Evaluation of Enzymatic Hydrolysis of Sugarcane Bagasse Using Combination of Enzymes or Co-Substrate to Boost Lytic Polysaccharide Monooxygenases Action
Abstract
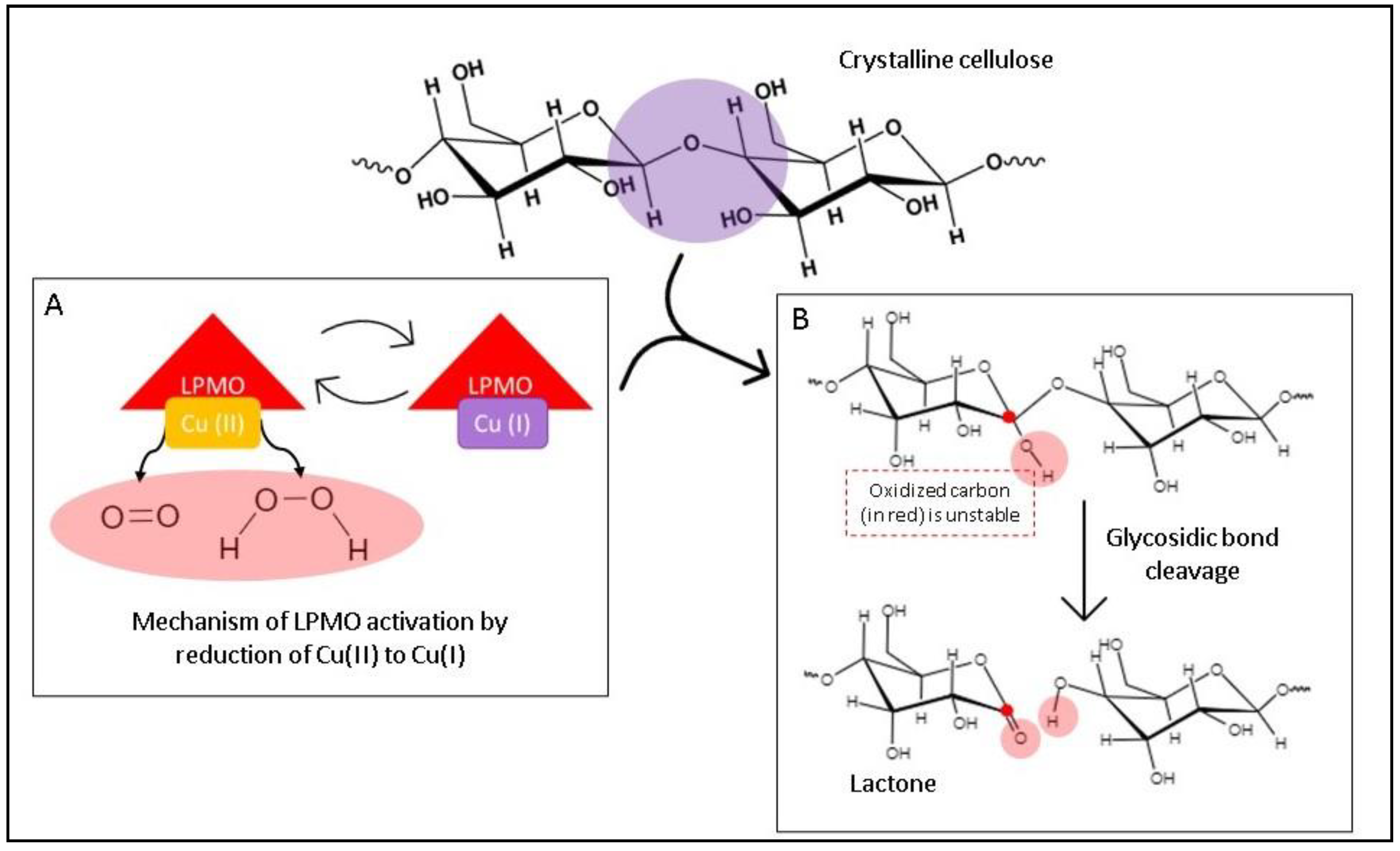
1. Introduction
2. Results and Discussion
2.1. Enzymatic Hydrolysis in Deep-Well Plates
2.2. Statistical Analysis
3. Materials and Methods
3.1. Biomass Pretreatment and Composition
3.2. Enzymatic Hydrolysis
3.3. Enzyme and Additives Supplementation
| Conditions | LPMO Activity ** (U/mL) | Added Volume of LPMO (μL) |
|---|---|---|
| CC2 * | 1.47 × 10−5 | 0.0 |
| CC2 * + 1x LPMO | 3.56 × 10−5 | 10.3 |
| CC2 * + 2x LPMO | 5.64 × 10−5 | 20.6 |
| CC2 * + 3x LPMO | 7.72 × 10−5 | 31.0 |
| CC2 * + 4x LPMO | 9.80 × 10−5 | 41.3 |
| CC2 * + 5x LPMO | 11.89 × 10−5 | 51.7 |
3.4. Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dragone, G.; Kerssemakers, A.A.; Driessen, J.L.; Yamakawa, C.K.; Brumano, L.P.; Mussatto, S.I. Innovation and strategic orientations for the development of advanced biorefineries. Bioresour. Technol. 2020, 302, 122847. [Google Scholar] [CrossRef] [PubMed]
- Ajala, E.O.; Ighalo, J.O.; Ajala, M.A.; Adeniyi, A.G.; Ayanshola, A.M. Sugarcane bagasse: A biomass sufficiently applied for improving global energy, environment and economic sustainability. Bioresour. Bioprocess. 2021, 8, 87. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Yamakawa, C.K.; van der Maas, L.; Dragone, G. New trends in bioprocesses for lignocellulosic biomass and CO2 utilization. Renew. Sustain. Energy Rev. 2021, 152, 111620. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.M. Biomass pretreatment, biorefineries and potential products for a bioeconomy development. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier Inc.: Waltham, MA, USA, 2016; pp. 1–22. [Google Scholar] [CrossRef]
- Hilares, R.T.; Santos, J.; Ahmed, M.A.; Jeon, S.H.; da Silva, S.S.; Han, J.-I. Hydrodynamic cavitation-assisted alkaline pretreatment as a new approach for sugarcane bagasse biorefineries. Bioresour. Technol. 2016, 214, 609–614. [Google Scholar] [CrossRef]
- Hilares, R.T.; Dionízio, R.; Prado, C.; Ahmed, M.; da Silva, S.; Santos, J. Pretreatment of sugarcane bagasse using hydrodynamic cavitation technology: Semi-continuous and continuous process. Bioresour. Technol. 2019, 290, 121777. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.; Antunes, F.; Rocha, T.; Sánchez-Muñoz, S.; Barbosa, F.; Terán-Hilares, R.; Cruz-Santos, M.; Arruda, G.; da Silva, S.; Santos, J. A review on recent developments in hydrodynamic cavitation and advanced oxidative processes for pretreatment of lignocellulosic materials. Bioresour. Technol. 2021, 345, 126458. [Google Scholar] [CrossRef] [PubMed]
