Glycerol Hydrogenolysis to 1,2-Propanediol over Novel Cu/ZrO2 Catalysts
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Characterizations
2.1.1. N2 Physisorption, Inductively Coupled Plasma Mass Spectrometry, X-ray Diffraction, and Scanning Electron Microscopy
2.1.2. Temperature-Programmed Reduction in Hydrogen (H2-TPR) and Temperature-Programmed Desorption of Ammonia (NH3-TPD)
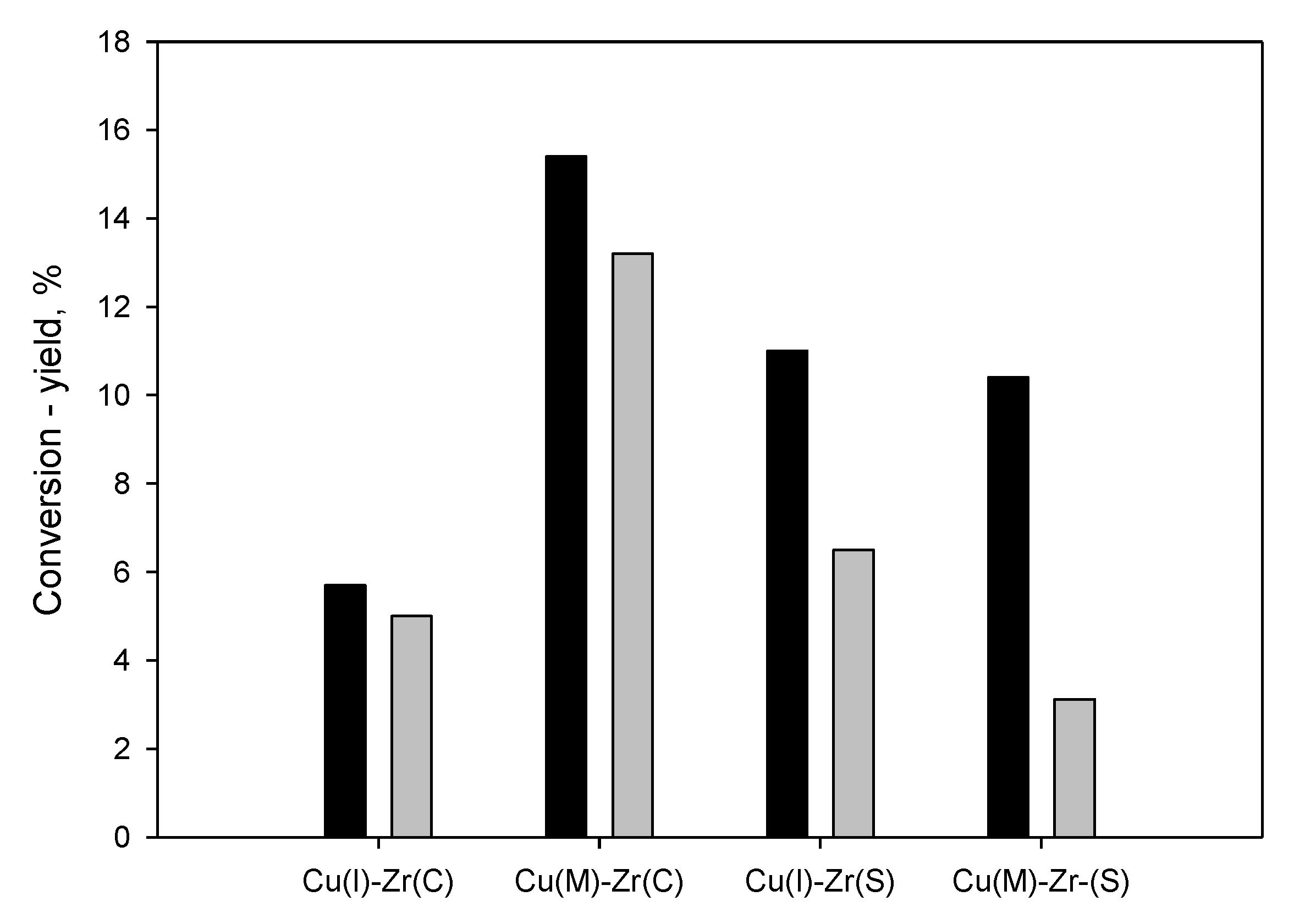
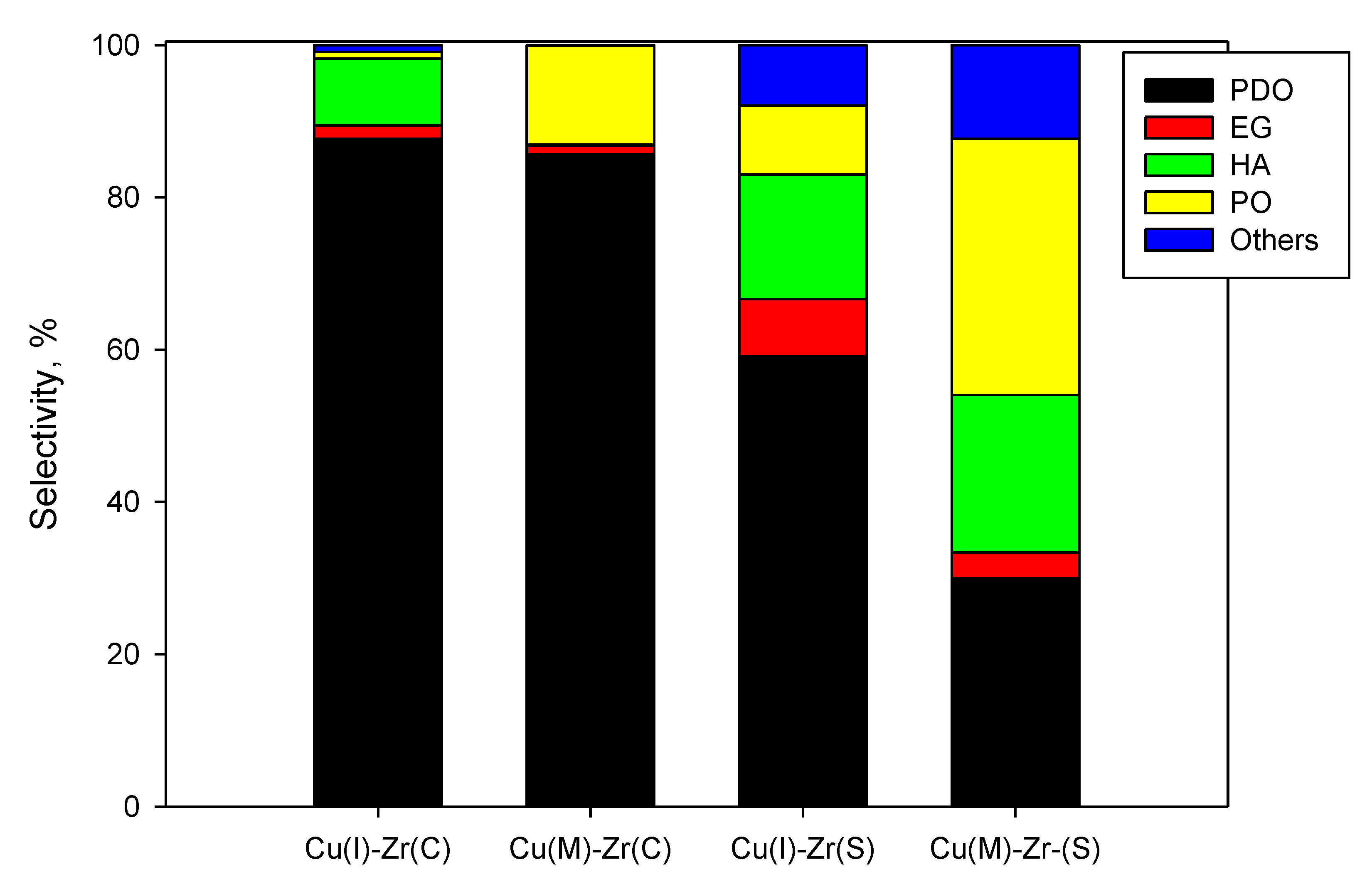
2.2. Activity Tests
2.2.1. Effect of Support and Preparation Routes
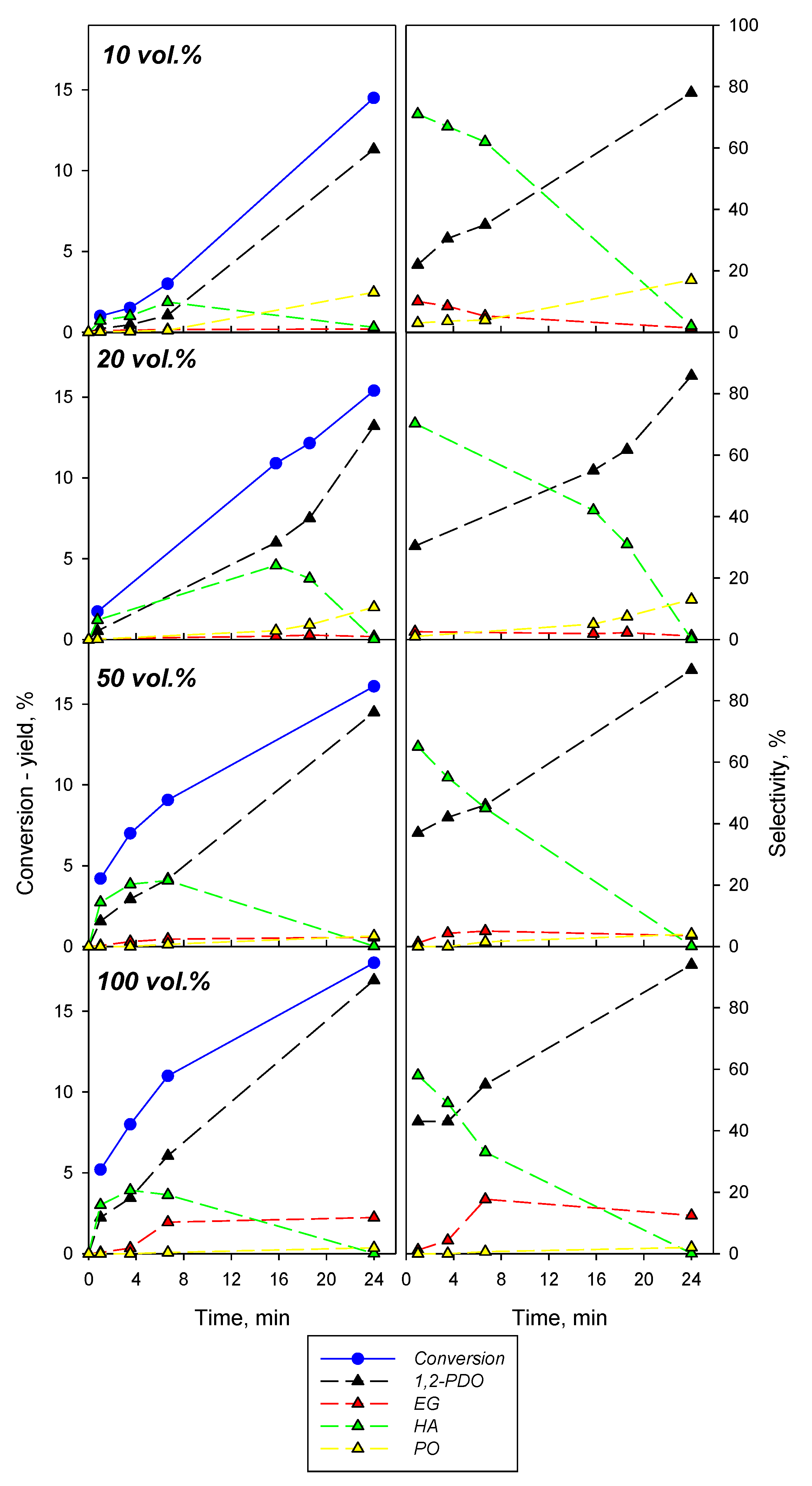
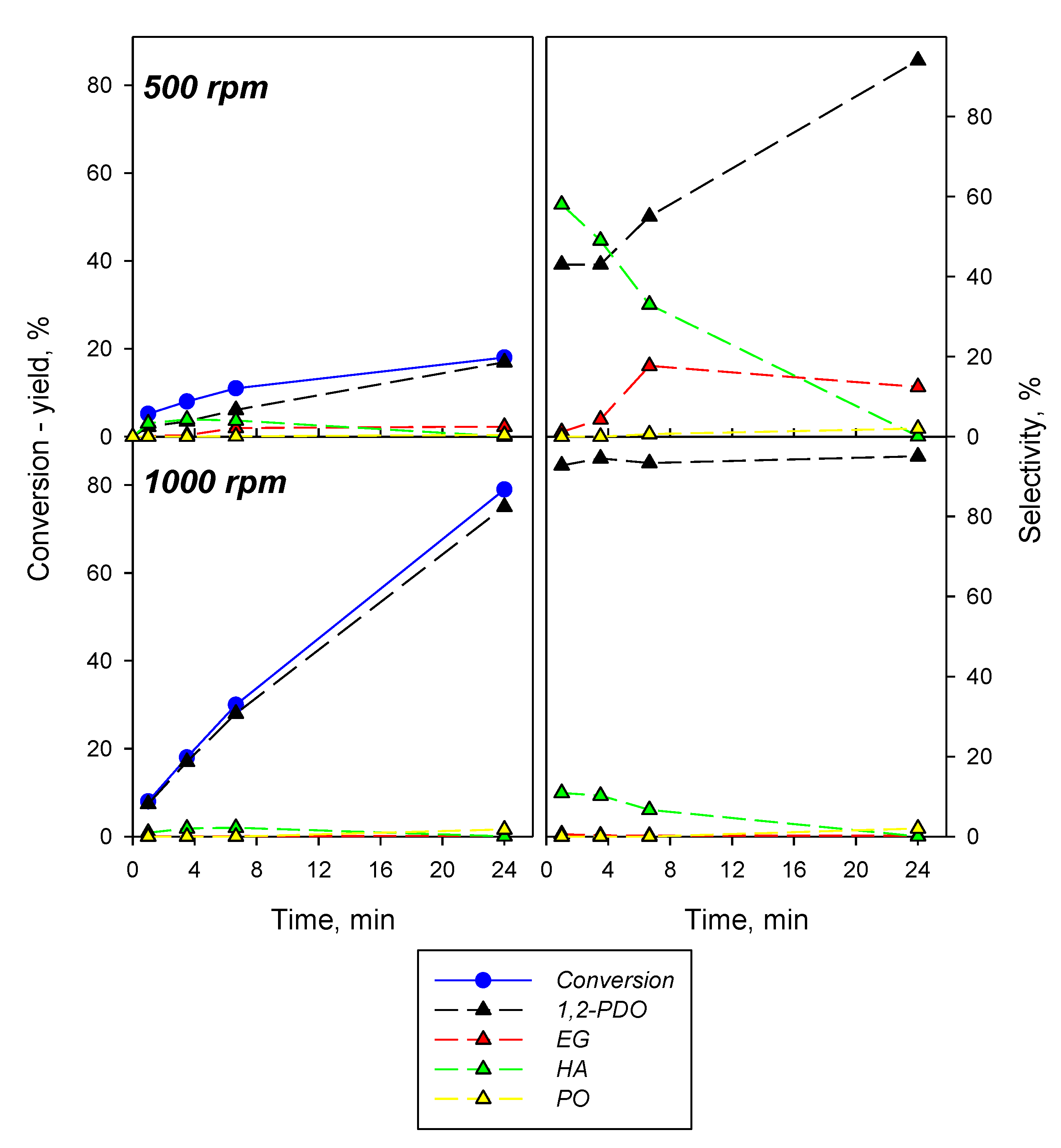
2.2.2. Effect of the Operating Conditions on Cu(M)-Zr(C)
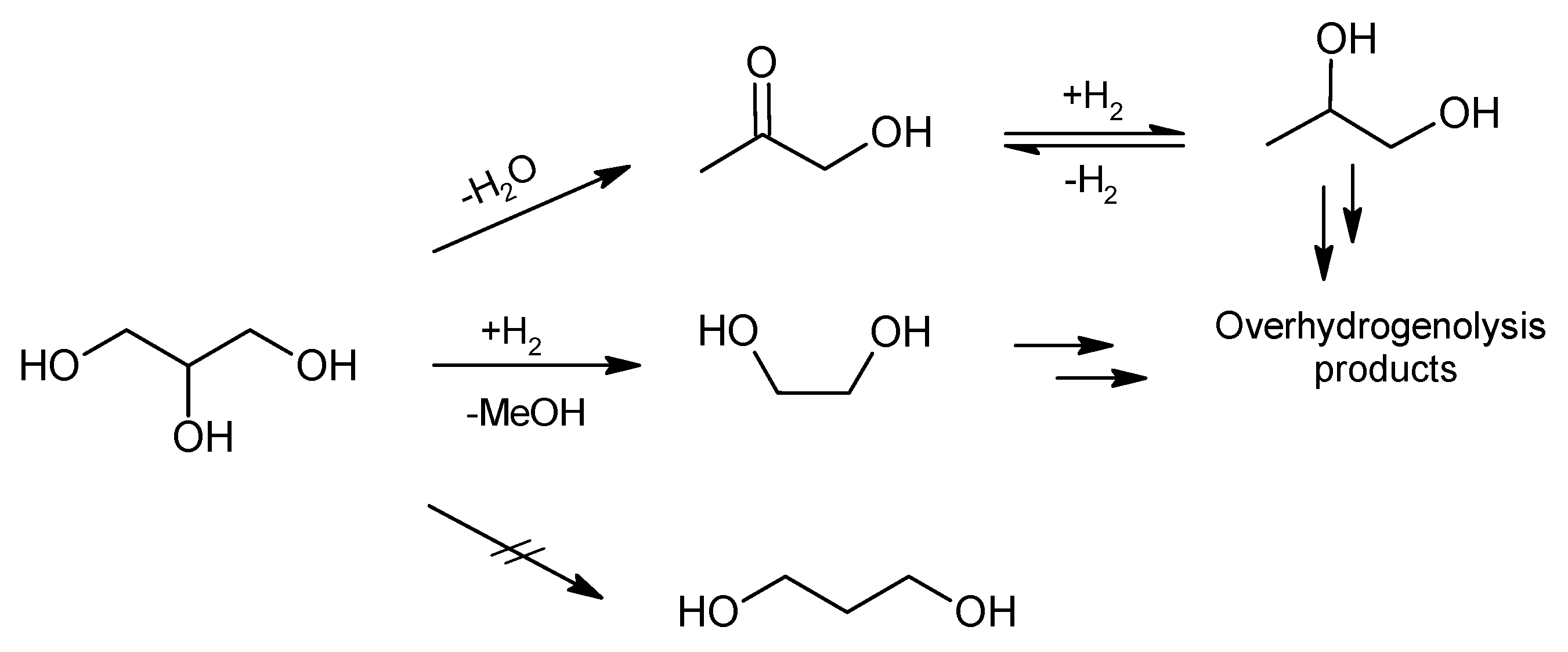
3. Discussion
4. Materials and Methods
4.1. Preparation of Catalysts
4.2. Physicochemical Characterizations
4.3. Catalytic Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ren, X.; Zhang, F.; Sudhakar, M.; Wang, N.; Dai, J.; Liu, L. Gas-phase dehydration of glycerol to acrolein catalyzed by hybrid acid sites derived from transition metal hydrogen phosphate and meso-HZSM-5. Catal. Today 2019, 332, 20–27. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Pina, C. Della Recent advances in the conversion of bioglycerol into value-added products. Eur. J. Lipid Sci. Technol. 2009, 111, 788–799. [Google Scholar] [CrossRef]
- Abreu, T.H.; Meyer, C.I.; Padró, C.; Martins, L. Acidic V-MCM-41 catalysts for the liquid-phase ketalization of glycerol with acetone. Microporous Mesoporous Mater. 2019, 273, 219–225. [Google Scholar] [CrossRef]
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The potential of glycerol as a value-added commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Basile, F.; Cavani, F.; Chieregato, A.; Concepción, P.; Liosi, G.; López Nieto, J.M.; Soriano, M.D.; Trevisanut, C. Glycerol oxidehydration into acrolein and acrylic acid over W/V/Nb bronzes with hexagonal structure. DGMK Tag. 2012, 2012, 189–193. [Google Scholar]
- Célerier, S.; Morisset, S.; Batonneau-Gener, I.; Belin, T.; Younes, K.; Batiot-Dupeyrat, C. Glycerol dehydration to hydroxyacetone in gas phase over copper supported on magnesium oxide (hydroxide) fluoride catalysts. Appl. Catal. A Gen. 2018, 557, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Christy, S.; Noschese, A.; Lomelí-Rodriguez, M.; Greeves, N.; Lopez-Sanchez, J.A. Recent progress in the synthesis and applications of glycerol carbonate. Curr. Opin. Green Sustain. Chem. 2018, 14, 99–107. [Google Scholar] [CrossRef]
