Construction of SrTiO3–LaCrO3 Solid Solutions with Consecutive Band Structures for Photocatalytic H2 Evolution under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Characterization
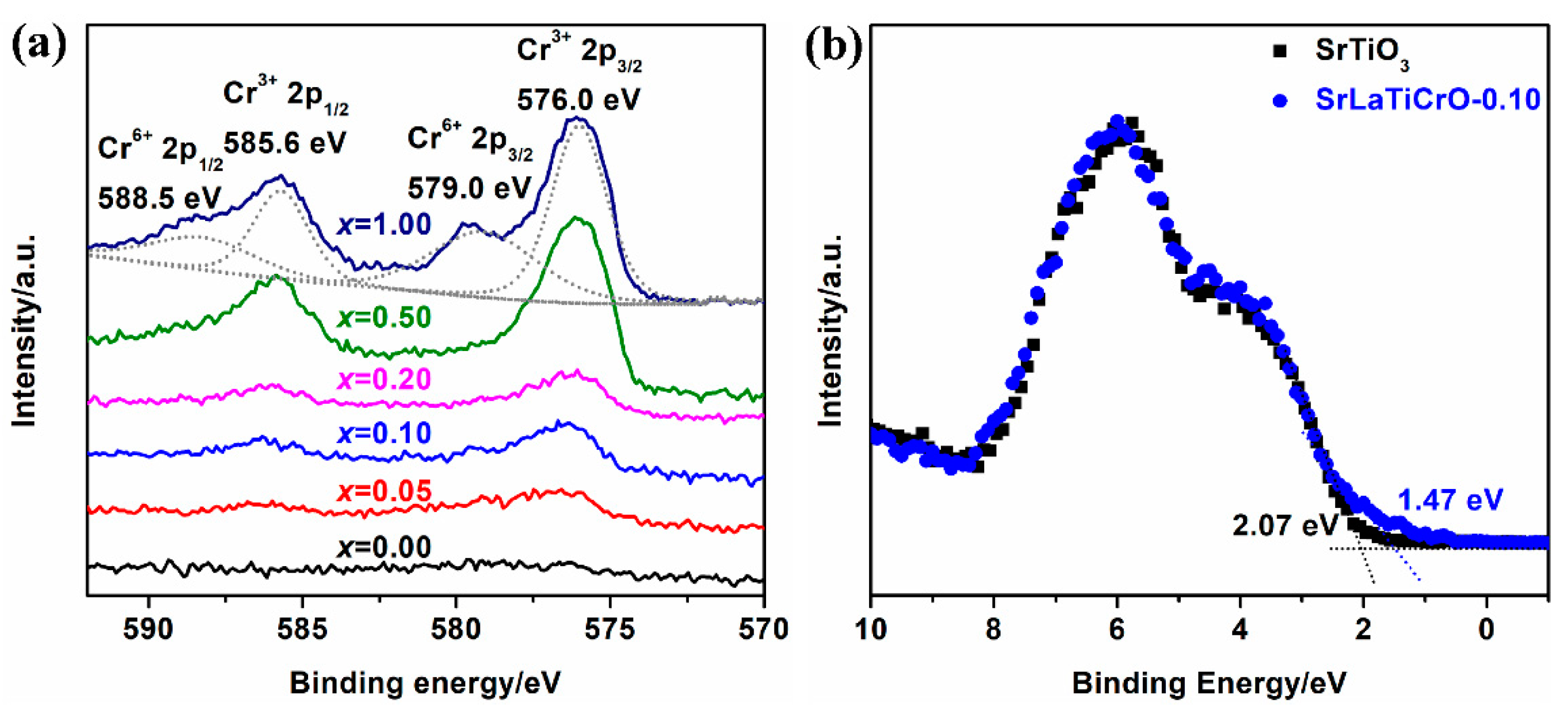
2.2. Chemical Composition and State
2.3. Optical Properties and Band Structure
2.4. Photocatalytic Performance
2.5. Photocatalytic Mechanism
3. Materials and Methods
3.1. Synthesis of Photocatalysts
3.2. Characterization
3.3. Electrochemical Measurements
3.4. Photocatalytic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, J.M. Carbon neutrality: Toward a sustainable future. Innovation 2021, 2, 100127. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-M.; Chen, K.; Kang, J.-N.; Chen, W.; Wang, X.-Y.; Zhang, X. Policy and management of carbon peaking and carbon neutrality: A literature review. Engineering 2022, 14, 52–63. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green carbon science: Efficient carbon resource processing, utilization, and recycling towards carbon neutrality. Angew. Chem. Int. Ed. 2022, 61, e202112835. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate photocatalysts for light-driven water splitting: Mechanisms, challenges, and design strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, J.; Kong, Y.; Zhao, Y.; Chen, S.; Li, D.; Liu, W.; Chen, Y.; Xie, T.; Cui, J.; et al. Unraveling of cocatalysts photodeposited selectively on facets of BiVO4 to boost solar water splitting. Nat. Commun. 2022, 13, 484. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Dong, C.-L.; Huang, Y.-C.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Jia, Q.; Tokudome, H.; Zhong, M.; Wang, C.; Pan, Z.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 2016, 15, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Chowdhury, F.A.; Wang, Y.; Pant, N.; Vanka, S.; Trudeau, M.L.; Guo, L.; Vayssieres, L.; Mi, Z. Making of an industry-friendly artificial photosynthesis device. ACS Energy Lett. 2018, 3, 2230–2231. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Y.; Su, J.; Liu, M.; Liu, Y. Obstacles of solar-powered photocatalytic water splitting for hydrogen production: A perspective from energy flow and mass flow. Energy 2019, 172, 1079–1086. [Google Scholar] [CrossRef]
- Shi, J.; Guo, L. ABO3-based photocatalysts for water splitting. Prog. Nat. Sci. 2012, 22, 592–615. [Google Scholar] [CrossRef]
- Phoon, B.L.; Lai, C.W.; Juan, J.C.; Show, P.-L.; Chen, W.-H. A review of synthesis and morphology of SrTiO3 for energy and other applications. Int. J. Energy Res. 2019, 43, 5151–5174. [Google Scholar] [CrossRef]
- Patial, S.; Hasija, V.; Raizada, P.; Singh, P.; Singh, A.A.P.K.; Asiri, A.M. Tunable photocatalytic activity of SrTiO3 for water splitting: Strategies and future scenario. J. Environ. Chem. Eng. 2020, 8, 103791. [Google Scholar] [CrossRef]
- Goto, Y.; Hisatomi, T.; Wang, Q.; Higashi, T.; Ishikiriyama, K.; Maeda, T.; Sakata, Y.; Okunaka, S.; Tokudome, H.; Katayama, M.; et al. A particulate photocatalyst water-splitting panel for large-scale solar hydrogen generation. Joule 2018, 2, 509–520. [Google Scholar] [CrossRef]
