ZrO2-Based Photocatalysts for Wastewater Treatment: From Novel Modification Strategies to Mechanistic Insights
Abstract
:1. Introduction
2. ZrO2 as Photocatalyst
- ZrO2 is rich in OVs, which exist on the surface or in the lattice’s interstitial sites, which further promotes the optoelectronic properties of ZrO2 leading to boosted light harvesting ability and superior isolation of photocarriers [22].
- The optical characteristics of ZrO2 are modified by the introduction of midgap states in the elemental doping, resulting in optimal visible light responsive photocatalyst [22]. By doping with ZrO2, ions diffuse throughout the lattice at interstitial sites, resulting in a new valance band maximum (VBM) or conduction band minimum (CBM), which narrows the optical bandgap of ZrO2 [33].
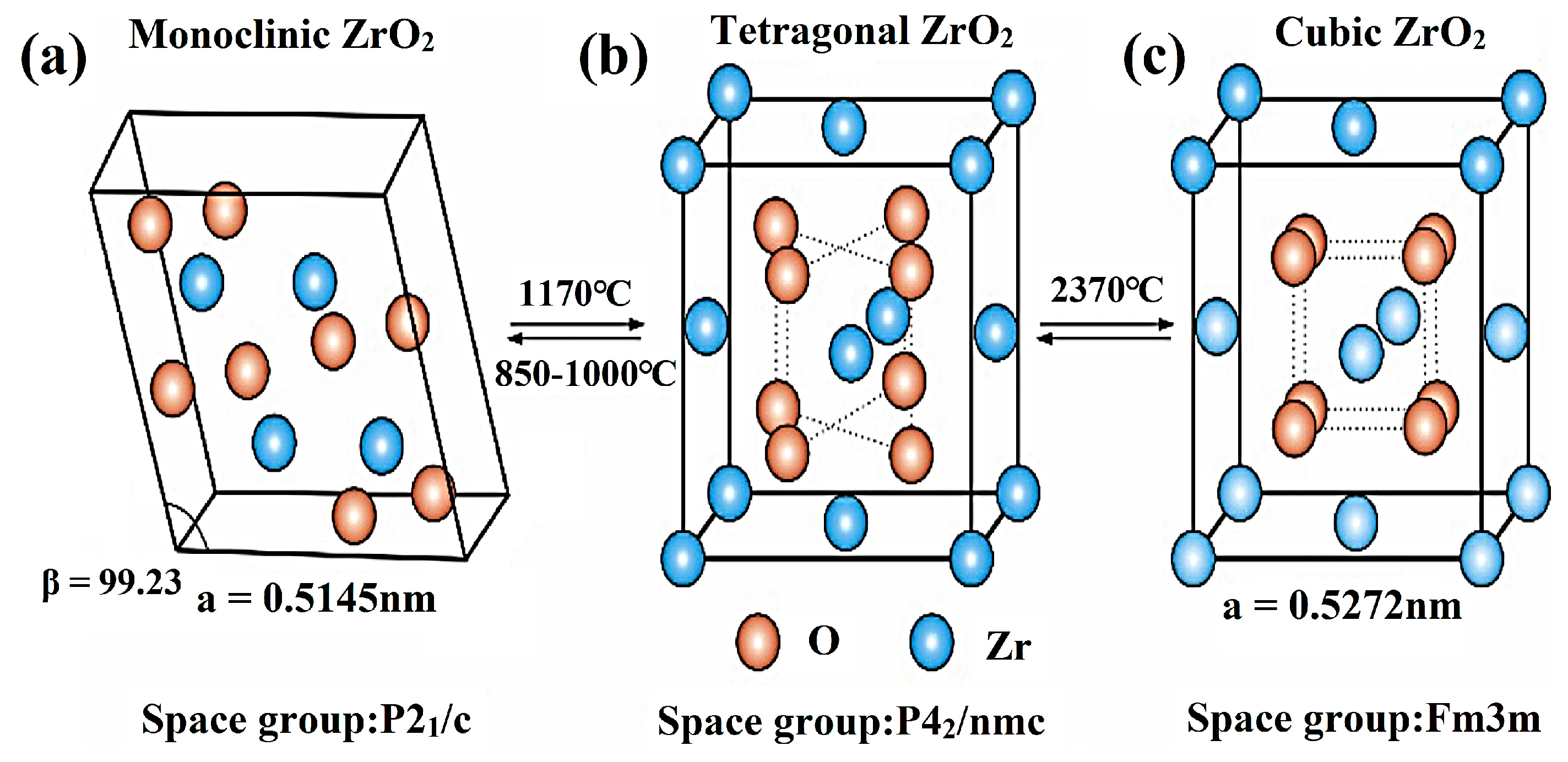
2.1. Crystal Structure
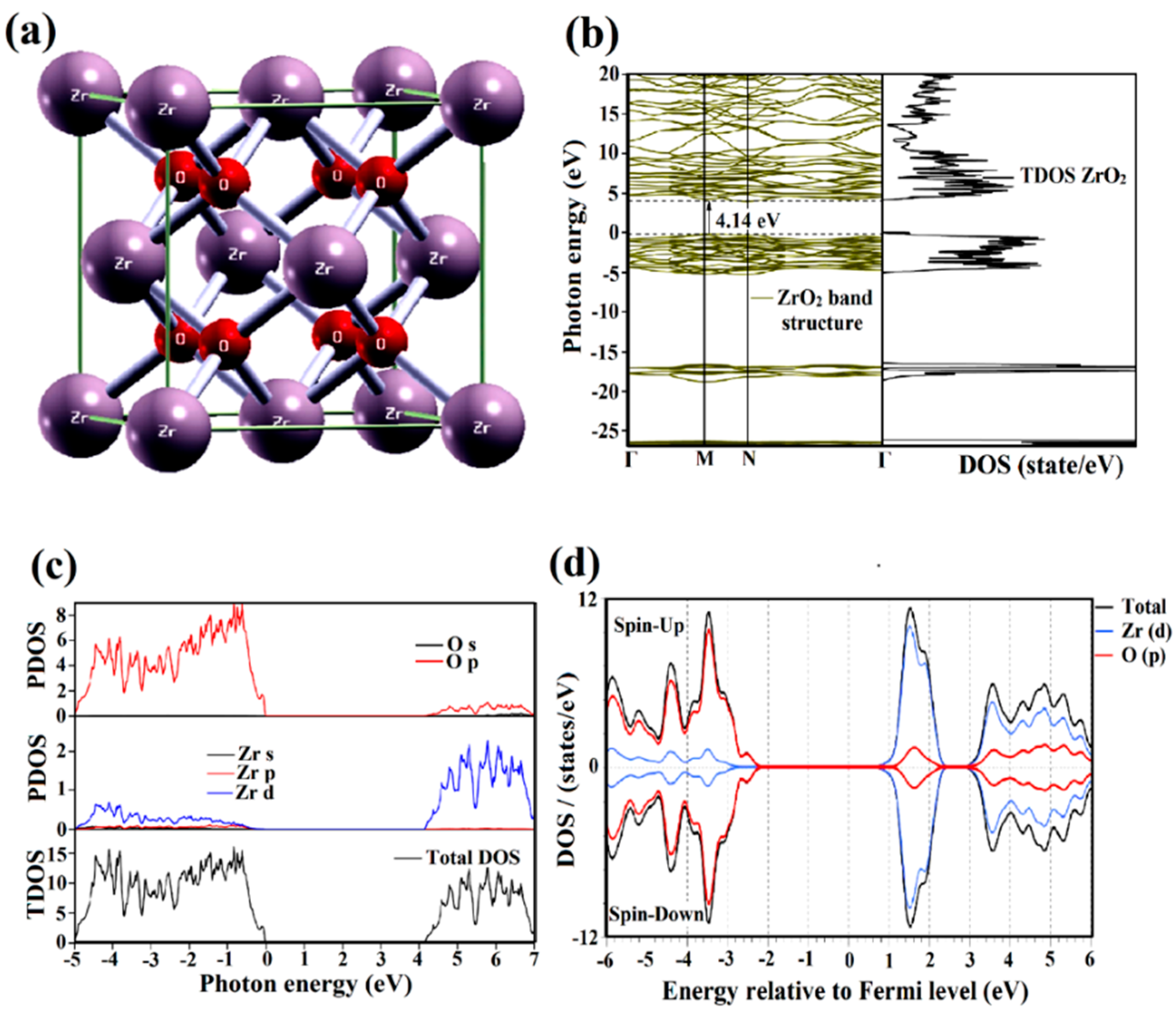
2.2. Bandgap Formation
2.3. Opto-Electronic Properties
3. Modification Strategies
3.1. Morphology and Structural Modulation
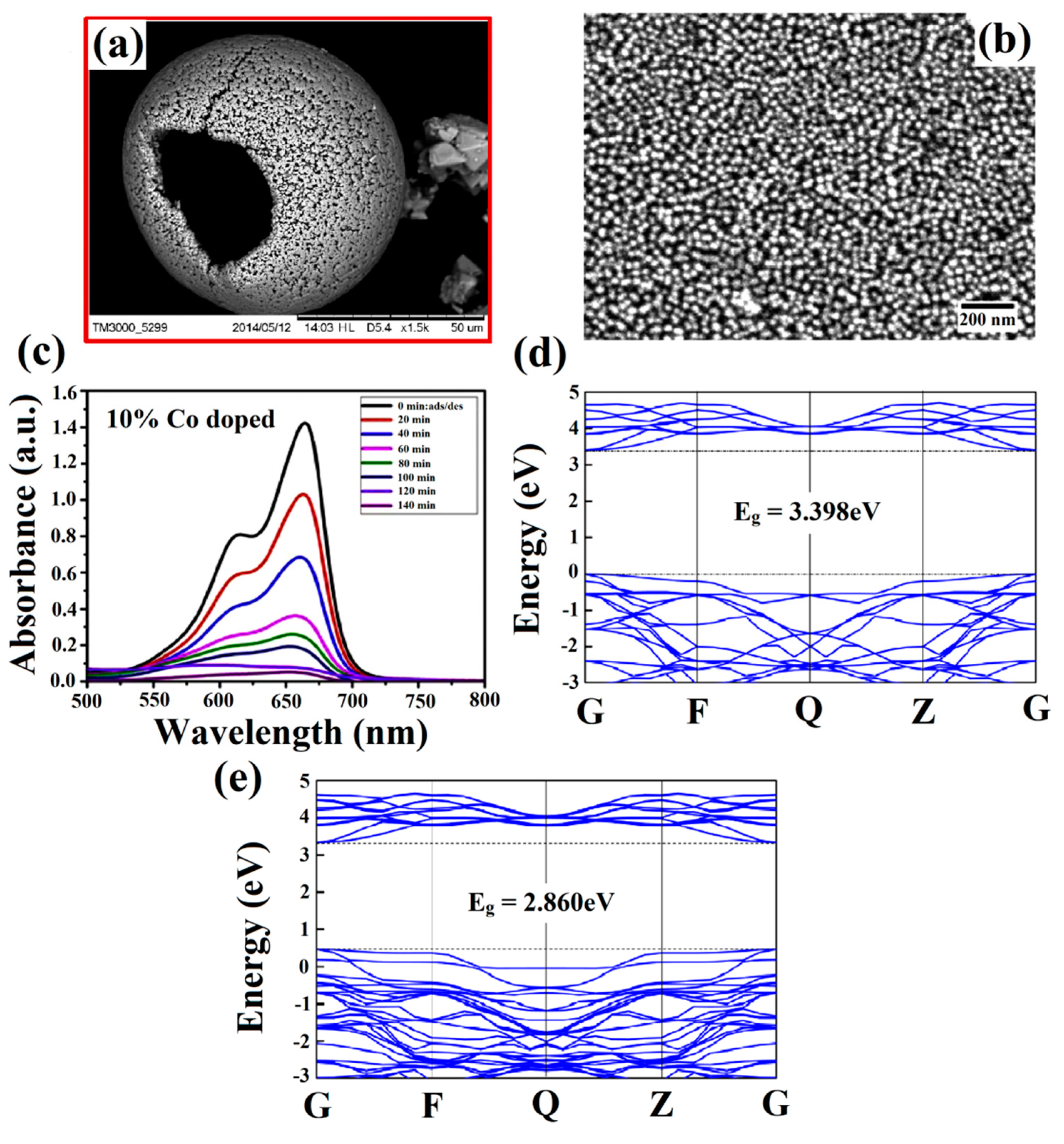
3.2. Bandgap Tuning via Doping
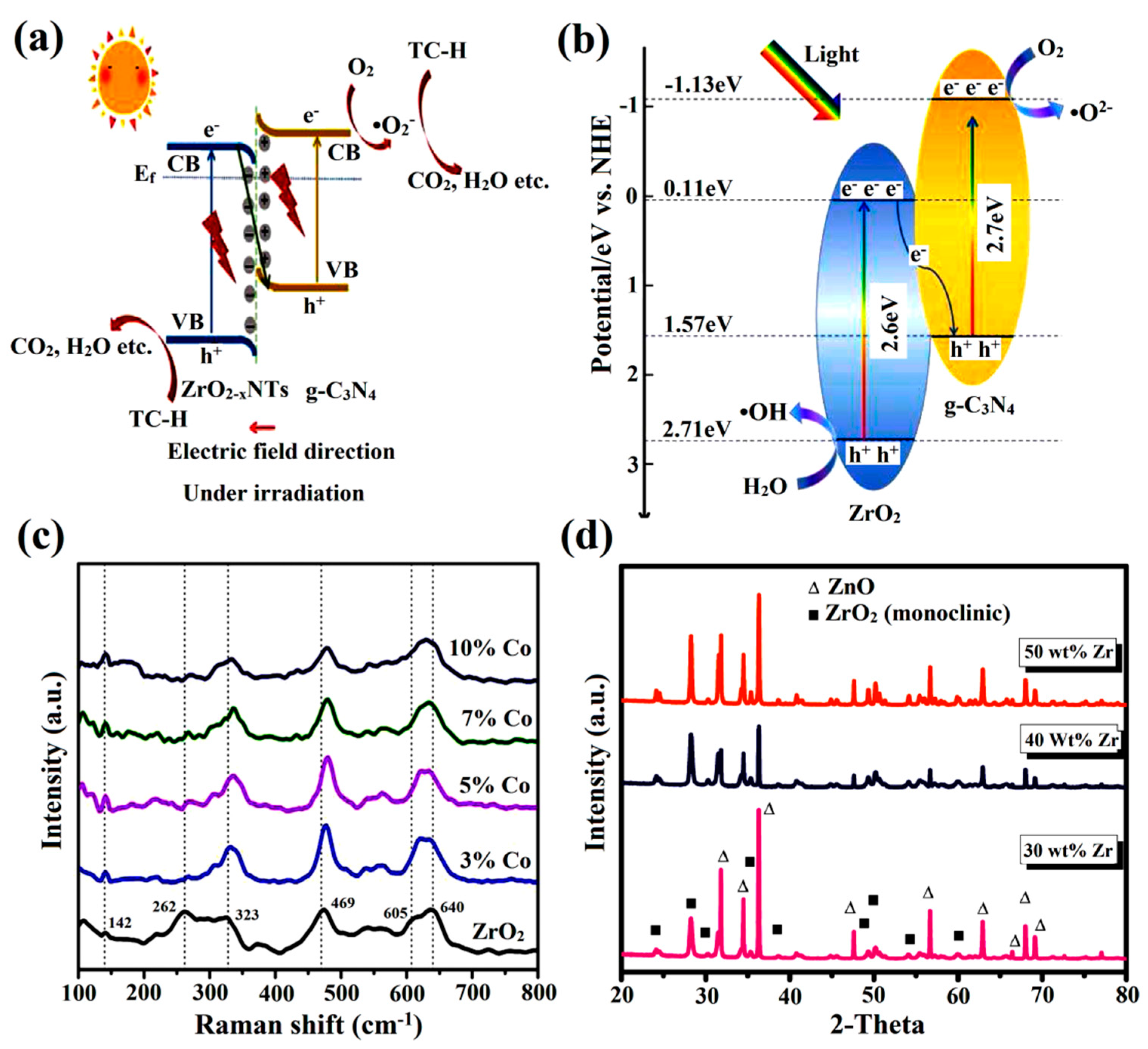
3.3. Heterojunction Formation
3.4. Generation of Oxygen Vacancies
4. Synthesis Methods
4.1. Bottom-Up Strategies
4.1.1. Hydrothermal/Solvothermal Method
4.1.2. Crystal Structure Engineering
4.2. Top-Down Strategies
4.2.1. Thermal Treatment
4.2.2. Ultrasonication
4.2.3. Chemical Reduction
5. Photodegradation and Mechanism
5.1. Organic Pollutant Degradation
5.2. Inorganic Pollutant Degradation
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hosseini-Bandegharaei, A. Adsorption and photocatalysis compiled toxic dyes mineralization using graphitic carbon nitride modified ZnFe2O4 and CoFe2O4 photocatalysts supported onto N-doped graphene. Desalination Water Treat 2020, 191, 381–399. [Google Scholar]
- Kumar, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Nguyen, V.H.; Singh, P. C-, N-Vacancy defect engineered polymeric carbon nitride towards photocatalysis: Viewpoints and challenges. J. Mater. Chem. A 2021, 9, 111–153. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Kumari, P.; Bahadur, N.; Kong, L.; O’Dell, L.A.; Merenda, A.; Dumee, L. Engineering Schottky-like and heterojunction material for enhanced photocatalysis performance-a review. Adv. Mater. 2022, 3, 2309–2323. [Google Scholar] [CrossRef]
- Almeida, F.; Grzebielucka, E.C.; Antunes, S.R.M.; Borges, C.P.F.; Andrade, A.V.C.; Souza, É.C.F. Visible light activated magnetic photocatalysts for water treatment. J. Environ. Manag. 2020, 273, 111143. [Google Scholar] [CrossRef] [PubMed]
- Alshorifi, F.T.; Ali, S.L.; Salama, R.S. Promotional Synergistic Effect of Cs–Au NPs on the Performance of Cs–Au/MgFe2O4 Catalysts in Catalysis 3, 4-Dihydropyrimidin-2 (1H)-Ones and Degradation of RhB Dye. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3765–3776. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Ubaidullah, M.; Alshehri, S. Fabrication of highly porous polymeric nanocomposite for the removal of radioactive U (VI) and Eu (III) ions from aqueous solution. Polym. J. 2020, 12, 2940. [Google Scholar] [CrossRef]
