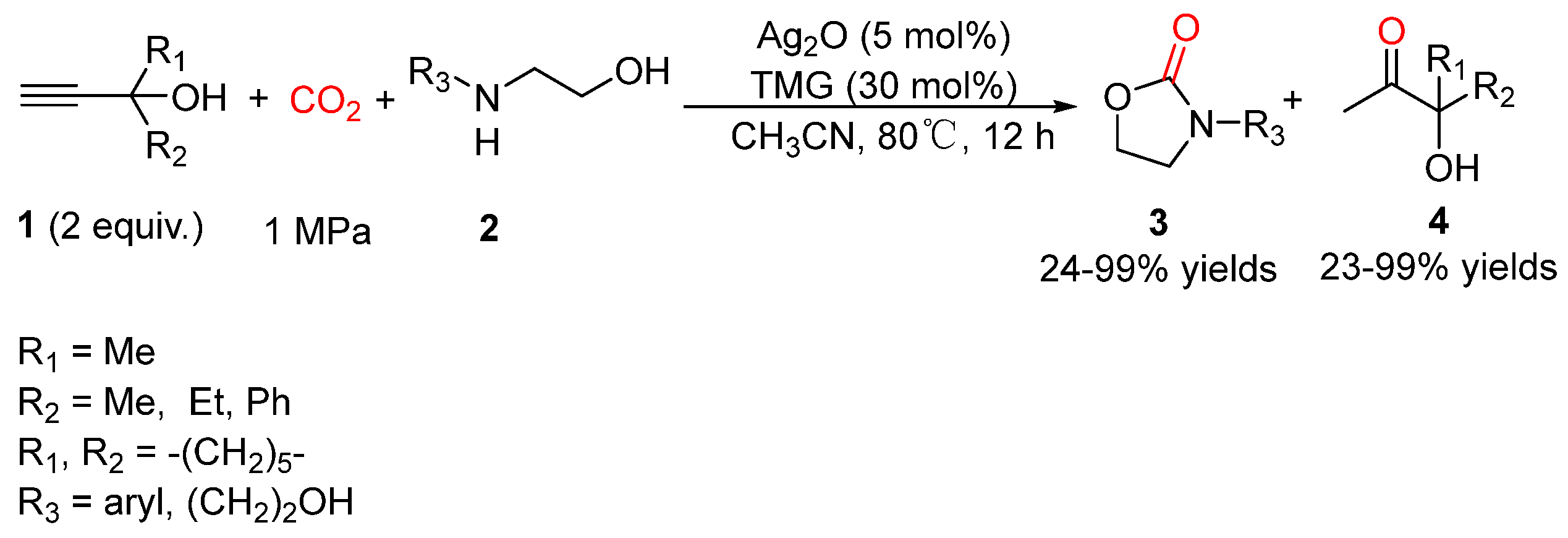
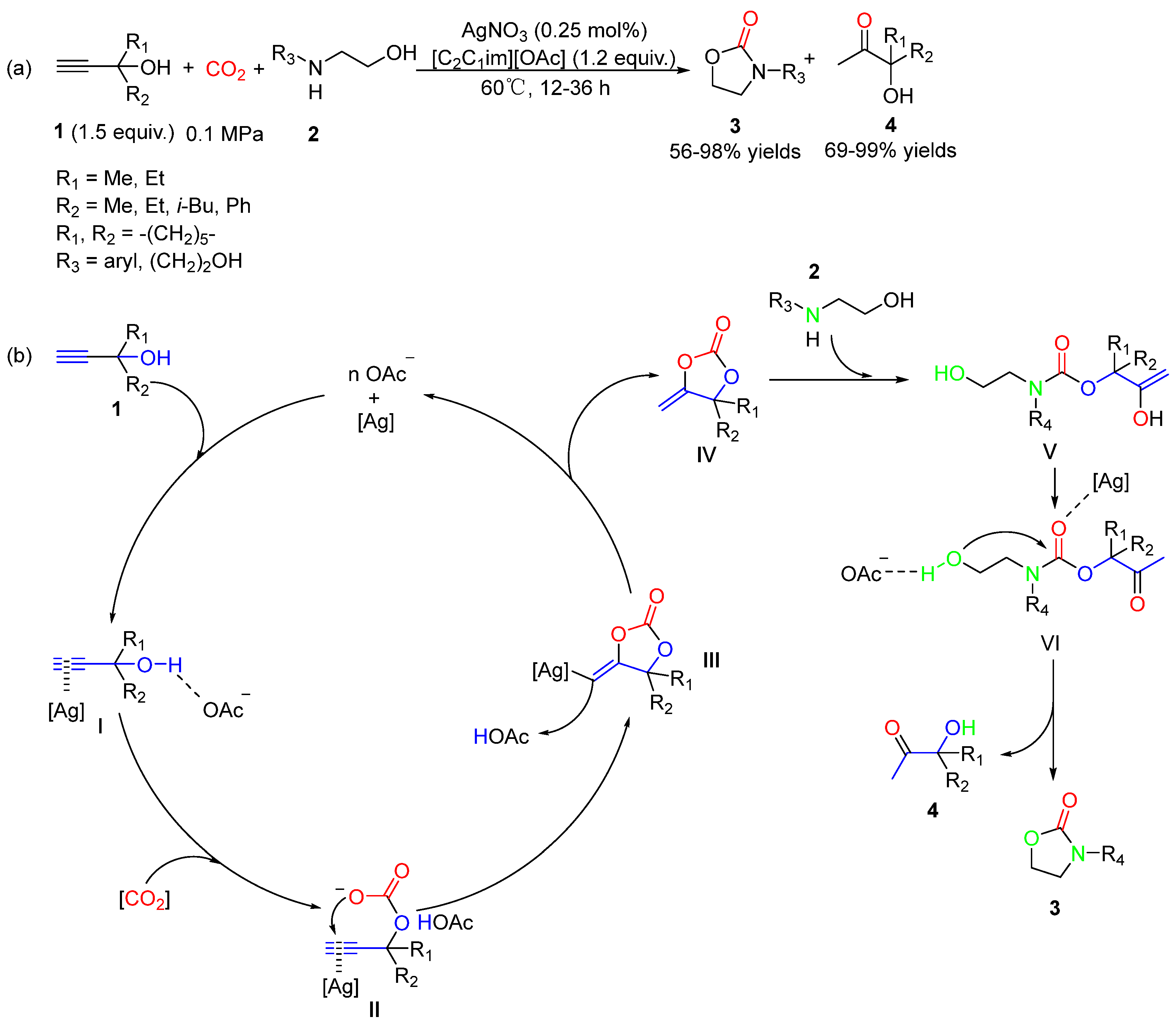
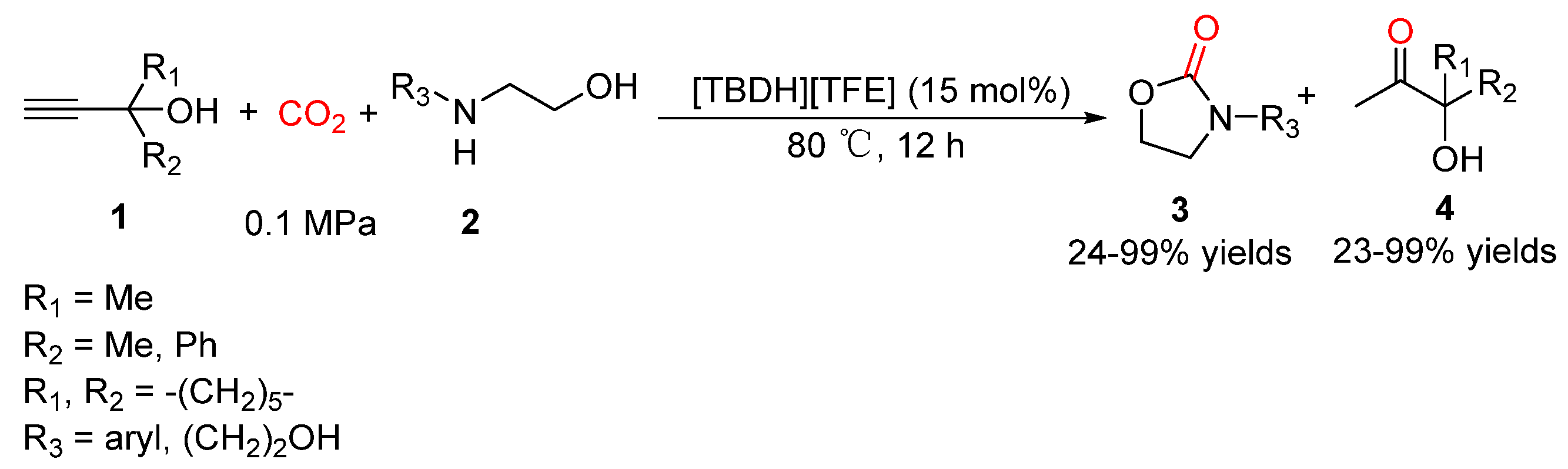
5.2. Aminoethanols as Bi-Nucleophiles
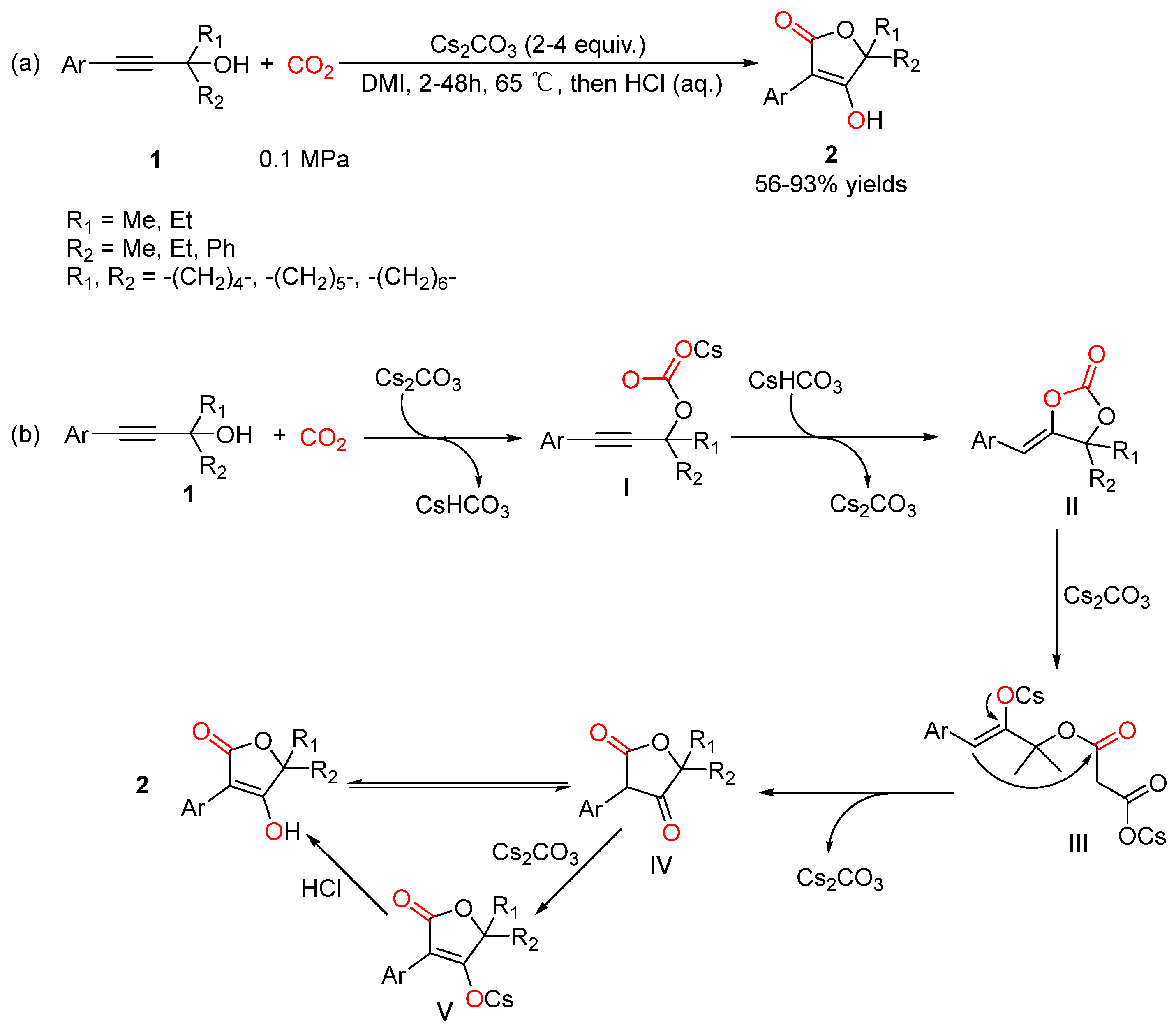
6. Isomerization of α-Alkylidene Cyclic Carbonates
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Federsel, C.; Jackstell, R.; Beller, M. State-of-the-Art Catalysts for Hydrogenation of Carbon Dioxide. Angew. Chem. Int. Ed. 2010, 49, 6254–6257. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Draper, A.M.; Weissburg, M.J. Impacts of Global Warming and Elevated CO2 on Sensory Behavior in Predator-Prey Interactions: A Review and Synthesis. Front. Ecol. Evol. 2019, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Burkart, M.D.; Hazari, N.; Tway, C.L.; Zeitler, E.L. Opportunities and Challenges for Catalysis in Carbon Dioxide Utilization. ACS Catal. 2019, 9, 7937–7956. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Scientific Basis for Integrating Carbon Resource Processing, Utilization, and Recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633. [Google Scholar] [CrossRef]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 2012, 5, 7281–7305. [Google Scholar] [CrossRef] [Green Version]
- Stolten, D. Efficient Carbon Capture for Coal Power Plants. Chem. Eng. Technol. 2012, 35, 407. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Prakash, G.K.S.; Olah, G.A. Air as the renewable carbon source of the future: An overview of CO2 capture from the atmosphere. Energy Environ. Sci. 2012, 5, 7833–7853. [Google Scholar] [CrossRef]
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Pieri, T.; Nikitas, A.; Castillo-Castillo, A.; Angelis-Dimakis, A. Holistic Assessment of Carbon Capture and Utilization Value Chains. Environments 2018, 5, 108. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for the Direct Capture of CO2 from Ambient Air. Angew. Chem. Int. Ed. 2020, 59, 6984–7006. [Google Scholar] [CrossRef] [PubMed]
- Olfe-Kräutlein, B. Advancing CCU Technologies Pursuant to the SDGs: A Challenge for Policy Making. Front. Energy Res. 2020, 8, 198. [Google Scholar] [CrossRef]
- Ghiat, I.; Al-Ansari, T. A review of carbon capture and utilisation as a CO2 abatement opportunity within the EWF nexus. J. CO2 Util. 2021, 45, 101432. [Google Scholar] [CrossRef]
- Dalpozzo, R.; Della Ca’, N.; Gabriele, B.; Mancuso, R. Recent Advances in the Chemical Fixation of Carbon Dioxide: A Green Route to Carbonylated Heterocycle Synthesis. Catalysts 2019, 9, 511. [Google Scholar] [CrossRef] [Green Version]
- Ding, P.; Zhao, H.; Li, T.; Luo, Y.; Fan, G.; Chen, G.; Gao, S.; Shi, X.; Lu, S.; Sun, X. Metal-based electrocatalytic conversion of CO2 to formic acid/formate. J. Mater. Chem. A 2020, 8, 21947–21960. [Google Scholar] [CrossRef]
- Wang, G.; Chen, J.; Ding, Y.; Cai, P.; Yi, L.; Li, Y.; Tu, C.; Hou, Y.; Wen, Z.; Dai, L. Electrocatalysis for CO2 conversion: From fundamentals to value-added products. Chem. Soc. Rev. 2021, 50, 4993–5061. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward Solar Fuels: Photocatalytic Conversion of Carbon Dioxide to Hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef]
- Joos, L.; Huck, J.M.; Van Speybroeck, V.; Smit, B. Cutting the cost of carbon capture: A case for carbon capture and utilization. Faraday Discuss. 2016, 192, 391–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, T.H.; Bisinella, V. Climate change impacts of introducing carbon capture and utilisation (CCU) in waste incineration. Waste Manag. 2021, 126, 754–770. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yang, J.; Kim, D.; Gim, H.; Choi, W.Y.; Lee, J.W. Review of recent technologies for transforming carbon dioxide to carbon materials. Chem. Eng. J. 2022, 427, 130980. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Wang, J.; Morsi, B.; Li, B. Carbon Dioxide Conversion to Nanomaterials: Methods, Applications, and Challenges. Energy Fuels 2021, 35, 11820–11834. [Google Scholar] [CrossRef]
- Mohsin, I.; Al-Attas, T.A.; Sumon, K.Z.; Bergerson, J.; McCoy, S.; Kibria, M.G. Economic and Environmental Assessment of Integrated Carbon Capture and Utilization. Cell Rep. Phys. Sci. 2020, 1, 100104. [Google Scholar] [CrossRef]
