In Situ Assembly of Well-Defined MoS2 Slabs on Shape-Tailored Anatase TiO2 Nanostructures: Heterojunctions Role in Phenol Photodegradation
Abstract
1. Introduction
2. Results and Discussion
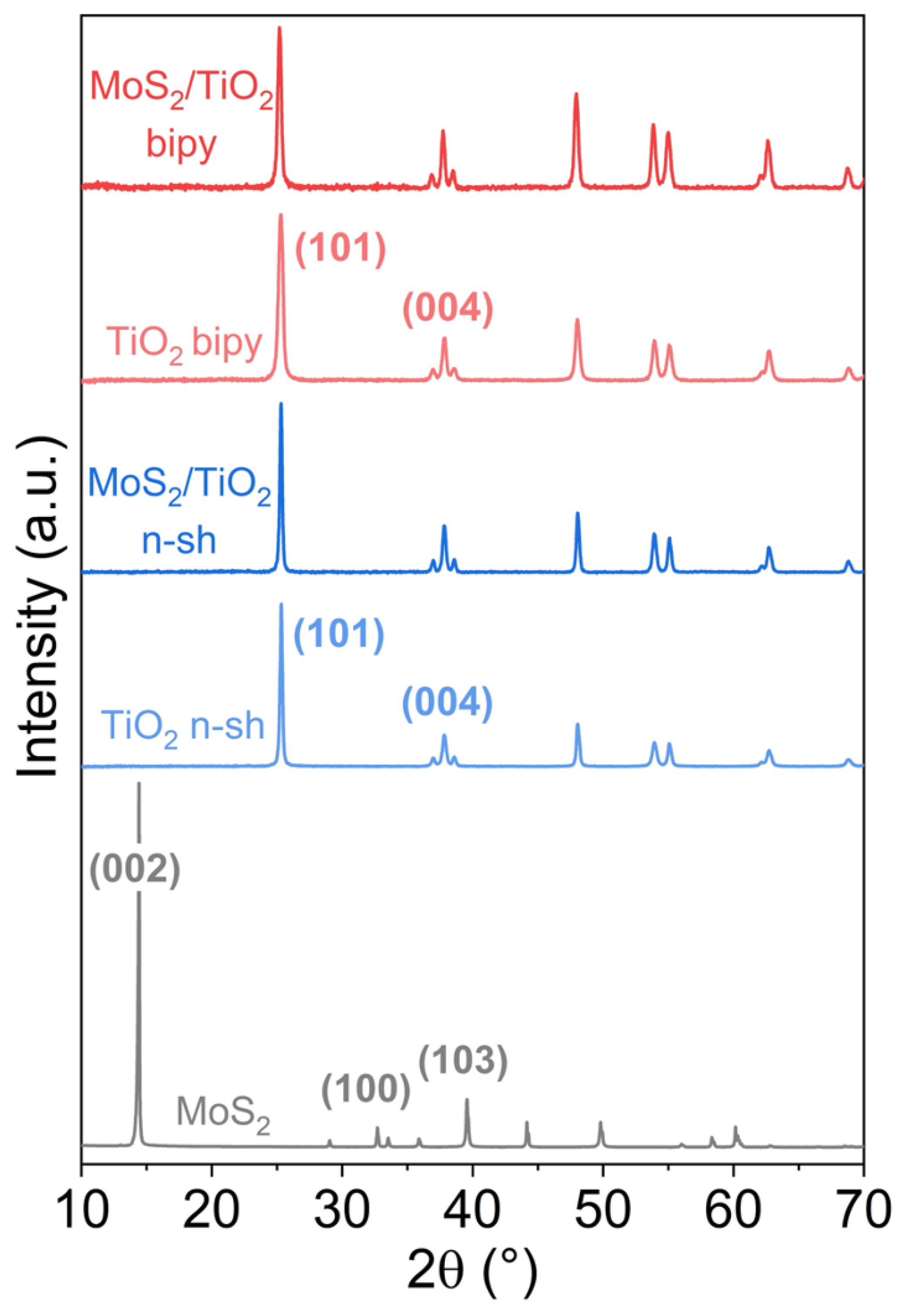
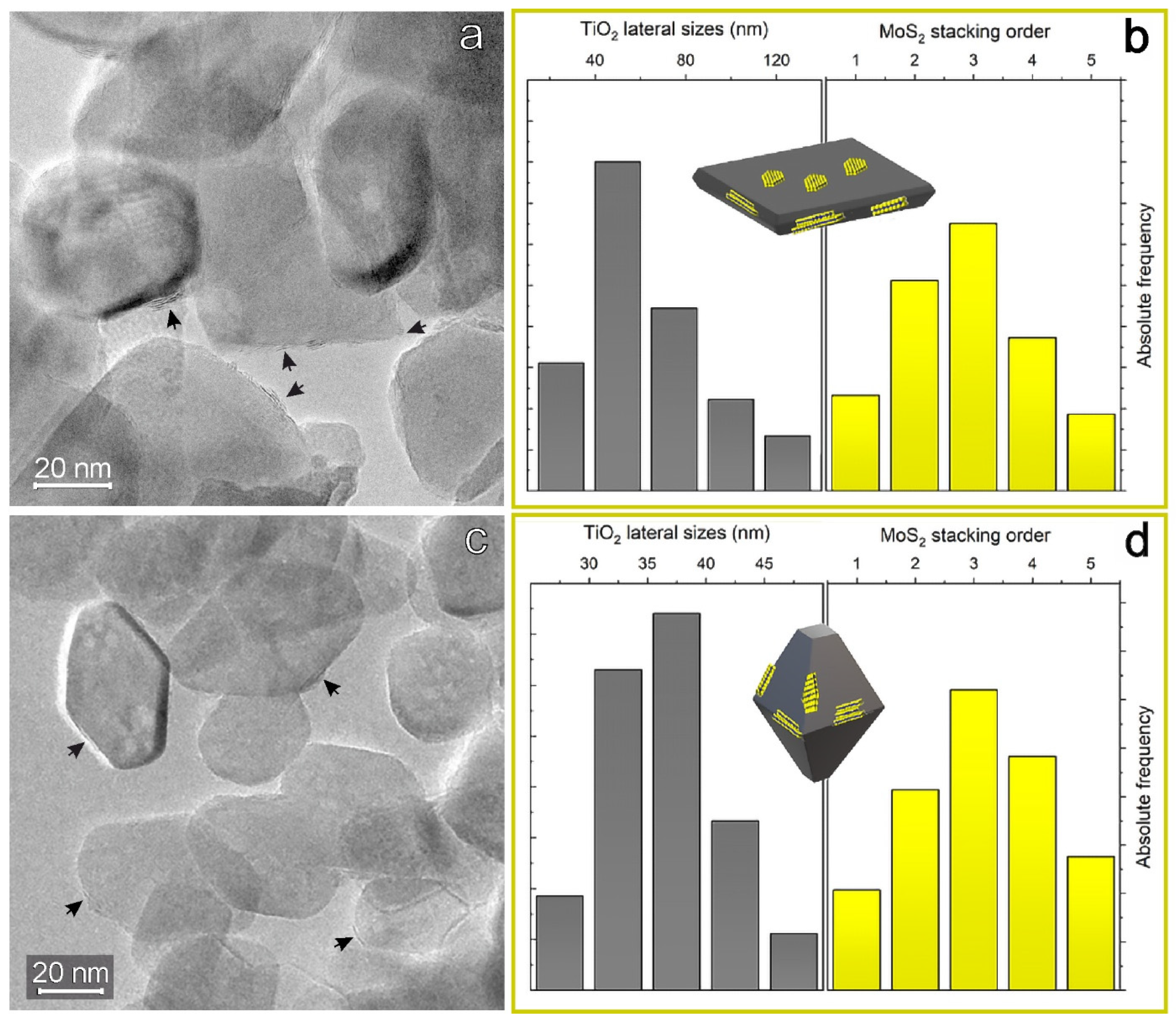
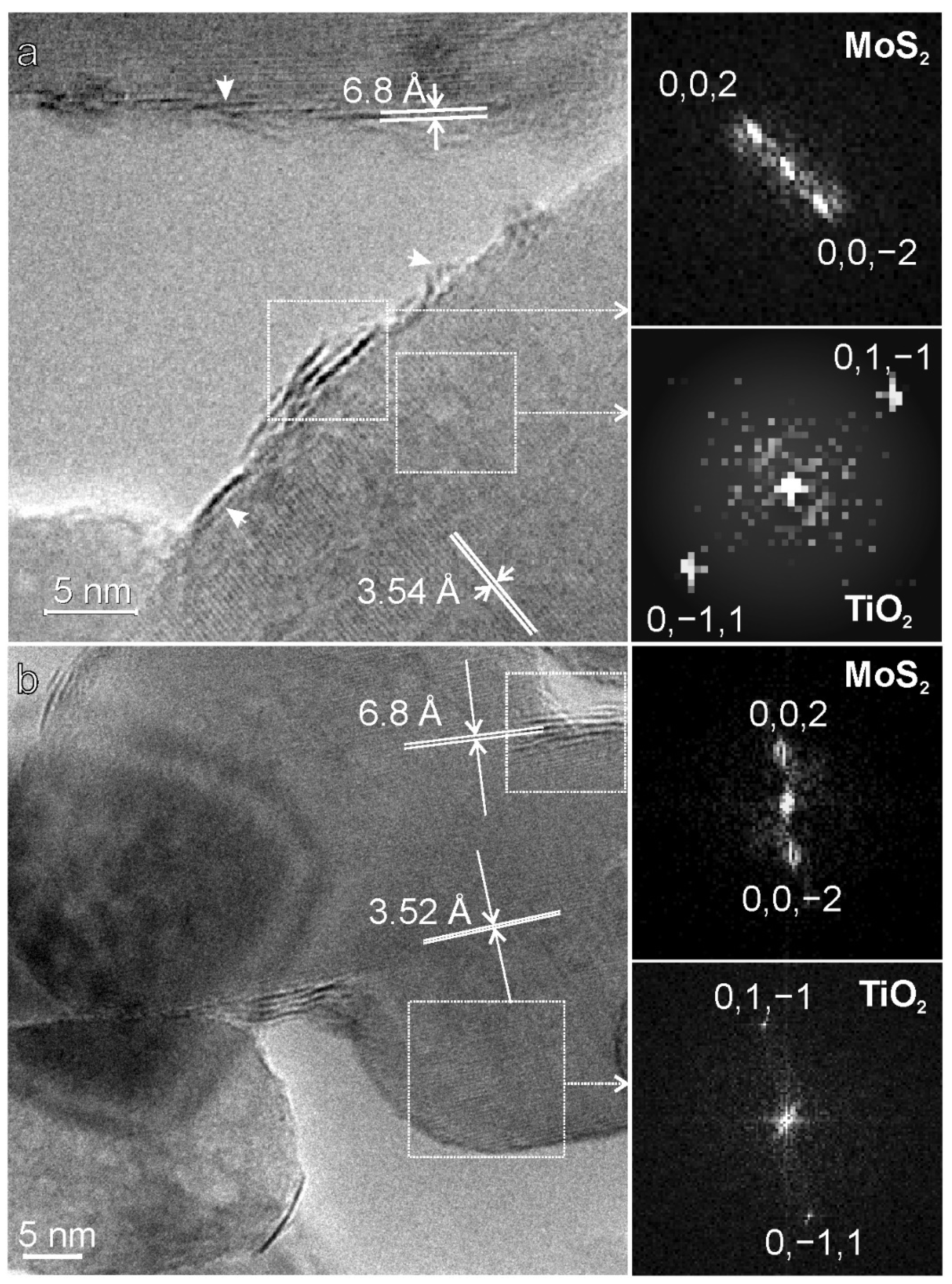
2.1. Structure and Morphology of the MoS2/TiO2 Nanoparticles by XRD, HRTEM, and Raman Analyses
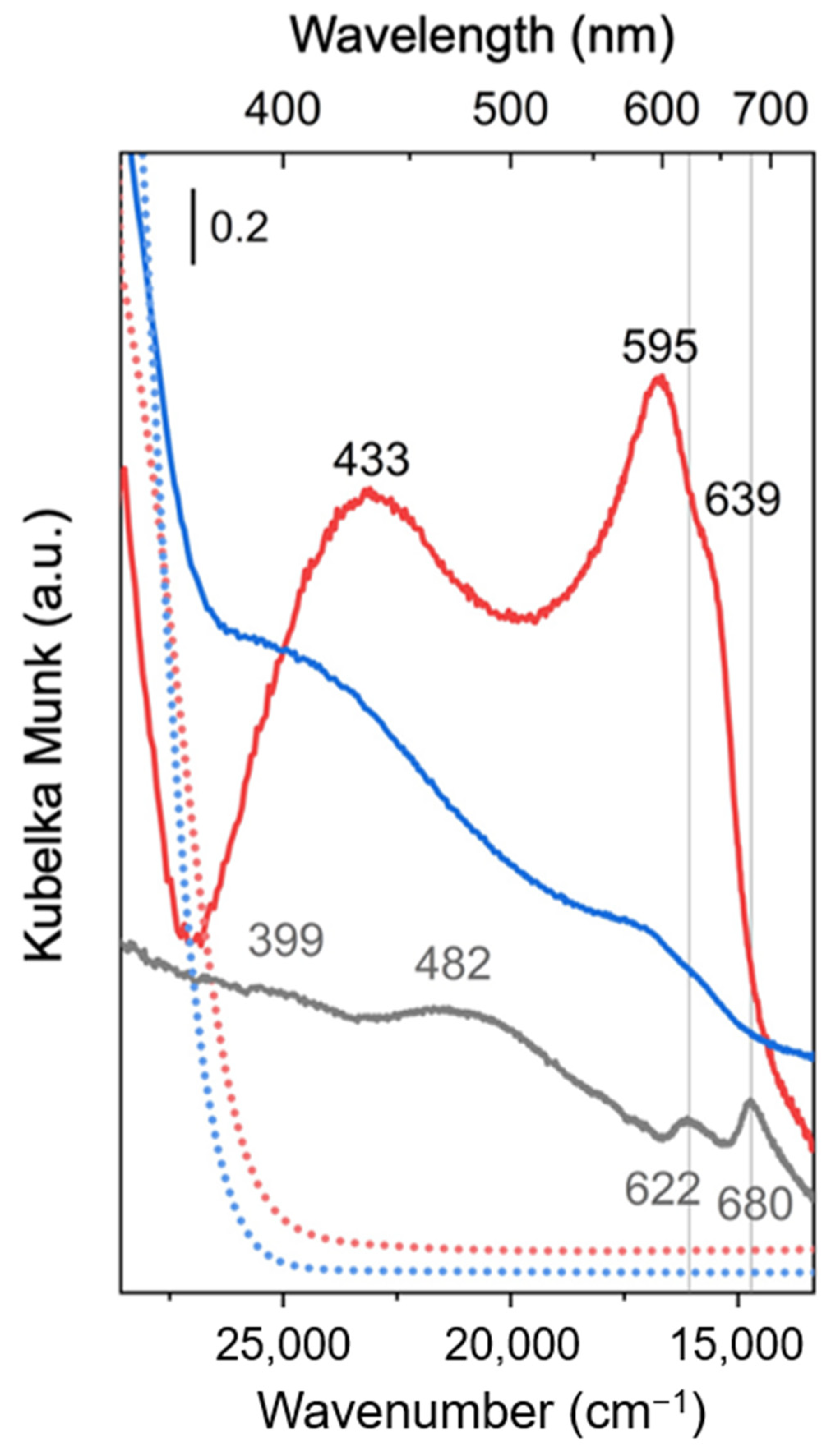
2.2. Optical Properties by UV-Vis Spectroscopy
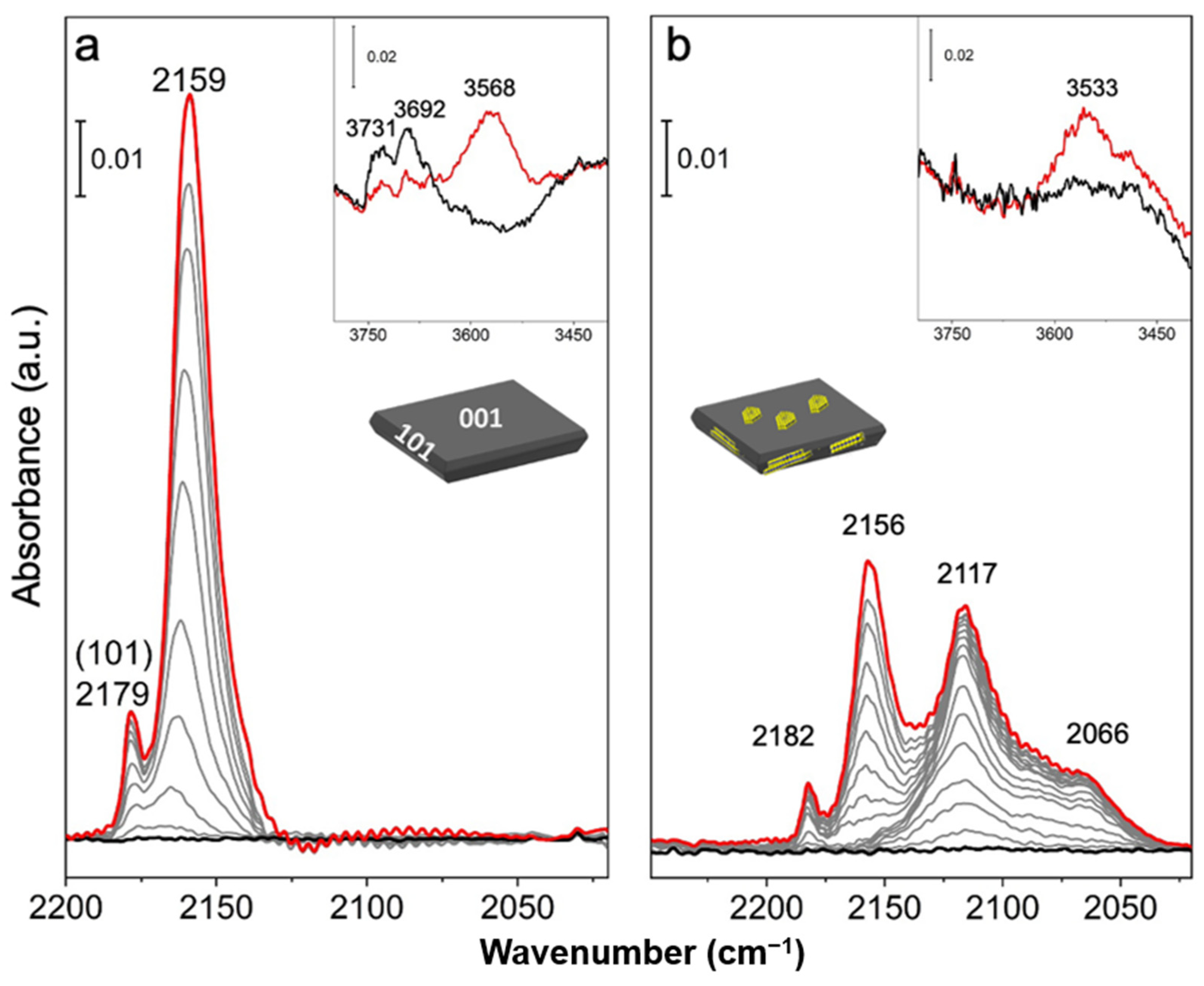
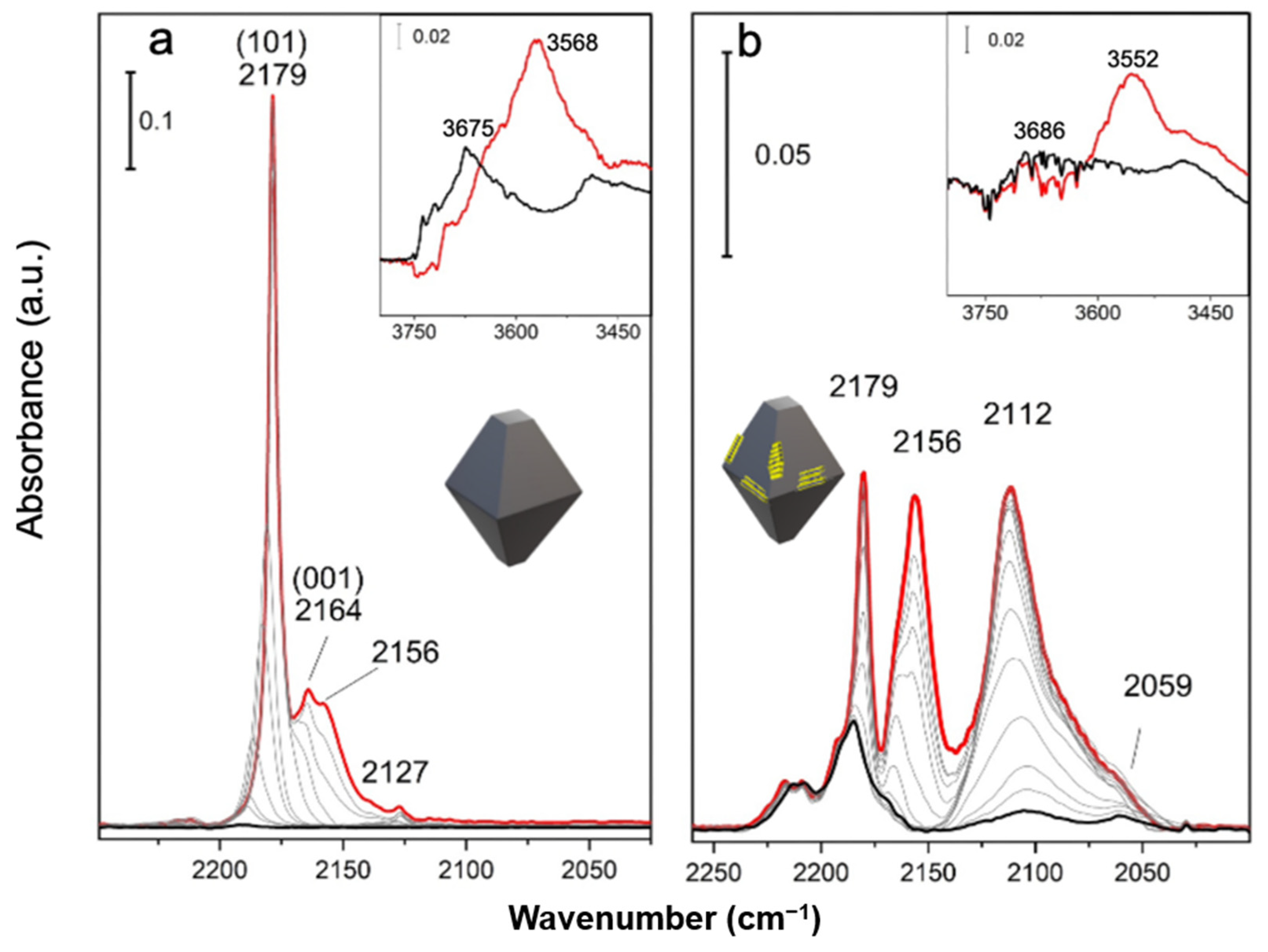
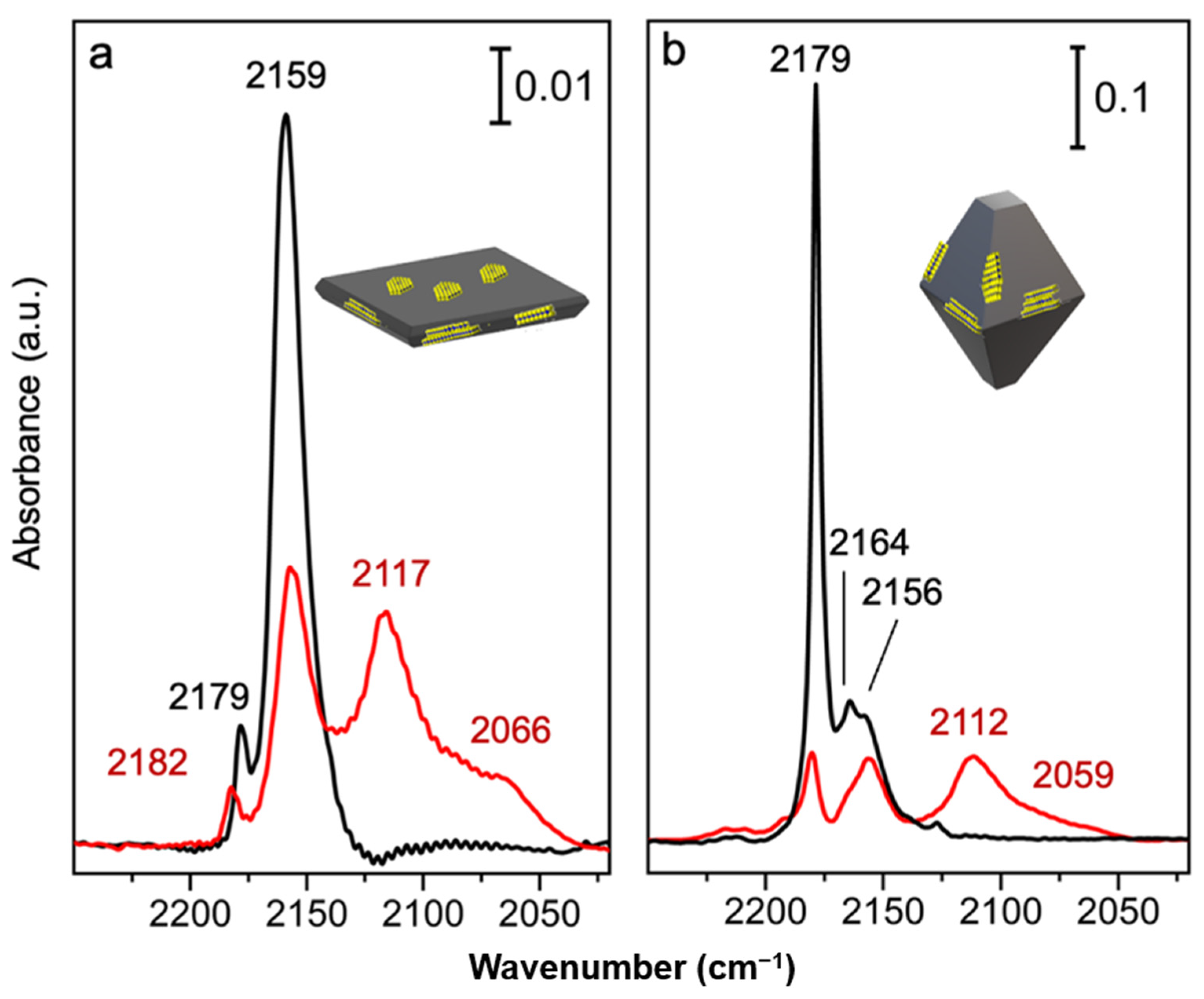
2.3. Surface Vibrational Properties by FTIR Spectra of CO Adsorbed on MoS2/TiO2 n-sh and on MoS2/TiO2 Bipy
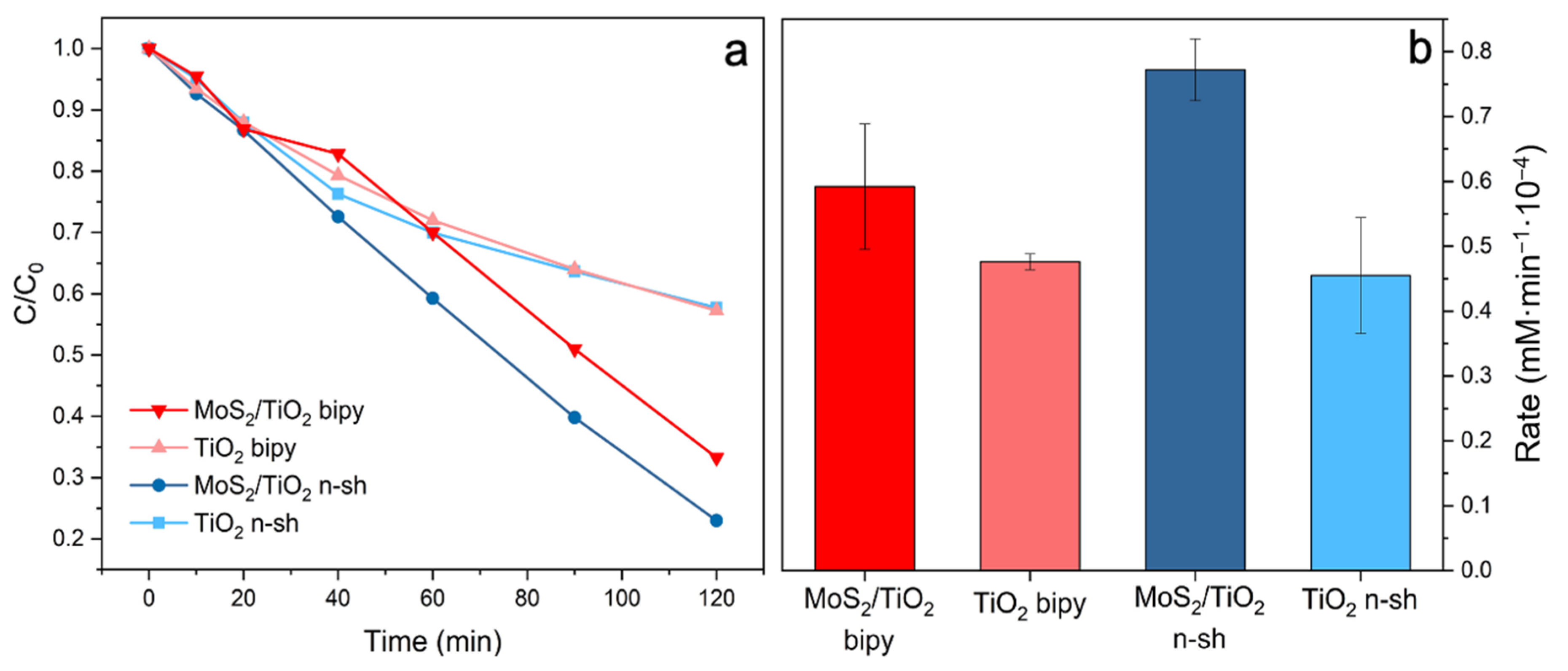
2.4. Photocatalytic Activity
3. Materials and Methods
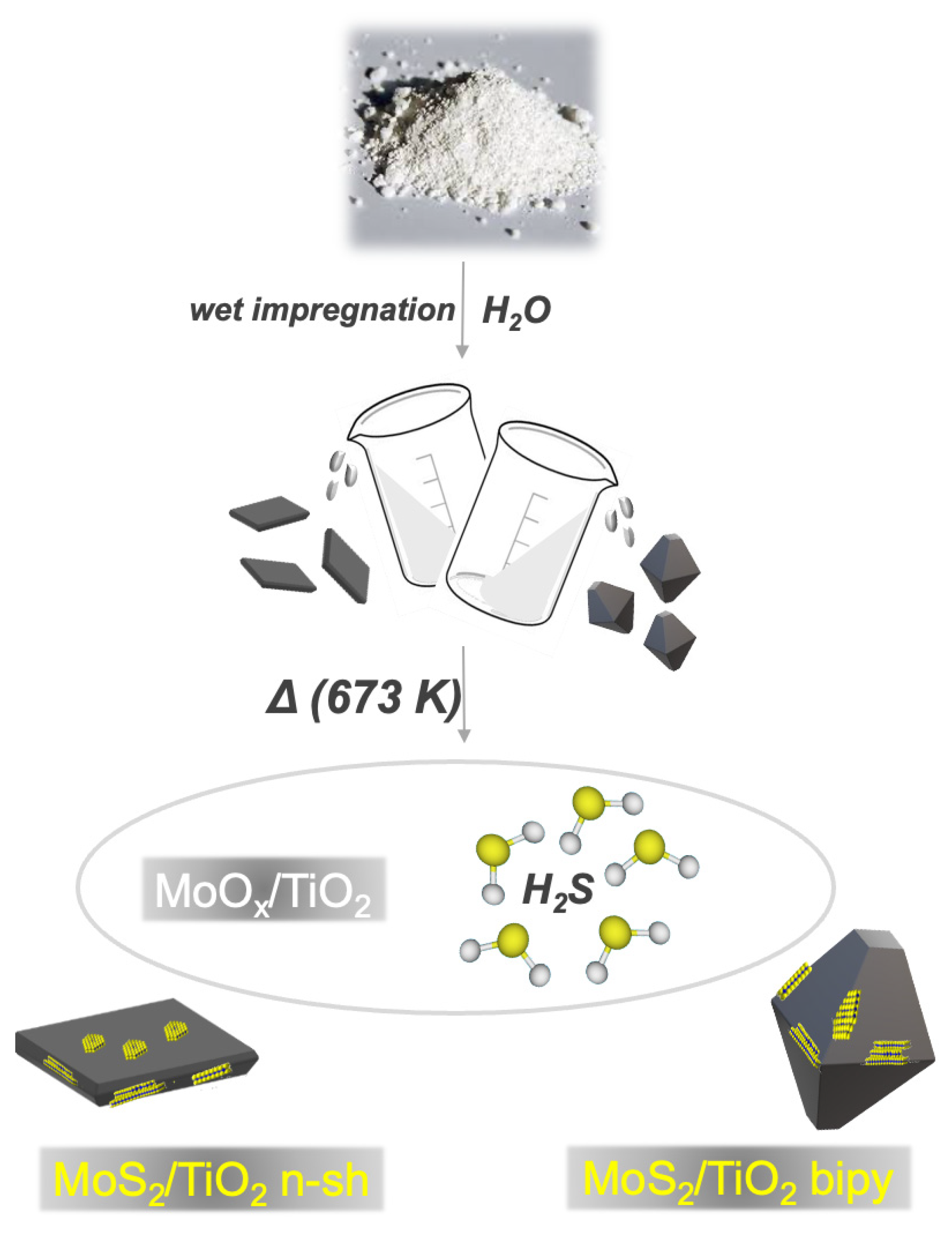
3.1. Synthesis of the Samples
3.1.1. TiO2 Nanosheets
3.1.2. TiO2 Bipyramidal Nanoparticles
3.1.3. Synthesis of the MoS2/TiO2 n-sh (Nanosheets) and MoS2/TiO2 Bipy (Bipyramids) by In Situ Method
3.2. Characterization Methods
3.3. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Huang, H.; Jin, B.; Luo, J.; Zhou, X. Facile synthesis of MoS2/B-TiO2 nanosheets with exposed {001} facets and enhanced visible-light-driven photocatalytic H2 production activity. RSC Adv. 2016, 6, 107075–107080. [Google Scholar] [CrossRef]
- Chen, C.; Xin, X.; Zhang, J.; Li, G.; Zhang, Y.; Lu, H.; Gao, J.; Yang, Z.; Wang, C.; He, Z. Few-Layered MoS2 Nanoparticles Loaded TiO2 Nanosheets with Exposed {001} Facets for Enhanced Photocatalytic Activity. Nano 2018, 13, 1850129. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; Lu, Z.; Jin, Z.; Wang, X.; Xu, G.; Zhang, E.; Wang, H.; Kong, Z.; Xi, J.; et al. Crystal face regulating MoS2/TiO2 (001) heterostructure for high photocatalytic activity. J. Alloys Compd. 2016, 688, 840–848. [Google Scholar] [CrossRef]
- Cao, L.; Wang, R.; Wang, D.; Li, X.; Jia, H. MoS2-hybridized TiO2 nanosheets with exposed {001} facets to enhance the visible-light photocatalytic activity. Mater. Lett. 2015, 160, 286–290. [Google Scholar] [CrossRef]
- Jia, P.-Y.; Guo, R.-T.; Pan, W.-G.; Huang, C.-Y.; Tang, J.-Y.; Liu, X.-Y.; Qin, H.; Xu, Q.-Y. The MoS2/TiO2 heterojunction composites with enhanced activity for CO2 photocatalytic reduction under visible light irradiation. Coll. Surf. A Phys. Engin. Asp. 2019, 570, 306–316. [Google Scholar] [CrossRef]
