Kinetic Modelling of Esterification and Transesterification Processes for Biodiesel Production Utilising Waste-Based Resource
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Relative Effect of Parameters on the Quantity of Acid Removal from the Esterification Process
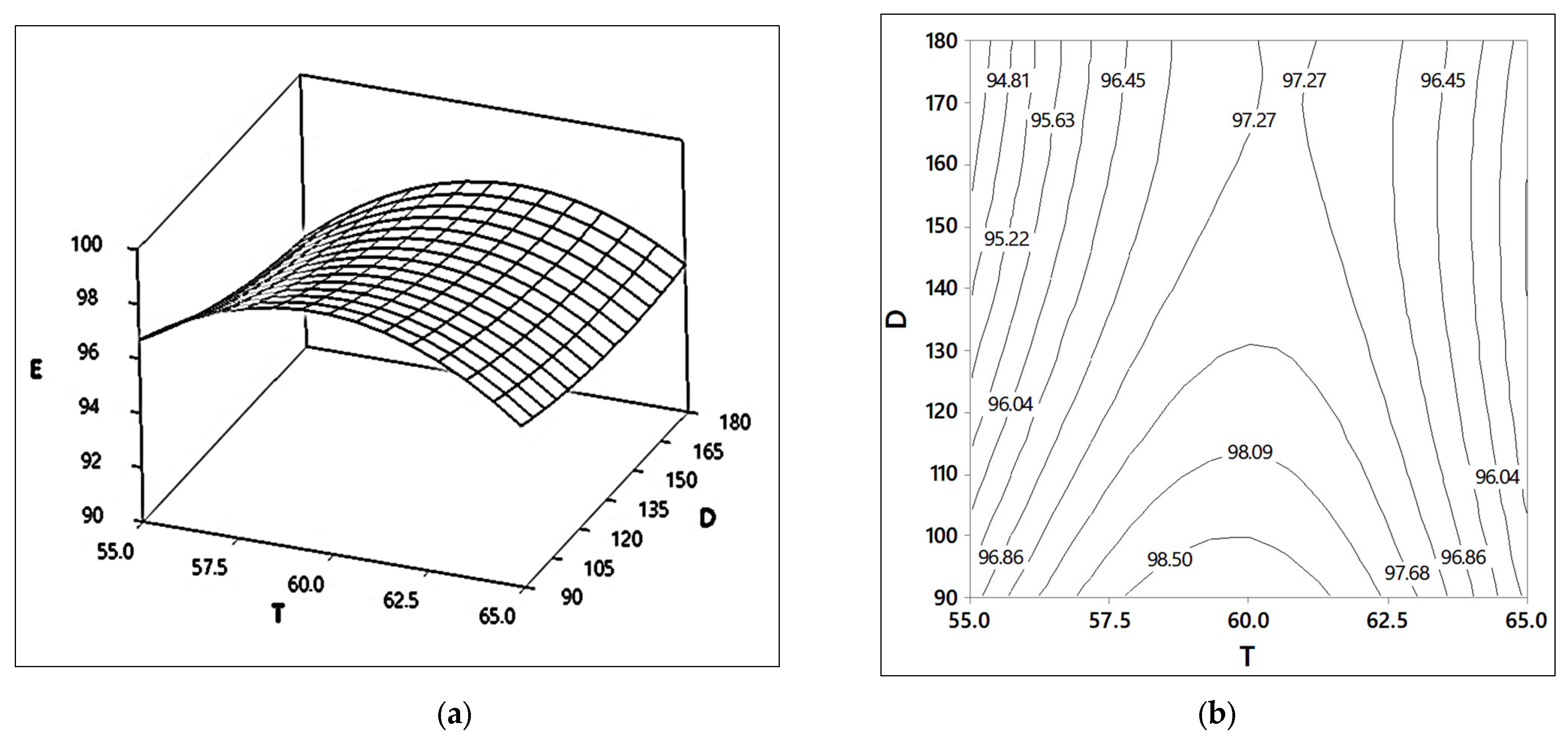
2.1.1. Effects of Reaction Time (D) and Reaction Temperature (T)
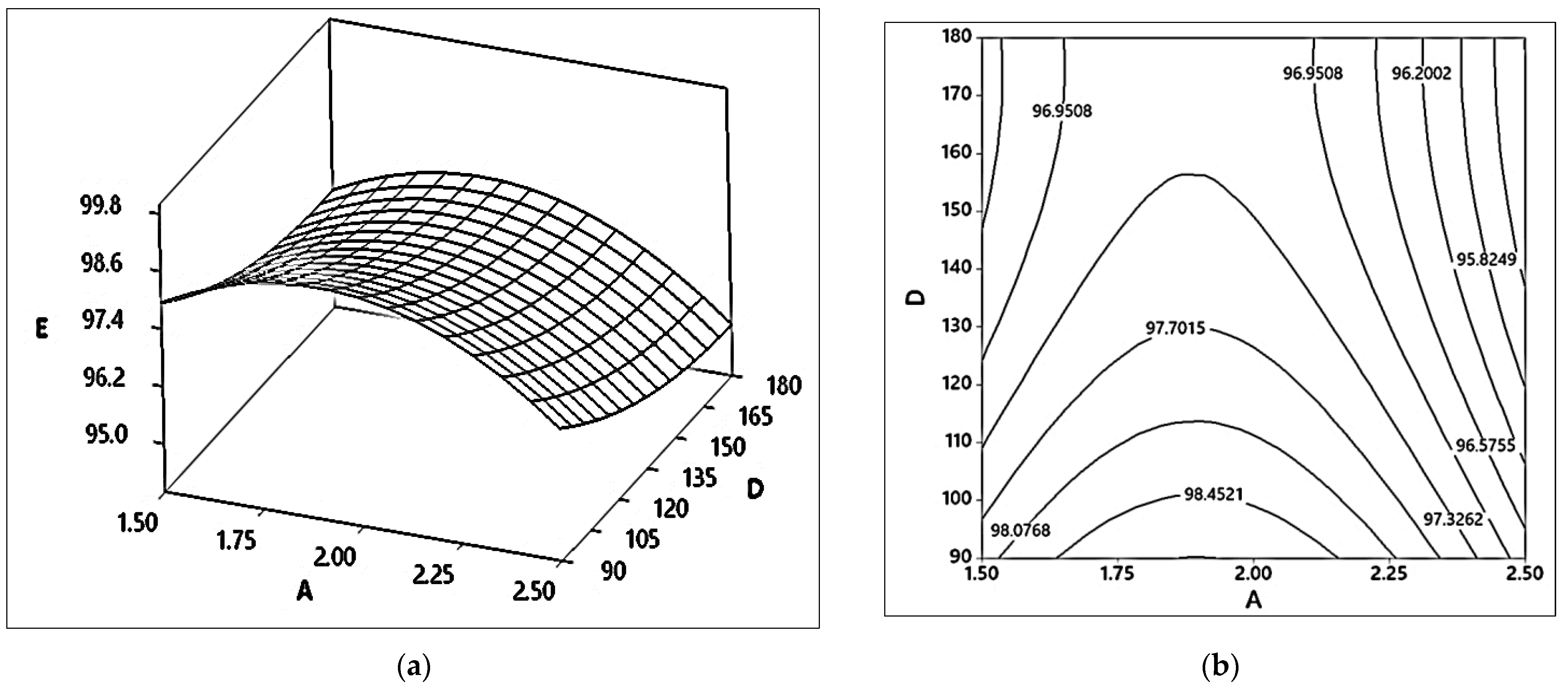
2.1.2. Effects of Methanol to Oil Ratio (M) and Catalyst Content (A)
2.1.3. Effects of Methanol to Oil Ratio (M) and Reaction Temperature (T)
2.1.4. Effects of Methanol to Oil Ratio (M) and Reaction Time (D)
2.1.5. Interaction between Acid Catalyst (A) and Reaction Temperature (T)
2.1.6. Interaction between Acid Catalyst (A) and Reaction Time (D)
2.2. Kinetic Parameter Determination for the Esterification Process of WCO
2.2.1. Determination of Kinetic Parameters for a Pseudo-Homogeneous Irreversible Esterification Process
