Rhodium-Catalyzed Dynamic Kinetic Resolution of Racemic Internal Allenes towards Chiral Allylated Triazoles and Tetrazoles
Abstract
:1. Introduction
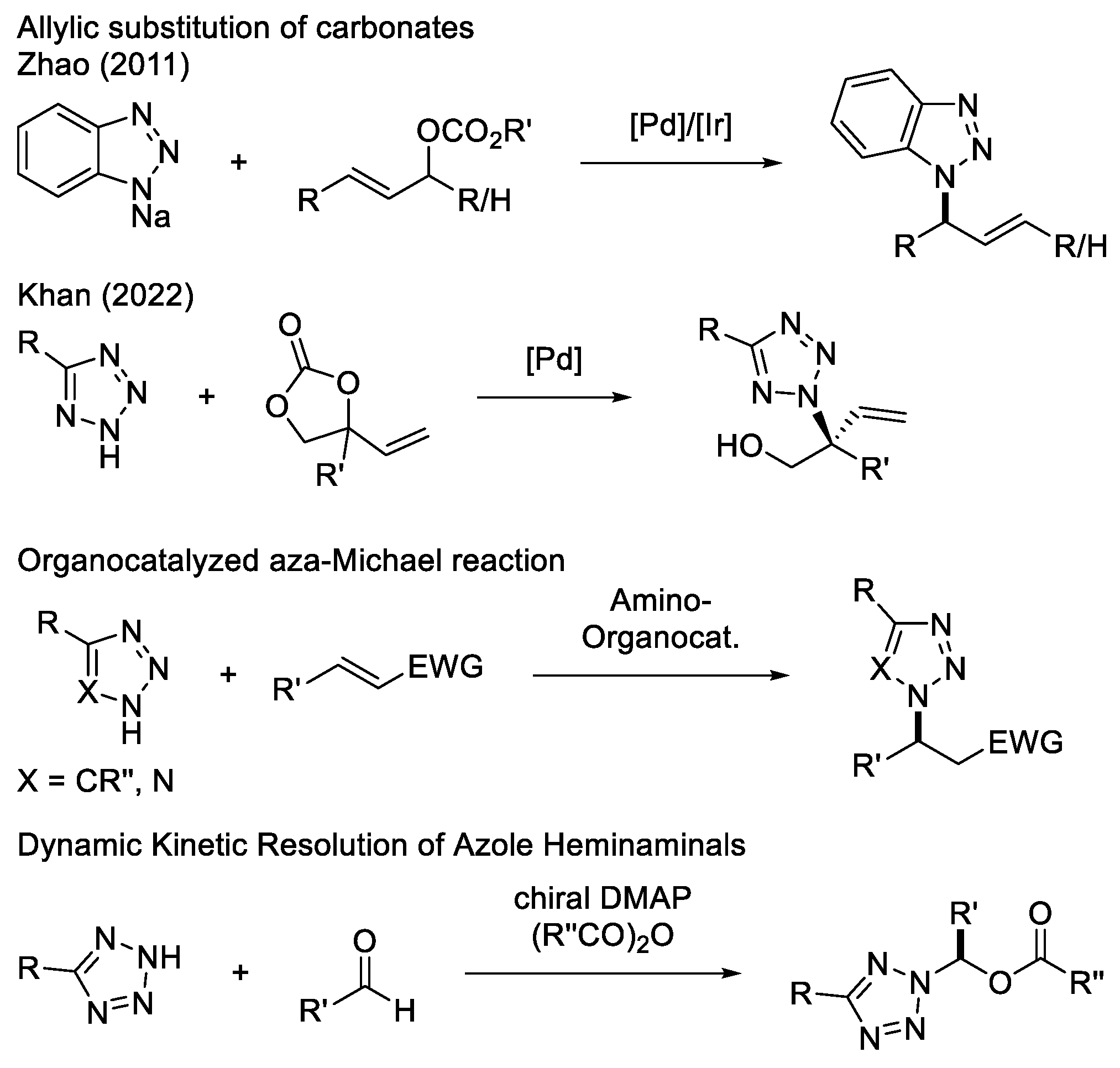
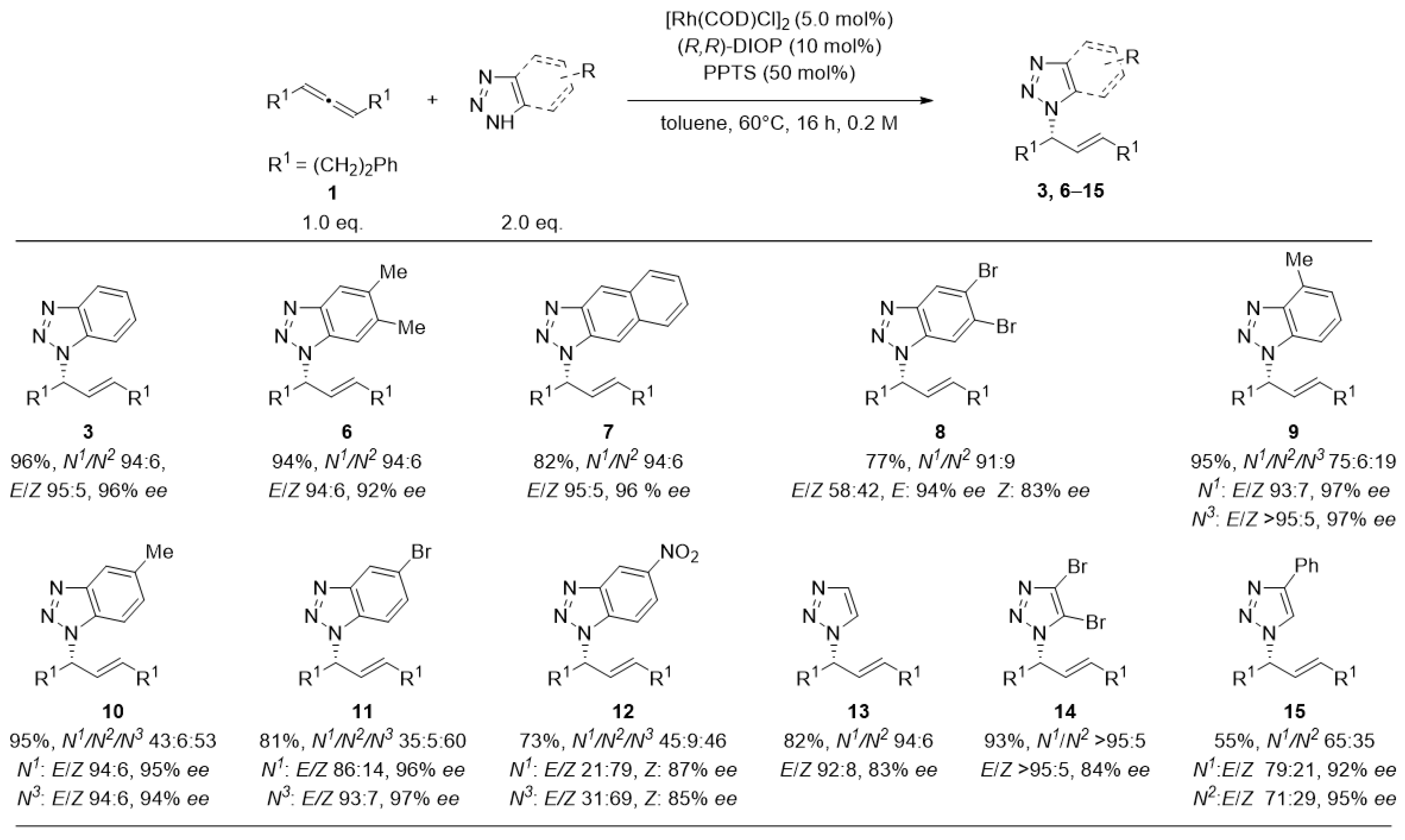
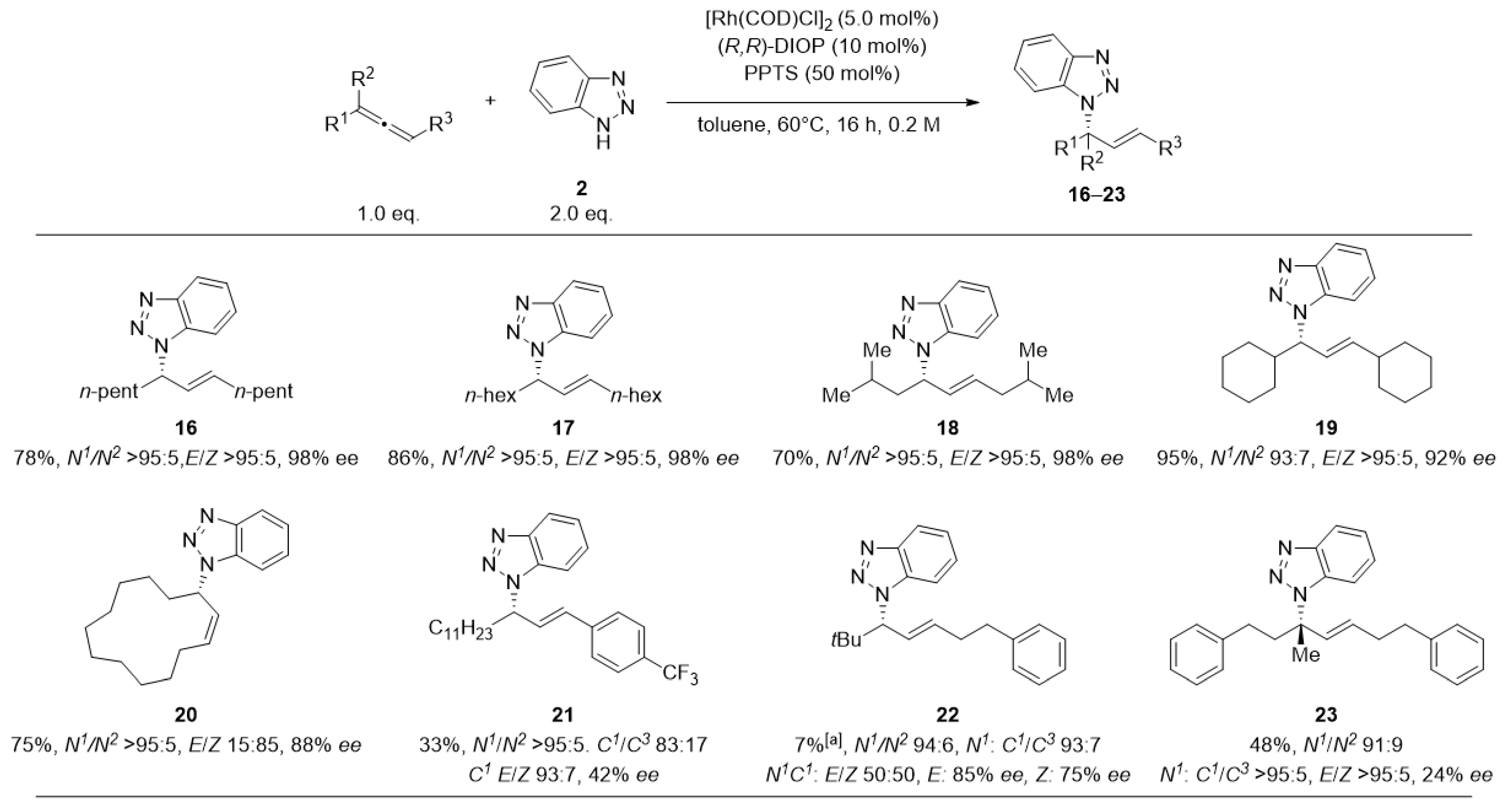
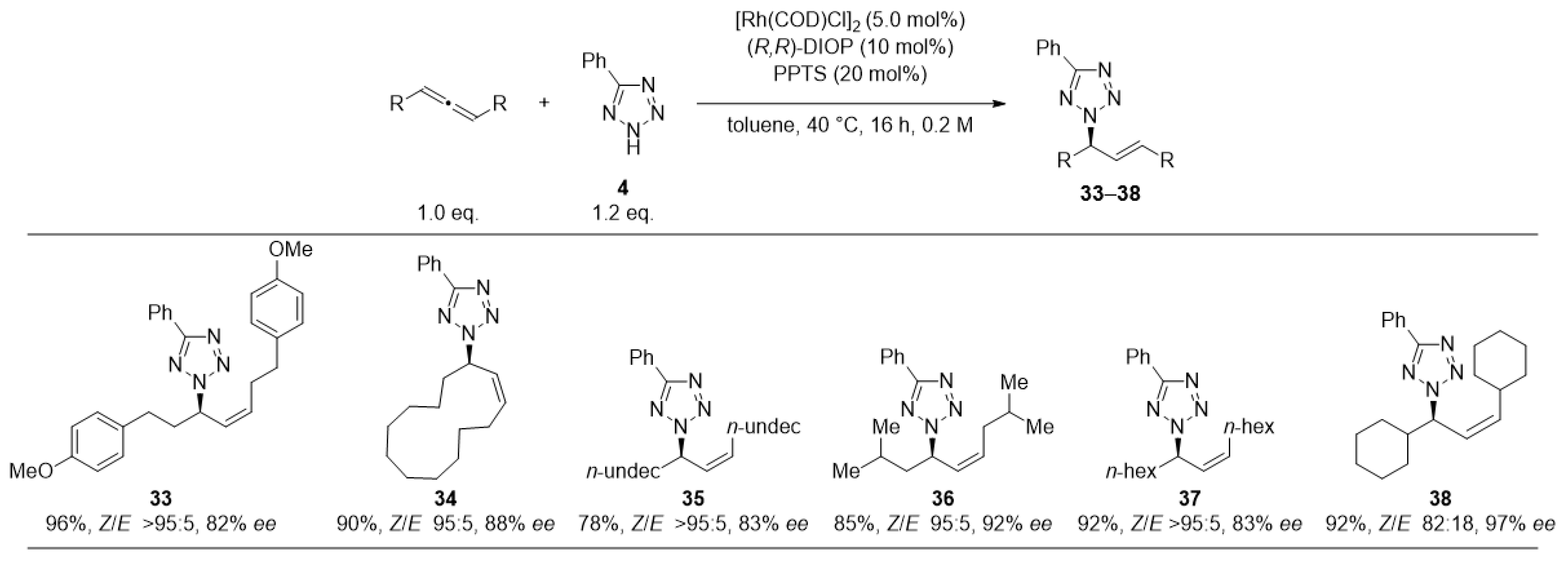
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rezaei, Z.; Khabnadideh, S.; Pakshir, K.; Hossaini, Z.; Amiri, F.; Assadpour, E. Design, synthesis, and antifungal activity of triazole and benzotriazole derivatives. Eur. J. Med. Chem. 2009, 44, 3064–3067. [Google Scholar] [CrossRef] [PubMed]
- Paluchowska, M.H.; Bugno, R.; Charakchieva-Minol, S.; Bojarski, A.J.; Tatarczyńska, E.; Chojnacka-Wójcik, E. Conformational restriction in novel NAN-190 and MP3022 analogs and their 5-HT(1A) receptor activity. Arch. Pharm. 2006, 339, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Letavic, M.A.; Bronk, B.S.; Bertsche, C.D.; Casavant, J.M.; Cheng, H.; Daniel, K.L.; George, D.M.; Hayashi, S.F.; Kamicker, B.J.; Kolosko, N.L.; et al. Synthesis and Activity of a Novel Class of Tribasic Macrocyclic Antibiotics: The Triamilides. Bioorg. Med. Chem. Lett. 2002, 12, 2771–2774. [Google Scholar] [CrossRef]
- Sambasiva Rao, P.; Kurumurthy, C.; Veeraswamy, B.; Santhosh Kumar, G.; Poornachandra, Y.; Ganesh Kumar, C.; Vasamsetti, S.B.; Kotamraju, S.; Narsaiah, B. Synthesis of novel 1,2,3-triazole substituted-N-alkyl/aryl nitrone derivatives, their anti-inflammatory and anticancer activity. Eur. J. Med. Chem. 2014, 80, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Upadhayaya, R.S.; Jain, S.; Sinha, N.; Kishore, N.; Chandra, R.; Arora, S.K. Synthesis of novel substituted tetrazoles having antifungal activity. Eur. J. Med. Chem. 2004, 39, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Mollison, K.W.; Liu, L.; Henry, C.L.; Rosenberg, T.A.; Bamaung, N.; Tu, N.; Wiedeman, P.E.; Or, Y.; Luly, J.R.; et al. Rapamycin analogs with reduced systemic exposure. Bioorg. Med. Chem. Lett. 2005, 15, 5340–5343. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Poliakov, A.; Åkerblom, E.; Wiklund, K.; Lindeberg, G.; Winiwarter, S.; Danielson, U.; Samuelsson, B.; Hallberg, A. Acyl sulfonamides as potent protease inhibitors of the hepatitis C virus full-Length NS3 (Protease-Helicase/NTPase): A comparative study of different C-terminals. Bioorg. Med. Chem. 2003, 11, 2551–2568. [Google Scholar] [CrossRef]
