Effect of Light and Heavy Rare Earth Doping on the Physical Structure of Bi2O2CO3 and Their Performance in Photocatalytic Degradation of Dimethyl Phthalate
Abstract
:1. Introduction
2. Results and Discussion
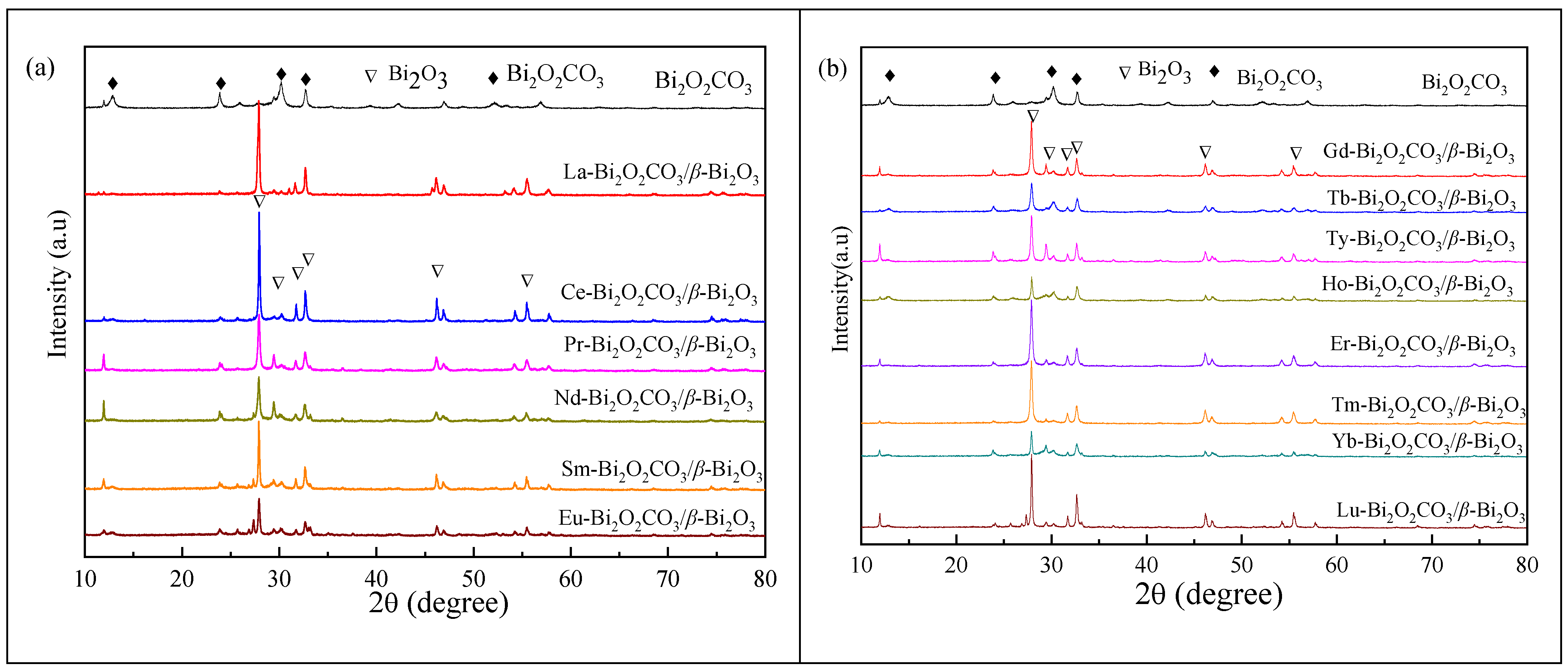
2.1. X-ray Powder Diffraction (XRD) Analysis
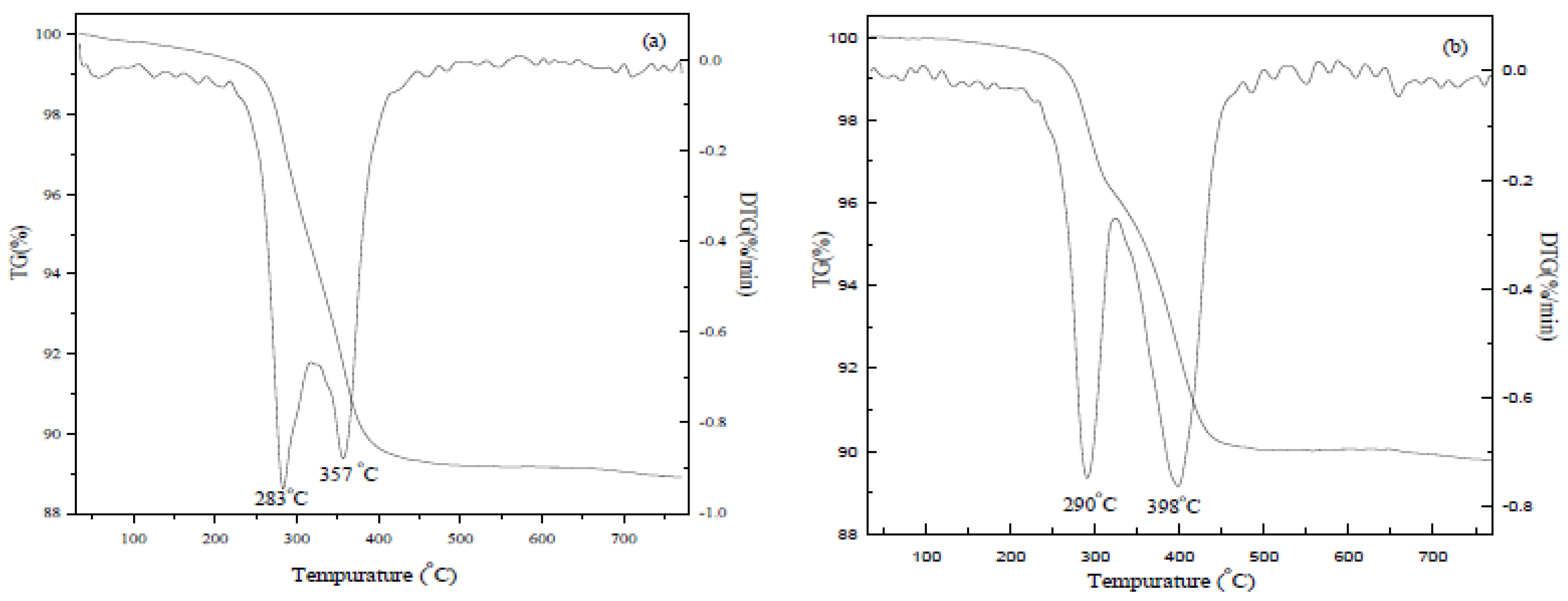
2.2. Thermogravimetric (TG) Analysis
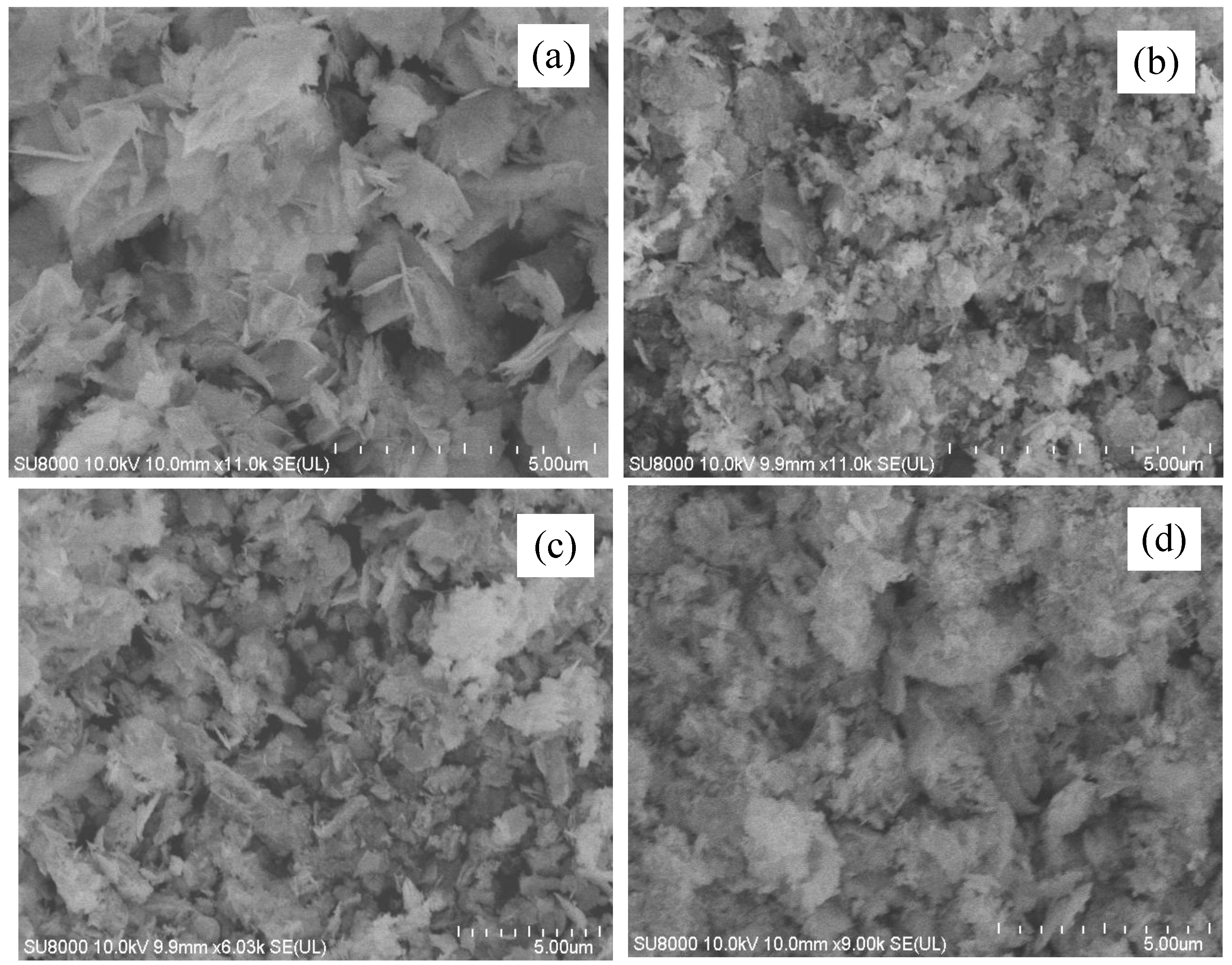
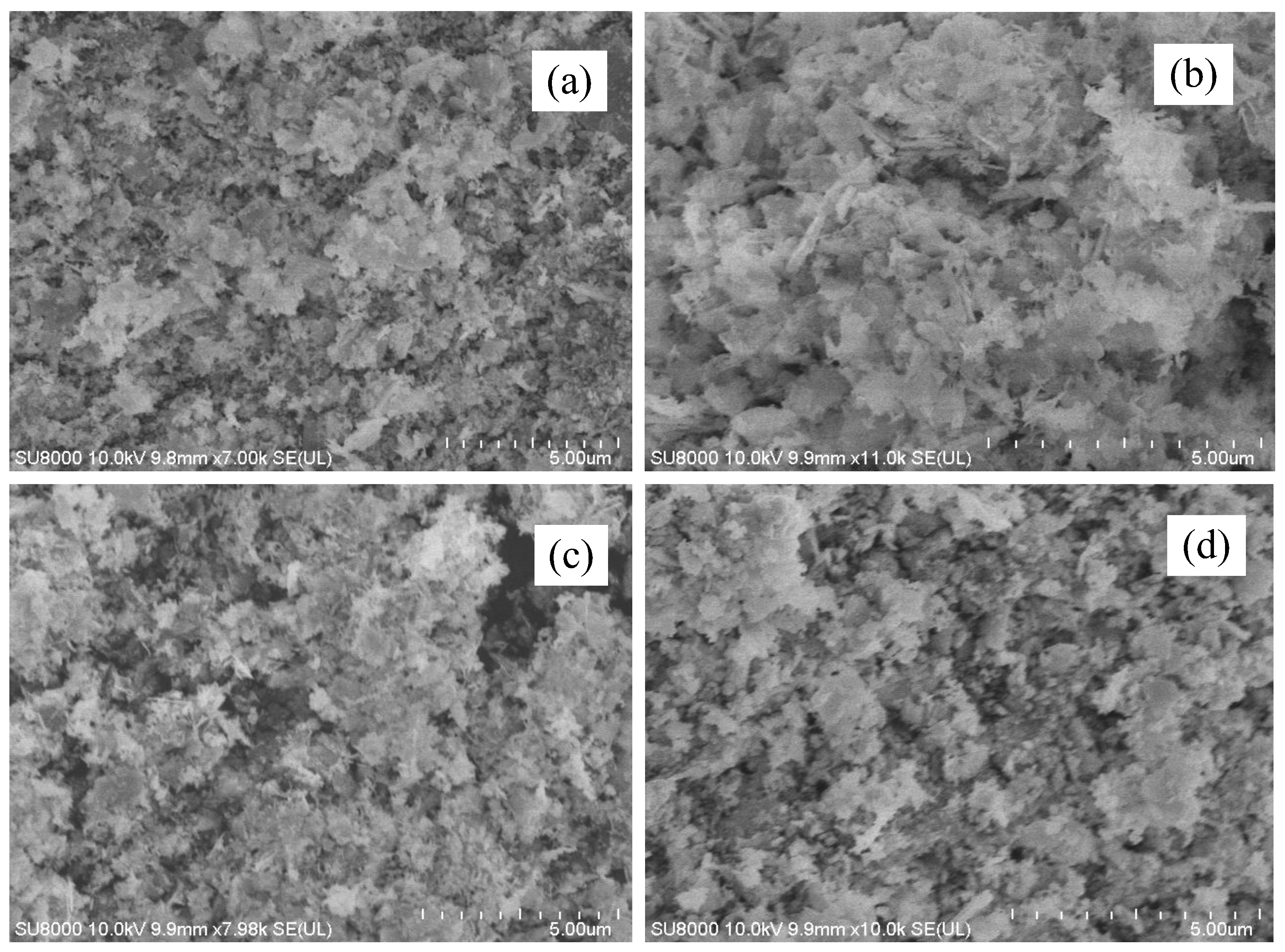
2.3. Scanning Electron Microscope (SEM) Analysis
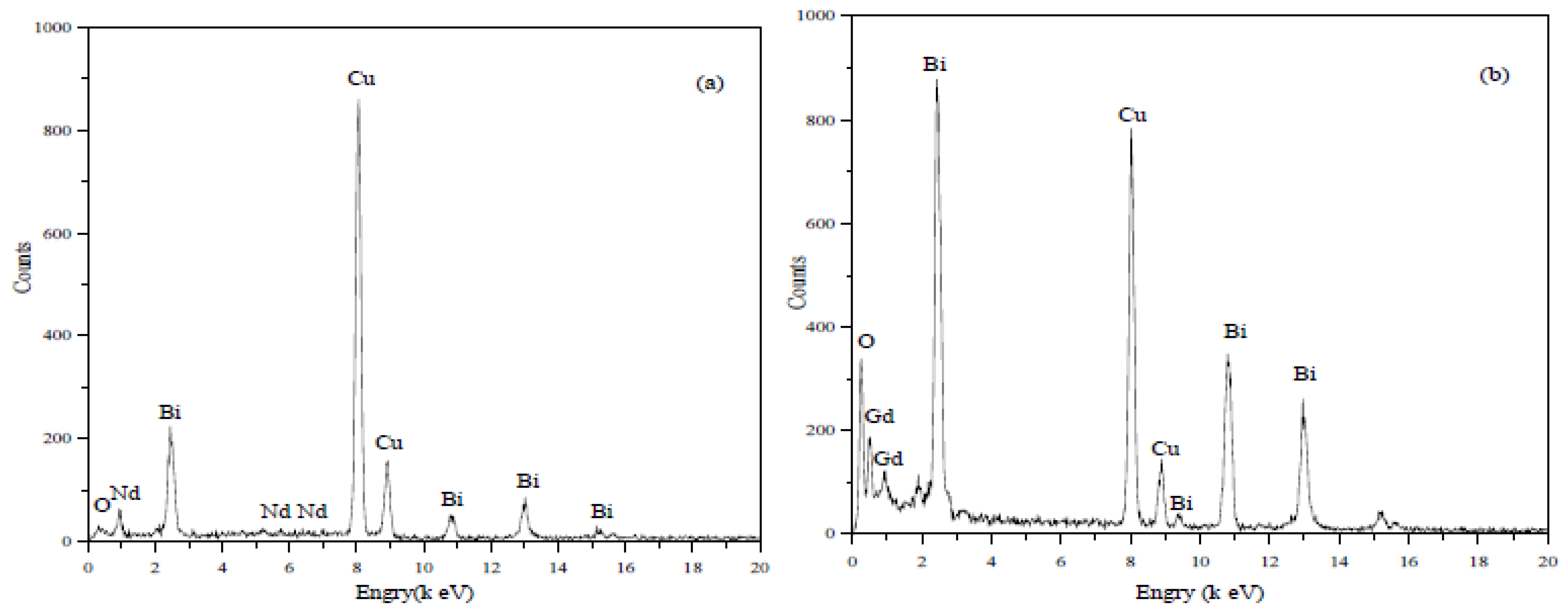
2.4. TEM and EDX Analysis
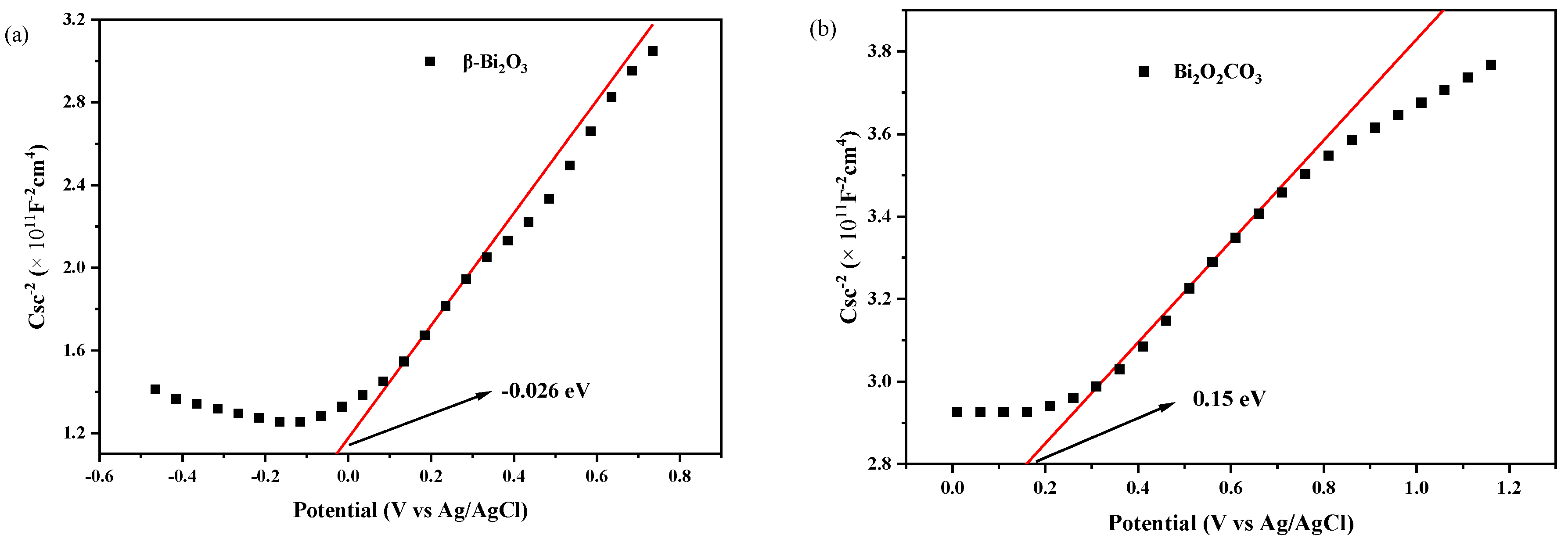
2.5. Light Absorption and Specific Surface Area Analysis
2.6. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
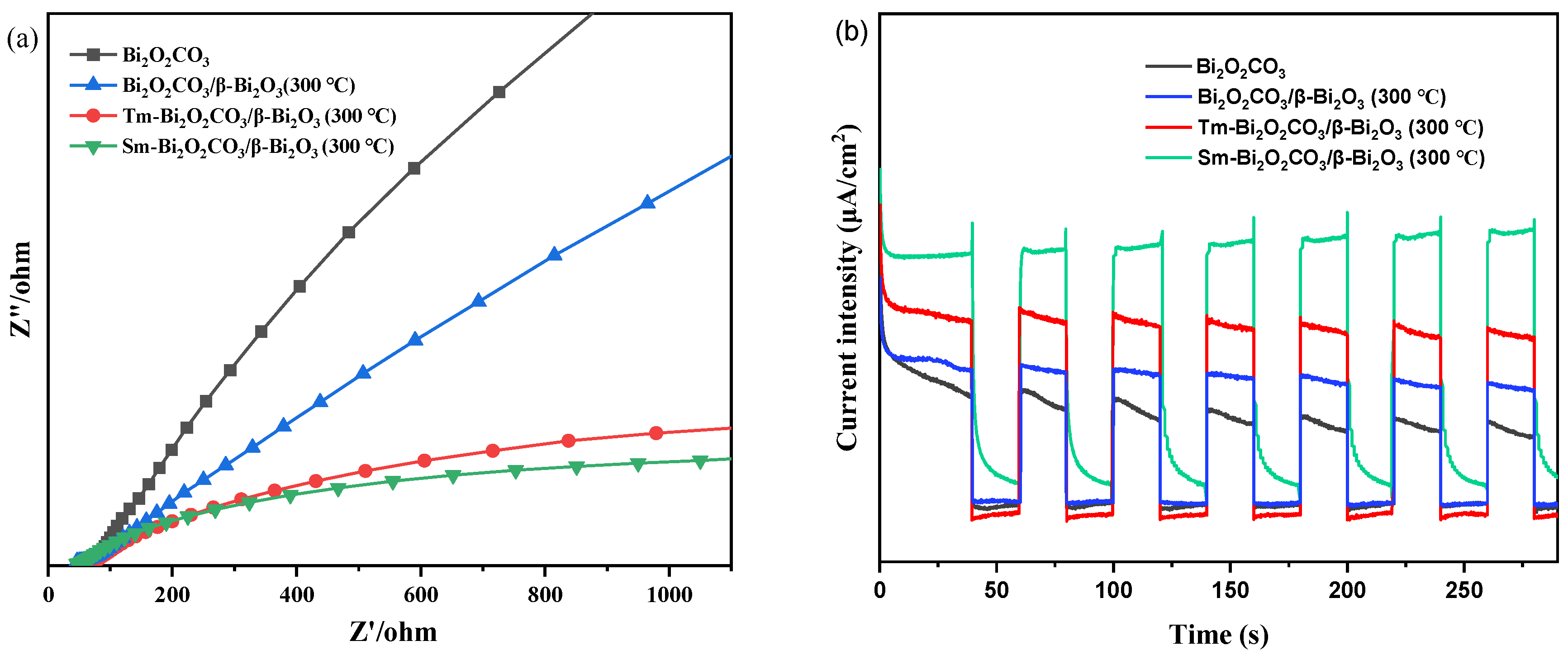
2.7. Photocatalytic Activity
3. Experimental
3.1. Materials
3.2. Sample Synthesis
3.3. Characterization
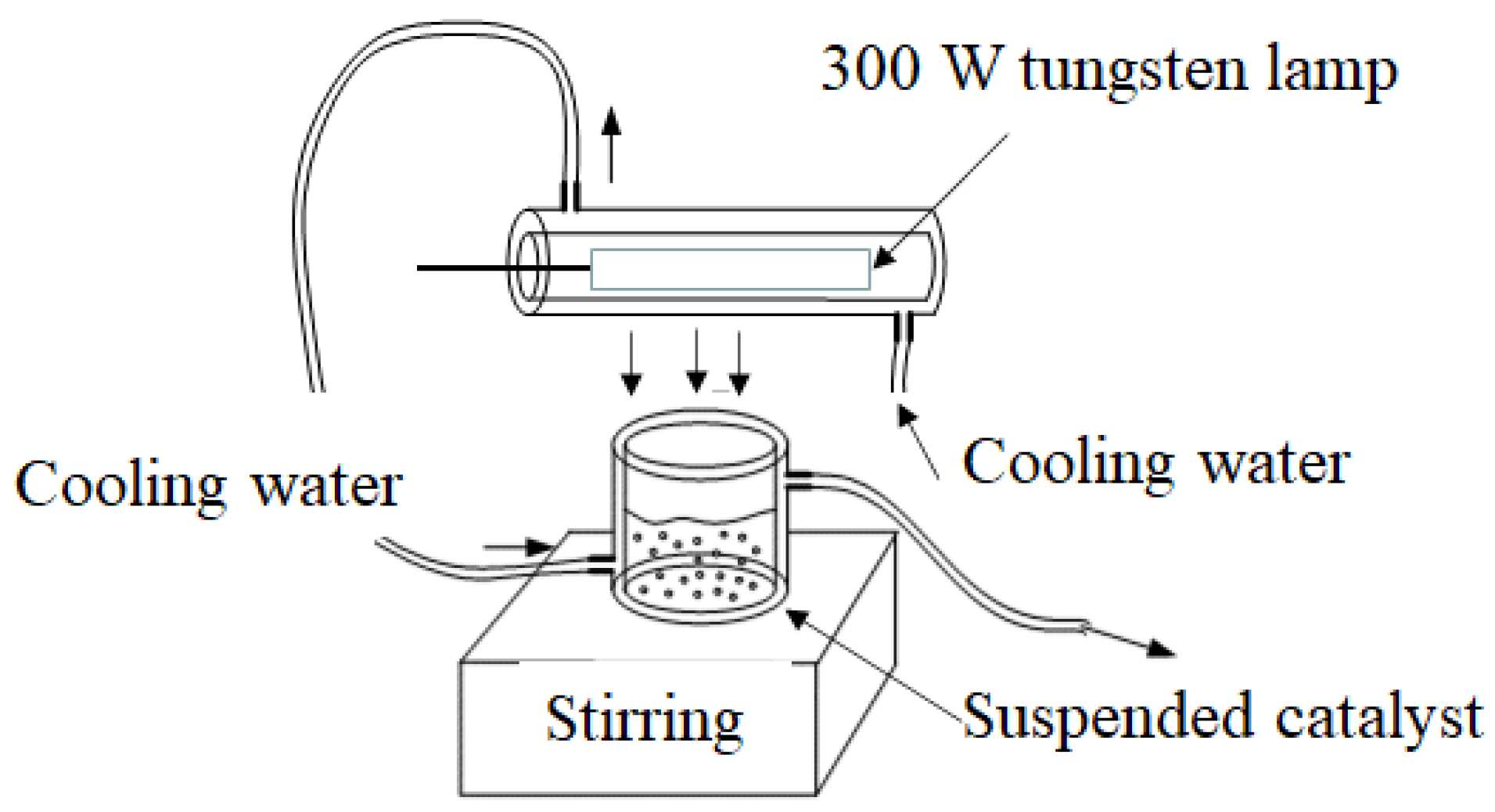
3.4. Photocatalytic Activity Evaluation
3.5. Photoelectrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.P.; Yang, Y.; Li, D.L. Research progress of DEHP pollution in water environment and its removal. Chem. Prog. 2014, 33, 498–504. (In Chinese) [Google Scholar]
- Pang, X.Z.; Skillen, N.; Gunaratne, N.; Rooney, D.W.; Robertson, P.K.J. Removal of phthalates from aqueous solution by semiconductor photocatalysis: A review. J. Hazard. Mater. 2021, 402, 123461–123481. [Google Scholar] [CrossRef]
