Graphene Oxide Decorated with Ag and CeO2 Nanoparticles as a Catalyst for Room-Temperature 4-Nitrophenol Reduction
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Support
2.1.1. N2 Sorption
2.1.2. XRD
2.1.3. TGA
2.2. Characterization of Catalysts
2.2.1. XRD
2.2.2. XPS
2.2.3. TGA
2.2.4. Raman Spectroscopy
2.2.5. UV–VIS Spectrometry
2.3. Catalytic Experiment
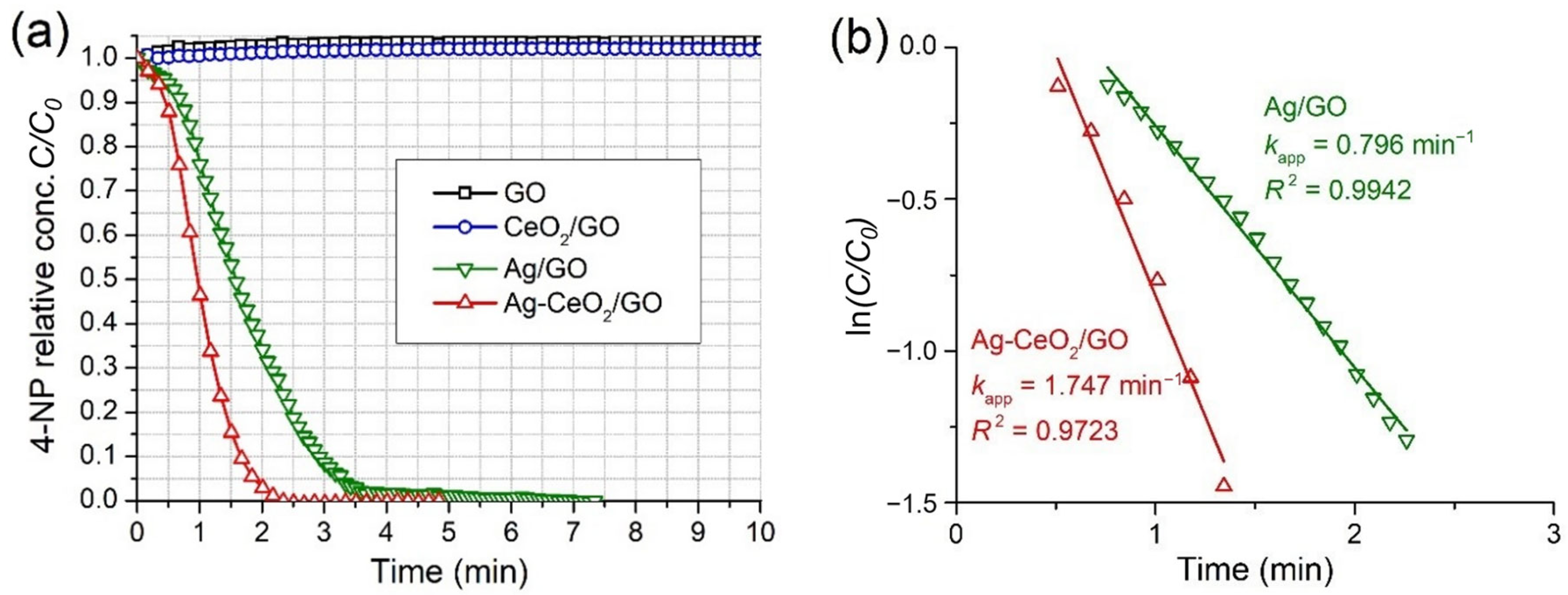
2.3.1. Activity of As-prepared Catalysts
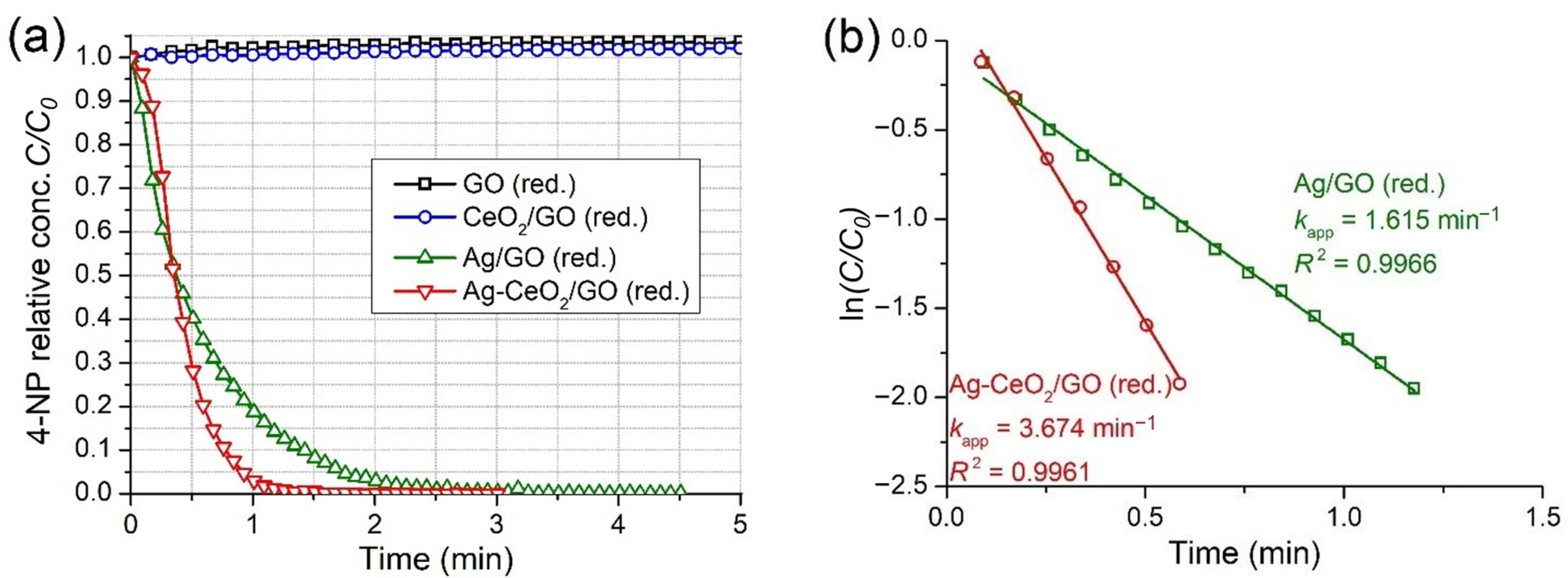
2.3.2. Pre-Reduced Catalysts
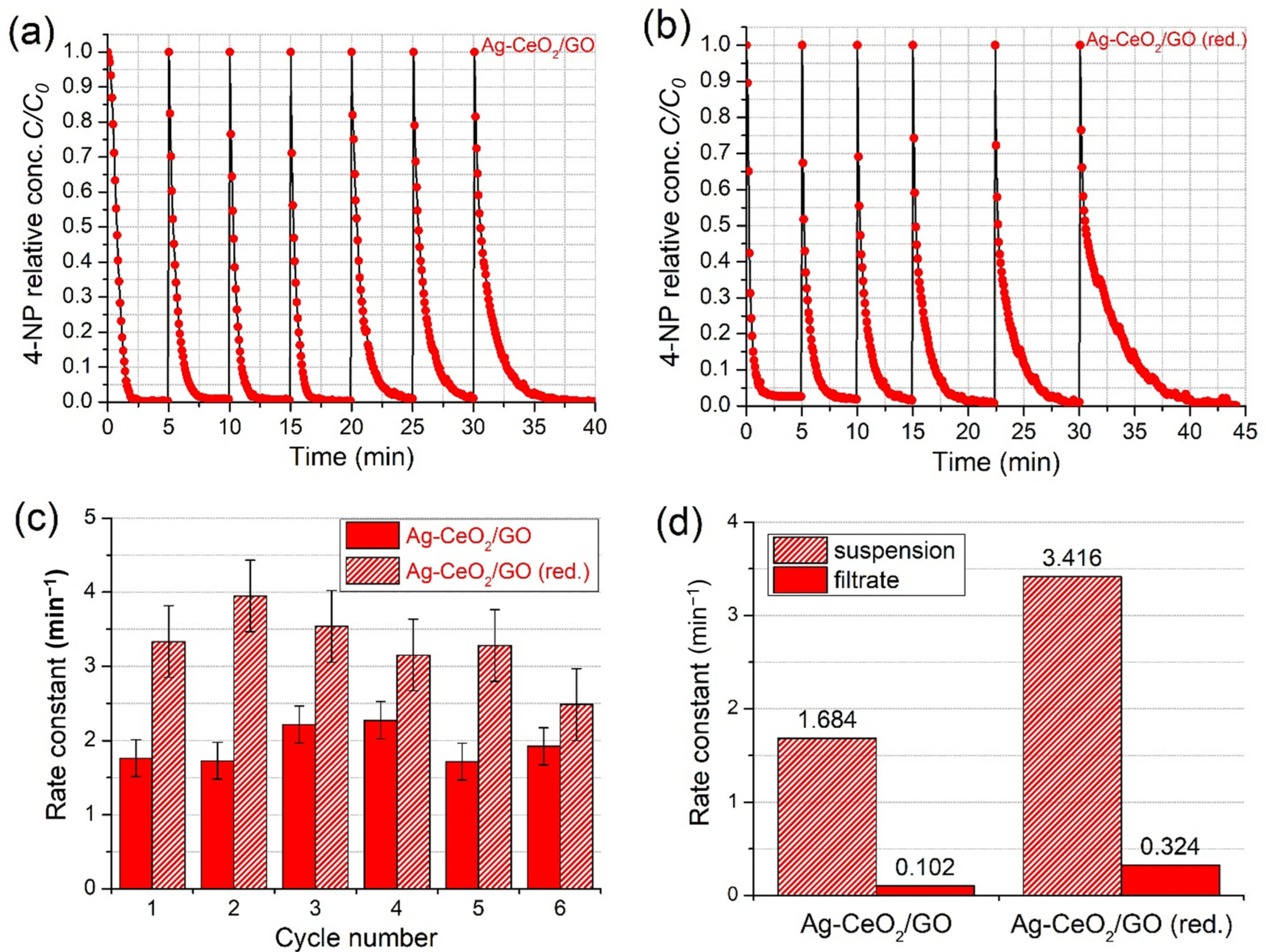
2.3.3. Stability and Leaching Tests
3. Materials and Methods
3.1. Synthesis of Graphene Oxide
3.2. Synthesis of Catalysts
3.3. Characterization of Samples
3.4. Catalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ju, K.-S.; Parales, R.E. Nitroaromatic Compounds, from Synthesis to Biodegradation. Microbiol. Mol. Biol. Rev. 2010, 74, 250–272. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Mishra, K.; Ramanthan, G. Bioremediation of Nitroaromatic Compounds. In Wastewater Treatment Engineering; Samer, M., Ed.; IntechOpen: London, UK, 2015; pp. 51–83. [Google Scholar] [CrossRef]
- Králik, M.; Turáková, M.; Mačák, I.; Lehocký, P. Aniline-catalysis and chemical engineering. In Proceedings of the 41st International Conference of Slovak Society of Chemical Engineering, Tatranské Matliare, Slovakia, 26–30 May 2014; pp. 723–733. [Google Scholar]
- Saha, B.; De, S.; Dutta, S. Recent Advancements of Replacing Existing Aniline Production Process with Environmentally Friendly One-Pot Process: An Overview. Crit. Rev. Env. Sci. Technol. 2013, 43, 84–120. [Google Scholar] [CrossRef]
- Couto, C.S.; Madeira, L.M.; Nunes, C.P.; Araújo, P. Commercial Catalysts Screening for Liquid Phase Nitrobenzene Hydrogenation. Appl. Catal. A: Gen. 2016, 522, 152–164. [Google Scholar] [CrossRef]
- Fishbein, L. Aromatic Amines. In Anthropogenic Compounds. The Handbook of Environmental Chemistry; Hutzinger, O., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 3/3C. [Google Scholar] [CrossRef]
- Samer, M. Biological and Chemical Wastewater Treatment Processes. In Wastewater Treatment Engineering; Samer, M., Ed.; IntechOpen: London, UK, 2015; pp. 1–50. [Google Scholar] [CrossRef]
- Kumar, B. Graphene- and Graphene Oxide-Bounded Metal Nanocomposite for Remediation of Organic Pollutants. In Carbon-Based Material for Environmental Protection and Remediation; Bartoli, M., Frediani, M., Rosi, L., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Zhang, K.; Suh, J.M.; Choi, J.-W.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Recent Advances in the Nanocatalyst-Assisted NaBH4 Reduction of Nitroaromatics in Water. ACS Omega 2019, 4, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, M.; Brenna, D.; Harms, R.; Jost, S.; Benaglia, M. Recent Developments in the Reduction of Aromatic and Aliphatic Nitro Compounds to Amines. Org. Process Res. Dev. 2018, 22, 430–445. [Google Scholar] [CrossRef]