- Deleplanque, J.; Dubois, J.L.; Devaux, J.F.; Ueda, W. Production of acrolein and acrylic acid through dehydration and oxydehydration of glycerol with mixed oxide catalysts. Catal. Today 2010, 157, 351–358. [Google Scholar] [CrossRef]
- Yfanti, V.-L.; Ipsakis, D.; Lemonidou, A.A. Kinetic study of liquid phase glycerol hydrodeoxygenation under inert conditions over a Cu-based catalyst. React. Chem. Eng. 2018, 3, 559–571. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Pourmohammadi-Mahunaki, M.; Haddadi-Asl, V.; Roghani-Mamaqani, H.; Koosha, M.; Yazdi, M. Effect of chain extender length and molecular architecture on phase separation and rheological properties of ether-based polyurethanes. Polym. Bull. 2021, 1–16. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, W.; Liao, Z. Preparation, Characterization and Properties of Flame Retardant Unsaturated Polyester Resin Based on r-PET. J. Polym. Environ. 2021, 1–11. [Google Scholar] [CrossRef]
- Kaur, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Valorisation of crude glycerol to value-added products: Perspectives of process technology, economics and environmental issues. Biotechnol. Rep. 2020, 27, e00487. [Google Scholar] [CrossRef] [PubMed]
- Pandhare, N.N.; Pudi, S.M.; Biswas, P.; Sinha, S. Selective hydrogenolysis of glycerol to 1,2-propanediol over highly active and stable Cu/MgO catalyst in the vapor phase. Org. Process Res. Dev. 2016, 20, 1059–1067. [Google Scholar] [CrossRef]
- Salgado, A.L.P.; Araújo, F.C.; Soares, A.V.H.; Xing, Y.; Passos, F.B. Glycerol hydrogenolysis over Ru-Cu bimetallic catalysts supported on modified zirconias. Appl. Catal. A Gen. 2021, 626, 118359. [Google Scholar] [CrossRef]
- Rodrigues, R.; Isoda, N.; Gonçalves, M.; Figueiredo, F.C.A.; Mandelli, D.; Carvalho, W.A. Effect of niobia and alumina as support for Pt catalysts in the hydrogenolysis of glycerol. Chem. Eng. J. 2012, 198–199, 457–467. [Google Scholar] [CrossRef]