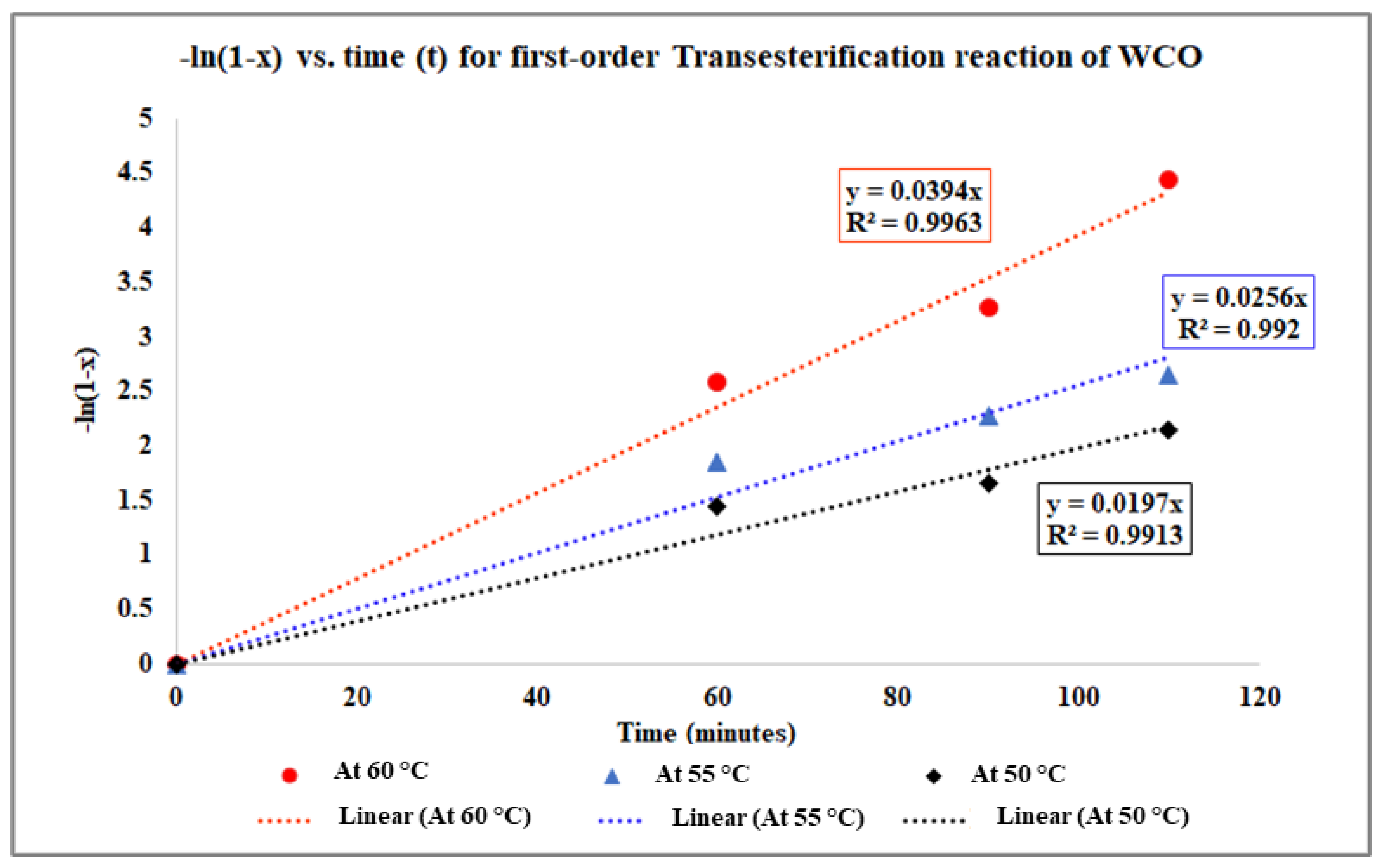
2.2.2. Determination of Reaction Kinetics When the Esterification Process Is the Pseudo-Homogeneous First-Order Irreversible Reaction Process
2.3. The Relative Effect of Parameters on Yield from the Transesterification Process
2.3.1. Effects of Catalyst Content (B) and Methanol to Oil Ratio (M)
2.3.2. Effects of Catalyst Content (B) and Reaction Time (D)
2.3.3. Effects of Reaction Temperature (T) and Reaction Time (D)
2.3.4. Effect of Methanol to Esterified WCO Content (M) and Reaction Temperature (T)
2.3.5. Effects of Methanol to Esterified Tallow Content (M) and Reaction Time (D)
2.3.6. Effects of Methanol to Reaction Temperature (T) and Catalyst Content (B)
2.4. Determination of Kinetic Model for Transesterification Process of WCO
2.4.1. Transesterification Process Kinetics for Pseudo-First-Order Homogeneous Irreversible Reaction
2.4.2. Transesterification Process Kinetic Modelling for WCO Biodiesel Production When the Reaction Is a Pseudo-Homogeneous Irreversible Process