- Zang, W.; Li, G.; Wang, L.; Zhang, X. Catalytic hydrogenation by noble-metal nanocrystals with well-defined facets: A review. Catal. Sci. Technol. 2015, 5, 2532–2553. [Google Scholar] [CrossRef]
- Torbina, V.V.; Vodyankin, A.A.; Ten, S.; Mamontov, G.V.; Salaev, M.A.; Sobolev, V.I.; Vodyankina, O.V. Ag-Based Catalysts in Heterogeneous Selective Oxidation of Alcohols: A Review. Catalysts 2018, 8, 447. [Google Scholar] [CrossRef]
- Vodyankina, O.V.; Mamontov, G.V.; Dutov, V.V.; Kharlamova, T.S.; Salaev, M.A. Ag-Containing Nanomaterials in Heterogeneous Catalysis: Advances and Recent Trends. In Advanced Nanomaterials for Catalysis and Energy: Synthesis, Characterization and Applications; Sadykov, V.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 143–175. [Google Scholar] [CrossRef]
- Wen, C.; Yin, A.; Dai, W.-L. Recent advances in silver-based heterogeneous catalysts for green chemistry processes. Appl. Catal. B: Environ. 2014, 160–161, 730–741. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhong, L.; Fang, J.; Zhang, L.; Xu, Z.; Gao, H.; Fang, B. Unlocking the door to highly efficient Ag-based nanoparticles catalysts for NaBH4-assisted nitrophenol reduction. Nano Res. 2019, 12, 2407–2436. [Google Scholar] [CrossRef]
- Pradhan, N.; Pal, A.; Pal, T. Silver nanoparticle catalyzed reduction of aromatic nitro compounds. Colloids Surf. A: Physicochem. Eng. Aspects 2002, 196, 247–257. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Salaev, M.A.; Salaeva, A.A.; Kharlamova, T.S.; Mamontov, G.V. Pt–CeO2-based composites in environmental catalysis: A review. Appl. Catal. B: Environ. 2021, 295, 120286. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mamontov, G.V.; Zaikovskii, V.I.; La Parola, V.; Liotta, L.F.; Vodyankina, O.V. The role of metal–support interaction in Ag/CeO2 catalysts for CO and soot oxidation. Appl. Catal. B: Environ. 2020, 260, 118148. [Google Scholar] [CrossRef]
- Kong, D.; Wang, G.; Pan, Y.; Hu, S.; Hou, J.; Pan, H.; Campbell, C.T.; Zhu, J. Growth, Structure, and Stability of Ag on CeO2(111): Synchrotron Radiation Photoemission Studies. J. Phys. Chem. C 2011, 115, 6715–6725. [Google Scholar] [CrossRef]
- Mamontov, G.V.; Grabchenko, M.V.; Sobolev, V.I.; Zaikovskii, V.I.; Vodyankina, O.V. Ethanol dehydrogenation over Ag-CeO2/SiO2 catalyst: Role of Ag-CeO2 interface. Appl. Catal. A: General 2016, 528, 161–167. [Google Scholar] [CrossRef]
