Migration Mechanism of Lattice Oxygen: Conversion of CO2 to CO Using NiFe2O4 Spinel Oxygen Carrier in Chemical Looping Reactions
Abstract
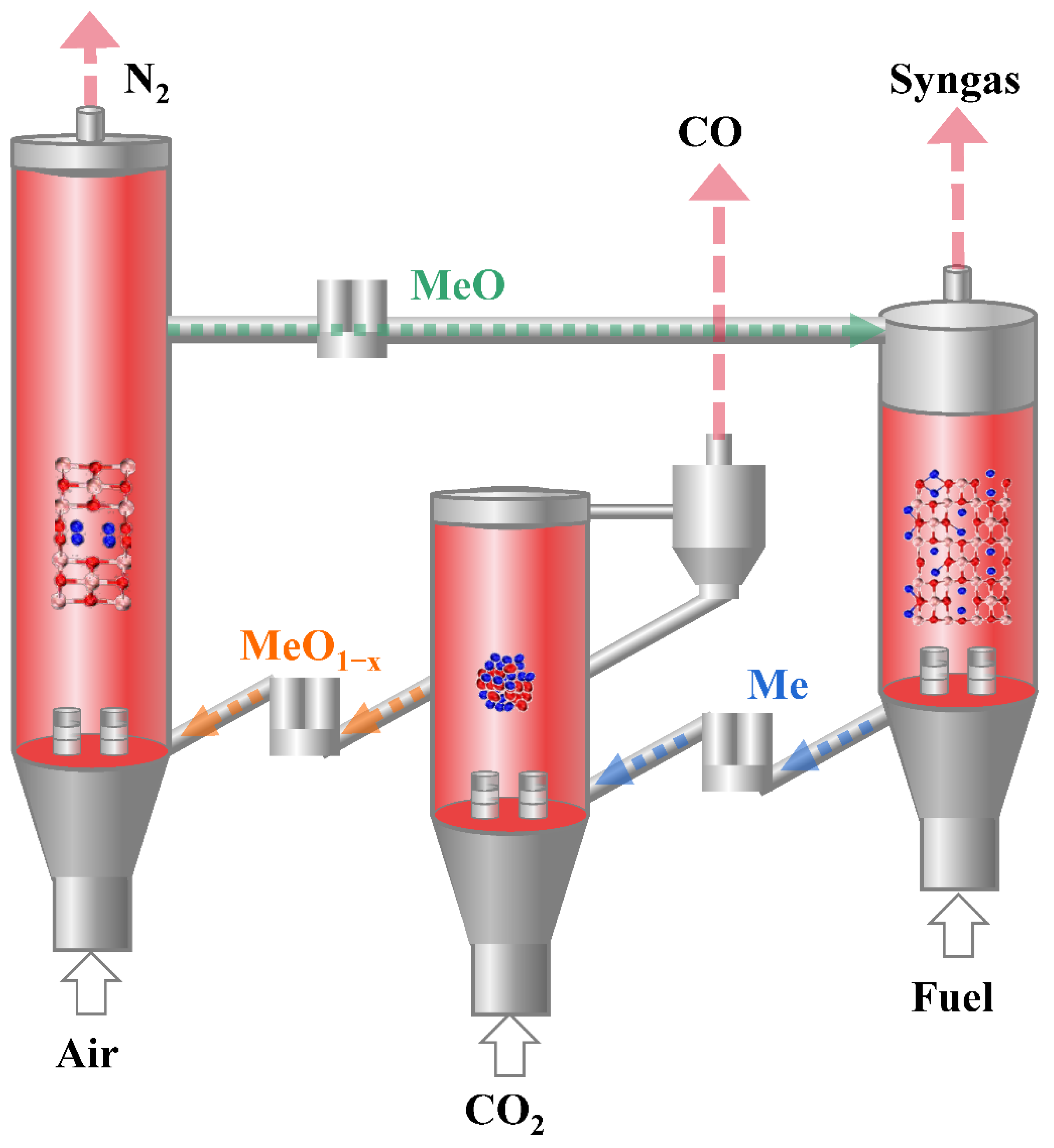
:1. Introduction
2. Results and Discussion
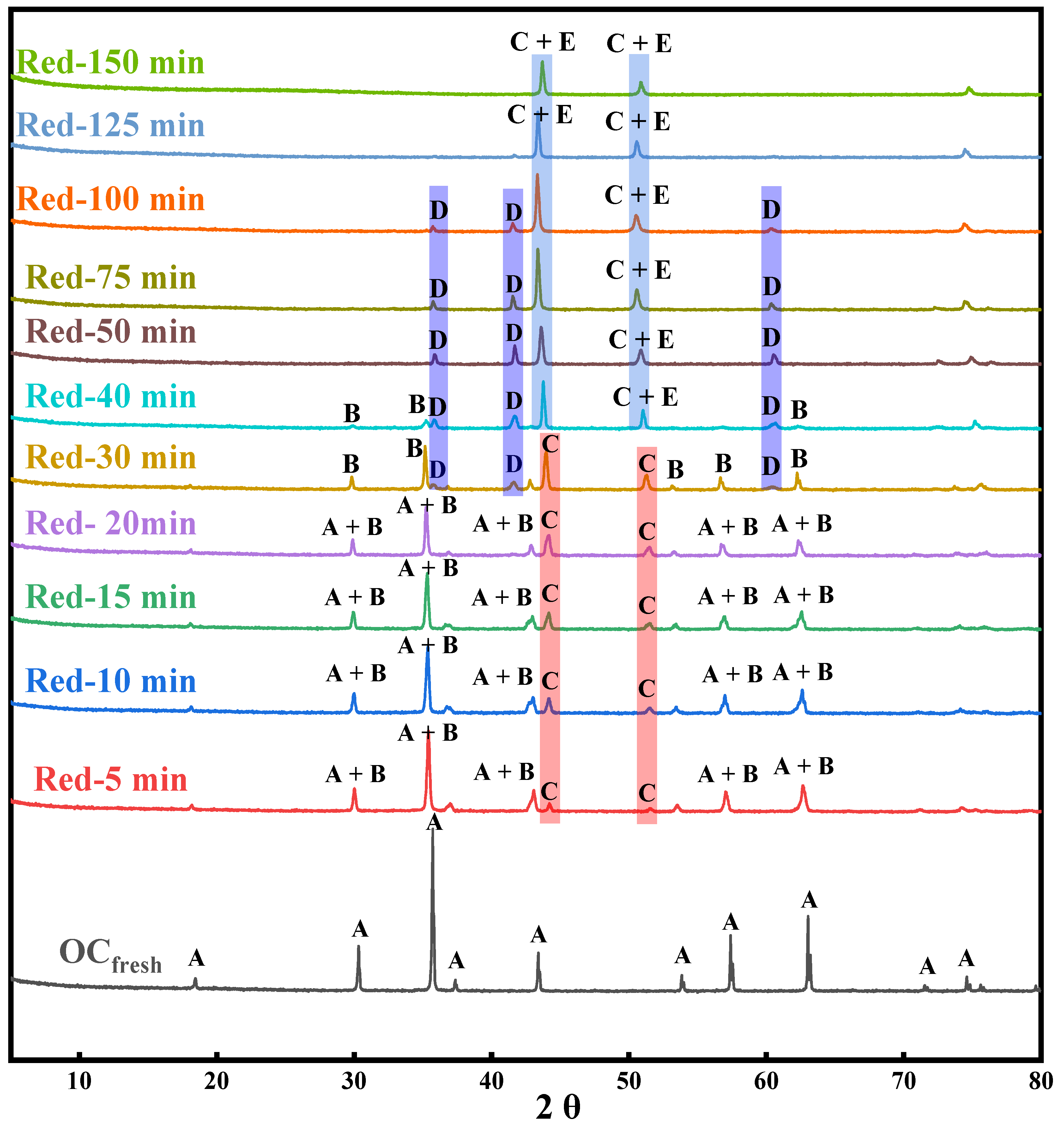
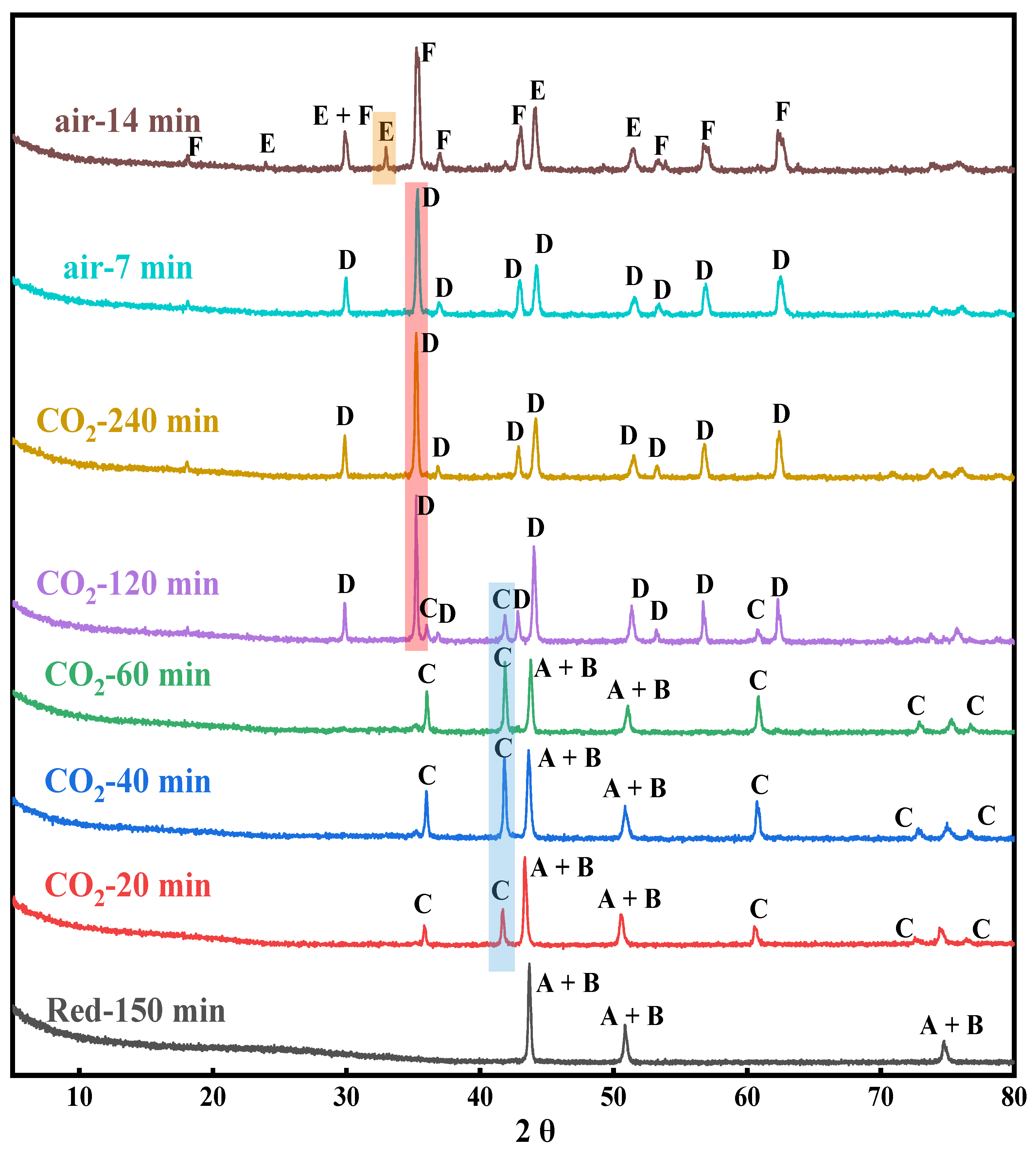
2.1. XRD Analysis
2.1.1. Crystalline Phase Evolution of the OC in Reduction
2.1.2. Crystalline Phase Evolution of the Reduced OC in Oxidation
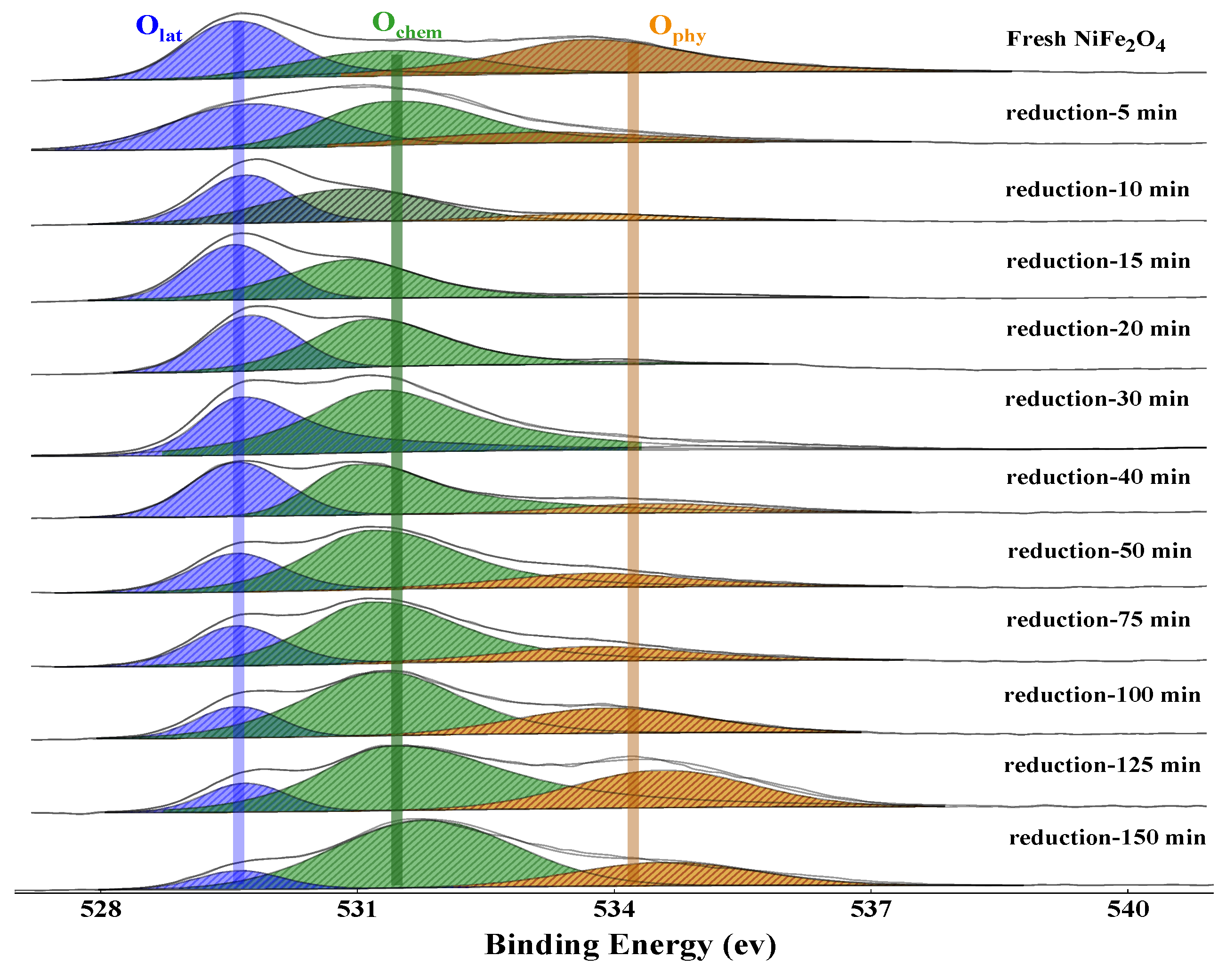
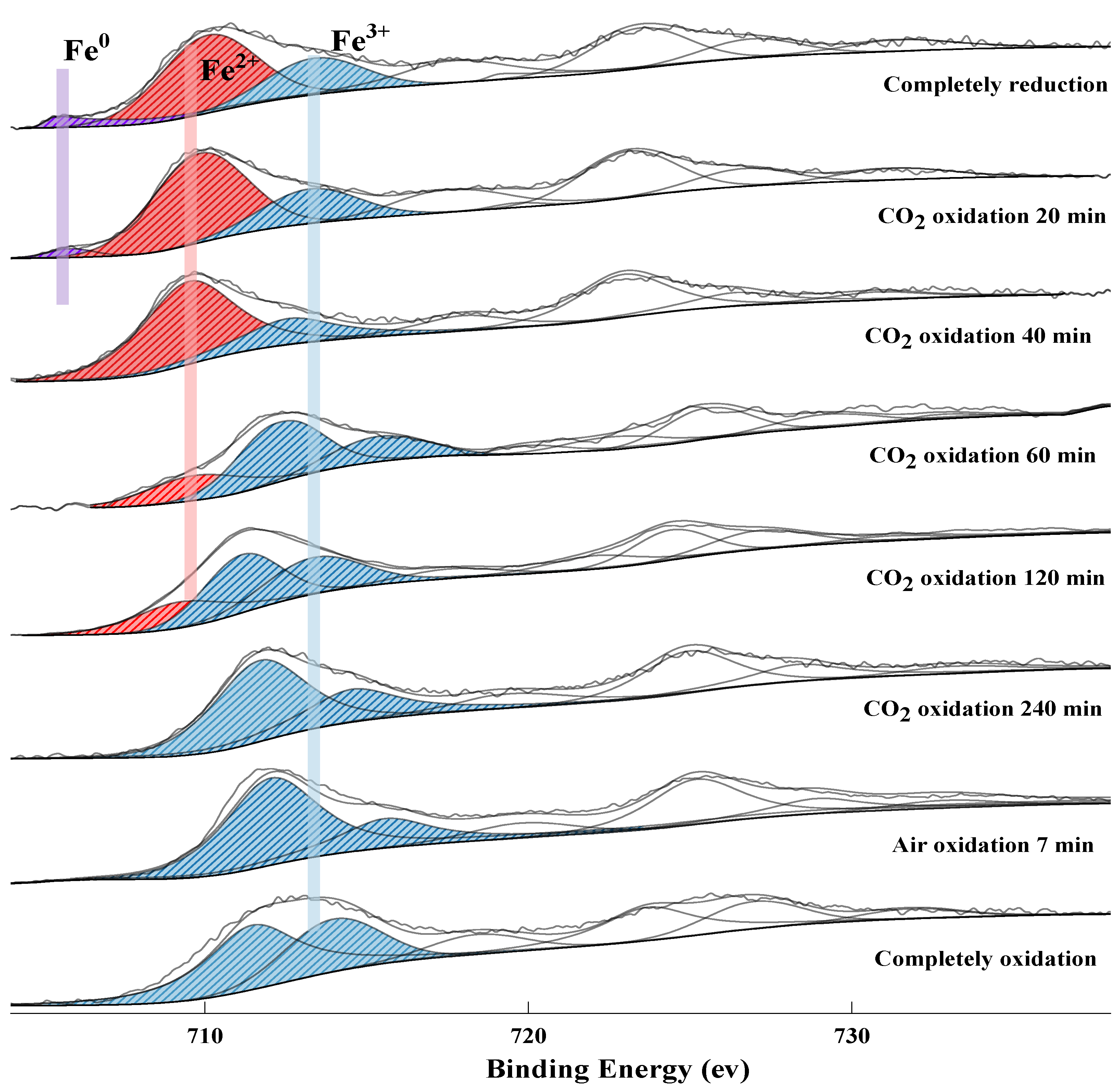
2.2. XPS Analysis
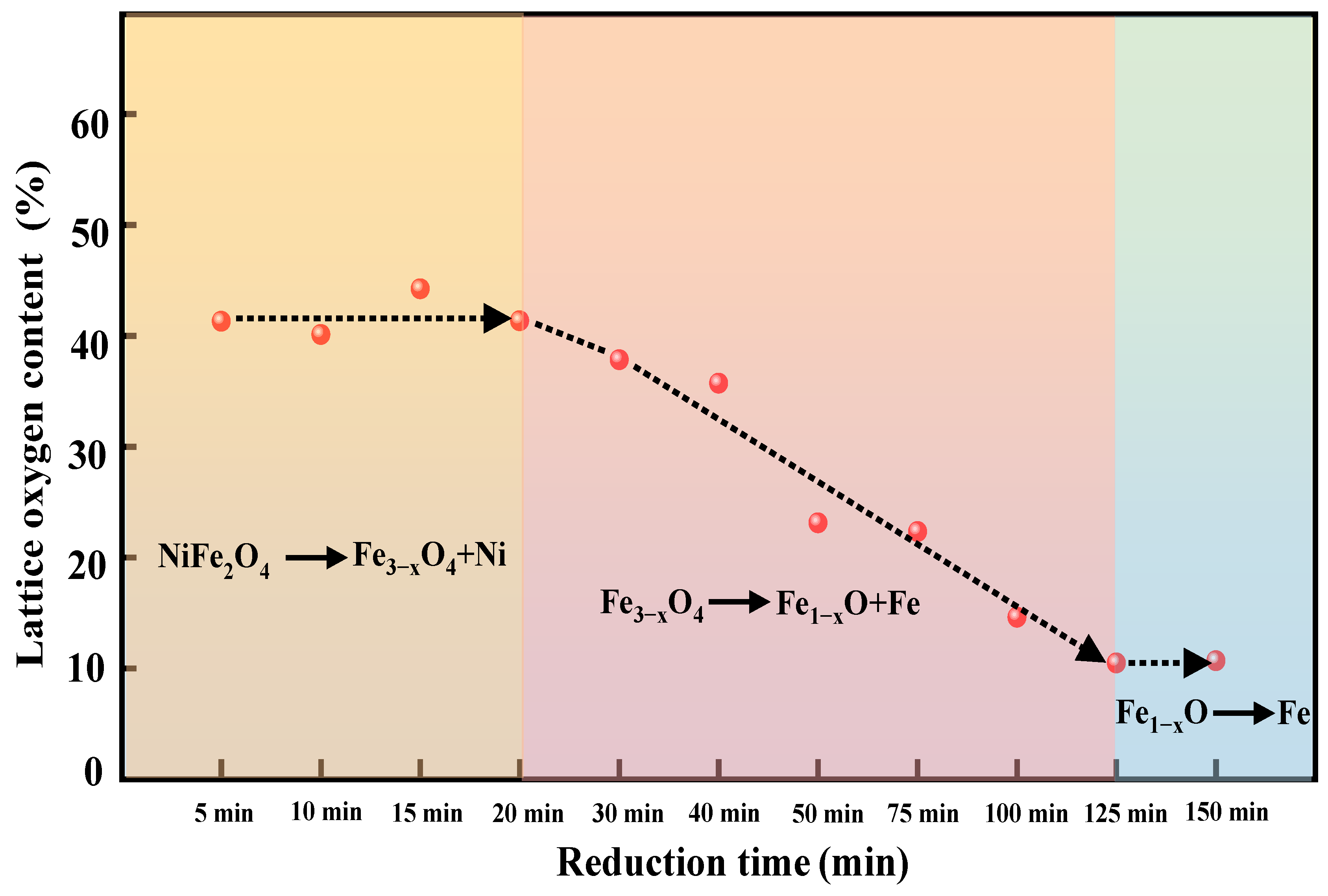
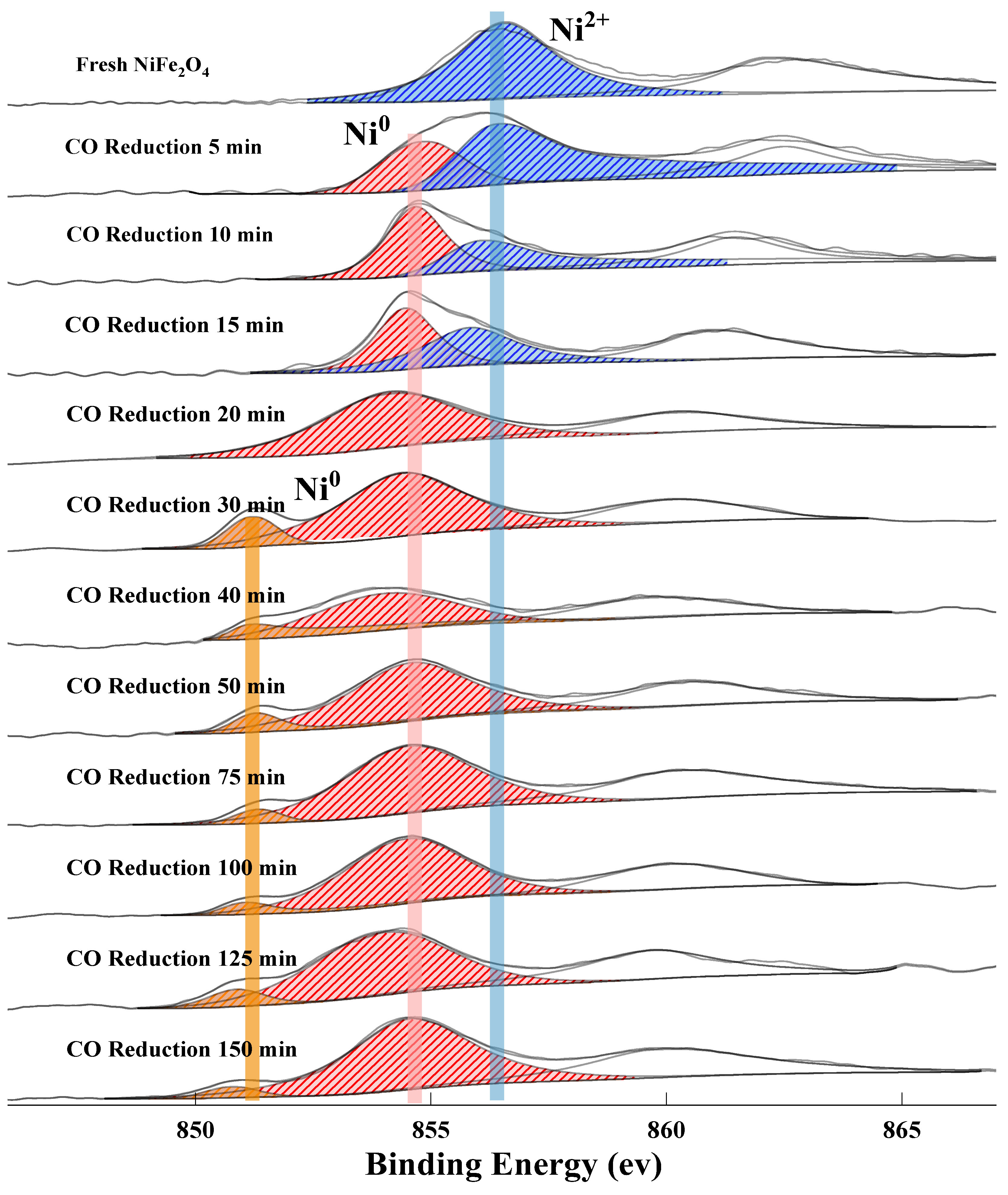
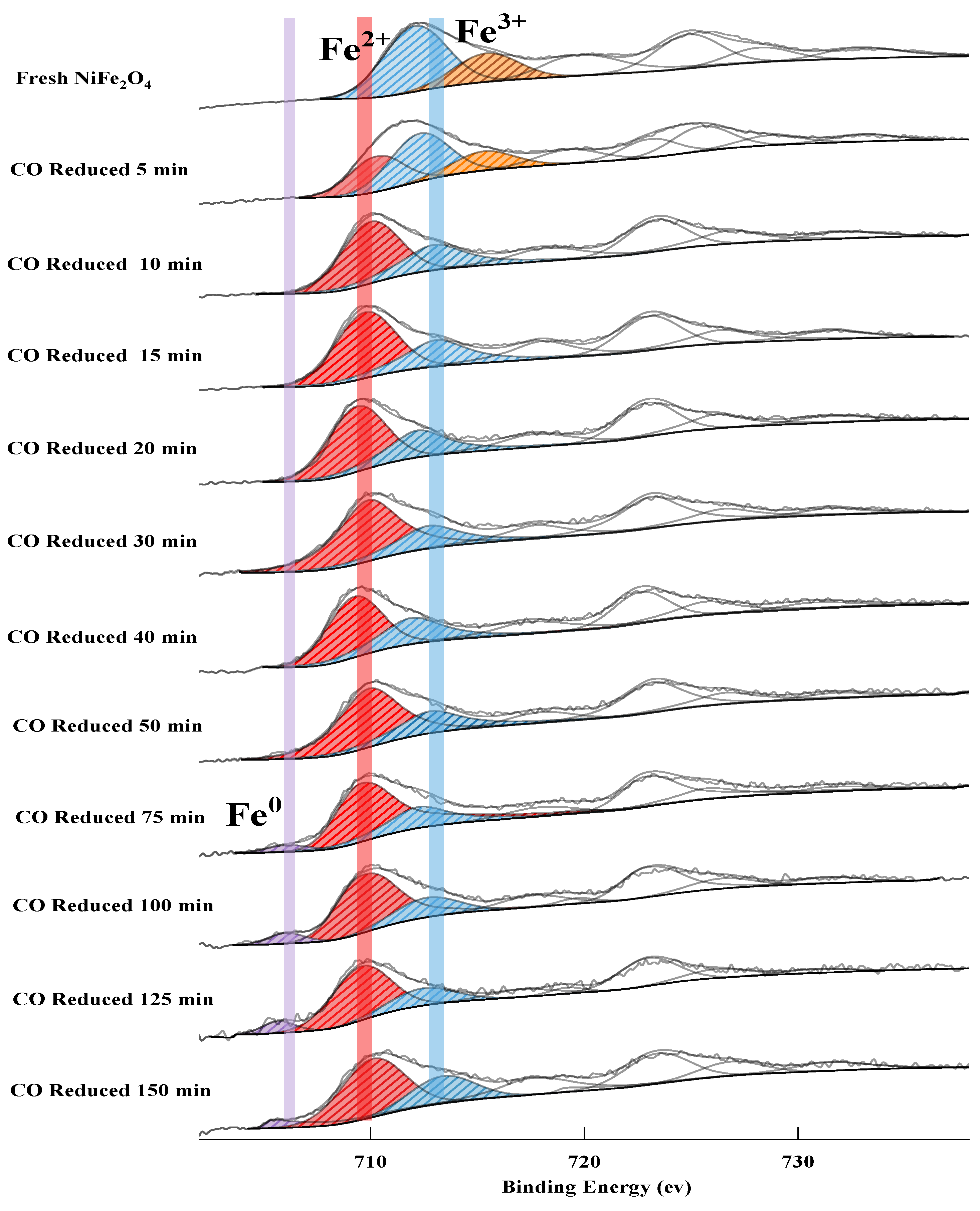
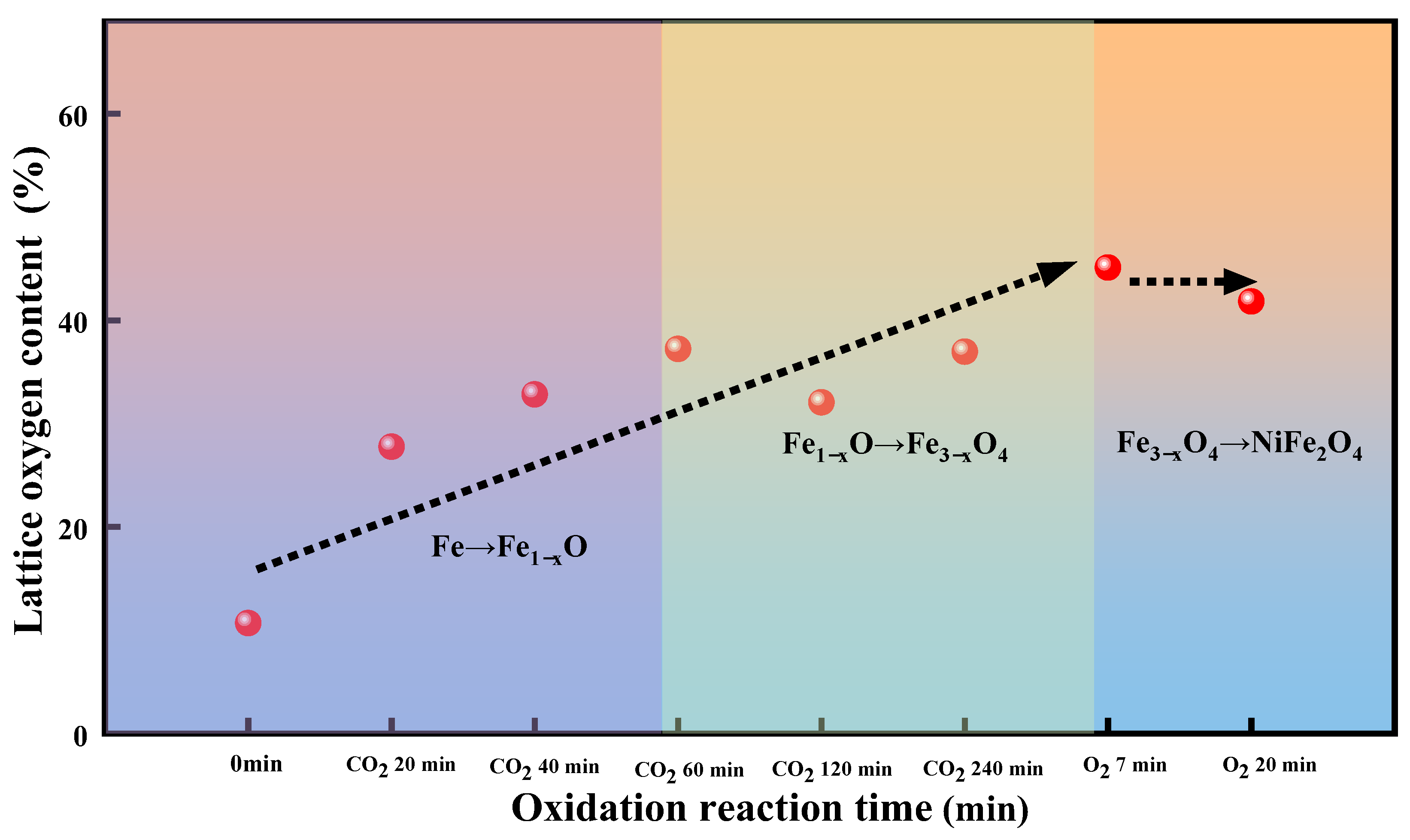
2.2.1. The Release of Lattice Oxygen
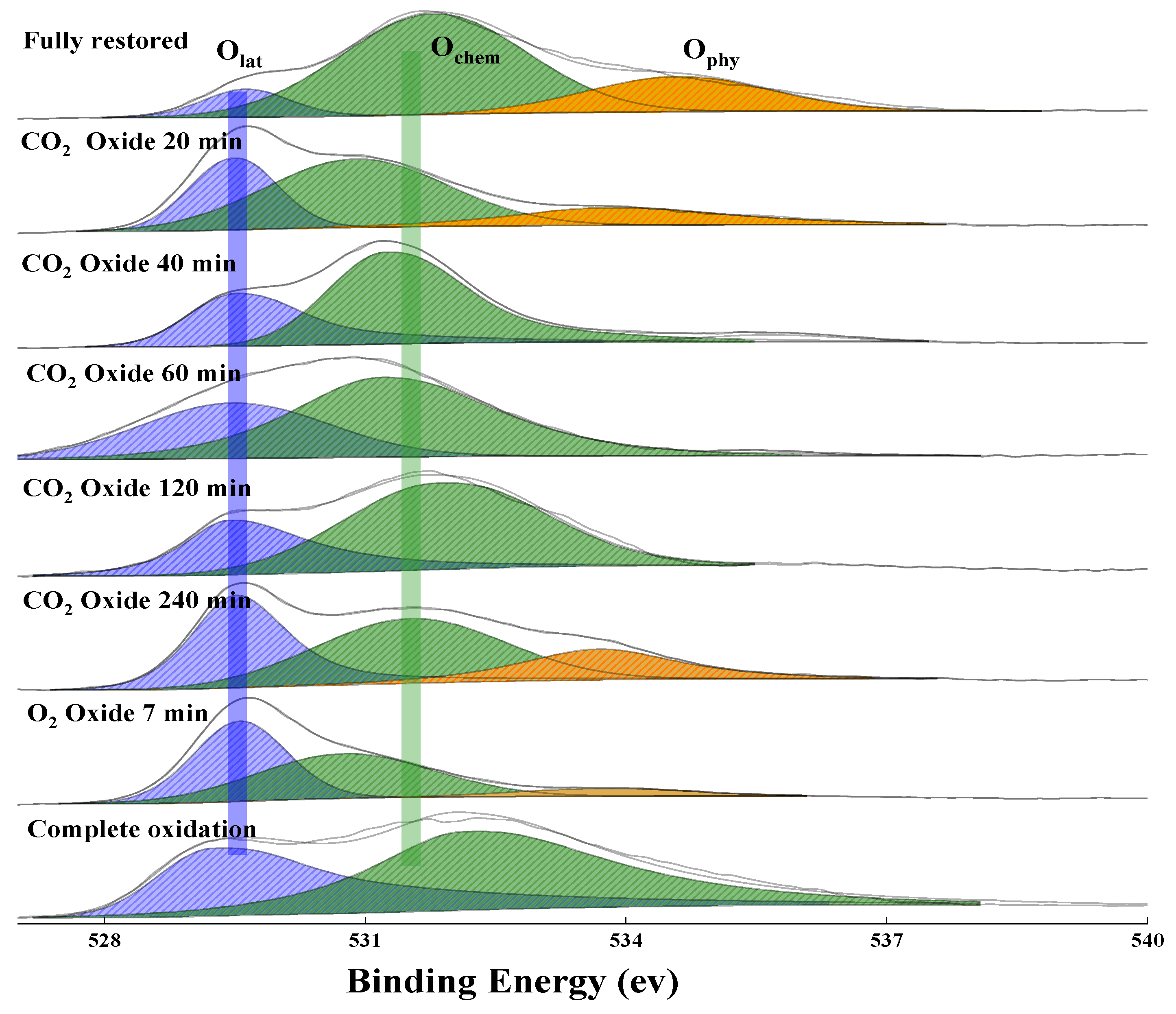
2.2.2. The Uptake of Lattice Oxygen
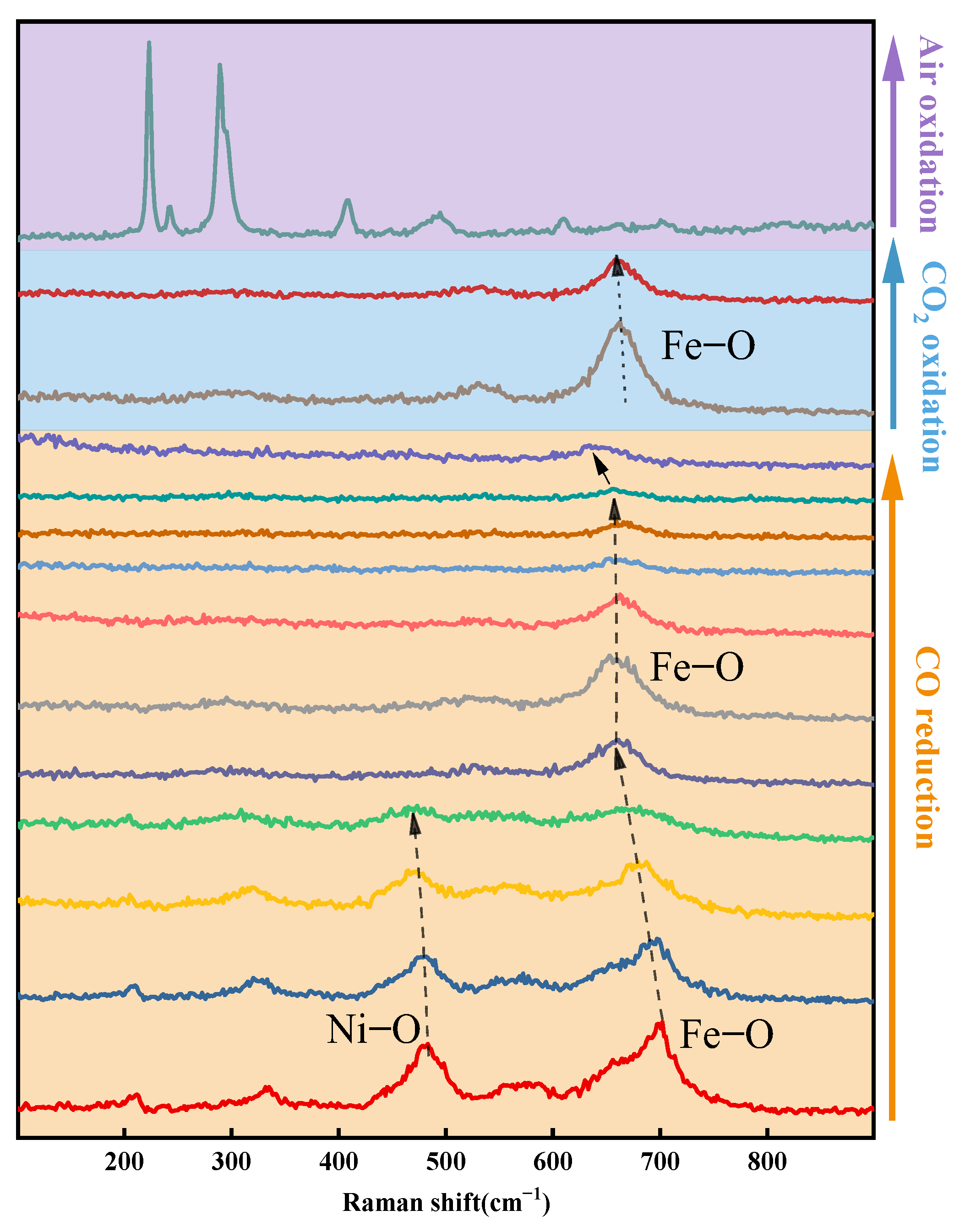
2.3. In Situ Raman Analysis
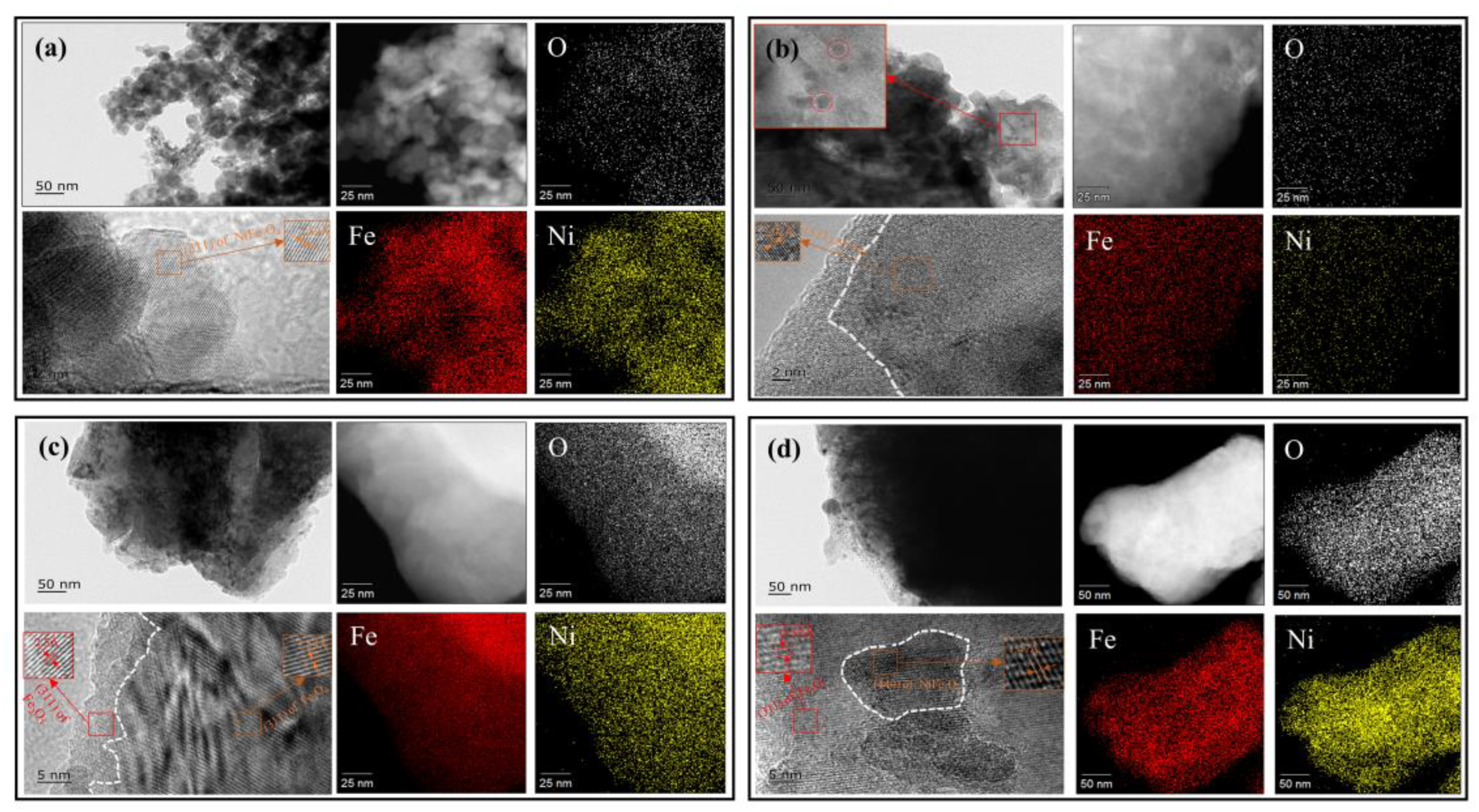
2.4. TEM Analysis
3. Materials and Methods
3.1. Preparation of Oxygen Carrier
3.2. Thermo-Gravimetric (TG) Analysis
3.3. Characterization
3.3.1. X-ray Photoelectron Spectroscopy (XPS)
3.3.2. X-ray Diffraction (XRD)
3.3.3. In Situ Raman
3.3.4. Transmission Electron Microscopy (TEM)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Donat, F.; Müller, C.R. CO2-free conversion of CH4 to syngas using chemical looping. Appl. Catal. B Environ. 2020, 278. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Johnson, T.A.; Miller, J.E.; Stechel, E.B.; Maravelias, C.T. Fuel production from CO2 using solar-thermal energy: System level analysis. Energy Environ. Sci. 2012, 5, 8417–8429. [Google Scholar] [CrossRef]