- Balakrishnan, T.; Kiran, K.U.V.; Kumar, S.M.; Raju, A.; Kumar, S.A.; Mayavan, S. Copper particles decorated boron carbon nitride, an efficient catalyst for methyl orange oxidation under sonication and sunlight irradiation. J. Environ. Chem. Eng. 2017, 5, 564–571. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Alswat, A.A.; Salama, R.S. Gold-selenide quantum dots supported onto cesium ferrite nanocomposites for the efficient degradation of rhodamine B. Heliyon 2022, 8, e09652. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Ubaidullah, M.; Ahmed, J.; Ahamad, T.; Ahmad, T.; Shaikh, S.F.; Naushad, M. Synthesis of NiOx@ NPC composite for high-performance supercapacitor via waste PET plastic-derived Ni-MOF. Compos. B. Eng. 2020, 183, 107655. [Google Scholar] [CrossRef]
- Hasija, V.; Raizada, P.; Sudhaik, A.; Sharma, K.; Kumar, A.; Singh, P.; Jonnalagadda, S.B.; Thakur, V.K. Recent advances in noble metal free doped graphitic carbon nitride based nanohybrids for photocatalysis of organic contaminants in water: A review. Appl. Mater. Today 2019, 15, 494–524. [Google Scholar] [CrossRef]
- El-Hakam, S.A.; ALShorifi, F.T.; Salama, R.S.; Gamal, S.; El-Yazeed, W.A.; Ibrahim, A.A.; Ahmed, A.I. Application of nanostructured mesoporous silica/bismuth vanadate composite catalysts for the degradation of methylene blue and brilliant green. J. Mater. Res. Technol. 2022, 18, 1963–1976. [Google Scholar] [CrossRef]
- Thakur, N.; Kumar, K.; Thakur, V.K.; Soni, S.; Kumar, A.; Samant, S.S. Antibacterial and photocatalytic activity of undoped and (Ag, Fe) co-doped CuO nanoparticles via microwave-assisted method. Nanofabrication 2022, 7. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Jangwan, J.; Kumar, S.S.; Kumar, V.; Kumar, A.; Kumar, D. A review on the capability of zinc oxide and iron oxides nanomaterials, as a water decontaminating agent: Adsorption and photocatalysis. Appl. Water Sci. 2022, 12, 1–14. [Google Scholar]
- Hussain, N.; Bilal, M.; Iqbal, H.M. Carbon-based nanomaterials with multipurpose attributes for water treatment: Greening the 21st-century nanostructure materials deployment. Biomater. Polym. Horiz. 2022, 1, 1–11. [Google Scholar] [CrossRef]
- Pournemati, K.; Habibi-Yangjeh, A.; Khataee, A. Rational design of TiO2/MnMoO4/MoO3 nanocomposites: Visible-light-promoted photocatalysts for decomposition of tetracycline with tandem nn heterojunctions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130315. [Google Scholar] [CrossRef]
- Pelosato, R.; Bolognino, I.; Fontana, F.; Sora, I.N. Applications of Heterogeneous Photocatalysis to the Degradation of Oxytetracycline in Water: A Review. Molecules 2022, 27, 2743. [Google Scholar] [CrossRef]