- Mikheeva, N.N.; Zaikovskii, V.I.; Larichev, Y.V.; Mamontov, G.V. Toluene abatement on Ag-CeO2/SBA-15 catalysts: Synergistic effect of silver and ceria. Mater. Today Chem. 2021, 21, 100530. [Google Scholar] [CrossRef]
- Ou, C.-C.; Chen, C.-H.; Chan, T.-S.; Chen, C.-S.; Cheng, S. Influence of pretreatment on the catalytic performance of Ag/CeO2 for formaldehyde removal at low temperature. J. Catal. 2019, 380, 43–54. [Google Scholar] [CrossRef]
- Taratayko, A.; Larichev, Y.; Zaikovskii, V.; Mikheeva, N.; Mamontov, G. Ag-CeO2/SBA-15 composite prepared from Pluronic P123@SBA-15 hybrid as catalyst for room-temperature reduction of 4-nitrophenol. Catal. Today 2021, 375, 576–584. [Google Scholar] [CrossRef]
- Qian, X.; Kuwahara, Y.; Mori, K.; Yamashita, H. Silver Nanoparticles Supported on CeO2-SBA-15 by Microwave Irradiation Possess Metal–Support Interactions and Enhanced Catalytic Activity. Chem. Eur. J. 2014, 20, 15746–15752. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Plasmonic catalysis of Ag nanoparticles deposited on CeO2 modified mesoporous silica for the nitrostyrene reduction under light irradiation conditions. Catal. Today 2019, 324, 83–89. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S. Graphene-Based Nanocomposites. In Physics and Applications of Graphene–Experiments; Mikhailov, S., Ed.; IntechOpen: London, UK, 2011; pp. 135–168. [Google Scholar] [CrossRef]
- Obodo, R.M.; Ahmad, I.; Ezema, F.I. Introductory Chapter: Graphene and Its Applications. In Graphene and Its Derivatives-Synthesis and Applications; Ahmad, I., Ezema, F.I., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Yusuf, M.; Kumar, M.; Khan, M.A.; Sillanpa, M.; Arafat, H. A review on exfoliation, characterization, environmental and energy applications of graphene and graphene-based composites. Adv. Colloid Interface Sci. 2019, 273, 102036. [Google Scholar] [CrossRef]
- Prasad, C.; Liu, Q.; Tang, H.; Yuvaraja, G.; Long, J.; Rammohan, A.; Zyryanov, G.V. An overview of graphene oxide supported semiconductors based photocatalysts: Properties, synthesis and photocatalytic applications. J. Mol. Liq. 2020, 297, 111826. [Google Scholar] [CrossRef]
- Shen, Y.; Fang, Q.; Chen, B. Environmental Applications of Three-Dimensional Graphene-Based Macrostructures: Adsorption, Transformation, and Detection. Environ. Sci. Technol. 2014, 49, 67–84. [Google Scholar] [CrossRef]
