PNP-Ligated Rare-Earth Metal Catalysts for Efficient Polymerization of Isoprene
Abstract
1. Introduction
2. Results
2.1. Preparation of the Tridentate PNP-Ligated Rare-Earth Metal Complexes
2.2. Polymerization of Isoprene with Borate [NH-BARF] Activator in Toluene
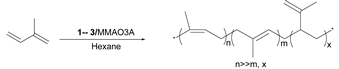
2.3. Polymerization of Isoprene with MMAO3A Activator in Hexane and Toluene
3. Discussion
4. Materials and Methods
4.1. Synthesis of Complexes 1, 2 and 3
4.2. Polymerizations in Toluene
4.3. Polymerizations in Hexane
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Senyek, M.L. Isoprene Polymers. In Encyclopedia of Polymer Science and Technology, 4th ed.; Herman, M.F., Ed.; John Welly & Sons Inc.: New York, NY, USA, 2014; Volume 7, pp. 270–348. [Google Scholar]
- Takeuchi, D. Stereoselective Polymerization of Conjugated Dienes. In Encyclopedia of Polymer Science and Technology, 4th ed.; Herman, M.F., Ed.; John Welly & Sons Inc.: New York, NY, USA, 2014; Volume 13, pp. 126–150. [Google Scholar]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well-defined transition metal complexes with phosphorus and nitrogen ligands for 1,3-dienes polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Puskas, J.E.; Gautriaud, E.; Deffifieux, A.; Kennedy, J.P. Natural rubber biosynthesis—A living carbocationic polymerization. Prog. Polym. Sci. 2006, 3, 533–548. [Google Scholar] [CrossRef]
- Ouardad, S.; Deffieux, A.; Peruch, F. Polyisoprene synthesized via cationic polymerization: State of the art. Pure Appl. Chem. 2012, 84, 2065–2080. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, N.; Wu, Y.X. Highly Cis-1,4 Selective Polymerization of Conjugated Dienes Catalyzed by N-heterocyclic Carbene-ligated Neodymium Complexes. Chin. J. Polym. Sci. 2020, 38, 1305–1312. [Google Scholar] [CrossRef]
- Guo, G.; Wu, X.L.; Yan, X.Q.; Yan, L.; Li, X.F.; Zhang, S.W.; Qiu, N.N. Unprecedentedly High Activity and/or High Regio-/Stereoselectivity of Fluorenyl-Based CGC Allyl-Type η3:η1-tert-Butyl(dimethylflfluorenylsilyl)amido Ligated Rare Earth Metal Monoalkyl Complexes in Olefin Polymerization. Polymer 2019, 11, 836. [Google Scholar] [CrossRef]
- Tan, J.M.; Li, C.Q.; Xu, L.; Zhang, J.; Liang, A.M.; Zhang, L.Q.; Wu, S.Z. Study on the Microsequence Structure and Crystallization Properties of Isoprene Rubber. Sci. Sin. Chim. 2014, 44, 1733–1739. [Google Scholar]
- Tanaka, Y.; Sato, H. Sequence distribution of polyisoprenes. Polymer 1976, 17, 413–418. [Google Scholar] [CrossRef]
- Sato, H.; Ono, A.; Tanaka, Y. Distribution of isomeric structures in polyisoprenes. Polymer 1977, 18, 580–586. [Google Scholar] [CrossRef]
- Tuampoemsab, S.; Nimpaiboon, A.; Sakdapipanich, J.T. Quantitative analysis of isoprene units in natural rubber and synthetic polyisoprene using 1H-NMR spectroscopy with an internal standard. Polym. Test. 2015, 43, 21–26. [Google Scholar] [CrossRef]
- FMI. Synthetic Polyisoprene Market Size, Industry Share & Trends–2032. Available online: https://www.futuremarketinsights.com/reports/synthetic-polyisoprene-rubber-market (accessed on 22 July 2022).