- Pendem, C.; Gupta, P.; Chaudhary, N.; Singh, S.; Kumar, J.; Sasaki, T.; Datta, A.; Bal, R. Aqueous phase reforming of glycerol to 1,2-propanediol over Pt-nanoparticles supported on hydrotalcite in the absence of hydrogen. Green Chem. 2012, 14, 3107–3113. [Google Scholar] [CrossRef]
- Checa, M.; Marinas, A.; Marinas, J.M.; Urbano, F.J. Deactivation study of supported Pt catalyst on glycerol hydrogenolysis. Appl. Catal. A Gen. 2015, 507, 34–43. [Google Scholar] [CrossRef]
- Mane, R.; Patil, S.; Shirai, M.; Rayalu, S.; Rode, C. Influence of carbon based supports on selectivity behavior of diols and propanol in Ru catalyzed glycerol hydrogenolysis. Appl. Catal. B Environ. 2017, 204, 134–146. [Google Scholar] [CrossRef]
- Balaraju, M.; Rekha, V.; Devi, B.L.A.P.; Prasad, R.B.N.; Prasad, P.S.S.; Lingaiah, N. Surface and structural properties of titania-supported Ru catalysts for hydrogenolysis of glycerol. Appl. Catal. A Gen. 2010, 384, 107–114. [Google Scholar] [CrossRef]
- Meena, M.L.; Pandey, D.K.; Malviya, H.; Biswas, P. Kinetic Model for the Manufacturing of 1,2-Propanediol (1,2-PDO) via Hydrogenolysis of Bio-glycerol Over Layered Double Hydroxide (LDH) Derived Cu0.45Zn0.15Mg5.4Al2O9 Catalyst in an Autoclave Reactor. Catal. Lett. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Azri, N.; Irmawati, R.; Nda-Umar, U.I.; Saiman, M.I.; Taufiq-Yap, Y.H. Promotional effect of transition metals (Cu, Ni, Co, Fe, Zn)–supported on dolomite for hydrogenolysis of glycerol into 1,2-propanediol. Arab. J. Chem. 2021, 14, 103047. [Google Scholar] [CrossRef]
- Freitas, I.C.; Manfro, R.L.; Souza, M.M.V.M. Hydrogenolysis of glycerol to propylene glycol in continuous system without hydrogen addition over Cu-Ni catalysts. Appl. Catal. B Environ. 2018, 220, 31–41. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, H.; Chen, C.; Chen, R.; Xing, W. Solvent effect on hydrogenolysis of glycerol to 1,2-propanediol over Cu-ZnO catalyst. Chem. Eng. J. 2015, 264, 344–350. [Google Scholar] [CrossRef]
- Guo, L.; Zhou, J.; Mao, J.; Guo, X.; Zhang, S. Supported Cu catalysts for the selective hydrogenolysis of glycerol to propanediols. Appl. Catal. A Gen. 2009, 367, 93–98. [Google Scholar] [CrossRef]