- Paramasivan, T.; Sivarajasekar, N.; Muthusaravanan, S.; Subashini, R.; Prakashmaran, J.; Sivamani, S.; Ajmal Koya, P. Graphene Family Materials for the Removal of Pesticides from Water. In A New Generation Material Graphene: Applications in Water Technology; Naushad, M., Ed.; Springer: Berlin, Germany, 2019; pp. 309–327. [Google Scholar] [CrossRef]
- Jimenez-Cervantes, E.; López-Barroso, J.; Martínez-Hernández, A.L.; Velasco-Santos, C. Graphene-Based Materials Functionalization with Natural Polymeric Biomolecules. In Recent Advances in Graphene Research; Nayak, P.K., Ed.; IntechOpen: London, UK, 2016; pp. 257–298. [Google Scholar] [CrossRef]
- Gemeay, A.; El-Halwagy, M. Immobilization Impact of Photocatalysts onto Graphene Oxide. In Graphene Oxide-Applications and Opportunities; Kamble, G., Ed.; IntechOpen: London, UK, 2018; pp. 107–126. [Google Scholar] [CrossRef]
- Romero, U.A.M.; Soto, M.Á.V.; Jiménez, L.L.; Quintana, J.Á.; García, S.A.P. Graphene Derivatives: Controlled Properties, Nanocomposites, and Energy Harvesting Applications. In Graphene Materials-Structure, Properties and Modifications; Kyzas, G.Z., Mitropoulos, A., Eds.; IntechOpen: London, UK, 2017; pp. 77–96. [Google Scholar] [CrossRef]
- Ji, Z.; Shen, X.; Yang, J.; Zhu, G.; Chen, K. A novel reduced graphene oxide/Ag/CeO2 ternary nanocomposite: Green synthesis and catalytic properties. Appl. Catal. B: Environ. 2014, 144, 454–461. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, C.; Yin, Z. Reduced graphene oxide decorated with Ag/CeO2 nanocomposite towards room temperature photocatalytic esterification of aldehydes. Mater. Lett. 2020, 270, 127723. [Google Scholar] [CrossRef]
- Tao, X.; Zhou, Y.; Xu, K.; Wu, Y.; Mi, J.; Li, Y.; Liu, Q.; Cheng, X.; Zhao, N.; Shi, H.; et al. Bifunctional Material with Organic Pollutant Removing and Antimicrobial Properties: Graphene Aerogel Decorated with Highly Dispersed Ag and CeO2 Nanoparticles. ACS Sustainable Chem. Eng. 2018, 6, 16907–16919. [Google Scholar] [CrossRef]
- Mardani, C.; Rizal, M.Y.; Saleh, R.; Taufik, A.; Yin, S. Synthesis and characterization of Ag/CeO2/graphene nanocomposites as catalysts for water-pollution treatment. Appl. Surf. Sci. 2020, 530, 147297. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Huh, S.H. Thermal Reduction of Graphene Oxide. In Physics and Applications of Graphene–Experiments; Mikhailov, S., Ed.; IntechOpen: London, UK, 2011; pp. 73–90. [Google Scholar] [CrossRef]
- Ossonon, B.D.; Bélanger, D. Synthesis and characterization of sulfophenyl-functionalized reduced graphene oxide sheet. RSC Adv. 2017, 7, 27224–27234. [Google Scholar] [CrossRef]
- He, Y.; Zhang, N.; Wu, F.; Xu, F.; Liu, Y.; Gao, J. Graphene oxide foams and their excellent adsorption ability for acetone gas. Mater. Res. Bull. 2013, 48, 3553–3558. [Google Scholar] [CrossRef]