- Murillo-Sierra, J.; Hernández-Ramírez, A.; Hinojosa-Reyes, L.; Guzmán-Mar, J. A review on the development of visible light-responsive WO3-based photocatalysts for environmental applications. Adv. Chem. Eng. 2021, 5, 100070. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, C.; Almatrafi, E.; Yan, M.; Liu, Y.; Fu, Y.; Wang, Z.; Li, L.; Zhou, C.; Xu, P. Photocatalytic water purification with graphitic C3N4-based composites: Enhancement, mechanisms, and performance. Appl. Mater. Today 2021, 24, 101118. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, H.; Umar, A. Photocatalysis from UV/Vis to near-infrared light: Towards full solar-light spectrum activity. ChemCatChem 2015, 7, 559–573. [Google Scholar] [CrossRef]
- Wahba, M.A.; Yakout, S.M.; Mohamed, W.A.; Galal, H.R. Remarkable photocatalytic activity of Zr doped ZnO and ZrO2/ZnO nanocomposites: Structural, morphological and photoluminescence properties. Mater. Chem. Phys. 2020, 256, 123754. [Google Scholar] [CrossRef]
- Pirzada, B.M.; Mir, N.A.; Qutub, N.; Mehraj, O.; Sabir, S.; Muneer, M. Synthesis, characterization and optimization of photocatalytic activity of TiO2/ZrO2 nanocomposite heterostructures. Mater. Sci. Eng. B 2015, 193, 137–145. [Google Scholar] [CrossRef]
- Aldeen, E.S.; Jalil, A.A.; Mim, R.S.; Alhebshi, A.; Hassan, N.S.; Saravanan, R. Altered zirconium dioxide based photocatalyst for enhancement of organic pollutants degradation: A review. Chemosphere 2022, 304, 135349. [Google Scholar] [CrossRef]
- Ahmed, W.; Iqbal, J. Co doped ZrO2 nanoparticles: An efficient visible light triggered photocatalyst with enhanced structural, optical and dielectric characteristics. Ceram. Int. 2020, 46, 25833–25844. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, M.; Yu, C.; Yang, K.; Li, X.; Dai, W.; Guan, J.; Shu, Q.; Huang, W. Construction of S-scheme g-C3N4/ZrO2 heterostructures for enhancing photocatalytic disposals of pollutants and electrocatalytic hydrogen evolution. Dyes Pigm. 2020, 180, 108525. [Google Scholar] [CrossRef]
- García-López, E.; Marcì, G.; Pomilla, F.; Paganini, M.; Gionco, C.; Giamello, E.; Palmisano, L. ZrO2 Based materials as photocatalysts for 2-propanol oxidation by using UV and solar light irradiation and tests for CO2 reduction. Catal. Today 2018, 313, 100–105. [Google Scholar] [CrossRef]
- Qin, Y.; Ding, Z.; Guo, W.; Guo, X.; Hou, C.; Jiang, B.-P.; Liu, C.-G.; Shen, X.-C. A full solar light spectrum responsive B@ ZrO2–OV photocatalyst: A synergistic strategy for visible-to-NIR photon harvesting. ACS Sustain. Chem. Eng. 2020, 8, 13039–13047. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Lu, H.; Chen, Y.; Liu, Z.; Su, S.; Xue, Y.; Yao, J.; Zeng, H. Novel N-doped ZrO2 with enhanced visible-light photocatalytic activity for hydrogen production and degradation of organic dyes. RSC Adv. 2018, 8, 6752–6758. [Google Scholar] [CrossRef] [Green Version]