- Chen, W.Q.; Wang, F.S. Synthetic rubbers prepared by lanthanide coordination catalysts. Sci. China B Chem. 2009, 52, 1520–1543. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Cui, D.M.; Wang, B.L.; Liu, B.; Yang, Y. Polymerization of 1,3-conjugated dienes with rare-earth metal precursors. In Structure and Bonding, Molecular Catalysis of Rare-Earth Elements; Mingos, D.M.P., Roesky, P.W., Eds.; Springer: Berlin, Germany, 2010; Volume 137, pp. 49–108. [Google Scholar]
- Hou, Z.M.; Suzuki, T. Scandium and yttrium complexes for well-controlled polymerization. Mater. Integr. 2007, 21, 34–42. [Google Scholar]
- Shen, Z.Q. Catalytic activities of rare-earth calixarene complexes in polymer syntheses. Chin. J. Polym. Sci. 2005, 23, 593–602. [Google Scholar] [CrossRef]
- Zhang, G.C.; Deng, B.J.; Wang, S.W.; Wei, Y.; Zhou, S.L.; Zhu, X.C.; Huang, Z.M.; Mu, X.L. Di and trinuclear rare-earth metal complexes supported by 3-amido appended indolyl ligands: Synthesis, characterization and catalytic activity towards isoprene 1,4-cis polymerization. Dalton Trans. 2016, 45, 15445–15456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Suzuki, T.; Luo, Y.; Nishiura, M.; Hou, Z.M. Cationic Alkyl Rare-Earth Metal Complexes Bearing an Ancillary Bis(phosphinophenyl)amido Ligand: A Catalytic System for Living cis-1,4-Polymerization and Copolymerization of Isoprene and Butadiene. Angew. Chem. Int. Ed. 2007, 46, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, M.; Hou, Z.M. Novel polymerization catalysts and hydride clusters from rare-earth metal dialkyls. Nat. Chem. 2010, 2, 257–268. [Google Scholar] [CrossRef]
- Suzuki, T.; Zhang, L.X.; Hou, Z.M. Metal Complex Containing Tridentate Ligand, and Polymerization Catalyst Comprising the Same. U.S. Patent Application 11/795,571, 15 May 2008. [Google Scholar]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-Based Ziegler/Natta Catalysts and Their Application in Diene Polymerization. In Neodymium Based Ziegler Catalysts—Fundamental Chemistry; Nuyken, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 204, pp. 1–154. [Google Scholar]
- Ren, W.H.; Liu, H.; You, F.; Mao, P.J.; So, Y.M.; Kang, X.H.; Shi, X.C. Unsymmetrical diarylamido-based rare-earth alkyl complexes: Their synthesis and catalytic performance in isoprene polymerization. Dalton Trans. 2021, 50, 1334–1343. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, D.H.; Yan, X.Q.; Gao, F.; Zhang, L.; Zhang, S.W.; Li, X.F. Cis-1,4-polymerization of isoprene by 1,3-bis(oxazolinymethylidene)isoindoline-ligated rare-earth metal dialkyl complexes. Polymer 2017, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Basalova, O.A.; Tolpygin, A.O.; Kovylina, T.A.; Cherkasov, A.V.; Fukin, G.K.; Trifonov, A.A. Bis(tetramethylaluminate) rare-earth metal Complexes Supported by Bi-and Tridentate Amidinate Ligands: Performance in Isoprene Polymerization. Organometallic 2021, 40, 979–988. [Google Scholar] [CrossRef]
