Abstract
Dynamic kinetic resolution allows for the synthesis of enantiomerically pure asymmetric alcohols. Cyclopentadienyl-derived ruthenium catalysts were immobilized with an ionic liquid, [BMIM][NTf2], on multiwall carbon nanotubes and used for the racemization of chiral secondary alcohols. This successful approach was combined with the enantioselective enzymatic acylation of secondary alcohols (1-phenylethanol and 1-(1-naphthyl)ethanol) using Novozyme® 435. The resulting catalytic system of the ruthenium racemization catalysts and enzymatic acylation led to chiral esters being obtained by dynamic kinetic resolution. The immobilized catalytic system in the ionic liquid gave the same activity of >96% yield within 6 h and a selectivity of 99% enantiomeric excess as the homogeneous system, while allowing for the convenient separation of the desired products from the catalyst. Additionally, the process can be regarded as green, since the efficient reuse of the catalytic system was demonstrated.
1. Introduction
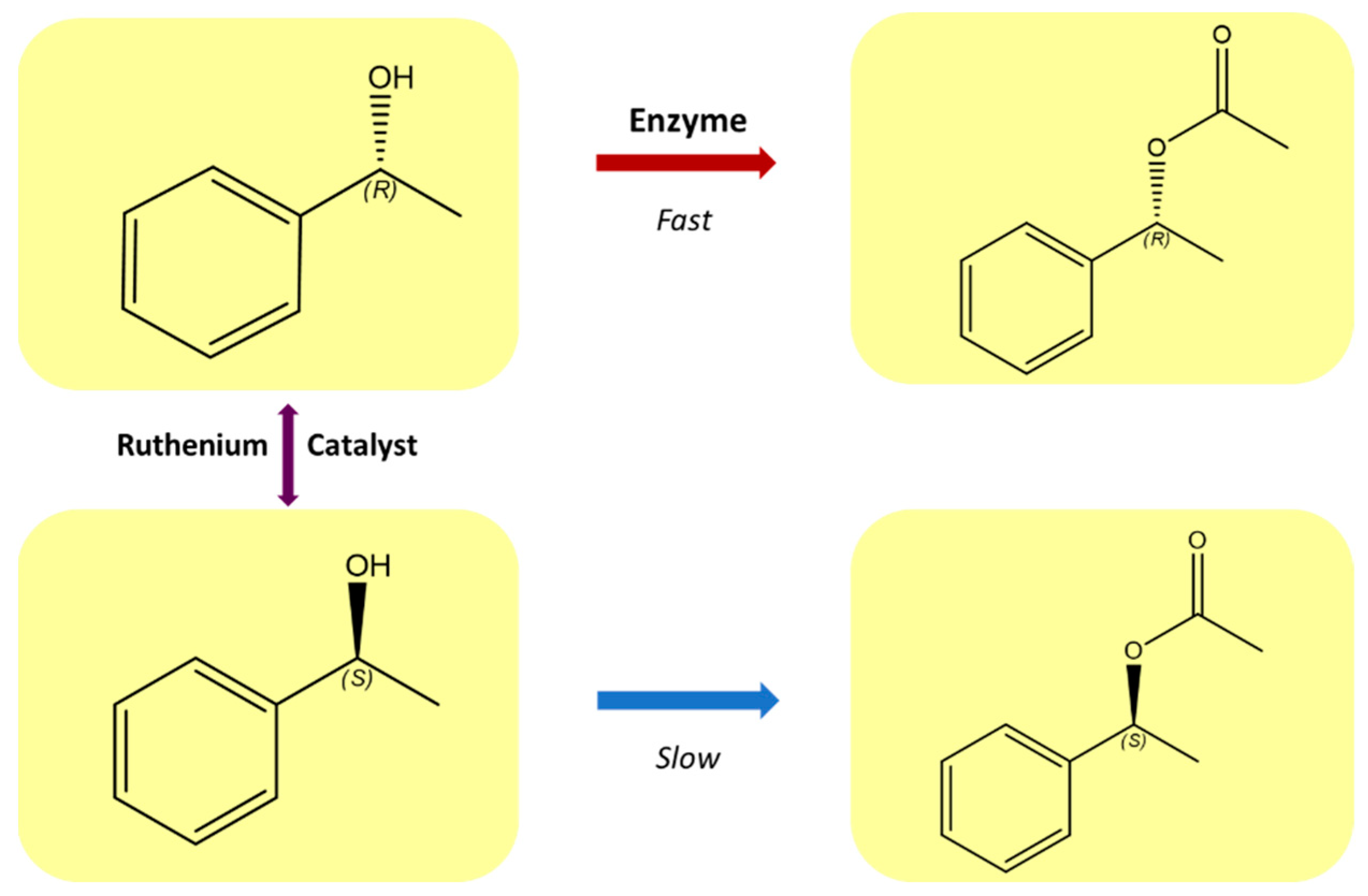
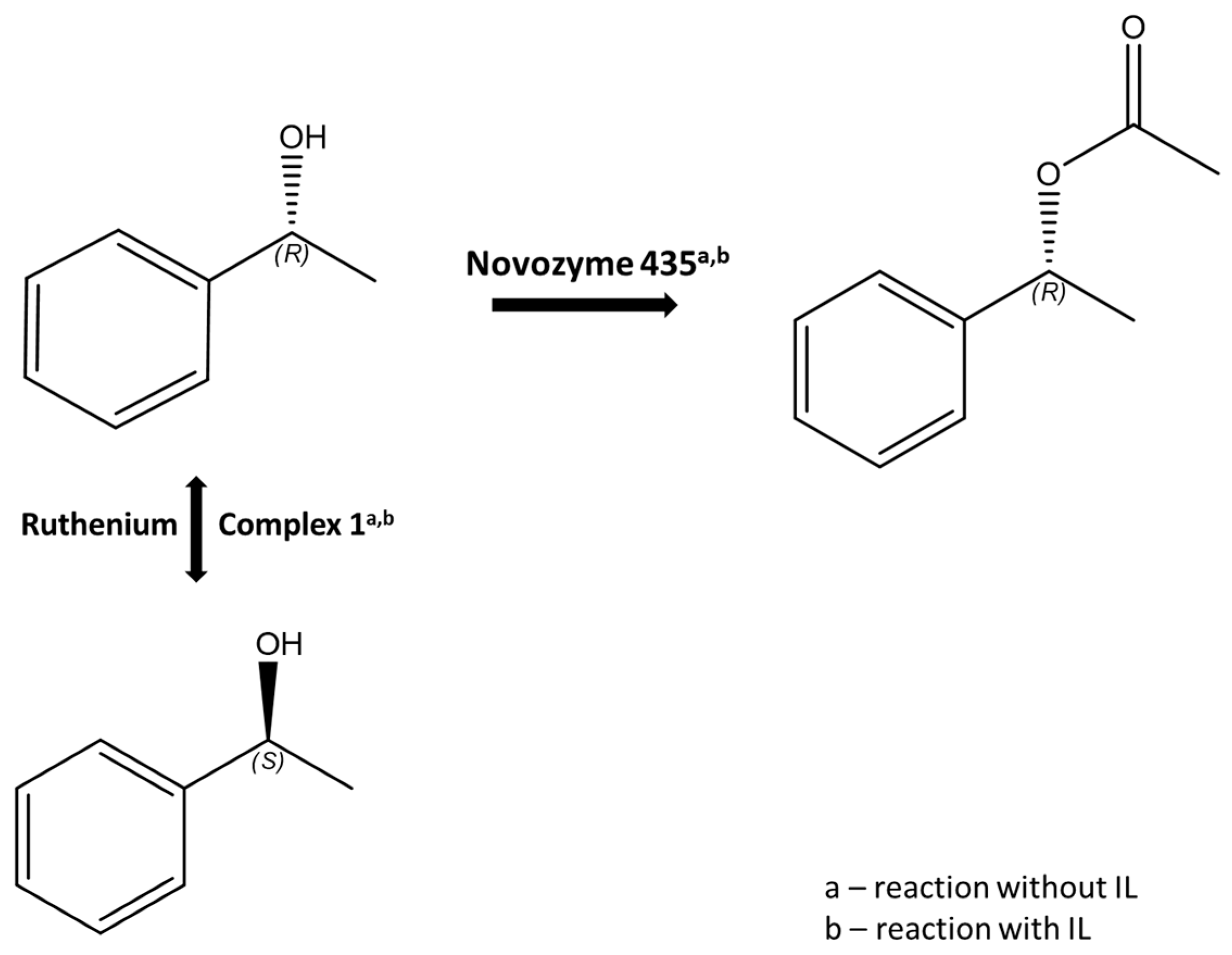
Continuous development of effective methods of asymmetric synthesis is necessary to meet the growing demand for enantiomerically pure compounds, which are used largely in the pharmaceutical, chemical, and agrochemical industries. The development of areas, such as the kinetic separation of racemates or dynamic kinetic separation, gives the possibility of efficiently obtaining enantiomerically pure compounds [1,2]. Dynamic kinetic resolution (DKR) is a powerful extension of the classical kinetic resolution of racemates [3]. Compared to traditional kinetic resolution, DKR shows a significant increase in yield from a maximum of 50% to almost 100%, while maintaining high enantioselectivity [4,5,6]. In this process, the slow-reacting enantiomer is continuously converted to the one that is preferred by an enzyme. The development of chiral catalysts containing transition metals has opened new possibilities that allow for the synthesis of pure enantiomers from achiral substrates [7]. Ruthenium complexes constitute an important group of catalysts in organic synthesis [8]. These compounds are among the most effective catalysts for the racemization of secondary alcohols and amines [1,6,9,10], and they can be employed in DKR homogeneous systems (Figure 1 [11,12]).