- Santalucia, R.; Vacca, T.; Cesano, F.; Martra, G.; Pellegrino, F.; Scarano, D. Few-layered MoS2 nanoparticles covering anatase TiO2 nanosheets: Comparison between ex situ and in situ synthesis approaches. Appl. Sci. 2021, 11, 143. [Google Scholar] [CrossRef]
- Chatterjee, D.; Mahata, A. Visible light induced photodegradation of organic pollutants on dye adsorbed TiO2 surface. J. Photochem. Photob. A Chem. 2002, 153, 199–204. [Google Scholar] [CrossRef]
- Matos, J.; García, A.; Zhao, L.; Titirici, M.M. Solvothermal carbon-doped TiO2 photocatalyst for the enhanced methylene blue degradation under visible light. Appl. Catal. A Gen. 2010, 390, 175–182. [Google Scholar] [CrossRef]
- Cheng, Y.; Geng, H.; Huang, X. Advanced water splitting electrocatalysts via the design of multicomponent heterostructures. Dalton Trans. 2020, 49, 2761–2765. [Google Scholar] [CrossRef]
- Uddin, M.J.; Daramola, D.; Velasquez, E.; Dickens, T.; Yan, J.; Hammel, E.; Cesano, F.; Okoli, O.I. A high efficiency 3D photovoltaic microwire with carbon nanotubes (CNT)-quantum dot (QD) hybrid interface. Phys. Status Solidi (RRL)—Rapid Res. Lett. 2014, 8, 898–903. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Fu, W.; Fang, Z.; Zhou, W.; Liu, Z. Two-dimensional heterostructures: Fabrication, characterization, and application. Nanoscale 2014, 6, 12250–12272. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mi, B. Environmental Applications of 2D Molybdenum Disulfide (MoS2) Nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-J.; Ye, Z.-J.; Lu, H.-W.; Hu, B.; Li, Y.-H.; Chen, D.-Q.; Zhong, J.-S.; Yu, Z.-T.; Zou, Z.-G. Constructing Anatase TiO2 Nanosheets with Exposed (001) Facets/Layered MoS2 Two-Dimensional Nanojunctions for Enhanced Solar Hydrogen Generation. ACS Catal. 2016, 6, 532–541. [Google Scholar] [CrossRef]
- Chen, B.; Meng, Y.; Sha, J.; Zhong, C.; Hu, W.; Zhao, N. Preparation of MoS2/TiO2 based nanocomposites for photocatalysis and rechargeable batteries: Progress, challenges, and perspective. Nanoscale 2018, 10, 34–68. [Google Scholar] [CrossRef] [PubMed]