- Omar, L.; Perret, N.; Daniele, S. Self-assembled hybrid zno nanostructures as supports for copper-based catalysts in the hydrogenolysis of glycerol. Catalysts 2021, 11, 516. [Google Scholar] [CrossRef]
- Poddar, M.K.; Pandey, A.; Jha, M.K.; Andola, S.C.; Ali, S.S.; Bhandari, S.; Sahani, G.K.; Bal, R. Aqueous phase hydrogenolysis of renewable glycerol to 1,2-propanediol over bimetallic highly stable and efficient Ni-Cu/Al2O3 catalyst. Mol. Catal. 2021, 515, 111943. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Zhu, Y.; Zheng, H.; Li, Y. Promoting effect of boron oxide on Cu/SiO2 catalyst for glycerol hydrogenolysis to 1,2-propanediol. J. Catal. 2013, 303, 70–79. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Fan, W.; Wang, J.; Li, Y. A highly efficient and robust Cu/SiO2 catalyst prepared by the ammonia evaporation hydrothermal method for glycerol hydrogenolysis to 1,2-propanediol. Catal. Sci. Technol. 2015, 5, 1169–1180. [Google Scholar] [CrossRef]
- Wu, Z.; Mao, Y.; Song, M.; Yin, X.; Zhang, M. Cu/boehmite: A highly active catalyst for hydrogenolysis of glycerol to 1,2-propanediol. Catal. Commun. 2013, 32, 52–57. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, F.; Xue, J.; Zuo, J.; Chen, J.; Xia, C. Cu/SiO2 catalysts prepared by hom- and heterogeneous deposition-precipitation methods: Texture, structure, and catalytic performance in the hydrogenolysis of glycerol to 1,2-propanediol. Catal. Today 2012, 183, 42–51. [Google Scholar] [CrossRef]
- Garcés, J.; Arrué, R.; Novoa, N.; Peixoto, A.F.; Chimentão, R.J. Glycerol valorization over zro2-supported copper nanoparticles catalysts prepared by chemical reduction method. Catalysts 2021, 11, 1040. [Google Scholar] [CrossRef]
- Balaraju, M.; Jagadeeswaraiah, K.; Prasad, P.S.S.; Lingaiah, N. Catalytic hydrogenolysis of biodiesel derived glycerol to 1,2-propanediol over Cu-MgO catalysts. Catal. Sci. Technol. 2012, 2, 1967–1976. [Google Scholar] [CrossRef]
- Yun, D.; Yun, Y.S.; Kim, T.Y.; Park, H.; Lee, J.M.; Han, J.W.; Yi, J. Mechanistic study of glycerol dehydration on Brønsted acidic amorphous aluminosilicate. J. Catal. 2016, 341, 33–43. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, J.; Wang, L.; Xie, W.; Chen, P.; Hou, Z.; Zheng, X. Biodiesel derived glycerol hydrogenolysis to 1,2-propanediol on Cu/MgO catalysts. Bioresour. Technol. 2010, 101, 7088–7092. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, S.; Yu, L.; Liu, Y.; Cao, Y. Efficient catalytic hydrogenolysis of glycerol using formic acid as hydrogen source. Cuihua Xuebao/Chin. J. Catal. 2013, 34, 2066–2074. [Google Scholar] [CrossRef]