- Verma, R.; Samdarshi, S.K. In-Situ Decorated Optimized CeO2 on Reduced Graphene Oxide with Enhanced Adsorptivity and Visible Light Photocatalytic Stability and Reusability. J. Phys. Chem. C 2016, 120, 22281–22290. [Google Scholar] [CrossRef]
- Hui, K.S.; Hui, K.N.; Dinh, D.A.; Tsang, C.H.; Cho, Y.R.; Zhou, W.; Hong, X.; Chun, H.-H. Green synthesis of dimension-controlled silver nanoparticle–graphene oxide with in situ ultrasonication. Acta Mater. 2014, 64, 326–332. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Ferraria, A.M.; Carapeto, A.P.; Botelho do Rego, A.M. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Khairy, M.; Mohamed, M.M.; Ibrahem, A. Enhanced the Catalytic activity of reduction of 4-nitrophenol on Ag/RGO nanocomposites. J. Bas. Environ. Sci. 2018, 5, 101–114. [Google Scholar]
- Bîru, E.I.; Iovu, H. Graphene Nanocomposites Studied by Raman Spectroscopy. In Raman Spectroscopy; Do Nascimento, G.M., Ed.; IntechOpen: London, UK, 2018; pp. 179–201. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Wu, X.; Jaatinen, E.; Sarina, S.; Zhu, H.Y. Direct photocatalysis of supported metal nanostructures for organic synthesis. J. Phys. D: Appl. Phys. 2017, 50, 283001. [Google Scholar] [CrossRef]
- Anandkumar, M.; Vinothkumar, G.; Babu, K.S. Synergistic effect of gold supported on redox active cerium oxide nanoparticles for the catalytic hydrogenation of 4-nitrophenol. New J. Chem. 2017, 41, 6720–6729. [Google Scholar] [CrossRef]
- Strachan, J.; Barnett, C.; Masters, A.F.; Maschmeyer, T. 4-Nitrophenol Reduction: Probing the Putative Mechanism of the Model Reaction. ACS Catal. 2020, 10, 5516–5521. [Google Scholar] [CrossRef]
- Karuppusamy, S.; Marken, F.; Kulandainathan, M.A. Role of Dissolved Oxygen in Nitroarene Reduction Catalyzed by a Heterogeneous Silver Textile Catalyst in Water. New J. Chem. 2020, 44, 17780–17790. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic Analysis of Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles Immobilized in Spherical Polyelectrolyte Brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, L.; Duan, Y.; An, J.; Luo, Q.; Zhang, Y.; Tang, Y.; Huang, J.; Zhang, B.; Liu, J.; et al. A novel strategy to construct supported silver nanocomposite as an ultrahigh efficient catalyst. Appl. Catal. B: Environ. 2021, 283, 119592. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, X.; Zhu, Y.; Tan, H.; Chen, X.; Lu, Z.-H. Core-shell structured nanocomposites Ag@CeO2 as catalyst for hydrogenation of 4-nitrophenol and 2-nitroaniline. RSC Adv. 2016, 6, 47966–47973. [Google Scholar] [CrossRef]