- Cesano, F.; Pellerej, D.; Scarano, D.; Ricchiardi, G.; Zecchina, A. Radially organized pillars of TiO2 nanoparticles: Synthesis, characterization and photocatalytic tests. J. Photochem. Photob. A Chem. 2012, 242, 51–58. [Google Scholar] [CrossRef]
- Haque, M.M.; Khan, A.; Umar, K.; Mir, N.A.; Muneer, M.; Harada, T.; Matsumura, M. Synthesis, Characterization and Photocatalytic Activity of Visible Light Induced Ni-Doped TiO2. Energy Environ. Focus 2013, 2, 73–78. [Google Scholar] [CrossRef]
- Cravanzola, S.; Cesano, F.; Gaziano, F.; Scarano, D. Sulfur-Doped TiO2: Structure and Surface Properties. Catalysts 2017, 7, 214. [Google Scholar] [CrossRef]
- Scarano, D.; Cesano, F.; Zecchina, A. MoS2 Domains on TiO2-Based Nanostructures: Role of Titanate/TiO2 Transformation and Sulfur Doping on the Interaction with the Support. J. Phys. Chem. C 2019, 123, 7799–7809. [Google Scholar] [CrossRef]
- Yashwanth, H.J.; Rondiya, S.R.; Dzade, N.Y.; Hoye, R.L.Z.; Choudhary, R.J.; Phase, D.M.; Dhole, S.D.; Hareesh, K. Improved photocatalytic activity of TiO2 nanoparticles through nitrogen and phosphorus co-doped carbon quantum dots: An experimental and theoretical study. Phys. Chem. Chem. Phys. 2022, 24, 15271–15279. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Z.; Arif, N.; Jiang, W.-C.; Zeng, Y.-J. 2D material based heterostructures for solar light driven photocatalytic H2 production. Mater. Adv. 2022, 3, 3389–3417. [Google Scholar] [CrossRef]
- Du, R.; Li, B.; Han, X.; Xiao, K.; Wang, X.; Zhang, C.; Arbiol, J.; Cabot, A. 2D/2D Heterojunction of TiO2 Nanoparticles and Ultrathin g–C3N4 Nanosheets for Efficient Photocatalytic Hydrogen Evolution. Nanomaterials 2022, 12, 1557. [Google Scholar] [CrossRef] [PubMed]
- Cravanzola, S.; Sarro, M.; Cesano, F.; Calza, P.; Scarano, D. Few-Layer MoS₂ Nanodomains Decorating TiO₂ Nanoparticles: A Case Study for the Photodegradation of Carbamazepine. Nanomaterials 2018, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Yan, Z.; Yang, L.; Du, P.; Zhang, J.; Xiang, B. MoS2 nanosheet/TiO2 nanowire hybrid nanostructures for enhanced visible-light photocatalytic activities. Chem. Commun. 2014, 50, 15447–15449. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.-D.; Heyde, M.; Freund, H.-J. Charge Control in Model Catalysis: The Decisive Role of the Oxide–Nanoparticle Interface. Chem. A Eur. J. 2018, 24, 2317–2327. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.H.; Hu, X.G.; Xu, Y.F.; Pan, X.Z. The effect of morphology and size on the photocatalytic properties of MoS2. React. Kinet. Mech. Catal. 2010, 100, 153–163. [Google Scholar] [CrossRef]