- Wang, B.; Shen, S.; Guo, L. SrTiO3 single crystals enclosed with high-indexed {023} facets and {001} facets for photocatalytic hydrogen and oxygen evolution. Appl. Catal. B-Environ. 2015, 166–167, 320–326. [Google Scholar] [CrossRef]
- Townsend, T.K.; Browning, N.D.; Osterloh, F.E. Overall photocatalytic water splitting with NiOx-SrTiO3—A revised mechanism. Energy Environ. Sci. 2012, 5, 9543–9550. [Google Scholar] [CrossRef]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic activities of noble metal ion doped SrTiO3 under visible light irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. [Google Scholar] [CrossRef]
- Kato, H.; Sasaki, Y.; Shirakura, N.; Kudo, A. Synthesis of highly active rhodium-doped SrTiO3 powders in Z-scheme systems for visible-light-driven photocatalytic overall water splitting. J. Mater. Chem. A 2013, 1, 12327–12333. [Google Scholar] [CrossRef]
- Wang, D.; Ye, J.; Kako, T.; Kimura, T. Photophysical and Photocatalytic Properties of SrTiO3 Doped with Cr Cations on Different Sites. J. Phys. Chem. B 2006, 110, 15824–15830. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lim, S.N.; Park, S.B. Co-doping schemes to enhance H2 evolution under visible light irradiation over SrTiO3:Ni/M (M = La or Ta) prepared by spray pyrolysis. Int. J. Hydrogen Energy 2012, 37, 5540–5549. [Google Scholar] [CrossRef]
- Shi, J.; Ye, J.; Ma, L.; Ouyang, S.; Jing, D.; Guo, L. Site-selected doping of upconversion luminescent Er3+ into SrTiO3 for visible-light-driven photocatalytic H2 or O2 evolution. Chem.-Eur. J. 2012, 18, 7543–7551. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Huang, C.-W.; Wu, J.C.S.; Lin, S.-T. Theoretical Investigation of the Metal-Doped SrTiO3 Photocatalysts for Water Splitting. J. Phys. Chem. C 2012, 116, 7897–7903. [Google Scholar] [CrossRef]
- Reunchan, P.; Ouyang, S.; Umezawa, N.; Xu, H.; Zhang, Y.; Ye, J. Theoretical design of highly active SrTiO3-based photocatalysts by a codoping scheme towards solar energy utilization for hydrogen production. J. Mater. Chem. A 2013, 1, 4221–4227. [Google Scholar] [CrossRef]
- Guan, X.; Zong, S.; Tian, L.; Liu, M. Efficient photocatalytic hydrogen production under visible-light irradiation on SrTiO3 without noble metal: Dye-sensitization and earth-abundant cocatalyst modification. Mater. Today Chem. 2022, 26, 101018. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Y.; Li, J.; Li, P. Efficient hydrogen evolution with ZnO/SrTiO3 S-scheme heterojunction photocatalyst sensitized by Eosin Y. Int. J. Hydrogen Energy 2021, 46, 18922–18935. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Lu, G.; Yao, X.; Guo, L. Twins in Cd1−xZnxS solid solution: Highly efficient photocatalyst for hydrogen generation from water. Energy Environ. Sci. 2011, 4, 1372–1378. [Google Scholar] [CrossRef]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN:ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Chowdhury, F.A.; Pant, N.; Guo, L.; Vayssieres, L.; Mi, Z. Efficient unassisted overall photocatalytic seawater splitting on GaN-based nanowire arrays. J. Phys. Chem. C 2018, 122, 13797–13802. [Google Scholar] [CrossRef]