- Chernykh, M.V.; Mikheeva, N.N.; Zaikovskii, V.I.; Mamontov, G.V. Influence of the Ag Content on the Activity of Ag/CeO2 Catalysts in the Reduction of 4-Nitrophenol at Room Temperature and Atmospheric Pressure. Kinet. Catal. 2020, 61, 708–715. [Google Scholar] [CrossRef]
- Kharlamova, T.S.; Salina, M.V.; Svetlichnyi, V.A.; Salaev, M.A.; Stadnichenko, A.I.; Mamontov, G.V. CeO2-supported Pt–Ag bimetallic catalysts for 4-nitrophenol reduction. Catal. Today 2022, 384–386, 12–24. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Gholami, M.R. MxNi100-x (M = Ag, and Co) Nanoparticles Supported on CeO2 Nanorods Derived from Ce-Metal Organic Frameworks as an Effective Catalyst for Reduction of Organic Pollutants: Langmuir-Hinshelwood Kinetics and Mechanism. New J. Chem. 2017, 41, 10948–10958. [Google Scholar] [CrossRef]
- Lei, G.; Ma, J.; Li, Z.; Fan, X.; Peng, W.; Zhang, G.; Zhang, F.; Li, Y. Magnetic Au-Ag-γ-Fe2O3/rGO Nanocomposites as an Efficient Catalyst for the Reduction of 4-Nitrophenol. Nanomaterials 2018, 8, 877. [Google Scholar] [CrossRef]
- Qu, J.; Ren, C.; Dong, Y.; Chang, Y.; Zhou, M.; Chen, X. Facile synthesis of multifunctional graphene oxide/AgNPs-Fe3O4 nanocomposite: A highly integrated catalysts. Chem. Eng. J. 2012, 211–212, 412–420. [Google Scholar] [CrossRef]
- Joshi, M.K.; Pant, H.R.; Kim, H.J.; Kim, J.H.; Kim, C.S. One-pot synthesis of Ag-iron oxide/reduced graphene oxide nanocomposite via hydrothermal treatment. Colloids Surf. A 2014, 446, 102–108. [Google Scholar] [CrossRef]
- Varshney, S.; Bar-Ziv, R.; Zidki, T. On the Remarkable Performance of Silver-based Alloy Nanoparticles in 4-nitrophenol Catalytic Reduction. ChemCatChem 2020, 12, 4680–4688. [Google Scholar] [CrossRef]
- Li, W.; Ge, X.; Zhang, H.; Ding, Q.; Ding, H.; Zhang, Y.; Wang, G.; Zhang, H.; Zhao, H. Hollow Mesoporous SiO2 Spheres Nanoarchitecture with Encapsulated Silver Nanoparticles for Catalytic Reduction of 4-nitrophenol. Inorg. Chem. Front. 2016, 3, 663–670. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, X.-L.; Feng, G.; Chen, X.; Lu, Z.-H. Ag-SiO2 nanocomposites with plum-pudding structure as catalyst for hydrogenation of 4-nitrophenol. Ceram. Int. 2015, 41, 14660–14667. [Google Scholar] [CrossRef]
- Xiao, Z.-Y.; Huang, S.-X.; Zhai, S.-R.; Zhai, B.; Zhang, F.; An, Q.-D. PMHS-reduced fabrication of hollow Ag–SiO2 composite spheres with developed porosity. J. Sol-Gel Sci. Technol. 2015, 75, 82–89. [Google Scholar] [CrossRef]
- Bano, M.; Ahirwar, D.; Thomas, M.; Naiko, G.A.; Sheikh, M.U.D.; Khan, F. Hierarchical synthesis of silver monoliths and their efficient catalytic activity for the reduction of 4-Nitrophenol to 4-Aminophenol. New J. Chem. 2016, 40, 6787–6795. [Google Scholar] [CrossRef]