2.5. WCO Fatty Acid Composition and Properties
3. Discussion
4. Materials and Method
4.1. Materials
4.2. Method for Biodiesel Production from WCO
4.3. Optimisation of Esterification Process of WCO
4.3.1. Design for Optimal Esterification Reaction Experiments
4.3.2. Design for Esterification Process RSM Analysis
4.3.3. Response Surface Regression
4.3.4. Response Optimisation
4.3.5. Analysis of Variance (ANOVA) for WCO Esterification Process
4.3.6. Effects Plots for WCO Esterification Process
4.4. Optimisation of the Transesterification Process of WCO
4.4.1. RSM Analysis and Optimisation of the Transesterification Process
4.4.2. Design for Transesterification Process RSM Analysis
4.4.3. Response Surface Regression
4.4.4. Response Optimisation
4.4.5. Analysis of Variance (ANOVA) for the Transesterification Process of WCO
4.5. Reaction Kinetic Models for Esterification and Transesterification Processes of WCO
- Excessive methanol was used to dominate the reaction rates of both the esterification and transesterification processes to be driven towards the product side only (i.e., esterified WCO oil and WCO biodiesel, respectively). Thus, the reversibility was negligible for these reactions.
- The higher molar ratio of methanol to triglycerides indicates that the change in methanol concentration due to the esterification reaction of the available FFA in the triglycerides and that of conversion of triglycerides into fatty acid methyl esters do not affect the rate of the reactions. Thus, it can be considered constant in a batch reactor.
- Sufficiently effective and higher stirring was conducted to lessen the hydrodynamic effect and mass transfer resistance effect between methanol and FFA as well as between methanol and WCO triglycerides.
- The overall reaction is considered to be a constant density and constant volume reaction system.