- Vitiello, G.; Clarizia, L.; Abdelraheem, W.; Esposito, S.; Bonelli, B.; Ditaranto, N.; Vergara, A.; Nadagouda, M.; Dionysiou, D.D.; Andreozzi, R.; et al. Near UV-Irradiation of CuOx-Impregnated TiO2 Providing Active Species for H2 Production Through Methanol Photoreforming. ChemCatChem 2019, 11, 4314–4326. [Google Scholar] [CrossRef]
- Alfè, M.; Gargiulo, V.; Amati, M.; Maraloiu, V.A.; Maddalena, P.; Lettieri, S. Mesoporous tio2 from metal-organic frameworks for photo-luminescence-based optical sensing of oxygen. Catalysts 2021, 11, 795. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Shao, Z.; Jiang, S.P. Metal-organic frameworks derived porous carbon, metal oxides and metal sulfides-based compounds for supercapacitors application. Energy Storage Mater. 2020, 26, 1–22. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Sun, L.; Wang, L. Metal/metal oxide nanostructures derived from metal-organic frameworks. RSC Adv. 2015, 5, 7267–7279. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-organic frameworks: Synthetic methods and potential applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef]
- Gonzalez, R.D.; Lopez, T.; Gomez, R. Sol-gel preparation of supported metal catalysts. Catal. Today 1997, 35, 293–317. [Google Scholar] [CrossRef]
- Alfè, M.; Gargiulo, V.; Lisi, L.; Di Capua, R. Synthesis and characterization of conductive copper-based metal-organic framework/graphene-like composites. Mater. Chem. Phys. 2014, 147, 744–750. [Google Scholar] [CrossRef]
- Alfe, M.; Policicchio, A.; Lisi, L.; Gargiulo, V. Solid sorbents for CO2 and CH4 adsorption: The effect of metal organic framework hybridization with graphene-like layers on the gas sorption capacities at high pressure. Renew. Sustain. Energy Rev. 2021, 141, 110816. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Landi, G.; Lisi, L.; Patrono, P.; Pinzari, F. Effect of the support on the catalytic properties of vanadyl phosphate in the oxidative dehydrogenation of propane. J. Mol. Catal. A Chem. 2010, 329, 50–56. [Google Scholar] [CrossRef]