- Ekström, C.; Schwendig, F.; Biede, O.; Franco, F.; Haupt, G.; de Koeijer, G.; Papapavlou, C.; Røkke, P.E. Techno-Economic Evaluations and Benchmarking of Pre-combustion CO2 Capture and Oxy-fuel Processes Developed in the European ENCAP Project. Energy Procedia 2009, 1, 4233–4240. [Google Scholar] [CrossRef] [Green Version]
- Meng, W.X.; Banerjee, S.; Zhang, X.; Agarwal, R.K. Process simulation of multi-stage chemical-looping combustion using Aspen Plus. Energy 2015, 90, 1869–1877. [Google Scholar] [CrossRef]
- Ksepko, E.; Lysowski, R. Reactivity Study of Bimetallic Fe-Mn Oxides with Addition of TiO2 for Chemical Looping Combustion Purposes. Catalysts 2021, 11, 1437. [Google Scholar] [CrossRef]
- Hossain, M.M.; de Lasa, H.I. Chemical-looping combustion (CLC) for inherent CO2 separations—A review. Chem. Eng. Sci. 2008, 63, 4433–4451. [Google Scholar] [CrossRef]
- Tang, M.C.; Xu, L.; Fan, M.H. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Bliznuk, V.; Detavernier, C.; Marin, G.B. CeO2-Modified Fe2O3 for CO2 Utilization via Chemical Looping. Ind. Eng. Chem. Res. 2013, 52, 8416–8426. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Catalyst-assisted chemical looping for CO2 conversion to CO. Appl. Catal. B Environ. 2015, 164, 184–191. [Google Scholar] [CrossRef]
- Song, T.; Shen, T.; Shen, L.; Xiao, J.; Gu, H.; Zhang, S. Evaluation of hematite oxygen carrier in chemical-looping combustion of coal. Fuel 2013, 104, 244–252. [Google Scholar] [CrossRef]