- Zhang, P.F.; Liao, H.Y.; Wang, H.H.; Li, X.F.; Yang, F.Z.; Zhang, S.W. Cis-1,4-Polymerization of Isoprene Catalyzed by 1,3-Bis(2-pyridylimino)isoindoline-Ligated Rare-Earth-Metal Dialkyl Complexes. Organometallic 2017, 36, 2446–2451. [Google Scholar] [CrossRef]
- Pan, Y.; Li, W.Q.; Wei, N.N.; So, Y.M.; Li, Y.; Jiang, K.; He, G.H. Anilido-oxazoline-ligated rare-earth metal complexes: Synthesis, characterization and highly cis-1,4-selective polymerization of isoprene. Dalton Trans. 2019, 48, 3583–3592. [Google Scholar] [CrossRef]
- Trifonov, A.A.; Lyubov, D.M.A. Quarter-century long story of bis(alkyl) rare-earth (III) complexes. Coord. Chem. Rev. 2017, 340, 10–61. [Google Scholar] [CrossRef]
- Zhang, L.X.; Hou, Z.M. Unprecedented Isospecific 3,4-Polymerization of Isoprene by Cationic Rare-Earth Metal Alkyl Species Resulting from a Binuclear Precursor. J. Am. Chem Soc. 2005, 127, 14562–14563. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.; Cordes, H.G.; Herwig, H.; Kaminsky, W.; Merk, A.; Mottweier, R.; Pein, J.; Sinn, H.; Vollmer, H.J. Halogen-Free Soluble Ziegler catalysts for the polymerization of Ethylene, Control of Molecular Weight by Choice of Temperature. Angew. Chem. Int. Ed. Engl. 1976, 15, 630–632. [Google Scholar] [CrossRef]
- Resconi, L.; Covallo, L.; Fait, A.; Piemontes, F. Selectivity in Propene Polymerization with Metallocene Catalysts. Chem. Rev. 2000, 100, 1253–1345. [Google Scholar] [CrossRef]
- Alt, H.G.; Koppl, A. Effect of the Nature of Metallocene Complexes of Group IV Metals on Their Performance in Catalytic Ethylene and propylene Polymerization. Chem. Rev. 2000, 100, 1205–1221. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, W. New Elastomers by Metallocene Catalysis. Macromol. Symp. 2001, 174, 269–276. [Google Scholar] [CrossRef]
- Luconi, L.; Lyubov, D.M.; Rossin, A.; Glukhova, T.A.; Cherkasov, A.V.; Tuci, G.; Fukin, G.K.; Trifonov, A.A.; Giambastiani, G. Organolanthanide complexes supported by thiazole-containing amidopyridinate ligands: Synthesis, characterization, and catalytic activity in isoprene polymerization. Organometallic 2014, 33, 7125–7134. [Google Scholar] [CrossRef]
- Bruaseth, I.; Rytte, E. Dual Site Ethene/1-Hexene Copolymerization with MAO Activated (1,2,4-Me3Cp)2ZrCl2 and (Me5Cp)2ZrCl2 Catalysts. Possible Transfer of Polymer Chains between the Sites. Macromolecules 2003, 36, 3026–3034. [Google Scholar] [CrossRef]
- Zhang, L.X.; Nishiura, M.; Yuki, M.; Luo, Y.; Hou, Z.M. Isoprene Polymerization with Yttrium Amidinate Catalysts: Switching the Regio- and Stereoselectivity by Addition of AlMe3. Angew. Chem. Int. Ed. 2008, 47, 2642–2645. [Google Scholar] [CrossRef]
- The Engineering Toolbox. Liquids Dielectric Constants. Available online: https://www.engineeringtoolbox.com (accessed on 29 July 2022).