- Hilares, R.T.; Kamoei, D.V.; Ahmed, M.A.; da Silva, S.S.; Han, J.-I.; dos Santos, J.C. A new approach for bioethanol production from sugarcane bagasse using hydrodynamic cavitation assisted-pretreatment and column reactors. Ultrason. Sonochem. 2018, 43, 219–226. [Google Scholar] [CrossRef]
- Laca, A.; Laca, A.; Díaz, M. Hydrolysis: From cellulose and hemicellulose to simple sugars. In Second and Third Generation of Feedstocks; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–240. [Google Scholar] [CrossRef]
- Gao, W.; Li, Z.; Liu, T.; Wang, Y. Production of high-concentration fermentable sugars from lignocellulosic biomass by using high solids fed-batch enzymatic hydrolysis. Biochem. Eng. J. 2021, 176, 108186. [Google Scholar] [CrossRef]
- Müller, G.; Chylenski, P.; Bissaro, B.; Eijsink, V.G.H.; Horn, S.J. The impact of hydrogen peroxide supply on LPMO activity and overall saccharification efficiency of a commercial cellulase cocktail. Biotechnol. Biofuels 2018, 11, 209. [Google Scholar] [CrossRef]
- Vaaje-Kolstad, G.; Westereng, B.; Horn, S.J.; Liu, Z.; Zhai, H.; Sørlie, M.; Eijsink, V.G.H. An Oxidative Enzyme Boosting the Enzymatic Conversion of Recalcitrant Polysaccharides. Science 2010, 330, 219–222. [Google Scholar] [CrossRef]
- Quinlan, R.J.; Sweeney, M.D.; Leggio, L.L.; Otten, H.; Poulsen, J.-C.N.; Johansen, K.S.; Krogh, K.B.R.M.; Jørgensen, C.I.; Tovborg, M.; Anthonsen, A.; et al. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl. Acad. Sci. USA 2011, 108, 15079–15084. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Arantes, V.; Pribowo, A.; Gourlay, K.; Saddler, J.N. Substrate factors that influence the synergistic interaction of AA9 and cellulases during the enzymatic hydrolysis of biomass. Energy Environ. Sci. 2014, 7, 2308–2315. [Google Scholar] [CrossRef]
- Rodríguez-Zúñiga, U.F.; Cannella, D.; Giordano, R.D.C.; Giordano, R.D.L.C.; Jørgensen, H.; Felby, C. Lignocellulose pretreatment technologies affect the level of enzymatic cellulose oxidation by LPMO. Green Chem. 2015, 17, 2896–2903. [Google Scholar] [CrossRef]
- Chylenski, P.; Bissaro, B.; Sørlie, M.; Røhr, Å.K.; Várnai, A.; Horn, S.J.; Eijsink, V.G. Lytic Polysaccharide Monooxygenases in Enzymatic Processing of Lignocellulosic Biomass. ACS Catal. 2019, 9, 4970–4991. [Google Scholar] [CrossRef]
- Bissaro, B.; Røhr, Å.K.; Müller, G.; Chylenski, P.; Skaugen, M.; Forsberg, Z.; Horn, S.J.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat. Chem. Biol. 2017, 13, 1123–1128. [Google Scholar] [CrossRef]
- Costa, T.H.F.; Kadic’, A.; Chylenski, P.; Várnai, A.; Bengtsson, O.; Lidén, G.; Eijsink, V.G.; Horn, S.J. Demonstration-scale enzymatic saccharification of sulfite-pulped spruce with addition of hydrogen peroxide for LPMO activation. Biofuels Bioprod. Biorefining 2020, 14, 734–745. [Google Scholar] [CrossRef]
- Müller, G.; Várnai, A.; Johansen, K.S.; Eijsink, V.G.H.; Horn, S.J. Harnessing the potential of LPMO-containing cellulase cocktails poses new demands on processing conditions. Biotechnol. Biofuels 2015, 8, 187. [Google Scholar] [CrossRef]