- Huang, K.; Sun, C.-L.; Shi, Z.-J. Transition-metal-catalyzed C–C bond formation through the fixation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 2435–2452. [Google Scholar] [CrossRef]
- Pulla, S.; Felton, C.M.; Ramidi, P.; Gartia, Y.; Ali, N.; Nasini, U.B.; Ghosh, A. Advancements in oxazolidinone synthesis utilizing carbon dioxide as a C1 source. J. CO2 Util. 2013, 2, 49–57. [Google Scholar] [CrossRef]
- Pescarmona, P.P.; Taherimehr, M. Challenges in the catalytic synthesis of cyclic and polymeric carbonates from epoxides and CO2. Catal. Sci. Technol. 2012, 2, 2169–2187. [Google Scholar] [CrossRef]
- Artz, J.; Müller, T.E.; Thenert, K.M.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef]
- Ganesh, K.N.; Zhang, D.; Miller, S.J.; Rossen, K.; Chirik, P.J.; Kozlowski, M.C.; Zimmerman, J.B.; Brooks, B.W.; Savage, P.E.; Allen, D.T.; et al. Green Chemistry: A Framework for a Sustainable Future. Environ. Sci. Technol. Lett. 2021, 8, 487–491. [Google Scholar] [CrossRef]
- Kuchurov, I.V.; Zharkov, M.N.; Fershtat, L.L.; Makhova, N.N.; Zlotin, S.G. Prospective Symbiosis of Green Chemistry and Energetic Materials. ChemSusChem 2017, 10, 3914–3946. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; He, L.-N.; Gao, J.; Liu, A.-H.; Yu, B. Carbon dioxide utilization with C–N bond formation: Carbon dioxide capture and subsequent conversion. Energy Environ. Sci. 2012, 5, 6602–6639. [Google Scholar] [CrossRef]
- Sekine, K.; Yamada, T. Silver-catalyzed carboxylation. Chem. Soc. Rev. 2016, 45, 4524–4532. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.-W.; Zhou, Z.-H.; He, L.-N. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707–3728. [Google Scholar] [CrossRef]
- Li, M.; Abdolmohammadi, S.; Hoseininezhad-Namin, M.S.; Behmagham, F.; Vessally, E. Carboxylative cyclization of propargylic alcohols with carbon dioxide: A facile and Green route to alpha-methylene cyclic carbonates. J. CO2 Util. 2020, 38, 220–231. [Google Scholar] [CrossRef]
- Comerford, J.W.; Ingram, I.D.V.; North, M.; Wu, X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem. 2015, 17, 1966–1987. [Google Scholar] [CrossRef]
- Gu, Y. Multicomponent reactions in unconventional solvents: State of the art. Green Chem. 2012, 14, 2091–2128. [Google Scholar] [CrossRef]
- Arshadi, S.; Vessally, E.; Hosseinian, A.; Soleimani-Amiri, S.; Edjlali, L. Three-component coupling of CO2, propargyl alcohols, and amines: An environmentally benign access to cyclic and acyclic carbamates (A Review). J. CO2 Util. 2017, 21, 108–118. [Google Scholar] [CrossRef]
- Gennen, S.; Grignard, B.; Tassaing, T.; Jérôme, C.; Detrembleur, C. CO2-Sourced α-Alkylidene Cyclic Carbonates: A Step Forward in the Quest for Functional Regioregular Poly(urethane)s and Poly(carbonate)s. Angew. Chem. Int. Ed. 2017, 56, 10394–10398. [Google Scholar] [CrossRef] [PubMed]
- Habets, T.; Siragusa, F.; Grignard, B.; Detrembleur, C. Advancing the Synthesis of Isocyanate-Free Poly(oxazolidones)s: Scope and Limitations. Macromolecules 2020, 53, 6396–6408. [Google Scholar] [CrossRef]
- He, H.; Qi, C.; Hu, X.; Guan, Y.; Jiang, H. Efficient synthesis of tertiary alpha -hydroxy ketones through CO2-promoted regioselective hydration of propargylic alcohols. Green Chem. 2014, 16, 3729–3733. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Z.; Yu, B.; Zhang, H.; Xu, H.; Hao, L.; Han, B.; Liu, Z. Task-specific ionic liquid and CO2-cocatalysed efficient hydration of propargylic alcohols to α-hydroxy ketones. Chem. Sci. 2015, 6, 2297–2301. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.-H.; Zhang, X.; Huang, Y.-F.; Chen, K.-H.; He, L.-N. Synthesis of α-hydroxy ketones by copper(I)-catalyzed hydration of propargylic alcohols: CO2 as a cocatalyst under atmospheric pressure. Chin. J. Catal. 2019, 40, 1345–1351. [Google Scholar] [CrossRef]
- Li, D.; Gong, Y.; Du, M.; Bu, C.; Chen, C.; Chaemcheun, S.; Hu, J.; Zhang, Y.; Yuan, Y.; Verpoort, F. CO2-Promoted Hydration of Propargylic Alcohols: Green Synthesis of α-Hydroxy Ketones by an Efficient and Recyclable AgOAc/Ionic Liquid System. ACS Sustain. Chem. Eng. 2020, 8, 8148–8155. [Google Scholar] [CrossRef]