- Ki, S.J.; Park, Y.-K.; Kim, J.-S.; Lee, W.-J.; Lee, H.; Jung, S.-C. Facile preparation of tungsten oxide doped TiO2 photocatalysts using liquid phase plasma process for enhanced degradation of diethyl phthalate. Chem. Eng. J. 2019, 377, 120087–120093. [Google Scholar] [CrossRef]
- Hung, C.-H.; Yuan, C.; Li, H.-W. Photodegradation of diethyl phthalate with PANi/CNT/TiO2 immobilized on glass plate irradiated with visible light and simulated sunlight—Effect of synthesized method and pH. J. Hazard. Mater. 2017, 322, 243–253. [Google Scholar] [CrossRef]
- He, G.Y.; Zhang, J.H.; Hu, Y.; Bai, Z.G.; Wei, C.H. Dual-template synthesis of mesoporous TiO2 nanotubes with structure-enhanced functional photocatalytic performance. Appl. Catal. B Environ. 2019, 250, 301–312. [Google Scholar] [CrossRef]
- Akbari-Adergani, B.; Saghi, M.H.; Eslami, A.; Mohseni-Bandpei, A.; Rabbani, M. Removal of dibutyl phthalate from aqueous environments using a nanophotocatalytic Fe, Ag-ZnO/VIS-LED system: Modeling and optimization. Environ. Technol. 2018, 39, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Chuai, H.Y.; Zhou, D.F.; Zhu, X.F.; Li, Z.H.; Huang, W.P. Characterization of V2O5/MoO3 composite photocatalysts prepared via electrospinning and their photodegradation activity for dimethyl phthalate. Chin. J. Catal. 2015, 36, 2194–2202. [Google Scholar] [CrossRef]