- Herr, R. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: Medicinal chemistry and synthetic methods. Bioorg. Med. Chem. 2002, 10, 3379–3393. [Google Scholar] [CrossRef]
- Vantikommu, J.; Palle, S.; Reddy, P.S.; Ramanatham, V.; Khagga, M.; Pallapothula, V.R. Synthesis and cytotoxicity evaluation of novel 1,4-disubstituted 1,2,3-triazoles via CuI catalysed 1,3-dipolar cycloaddition. Eur. J. Med. Chem. 2010, 45, 5044–5050. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, Y.; Chen, H.; Wild, C.; Wang, T.; White, M.A.; Shen, Q.; Zhou, J. Overcoming synthetic challenges of oridonin A-ring structural diversification: Regio- and stereoselective installation of azides and 1,2,3-triazoles at the C-1, C-2, or C-3 position. Org. Lett. 2013, 15, 3718–3721. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.S.; Swartz, S.G.; Slusarchyk, D.; Yan, M.; Seethala, R.K.; Sleph, P.; Grover, G.; Dickinson, K.; Giupponi, L.; Harper, T.W.; et al. Optimization of 1H-tetrazole-1-alkanenitriles as potent orally bioavailable growth hormone secretagogues. Bioorg. Med. Chem. Lett. 2008, 18, 2067–2072. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Jiang, F.; Shi, F. Organocatalytic Asymmetric Synthesis of Indole-Based Chiral Heterocycles: Strategies, Reactions, and Outreach. Acc. Chem. Res. 2020, 53, 425–446. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, D.; Zheng, S.; Yue, Y.; Liu, D.; Zhao, X. Enantioselective Palladium-Catalyzed Allylic Substitution of Sodium Benzotriazolide. Eur. J. Org. Chem. 2011, 2011, 6288–6293. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, X.-W.; Zheng, S.-C.; Zhao, X.-M. Enantioselective iridium-catalyzed allylation of sodium benzotriazolide: An efficient way to chiral allylbenzotriazoles. Tetrahedron Lett. 2012, 53, 6995–6998. [Google Scholar] [CrossRef]