- Kaviyarasu, K.; Kotsedi, L.; Simo, A.; Fuku, X.; Mola, G.T.; Kennedy, J.; Maaza, M. Photocatalytic activity of ZrO2 doped lead dioxide nanocomposites: Investigation of structural and optical microscopy of RhB organic dye. Appl. Surf. Sci. 2017, 421, 234–239. [Google Scholar] [CrossRef]
- Renuka, L.; Anantharaju, K.; Vidya, Y.; Nagabhushana, H.; Uma, B.; Malini, S.; More, S.S.; Koppad, P. Porous network ZrO2/ZnFe2O4 nanocomposite with heterojunction towards industrial water purification under sunlight: Enhanced charge separation and elucidation of photo-mechanism. Ceram. Int. 2021, 47, 14845–14861. [Google Scholar] [CrossRef]
- Idrissi, S.; Ziti, S.; Labrim, H.; Bahmad, L. Sulfur doping effect on the electronic properties of zirconium dioxide ZrO2. Mater. Sci. Eng. B 2021, 270, 115200. [Google Scholar] [CrossRef]
- Faizan, M.; Siddique, M.N.; Ahmad, S.; Tripathi, P.; Riyajuddin, S. Tunable luminescence in Ce3+/Mn2+ co-doped ZrO2 nanophosphor integrated with theoretical studies on possible (ZrO2) n clusters using DFT method. J. Alloys Compd. 2021, 853, 157378. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, P.; Gong, T.; Xiao, Y.; Zhang, Z.; Zhao, L.; Fan, K.; Deng, M. Effects of ZrO2 crystal structure on the tribological properties of copper metal matrix composites. Tribol. Int. 2019, 138, 380–391. [Google Scholar] [CrossRef]
- Lamas, D.; Rosso, A.; Anzorena, M.S.; Fernández, A.; Bellino, M.; Cabezas, M.; de Reca, N.W.; Craievich, A. Crystal structure of pure ZrO2 nanopowders. Scr. Mater. 2006, 55, 553–556. [Google Scholar] [CrossRef]
- McCullough, J.T.; Trueblood, K. The crystal structure of baddeleyite (monoclinic ZrO2). Acta Crystallogr. 1959, 12, 507–511. [Google Scholar] [CrossRef]
- Singh, H.; Yadav, K.K.; Bajpai, V.K.; Jha, M. Tuning the bandgap of m-ZrO2 by incorporation of copper nanoparticles into visible region for the treatment of organic pollutants. Mater. Res. Bull. 2020, 123, 110698. [Google Scholar] [CrossRef]
- Teufer, G. The crystal structure of tetragonal ZrO2. Acta Crystallogr. 1962, 15, 1187. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Liang, F.-L. ZrO2/carbon aerogel composites: A study on the effect of the crystal ZrO2 structure on cationic dye adsorption. J. Taiwan Inst. Chem. Eng. 2016, 65, 78–82. [Google Scholar] [CrossRef]
- Morinaga, M.; Adachi, H.; Tsukada, M. Electronic structure and phase stability of ZrO2. J. Phys. Chem. Solids 1983, 44, 301–306. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Meng, J.; Li, Q.; Yang, J. Double-hole codoped huge-gap semiconductor ZrO2 for visible-light photocatalysis. Phys. Chem. Chem. Phys. 2016, 18, 17517–17524. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, J.T.; Mayengbam, R.; Tripathy, S. Theoretical investigation on structural, electronic, optical and elastic properties of TiO2, SnO2, ZrO2 and HfO2 using SCAN meta-GGA functional: A DFT study. Mater. Chem. Phys. 2020, 254, 123474. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Y.; Fan, Y.; Wang, R.; Zhu, R.; Tang, Y.; Yin, Z.; Zeng, Z. InVO4-based photocatalysts for energy and environmental applications. Chem. Eng. 2022, 428, 131145. [Google Scholar] [CrossRef]