- Zhou, Z.-H.; Song, Q.-W.; Xie, J.-N.; Ma, R.; He, L.-N. Silver(I)-Catalyzed Three-Component Reaction of Propargylic Alcohols, Carbon Dioxide and Monohydric Alcohols: Thermodynamically Feasible Access to beta-Oxopropyl Carbonates. Chem.—Asian J. 2016, 11, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-H.; Song, Q.-W.; He, L.-N. Silver(I)-Promoted Cascade Reaction of Propargylic Alcohols, Carbon Dioxide, and Vicinal Diols: Thermodynamically Favorable Route to Cyclic Carbonates. ACS Omega 2017, 2, 337–345. [Google Scholar] [CrossRef]
- Song, Q.-W.; Liu, P.; Zhao, Q.-N.; Li, J.-Y.; Zhang, K. Selective Conversion of CO2 and Switchable Alcohols into Linear or Cyclic Carbonates via Versatile Zinc Catalysis. Synthesis 2018, 51, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Song, Q.; Zhang, H.; Liu, P.; Zhang, K.; Wang, J.; Zhang, D. Synergistic Ag(I)/ Bu4NBr-catalyzed fixation of CO2 to β-oxopropyl carbonates via propargylic alcohols and monohydric alcohols. Tetrahedron 2019, 75, 2343–2349. [Google Scholar] [CrossRef]
- Li, J.-Y.; Han, L.-H.; Xu, Q.-C.; Song, Q.-W.; Liu, P.; Zhang, K. Cascade Strategy for Atmospheric Pressure CO2 Fixation to Cyclic Carbonates via Silver Sulfadiazine and Et4NBr Synergistic Catalysis. ACS Sustain. Chem. Eng. 2019, 7, 3378–3388. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, H.; Mu, S.; Zhang, W.-Z.; Ren, W.-M.; Lu, X.-B. Highly regio- and stereoselective synthesis of cyclic carbonates from biomass-derived polyolsviaorganocatalytic cascade reaction. Green Chem. 2019, 21, 6335–6341. [Google Scholar] [CrossRef]
- Guo, R.; Wang, G.; Liu, W. Clever use of natural clay materials in the synthesis of non-symmetric carbonates by utilizing CO2 as a feedstock: Ag/attapulgite nano-catalyst. Dalton Trans. 2020, 49, 10232–10239. [Google Scholar] [CrossRef]
- Ngassam Tounzoua, C.; Grignard, B.; Brege, A.; Jerome, C.; Tassaing, T.; Mereau, R.; Detrembleur, C. A Catalytic Domino Approach toward Oxo-Alkyl Carbonates and Polycarbonates from CO2, Propargylic Alcohols, and (Mono- and Di-)Alcohols. ACS Sustain. Chem. Eng. 2020, 8, 9698–9710. [Google Scholar] [CrossRef]
- Han, L.-H.; Li, J.-Y.; Song, Q.-W.; Zhang, K.; Zhang, Q.-X.; Sun, X.-F.; Liu, P. Thermodynamic favorable CO2 conversion via vicinal diols and propargylic alcohols: A metal-free catalytic method. Chin. Chem. Lett. 2020, 31, 341–344. [Google Scholar] [CrossRef]
- Song, Q.-W.; Zhou, Z.-H.; Wang, M.-Y.; Zhang, K.; Liu, P.; Xun, J.-Y.; He, L.-N. Thermodynamically Favorable Synthesis of 2-Oxazolidinones through Silver-Catalyzed Reaction of Propargylic Alcohols, CO2, and 2-Aminoethanols. ChemSusChem 2016, 9, 2054–2058. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.-N.; Liu, X.-H.; Li, T.; Ma, J.-G.; Cheng, P.; Yang, G.-M. Composite System of Ag Nanoparticles and Metal–Organic Frameworks for the Capture and Conversion of Carbon Dioxide under Mild Conditions. Inorg. Chem. 2017, 56, 3414–3420. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Cao, Y.; Ma, R.; He, L.-N. Thermodynamically favorable protocol for the synthesis of 2-oxazolidinones via Cu(I)-catalyzed three-component reaction of propargylic alcohols, CO2 and 2-aminoethanols. J. CO2 Util. 2018, 25, 338–345. [Google Scholar] [CrossRef]
- Xia, S.; Song, Y.; Li, X.; Li, H.; He, L.-N. Ionic Liquid-Promoted Three-Component Domino Reaction of Propargyl Alcohols, Carbon Dioxide and 2-Aminoethanols: A Thermodynamically Favorable Synthesis of 2-Oxazolidinones. Molecules 2018, 23, 3033. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Gong, Y.; Bu, C.; Hu, J.; Zhang, Y.; Chen, C.; Chaemchuen, S.; Yuan, Y.; Verpoort, F. An efficient and recyclable AgNO3/ionic liquid system catalyzed atmospheric CO2 utilization: Simultaneous synthesis of 2-oxazolidinones and α-hydroxyl ketones. J. Catal. 2021, 393, 70–82. [Google Scholar] [CrossRef]
- Bu, C.; Gong, Y.; Du, M.; Chen, C.; Chaemchuen, S.; Hu, J.; Zhang, Y.; Díaz Velázquez, H.; Yuan, Y.; Verpoort, F. Green Synthesis of 2-Oxazolidinones by an Efficient and Recyclable CuBr/Ionic Liquid System via CO2, Propargylic Alcohols and 2-Aminoethanols. Catalysts 2021, 11, 233. [Google Scholar] [CrossRef]