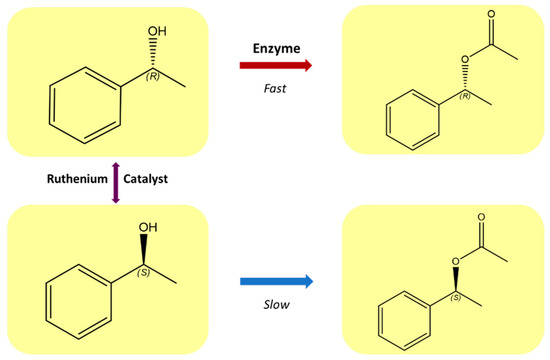
Figure 1.
Dynamic kinetic resolution of secondary alcohols.
However, there are difficulties in separating products from the reaction mixture and catalyst recycling. Therefore, developing recyclable catalysts for the racemization of secondary alcohols is an attractive subject for investigation [13]. Homogeneous catalysts generate large amounts of waste during processes, thus disturbing the environmental and ecological balance [14]. The use of heterogeneous catalysis is also beneficial from an industrial point of view due to the convenient isolation of the product and the recycling of the catalyst [15]. Currently, carbon, zeolite, polymers, silica, and many other mesoporous materials are used as solid supports [16,17]. Ionic liquids (ILs), which are known as green solvents, are organic salts with negligible vapor pressure. ILs with unique properties, such as the capability of creating hydrogen bonds, non-/polarity, and the possibility of cation and anion selection, are found to support enzymes’ active conformations [18,19]. A supported IL phase (SILP) system allows for the immobilization on a solid carrier of both an IL and an enzyme, where the IL forms a thin layer on the matrix, and the enzyme is adsorbed. Moreover, a heterogeneous SILP biocatalytic system consequently reduces the amount of IL in the process and simplifies biocatalyst recycling [20]. So far, the issue of the immobilization of ruthenium complexes in green supports, such as ILs for DKR, has not been widely explored. There has also been little work on the use of immobilized ruthenium complexes in DKR, especially regarding non-covalent immobilization. This study addresses both issues by showing the possibilities of the non-covalent immobilization of one of the best-performing ruthenium catalysts. On the other hand, it shows the effective use of a combination of the ruthenium complex, an IL, and an enzyme as a heterogeneous DKR catalytic system.
2. Results and Discussion
Encouraged by our previous studies on the immobilization of racemization catalysts for secondary alcohols on mesoporous cellular foams (MCF) [21], we undertook a further search for better heterogeneous catalytic systems that could be paired with an enzyme and used in DKR. The possibility of creating an efficient heterogeneous catalyst in which the ruthenium complex was non-covalently attached to a support was also considered, due to the simplicity of the system and the support having the least influence on the catalytic properties. Non-covalent immobilization of an efficient ruthenium catalyst would give a possibility of maintaining its catalytic activity and make its preparation as simple as possible. We hypothesized that a SILP could serve as a promising platform for both Ru racemization catalysts and enzymatic kinetic resolution. Moreover, the resulting immobilized DKR system could benefit from its heterogeneous character for the separation and recycling goals.
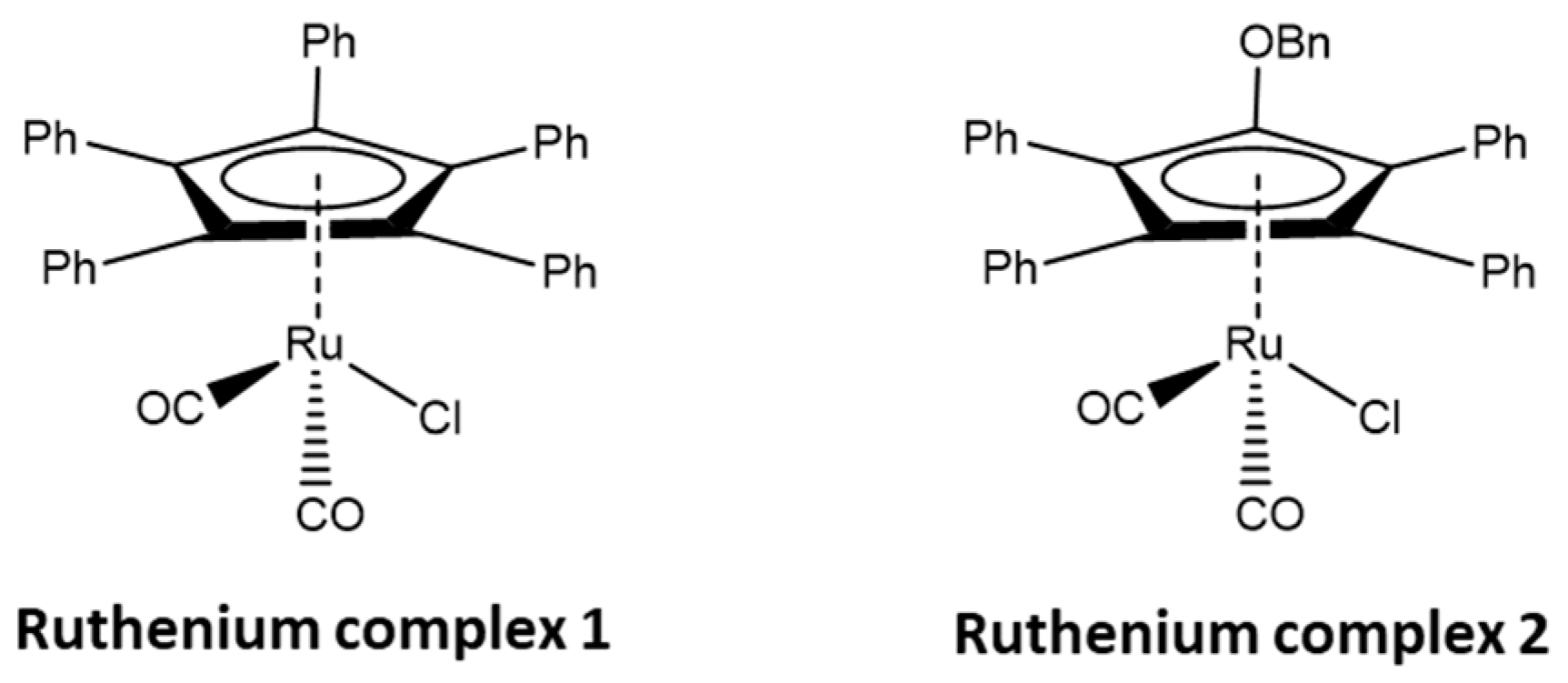
The ruthenium complexes selected by us, [Ru(1)] and [Ru(2)] (Figure 2), belong to the group of cyclopentadienyl carbonyl complexes, where cyclopentadienyl ligands are effective in stabilizing alkyl-ruthenium bonds [22]. These complexes have been repeatedly tested both in the racemization reaction of secondary alcohols and in DKR [7]. Moreover, such complexes could cooperate with lipase B, commercially available on a support as Novozyme® 435, as well as possibly being immobilized on a silica support, as demonstrated in a previous study [23].