- Khan, S.; Wang, Y.; Zhang, M.-N.; Perveen, S.; Zhang, J.; Khan, A. Regio- and enantioselective formation of tetrazole-bearing quaternary stereocenters via palladium-catalyzed allylic amination. Org. Chem. Front. 2022, 9, 456–461. [Google Scholar] [CrossRef]
- Trost, B.M. The atom economy—A search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Dinér, P.; Nielsen, M.; Marigo, M.; Jørgensen, K.A. Enantioselective organocatalytic conjugate addition of N heterocycles to alpha, beta-unsaturated aldehydes. Angew. Chem. Int. Ed. 2007, 46, 1983–1987. [Google Scholar] [CrossRef]
- Gandelman, M.; Jacobsen, E.N. Highly enantioselective catalytic conjugate addition of N-heterocycles to alpha, beta-unsaturated ketones and imides. Angew. Chem. Int. Ed. 2005, 44, 2393–2397. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, S.; Duan, W.; Wang, W. Enantioselective Conjugate Addition of N-Heterocycles to α,β-Unsaturated Ketones Catalyzed by Chiral Primary Amines. Synthesis 2009, 2009, 1564–1572. [Google Scholar] [CrossRef]
- Uria, U.; Vicario, J.L.; Badía, D.; Carrillo, L. Organocatalytic enantioselective aza-Michael reaction of nitrogen heterocycles and alpha, beta-unsaturated aldehydes. Chem. Commun. 2007, 24, 2509–2511. [Google Scholar] [CrossRef]
- Wang, J.; Zu, L.; Li, H.; Xie, H.; Wang, W. Cinchona Alkaloid Based Thiourea Promoted Enantioselective Conjugate Addition of N-Heterocycles to Enones. Synthesis 2007, 2007, 2576–2580. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Zu, L.; Wang, W. Enantioselective organocatalytic Michael addition reactions between N-heterocycles and nitroolefins. Org. Lett. 2006, 8, 1391–1394. [Google Scholar] [CrossRef]
- Piotrowski, D.W.; Kamlet, A.S.; Dechert-Schmitt, A.-M.R.; Yan, J.; Brandt, T.A.; Xiao, J.; Wei, L.; Barrila, M.T. Regio- and Enantioselective Synthesis of Azole Hemiaminal Esters by Lewis Base Catalyzed Dynamic Kinetic Resolution. J. Am. Chem. Soc. 2016, 138, 4818–4823. [Google Scholar] [CrossRef]
- Kinens, A.; Sejejs, M.; Kamlet, A.S.; Piotrowski, D.W.; Vedejs, E.; Suna, E. Development of a Chiral DMAP Catalyst for the Dynamic Kinetic Resolution of Azole Hemiaminals. J. Org. Chem. 2017, 82, 869–886. [Google Scholar] [CrossRef]