- Goswami, N.; Giri, A.; Pal, S.K. MoS2 Nanocrystals Confined in a DNA Matrix Exhibiting Energy Transfer. Langmuir 2013, 29, 11471–11478. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Zhang, X.-Q.; Zhang, W.; Chang, M.-T.; Lin, C.-T.; Chang, K.-D.; Yu, Y.-C.; Wang, J.T.-W.; Chang, C.-S.; Li, L.-J.; et al. Synthesis of large-area MoS2 atomic layers with chemical vapor deposition. Adv. Mater. 2012, 24, 2320–2325. [Google Scholar] [CrossRef]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209–231. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, P.; Wei, C. Fabrication of MoS2/TiO2 heterostructures with enhanced photocatalytic activity. CrystEngComm 2019, 21, 3439–3450. [Google Scholar] [CrossRef]
- Wan, J.; Lacey, S.D.; Dai, J.; Bao, W.; Fuhrer, M.S.; Hu, L. Tuning two-dimensional nanomaterials by intercalation: Materials, properties and applications. Chem. Soc. Rev. 2016, 45, 6742–6765. [Google Scholar] [CrossRef] [PubMed]
- Sreepal, V.; Yagmurcukardes, M.; Vasu, K.S.; Kelly, D.J.; Taylor, S.F.R.; Kravets, V.G.; Kudrynskyi, Z.; Kovalyuk, Z.D.; Patanè, A.; Grigorenko, A.N.; et al. Two-Dimensional Covalent Crystals by Chemical Conversion of Thin van der Waals Materials. Nano Lett. 2019, 19, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Song, P.; Wang, C.; Riis-Jensen, A.C.; Fu, W.; Deng, Y.; Wan, D.; Kang, L.; Ning, S.; Dan, J.; et al. Engineering covalently bonded 2D layered materials by self-intercalation. Nature 2020, 581, 171–177. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wong, S.S. Size- and Shape-Dependent Transformation of Nanosized Titanate into Analogous Anatase Titania Nanostructures. JACS 2006, 128, 8217–8226. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Wada, E.; Nakajima, K.; Hayashi, S.; Miyazaki, S.; Kobayashi, H.; Hara, M. Protonated Titanate Nanotubes with Lewis and Brønsted Acidity: Relationship between Nanotube Structure and Catalytic Activity. Chem. Mater. 2013, 25, 385–393. [Google Scholar] [CrossRef]