- Soysal, F.; Çıplak, Z.; Gökalp, C.; Getiren, B.; Yildiz, N. One-step hydrothermal synthesis of nitrogen doped reduced graphene oxide-silver nanocomposites: Catalytic performance. Appl. Organomet. Chem. 2020, 34, e5621. [Google Scholar] [CrossRef]
- Duan, C.; Liu, C.; Meng, X.; Lu, W.; Ni, Y. Fabrication of carboxymethylated cellulose fibers supporting Ag NPs@MOF-199s nanocatalysts for catalytic reduction of 4-nitrophenol. Appl. Organomet. Chem. 2019, 33, e4865. [Google Scholar] [CrossRef]
- Chang, S.; Liu, C.; Sun, Y.; Yan, Z.; Zhang, X.; Hu, X.; Zhang, H. Fe3O4 Nanoparticles Coated with Ag-Nanoparticle-Embedded Metal−Organic Framework MIL-100(Fe) for the Catalytic Reduction of 4-Nitrophenol. ACS Appl. Nano Mater. 2020, 3, 2302–2309. [Google Scholar] [CrossRef]
- Liu, G.-F.; Qiao, X.-X.; Cai, Y.-L.; Xu, J.-Y.; Yan, Y.; Karadeniz, B.; Lu, J.; Cao, R. Aluminum Metal−Organic Framework−Silver Nanoparticle Composites for Catalytic Reduction of Nitrophenols. ACS Appl. Nano Mater. 2020, 3, 11426–11433. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P. A review: Hydrogen generation from borohydride hydrolysis reaction. J. Power Sources 2009, 187, 527–534. [Google Scholar] [CrossRef]
- Pinheiro, D.; Devi, K.R.S.; Jose, A.; Karthik, K.; Sugunan, S.; Mohan, M.K. Experimental Design for Optimization of 4-Nitrophenol Reduction by Green Synthesized CeO2/g-C3N4/Ag Catalyst Using Response Surface Methodology. J. Rare Earths 2020, 38, 1171–1177. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
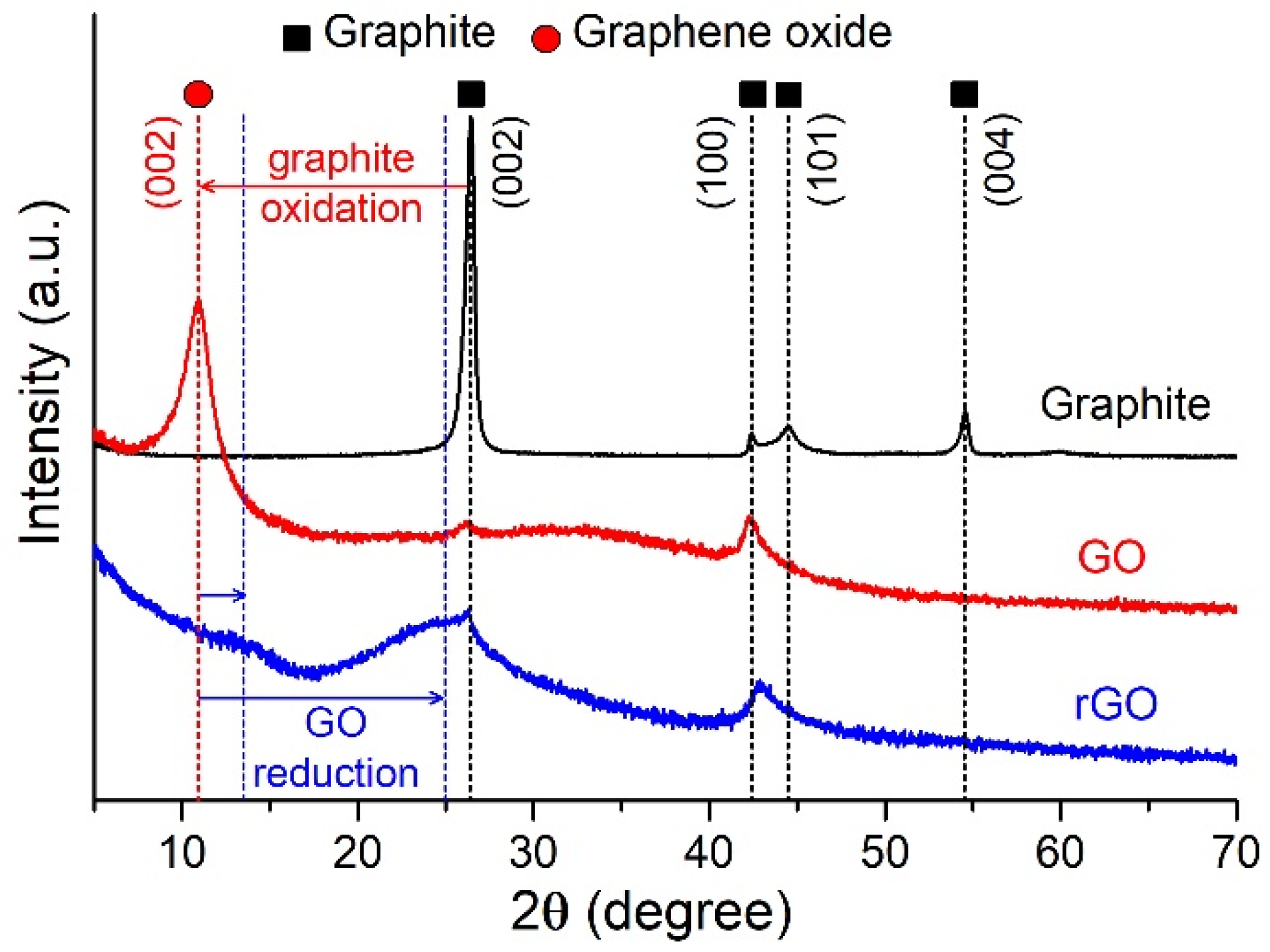
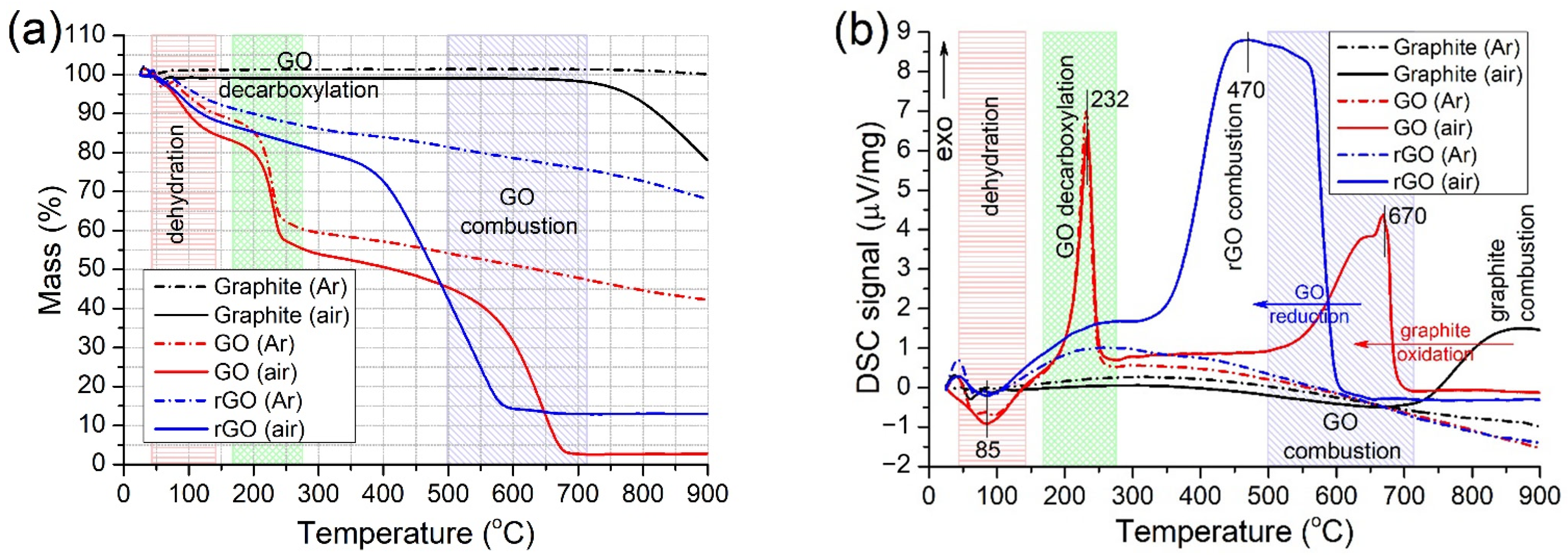

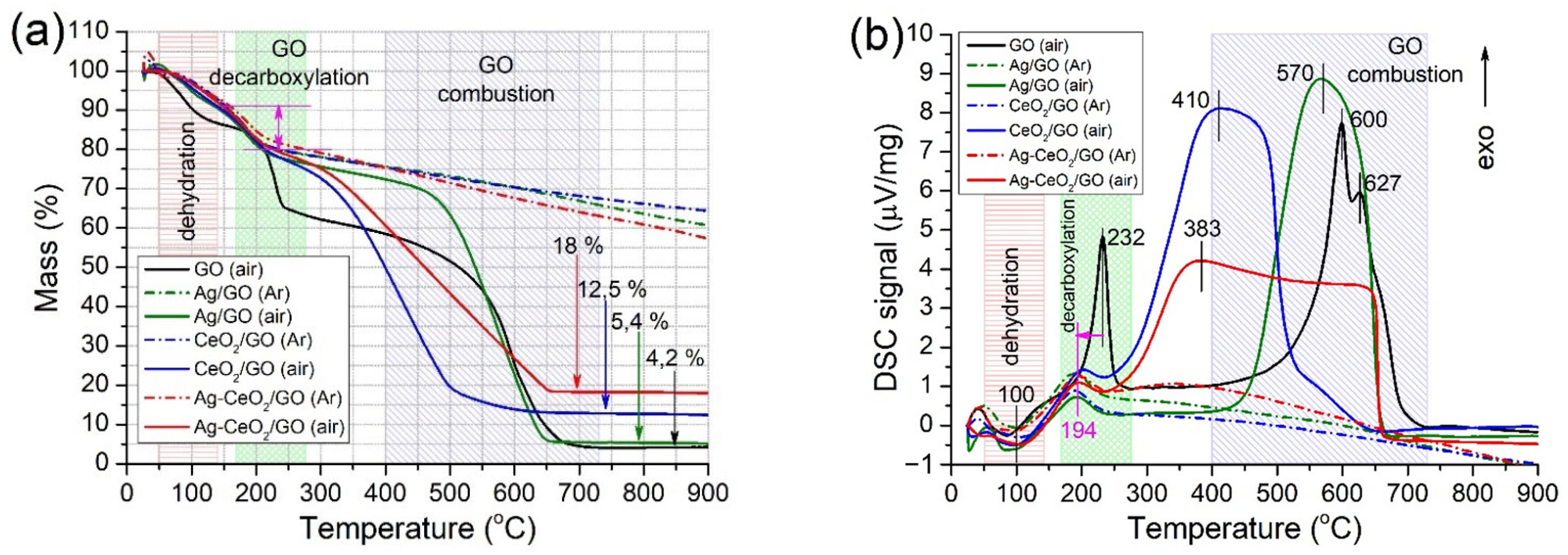
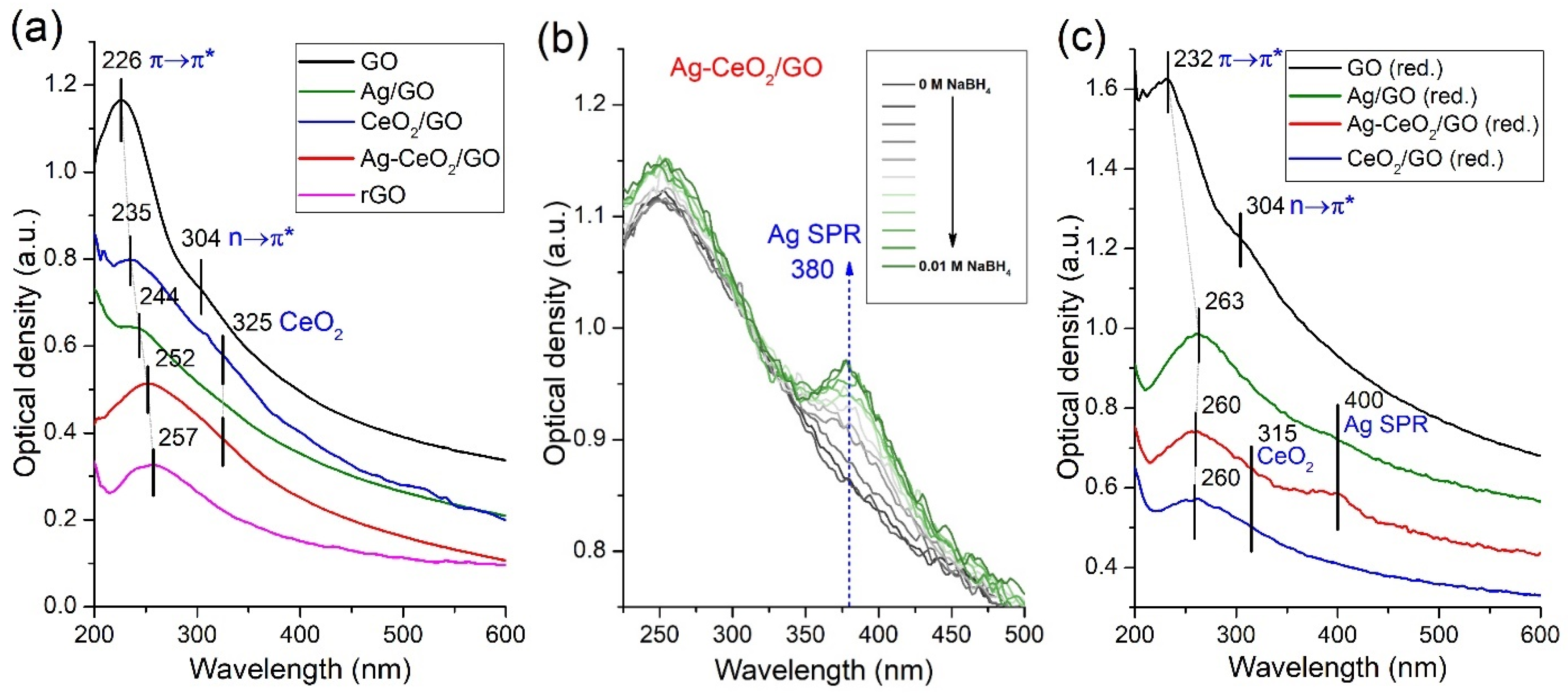


| Sample | SBET (m2/g) | Vpore (cm3/g) | Pore Width (nm) | Smicro* (m2/g) | Vmicro* (cm3/g) | Dmicro* (nm) |
|---|---|---|---|---|---|---|
| GO | 54.6 | 0.10 | 5.23 | 46 | 0.02 | 0.45 |
| Graphite | 0.46 | 0.02 | - | - | - | - |
| Sample | Phase | Calculated Reflection (hkl) | Interplanar Distance d (Å) | Particle Size D (nm) |
|---|---|---|---|---|
| GO | GO (hex.) | (002) | 7.98 | 5.0 |
| Ag/GO | Ag (cub.) | (111) | 2.36 | 27.5 |
| CeO2/GO | CeO2 (cub.) | (111) | 3.12 | 5.0 |
| Ag-CeO2/GO | Ag (cub.) | (111) | 2.36 | 29.3 |
| CeO2 (cub.) | (111) | 3.12 | 5.3 |
| Sample | Calculated XPS Peaks | ||||
|---|---|---|---|---|---|
| C 1s (at.%) | O 1s (at.%) | N 1s (at.%) | Ag 3d (at.%) | Ce 3d (at.%) | |
| GO | 73.36 | 26.64 | - | - | - |
| rGO | 86.79 | 13.21 | - | - | - |
| Ag/GO | 83.57 | 12.62 | 3.46 | 0.35 | - |
| CeO2/GO | 77.44 | 17.74 | 4.14 | - | 0.67 |