- Shen, G.; Zhou, W.-J.; Zhang, X.-B.; Cao, G.-M.; Zhang, Z.; Ye, J.-H.; Liao, L.-L.; Li, J.; Yu, D.-G. Synthesis of tetronic acids from propargylic alcohols and CO2. Chem. Commun. 2018, 54, 5610–5613. [Google Scholar] [CrossRef] [Green Version]
- Roger, C.; Roberts, J.A.; Muller, L. Clinical Pharmacokinetics and Pharmacodynamics of Oxazolidinones. Clin. Pharmacokinet. 2018, 57, 559–575. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, H.; Zhao, H.; Zhang, K.; Tian, Y. Application of carbamyl in structural optimization. Bioorgan. Chem. 2020, 98, 103757. [Google Scholar] [CrossRef]
- Bosak, A.; Bavec, A.; Konte, T.; Šinko, G.; Kovarik, Z.; Goličnik, M. Interactions of Paraoxonase-1 with Pharmacologically Relevant Carbamates. Molecules 2020, 25, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schilling, W.; Das, S. Transition Metal-Free Synthesis of Carbamates Using CO2 as the Carbon Source. ChemSusChem 2020, 13, 6246–6258. [Google Scholar] [CrossRef] [PubMed]
- Foti, C.; Piperno, A.; Scala, A.; Giuffrè, O. Oxazolidinone Antibiotics: Chemical, Biological and Analytical Aspects. Molecules 2021, 26, 4280. [Google Scholar] [CrossRef]
- Sasaki, Y.; Dixneuf, P.H. Ruthenium-catalyzed reaction of carbon dioxide, amine, and acetylenic alcohol. J. Org. Chem. 1987, 52, 4389–4391. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Q.; Duan, Z.; Zhang, J.; Zhang, A.S.; Deng, Y. Ionic Liquid as an Efficient Promoting Medium for Fixation of Carbon Dioxide: A Clean Method for the Synthesis of 5-Methylene-1,3-oxazolidin-2-ones from Propargylic Alcohols, Amines, and Carbon Dioxide Catalyzed by Cu(I) under Mild Conditions. J. Org. Chem. 2005, 70, 7376–7380. [Google Scholar] [CrossRef]
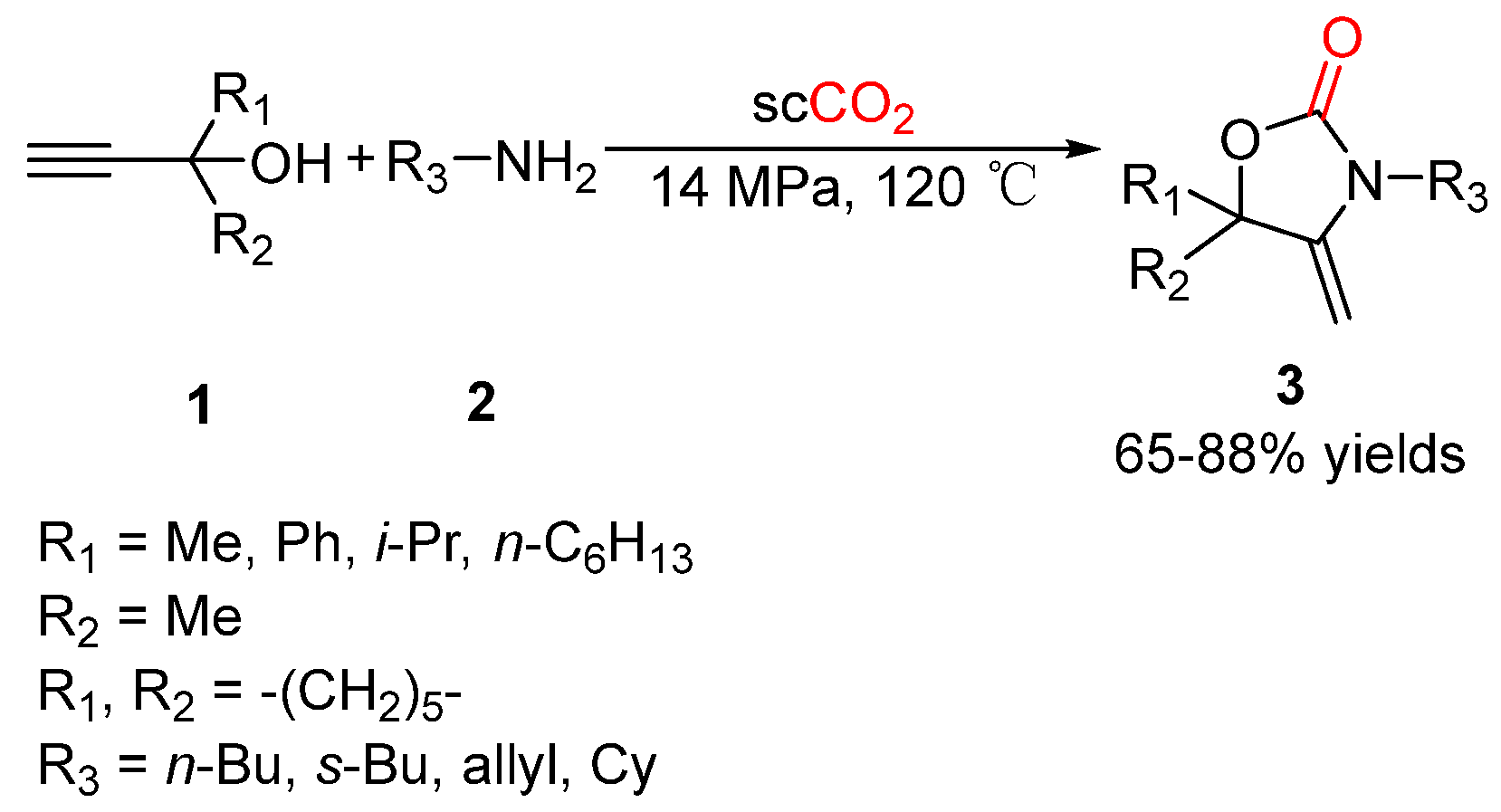
- Jiang, H.; Zhao, J.; Wang, A. An efficient and eco-friendly process for the conversion of carbon dioxide into oxazolones and oxazolidinones under Supercritical conditions. Synthesis 2008, 5, 763–769. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, J.; Jia, Z.; Zhang, J. Facile and Mild Process for Chemical Fixation of CO2 to 4-Methylene-1,3-oxazolidin-2-ones Under Solvent-Free Conditions. Synth. Commun. 2011, 41, 858–863. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Zhao, Y.; Wang, H.; Li, Z.; Wang, J.; Jiao, T. Cu(I)/Ionic Liquids Promote the Conversion of Carbon Dioxide into Oxazolidinones at Room Temperature. Molecules 2019, 24, 1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.-F.; Zhao, J.-W. Silver-catalyzed activation of internal propargylic alcohols in supercritical carbon dioxide: Efficient and eco-friendly synthesis of 4-alkylidene-1,3-oxazolidin-2-ones. Tetrahedron Lett. 2009, 50, 60–62. [Google Scholar] [CrossRef]
- Song, Q.-W.; Yu, B.; Li, X.-D.; Ma, R.; Diao, Z.-F.; Li, R.-G.; Li, W.; He, L.-N. Efficient chemical fixation of CO2 promoted by a bifunctional Ag2WO4/Ph3P system. Green Chem. 2014, 16, 1633–1638. [Google Scholar] [CrossRef]
- Song, Q.-W.; Liu, P.; Han, L.-H.; Zhang, K.; He, L.-N. Upgrading CO2 by Incorporation into Urethanes through Silver-Catalyzed One-Pot Stepwise Amidation Reaction. Chin. J. Chem. 2018, 36, 147–152. [Google Scholar] [CrossRef]
- Cokoja, M.; Wilhelm, M.E.; Anthofer, M.H.; Herrmann, W.A.; Kuehn, F.E. Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide by Using Organocatalysts. ChemSusChem 2015, 8, 2436–2454. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, G.; Guo, W.; Kleij, A.W. Sustainable conversion of carbon dioxide: The advent of organocatalysis. Green Chem. 2015, 17, 1375–1389. [Google Scholar] [CrossRef]
- Fournier, J.; Bruneau, C.; Dixneuf, P.H. A simple synthesis of oxazolidinones in one step from carbon dioxide. Tetrahedron Lett. 1990, 31, 1721–1722. [Google Scholar] [CrossRef]
- Della Ca’, N.; Gabriele, B.; Ruffolo, G.; Veltri, L.; Zanetta, T.; Costa, M. Effective Guanidine-Catalyzed Synthesis of Carbonate and Carbamate Derivatives from Propargyl Alcohols in Supercritical Carbon Dioxide. Adv. Synth. Catal. 2011, 353, 133–146. [Google Scholar] [CrossRef]