- Linderholm, C.; Knutsson, P.; Schmitz, M.; Markström, P.; Lyngfelt, A. Material balances of carbon, sulfur, nitrogen and ilmenite in a 100 kW CLC reactor system. Int. J. Greenh. Gas Control 2014, 27, 188–202. [Google Scholar] [CrossRef]
- Cocchi, S.; Mari, M.; Cavani, F.; Millet, J.-M.M. Chemical and physical behavior of CoFe2O4 in steam–iron process with methanol. Appl. Catal. B Environ. 2014, 152, 250–261. [Google Scholar] [CrossRef]
- Siriwardane, R.; Tian, H.; Simonyi, T.; Poston, J. Synergetic effects of mixed copper–iron oxides oxygen carriers in chemical looping combustion. Fuel 2013, 108, 319–333. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Ma, J.-L.; Chin, C.-P.; Kuo, Y.-L.; Ku, Y. Preparation of composite nickel–iron oxide as highly reactive oxygen carrier for chemical-looping combustion process. J. Taiwan Inst. Chem. Eng. 2014, 45, 174–179. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, S.; Cui, D.; Li, M.; Zeng, J.; Zeng, D.; Xiao, R. Enhanced hydrogen production performance at intermediate temperatures through the synergistic effects of binary oxygen carriers. Appl. Energy 2019, 252, 113454. [Google Scholar] [CrossRef]
- Qin, L.; Guo, M.; Liu, Y.; Cheng, Z.; Fan, J.A.; Fan, L.-S. Enhanced methane conversion in chemical looping partial oxidation systems using a copper doping modification. Appl. Catal. B Environ. 2018, 235, 143–149. [Google Scholar] [CrossRef]
- Zhou, H.; Yi, Q.; Wei, G.; Zhang, Y.; Hou, Y.; Huang, Z.; Zheng, A.; Zhao, Z.; Li, H. Reaction performance and lattice oxygen migration of MnFe2O4 oxygen carrier in methane-carbon dioxide reaction system. Int. J. Hydrogen Energy 2020, 45, 30254–30266. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, X.; Russell, C.K.; Dyar, M.D.; Sklute, E.C.; Toan, S.; Fan, M.; Duan, L.; Xiang, W. Synergistic enhancement of chemical looping-based CO2 splitting with biomass cascade utilization using cyclic stabilized Ca2Fe2O5 aerogel. J. Mater. Chem. A 2018, 7, 1216–1226. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; Chen, D.; Wei, G.; He, F.; Zhao, K.; Zheng, A.; Zhao, Z.; Li, H. Exploration of Reaction Mechanisms on Hydrogen Production through Chemical Looping Steam Reforming Using NiFe2O4 Oxygen Carrier. ACS Sustain. Chem. Eng. 2019, 7, 11621–11632. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.; Zhao, Z.; He, F.; Huang, Z.; Wei, G. Identifying the roles of MFe2O4 (M=Cu, Ba, Ni, and Co) in the chemical looping reforming of char, pyrolysis gas and tar resulting from biomass pyrolysis. Int. J. Hydrogen Energy 2019, 44, 4674–4687. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kent, R.A.; Yung, M.M.; Kuhn, J.N. Carbon Dioxide Conversion by Reverse Water–Gas Shift Chemical Looping on Perovskite-Type Oxides. Ind. Eng. Chem. Res. 2014, 53, 5828–5837. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Julbe, A. Non-Stoichiometric Redox Active Perovskite Materials for Solar Thermochemical Fuel Production: A Review. Catalysts 2018, 8, 611. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, A.J.; Bork, A.H.; Moser, T.; Sediva, E.; Hood, Z.D.; Rupp, J.L.M. Modifying La0.6Sr0.4MnO3 Perovskites with Cr Incorporation for Fast Isothermal CO2-Splitting Kinetics in Solar-Driven Thermochemical Cycles. Adv. Energy Mater. 2019, 9, 13. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, H.; He, F.; Chen, D.; Wei, G.; Zhao, K.; Zheng, A.; Feng, Y.; Zhao, Z.; Li, H. Evaluation of multi-cycle performance of chemical looping dry reforming using CO2 as an oxidant with Fe–Ni bimetallic oxides. J. Energy Chem. 2016, 25, 62–70. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Huang, Z.; Liu, M.; Wei, G.; Zhao, Z.; Li, H.; Fang, Y. Chemical looping gasification coupled with steam reforming of biomass using NiFe2O4: Kinetic analysis of DAEM-TI, thermodynamic simulation of OC redox, and a loop test. Chem. Eng. J. 2020, 395, 125046. [Google Scholar] [CrossRef]
- Liu, W. Controlling lattice oxygen activity of oxygen carrier materials by design: A review and perspective. React. Chem. Eng. 2021, 6, 1527–1537. [Google Scholar] [CrossRef]
- Chen, D.; He, D.; Lu, J.; Zhong, L.; Liu, F.; Liu, J.; Yu, J.; Wan, G.; He, S.; Luo, Y. Investigation of the role of surface lattice oxygen and bulk lattice oxygen migration of cerium-based oxygen carriers: XPS and designed H2-TPR characterization. Appl. Catal. B Environ. 2017, 218, 249–259. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, J.; Xuan, G.; Zhang, F.; Yang, L. Spatial evolution characteristics of active components of copper-iron based oxygen carrier in chemical looping combustion. Fuel 2021, 306, 121650. [Google Scholar] [CrossRef]
- Zhao, K.; Zheng, A.; Li, H.; He, F.; Huang, Z.; Wei, G.; Shen, Y.; Zhao, Z. Exploration of the mechanism of chemical looping steam methane reforming using double perovskite-type oxides La1.6Sr0.4FeCoO6. Appl. Catal. B Environ. 2017, 219, 672–682. [Google Scholar] [CrossRef]