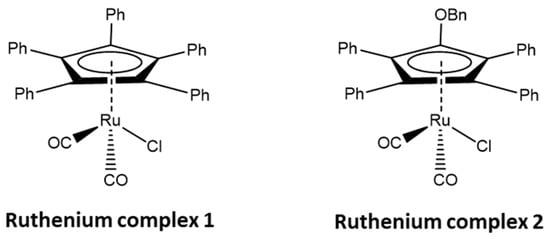
Figure 2.
Ruthenium complexes [Ru(1)] and [Ru(2)] used in this study, Bn is the -CH2Ph group.
The influence of an IL on enzyme stabilization is dependent on various factors [18,19]. The IL 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [BMIM][NTf2] was found by many studies to have a strong effect on lipase stabilization [20,24]. Moreover, ILs allow for covalent and non-covalent modifications of multiwall carbon nanotubes (MWCNT) without damaging the π-conjugated nanotube structure. The non-covalent interactions between imidazolium ILs and hexagonally arranged sp2-hybridized aromatic carbon atoms are mostly based on van der Waals forces and π-electronic interactions, which are also found in similar systems [25,26].
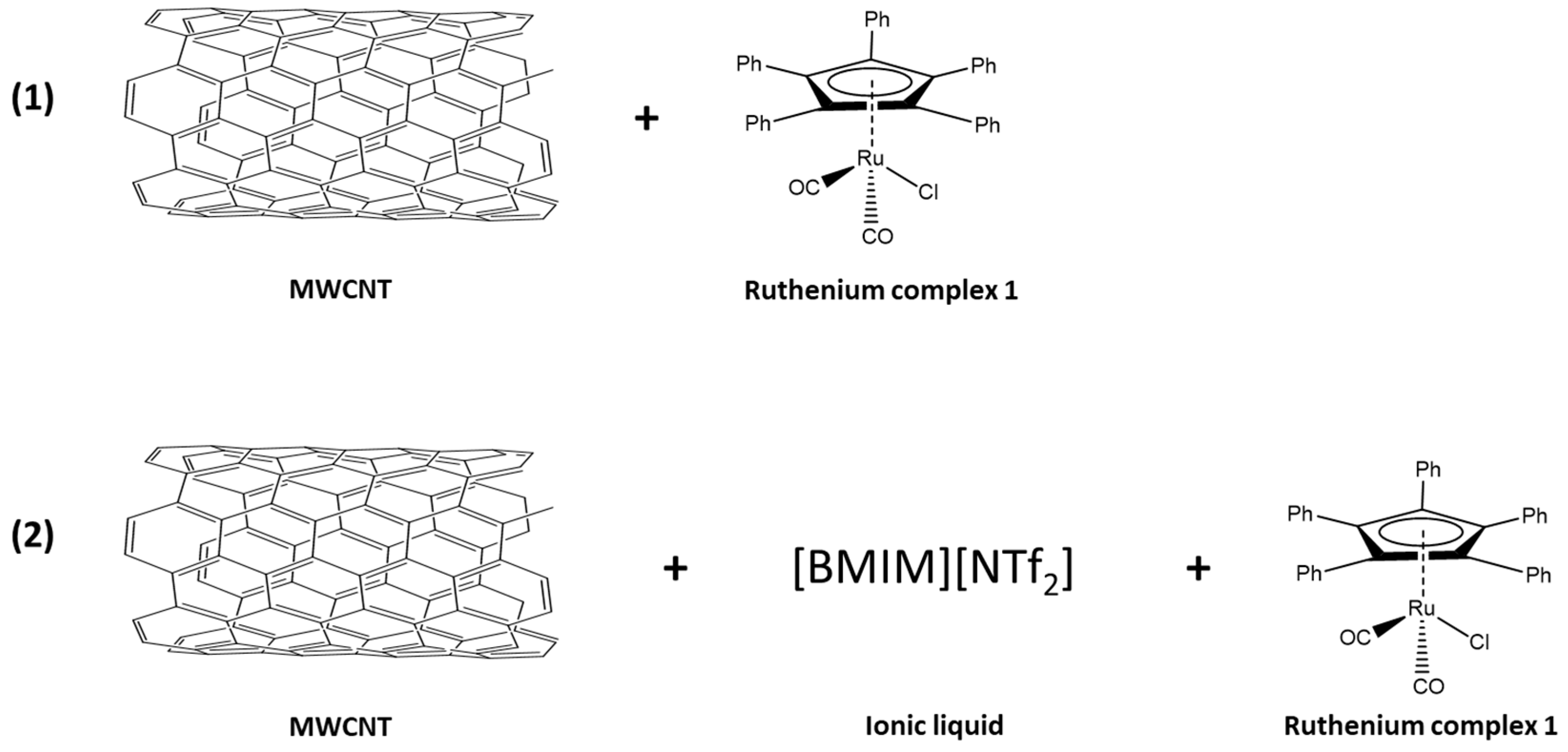
Kim’s research [27] on the application of ILs prompted us to explore such media. However, the first tests showed a relatively low stereoselectivity of the DKR. The ruthenium complex [Ru(1)] was immobilized directly on MWCNT to give [Ru(1)-MWCNT]. The structural features of the complex were exploited to bind to the nanotube by π-stacking. In a second variant, the same complex was immobilized on MWCNT with an IL as a SILP system [Ru(1)-IL-MWCNT] (Figure 3).
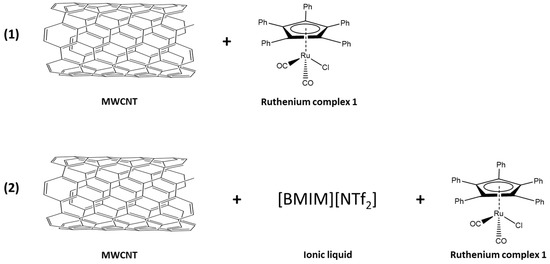
Figure 3.
Two variants of non-covalent immobilization of the ruthenium complex [Ru(1)] on MWCNT; (1) [Ru(1)-MWCNT] (2) [Ru(1)-IL-MWCNT] is a SILP system.
2.1. Racemization Studies
The amount of IL and complex in both variants was measured by TGA. For the material without any IL, the Ru complex loading was 4.4% (Figure S4, SI), while for the SILP it was 2.2% (Figure S6, SI). TGA analysis showed 0.7% IL loading on the SILP (Figure S5, SI). SEM-EDS analysis of the first catalyst indicated elements of the Ru complex on the surface (Figure S9, SI).
First, tests of the racemization of 1-S-phenylethanol (alcohol 1) in the presence of MWCNT were performed with [Ru(1)] (Table 1). It was found that neither the MWCNT support nor the IL affected the activity of the ruthenium catalyst. A blank test was then performed which confirmed that neither the MWCNT nor the IL could racemize secondary alcohols on their own. Tests on the racemization ability of the immobilized complexes were carried out on two alcohols: 1-S-phenylethanol (alcohol 1) and 1-S-(1-naphthyl)ethanol (alcohol 2).