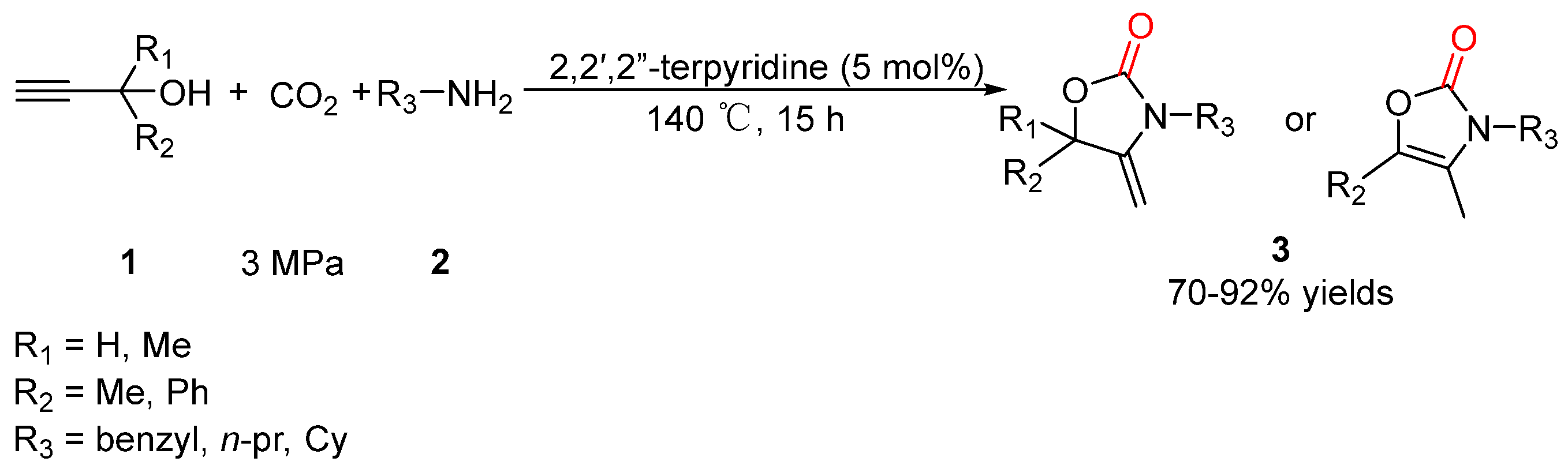
- Liu, H.; Hua, R. Conversion of carbon dioxide into 2-oxazolidinones and 2(3H)-oxazolones catalyzed by 2,2′,2″-terpyridine. Tetrahedron 2016, 72, 1200–1204. [Google Scholar] [CrossRef]
- Antonietti, M.; Kuang, D.-B.; Smarsly, B.; Yong, Z. Ionic Liquids for the Convenient Synthesis of Functional Nanoparticles and Other Inorganic Nanostructures. Angew. Chem. Int. Ed. 2004, 43, 4988–4992. [Google Scholar] [CrossRef] [PubMed]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Shi, F.; Gu, Y.L.; Yang, J.; Deng, Y.Q. Efficient and eco-friendly process for the synthesis of N-substituted 4-methylene-2-oxazolidinones in ionic liquids. Tetrahedron Lett. 2005, 46, 5907–5911. [Google Scholar] [CrossRef]
- Xu, J.X.; Zhao, J.W.; Jia, Z.B. Efficient catalyst-free chemical fixation of carbon dioxide into 2-oxazolidinones under supercritical condition. Chin. Chem. Lett. 2011, 22, 1063–1066. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z. Recent Advances in Photocatalytic CO2 Reduction Using Earth-Abundant Metal Complexes-Derived Photocatalysts. Chin. J. Chem. 2018, 36, 455–460. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Fujiwara, K.; Sugano, Y.; Ichikawa, S.; Hirai, T. N-Monoalkylation of Amines with Alcohols by Tandem Photocatalytic and Catalytic Reactions on TiO2 Loaded with Pd Nanoparticles. ACS Catal. 2013, 3, 312–320. [Google Scholar] [CrossRef]
- Calabrese, C.; Liotta, L.F.; Giacalone, F.; Gruttadauria, M.; Aprile, C. Supported Polyhedral Oligomeric Silsesquioxane-Based (POSS) Materials as Highly Active Organocatalysts for the Conversion of CO2. ChemCatChem 2019, 11, 560–567. [Google Scholar] [CrossRef]
- He, Q.; O’Brien, J.W.; Kitselman, K.A.; Tompkins, L.E.; Curtis, G.C.T.; Kerton, F.M. Synthesis of cyclic carbonates from CO2 and epoxides using ionic liquids and related catalysts including choline chloride–metal halide mixtures. Catal. Sci. Technol. 2014, 4, 1513–1528. [Google Scholar] [CrossRef]
- Zhao, Q.-N.; Song, Q.-W.; Liu, P.; Zhang, Q.-X.; Gao, J.-H.; Zhang, K. Catalytic Conversion of CO2 to Cyclic Carbonates through Multifunctional Zinc-Modified ZSM-5 Zeolite. Chin. J. Chem. 2018, 36, 187–193. [Google Scholar] [CrossRef]
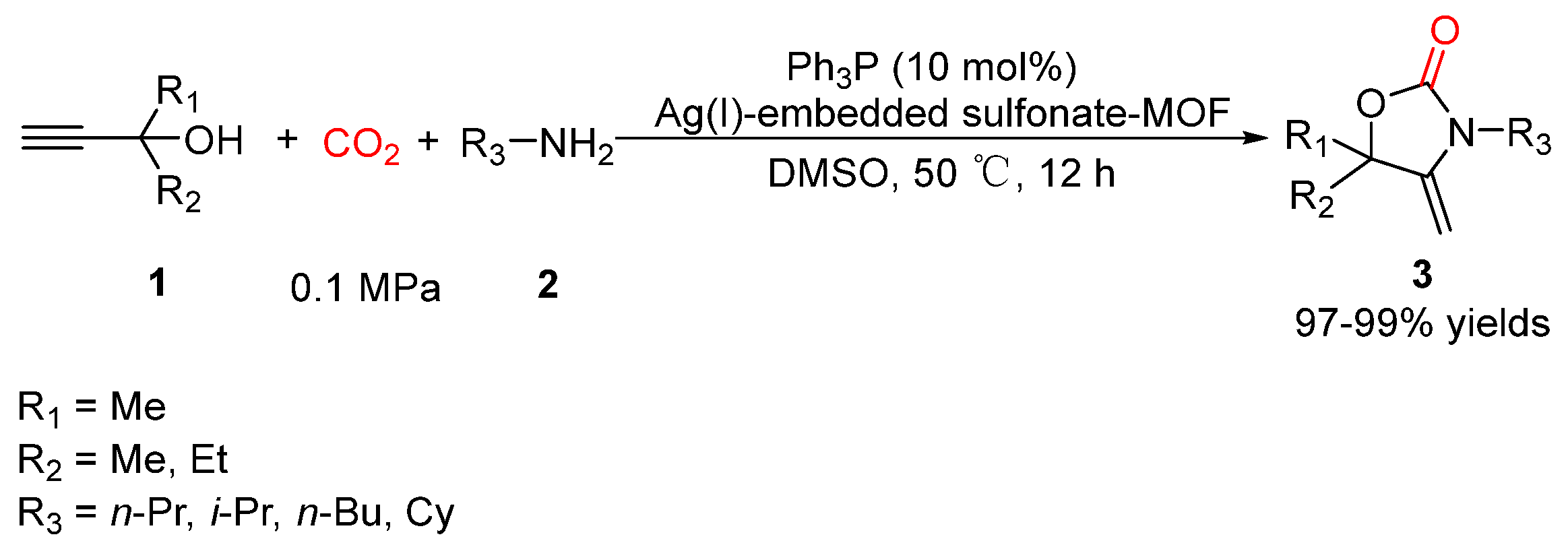
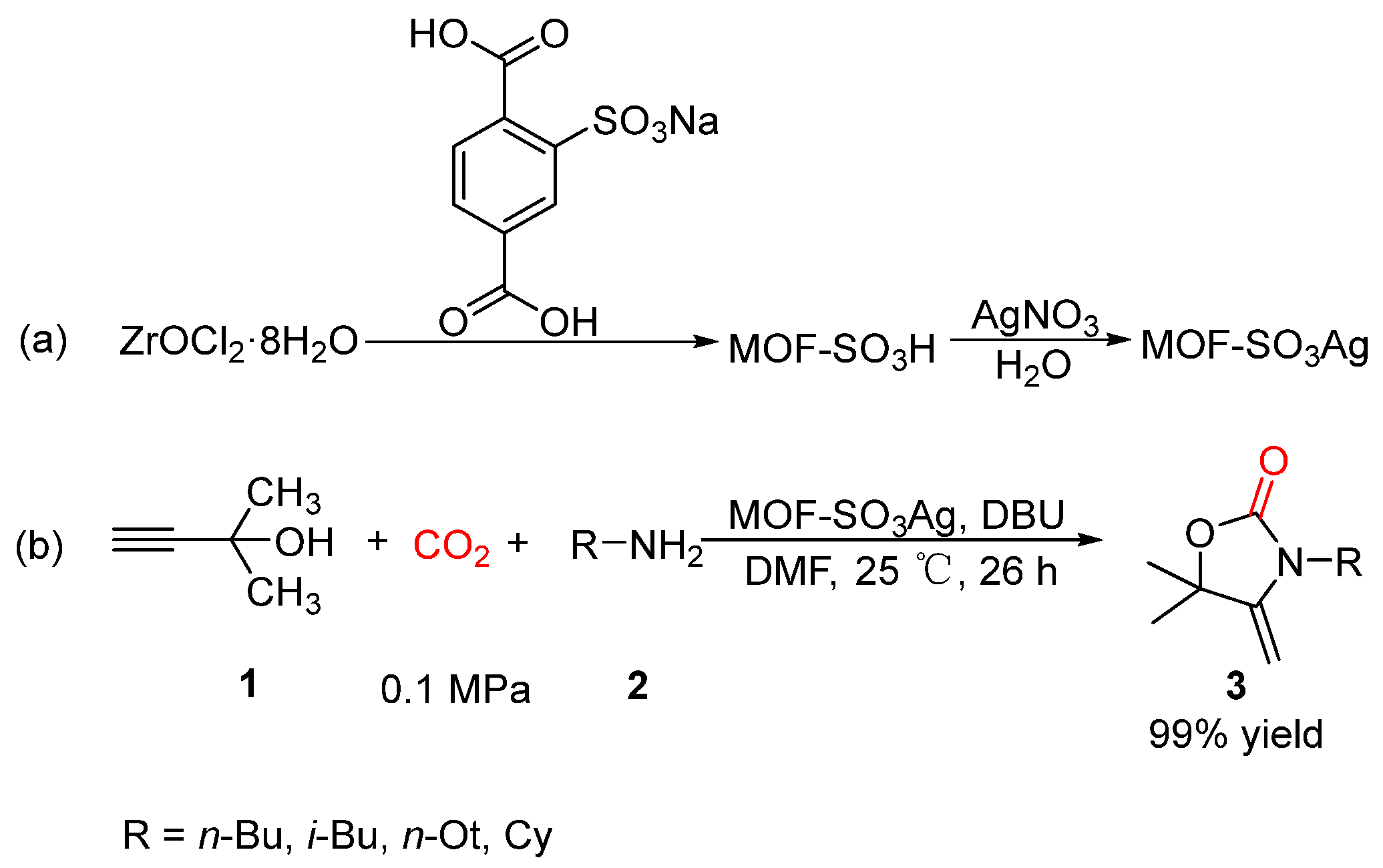
- Zhang, G.; Yang, H.; Fei, H. Unusual Missing Linkers in an Organosulfonate-Based Primitive–Cubic (pcu)-Type Metal–Organic Framework for CO2 Capture and Conversion under Ambient Conditions. ACS Catal. 2018, 8, 2519–2525. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Li, N.; Li, B.; Bu, X.-H. Recent Advances on Metal-Organic Frameworks in the Conversion of Carbon Dioxide. Chin. J. Chem. 2021, 39, 440–462. [Google Scholar] [CrossRef]
- Das, R.; Nagaraja, C.M. Highly Efficient Fixation of Carbon Dioxide at RT and Atmospheric Pressure Conditions: Influence of Polar Functionality on Selective Capture and Conversion of CO2. Inorg. Chem. 2020, 59, 9765–9773. [Google Scholar] [CrossRef]
- Bruncau, C.; Dixncuf, P.H. Catalytic synthesis of O-beta-oxoalkylcarbamates. Tetrahedron Lett. 1987, 28, 2005–2008. [Google Scholar] [CrossRef]
- Kim, T.-J.; Kwon, K.-H.; Kwon, S.-C.; Baeg, J.-O.; Shim, S.-C.; Lee, D.-H. Iron complexes of 1,1′-bis(diphenylphosphino)ferrocene (BPPF) as efficient catalysts in the synthesis of carbamates. X-ray crystal structure of (BPPF)Fe(CO)3. J. Organomet. Chem. 1990, 389, 205–217. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, J.-W.; Kwon, S.-C.; Shim, S.-C.; Kim, T.-J. Catalytic formation of carbamates and cyclic carbonates by copper complex of 2,5,19,22-tetraaza[6,6](1,1′)ferrocenophane-1,5-diene X-ray crystal structure of [Cu(1)]PF6. J. Organomet. Chem. 1997, 545, 337–344. [Google Scholar] [CrossRef]
- Qi, C.; Huang, L.; Jiang, H. Efficient Synthesis of beta-Oxoalkyl Carbamates from Carbon Dioxide, Internal Propargylic Al-cohols, and Secondary Amines Catalyzed by Silver Salts and DBU. Synthesis 2010, 9, 1433–1440. [Google Scholar] [CrossRef]