- Davies, N.W.; Frey, A.S.P.; Gardiner, M.G.; Wang, J. Reductive disproportionation of carbon dioxide by a Sm(II) complex: Unprecedented f-block element reactivity giving a carbonate complex. Chem. Commun. 2006, 46, 4853–4855. [Google Scholar] [CrossRef]
- Xin, S.X.; Aitken, C.; Harrod, J.F.; Mu, Y.; Samuel, E. Redistribution Reactions of Alkoxy- and Siloxysilanes, Catalyzed by Dimethyltitanocene. Can. J. Chem. 1990, 68, 471–476. [Google Scholar] [CrossRef]
- Fink, G.; Steinmetz, B.; Zechlin, J.; Przybyla, C.; Tesche, B. Propene Polymerization with Silica-Supported Metallocene/MAO Catalysts. Chem. Rev. 2000, 100, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Dunkai, N.; Nakazawa, A.; Inagaki, H. A Universal Calibration in Gel Permeation Chromatography. Bull. Inst. Chem. Res. Kyoto Univ. 1970, 48, 79–87. [Google Scholar]
- Dawkins, J.V. Calibration Procedures in Gel Permeation Chromatography. Br. Polym. J. 1972, 4, 87–101. [Google Scholar] [CrossRef]
- Crouzet, P.; Martens, P.; Mangin, P. Universal Calibration in Permeation Chromatography. Application to Polyethylene, Polypropylene, and Ethylene-Propylene Copolymers. J. Chromatogr. Sci. 1971, 9, 525–530. [Google Scholar] [CrossRef]
- Dyson, P.J.; McIndoe, J.S. Analysis of organometallic compounds using ion trap mass spectrometry. Inorg. Chim. Acta 2003, 354, 68–74. [Google Scholar] [CrossRef]
- Johnstone, R.A.W.; Cragg, R.H. Organometallic, co-ordination, and inorganic compounds investigated by mass spectrometry Mass Spectrometry. In Mass Spectrometry of Inorganic and Organometallic Compounds; Hendersen, W., McIndoe, J.S., Eds.; Welly: New York, NY, USA, 2005; Volume 6, pp. 294–328. [Google Scholar]

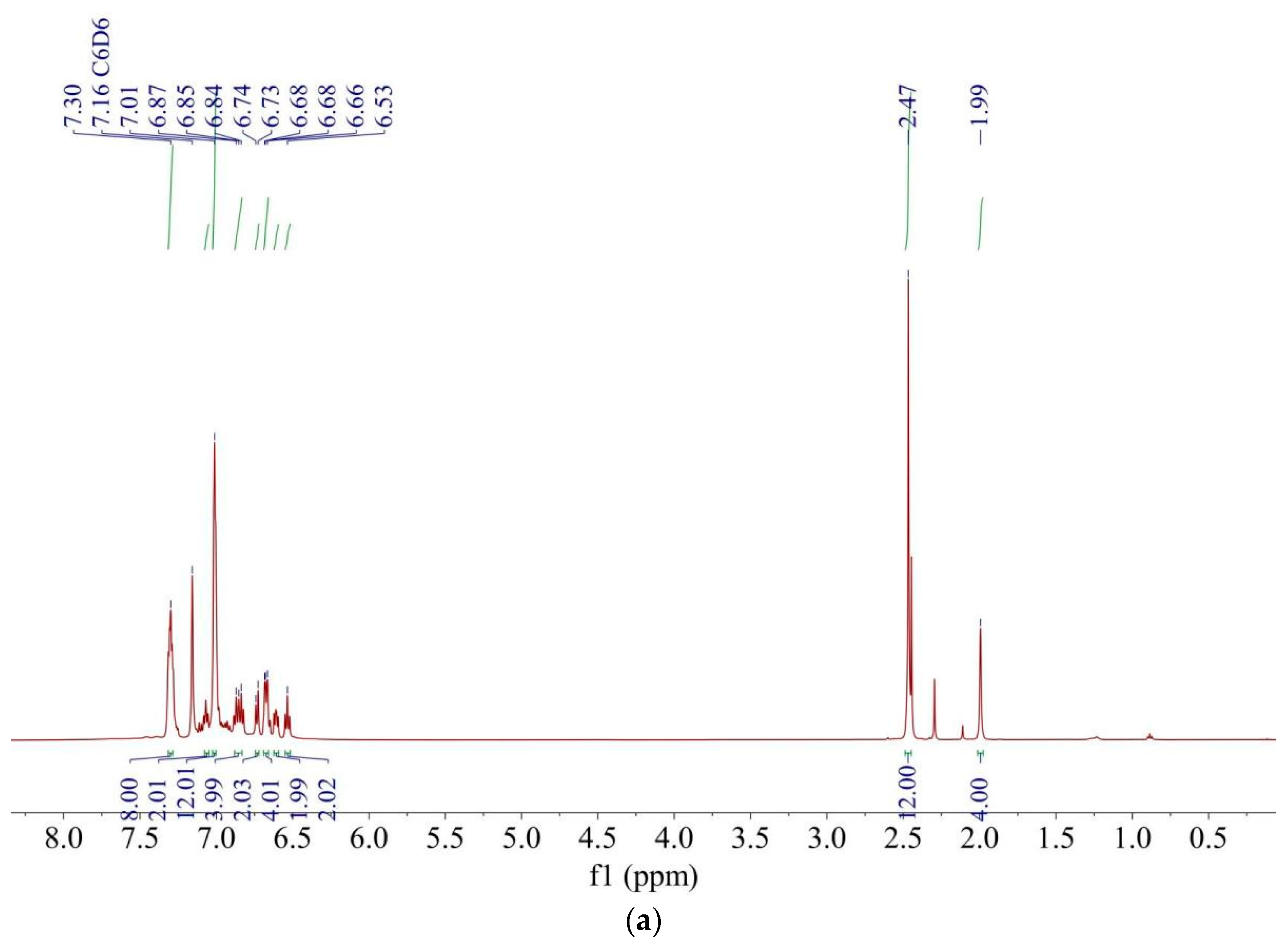

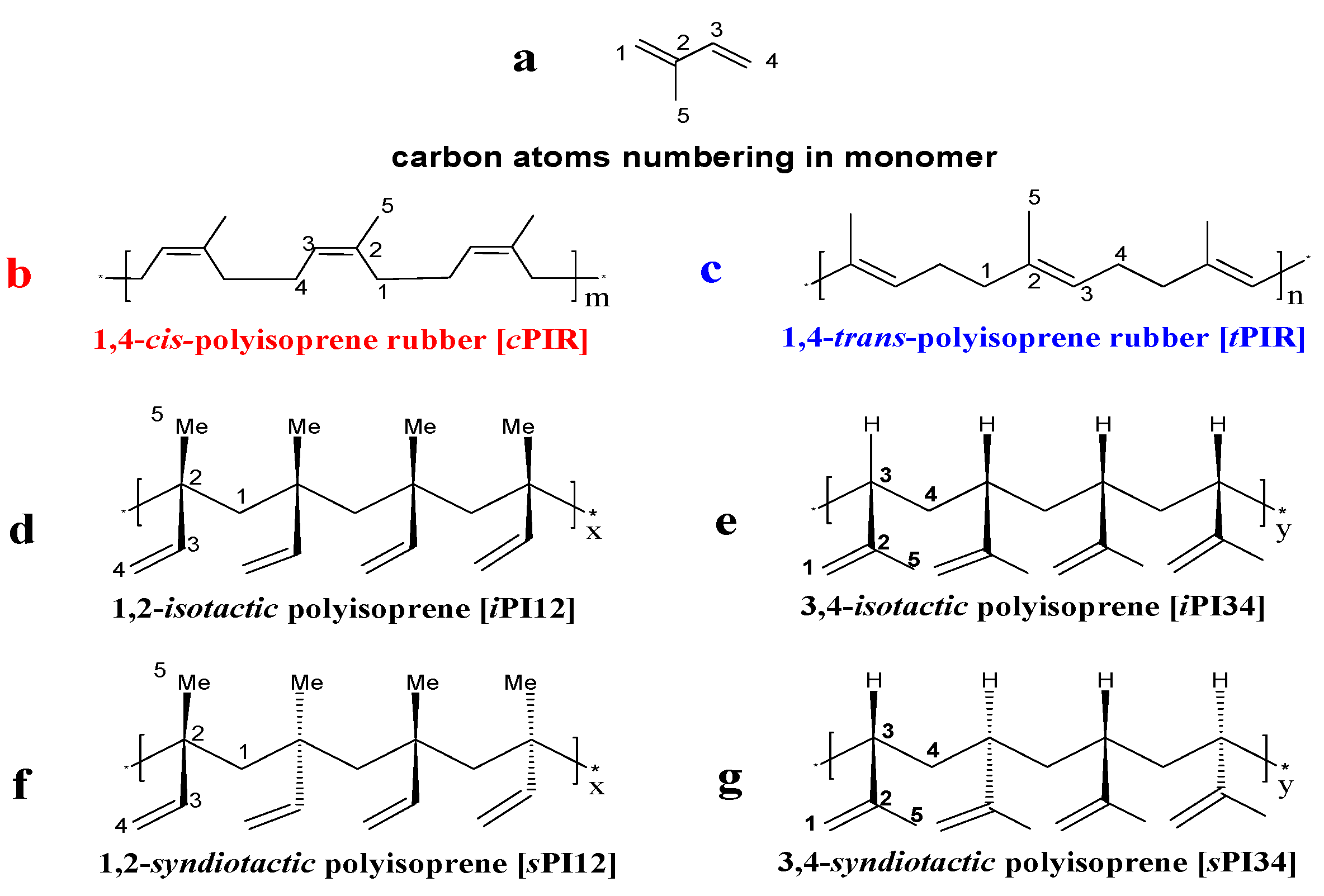



 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Run | Cat. | Pt (min) | Tp (°C) | Conversion% | Mw a (kg/moL) | PDI a | Microstructure b | Tg c | |
| cis-1,4% | 3,4% | ||||||||
| 1 | 1 | 5 | 20 | 93.6 | 777 | 1.54 | (98.69) | 1.31 | |
| 2 | 2 | 780 | 20 | trace | --- | --- | --- | --- | --- |
| 3 | 3 | 5 | 20 | 100 | 369 | 1.28 | (98.58) | 1.42 | --- |