- Stepnov, A.A.; Eijsink, V.G.H.; Forsberg, Z. Enhanced in situ H2O2 production explains synergy between an LPMO with a cellulose-binding domain and a single-domain LPMO. Sci. Rep. 2022, 12, 6129. [Google Scholar] [CrossRef]
- Kadić, A.; Chylenski, P.; Hansen, M.A.T.; Bengtsson, O.; Eijsink, V.G.; Lidén, G. Oxidation-reduction potential (ORP) as a tool for process monitoring of H2O2/LPMO assisted enzymatic hydrolysis of cellulose. Process Biochem. 2019, 86, 89–97. [Google Scholar] [CrossRef]
- Haddad Momeni, M.; Fredslund, F.; Bissaro, B.; Raji, O.; Vuong, T.V.; Meier, S.; Nielsen, T.S.; Lombard, V.; Guigliarelli, B.; Biaso, F.; et al. Discovery of fungal oligosaccharide-oxidising flavo-enzymes with previously unknown substrates, redox-activity profiles and interplay with LPMOs. Nat. Commun. 2021, 12, 2132. [Google Scholar] [CrossRef]
- Berrin, J.-G.; Rosso, M.-N.; Hachem, M.A. Fungal secretomics to probe the biological functions of lytic polysaccharide monooxygenases. Carbohydr. Res. 2017, 448, 155–160. [Google Scholar] [CrossRef]
- Ladevèze, S.; Haon, M.; Villares, A.; Cathala, B.; Grisel, S.; Herpoël-Gimbert, I.; Henrissat, B.; Berrin, J.-G. The yeast Geotrichum candidum encodes functional lytic polysaccharide monooxygenases. Biotechnol. Biofuels 2017, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, F.; Keser, M.; Akeroyd, M.; Bevers, L.E.; Eijsink, V.G.H.; Várnai, A.; Berg, M.A.V.D. Characterization of an AA9 LPMO from Thielavia australiensis, TausLPMO9B, under industrially relevant lignocellulose saccharification conditions. Biotechnol. Biofuels 2020, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Hedison, T.M.; Breslmayr, E.; Shanmugam, M.; Karnpakdee, K.; Heyes, D.J.; Green, A.P.; Ludwig, R.; Scrutton, N.S.; Kracher, D. Insights into the H2O2—driven catalytic mechanism of fungal lytic polysaccharide monooxygenases. FEBS J. 2021, 288, 4115–4128. [Google Scholar] [CrossRef] [PubMed]
- Bissaro, B.; Várnai, A.; Røhr, K.; Eijsink, V.G.H. Oxidoreductases and Reactive Oxygen Species in Conversion of Lignocellulosic Biomass. Microbiol. Mol. Biol. Rev. 2018, 82, e00029-18. [Google Scholar] [CrossRef]
- Barbosa, F.C.; Kendrick, E.; Brenelli, L.B.; Arruda, H.S.; Pastore, G.M.; Rabelo, S.C.; Damasio, A.; Franco, T.T.; Leak, D.; Goldbeck, R. Optimization of cello-oligosaccharides production by enzymatic hydrolysis of hydrothermally pretreated sugarcane straw using cellulolytic and oxidative enzymes. Biomass-Bioenergy 2020, 141, 105697. [Google Scholar] [CrossRef]
- Hilares, R.T.; de Almeida, G.F.; Ahmed, M.A.; Antunes, F.A.; da Silva, S.S.; Han, J.-I.; Santos, J. Hydrodynamic cavitation as an efficient pretreatment method for lignocellulosic biomass: A parametric study. Bioresour. Technol. 2017, 235, 301–308. [Google Scholar] [CrossRef]