- Song, Q.-W.; Chen, W.-Q.; Ma, R.; Yu, A.; Li, Q.-Y.; Chang, Y.; He, L.-N. Bifunctional Silver(I) Complex-Catalyzed CO2 Conversion at Ambient Conditions: Synthesis of alpha-Methylene Cyclic Carbonates and Derivatives. ChemSusChem 2015, 8, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-N.; Song, Q.-W.; Liu, P.; Zhang, K.; Hao, J. Ag(I)/(C2 H5 )4 NCl Cooperation Catalysis for Fixing CO2 or Its Derivatives into beta-Oxopropylcarbamates. Chemistryselect 2018, 3, 6897–6901. [Google Scholar] [CrossRef]
- Song, D.; Li, D.; Xiao, X.; Cheng, C.; Chaemchuen, S.; Yuan, Y.; Verpoort, F. Synthesis of β-oxopropylcarbamates in a recyclable AgBr/ionic liquid catalytic system: An efficient assembly of CO2 under ambient pressure. J. CO2 Util. 2018, 27, 217–222. [Google Scholar] [CrossRef]
- Qi, C.-R.; Jiang, H.-F. Efficient synthesis of β-oxopropylcarbamates in compressed CO2 without any additional catalyst and solvent. Green Chem. 2007, 9, 1284–1286. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, W.; Deng, X.; Zhou, X.; Qi, C.; Hu, J. Silver-Nanowire-Catalyzed Three-Component Coupling of Carbon Dioxide, Amines and Propargylic Alcohols for the Synthesis of beta-Oxopropyl Carbamates. Asian J. Org. Chem. 2018, 8, 179–184. [Google Scholar] [CrossRef]
- Cui, M.; Qian, Q.; He, Z.; Ma, J.; Kang, X.; Hu, J.; Liu, Z.; Han, B. Synthesizing Ag Nanoparticles of Small Size on a Hierarchical Porosity Support for the Carboxylative Cyclization of Propargyl Alcohols with CO2 under Ambient Conditions. Chem. Eur. J. 2015, 21, 15924–15928. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-H.; Ma, J.-G.; Niu, Z.; Yang, G.-M.; Cheng, P. An Efficient Nanoscale Heterogeneous Catalyst for the Capture and Conversion of Carbon Dioxide at Ambient Pressure. Angew. Chem. Int. Ed. 2015, 54, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhiani, R.; Sadeghzadeh, S.M. Fixing CO2 into beta-oxopropylcarbamates in neat condition by ionic gelation/Ag(i) supported on dendritic fibrous nanosilica. RSC Adv. 2019, 9, 16955–16965. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Wang, J.; Zhang, X.; Sadeghzadeh, S.M.; Zhiani, R.; Shahroudi, M.; Amarloo, F. Co-immobilization of Laccase and TEMPO onto Glycidyloxypropyl Functionalized Fibrous Phosphosilicate Nanoparticles for Fixing CO2 into β-Oxopropylcarbamatesin. Catal. Lett. 2019, 149, 3465–3475. [Google Scholar] [CrossRef]
- Asadi Zeydabadi, H.; Mehrzad, J.; Motavalizadehkakhky, A.; Zhiani, R. Fixing CO2 into β-Oxopropylcarbamatesin by Palladium NPs Supported on Magnetic Fibrous Silica Ionic Gelation. Catal. Lett. 2021, 151, 582–592. [Google Scholar] [CrossRef]
- Zhao, R.; Lv, M.; Li, Y.; Sun, M.; Kong, W.; Wang, L.; Song, S.; Fan, C.; Jia, L.; Qiu, S.; et al. Stable Nanocomposite Based on PEGylated and Silver Nanoparticles Loaded Graphene Oxide for Long-Term Antibacterial Activity. ACS Appl. Mater. Interfaces 2017, 9, 15328–15341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Kong, W.; Sun, M.; Yang, Y.; Liu, W.; Lv, M.; Song, S.; Wang, L.; Song, H.; Hao, R. Highly Stable Graphene-Based Nanocomposite (GO–PEI–Ag) with Broad-Spectrum, Long-Term Antimicrobial Activity and Antibiofilm Effects. ACS Appl. Mater. Interfaces 2018, 10, 17617–17629. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, F.; Yang, W.; Guo, M.; Wang, X.; Zhanga, B.; Tang, J. A facile one-pot method to high-quality Ag-graphene composite nanosheets for efficient surface-enhanced Raman scattering. Chem. Commun. 2011, 47, 6440–6442. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Z.; Cao, Z.; Zhang, H.-B.; Liu, J.; Yu, Z.-Z. Growth of silver nanocrystals on graphene by simultaneous reduction of graphene oxide and silver ions with a rapid and efficient one-step approach. Chem. Commun. 2011, 47, 3084–3086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, K.-H.; Zhou, Z.-H.; He, L.-N. Reduced Graphene Oxide Supported Ag Nanoparticles: An Efficient Catalyst for CO2 Conversion at Ambient Conditions. ChemCatChem 2020, 12, 4825–4830. [Google Scholar] [CrossRef]
- Kutscheroff, M. Ueber eine neue Methode direkter Addition von Wasser (Hydratation) an die Kohlenwasserstoffe der Acetylenreihe. Ber. Dtsch. Chem. Ges. 1881, 14, 1540–1542. [Google Scholar] [CrossRef] [Green Version]