- Liao, W.C.; Zheng, T.; Wang, P.; Tu, S.S.; Pan, W.Q. Efficient microwave-assisted photocatalytic degradation of endocrine disruptor dimethyl phthalate over composite catalyst ZrOx/ZnO. J. Environ. Sci. 2010, 22, 1800–1806. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, N.; Hu, J.S.; Li, S.; Qin, G.W. Photocatalytic degradation properties of α-Fe2O3 nanoparticles for dibutyl phthalate in aqueous solution system. Roy. Soc. Open Sci. 2018, 5, 172196–172204. [Google Scholar] [CrossRef] [Green Version]
- Shan, W.J.; Hu, Y.; Bai, Z.G.; Zheng, M.M.; Wei, C.H. In situ preparation of g-C3N4/bismuth-based oxide nanocomposites with enhanced photocatalytic activity. Appl. Cataly. B Environ. 2016, 188, 1–12. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.H.; Xi, L.L.; Zhu, G.F.; Li, X.; Shi, D.Y.; Fan, J. Highly efficient photocatalytic removal of multiple refractory organic pollutants by BiVO4/CH3COO(BiO) heterostructured nanocomposite. Sci. Total. Environ. 2019, 647, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Samanta, P.; Dutta, S.; Ghosh, S.K. Fluorescent “Turn-on” Sensing Based on Metal-Organic Frameworks (MOFs). Chem. Asian J. 2019, 14, 4506–4519. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, Y.-M.; Guo, W.W.; Yin, X.