- Cravanzola, S.; Muscuso, L.; Cesano, F.; Agostini, G.; Damin, A.; Scarano, D.; Zecchina, A. MoS2 Nanoparticles Decorating Titanate-Nanotube Surfaces: Combined Microscopy, Spectroscopy, and Catalytic Studies. Langmuir 2015, 31, 5469–5478. [Google Scholar] [CrossRef]
- Cesano, F.; Bertarione, S.; Piovano, A.; Agostini, G.; Rahman, M.M.; Groppo, E.; Bonino, F.; Scarano, D.; Lamberti, C.; Bordiga, S.; et al. Model oxide supported MoS2 HDS catalysts: Structure and surface properties. Catal. Sci. Technol. 2011, 1, 123–136. [Google Scholar] [CrossRef]
- Cravanzola, S.; Cesano, F.; Gaziano, F.; Scarano, D. Carbon Domains on MoS2/TiO2 System via Catalytic Acetylene Oligomerization: Synthesis, Structure, and Surface Properties. Front. Chem. 2017, 5, 91. [Google Scholar] [CrossRef]
- Cesano, F.; Bertarione, S.; Uddin, M.J.; Agostini, G.; Scarano, D.; Zecchina, A. Designing TiO2 Based Nanostructures by Control of Surface Morphology of Pure and Silver Loaded Titanate Nanotubes. J. Phys. Chem. C 2010, 114, 169–178. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties, and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Cesano, F.; Cravanzola, S.; Rahman, M.M.; Scarano, D. Interplay between Fe-Titanate Nanotube Fragmentation and Catalytic Decomposition of C2H4: Formation of C/TiO2 Hybrid Interfaces. Inorganics 2018, 6, 55. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Lu, G.Q.M.; Cheng, H.-M. On the True Photoreactivity Order of {001}, {010}, and {101} Facets of Anatase TiO2 Crystals. Angew. Chem. Int. Ed. 2011, 50, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Yu, W.; Jiang, F.; Zhang, E.; Wang, H.; Kong, Z.; Xi, J.; Ji, Z. Novel dual heterojunction between MoS2 and anatase TiO2 with coexposed {101} and {001} facets. J. Am. Ceram. Soc. 2017, 100, 5274–5285. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Ma, X.; Ji, Z. A study of constructing heterojunction between two-dimensional transition metal sulfides (MoS2 and WS2) and (101), (001) faces of TiO2. Appl. Surf. Sci. 2018, 430, 424–437. [Google Scholar] [CrossRef]
- Mino, L.; Pellegrino, F.; Rades, S.; Radnik, J.; Hodoroaba, V.-D.; Spoto, G.; Maurino, V.; Martra, G. Beyond Shape Engineering of TiO2 Nanoparticles: Post-Synthesis Treatment Dependence of Surface Hydration, Hydroxylation, Lewis Acidity and Photocatalytic Activity of TiO2 Anatase Nanoparticles with Dominant {001} or {101} Facets. ACS Appl. Nano Mater. 2018, 1, 5355–5365. [Google Scholar] [CrossRef]