| 4 | 3 | 30 | 0 | 78 | 282 | 1.27 | 98.63 (98.63) | 1.37 | |
| 5 | 3 | 60 | 0 | 88 | 480 | 1.24 | 98.45 (98.63) | 1.55 | −72 |
| 6 | 3 | 60 | 0 | 92 | 440 | 1.32 | 98.44 (98.58) | 1.56 | --- |
| 7 | 3 | 90 | 0 | 99 | 376 | 1.39 | 98.51 (98.55) | 1.49 | --- |
| 8 | 3 | 120 | 0 | 100 | 387 | 1.29 | 98.78 (98.53) | 1.22 | --- |
| 9 | 3 | 2 | 20 | 52 | 368 | 1.30 | 98.66 (98.50) | 1.34 | --- |
| 10 | 3 | 2 | 40 | 92 | 468 | 1.27 | 98.44 (98.52) | 1.56 | --- |
| 11 | 3 | 2 | 60 | 99.5 | 452 | 1.43 | 98.30 (98.47) | 1.70 | --- |
| 12 | 3 | 2 | 80 | 100 | 476 | 1.32 | 98.60 (98.42) | 1.40 | --- |
| 13 | 3 d | 30 | 20 | 100 | 718 | 1.68 | (98.64) | 1.36 | ---- |
| 14 | 3 e | 30 | 20 | 100 | 836 | 1.57 | (98.54) | 1.46 | --- |
| 15 | 3 f | 30 | 20 | 100 | 886 | 1.58 | (98.65) | 1.35 | --- |
| 16 | 3 g | 30 | 20 | 90.2 | 1356 | 2.07 | (98.67) | 1.33 | --- |
| 17 | 3 | 8 | 20 | 99 | 357 | 1.34 | (98.56) | 1.44 | |
| 18 | 3 | 10 | 20 | 100 | 348 | 1.18 | 98.66 (98.50) | 1.34 | −70 |
| 19 | 3 | 30 | 20 | 100 | 625 | 2.29 | (98.64) | 1.36 | |