- Gabrysch, T.; Peng, B.; Bunea, S.; Dyker, G.; Muhler, M. The Role of Metallic Copper in the Selective Hydrodeoxygenation of Glycerol to 1,2-Propanediol over Cu/ZrO2. ChemCatChem 2018, 10, 1344–1350. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Lemonidou, A.A. Investigating the performance and deactivation behaviour of silica-supported copper catalysts in glycerol hydrogenolysis. Appl. Catal. A Gen. 2011, 396, 177–185. [Google Scholar] [CrossRef]
- Sherafat, Z.; Antunes, I.; Almeida, C.; Frade, J.R.; Paydar, M.H.; Mather, G.C.; Fagg, D.P. Enhanced BaZrO3 mechanosynthesis by the use of metastable ZrO2 precursors. Dalt. Trans. 2014, 43, 9324–9333. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.F.; Zhong, Y.J.; Yuan, X.X.; Zheng, X.M. TPR and TPD studies of CuO/CeO2 catalysts for low temperature CO oxidation. Appl. Catal. A Gen. 1997, 162, 121–131. [Google Scholar] [CrossRef]
- Barbato, P.S.; Colussi, S.; Di Benedetto, A.; Landi, G.; Lisi, L.; Llorca, J.; Trovarelli, A. CO preferential oxidation under H2-rich streams on copper oxide supported on Fe promoted CeO2. Appl. Catal. A Gen. 2015, 506, 268–277. [Google Scholar] [CrossRef]
- Barbato, P.S.; Colussi, S.; Di Benedetto, A.; Landi, G.; Lisi, L.; Llorca, J.; Trovarelli, A. Origin of High Activity and Selectivity of CuO/CeO2 Catalysts Prepared by Solution Combustion Synthesis in CO-PROX Reaction. J. Phys. Chem. C 2016, 120, 13039–13048. [Google Scholar] [CrossRef]
- Landi, G.; Barbato, P.S.; Di Benedetto, A.; Lisi, L. Optimization of the preparation method of CuO/CeO2 structured catalytic monolith for CO preferential oxidation in H2-rich streams. Appl. Catal. B Environ. 2016, 181, 727–737. [Google Scholar] [CrossRef]
- Shimokawabe, M.; Asakawa, H.; Takezawa, N. Characterization of copper/zirconia catalysts prepared by an impregnation method. Appl. Catal. 1990, 59, 45–58. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Sagar, G.V.; Srikanth, C.S.; Rao, V.V. Characterization and catalytic functionalities of copper oxide catalysts supported on zirconia. J. Phys. Chem. B 2007, 111, 543–550. [Google Scholar] [CrossRef]