Table 1.
Results of racemization tests for the obtained heterogeneous catalysts.
The racemization studies proved that catalytic activity was maintained in the racemization reaction of the immobilized systems. Additionally, in the case of the aromatic non-fused alcohol 1, the SILP system led to faster racemization than the system without IL.
Encouraged by the good results of the preliminary racemization tests, we decided to check the possibility of reusing heterogeneous catalysts in the reaction (Table 2).

Table 2.
Recycling of [Ru(1)-IL-MWCNT] catalysts in the racemization reaction with alcohol 1.
The experiments demonstrate moderate changes in the catalytic activity during the recycling of the catalytic system, which might be explained by the solvation of the ruthenium complex from the carrier and some deactivation due to exposure to air during work-up. Nevertheless, the immobilization approach opened new possibilities for the reuse of the catalytic system.
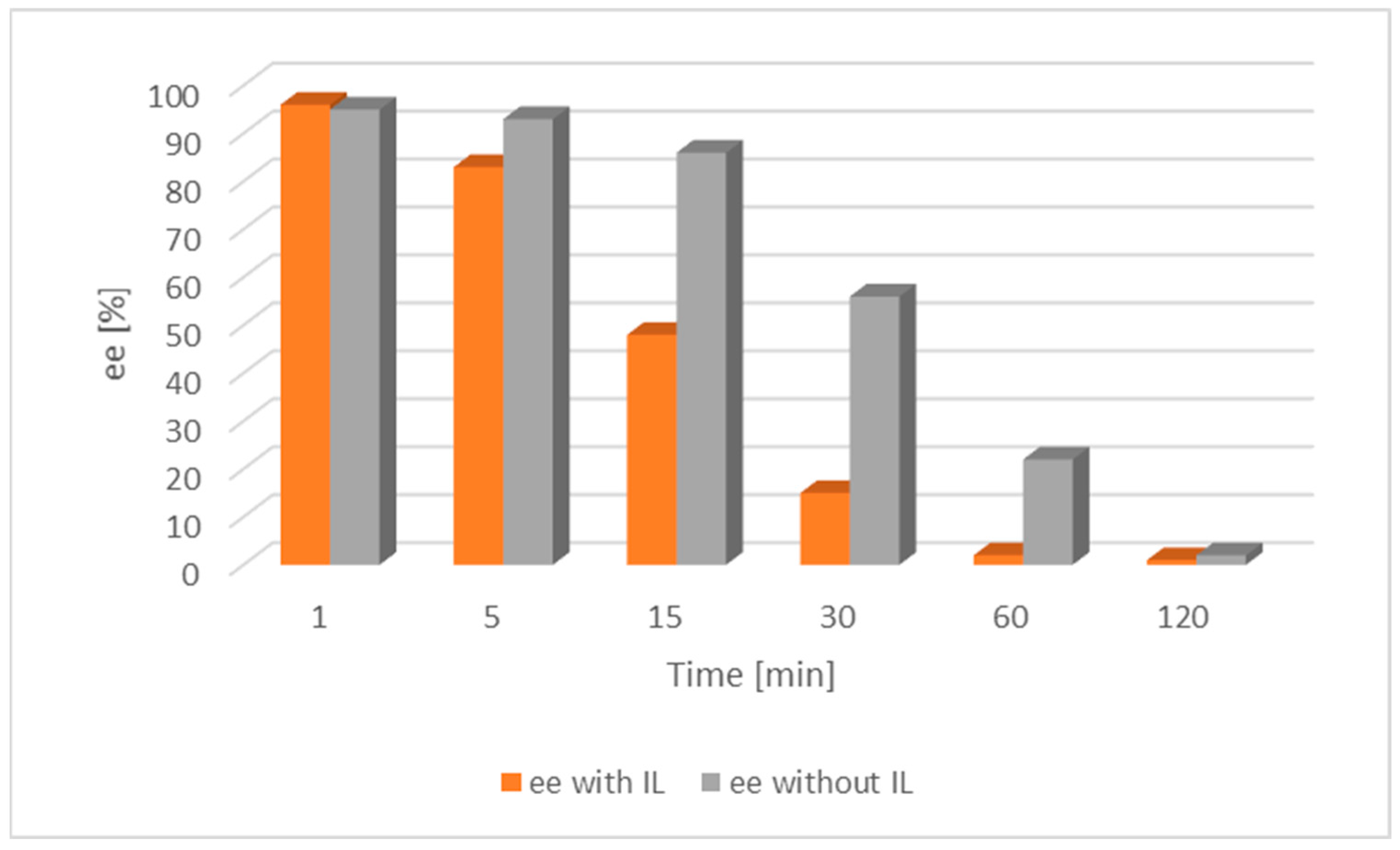
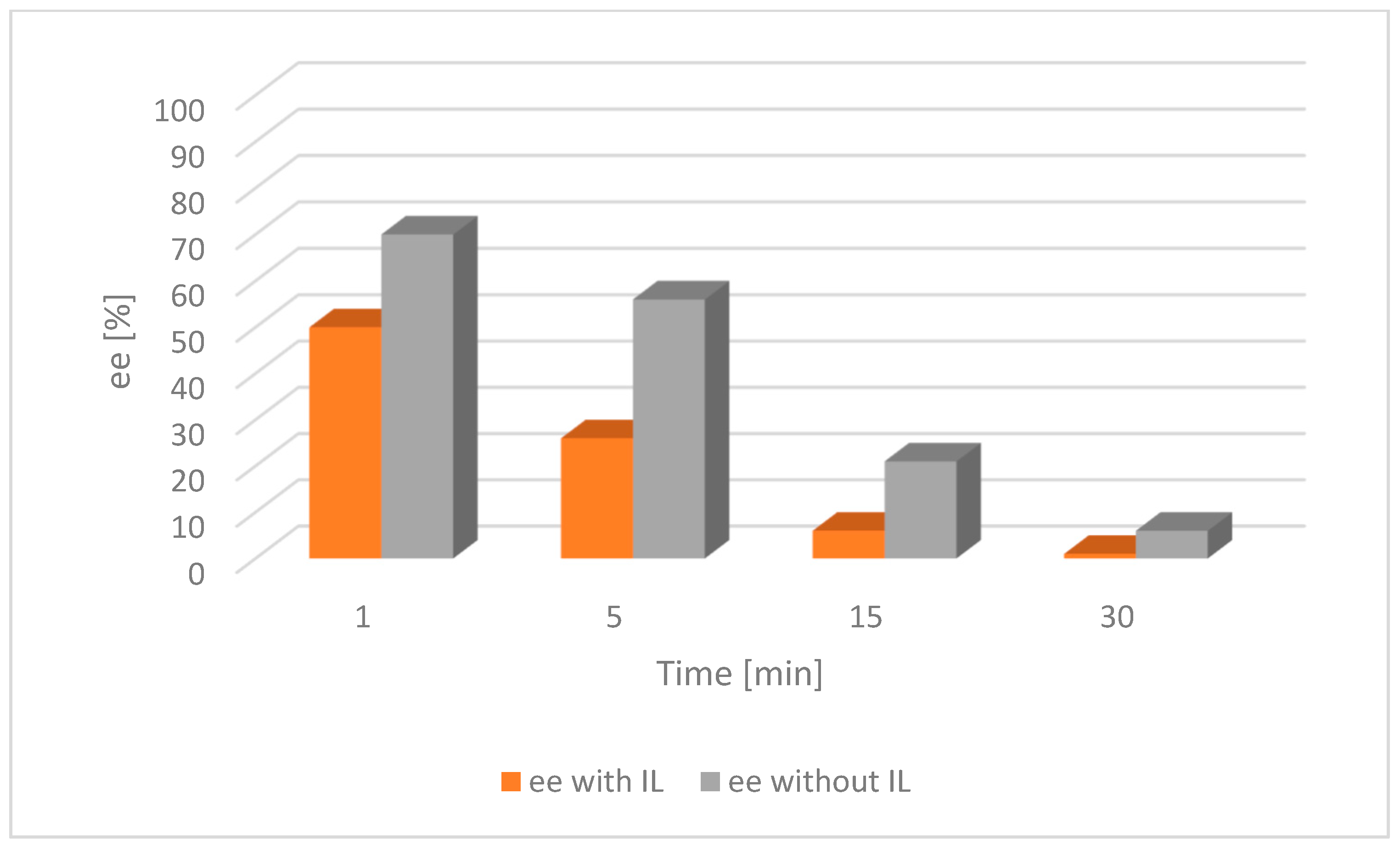
Further investigations focused on the exploration of the impact of IL on racemization were carried out on both [Ru(1)] and [Ru(2)] along with alcohol 1 (Figure 4 and Figure 5).