- Koschker, P.; Breit, B. Branching Out: Rhodium-Catalyzed Allylation with Alkynes and Allenes. Acc. Chem. Res. 2016, 49, 1524–1536. [Google Scholar] [CrossRef]
- Haydl, A.M.; Breit, B.; Liang, T.; Krische, M.J. Alkynes as Electrophilic or Nucleophilic Allylmetal Precursors in Transition-Metal Catalysis. Angew. Chem. Int. Ed. 2017, 56, 11312–11325. [Google Scholar] [CrossRef]
- Berthold, D.; Klett, J.; Breit, B. Rhodium-catalyzed asymmetric intramolecular hydroarylation of allenes: Access to functionalized benzocycles. Chem. Sci. 2019, 10, 10048–10052. [Google Scholar] [CrossRef] [Green Version]
- Grugel, C.P.; Breit, B. Rhodium-Catalyzed Enantioselective Cyclization of 3-Allenyl-indoles: Access to Functionalized Tetrahydrocarbazoles. Org. Lett. 2019, 21, 5798–5802. [Google Scholar] [CrossRef]
- Hilpert, L.J.; Breit, B. Rhodium-Catalyzed Parallel Kinetic Resolution of Racemic Internal Allenes Towards Enantiopure Allylic 1,3-Diketones. Angew. Chem. Int. Ed. 2019, 58, 9939–9943. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Breit, B. Transition metal catalyzed stereodivergent synthesis of syn- and anti-δ-vinyl-lactams: Formal total synthesis of (-)-cermizine C and (-)-senepodine G. Chem. Sci. 2019, 10, 3074–3079. [Google Scholar] [CrossRef]
- Berthold, D.; Geissler, A.G.A.; Giofré, S.; Breit, B. Rhodium-Catalyzed Asymmetric Intramolecular Hydroamination of Allenes. Angew. Chem. 2019, 131, 10099–10102. [Google Scholar] [CrossRef]
- Berthold, D.; Breit, B. Asymmetric Total Syntheses of (-)-Angustureine and (-)-Cuspareine via Rhodium-Catalyzed Hydroamination. Org. Lett. 2020, 22, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Koschker, P.; Lumbroso, A.; Breit, B. Enantioselective synthesis of branched allylic esters via rhodium-catalyzed coupling of allenes with carboxylic acids. J. Am. Chem. Soc. 2011, 133, 20746–20749. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Breit, B. Rhodium-Catalyzed Enantioselective Intermolecular Hydroalkoxylation of Allenes and Alkynes with Alcohols: Synthesis of Branched Allylic Ethers. Angew. Chem. Int. Ed. 2016, 55, 8440–8443. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.P.; Breit, B. Rhodium-Catalyzed Cyclization of Terminal and Internal Allenols: An Atom Economic and Highly Stereoselective Access Towards Tetrahydropyrans. Angew. Chem. Int. Ed. 2020, 59, 23485–23490. [Google Scholar] [CrossRef] [PubMed]