- Wang, M.J.; Zhang, S.X.; Xia, M.; Wang, M.K. A Theoretical Study of the Oxygen Release Mechanisms of a Cu-Based Oxygen Carrier during Chemical Looping with Oxygen Uncoupling. Catalysts 2022, 12, 332. [Google Scholar] [CrossRef]
- Cheng, Z.; Baser, D.S.; Nadgouda, S.G.; Qin, L.; Fan, J.A.; Fan, L.-S. C2 Selectivity Enhancement in Chemical Looping Oxidative Coupling of Methane over a Mg–Mn Composite Oxygen Carrier by Li-Doping-Induced Oxygen Vacancies. ACS Energy Lett. 2018, 3, 1730–1736. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Chen, D.; Zhao, K.; Wei, G.; Zheng, A.; Zhao, Z.; Li, H. Investigation on reactivity of iron nickel oxides in chemical looping dry reforming. Energy 2016, 116, 53–63. [Google Scholar] [CrossRef]
- Cao, J.-L.; Wang, Y.; Yu, X.-L.; Wang, S.-R.; Wu, S.-H.; Yuan, Z.-Y. Mesoporous CuO–Fe2O3 composite catalysts for low-temperature carbon monoxide oxidation. Appl. Catal. B Environ. 2008, 79, 26–34. [Google Scholar] [CrossRef]
- Cao, W.; Tan, O.; Pan, J.; Zhu, W.; Reddy, C.V.G. XPS characterization of xα-Fe2O3–(1−x)ZrO2 for oxygen gas sensing application. Mater. Chem. Phys. 2002, 75, 67–70. [Google Scholar] [CrossRef]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Wang, D.; Jin, L.; Li, Y.; Hu, H. Partial oxidation of vacuum residue over Al and Zr-doped α-Fe2O3 catalysts. Fuel 2017, 210, 803–810. [Google Scholar] [CrossRef]
- Aronniemi, M.; Sainio, J.; Lahtinen, J. XPS study on the correlation between chemical state and oxygen-sensing properties of an iron oxide thin film. Appl. Surf. Sci. 2007, 253, 9476–9482. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, H.; Li, K. Ce-Fe-O mixed oxide as oxygen carrier for the direct partial oxidation of methane to syngas. J. Rare Earths 2010, 28, 560–565. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Kuhn, J.N.; Zhao, Z.; Felix, L.G.; Slimane, R.B.; Choi, C.W.; Ozkan, U.S. Olivine catalysts for methane- and tar-steam reforming. Appl. Catal. B Environ. 2008, 81, 14–26. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Wang, D.; Rosynek, M.P.; Lunsford, J.H. Conversion of Methane to Benzene over Transition Metal Ion ZSM-5 Zeolites: II. Catalyst Characterization by X-ray Photoelectron Spectroscopy. J. Catal. 1998, 175, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Cheng, Z.; Fan, J.A.; Fan, L.-S.; Gong, J. Metal oxide redox chemistry for chemical looping processes. Nat. Rev. Chem. 2018, 2, 349–364. [Google Scholar] [CrossRef]
- Liu, S.; He, F.; Huang, Z.; Zheng, A.; Feng, Y.; Shen, Y.; Li, H.; Wu, H.; Glarborg, P. Screening of NiFe2O4 Nanoparticles as Oxygen Carrier in Chemical Looping Hydrogen Production. Energy Fuels 2016, 30, 4251–4262. [Google Scholar] [CrossRef]
- Yang, H.; Gong, L.; Wang, H.; Dong, C.; Wang, J.; Qi, K.; Liu, H.; Guo, X.; Xia, B.Y. Preparation of nickel-iron hydroxides by microorganism corrosion for efficient oxygen evolution. Nat. Commun. 2020, 11, 5075. [Google Scholar] [CrossRef]
- Louie, M.W.; Bell, A.T. An Investigation of Thin-Film Ni–Fe Oxide Catalysts for the Electrochemical Evolution of Oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337. [Google Scholar] [CrossRef] [Green Version]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman spectroscopy to unravel the magnetic properties of iron oxide nanocrystals for bio-related applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Zeng, D.; Zhang, S.; Xiao, R. Effect of Supports on the Redox Performance of NiFe2O4 in a Chemical Looping Process. Energy Technol. 