- Mesquita, J.F.; Ferraz, A.; Aguiar, A. Alkaline-sulfite pretreatment and use of surfactants during enzymatic hydrolysis to enhance ethanol production from sugarcane bagasse. Bioprocess Biosyst. Eng. 2015, 39, 441–448. [Google Scholar] [CrossRef]
- Haven, M.; Jørgensen, H. The Challenging Measurement of Protein in Complex Biomass-Derived Samples. Appl. Biochem. Biotechnol. 2014, 172, 87–101. [Google Scholar] [CrossRef]
- Adney, B.; Baker, J. Measurement of Cellulase Activities. Laboratory Analytical Procedure (LAP). National Renewable Energy Laboratory (NREL). Technical Report NREL/TP-510-42628. Golden, CO, USA. NREL. 1996. Available online: https://www.nrel.gov/docs/gen/fy08/42628.pdf (accessed on 19 August 2021).
- Breslmayr, E.; Daly, S.; Požgajčić, A.; Chang, H.-C.; Rezić, T.; Oostenbrink, C.; Ludwig, R. Improved spectrophotometric assay for lytic polysaccharide monooxygenase. Biotechnol. Biofuels 2019, 12, 283. [Google Scholar] [CrossRef]

| Source of Variation | SQ | gl | MQ | F | p-Value | F Crit |
|---|---|---|---|---|---|---|
| Between groups | 8121.72 | 8 | 1015.22 | 2.39 | 0.11 | 3.23 |
| Within groups | 3826.22 | 9 | 425.14 | |||
| Total | 11,947.94 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaguer Moya, E.; Cunha, M.L.S.; Prado, C.A.; Turella, S.; da Silva, S.S.; Abou-Hachem, M.; Dragone, G.; dos Santos, J.C.; Mussatto, S.I. Evaluation of Enzymatic Hydrolysis of Sugarcane Bagasse Using Combination of Enzymes or Co-Substrate to Boost Lytic Polysaccharide Monooxygenases Action. Catalysts 2022, 12, 1158. https://doi.org/10.3390/catal12101158
Balaguer Moya E, Cunha MLS, Prado CA, Turella S, da Silva SS, Abou-Hachem M, Dragone G, dos Santos JC, Mussatto SI. Evaluation of Enzymatic Hydrolysis of Sugarcane Bagasse Using Combination of Enzymes or Co-Substrate to Boost Lytic Polysaccharide Monooxygenases Action. Catalysts. 2022; 12(10):1158. https://doi.org/10.3390/catal12101158
Chicago/Turabian StyleBalaguer Moya, Eva, Maria Laura Silva Cunha, Carina Aline Prado, Simone Turella, Silvio Silvério da Silva, Maher Abou-Hachem, Giuliano Dragone, Júlio César dos Santos, and Solange Inês Mussatto. 2022. "Evaluation of Enzymatic Hydrolysis of Sugarcane Bagasse Using Combination of Enzymes or Co-Substrate to Boost Lytic Polysaccharide Monooxygenases Action" Catalysts 12, no. 10: 1158. https://doi.org/10.3390/catal12101158
APA StyleBalaguer Moya, E., Cunha, M. L. S., Prado, C. A., Turella, S., da Silva, S. S., Abou-Hachem, M., Dragone, G., dos Santos, J. C., & Mussatto, S. I. (2022). Evaluation of Enzymatic Hydrolysis of Sugarcane Bagasse Using Combination of Enzymes or Co-Substrate to Boost Lytic Polysaccharide Monooxygenases Action. Catalysts, 12(10), 1158. https://doi.org/10.3390/catal12101158