- Kumar, S.; Shakya, J.; Mohanty, T. Probing interfacial charge transfer dynamics in MoS2/TiO2 nanocomposites using scanning Kelvin probe for improved photocatalytic response. Surf. Sci. 2020, 693, 121530. [Google Scholar] [CrossRef]
- Zhu, K.-R.; Zhang, M.-S.; Chen, Q.; Yin, Z. Size and phonon-confinement effects on low-frequency Raman mode of anatase TiO2 nanocrystal. Phys. Lett. A 2005, 340, 220–227. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Mignuzzi, S.; Pollard, A.J.; Bonini, N.; Brennan, B.; Gilmore, I.S.; Pimenta, M.A.; Richards, D.; Roy, D. Effect of disorder on Raman scattering of single-layer MoS2. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 91, 195411. [Google Scholar] [CrossRef]
- Muscuso, L.; Cravanzola, S.; Cesano, F.; Scarano, D.; Zecchina, A. Optical, Vibrational, and Structural Properties of MoS2 Nanoparticles Obtained by Exfoliation and Fragmentation via Ultrasound Cavitation in Isopropyl Alcohol. J. Phys. Chem. C 2015, 119, 3791–3801. [Google Scholar] [CrossRef]
- Wilcoxon, J.P.; Newcomer, P.P.; Samara, G.A. Synthesis and optical properties of MoS2 and isomorphous nanoclusters in the quantum confinement regime. J. Appl. Phys. 1997, 81, 7934–7944. [Google Scholar] [CrossRef]
- Shi, H.; Yan, R.; Bertolazzi, S.; Brivio, J.; Gao, B.; Kis, A.; Jena, D.; Xing, H.G.; Huang, L. Exciton Dynamics in Suspended Monolayer and Few-Layer MoS2 2D Crystals. ACS Nano 2013, 7, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Tsyganenko, A.A.; Can, F.; Travert, A.; Maugé, F. FTIR study of unsupported molybdenum sulfide—In situ synthesis and surface properties characterization. Appl. Catal. A Gen. 2004, 268, 189–197. [Google Scholar] [CrossRef]
- Bolis, V.; Barbaglia, A.; Bordiga, S.; Lamberti, C.; Zecchina, A. Heterogeneous Nonclassical Carbonyls Stabilized in Cu(I)− and Ag(I)−ZSM-5 Zeolites: Thermodynamic and Spectroscopic Features. J. Phys. Chem. B 2004, 108, 9970–9983. [Google Scholar] [CrossRef]