-B. Color-tunable lanthanide metal–organic framework gels. Chem. Sci. 2019, 10, 1644–1650. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Lei, B.; Dong, X.; Wang, H.; Sheng, J.P.; Cui, W.; Li, J.Y.; Sun, Y.J.; Wang, Z.M.; Dong, F. Rare-Earth Single-Atom La–N Charge-Transfer Bridge on Carbon Nitride for Highly Efficient and Selective Photocatalytic CO2 Reduction. ACS Nano 2020, 14, 15841–15852. [Google Scholar] [CrossRef]
- Du, J.M.; Zhao, G.Y.; Pang, H.; Qian, Y.T.; Liu, H.Q.; Kang, D.J. A template method for synthesis of porous Sn-doped TiO2 monolith and its enhanced photocatalytic activity. Mater. Lett. 2013, 93, 419–422. [Google Scholar] [CrossRef]
- Li, Y.R.; Kong, T.T.; Shen, S.H. Artificial Photosynthesis with Polymeric Carbon Nitride: When Meeting Metal Nanoparticles, Single Atoms, and Molecular Complexes. Small 2019, 15, 1900772–1900789. [Google Scholar] [CrossRef]
- Tang, T.; Yin, Z.L.; Chen, J.R.; Zhang, S.; Sheng, W.C.; Wei, W.X.; Xiao, Y.G.; Shi, Q.Y.; Cao, S.S. Novel p-n heterojunction Bi2O3/Ti3+-TiO2 photocatalyst enables the complete removal of tetracyclines under visible light. Chem. Eng. J. 2021, 417, 128058. [Google Scholar] [CrossRef]
- Zeng, Z.C.; Xu, Y.S.; Zhang, Z.S.; Gao, Z.S.; Luo, M.; Yin, Z.Y.; Zhang, C.; Xu, J.; Huang, B.L.; Luo, F.; et al. Rare-earth-containing perovskite nanomaterials: Design, synthesis, properties and applications. Chem. Soc. Rev. 2020, 49, 1109–1143. [Google Scholar] [CrossRef]