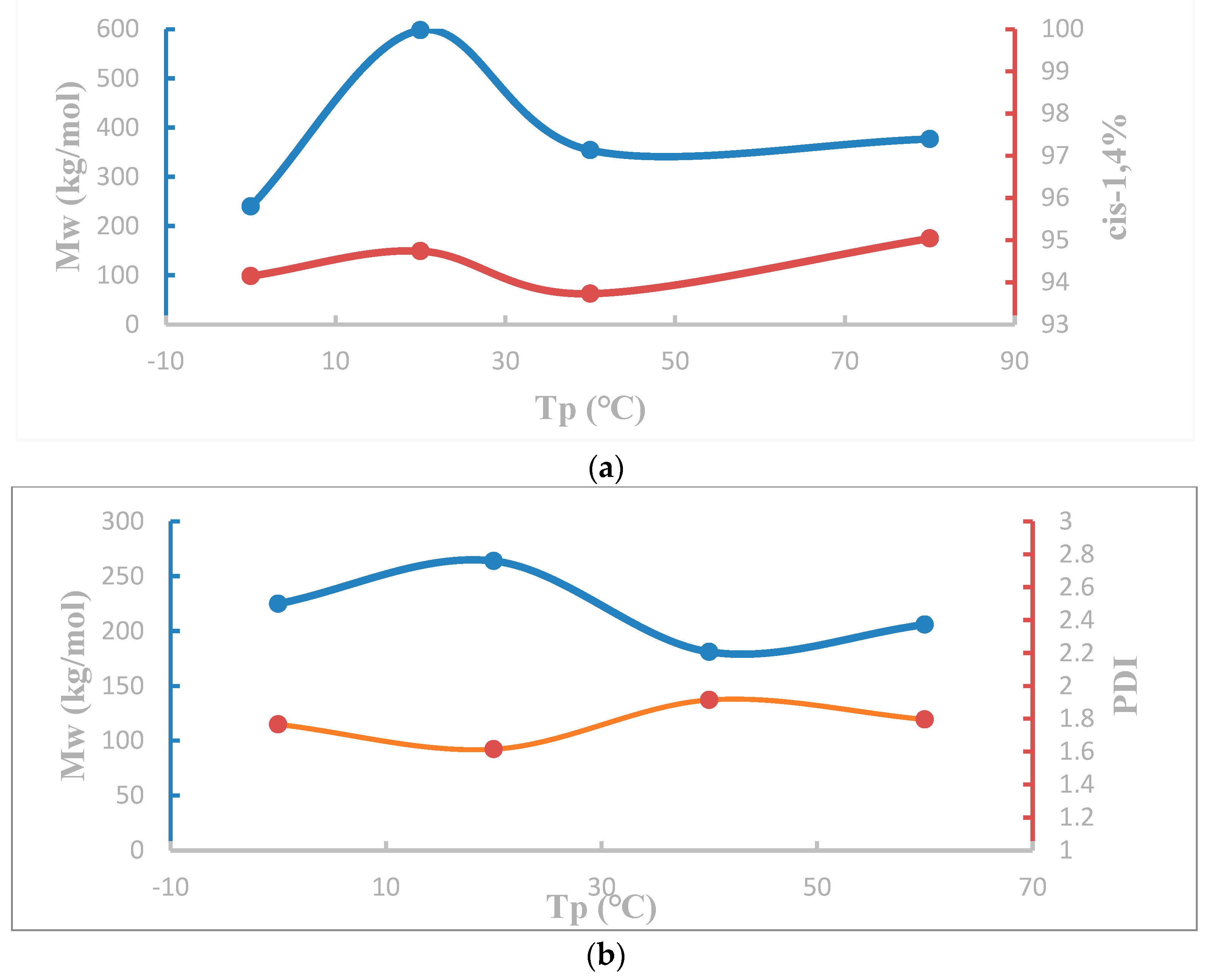
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Run | Cat. | Pt (min) | Tp (°C) | Conversion % | Mw a (kg/moL) | PDI a | Microstructure b | ||
| cis-1,4% | trans-1,4% | 3,4-cont.% | |||||||
| 1 | 1 | 5 | 80 | 96 | 270 | 1.69 | 91.83 (97.60) | 4.66 | 3.51 |
| 2 | 2 | 5 | 80 | 100 | 259 | 1.81 | 92.05 (97.39) | 4.54 | 3.41 |
| 3 | 3 | 5 | 80 | 100 | 377 | 1.56 | 95.04 (97.73) | 2.32 | 2.65 |
| 4 | 3 | 60 | 20 | 62 | 1163 d | 2.04 | 95.12 (97.84) | 2.04 | 2.84 |
| 5 | 3 | 60 | 20 | 85 | 598 | 1.58 | 94.74 (97.79) | 2.37 | 2.89 |
| 6 | 3 | 20 | 40 | 99.7 | 354 | 1.66 | 93.73 (97.51) | 3.14 | 3.14 |
| 7 | 3 | 30 | 40 | 100 | 401 | 2.12 | 94.44 (97.64) | 2.78 | 2.78 |
| 8 | 3 | 10 | 80 | 100 | 428 | 2.10 | 94.81 (97.69) | 2.57 | 2.63 |
| 9 | 3 | 120 | 0 | 100 | 240 | 1.78 | 94.15 (97.53) | 2.96 | 2.89 |
| 10 | 3 | 180 | 0 | 100 | 313 | 1.75 | 93.99 (97.44) | 3.04 | 2.97 |
| 11 c | 3 | 60 | 0 | 93.2 | 225 | 1.77 | (98.20) | ||
| 12 c | 3 | 30 | 20 | 100 | 264 | 1.62 | (98.13) | ||
| 13 c | 3 | 30 | 40 | 100 | 181 | 1.92 | (98.12) | ||