- Wang, D.; Kako, T.; Ye, J. New series of solid-solution semiconductors (AgNbO3)1−x(SrTiO3)x with modulated band structure and enhanced visible-light photocatalytic activity. J. Phys. Chem. C 2009, 113, 3785–3792. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.-W.; Zhang, W.; Suwardi, A.; Wang, H.; Wang, D.J.L. Single-crystalline thin films for studying intrinsic properties of BiFeO3–SrTiO3 solid solution photoelectrodes in solar energy conversion. Chem. Mater. 2015, 27, 6635–6641. [Google Scholar] [CrossRef]
- Lu, L.; Lv, M.; Liu, G.; Xu, X. Photocatalytic hydrogen production over solid solutions between BiFeO3 and SrTiO3. Appl. Surf. Sci. 2017, 391, 535–541. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L.; Qian, J.; Zhu, Z.; Ni, S.; Liu, G.; Xu, X. In situ exsolution of silver nanoparticles on AgTaO3-SrTiO3 solid solutions as efficient plasmonic photocatalysts for water splitting. Appl. Catal. B-Environ. 2019, 256, 117818. [Google Scholar] [CrossRef]
- Shi, J.; Zong, S.; Hu, Y.; Guan, X.; Luo, J.; Shang, Y.; Li, G.; Liu, D.; Wang, X.; Guo, P. Continuous solid solutions of Na0.5La0.5TiO3-LaCrO3 for photocatalytic H2 evolution under visible-light irradiation. RSC Adv. 2016, 6, 51801–51806. [Google Scholar] [CrossRef]
- Li, G.; Kuang, X.; Tian, S.; Liao, F.; Jing, X.; Uesu, Y.; Kohn, K. Structure and conductivity of perovskites Sr1−xLaxTi1−xCrxO3. J. Solid State Chem. 2002, 165, 381–392. [Google Scholar] [CrossRef]
- Chen, H.; Umezawa, N. Effect of cation arrangement on the electronic structures of the perovskite solid solutions (SrTiO3)1−x(LaCrO3)x from first principles. Phys. Rev. B 2014, 90, 045119. [Google Scholar] [CrossRef]
- Sudrajat, H.; Zhou, Y.; Sasaki, T.; Ichikuni, N.; Onishi, H. The atomic-scale structure of LaCrO3–NaTaO3 solid solution photocatalysts with enhanced electron population. Phys. Chem. Chem. Phys. 2019, 21, 5148–5157. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J. Band gap tuning of Na1−xLaxTa1−xCrxO3 for H2 generation from water under visible light irradiation. J. Appl. Phys. 2009, 106, 074910. [Google Scholar] [CrossRef]
- Ouyang, S.; Tong, H.; Umezawa, N.; Cao, J.; Li, P.; Bi, Y.; Zhang, Y.; Ye, J. Surface-alkalinization-induced enhancement of photocatalytic H2 evolution over SrTiO3-based photocatalysts. J. Am. Chem. Soc. 2012, 134, 1974–1977. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Wu, C.; Li, X.; Shao, G.; Zhang, P. Tracking charge transfer pathways in SrTiO3/CoP/Mo2C nanofibers for enhanced photocatalytic solar fuel production. Chin. J. Catal. 2022, 43, 507–518. [Google Scholar] [CrossRef]
- Liu, J.W.; Chen, G.; Li, Z.H.; Zhang, Z.G. Electronic structure and visible light photocatalysis water splitting property of chromium-doped SrTiO3. J. Solid State Chem. 2006, 179, 3704–3708. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, R.K.; Sinha, A.S.K.; Singh, P. Effect of isovalent ion substitution on electrical and dielectric properties of LaCrO3. J. Alloys Compd. 2013, 576, 154–160. [Google Scholar] [CrossRef]