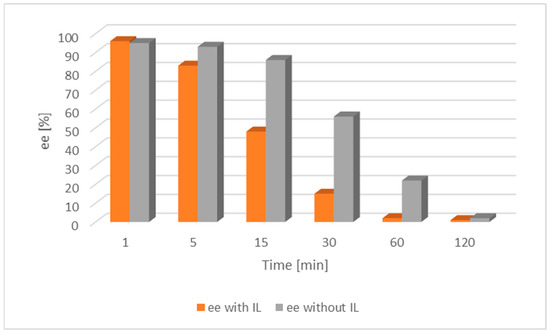
Figure 4.
Comparison of the catalytic activity of [Ru(1)] with and without an IL for alcohol 1. [Ru(1)] 0.003 mmol, tBuOK 0.009 mmol, alcohol 0.25 mmol, [BMIM][NTf2] 0.07 mmol, toluene 2 mL, a nitrogen atmosphere, RT; ee—enantiomeric excess of chiral alcohol in the mixture; the lower the ee, the nearer completion the desired racemization reaction.
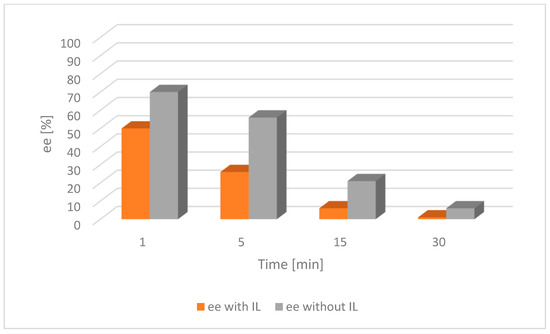
Figure 5.
Comparison of the catalytic activity of [Ru(2)] with and without an IL for alcohol 1. [Ru(2)] 0.004 mmol, tBuOK 0.02 mmol, alcohol 0.25 mmol, [BMIM][NTf2] 0.07 mmol, toluene 2 mL, a nitrogen atmosphere, RT; ee—enantiomeric excess of chiral alcohol in the mixture; the lower the ee, the nearer completion the desired racemization reaction.
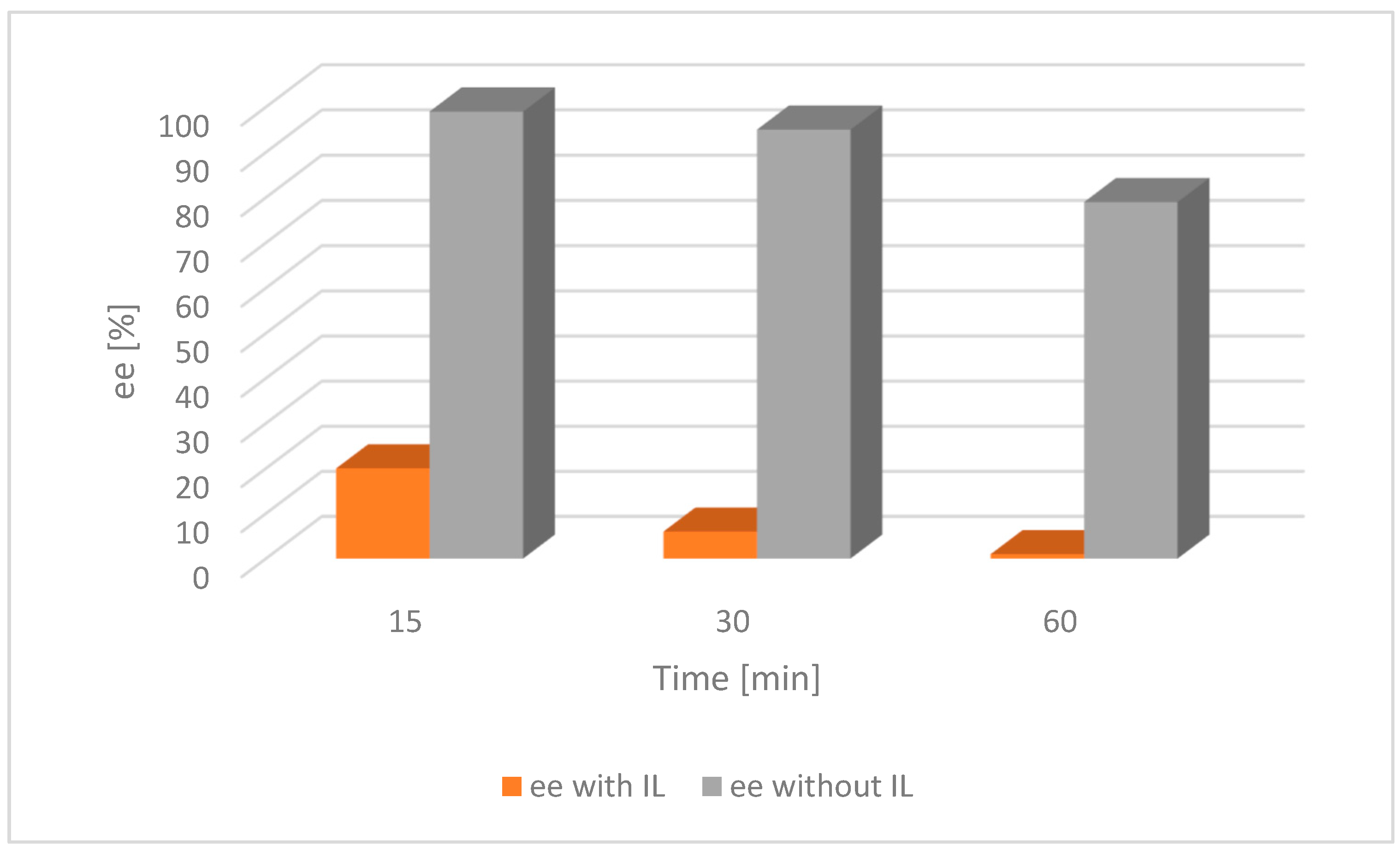
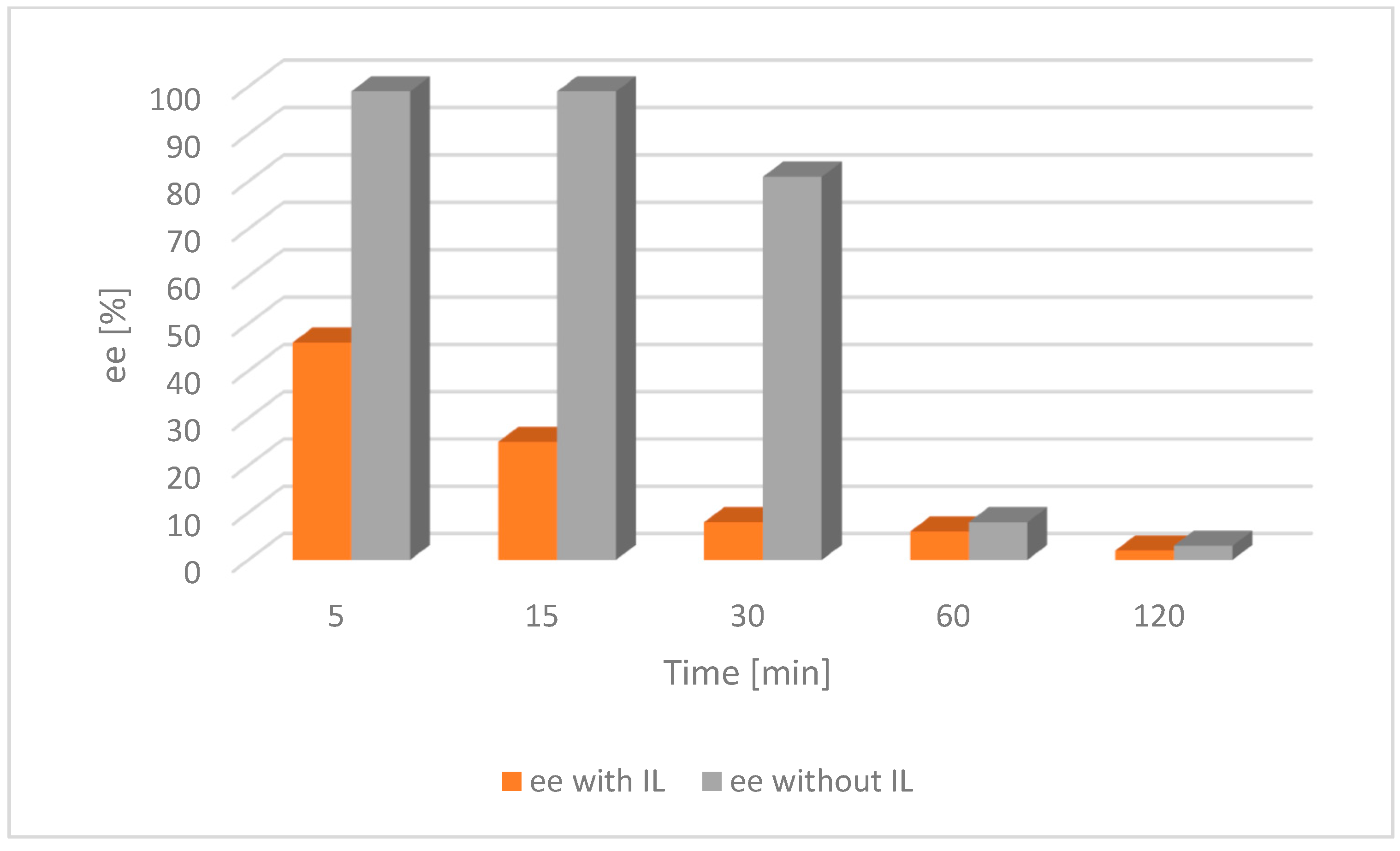
The result of the experiment shows that the addition of the IL had a significant impact on the acceleration of the racemization process. It was then compared with the appropriate tests on alcohol 2 (Figure 6 and Figure 7).
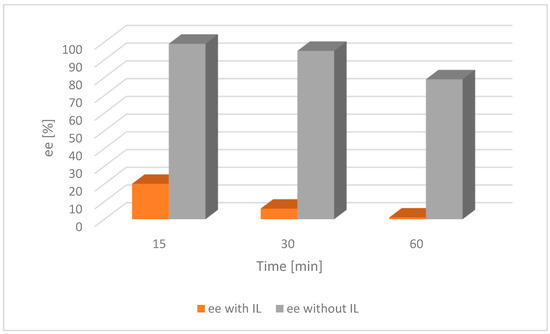
Figure 6.
Comparison of the catalytic activity of [Ru(1)] with and without an IL for alcohol 2. [Ru(1)] 0.003 mmol, tBuOK 0.009 mmol, alcohol 0.25 mmol, [BMIM][NTf2] 0.07 mmol, toluene 2 mL, a nitrogen atmosphere, RT; ee—enantiomeric excess of chiral alcohol in the mixture; the lower the ee, the nearer completion the desired racemization reaction.
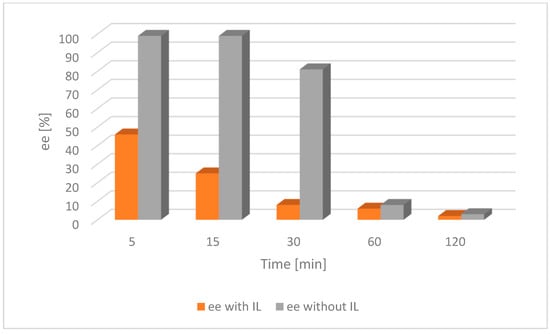
Figure 7.
Comparison of the catalytic activity of [Ru(2)] with and without an IL for alcohol 2. Ruthenium [Ru(2)] 0.004 mmol, tBuOK 0.02 mmol, alcohol 0.25 mmol, [BMIM][NTf2] 0.07 mmol, toluene 2 mL, a nitrogen atmosphere, RT; ee—enantiomeric excess of chiral alcohol in the mixture; the lower the ee, the nearer completion the desired racemization reaction.