- Pritzius, A.B.; Breit, B. Asymmetric rhodium-catalyzed addition of thiols to allenes: Synthesis of branched allylic thioethers and sulfones. Angew. Chem. 2015, 127, 3164–3168. [Google Scholar] [CrossRef]
- Khakyzadeh, V.; Wang, Y.-H.; Breit, B. Rhodium-catalyzed addition of sulfonyl hydrazides to allenes: Regioselective synthesis of branched allylic sulfones. Chem. Commun. 2017, 53, 4966–4968. [Google Scholar] [CrossRef] [Green Version]
- Evans, P.A.; Leahy, D.K. Regio- and enantiospecific rhodium-catalyzed allylic etherification reactions using copper(I) alkoxides: Influence of the copper halide salt on selectivity. J. Am. Chem. Soc. 2002, 124, 7882–7883. [Google Scholar] [CrossRef]
- Hayashi, T.; Okada, A.; Suzuka, T.; Kawatsura, M. High enantioselectivity in rhodium-catalyzed allylic alkylation of 1-substituted 2-propenyl acetates. Org. Lett. 2003, 5, 1713–1715. [Google Scholar] [CrossRef]
- Lu, Z.; Ma, S. Metal-catalyzed enantioselective allylation in asymmetric synthesis. Angew. Chem. 2008, 120, 264–303. [Google Scholar] [CrossRef]
- Madrahimov, S.T.; Li, Q.; Sharma, A.; Hartwig, J.F. Origins of Regioselectivity in Iridium Catalyzed Allylic Substitution. J. Am. Chem. Soc. 2015, 137, 14968–14981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrone, D.A.; Isomura, M.; Franzoni, I.; Rössler, S.L.; Carreira, E.M. Allenylic Carbonates in Enantioselective Iridium-Catalyzed Alkylations. J. Am. Chem. Soc. 2018, 140, 4697–4704. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Helmchen, G. Applications of Iridium-Catalyzed Asymmetric Allylic Substitution Reactions in Target-Oriented Synthesis. Acc. Chem. Res. 2017, 50, 2539–2555. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Crawley, M.L. Asymmetric transition-metal-catalyzed allylic alkylations: Applications in total synthesis. Chem. Rev. 2003, 103, 2921–2944. [Google Scholar] [CrossRef]