- Kutscheroff, M. Ueber die Einwirkung der Kohlenwasserstoffe der Acetylenreihe auf Quecksilberoxyd und dessen Salze. Ber. Dtsch. Chem. Ges. 1884, 17, 13–29. [Google Scholar] [CrossRef]
- Niu, T.-F.; Jiang, D.-Y.; Li, S.-Y.; Shu, X.-G.; Li, H.; Zhang, A.-L.; Xu, J.-Y.; Ni, B.-Q. Visible light promoted copper-catalyzed Markovnikov hydration of alkynes at room temperature. Tetrahedron Lett. 2017, 58, 1156–1159. [Google Scholar] [CrossRef]
- Mei, Q.; Liu, H.; Hou, M.; Liu, H.; Han, B. Selective hydration of asymmetric internal aryl alkynes without directing groups to α-aryl ketones over Cu-based catalyst. New J. Chem. 2017, 41, 6290–6295. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, M.; Ciccarelli, S.; Lee, J.; Catano, B. AuIII-Catalyzed Formation of α-Halomethyl Ketones from Terminal Alkynes. Eur. J. Org. Chem. 2017, 2017, 781–785. [Google Scholar] [CrossRef]
- Hoyos, P.; Sinisterra, J.-V.; Molinari, F.; Alcántara, A.R.; domínguez de María, P. Biocatalytic Strategies for the Asymmetric Synthesis of α-Hydroxy Ketones. Acc. Chem. Res. 2010, 43, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wu, P.; Xue, J.; Wei, X. Cytotoxic and Antibacterial Quinone Sesquiterpenes from a Myrothecium Fungus. J. Nat. Prod. 2014, 77, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Macabeo, A.P.G.; Martinez, F.P.A.; Kurtán, T.; Tóth, L.; Mándi, A.; Schmidt, S.; Heilmann, J.; Alejandro, G.J.D.; Knorn, M.; Dahse, H.-M.; et al. Tetrahydroxanthene-1,3(2H)-dione Derivatives from Uvaria valderramensis. J. Nat. Prod. 2014, 77, 2711–2715. [Google Scholar] [CrossRef] [PubMed]
- Engel, D.A.; Dudley, G.B. The Meyer–Schuster rearrangement for the synthesis of α,β-unsaturated carbonyl compounds. Org. Biomol. Chem. 2009, 7, 4149–4158. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Narayanan, K.V. Rupe and Meyer-Schuster rearrangements. Chem. Rev. 1971, 71, 429–438. [Google Scholar] [CrossRef]
- Ogoshi, S.; Morimoto, T.; Nishio, K.; Ohe, K.; Murai, S. Palladium-catalyzed reactions of ketone.alpha.-carbonates with norbornenes. An unusual cyclopropanation. J. Org. Chem. 1993, 58, 9–10. [Google Scholar] [CrossRef]
- Stainforth, N.E.; Cutting, G.A.; John, M.P.; Willis, M.C. Direct catalytic diastereoselective Mannich reactions: The synthesis of protected alpha-hydroxy-beta-aminoketones. Tetrahedron Asymmetry 2009, 20, 741–743. [Google Scholar] [CrossRef]
- Joumier, J.M.; Fournier, J.; Bruneau, C.; Dixneuf, P.H. Functional carbonates: Cyclic α-methylene and β-oxopropyl carbonates from prop-2-ynyl alcohol derivatives and CO2. J. Chem. Soc. Perkin Trans. 1 1991, 1, 3271–3274. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Lu, L.; Qian, Q.; Zhang, Z.; Xie, C.; Han, B. Synthesis of Asymmetrical Organic Carbonates using CO2 as a Feedstock in AgCl/Ionic Liquid System at Ambient Conditions. ChemSusChem 2017, 10, 1292–1297. [Google Scholar] [CrossRef]
- Tomishige, K.; Yasuda, H.; Yoshida, Y.; Nurunnabi, M.; Li, B.; Kunimori, K. Catalytic performance and properties of ceria based catalysts for cyclic carbonate synthesis from glycol and carbon dioxide. Green Chem. 2004, 6, 206–214. [Google Scholar] [CrossRef]
- Du, Y.; He, L.-N.; Kong, D.-L. Magnesium-catalyzed synthesis of organic carbonate from 1,2-diol/alcohol and carbon dioxide. Catal. Commun. 2008, 9, 1754–1758. [Google Scholar] [CrossRef]
- Da Silva, E.; Dayoub, W.; Mignani, G.; Raoul, Y.; Lemaire, M. Propylene carbonate synthesis from propylene glycol, carbon dioxide and benzonitrile by alkali carbonate catalysts. Catal. Commun. 2012, 29, 58–62. [Google Scholar] [CrossRef]
- Honda, M.; Tamura, M.; Nakao, K.; Suzuki, K.; Nakagawa, Y.; Tomishige, K. Direct Cyclic Carbonate Synthesis from CO2 and Diol over Carboxylation/Hydration Cascade Catalyst of CeO2 with 2-Cyanopyridine. ACS Catal. 2014, 4, 1893–1896. [Google Scholar] [CrossRef]
- Juárez, R.; Concepción, P.; Corma, A.; García, H. Ceria nanoparticles as heterogeneous catalyst for CO2 fixation by omega-aminoalcohols. Chem. Commun. 2010, 46, 4181–4183. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Foo, S.W.; Yamazaki, Y.; Saito, S. Catalytic fluoride triggers dehydrative oxazolidinone synthesis from CO2. RSC Adv. 2014, 4, 50851–50857. [Google Scholar] [CrossRef]