- Yan, X.-H.; Fang, M.-F.; Qian, Z.-Q.; Liu, Z.-L.; Wang, M.-L.; Tian, C.-P.; Du, M.-H.; Li, Y.-J.; Li, Z.-H.; Zhao, G.-F. Isolation and characterization of polymorphic microsatellites in the perennial herb Euphorbia kansui using paired-end Illumina shotgun sequencing. Conserv. Genet. Resour. 2014, 6, 841–843. [Google Scholar] [CrossRef]
- Yu, C.L.; Wu, Z.; Liu, R.Y.; He, H.B.; Fan, W.H.; Xue, S.S. The effects of Gd3+ doping on the physical structure and photocatalytic performance of Bi2MoO6 nanoplate crystals. J. Phys. Chem. Solids 2016, 93, 7–13. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.Q.; Wei, L.F.; Yu, C.L.; Yi, J.H.; Ji, H.B. Regulate the crystal and optoelectronic properties of Bi2WO6 nanosheet crystals by Sm3+ doping for superior visible-light-driven photocatalytic performance. Appl. Surf. Sci. 2020, 508, 145309–145318. [Google Scholar] [CrossRef]
- Fan, Q.Z.; Chen, X.; Chen, F.Y.; Tian, J.; Yu, C.L.; Liao, C.F. Regulating the stability and bandgap structure of BiOBr during thermo-transformation via La doping. Appl. Surf. Sci. 2019, 481, 564–575. [Google Scholar] [CrossRef]
- Xue, S.S.; He, H.B.; Wu, Z.; Yu, C.L.; Fan, Q.Z.; Peng, G.M.; Yang, K. An interesting Eu,F-codoped BiVO4 microsphere with enhanced photocatalytic performance. J. Alloys Compd. 2017, 694, 989–997. [Google Scholar] [CrossRef]