- Martin, A.; Armbruster, U.; Atia, H. Recent developments in dehydration of glycerol toward acrolein over heteropolyacids. Eur. J. Lipid Sci. Technol. 2012, 114, 10–23. [Google Scholar] [CrossRef]
- Kim, Y.T.; Jung, K.D.; Park, E.D. A comparative study for gas-phase dehydration of glycerol over H-zeolites. Appl. Catal. A Gen. 2011, 393, 275–287. [Google Scholar] [CrossRef]
- Samson, K.; Sliwa, M.; Socha, R.P.; Góra-Marek, K.; Mucha, D.; Rutkowska-Zbik, D.; Paul, J.F.; Ruggiero-Mikoajczyk, M.; Grabowski, R.; Soczyński, J. Influence of ZrO2 structure and copper electronic state on activity of Cu/ZrO2 catalysts in methanol synthesis from CO2. ACS Catal. 2014, 4, 3730–3741. [Google Scholar] [CrossRef]
- Yu, H.; Fang, H.; Zhang, H.; Li, B.; Deng, F. Acidity of sulfated tin oxide and sulfated zirconia: A view from solid-state NMR spectroscopy. Catal. Commun. 2009, 10, 920–924. [Google Scholar] [CrossRef]
- Mane, R.B.; Yamaguchi, A.; Malawadkar, A.; Shirai, M.; Rode, C.V. Active sites in modified copper catalysts for selective liquid phase dehydration of aqueous glycerol to acetol. RSC Adv. 2013, 3, 16499–16508. [Google Scholar] [CrossRef]
- Sato, S.; Akiyama, M.; Takahashi, R.; Hara, T.; Inui, K.; Yokota, M. Vapor-phase reaction of polyols over copper catalysts. Appl. Catal. A Gen. 2008, 347, 186–191. [Google Scholar] [CrossRef]
- Montassier, C.; Ménézo, J.C.; Hoang, L.C.; Renaud, C.; Barbier, J. Aqueous polyol conversions on ruthenium and on sulfur-modified ruthenium. J. Mol. Catal. 1991, 70, 99–110. [Google Scholar] [CrossRef]
- Dasari, M.A.; Kiatsimkul, P.P.; Sutterlin, W.R.; Suppes, G.J. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A Gen. 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tomishige, K. Heterogeneous catalysis of the glycerol hydrogenolysis. Catal. Sci. Technol. 2011, 1, 179–190. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ning, X.; Amada, Y.; Tomishige, K. Solid acid co-catalyst for the hydrogenolysis of glycerol to 1,3-propanediol over Ir-ReOx/SiO2. Appl. Catal. A Gen. 2012, 433–434, 128–134. [Google Scholar] [CrossRef]
- Balaraju, M.; Rekha, V.; Sai Prasad, P.S.; Prasad, R.B.N.; Lingaiah, N. Selective hydrogenolysis of glycerol to 1,2-propanediol over Cu-ZnO catalysts. Catal. Lett. 2008, 126, 119–124. [Google Scholar] [CrossRef]