- Niemi, T.; Fernández, I.; Steadman, B.; Mannisto, J.K.; Repo, T. Carbon dioxide-based facile synthesis of cyclic carbamates from amino alcohols. Chem. Commun. 2018, 54, 3166–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foden, F.R.; McCormick, J.; O’Mant, D.M. Vulpinic acids as potential antiinflammatory agents. 1. Vulpinic acids with substituents in the aromatic rings. J. Med. Chem. 1975, 18, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Ji, M.H.; Xu, X.H.; Cheng, J.L.; Zhu, G.N. Synthetic derivatives of spiromesifen and their bioactivity research. Chin. Chem. Lett. 2009, 20, 1307–1310. [Google Scholar] [CrossRef]
- Vieweg, L.; Reichau, S.; Schobert, R.; Leadlay, P.F.; Süssmuth, R.D. Recent advances in the field of bioactive tetronates. Nat. Prod. Rep. 2014, 31, 1554–1584. [Google Scholar] [CrossRef] [Green Version]
- Campbell, A.C.; Maidment, M.S.; Pick, J.H.; Stevenson, D.F.M. Synthesis of (E)- and (Z)-pulvinones. J. Chem. Soc. Perkin Trans. 1 1985, 1, 1567–1576. [Google Scholar] [CrossRef]
- Effenberger, F.; Syed, J. Stereoselective synthesis of biologically active tetronic acids. Tetrahedron Asymmetry 1998, 9, 817–825. [Google Scholar] [CrossRef]
- Mallinger, A.; Le Gall, T.; Mioskowski, C. 3-Aryltetronic Acids: Efficient Preparation and Use as Precursors for Vulpinic Acids. J. Org. Chem. 2009, 74, 1124–1129. [Google Scholar] [CrossRef]
- Yang, W.; Liu, J.; Zhang, H. Total synthesis of pulverolide: Revision of its structure. Tetrahedron Lett. 2010, 51, 4874–4876. [Google Scholar] [CrossRef]
- Manchoju, A.; Pansare, S.V. Catalytic Undirected Intermolecular C–H Functionalization of Arenes with 3-Diazofuran-2,4-dione: Synthesis of 3-Aryl Tetronic Acids, Vulpinic Acid, Pinastric Acid, and Methyl Isoxerocomate. Org. Lett. 2016, 18, 5952–5955. [Google Scholar] [CrossRef]
- Sadamitsu, Y.; Komatsuki, K.; Saito, K.; Yamada, T. Access to Tetronic Acids via Silver-Catalyzed CO2 Incorporation into Conjugated Ynones. Org. Lett. 2017, 19, 3191–3194. [Google Scholar] [CrossRef] [PubMed]
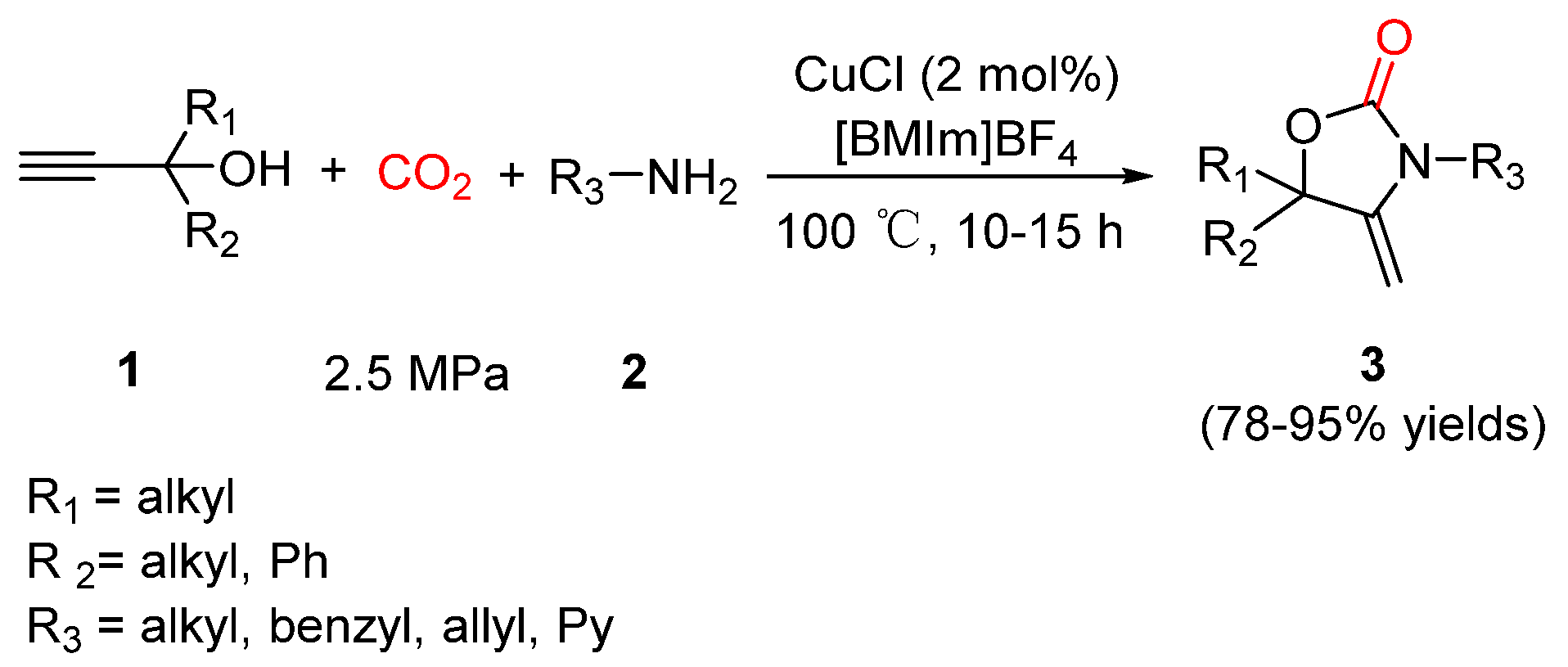
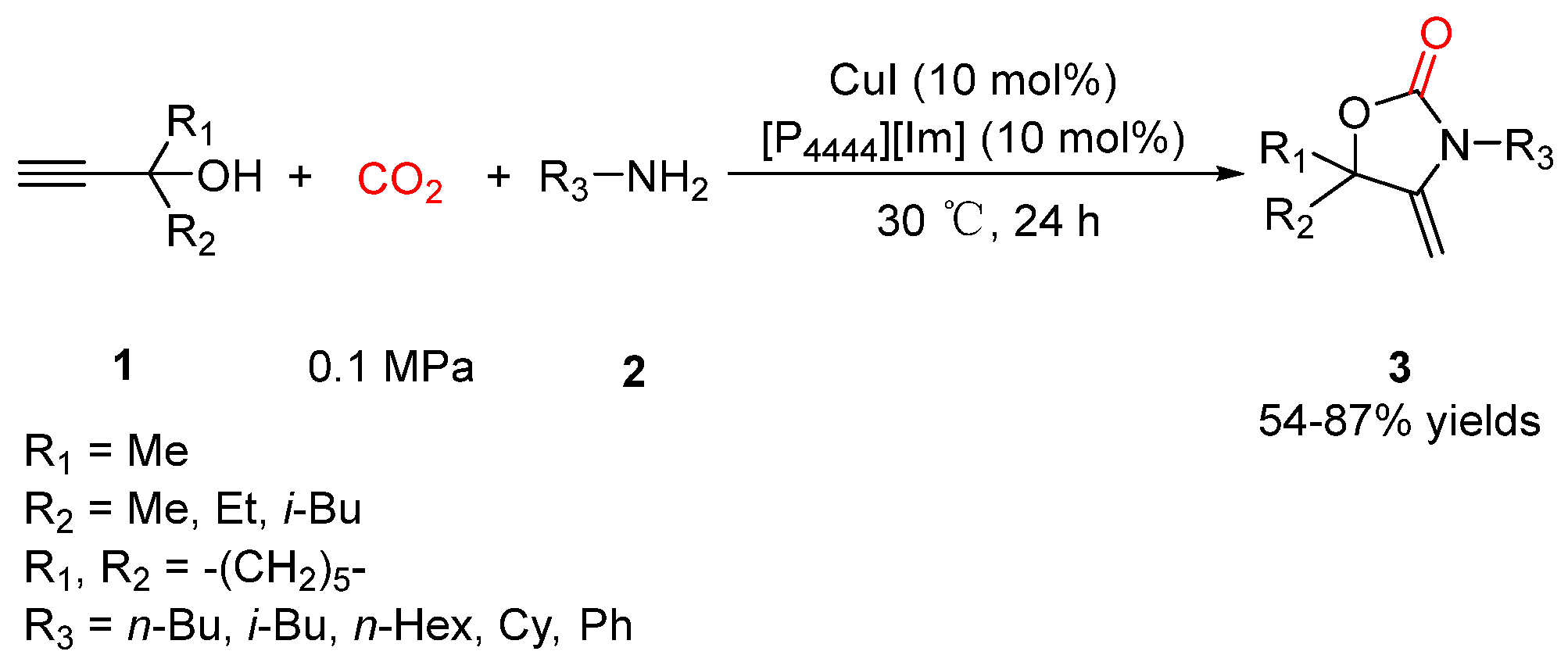
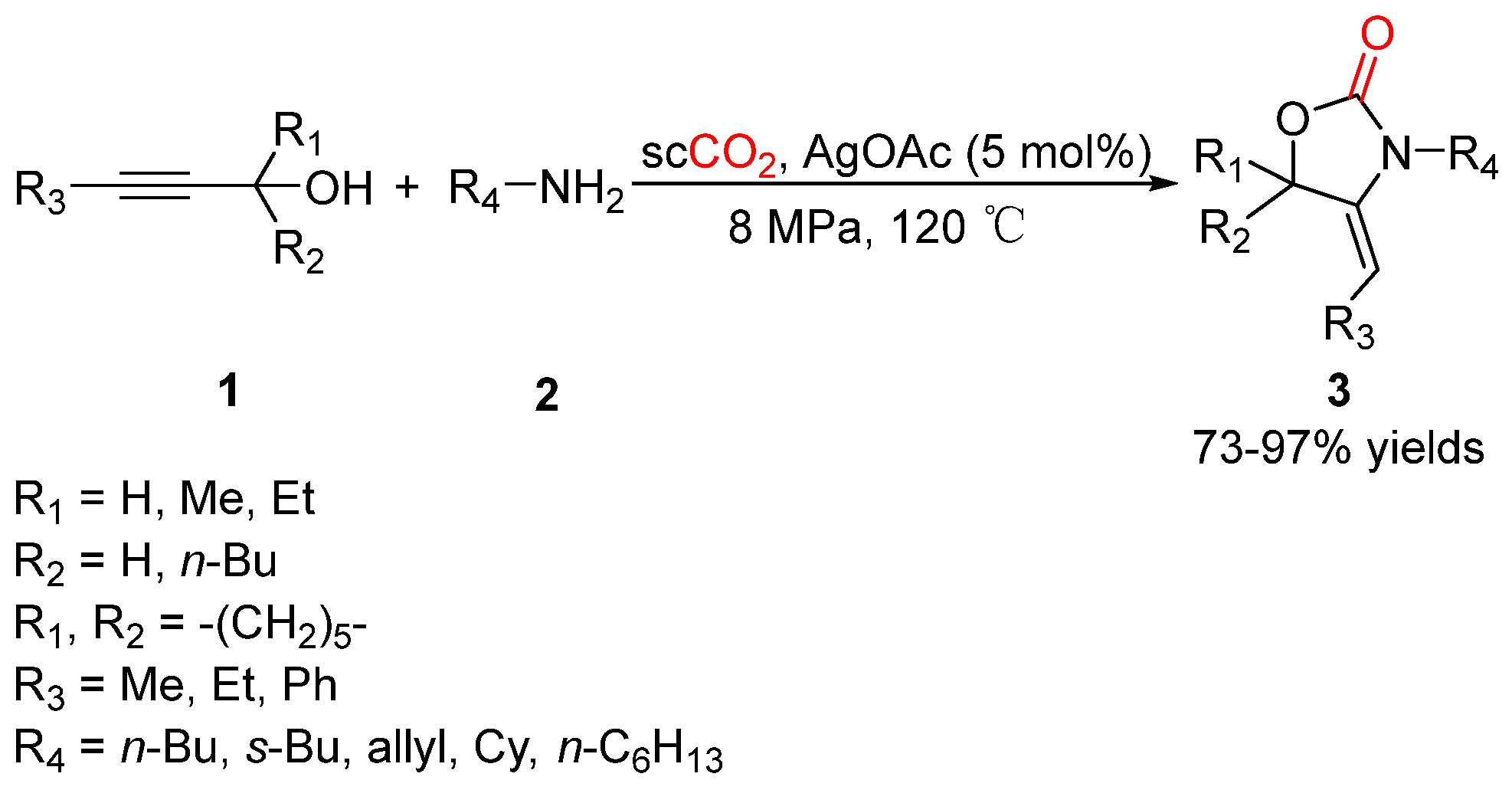
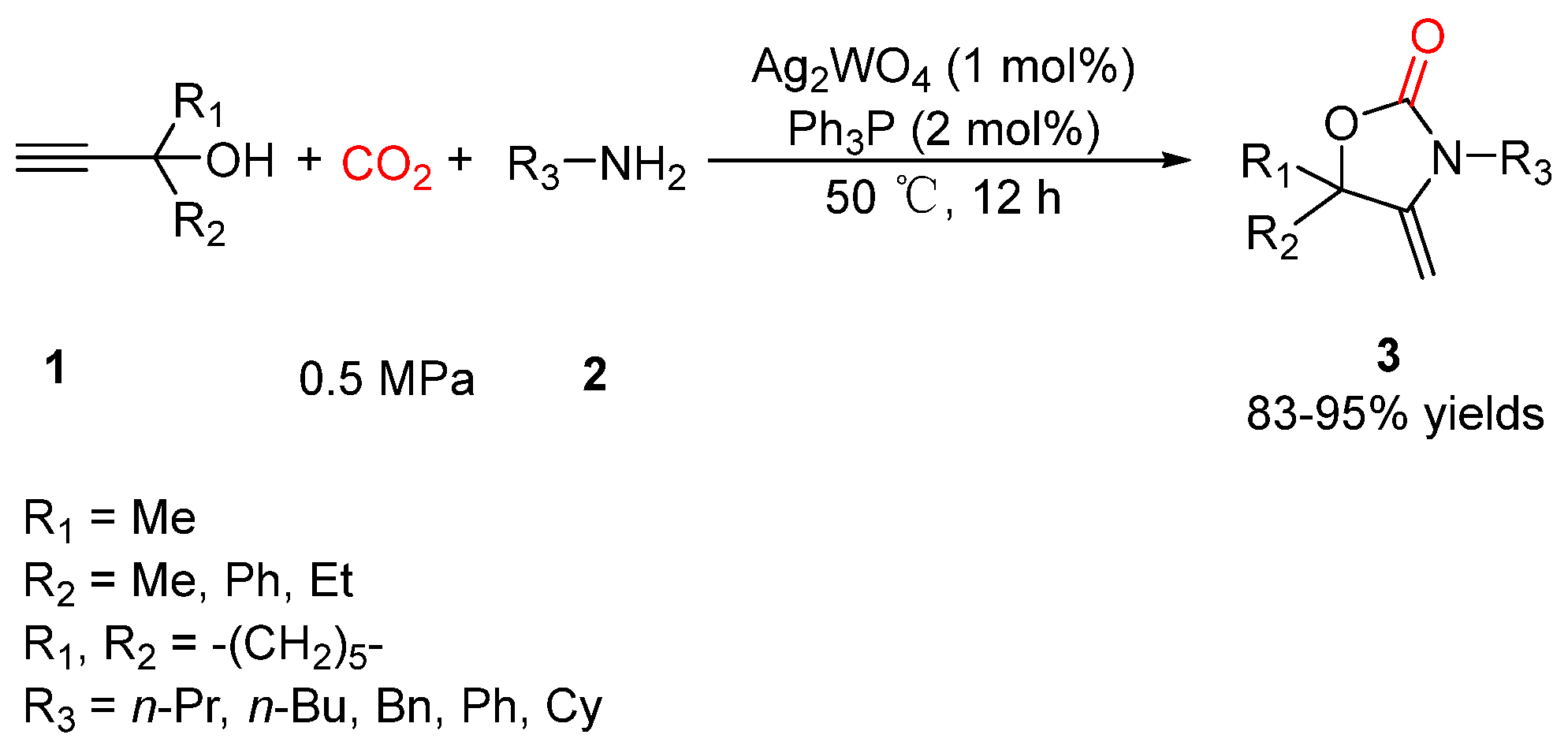
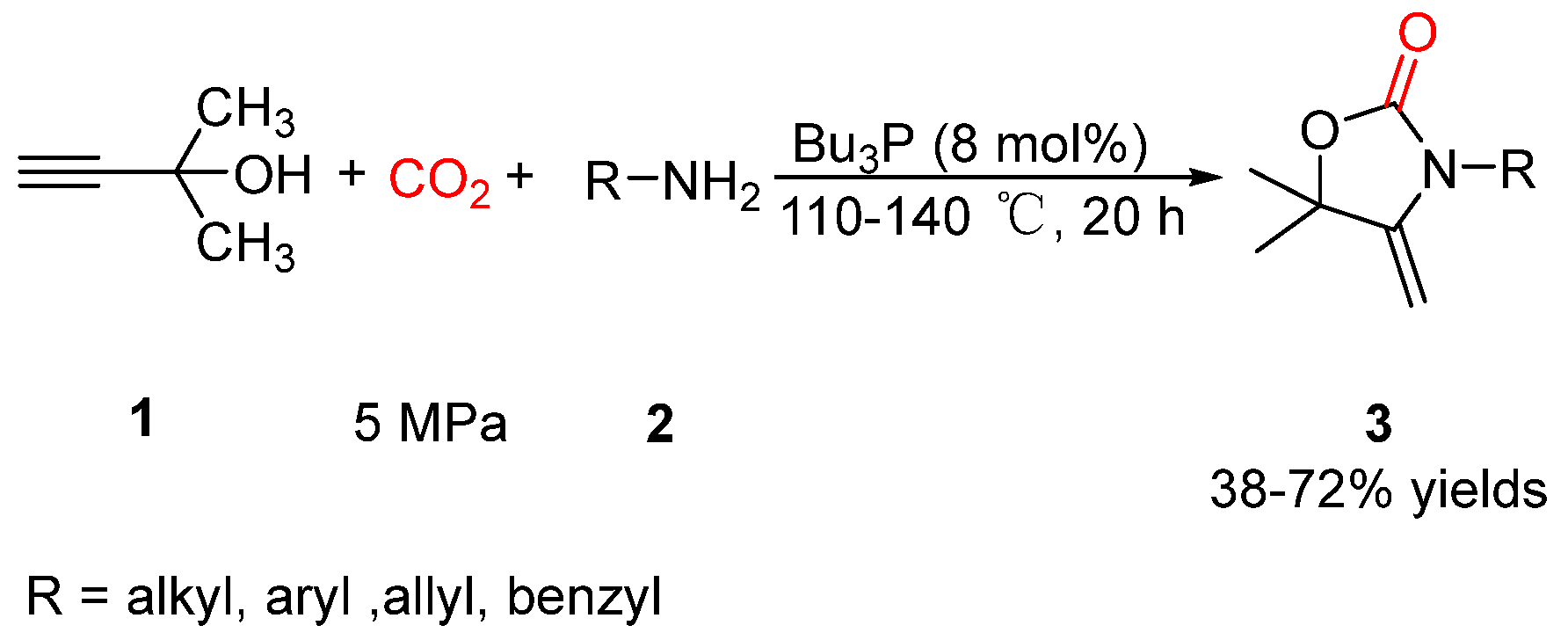
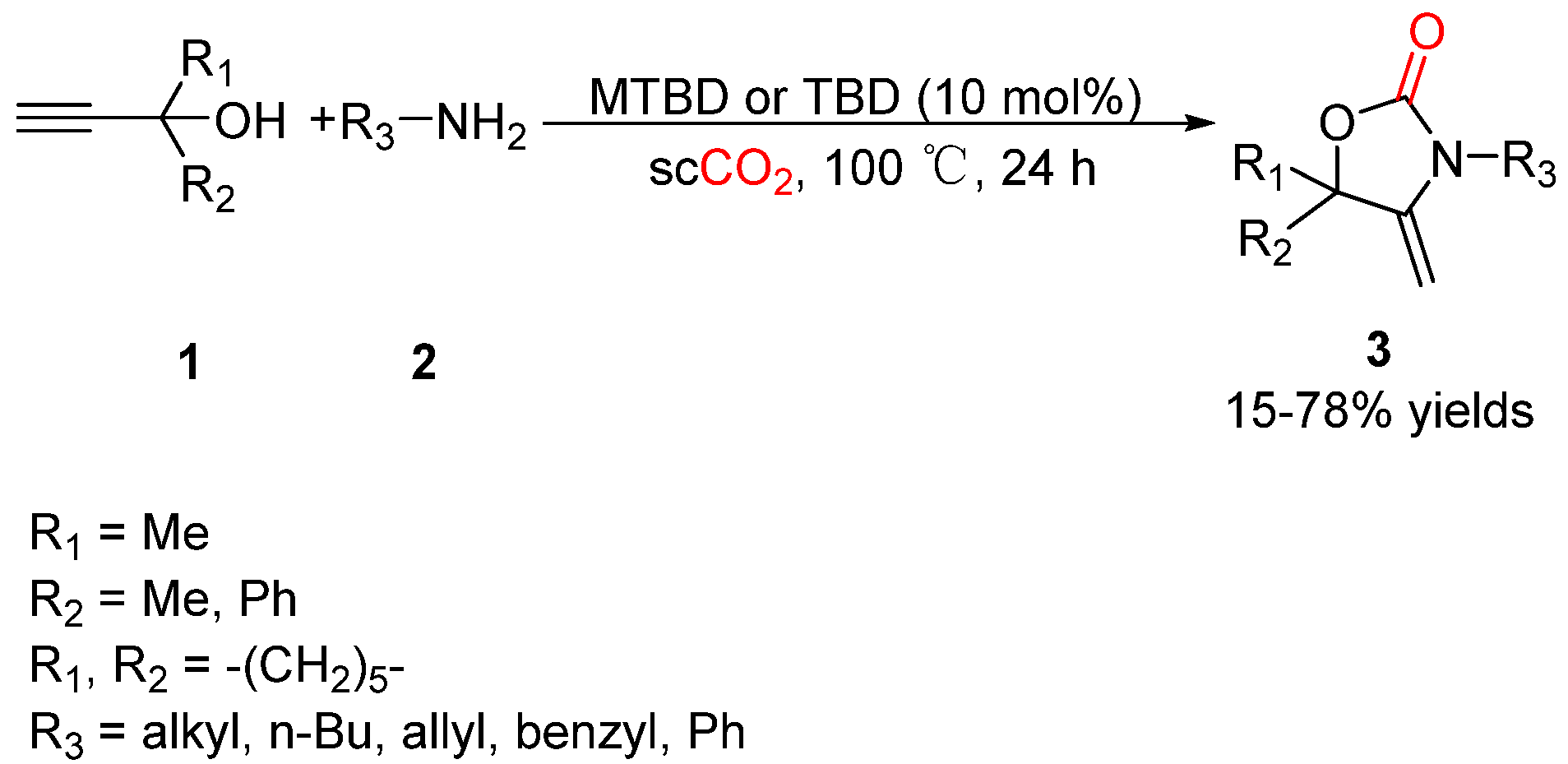

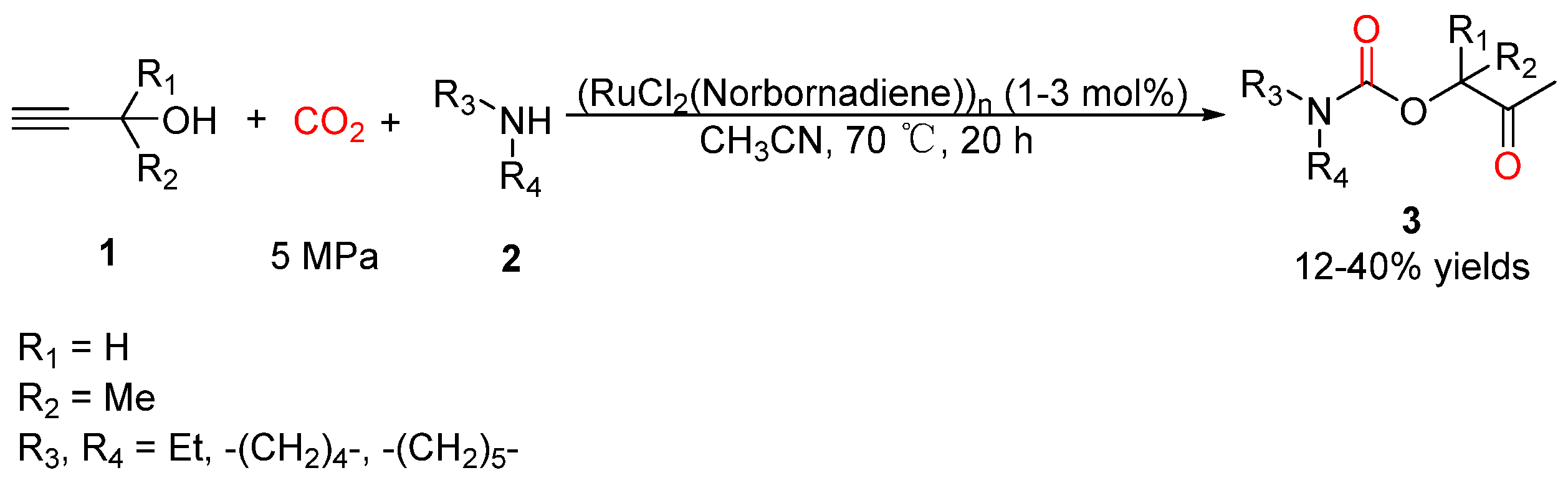
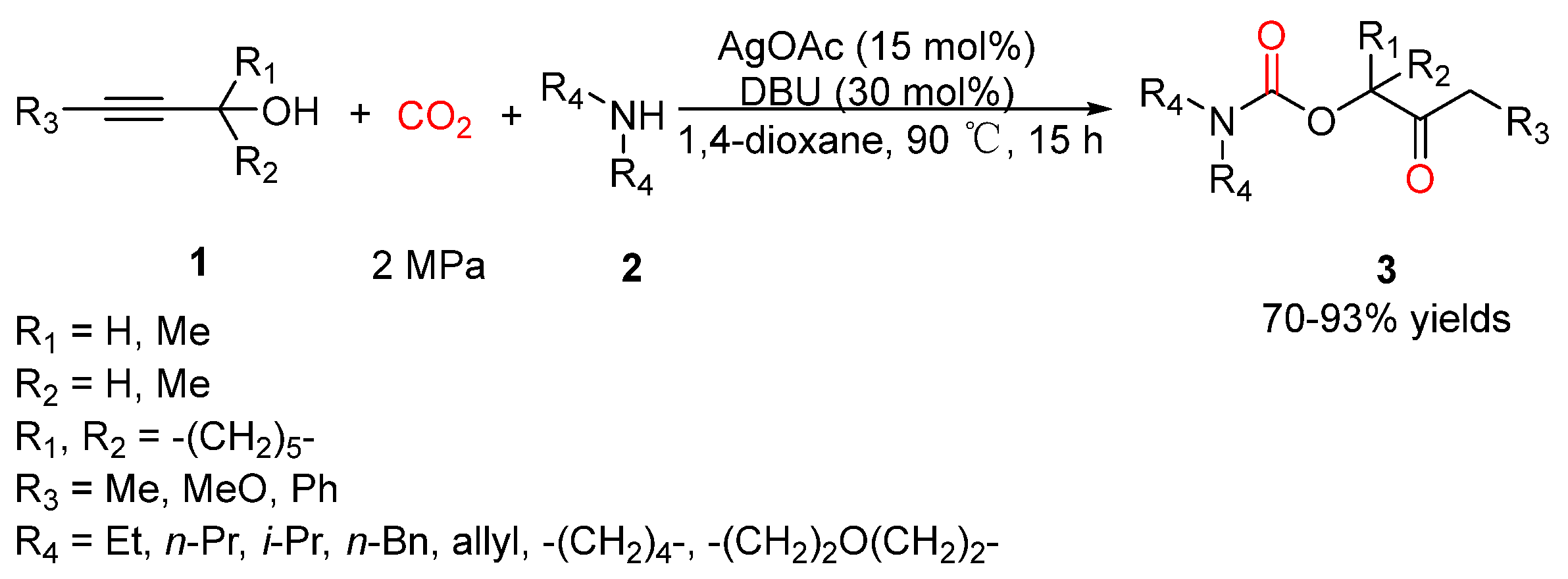
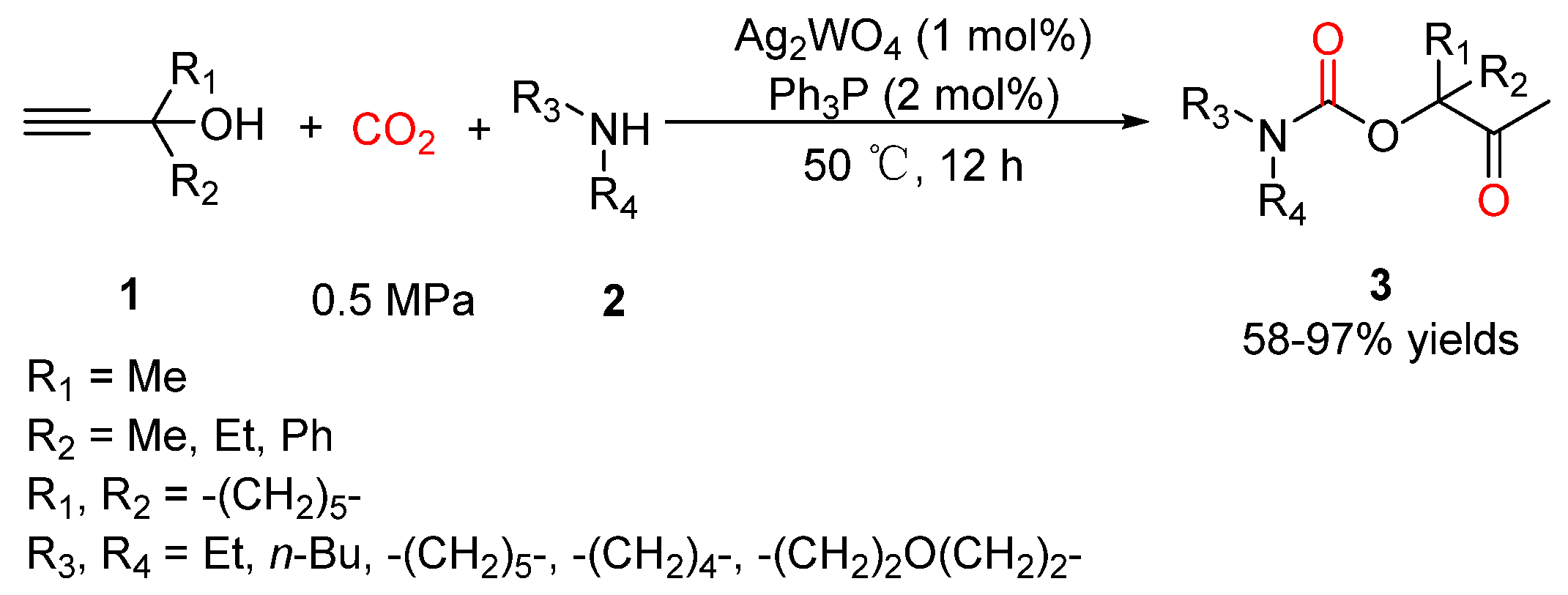
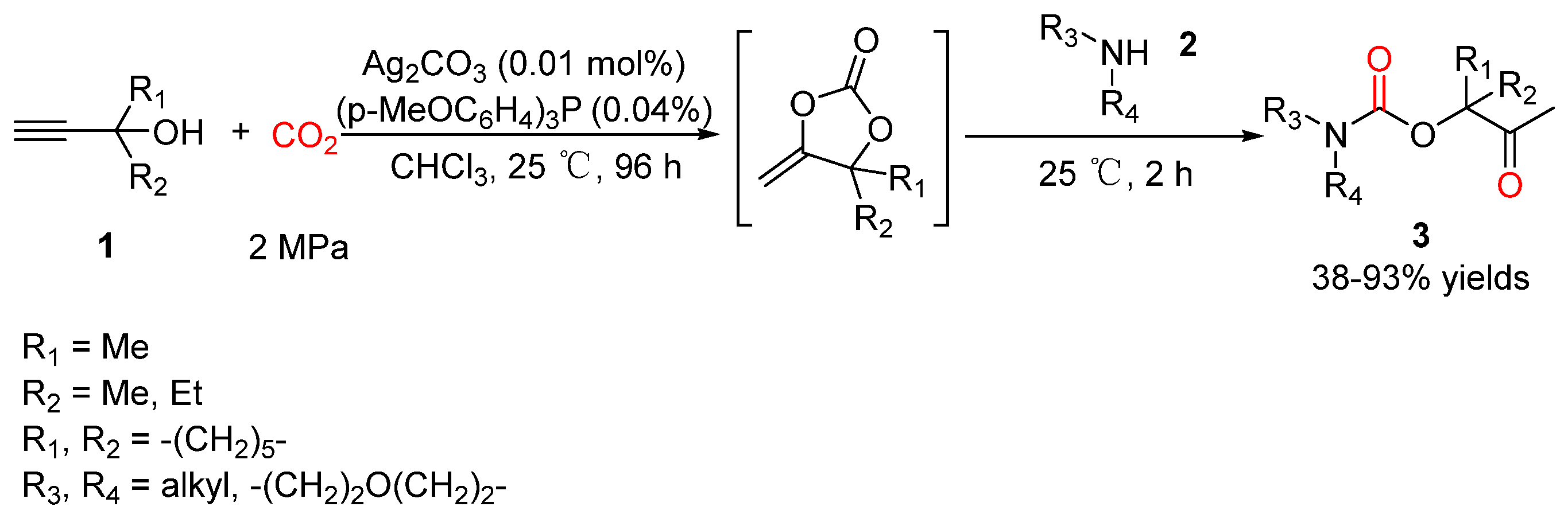
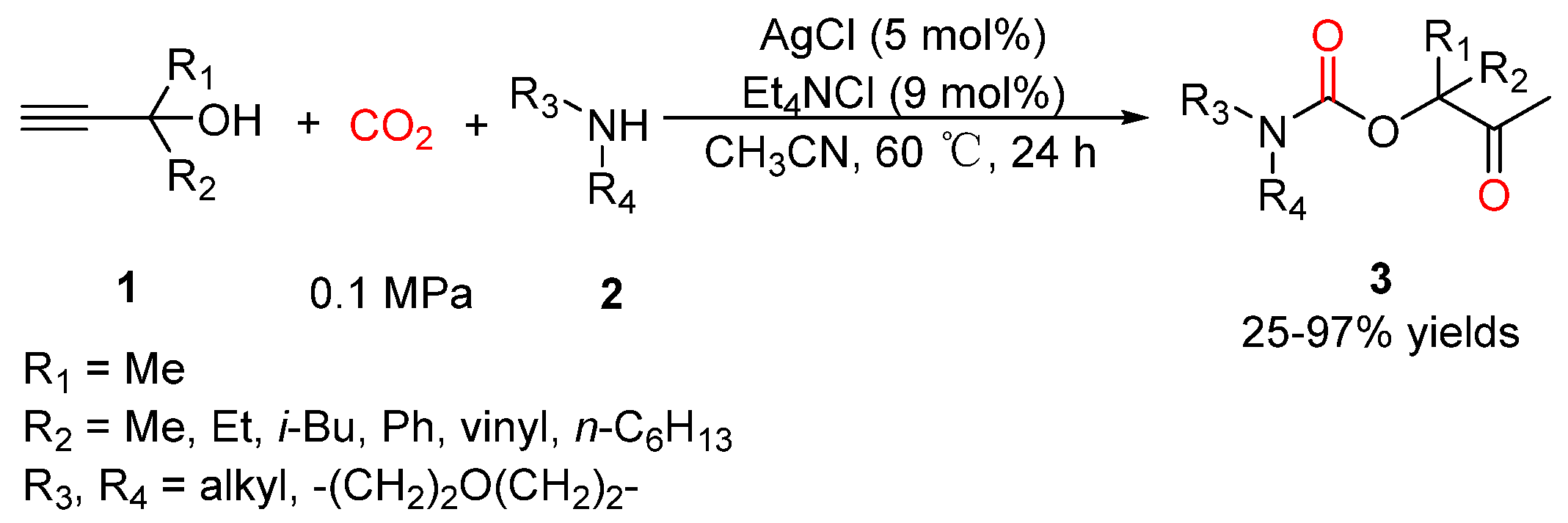
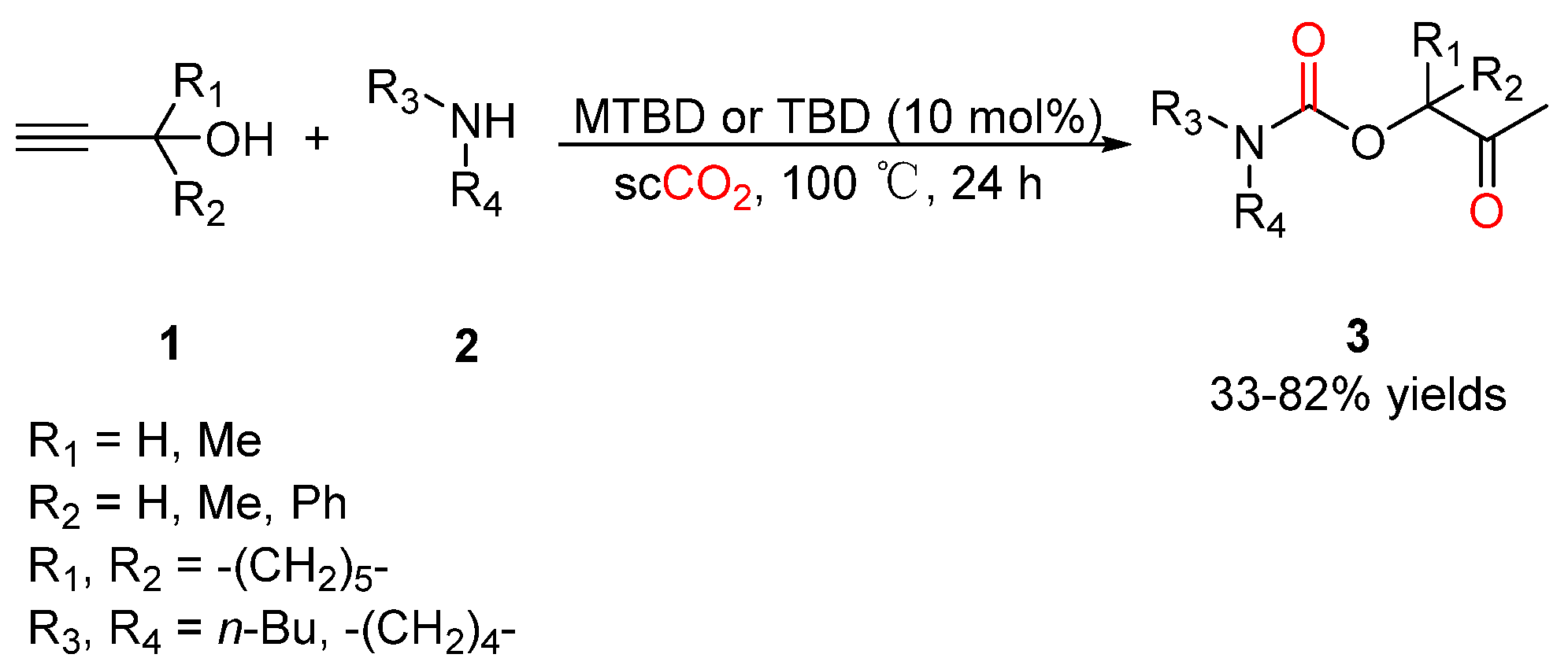

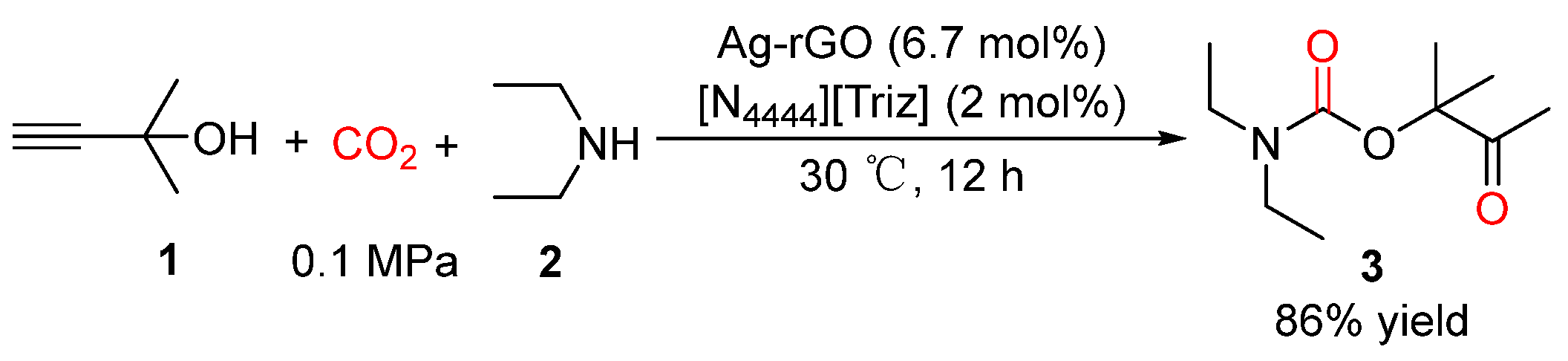
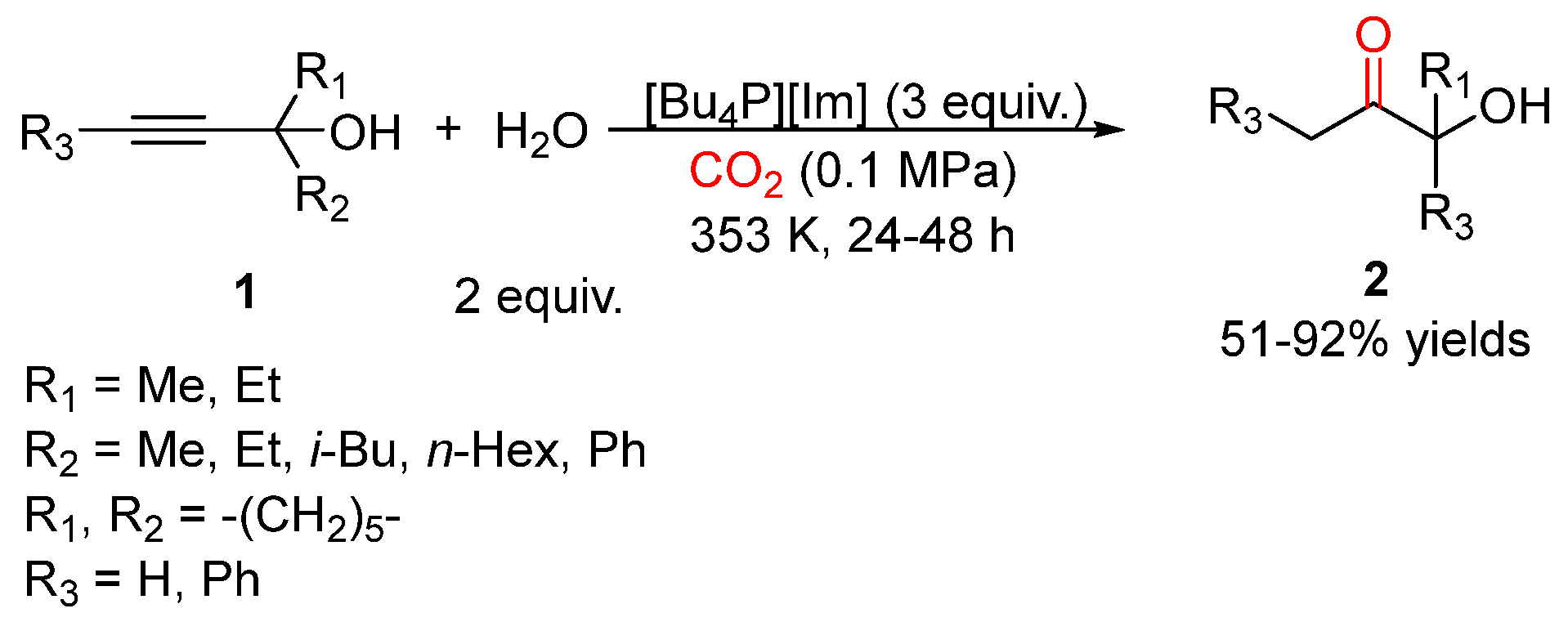

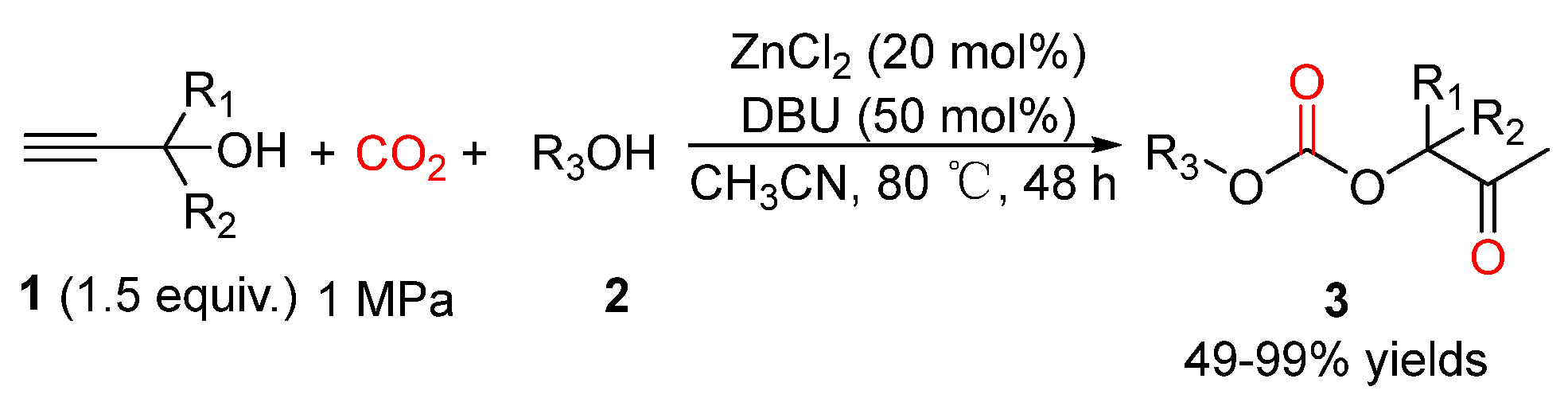

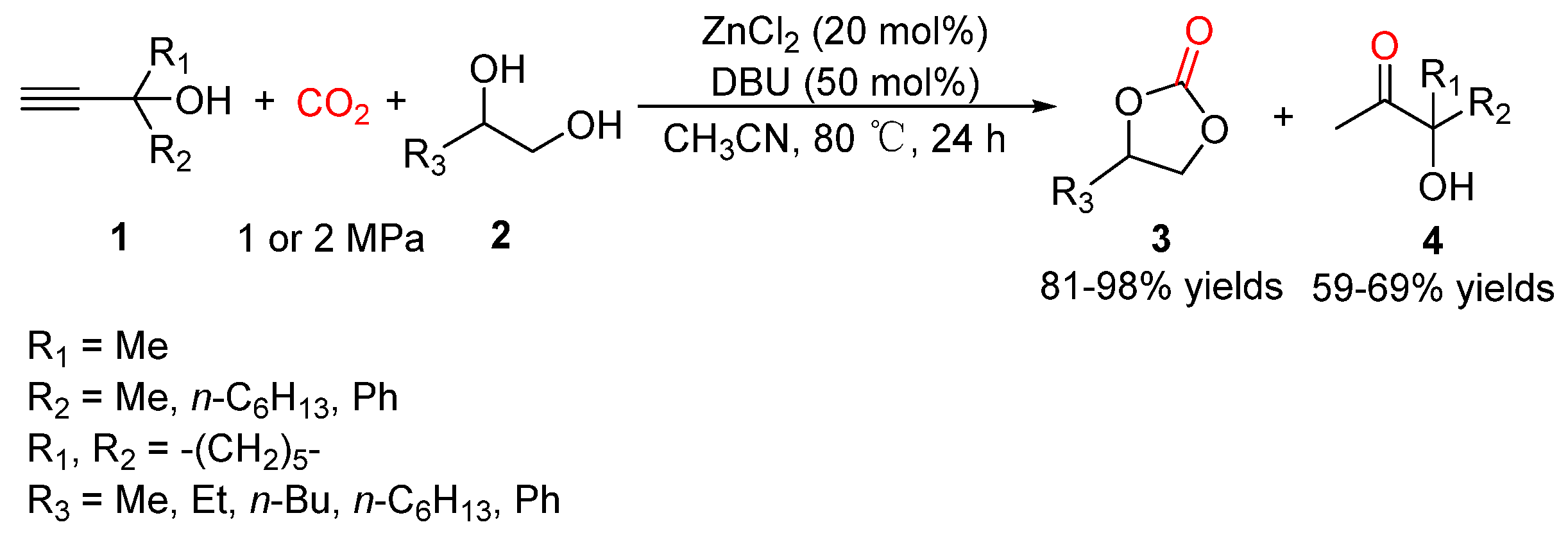



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Yan, X.; Xu, Y.; Likhanova, N.; Velázquez, H.D.; Gong, Y.; Yuan, Y.; Verpoort, F. Tandem Reactions Based on the Cyclization of Carbon Dioxide and Propargylic Alcohols: Derivative Applications of α-Alkylidene Carbonates. Catalysts 2022, 12, 73. https://doi.org/10.3390/catal12010073
Jiang B, Yan X, Xu Y, Likhanova N, Velázquez HD, Gong Y, Yuan Y, Verpoort F. Tandem Reactions Based on the Cyclization of Carbon Dioxide and Propargylic Alcohols: Derivative Applications of α-Alkylidene Carbonates. Catalysts. 2022; 12(1):73. https://doi.org/10.3390/catal12010073
Chicago/Turabian StyleJiang, Bowen, Xiangyu Yan, Yong Xu, Natalya Likhanova, Heriberto Díaz Velázquez, Yanyan Gong, Ye Yuan, and Francis Verpoort. 2022. "Tandem Reactions Based on the Cyclization of Carbon Dioxide and Propargylic Alcohols: Derivative Applications of α-Alkylidene Carbonates" Catalysts 12, no. 1: 73. https://doi.org/10.3390/catal12010073
APA StyleJiang, B., Yan, X., Xu, Y., Likhanova, N., Velázquez, H. D., Gong, Y., Yuan, Y., & Verpoort, F. (2022). Tandem Reactions Based on the Cyclization of Carbon Dioxide and Propargylic Alcohols: Derivative Applications of α-Alkylidene Carbonates. Catalysts, 12(1), 73. https://doi.org/10.3390/catal12010073