- Trost, B.M.; van Vranken, D.L. Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev. 1996, 96, 395–422. [Google Scholar] [CrossRef]
- Liu, G.; Stahl, S.S. Two-faced reactivity of alkenes: Cis- versus trans-aminopalladation in aerobic Pd-catalyzed intramolecular aza-Wacker reactions. J. Am. Chem. Soc. 2007, 129, 6328–6335. [Google Scholar] [CrossRef]
- Ma, R.; Young, J.; Promontorio, R.; Dannheim, F.M.; Pattillo, C.C.; White, M.C. Synthesis of anti-1,3 Amino Alcohol Motifs via Pd(II)/SOX Catalysis with the Capacity for Stereodivergence. J. Am. Chem. Soc. 2019, 141, 9468–9473. [Google Scholar] [CrossRef]
- Liu, G.; Wu, Y. Palladium-catalyzed allylic C-H bond functionalization of olefins. Top. Curr. Chem. 2010, 292, 195–209. [Google Scholar] [CrossRef]
- Steib, P.; Breit, B. Concise Total Synthesis of (-)-Vermiculine through a Rhodium-Catalyzed C2 -Symmetric Dimerization Strategy. Chem. Eur. J. 2019, 25, 3532–3535. [Google Scholar] [CrossRef]
- Schotes, C.; Ostrovskyi, D.; Senger, J.; Schmidtkunz, K.; Jung, M.; Breit, B. Total synthesis of (18S)- and (18R)-homolargazole by rhodium-catalyzed hydrocarboxylation. Chem. Eur. J. 2014, 20, 2164–2168. [Google Scholar] [CrossRef]
- Brosowsky, J.; Lutterbeck, M.; Liebich, A.; Keller, M.; Herp, D.; Vogelmann, A.; Jung, M.; Breit, B. Syntheses of Thailandepsin B Pseudo-Natural Products: Access to New Highly Potent HDAC Inhibitors via Late-Stage Modification. Chem. Eur. J. 2020, 26, 16241–16245. [Google Scholar] [CrossRef] [PubMed]
- Hilpert, L.J.; Sieger, S.V.; Haydl, A.M.; Breit, B. Palladium- and Rhodium-Catalyzed Dynamic Kinetic Resolution of Racemic Internal Allenes Towards Chiral Pyrazoles. Angew. Chem. 2019, 131, 3416–3419. [Google Scholar] [CrossRef]
- Hang, Q.-Q.; Wu, S.-F.; Yang, S.; Wang, X.; Zhong, Z.; Zhang, Y.-C.; Shi, F. Design and catalytic atroposelective synthesis of axially chiral isochromenone-indoles. Sci. China Chem. 2022, 65, 1929–1937. [Google Scholar] [CrossRef]