- Dai, R.; Zhang, A.; Pan, Z.; Al-Enizi, A.M.; Elzatahry, A.A.; Hu, L.; Zheng, G. Epitaxial Growth of Lattice-Mismatched Core–Shell TiO2@MoS2 for Enhanced Lithium-Ion Storage. Small 2016, 12, 2792–2799. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Clausen, B.S.; Topsøe, H.; Lægsgaard, E.; Lauritsen, J.V.; Besenbacher, F. Scanning tunneling microscopy studies of TiO2-supported hydrotreating catalysts: Anisotropic particle shapes by edge-specific MoS2–support bonding. J. Catal. 2009, 263, 98–103. [Google Scholar] [CrossRef]
- Arrouvel, C.; Breysse, M.; Toulhoat, H.; Raybaud, P. A density functional theory comparison of anatase (TiO2)- and γ-Al2O3-supported MoS2 catalysts. J. Catal. 2005, 232, 161–178. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced Photocatalytic CO2-Reduction Activity of Anatase TiO2 by Coexposed {001} and {101} Facets. JACS 2014, 136, 8839–8842. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, J.; Ma, Y.; Qi, L. Mesocrystalline TiO2 nanosheet arrays with exposed {001} facets: Synthesis via topotactic transformation and applications in dye-sensitized solar cells. Nano Res. 2017, 10, 2610–2625. [Google Scholar] [CrossRef]
- Deiana, C.; Minella, M.; Tabacchi, G.; Maurino, V.; Fois, E.; Martra, G. Shape-controlled TiO2 nanoparticles and TiO2 P25 interacting with CO and H2O2 molecular probes: A synergic approach for surface structure recognition and physico-chemical understanding. Phys. Chem. Chem. Phys. 2013, 15, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Panayotov, D.A.; Burrows, S.P.; Morris, J.R. Infrared Spectroscopic Studies of Conduction Band and Trapped Electrons in UV-Photoexcited, H-Atom n-Doped, and Thermally Reduced TiO2. J. Phys. Chem. C 2012, 116, 4535–4544. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, A.; Yamaguchi, S.; Ichikawa, T.; Lal, C.; Jain, I.P. Catalytic effect of TiF4 in improving hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2016, 41, 14178–14183. [Google Scholar] [CrossRef]
- Radnik, J.; Mohr, C.; Claus, P. On the origin of binding energy shifts of core levels of supported gold nanoparticles and dependence of pretreatment and material synthesis. Phys. Chem. Chem. Phys. 2003, 5, 172–177. [Google Scholar] [CrossRef]
- Sugimoto, T.; Zhou, X.; Muramatsu, A. Synthesis of uniform anatase TiO2 nanoparticles by gel–sol method: 3. Formation process and size control. J. Colloid Interface Sci. 2003, 259, 43–52. [Google Scholar] [CrossRef]