| 14 c | 3 | 30 | 60 | 100 | 206 | 1.80 | (98.10) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Hu, H.; Li, X.; Zhou, Y.; Li, H.; Sun, X.; Zhang, X.; Mao, G.; Xin, S. PNP-Ligated Rare-Earth Metal Catalysts for Efficient Polymerization of Isoprene. Catalysts 2022, 12, 1131. https://doi.org/10.3390/catal12101131
Ma R, Hu H, Li X, Zhou Y, Li H, Sun X, Zhang X, Mao G, Xin S. PNP-Ligated Rare-Earth Metal Catalysts for Efficient Polymerization of Isoprene. Catalysts. 2022; 12(10):1131. https://doi.org/10.3390/catal12101131
Chicago/Turabian StyleMa, Rongqing, Hongfan Hu, Xinle Li, Yi Zhou, Huashu Li, Xin Sun, Xueqin Zhang, Guoliang Mao, and Shixuan Xin. 2022. "PNP-Ligated Rare-Earth Metal Catalysts for Efficient Polymerization of Isoprene" Catalysts 12, no. 10: 1131. https://doi.org/10.3390/catal12101131
APA StyleMa, R., Hu, H., Li, X., Zhou, Y., Li, H., Sun, X., Zhang, X., Mao, G., & Xin, S. (2022). PNP-Ligated Rare-Earth Metal Catalysts for Efficient Polymerization of Isoprene. Catalysts, 12(10), 1131. https://doi.org/10.3390/catal12101131