- Yu, Z.Z.; Yang, K.; Yu, C.L.; Lu, K.Q.; Huang, W.Y.; Xu, L.; Zou, L.X.; Wang, S.B.; Chen, Z.; Hu, J.; et al. Steering Unit Cell Dipole and Internal Electric Field by Highly Dispersed Er atoms Embedded into NiO for Efficient CO2 Photoreduction. Adv. Funct. Mater. 2022, 32, 2111999–2112008. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Y.Y.; Ding, H.M.; Shan, Y.K. Improved structural stability of titanium-doped β-Bi2O3 during visible-light-activated photocatalytic processes. J. Mater. Sci. 2009, 45, 1385–1392. [Google Scholar] [CrossRef]
- Zeng, Y.F.; Qin, Y.Z.; Zhou, Z. Preparation of Y2O3-Bi2O3 Catalyst and Its Visible Light Catalytic Degradation of 2,4-Dichlorophenol. Chem. World 2011, 52, 257–261. (In Chinese) [Google Scholar]
- Muruganandham, M.; Amutha, R.; Lee, G.J.; Hsieh, S.H.; Wu, J.J.; Sillanpää, M. Facile Fabrication of Tunable Bi2O3 Self-Assembly and Its Visible Light Photocatalytic Activity. J. Phys. Chem. C 2012, 116, 12906–12915. [Google Scholar] [CrossRef]
- Liu, X.Q.; Fan, Q.Z.; Li, F.; Wei, L.F.; Yu, C.L. Nd3+-doped β-Bi2O3/Bi2O2CO3 composite nanoplates fabricated via phase transformation and their superior visible light photocatalytic performance for removing phenols and dyes. J. Nanopart. Res. 2021, 23, 233–248. [Google Scholar] [CrossRef]