- Renuka, L.; Anantharaju, K.; Sharma, S.; Nagaswarupa, H.; Prashantha, S.; Nagabhushana, H.; Vidya, Y. Hollow microspheres Mg-doped ZrO2 nanoparticles: Green assisted synthesis and applications in photocatalysis and photoluminescence. J. Alloys Compd. 2016, 672, 609–622. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, P.; Kuang, Y.; Ding, Y.; Yao, J.; Xu, J.; Cao, Y. Adjustment and Control of Energy Levels for TiO2–N/ZrO2–xNx with Enhanced Visible Light Photocatalytic Activity. J. Phys. Chem. C 2014, 118, 20982–20988. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, Q.; Wang, D.; Zeng, X.-F.; Wang, J.-X.; Chen, J.-F. Surfactant-free aqueous dispersions of shape-and size-controlled zirconia colloidal nanocrystal clusters with enhanced photocatalytic activity. Langmuir 2019, 35, 11755–11763. [Google Scholar] [CrossRef]
- Fu, X.; Clark, L.A.; Yang, Q.; Anderson, M.A. Enhanced photocatalytic performance of titania-based binary metal oxides: TiO2/SiO2 and TiO2/ZrO2. Environ. Sci. Technol. 1996, 30, 647–653. [Google Scholar] [CrossRef]
- Linnik, O.; Shestopal, N.; Smirnova, N.; Eremenko, A.; Korduban, O.; Kandyba, V.; Kryshchuk, T.; Socol, G.; Stefan, N.; Popescu-Pelin, G. Correlation between electronic structure and photocatalytic properties of non-metal doped TiO2/ZrO2 thin films obtained by pulsed laser deposition method. Vacuum 2015, 114, 166–171. [Google Scholar] [CrossRef]
- Berlin, I.J.; Joy, K. Optical enhancement of Au doped ZrO2 thin films by sol–gel dip coating method. Physica B Condens. Matter 2015, 457, 182–187. [Google Scholar] [CrossRef]
- Anwer, H.; Park, J.-W. Synthesis and characterization of a heterojunction rGO/ZrO2/Ag3PO4 nanocomposite for degradation of organic contaminants. J. Hazard. Mater. 2018, 358, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Gurushantha, K.; Renuka, L.; Anantharaju, K.; Vidya, Y.; Nagaswarupa, H.; Prashantha, S.; Nagabhushana, H. Photocatalytic and photoluminescence studies of ZrO2/ZnO nanocomposite for LED and waste water treatment applications. Mater. Today Proc. 2017, 4, 11747–11755. [Google Scholar] [CrossRef]
- Parveen, A.; Surumbarkuzhali, N. Spatial separation of photo-generated carriers and enhanced photocatalytic performance on ZrO2 catalysts via coupling with PPy. Inorg. Chem. Commun. 2020, 120, 108153. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Basharnavaz, H. Ni/P, Pt/P and Pd/P-modified graphitic carbon nitride nanosheets for hydrogen storage application using a DFT investigation. Mol. Phy. 2022, e2124934. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Enhanced visible light photocatalytic activity of g-C3N4 decorated ZrO2−x nanotubes heterostructure for degradation of tetracycline hydrochloride. J. Hazard. Mater. 2020, 384, 121275. [Google Scholar] [CrossRef]