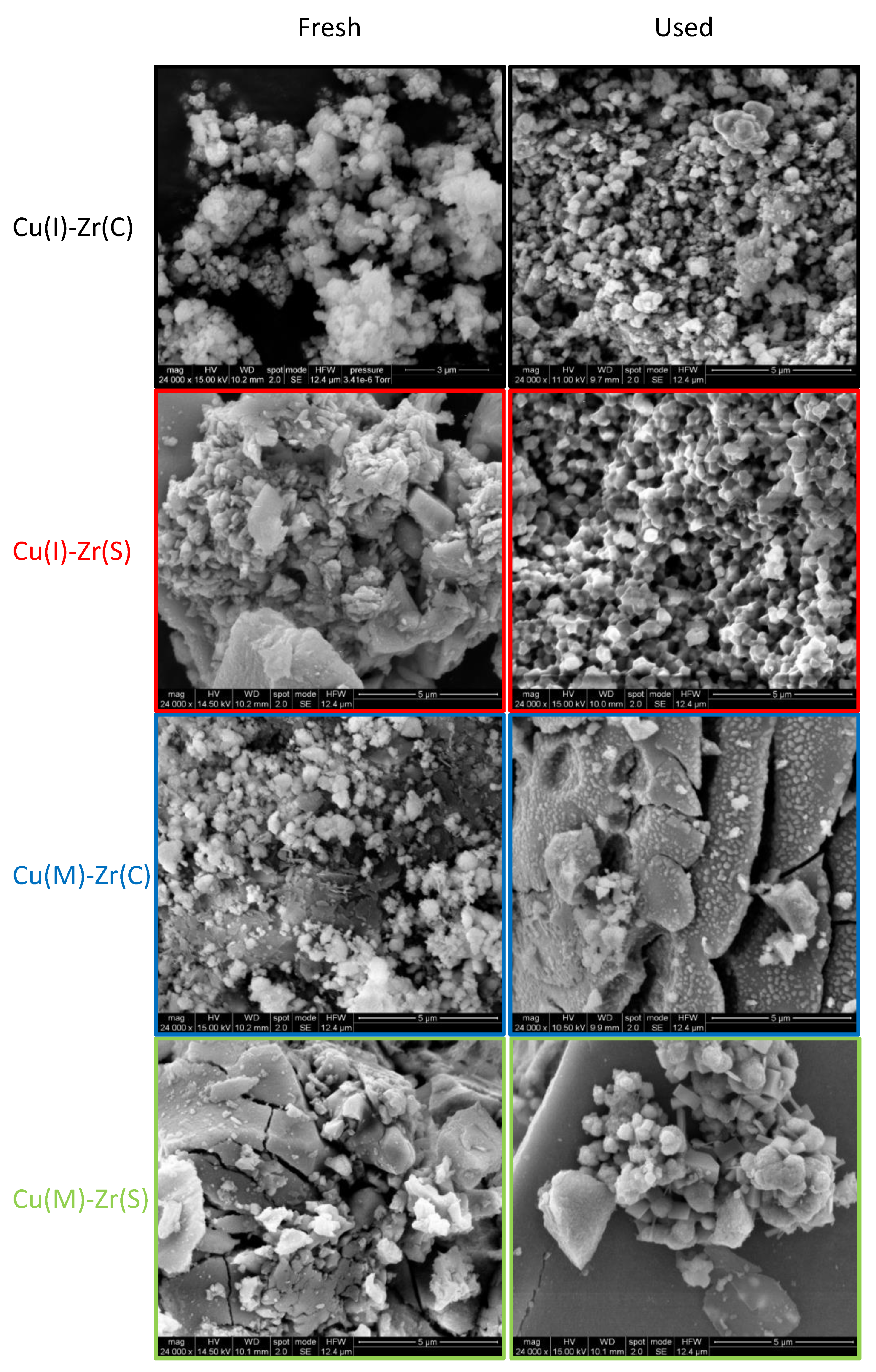


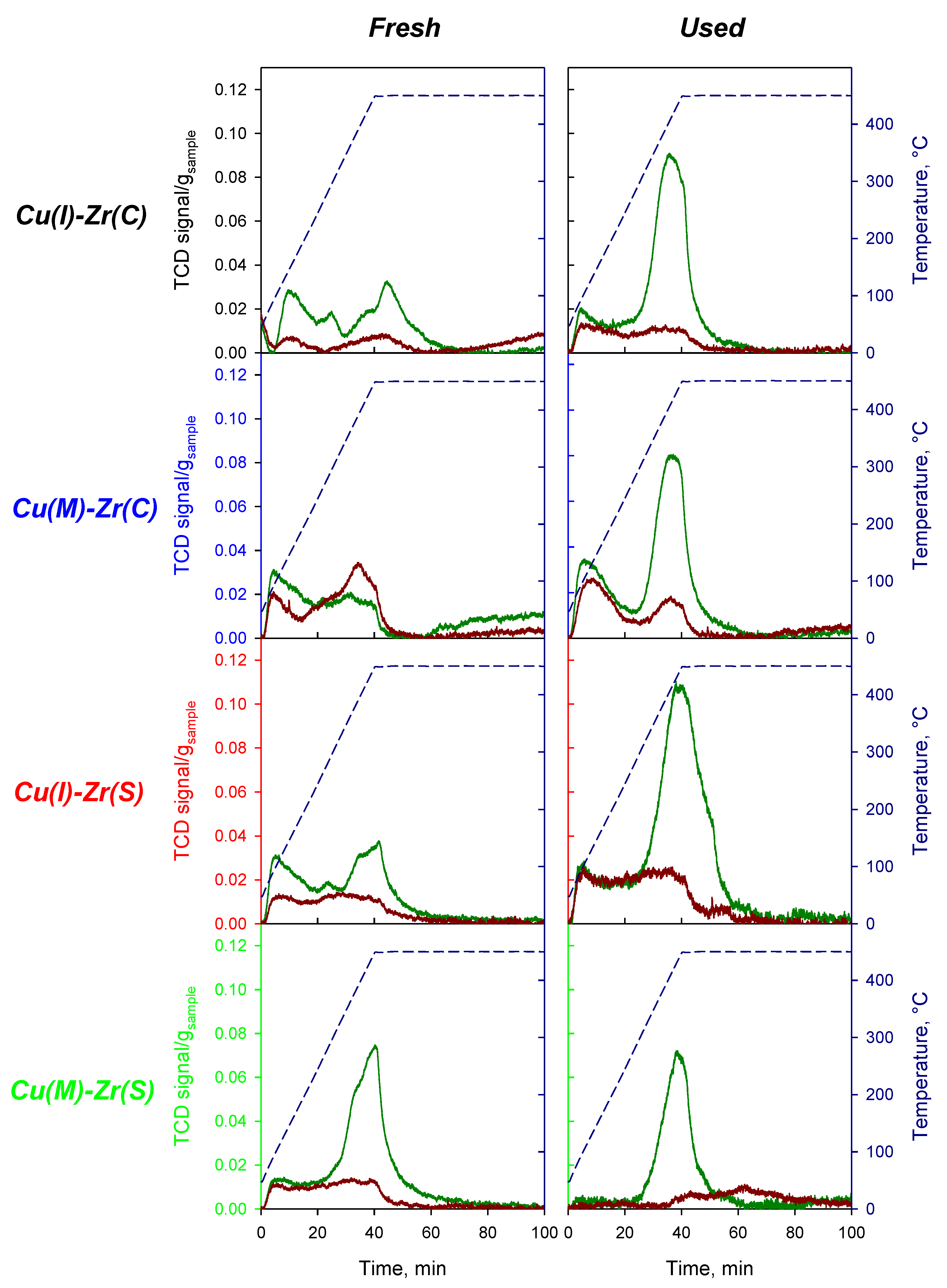



| Sample | Cu Source | Approach | ZrO2 Source | SSA (Fresh) | SSA (Used) |
|---|---|---|---|---|---|
| Cu(I)-Zr(C) | Cu(CH3COO)2 | Impregnation | Commercial | 18.1 | 8.4 |
| Cu(I)-Zr(S) | Cu(CH3COO)2 | Impregnation | Sol–Gel | 91.4 | 11.5 |
| Cu(M)-Zr(C) | HKUST-1 | MOFMS | Commercial | 15.6 | 6.4 |
| Cu(M)-Zr(S) | HKUST-1 | MOFMS | Sol–Gel | 25.8 | 23.2 |
| Sample | NH3—First TPD | H2 | H2/Cu | NH3—Second TPD |
|---|---|---|---|---|
| Fresh | ||||
| Cu(I)-Zr(C) | 1.64 | 1.338 | 0.74 | 0.37 |
| Cu(M)-Zr(C) | 1.79 | 1.777 | 0.92 | 1.91 |
| Cu(I)-Zr(S) | 2.02 | 1.901 | 1.02 | 0.99 |
| Cu(M)-Zr(S) | 2.38 | 1.980 | 1.02 | 0.86 |
| Used | ||||
| Cu(I)-Zr(C) | 3.83 | 0.113 | 0.06 | 1.02 |
| Cu(M)-Zr(C) | 3.33 | 0.379 | 0.19 | 1.23 |
| Cu(I)-Zr(S) | 9.69 | 0.065 | 0.04 | 3.89 |
| Cu(M)-Zr(S) | 2.80 | 0.005 | 0.003 | 0.63 |
| Catalyst | τ | x | s | T | xgly | Ref. |
|---|---|---|---|---|---|---|
| 18%Cu/ZrO2 | 160 | 80 | 92 | 200 | 4 | [46] |
| Cu:Zn 50:50 | 192 | 37 | 92 | 200 | 20 | [65] |
| 5%(Cu-Ru)/SiO2 | 37.5 | 39.2 | 85.9 | 240 | 100 | [47] |
| Pt/Nb2O5/Al2O3 | 60 | 75 | 85 | 140 | 20 | [16] |
| Cu(M)-Zr(C) | 120 | 79 | 95 | 240 | 4 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luciani, G.; Ruoppolo, G.; Landi, G.; Gargiulo, V.; Alfè, M.; Di Benedetto, A. Glycerol Hydrogenolysis to 1,2-Propanediol over Novel Cu/ZrO2 Catalysts. Catalysts 2022, 12, 72. https://doi.org/10.3390/catal12010072
Luciani G, Ruoppolo G, Landi G, Gargiulo V, Alfè M, Di Benedetto A. Glycerol Hydrogenolysis to 1,2-Propanediol over Novel Cu/ZrO2 Catalysts. Catalysts. 2022; 12(1):72. https://doi.org/10.3390/catal12010072
Chicago/Turabian StyleLuciani, Giuseppina, Giovanna Ruoppolo, Gianluca Landi, Valentina Gargiulo, Michela Alfè, and Almerinda Di Benedetto. 2022. "Glycerol Hydrogenolysis to 1,2-Propanediol over Novel Cu/ZrO2 Catalysts" Catalysts 12, no. 1: 72. https://doi.org/10.3390/catal12010072
APA StyleLuciani, G., Ruoppolo, G., Landi, G., Gargiulo, V., Alfè, M., & Di Benedetto, A. (2022). Glycerol Hydrogenolysis to 1,2-Propanediol over Novel Cu/ZrO2 Catalysts. Catalysts, 12(1), 72. https://doi.org/10.3390/catal12010072