For both catalysts and substrates, a significant increase in the rate of racemization was observed due to the addition of the IL, as described in the literature [28]. This trend was because the catalyst was confined in the small volume of the IL as opposed to being dispersed throughout the entire solvent volume in the absence of an IL.
2.2. DKR Studies
Intending to take advantage of all the benefits of the heterogeneous catalytic system, we designed the following system: Ru complex and IL (Ru(1)-IL), an enzyme, and a basic activator in toluene, which could be tested in a DKR reaction (Figure 8 and Table 3).
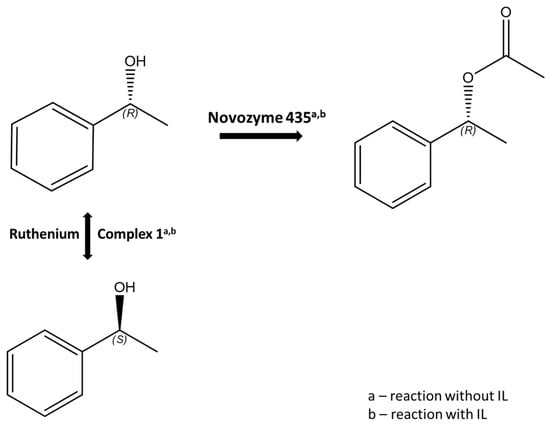
Figure 8.
The process of DKR with and without the addition of an IL.

Table 3.
DKR results.
The experiments on the immobilization of the DKR catalytic system ([Ru(1)] + Novozyme® 435) prove that it is possible to maintain a high catalytic activity in obtaining the chiral (99%) ester from the alcohol 1 racemate. The proposed catalytic system shows much higher activity than the previously reported system based on [RuCl2(cymene)]2, which in similar conditions led to enantiomeric esters over at least 48 h [27]. On the other hand, the higher activity in the DKR of secondary alcohols was only obtained in the homogeneous catalytic systems [29].
To demonstrate the advantages of the heterogeneous system, the recycling of the DKR catalytic system was studied. First, it was noted that it was possible to easily separate the two phases formed in the reaction mixture. In addition, the IL could be reused together with the ruthenium catalyst and the enzyme as demonstrated in Table 4.

Table 4.
The recycling of the catalytic DKR system.
3. Materials and Methods
Chemicals were purchased in the highest available purity from Acros Organics, Sigma-Aldrich, or Avantor Poland, and used without purification unless reported otherwise. Solvents were dried and distilled no earlier than two weeks prior to their use. All syntheses were carried out under a nitrogen atmosphere.