| Ag-CeO2/GO | 78.09 | 16.71 | 3.94 | 0.60 | 0.66 |
| Catalyst | C(NaBH4) (mM) | C(4-NP) (mM) | C(catalyst) (g/L) | kapp (min−1) | krel (L/g·min) | Ref. |
|---|---|---|---|---|---|---|
| Ag/GO | 15.0 | 0.075 | 0.030 | 0.796 | 26.5 | This work |
| Ag-CeO2/GO | 15.0 | 0.075 | 0.030 | 1.747 | 58.2 | |
| Ag/GO (red.) | 15.0 | 0.075 | 0.030 | 1.615 | 53.8 | |
| Ag-CeO2/GO (red.) | 15.0 | 0.075 | 0.030 | 3.674 | 122.5 | |
| RGO/Ag/CeO2-3 | 15.0 | 0.075 | - | 0.269 | - | [37] |
| Ag-CeO2/SBA-15 | 15.0 | 0.150 | 0.060 | 0.960 | 16.0 | [24] |
| Ag@CeO2 | 20.0 | 0.130 | 0.010 | 1.920 | 192.0 | [60] |
| 10Ag/CeO2 | 15.0 | 0.150 | 0.120 | 3.793 | 31.6 | [61] |
| 1Pt1Ag/CeO2 | 6.0 | 0.030 | 0.200 | 1.403 | 7.0 | [62] |
| Ag80Ni20@CeO2 | 10.0 | 0.080 | 0.400 | 6.231 | 15.6 | [63] |
| Au-Ag-γ-Fe2O3/rGO | 10.0 | 0.093 | 0.010 | 0.798 | 79.8 | [64] |
| GO/Ag-Fe3O4 | 6.7 | 0.100 | 0.033 | 1.602 | 48.5 | [65] |
| Ag-Fe3O4/RGO | 100.0 | 0.100 | 1.600 | 2.640 | 1.7 | [66] |
| Ag/MR-3 | 62.5 | 0.125 | 0.020 | 1.902 | 95.1 | [59] |
| Ag-Pt (9:1) | 0.6 | 0.100 | 0.0014 | 3.540 | 2528.6 | [67] |
| Ag@hm-SiO2 | 2.0 | 0.016 | 0.010 | 1.080 | 108.0 | [68] |
| Ag-SiO2 | 32.0 | 0.216 | 0.010 | 1.068 | 106.8 | [69] |
| Ag-SiO2 | 120.0 | 2.000 | 0.200 | 1.020 | 5.1 | [70] |
| Ag/Triton X-705/SiNPs | 100.0 | 0.100 | 2.400 | 3.636 | 1.5 | [71] |
| NrGO1-Ag1-Gly5 | 0.4 | 0.085 | 0.172 | 0.426 | 2.5 | [72] |
| Ag NPs@MOF-199 s/CCFs | 10.0 | 0.100 | 0.002 | 0.149 | 74.4 | [73] |
| Fe3O4@MIL-100(Fe)/Ag | 38.7 | 0.096 | 0.032 | 2.830 | 87.7 | [74] |
| Ag@NOTT-300(Al) | 100.0 | 0.144 | 0.100 | 2.670 | 26.7 | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taratayko, A.; Kolobova, E.; Mamontov, G. Graphene Oxide Decorated with Ag and CeO2 Nanoparticles as a Catalyst for Room-Temperature 4-Nitrophenol Reduction. Catalysts 2022, 12, 1393. https://doi.org/10.3390/catal12111393
Taratayko A, Kolobova E, Mamontov G. Graphene Oxide Decorated with Ag and CeO2 Nanoparticles as a Catalyst for Room-Temperature 4-Nitrophenol Reduction. Catalysts. 2022; 12(11):1393. https://doi.org/10.3390/catal12111393
Chicago/Turabian StyleTaratayko, Aleksey, Ekaterina Kolobova, and Grigory Mamontov. 2022. "Graphene Oxide Decorated with Ag and CeO2 Nanoparticles as a Catalyst for Room-Temperature 4-Nitrophenol Reduction" Catalysts 12, no. 11: 1393. https://doi.org/10.3390/catal12111393
APA StyleTaratayko, A., Kolobova, E., & Mamontov, G. (2022). Graphene Oxide Decorated with Ag and CeO2 Nanoparticles as a Catalyst for Room-Temperature 4-Nitrophenol Reduction. Catalysts, 12(11), 1393. https://doi.org/10.3390/catal12111393