- Guan, X.; Guo, L. Cocatalytic Effect of SrTiO3 on Ag3PO4 toward enhanced photocatalytic water oxidation. ACS Catal. 2014, 4, 3020–3026. [Google Scholar] [CrossRef]
- Shi, J.; Ye, J.; Li, Q.; Zhou, Z.; Tong, H.; Xi, G.; Guo, L. Single-crystal nanosheet-based hierarchical AgSbO3 with exposed {001} facets: Topotactic synthesis and enhanced photocatalytic activity. Chem.-Eur. J. 2012, 18, 3157–3162. [Google Scholar] [CrossRef]
- Nashim, A.; Parida, K. n-La2Ti2O7/p-LaCrO3: A novel heterojunction based composite photocatalyst with enhanced photoactivity towards hydrogen production. J. Mater. Chem. A 2014, 2, 18405–18412. [Google Scholar] [CrossRef]
- Cardon, F.; Gomes, W.P. On the determination of the flat-band potential of a semiconductor in contact with a metal or an electrolyte from the Mott-Schottky plot. J. Phys. D Appl. Phys. 1978, 11, L63–L67. [Google Scholar] [CrossRef]
- Ueda, K.; Kato, H.; Kobayashi, M.; Hara, M.; Kakihana, M. Control of valence band potential and photocatalytic properties of NaxLa1−xTaO1+2xN2−2x oxynitride solid solutions. J. Mater. Chem. A 2013, 1, 3667–3674. [Google Scholar] [CrossRef]
- Maeda, K.; Hirayama, N.; Nakata, H.; Wakayama, H.; Oka, K. Oxyfluoride Pb2Ti4O9F2 as a stable anode material for photoelectrochemical water oxidation. J. Phys. Chem. C 2019, 124, 1844–1850. [Google Scholar] [CrossRef]
- Murcia, J.J.; Hidalgo, M.C.; Navío, J.A.; Araña, J.; Doña-Rodríguez, J.M. Study of the phenol photocatalytic degradation over TiO2 modified by sulfation, fluorination, and platinum nanoparticles photodeposition. Appl. Catal. B-Environ. 2015, 179, 305–312. [Google Scholar] [CrossRef]
- Hu, C.-C.; Lee, Y.-L.; Teng, H. Efficient water splitting over Na1−xKxTaO3 photocatalysts with cubic perovskite structure. J. Mater. Chem. 2011, 21, 3824–3830. [Google Scholar] [CrossRef]
- Malik, J.; Kumar, S.; Srivastava, P.; Bag, M.; Mandal, T.K. Cation disorder and octahedral distortion control of internal electric field, band bending and carrier lifetime in Aurivillius perovskite solid solutions for enhanced photocatalytic activity. Mater. Adv. 2021, 2, 4832–4842. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, X.; Zong, S.; Tian, L.; Zhang, Y.; Shi, J. Construction of SrTiO3–LaCrO3 Solid Solutions with Consecutive Band Structures for Photocatalytic H2 Evolution under Visible Light Irradiation. Catalysts 2022, 12, 1123. https://doi.org/10.3390/catal12101123
Guan X, Zong S, Tian L, Zhang Y, Shi J. Construction of SrTiO3–LaCrO3 Solid Solutions with Consecutive Band Structures for Photocatalytic H2 Evolution under Visible Light Irradiation. Catalysts. 2022; 12(10):1123. https://doi.org/10.3390/catal12101123
Chicago/Turabian StyleGuan, Xiangjiu, Shichao Zong, Li Tian, Yazhou Zhang, and Jinwen Shi. 2022. "Construction of SrTiO3–LaCrO3 Solid Solutions with Consecutive Band Structures for Photocatalytic H2 Evolution under Visible Light Irradiation" Catalysts 12, no. 10: 1123. https://doi.org/10.3390/catal12101123