Spectroscopy—instrumentation details: 1HNMR and 13CNMR spectra were recorded with a Varian Unity Inova 300 MHz and an Agilent Technologies 400 MHz. GC analysis was performed on a chiral GC system (Agilent Technologies 6890N) equipped with a MEGA-DEX DMP-Beta (25 m × 0.25 mm × 0.25 μm) capillary column. The injection was carried out in split mode (100:1); the injector and FID detector temperatures were 250 °C. Analysis was carried out at a constant pressure (19.91 psi). Oven program: initial temperature 100 °C, then 10 °C min−1 to 150 °C (held at the latter temperature for 1.5 min). TGA was obtained on a Mettler Toledo (TGA851e) thermobalance. Samples were heated from 25 °C to 800 °C at a rate of 10 °C/min under a nitrogen flow of 60 mL/min. SEM-EDS analysis was performed on a Phenom Pro Desktop SEM instrument equipped with an EDS detector (15 kV) (Thermo Fisher Scientific).
3.1. Synthesis of [Ru(1)]
[Ru(1)] was synthesized according to the literature [30]. 1HNMR (CDCl3) ẟ: 7.17–7.20 (m, 5 H), 7.08–7.12 (dd, J = 7,10 Hz, 10 H), 7.02–7.06 (m, 10 H) ppm. 13CNMR (CDCl3) ẟ: 196.9, 132.2, 128.4, 127.8, 127.5, 106.5 ppm.
3.2. Synthesis of [Ru(2)]
[Ru(2)] was synthesized according to the literature [31], but with minor modifications. The synthesis time was reduced to 24 h, and the crude complex was purified by column chromatography using methylene chloride/petroleum ether (7:3). 1HNMR (CD3COCD3) ẟ: 7.59–7.64 (m, 4 H), 7.00–7.28 (m, 21 H), 4.81 (s, 2 H, CH2) ppm. 13CNMR (CD3COCD3) ẟ: 197.5 (CO), 135.9, 132.9, 132.2, 129.9, 129.9, 128.6, 128.5, 128.3, 128.3, 127.8, 102.6, 88.8, 75.3 (CH2) ppm.
3.3. Synthesis of IL
1-Butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([BMIM][NTf2]) was synthesized according to the literature [32]. 1H NMR (400 MHz, CDCl3): δ [ppm] 8.9 (s, 1 H), 7.8 (d, 2 H), 4.3 (t, 2 H), 3.8 (s, 3 H), 1.9 (m, 2 H), 1.4 (m, 2 H), 0.9 (t, 3 H). 13C NMR (101 MHz, CDCl3): δ [ppm] 136.3, 135.9, 124, 123.9, 122.6, 50.2, 36.5, 32.1, 19.5, 13.3.
3.4. Immobilization of IL on MWCNT
The immobilization of [BMIM][NTf2] was performed according to the literature [33]. TGA and SEM-EDS analyses were obtained (Figures S5 and S10, SI).
3.5. General Procedure for Immobilization of Ruthenium Complex 1 on Carbon Nanotubes
MWCNT (500 mg) were mixed under nitrogen with [Ru(1)] (0.03 mmol) in dry toluene (35 mL) for 24 h. The mixture was then filtered, washed with acetone and dichloromethane, and dried under vacuum for 2 days. A racemization test was carried out, and GC traces of the racemate were obtained (Figures S12 and S13, SI).
3.6. General Synthesis with Dynamic Kinetic Resolution
To a 2 mL vial, 0.01 mmol of ruthenium complex, 0.047 mmol K3PO4, 1 mL of toluene, 0.25 mmol alcohol, and 0.175 mmol IL were added. The vial was purged with nitrogen and tightly capped. The complex was activated for 18 h at 25 °C on a magnetic stirrer. An additional 0.75 mmol of isopropenyl acetate and 2.5 mg of Novozyme® 435 were then added. The mixture was purged with nitrogen and the vial was sealed with a septum. The reaction was carried out at 25 °C with continuous stirring over 6 to 24 h. The progress of the reaction was monitored by GC, with chromatographs showing enantiomerically pure product obtained (Figure S14, SI).
3.7. General Procedure for the DKR Recycle Test
After the DKR process was completed, the toluene phase was separated from the IL phase. New portions of alcohol (0.25 mmol), acyl donor (0.75 mmol), and toluene (1 mL) were added to the IL phase, and a new DKR cycle was started. The reaction was carried out over 6 to 24 h. The progress of the reaction was monitored by GC.
4. Conclusions
The obtained results reveal that even non-covalent immobilization of the ruthenium complexes on MWCNT and in the SILP system (MWCNT on IL) gives a predominantly heterogeneous catalytic reaction. The SILP system was efficient in the racemization of secondary alcohols. Further studies demonstrate the direct positive impact of the IL on the activity of ruthenium complexes in racemization. Addition of the IL shortened the complete racemization time from 60 to 30 min. The acceleration of the racemization reaction was observed both for 1-phenylethanol and 1-(1-naphthyl)ethanol. These findings were then transferred to the DKR involving an acylase enzyme (Novozyme® 435). The non-covalent immobilization of the DKR catalytic system (the Ru complex and the enzyme) in IL led to comparable activity to the homogeneous system but benefited from the possibility of easy separation of the desired products and catalyst reuse. The catalytic activity of the proposed immobilized DKR system was higher (ca. 4 times) than that reported earlier in the literature. Moreover, the reuse of the catalytic system was studied, and proved to maintain its activity over at least three cycles. The recycle tests and convenient separation of the products demonstrate the green character of the catalytic system in the DKR of secondary alcohols, featuring high activity and selectivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12111395/s1. Figures S1–S6: TGA analysis of the materials, and Figures S7–S11: SEM-EDS analysis.
Author Contributions
Conceptualization M.H., A.C. and N.K.; methodology M.H., A.W., D.S., A.C. and N.K.; investigation M.H., A.K.-H. and S.J.; resources M.H., A.W. and D.S.; data curation M.H., D.S. and S.J.; writing—original draft preparation M.H., A.W. and N.K.; writing—review and editing M.H. and N.K.; visualization M.H.; supervision A.C. and N.K.; project administration M.H., A.W., A.C. and N.K.; funding acquisition M.H., A.W., A.C. and N.K. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support given by the Silesian University of Technology, grant No. BKM /RCH2/2021 04/020/BKM21/, and Poland National Science Centre of Poland, grant. No. OPUS UMO-2020/39/B/ST8/00693 is gratefully acknowledged.
Data Availability Statement
The data presented in this study are available in supplementary material.
Conflicts of Interest
There are no conflict to declare.