- Hao, Y.; Li, L.; Liu, D.; Yu, H.; Zhou, Q. The synergy of SPR effect and Z-scheme of Ag on enhanced photocatalytic performance of 3DOM Ag/CeO2-ZrO2 composite. J. Mol. Catal. 2018, 447, 37–46. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Sobolev, A.; Varaksin, A.; Keda, O.; Khaimenov, A. Electronic structure and charge state of oxygen vacancies in perfect ZrO2 crystals. Phys. Status Solidi B Basic Res. 1990, 162, 165–171. [Google Scholar] [CrossRef]
- Reddy, C.V.; Reddy, I.N.; Ravindranadh, K.; Reddy, K.R.; Kim, D.; Shim, J. Ni-dopant concentration effect of ZrO2 photocatalyst on photoelectrochemical water splitting and efficient removal of toxic organic pollutants. Sep. Purif. Technol. 2020, 252, 117352. [Google Scholar] [CrossRef]
- Ma, H.; He, Y.; Chen, P.; Wang, H.; Sun, Y.; Li, J.; Dong, F.; Xie, G.; Sheng, J. Ultrathin Two-Dimensional Bi-Based photocatalysts: Synthetic strategies, surface defects, and reaction mechanisms. Chem. Eng. J. 2021, 417, 129305. [Google Scholar] [CrossRef]
- Rashid, J.; Barakat, M.; Mohamed, R.; Ibrahim, I. Enhancement of photocatalytic activity of zinc/cobalt spinel oxides by doping with ZrO2 for visible light photocatalytic degradation of 2-chlorophenol in wastewater. J. Photochem. Photobiol. 2014, 284, 1–7. [Google Scholar] [CrossRef]
- Navio, J.; Hidalgo, M.; Colon, G.; Botta, S.; Litter, M. Preparation and physicochemical properties of ZrO2 and Fe/ZrO2 prepared by a sol− gel technique. Langmuir 2001, 17, 202–210. [Google Scholar] [CrossRef]
- Agorku, E.; Pandey, A.; Mamba, B.; Mishra, A. Gd, C, N, S multi-doped ZrO2 for photocatalytic degradation of indigo carmine dye from synthetic water under simulated solar light. Mater. Today Proc. 2015, 2, 3909–3920. [Google Scholar] [CrossRef]
- Vignesh, K.; Priyanka, R.; Rajarajan, M.; Suganthi, A. Photoreduction of Cr (VI) in water using Bi2O3–ZrO2 nanocomposite under visible light irradiation. J. Mater. Sci. Eng. B 2013, 178, 149–157. [Google Scholar] [CrossRef]
- Zarei, M. Ultrasonic-assisted preparation of ZrO2/g-C3N4 nanocomposites with high visible-light photocatalytic activity for degradation of 4-chlorophenol in water. Water-Energy Nexus 2020, 3, 135–142. [Google Scholar] [CrossRef]
- Zhao, W.; Adeel, M.; Zhang, P.; Zhou, P.; Huang, L.; Zhao, Y.; Ahmad, M.A.; Shakoor, N.; Lou, B.; Jiang, Y.; et al. A critical review on surface modified nano-catalysts application for photocatalytic degradation of volatile organic compounds. Environ. Sci. Nano 2022, 9, 61–80. [Google Scholar] [CrossRef]