- Wu, P.; Yu, L.; Gao, C.-H.; Cheng, Q.; Deng, S.; Jiao, Y.; Tan, W.; Shi, F. Design and synthesis of axially chiral aryl-pyrroloindoles via the strategy of organocatalytic asymmetric (2 + 3) cyclization. Fundam. Res. 2022; in press. [Google Scholar] [CrossRef]
- Deska, J.; Del Pozo Ochoa, C.; Bäckvall, J.-E. Chemoenzymatic dynamic kinetic resolution of axially chiral allenes. Chem. Eur. J. 2010, 16, 4447–4451. [Google Scholar] [CrossRef]
- Huerta, F.F.; Minidis, A.B.E.; Bäckvall, J.-E. Racemisation in asymmetric synthesis. Dynamic kinetic resolution and related processes in enzyme and metal catalysis. Chem. Soc. Rev. 2001, 30, 321–331. [Google Scholar] [CrossRef]
- Ward, R.S. Dynamic kinetic resolution. Tetrahedron Asymm. 1995, 6, 1475–1490. [Google Scholar] [CrossRef]
- Cooke, M.L.; Xu, K.; Breit, B. Enantioselective rhodium-catalyzed synthesis of branched allylic amines by intermolecular hydroamination of terminal allenes. Angew. Chem. Int. Ed. 2012, 51, 10876–10879. [Google Scholar] [CrossRef]
- Xu, K.; Thieme, N.; Breit, B. Atom-economic, regiodivergent, and stereoselective coupling of imidazole derivatives with terminal allenes. Angew. Chem. Int. Ed. 2014, 53, 2162–2165. [Google Scholar] [CrossRef]
- Haydl, A.M.; Xu, K.; Breit, B. Regio- and enantioselective synthesis of N-substituted pyrazoles by rhodium-catalyzed asymmetric addition to allenes. Angew. Chem. Int. Ed. 2015, 54, 7149–7153. [Google Scholar] [CrossRef]
- Fürstner, A.; Wuchrer, M. Concise approach to the “higher sugar” core of the nucleoside antibiotic hikizimycin. Chem. Eur. J. 2005, 12, 76–89. [Google Scholar] [CrossRef]
- Kielbasinski, P.; Albrycht, M.; Mikolajczyk, M.; Wieczorek, M.W.; Majzner, W.R.; Filipczak, A.; Ciolkiewicz, P. Synthesis of chiral hydroxythiolanes as potential catalysts for asymmetric organozinc additions to carbonyl compounds. Heteroatom Chem. 2005, 16, 93–103. [Google Scholar] [CrossRef]
- Hobbs, C.F.; Knowles, W.S. Asymmetric hydroformylation of vinyl acetate with DIOP-type ligands. J. Org. Chem. 1981, 46, 4422–4427. [Google Scholar] [CrossRef]
- Johansson, M.J.; Gorin, D.J.; Staben, S.T.; Toste, F.D. Gold(I)-catalyzed stereoselective olefin cyclopropanation. J. Am. Chem. Soc. 2005, 127, 18002–18003. [Google Scholar] [CrossRef] [PubMed]