- Xu, J.J.; Ao, Y.H.; Fu, D.G.; Yuan, C.W. A simple route for the preparation of Eu, N-codoped TiO2 nanoparticles with enhanced visible light-induced photocatalytic activity. J. Colloid Interface Sci. 2008, 328, 447–451. [Google Scholar] [CrossRef]
- Wu, X.H.; Qin, W.; Ding, X.B.; Wen, Y.Y.; Liu, H.L.; Jiang, Z.H. Photocatalytic activity of Eu-doped TiO2 ceramic films prepared by microplasma oxidation method. J. Phys. Chem. Solids 2007, 68, 2387–2393. [Google Scholar]
- Miao, Y.C.; Pan, G.F.; Huo, Y.N.; Li, H.X. In situ rare earth modified Bi2MoO6 efficient visible light catalyst. Acta Inorg. Chem. 2014, 30, 1587–1592. (In Chinese) [Google Scholar]







| Sample | Eg (eV) | SBET (m2/g) | Sample | Eg (eV) | SBET (m2/g) |
|---|---|---|---|---|---|
| Bi2O2CO3 | 3.18 | 5.4 | Gd-Bi2O2CO3/β-Bi2O3 | 2.49 | 16.1 |
| La-Bi2O2CO3/β-Bi2O3 | 2.53 | 11.2 | Tb-Bi2O2CO3/β-Bi2O3 | 2.52 | 15.2 |
| Ce-Bi2O2CO3/β-Bi2O3 | 2.42 | 10.9 | Dy-Bi2O2CO3/β-Bi2O3 | 2.50 | 16.8 |
| Pr-Bi2O2CO3/β-Bi2O3 | 2.48 | 11.1 | Ho-Bi2O2CO3/β-Bi2O3 | 2.56 | 17.3 |
| Nd-Bi2O2CO3/β-Bi2O3 | 2.57 | 14.2 | Er-Bi2O2CO3/β-Bi2O3 | 2.54 | 14.6 |
| Sm-Bi2O2CO3/β-Bi2O3 | 2.42 | 13.6 | Tm-Bi2O2CO3/β-Bi2O3 | 2.48 | 15.3 |
| Eu-Bi2O2CO3/β-Bi2O3 | 2.57 | 14.5 | Yb-Bi2O2CO3/β-Bi2O3 | 2.47 | 14.9 |
| Lu-Bi2O2CO3/β-Bi2O3 | 2.44 | 13.9 |
| Sample | D (%) | R (%) | Sample | D (%) | R (%) |
|---|---|---|---|---|---|
| Bi2O2CO3 | 17 | 14 | Gd-Bi2O2CO3/β-Bi2O3 | 69 | 62 |