- Xu, F.; Lai, C.; Zhang, M.; Li, B.; Liu, S.; Chen, M.; Li, L.; Xu, Y.; Qin, L.; Fu, Y. Facile one-pot synthesis of carbon self-doped graphitic carbon nitride loaded with ultra-low ceric dioxide for high-efficiency environmental photocatalysis: Organic pollutants degradation and hexavalent chromium reduction. J. Colloid Interface Sci. 2021, 601, 196–208. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, K.; Cao, H.; Zhang, X. L-Lysine-assisted synthesis of ZrO2 nanocrystals and their application in photocatalysis. J. Phys. Chem. C 2009, 113, 18259–18263. [Google Scholar] [CrossRef]
- Polisetti, S.; Deshpande, P.A.; Madras, G. Photocatalytic activity of combustion synthesized ZrO2 and ZrO2–TiO2 mixed oxides. Ind. Eng. Chem. Res. 2011, 50, 12915–12924. [Google Scholar] [CrossRef]
- de Moraes, N.P.; de Azeredo, C.A.S.H.; Bacetto, L.A.; da Silva, M.L.C.P.; Rodrigues, L.A. The effect of C-doping on the properties and photocatalytic activity of ZrO2 prepared via sol-gel route. Optik 2018, 165, 302–309. [Google Scholar] [CrossRef]
- Keerthana, S.P.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Velauthapillai, D. Nd doped ZrO2 photocatalyst for organic pollutants degradation in wastewater. Environ. Technol. Innov. 2022, 28, 102851. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Ramazani, A.; Taghavi Fardood, S. Synthesis of Al2O3/ZrO2 nanocomposite and the study of its effects on photocatalytic degradation of reactive blue 222 and reactive yellow 145 dyes. ChemistrySelect 2020, 5, 9966–9973. [Google Scholar] [CrossRef]
- Du, W.; Zhu, Z.; Zhang, X.; Wang, D.; Liu, D.; Qian, X.; Du, J. RE/ZrO2 (RE= Sm, Eu) composite oxide nano-materials: Synthesis and applications in photocatalysis. Mater. Res. Bull. 2013, 48, 3735–3742. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, L.; Zhang, X.; Yang, H.; Cheng, Y.; Che, H.; Wang, L.; Cao, Y. Construction of heterojunction and homojunction to improve the photocatalytic performance of ZnO quantum dots sensitization three-dimensional ordered hollow sphere ZrO2–TiO2 arrays. Int. J. Hydrog. Energy 2020, 45, 31812–31824. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Li, H.; Ma, D.; Hou, S.; Zhang, J.; Qian, X.; Pang, H. ZrO2/Dy2O3 Solid Solution Nano-Materials: Tunable Composition, Visible light–Responsive Photocatalytic Activities and Reaction Mechanism. J. Am. Ceram. Soc. 2013, 96, 2979–2986. [Google Scholar] [CrossRef]
- Carbuloni, C.F.; Savoia, J.E.; Santos, J.S.; Pereira, C.A.; Marques, R.G.; Ribeiro, V.A.; Ferrari, A.M. Degradation of metformin in water by TiO2–ZrO2 photocatalysis. J. Environ. Manag. 2020, 262, 110347. [Google Scholar] [CrossRef]
- Tanzifi, M.; Jahanshahi, M.; Peyravi, M.; Khalili, S. A morphological decoration of g-C3N4/ZrO2 heterojunctions as a visible light activated photocatalyst for degradation of various organic pollutants. J. Environ. Chem. Eng. 2022, 10, 108600. [Google Scholar] [CrossRef]
- Alotaibi, M.R.; Mahmoud, M.H.H. Promptness of tetracycline pollutant degradation via CuCo2O4@ZrO2 nanocomposites photocatalyst. Opt. Mater. 2022, 126, 112200. [Google Scholar] [CrossRef]