References
- Choi, J.H.; Choi, Y.K.; Kim, Y.H.; Park, E.S.; Kim, E.J.; Kim, M.J.; Park, J. Aminocyclopentadienyl Ruthenium Complexes as Racemization Catalysts for Dynamic Kinetic Resolution of Secondary Alcohols at Ambient Temperature. J. Org. Chem. 2004, 69, 1972–1977. [Google Scholar] [CrossRef]
- Karvembu, R.; Prabhakaran, R.; Muthu Tamizh, M.; Natarajan, K. Ruthenium and Enzyme-Catalyzed Dynamic Kinetic Resolution of Alcohols. Comptes Rendus. Chim. 2009, 12, 951–962. [Google Scholar] [CrossRef]
- Fernández-Salas, J.A.; Manzini, S.; Nolan, S.P. A Cationic Ruthenium Complex for the Dynamic Kinetic Resolution of Secondary Alcohols. Chem. Eur. J. 2014, 20, 13132–13135. [Google Scholar] [CrossRef]
- Huerta, F.F.; Minidis, A.B.E.; Bäckvall, J.E. Racemisation in Asymmetric Synthesis. Dynamic Kinetic Resolution and Related Processes in Enzyme and Metal Catalysis. Chem. Soc. Rev. 2001, 30, 321–331. [Google Scholar] [CrossRef]
- Pellissier, H. Recent Developments in Dynamic Kinetic Resolution. Tetrahedron 2008, 64, 1563–1601. [Google Scholar] [CrossRef]
- Eckert, M.; Brethon, A.; Li, Y.X.; Sheldon, R.A.; Arends, I.W.C.E. Study of the Efficiency of Amino-Functionalized Ruthenium and Ruthenacycle Complexes as Racemization Catalysts in the Dynamic Kinetic Resolution of 1-Phenylethanol. Adv. Synth. Catal. 2007, 349, 2603–2609. [Google Scholar] [CrossRef]
- Martín-Matute, B.; Bäckvall, J.E. Dynamic Kinetic Resolution Catalyzed by Enzymes and Metals. Curr. Opin. Chem. Biol. 2007, 11, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, L.S.; Söderberg, B.C.G. Transition Metals in the Synthesis of Complex Organic Molecules, 3rd ed.; University Science Books: Sausalito, CA, USA, 2009; ISBN 978-1-891389-59-7. [Google Scholar]
- Mavrynsky, D.; Päiviö, M.; Lundell, K.; Sillanpää, R.; Kanerva, L.T.; Leino, R. Dicarbonylchloro(Pentabenzylcyclopentadienyl)Rutheniuni as Racemization Catalyst in the Dynamic Kinetic Resolution of Secondary Alcohols. Eur. J. Org. Chem. 2009, 2009, 1317–1320. [Google Scholar] [CrossRef]
- Gustafson, K.P.J.; Guðmundsson, A.; Bajnóczi, É.G.; Yuan, N.; Zou, X.; Persson, I.; Bäckvall, J.E. In Situ Structural Determination of a Homogeneous Ruthenium Racemization Catalyst and Its Activated Intermediates Using X-Ray Absorption Spectroscopy. Chem. Eur. J. 2020, 26, 3411–3419. [Google Scholar] [CrossRef]
- Pàmies, O.; Bäckvall, J.E. Combination of Enzymes and Metal Catalysts. A Powerful Approach in Asymmetric Catalysis. Chem. Rev. 2003, 103, 3247–3261. [Google Scholar] [CrossRef]
- Martín-Matute, B.; Edin, M.; Bogár, K.; Bäckvall, J.-E. Highly Compatible Metal and Enzyme Catalysts for Efficient Dynamic Kinetic Resolution of Alcohols at Ambient Temperature. Angew. Chem. Int. Ed. Engl. 2004, 116, 6697–6701. [Google Scholar] [CrossRef]
- Wuyts, S.; de Vos, D.E.; Verpoort, F.; Depla, D.; de Gryse, R.; Jacobs, P.A. A Heterogeneous Ru-Hydroxyapatite Catalyst for Mild Racemization of Alcohols. J. Catal. 2003, 219, 417–424. [Google Scholar] [CrossRef]
- Maurya, M.R.; Kumar, A.; Costa Pessoa, J. Vanadium Complexes Immobilized on Solid Supports and Their Use as Catalysts for Oxidation and Functionalization of Alkanes and Alkenes. Coord. Chem. Rev. 2011, 255, 2315–2344. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Heterogeneous Catalysts for the One-Pot Synthesis of Chemicals and Fine Chemicals. Chem. Rev. 2011, 111, 1072–1133. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhu, X.H.; Wang, D.; Sun, Z.; Deng, Y.; Hou, X.F.; Zhao, D. Selectivity Enhancement in Dynamic Kinetic Resolution of Secondary Alcohols through Adjusting the Micro-Environment of Metal Complex Confined in Nanochannels: A Promising Strategy for Tandem Reactions. ACS Catal. 2015, 5, 27–33. [Google Scholar] [CrossRef]
- Corma, A.; García, H.; Llabrés, I.; Xamena, F.X. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef]
- Zhao, H. Protein Stabilization and Enzyme Activation in Ionic Liquids. Specific Ion Effects. J. Chem. Technol. Biotechnol. 2016, 91, 25–50. [Google Scholar] [CrossRef]
- Itoh, T. Ionic Liquids as Tool to Improve Enzymatic Organic Synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef]
- Wolny, A.; Chrobok, A. Ionic Liquids for Development of Heterogeneous Catalysts Based on Nanomaterials for Biocatalysis. Nanomaterials 2021, 11, 2030. [Google Scholar] [CrossRef]
- Heba, M.; Stradomska, D.; Szymańska, K.; Jarzębski, A.; Ambroziak, K.; Masternak, M.; Kolanowska, A.; Pudło, W.; Kuźnik, N. Engineering and Performance of Ruthenium Complexes Immobilized on Mesoporous Siliceous Materials as Racemization Catalysts. Catalysts 2021, 11, 316. [Google Scholar] [CrossRef]
- Graf, D.D.; Day, N.C.; Mann, K.R. Synthesis and Characterization of Cyclopentadienyl and Pentamethylcyclopentadienyl Ruthenium Complexes of Oligothiophenes. Inorg. Chem. 1995, 34, 1562–1575. [Google Scholar] [CrossRef]
- Stradomska, D.; Heba, M.; Czernek, A.; Kuźnik, N.; Gillner, D.; Maresz, K.; Pudło, W.; Jarzębski, A.; Szymańska, K. Lipase Immobilized on Mcfs as Biocatalysts for Kinetic and Dynamic Kinetic Resolution of Sec-Alcohols. Catalysts 2021, 11, 518. [Google Scholar] [CrossRef]
- Lozano, P.; de Diego, T.; Gmouh, S.; Vaultier, M.; Iborra, J.L. Dynamic Structure-Function Relationships in Enzyme Stabilization by Ionic Liquids. Biocatal. Biotransform. 2005, 23, 169–176. [Google Scholar] [CrossRef]
- Fukushima, T.; Aida, T. Ionic Liquids for Soft Functional Materials with Carbon Nanotubes. Chem. Eur. J. 2007, 13, 5048–5058. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Iqbal, S.; Shah, M.; Maalik, A.; Hussain, R.; Khan, S.; Khan, I.; Pashameah, R.A.; Alzahrani, E.; Farouk, A.-E.; et al. New Quinoline-Based Triazole Hybrid Analogs as Effective Inhibitors of α-Amylase and α-Glucosidase: Preparation, in Vitro Evaluation, and Molecular Docking along with in Silico Studies. Front. Chem. 2022, 10, 995820. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Hyun, M.K.; Kim, D.; Ahn, Y.; Park, J. Dynamic Kinetic Resolution of Secondary Alcohols by Enzyme-Metal Combinations in Ionic Liquid. Green Chem. 2004, 6, 471–474. [Google Scholar] [CrossRef]
- van Rantwijk, F.; Sheldon, R.A. Biocatalysis in Ionic Liquids. Chem. Rev. 2007, 107, 2757–2785. [Google Scholar] [CrossRef] [PubMed]
- Verho, O.; Bäckvall, J.E. Chemoenzymatic Dynamic Kinetic Resolution: A Powerful Tool for the Preparation of Enantiomerically Pure Alcohols and Amines. J. Am. Chem. Soc. 2015, 137, 3996–4009. [Google Scholar] [CrossRef]
- Martín-Matute, B.; Edin, M.; Bogár, K.; Kaynak, F.B.; Bäckvall, J.E. Combined Ruthenium(II) and Lipase Catalysis for Efficient Dynamic Kinetic Resolution of Secondary Alcohols. Insight into the Racemization Mechanism. J. Am. Chem. Soc. 2005, 127, 8817–8825. [Google Scholar] [CrossRef]
- Kim, N.; Ko, S.B.; Min, S.K.; Kim, M.J.; Park, J. Air-Stable Racemization Catalyst for Dynamic Kinetic Resolution of Secondary Alcohols at Room Temperature. Org. Lett. 2005, 7, 4523–4526. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and Comparison of Hydrophilic and Hydrophobic Room Temperature Ionic Liquids Incorporating the Imidazolium Cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Szelwicka, A.; Wolny, A.; Grymel, M.; Jurczyk, S.; Boncel, S.; Chrobok, A. Chemo-Enzymatic Baeyer–Villiger Oxidation Facilitated with Lipases Immobilized in the Supported Ionic Liquid Phase. Materials 2021, 14, 3443. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).