- Berthold, D.; Breit, B. Chemo-, Regio-, and Enantioselective Rhodium-Catalyzed Allylation of Triazoles with Internal Alkynes and Terminal Allenes. Org. Lett. 2018, 20, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Raimondi, W.; Bury, T.; Breit, B. Enantioselective formation of tertiary and quaternary allylic C-N bonds via allylation of tetrazoles. Chem. Commun. 2015, 51, 10861–10863. [Google Scholar] [CrossRef]



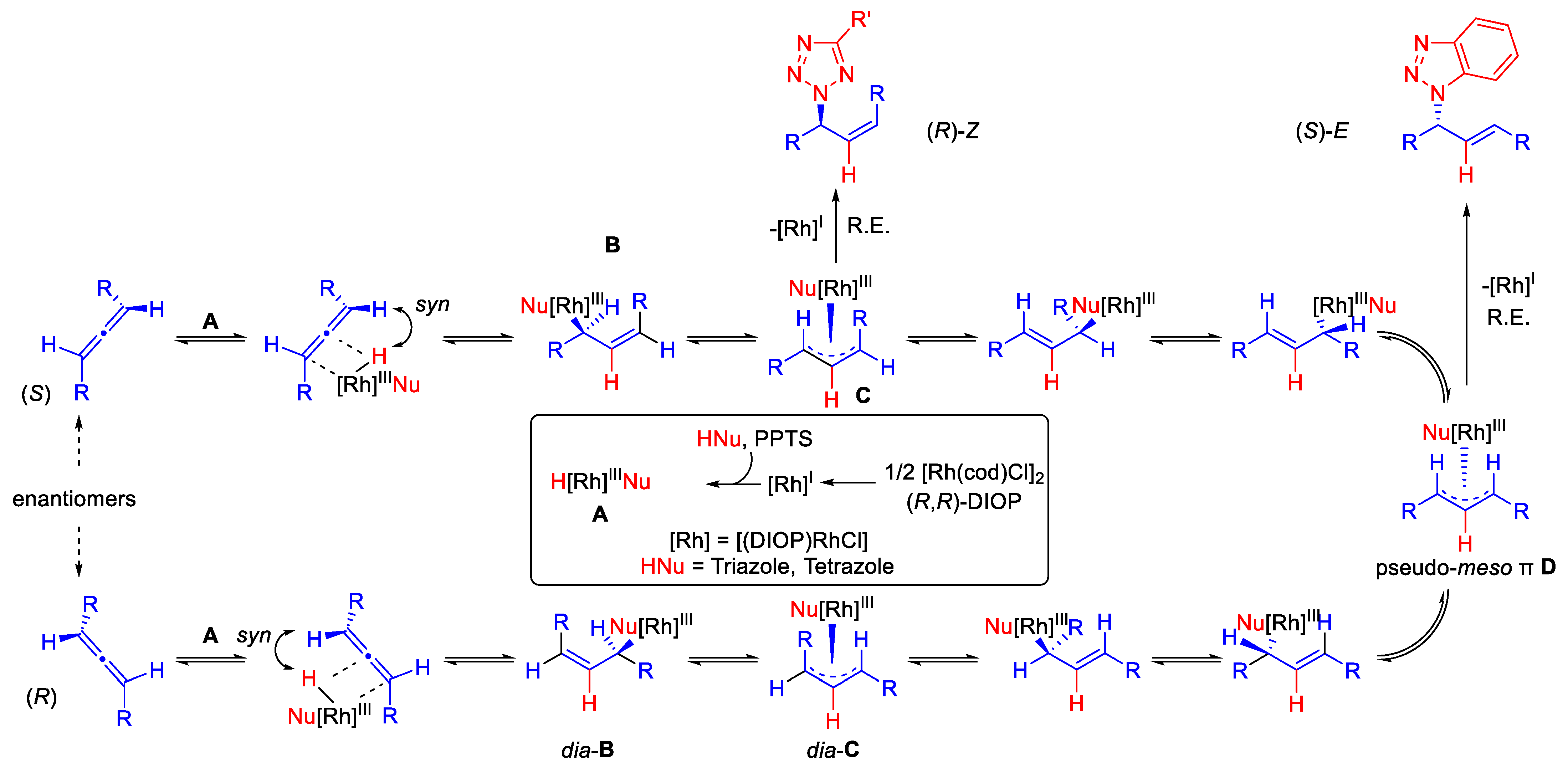
| # | Allene | Ligand | Product |
|---|---|---|---|
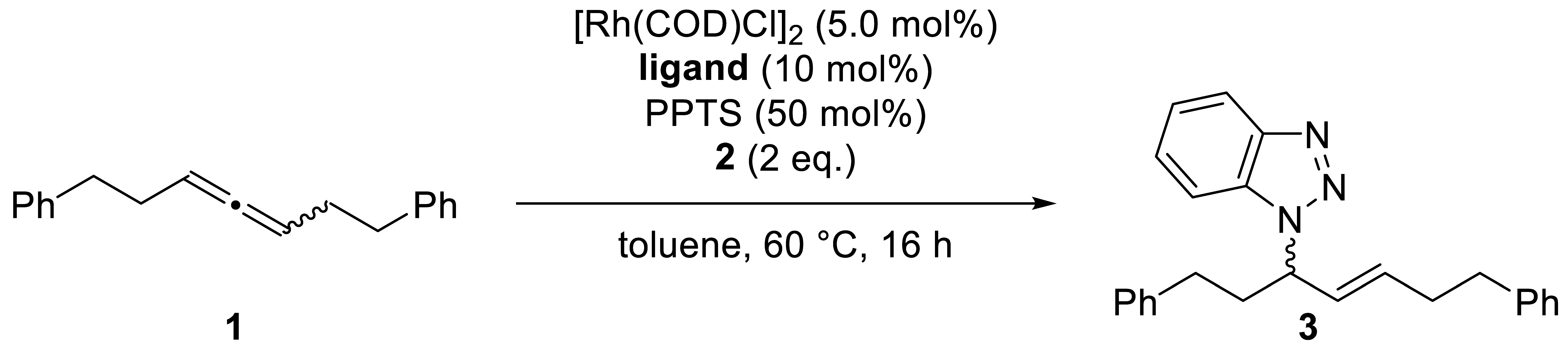 | |||
| 1 | (S)-1 99% ee | (R,R)-DIOP | (S)-3, 91%, N1/N2 95:5, E/Z 95:5, 94% ee |
| 2 | (R)-1 90% ee | (R,R)-DIOP | (S)-3, 89%, N1/N2 94:6, E/Z 95:5, 96% ee |
| 3 | (S)-1 99% ee | dppb | rac-3, 87%, N1/N2 88:12, E/Z 95:5 |
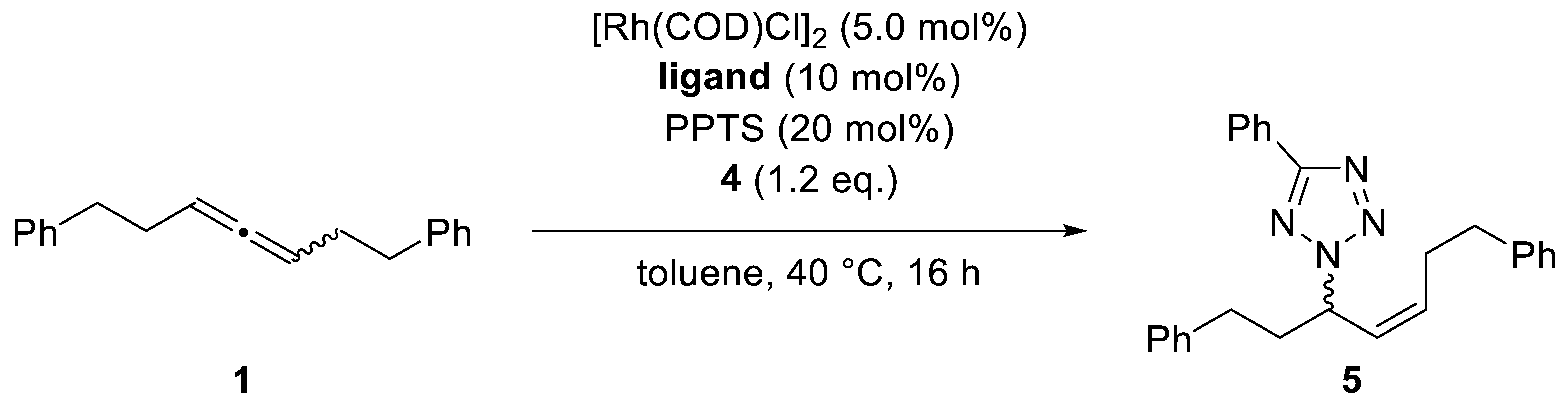 | |||
| # | Allene | Ligand | Product |
| 4 | (S)-1 99% ee | (R,R)-DIOP | (R)-5, 95%, N2/N1 >95:5, Z/E >95:5, 84% ee |
| 5 | (R)-1 90% ee | (R,R)-DIOP | (R)-5, 93%, N2/N1 >95:5, Z/E >95:5, 81% ee |
| 6 | (S)-1 99% ee | dppb | rac-5, 95%, N2/N1 >95:5, Z/E >95:5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sieger, S.V.; Lubins, I.; Breit, B. Rhodium-Catalyzed Dynamic Kinetic Resolution of Racemic Internal Allenes towards Chiral Allylated Triazoles and Tetrazoles. Catalysts 2022, 12, 1209. https://doi.org/10.3390/catal12101209
Sieger SV, Lubins I, Breit B. Rhodium-Catalyzed Dynamic Kinetic Resolution of Racemic Internal Allenes towards Chiral Allylated Triazoles and Tetrazoles. Catalysts. 2022; 12(10):1209. https://doi.org/10.3390/catal12101209
Chicago/Turabian StyleSieger, Simon V., Ilja Lubins, and Bernhard Breit. 2022. "Rhodium-Catalyzed Dynamic Kinetic Resolution of Racemic Internal Allenes towards Chiral Allylated Triazoles and Tetrazoles" Catalysts 12, no. 10: 1209. https://doi.org/10.3390/catal12101209
APA StyleSieger, S. V., Lubins, I., & Breit, B. (2022). Rhodium-Catalyzed Dynamic Kinetic Resolution of Racemic Internal Allenes towards Chiral Allylated Triazoles and Tetrazoles. Catalysts, 12(10), 1209. https://doi.org/10.3390/catal12101209