- Abdi, J.; Yahyanezhad, M.; Sakhaie, S.; Vossoughi, M.; Alemzadeh, I. Synthesis of porous TiO2/ZrO2 photocatalyst derived from zirconium metal organic framework for degradation of organic pollutants under visible light irradiation. J. Environ. Chem. Eng. 2019, 7, 103096. [Google Scholar] [CrossRef]

| Photocatalyst | Enhancement Strategies | Reaction Parameter | Targeted Pollutant | Photocatalyst Efficiency | Ref. |
|---|---|---|---|---|---|
| Ni doped ZrO2 | Doping | Visible light lamp (>400 nm), 15 mg photocatalyst | Methylene blue | 90.2% in 100 min. | [59] |
| C-doped ZrO2 | Doping | PL-L lamp, 0.2 g/L photocatalyst | Methylene blue | 75% | [70] |
| Nd doped ZrO2 | Doping | pH = 7 | Methylene blue, Rhodamine B, and acetophenone | 90%, 77%, and 60% | [71] |
| Al2O3/ZrO2 | Heterojunction | Visible light, 0.04 g catalyst | Reactive blue 222 and Reactive yellow 145 | 91.4% and 94.6% in 60 min. | [72] |
| RE/ZrO2 (RE = Sm, Eu) | Heterojunction | 350 W Xenon lamp | Methylene blue and Rhodamine B | 100% in 30 min. and 96.3% in 90 min. | [73] |
| ZnO QDs@ZrO2-TiO2 | Heterojunction | Ultra violet light | Congo Red | 94.62% | [74] |
| ZrO2/Dy2O3 | Heterojunction | Xenon lamp with cut-off UV filter | Rhodamine B, and Methylene blue | 100% in 30 min. and 87.79% | [75] |
| TiO2-ZrO2 | Heterojunction | 125 W Mercury lamp, pH = 7.6, 10 mg/L photocatalyst | Metformin | 92% in 150 min. | [76] |
| g-C3N4/ZrO2 | Heterojunction | 50 W LED lamp, 30 mg photocatalyst | Methylene blue, Rhodamine B, Congo Red, and Tetracycline | 96%, 98%, 90%, and 83% | [77] |
| CuCo2O4@ZrO2 | Heterojunction | Visible light | Tetracycline | 95% | [78] |
| TiO2/ZrO2 | Heterojunction | 100 W LED lamp | Rhodamine B | 90% | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, V.; Sharma, A.; Kumar, A.; Singh, P.; Thakur, S.; Singh, A.; Le, Q.V.; Nguyen, V.H.; Raizada, P. ZrO2-Based Photocatalysts for Wastewater Treatment: From Novel Modification Strategies to Mechanistic Insights. Catalysts 2022, 12, 1418. https://doi.org/10.3390/catal12111418
Rani V, Sharma A, Kumar A, Singh P, Thakur S, Singh A, Le QV, Nguyen VH, Raizada P. ZrO2-Based Photocatalysts for Wastewater Treatment: From Novel Modification Strategies to Mechanistic Insights. Catalysts. 2022; 12(11):1418. https://doi.org/10.3390/catal12111418
Chicago/Turabian StyleRani, Vandna, Amit Sharma, Abhinandan Kumar, Pardeep Singh, Sourbh Thakur, Archana Singh, Quyet Van Le, Van Huy Nguyen, and Pankaj Raizada. 2022. "ZrO2-Based Photocatalysts for Wastewater Treatment: From Novel Modification Strategies to Mechanistic Insights" Catalysts 12, no. 11: 1418. https://doi.org/10.3390/catal12111418
APA StyleRani, V., Sharma, A., Kumar, A., Singh, P., Thakur, S., Singh, A., Le, Q. V., Nguyen, V. H., & Raizada, P. (2022). ZrO2-Based Photocatalysts for Wastewater Treatment: From Novel Modification Strategies to Mechanistic Insights. Catalysts, 12(11), 1418. https://doi.org/10.3390/catal12111418