| La-Bi2O2CO3/β-Bi2O3 | 35 | 30 | Tb-Bi2O2CO3/β-Bi2O3 | 40 | 34 |
| Ce-Bi2O2CO3/β-Bi2O3 | 38 | 34 | Dy-Bi2O2CO3/β-Bi2O3 | 52 | 47 |
| Pr-Bi2O2CO3/β-Bi2O3 | 66 | 59 | Ho-Bi2O2CO3/β-Bi2O3 | 35 | 32 |
| Nd-Bi2O2CO3/β-Bi2O3 | 72 | 67 | Er-Bi2O2CO3/β-Bi2O3 | 78 | 69 |
| Sm-Bi2O2CO3/β-Bi2O3 | 53 | 48 | Tm-Bi2O2CO3/β-Bi2O3 | 46 | 41 |
| Eu-Bi2O2CO3/β-Bi2O3 | 40 | 31 | Yb-Bi2O2CO3/β-Bi2O3 | 57 | 49 |
| Lu-Bi2O2CO3/β-Bi2O3 | 30 | 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Liu, X.; Li, F.; Li, F.; Tao, L.; Yu, C. Effect of Light and Heavy Rare Earth Doping on the Physical Structure of Bi2O2CO3 and Their Performance in Photocatalytic Degradation of Dimethyl Phthalate. Catalysts 2022, 12, 1295. https://doi.org/10.3390/catal12111295
He Q, Liu X, Li F, Li F, Tao L, Yu C. Effect of Light and Heavy Rare Earth Doping on the Physical Structure of Bi2O2CO3 and Their Performance in Photocatalytic Degradation of Dimethyl Phthalate. Catalysts. 2022; 12(11):1295. https://doi.org/10.3390/catal12111295
Chicago/Turabian StyleHe, Qingyun, Xingqiang Liu, Feng Li, Fang Li, Leiming Tao, and Changlin Yu. 2022. "Effect of Light and Heavy Rare Earth Doping on the Physical Structure of Bi2O2CO3 and Their Performance in Photocatalytic Degradation of Dimethyl Phthalate" Catalysts 12, no. 11: 1295. https://doi.org/10.3390/catal12111295