- Pellegrino, F.; Isopescu, R.; Pellutiè, L.; Sordello, F.; Rossi, A.M.; Ortel, E.; Martra, G.; Hodoroaba, V.D.; Maurino, V. Machine learning approach for elucidating and predicting the role of synthesis parameters on the shape and size of TiO2 nanoparticles. Sci. Rep. 2020, 10, 18910. [Google Scholar] [CrossRef]
- Signorile, M.; Bonino, F.; Damin, A.; Bordiga, S. A Novel Raman Setup Based on Magnetic-Driven Rotation of Sample. Top. Catal. 2018, 61, 1491–1498. [Google Scholar] [CrossRef]
- Menżyk, A.; Damin, A.; Martyna, A.; Alladio, E.; Vincenti, M.; Martra, G.; Zadora, G. Toward a novel framework for bloodstains dating by Raman spectroscopy: How to avoid sample photodamage and subsampling errors. Talanta 2020, 209, 120565. [Google Scholar] [CrossRef]
- Pellegrino, F.; Pellutiè, L.; Sordello, F.; Minero, C.; Ortel, E.; Hodoroaba, V.-D.; Maurino, V. Influence of agglomeration and aggregation on the photocatalytic activity of TiO2 nanoparticles. Appl. Catal. B Environm. 2017, 216, 80–87. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santalucia, R.; Negro, P.; Vacca, T.; Pellegrino, F.; Damin, A.; Cesano, F.; Scarano, D. In Situ Assembly of Well-Defined MoS2 Slabs on Shape-Tailored Anatase TiO2 Nanostructures: Heterojunctions Role in Phenol Photodegradation. Catalysts 2022, 12, 1414. https://doi.org/10.3390/catal12111414
Santalucia R, Negro P, Vacca T, Pellegrino F, Damin A, Cesano F, Scarano D. In Situ Assembly of Well-Defined MoS2 Slabs on Shape-Tailored Anatase TiO2 Nanostructures: Heterojunctions Role in Phenol Photodegradation. Catalysts. 2022; 12(11):1414. https://doi.org/10.3390/catal12111414
Chicago/Turabian StyleSantalucia, Rosangela, Paolo Negro, Tiziano Vacca, Francesco Pellegrino, Alessandro Damin, Federico Cesano, and Domenica Scarano. 2022. "In Situ Assembly of Well-Defined MoS2 Slabs on Shape-Tailored Anatase TiO2 Nanostructures: Heterojunctions Role in Phenol Photodegradation" Catalysts 12, no. 11: 1414. https://doi.org/10.3390/catal12111414
APA StyleSantalucia, R., Negro, P., Vacca, T., Pellegrino, F., Damin, A., Cesano, F., & Scarano, D. (2022). In Situ Assembly of Well-Defined MoS2 Slabs on Shape-Tailored Anatase TiO2 Nanostructures: Heterojunctions Role in Phenol Photodegradation. Catalysts, 12(11), 1414. https://doi.org/10.3390/catal12111414