2019, 7, 1900374. [Google Scholar] [CrossRef]


| Element (Atom %) | Fresh OC | Completely Reduced OC | Completely Oxidized OC by CO2 | Completely Oxidized OC by Air |
|---|---|---|---|---|
| Fe | 47.71% | 59.90% | 38.19% | 37.49% |
| Ni | 25.98% | 9.53% | 3.67% | 3.43% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Lin, Y.; Zhao, K.; Huang, Z.; He, F.; Xiong, Y. Migration Mechanism of Lattice Oxygen: Conversion of CO2 to CO Using NiFe2O4 Spinel Oxygen Carrier in Chemical Looping Reactions. Catalysts 2022, 12, 1181. https://doi.org/10.3390/catal12101181
Song D, Lin Y, Zhao K, Huang Z, He F, Xiong Y. Migration Mechanism of Lattice Oxygen: Conversion of CO2 to CO Using NiFe2O4 Spinel Oxygen Carrier in Chemical Looping Reactions. Catalysts. 2022; 12(10):1181. https://doi.org/10.3390/catal12101181
Chicago/Turabian StyleSong, Da, Yan Lin, Kun Zhao, Zhen Huang, Fang He, and Ya Xiong. 2022. "Migration Mechanism of Lattice Oxygen: Conversion of CO2 to CO Using NiFe2O4 Spinel Oxygen Carrier in Chemical Looping Reactions" Catalysts 12, no. 10: 1181. https://doi.org/10.3390/catal12101181
APA StyleSong, D., Lin, Y., Zhao, K., Huang, Z., He, F., & Xiong, Y. (2022). Migration Mechanism of Lattice Oxygen: Conversion of CO2 to CO Using NiFe2O4 Spinel Oxygen Carrier in Chemical Looping Reactions. Catalysts, 12(10), 1181. https://doi.org/10.3390/catal12101181