- These models can also be used for non-catalytic processes, as the catalyst contents were not used during the determination of the kinetics.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ampah, J.D.; Yusuf, A.A.; Agyekum, E.B.; Afrane, S.; Jin, C.; Liu, H.; Fattah, I.M.R.; Show, P.L.; Shouran, M.; Habil, M.; et al. Progress and Recent Trends in the Application of Nanoparticles as Low Carbon Fuel Additives—A State of the Art Review. Nanomaterials 2022, 12, 1515. [Google Scholar] [CrossRef] [PubMed]
- Ampah, J.D.; Jin, C.; Rizwanul Fattah, I.M.; Appiah-Otoo, I.; Afrane, S.; Geng, Z.; Yusuf, A.A.; Li, T.; Mahlia, T.M.I.; Liu, H. Investigating the evolutionary trends and key enablers of hydrogen production technologies: A patent-life cycle and econometric analysis. Int. J. Hydrog. Energy 2022. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Fattah, I.M.R.; Yousaf, B.; Periyasamy, S. Effects of the COVID-19 pandemic on the environment, waste management, and energy sectors: A deeper look into the long-term impacts. Environ. Sci. Pollut. Res. 2022, 29, 46438–46457. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Ong, H.C.; Ibrahim, S.; Fattah, I.M.R.; Mofijur, M.; Chong, C.T. Valorisation of medical waste through pyrolysis for a cleaner environment: Progress and challenges. Environ. Pollut. 2021, 279, 116934. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Masjuki, H.H.; Kalam, M.A.; Hazrat, M.A.; Masum, B.M.; Imtenan, S.; Ashraful, A.M. Effect of antioxidants on oxidation stability of biodiesel derived from vegetable and animal based feedstocks. Renew. Sustain. Energy Rev. 2014, 30, 356–370. [Google Scholar] [CrossRef]
- Awogbemi, O.; Onuh, E.I.; Inambao, F.L. Comparative study of properties and fatty acid composition of some neat vegetable oils and waste cooking oils. Int. J. Low Carbon Technol. 2019, 14, 417–425. [Google Scholar] [CrossRef]
- He, C.; Mei, Y.; Zhang, Y.; Liu, L.; Li, P.; Zhang, Z.; Jing, Y.; Li, G.; Jiao, Y. Enhanced biodiesel production from diseased swine fat by ultrasound-assisted two-step catalyzed process. Bioresour. Technol. 2020, 304, 123017. [Google Scholar] [CrossRef]
- AbuKhadra, M.R.; Basyouny, M.G.; El-Sherbeeny, A.M.; El-Meligy, M.A.; Abd Elgawad, A.E.E. Transesterification of commercial waste cooking oil into biodiesel over innovative alkali trapped zeolite nanocomposite as green and environmental catalysts. Sustain. Chem. Pharm. 2020, 17, 100289. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Y.; Gu, J.; Xin, Z.; Sun, S. Butyl-biodiesel production from waste cooking oil: Kinetics, fuel properties and emission performance. Fuel 2019, 236, 1489–1495. [Google Scholar] [CrossRef]
- Sanjel, N.; Gu, H.J.; Oh, C.S. Transesterification Kinetics of Waste Vegetable Oil in Supercritical Alcohols. Energies 2014, 7, 2095–2106. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Rahman, S.M.A.; Ahmad, A. State of the Art of Catalysts for Biodiesel Production. Front. Energy Res. 2020, 8, 101. [Google Scholar] [CrossRef]
- Malvis, A.; Simon, P.; Dubaj, T.; Sladkova, A.; Haz, A.; Jablonsky, M.; Sekretar, S.; Schmidt, S.; Kreps, F.; Burcova, Z.; et al. Determination of the Thermal Oxidation Stability and the Kinetic Parameters of Commercial Extra Virgin Olive Oils from Different Varieties. J. Chem. 2019, 2019, 8. [Google Scholar] [CrossRef]
- Ruhul, A.M.; Kalam, M.A.; Masjuki, H.H.; Fattah, I.M.R.; Reham, S.S.; Rashed, M.M. State of the art of biodiesel production processes: A review of the heterogeneous catalyst. RSC Adv. 2015, 5, 101023–101044. [Google Scholar] [CrossRef]
- Liow, M.Y.; Gourich, W.; Chang, M.Y.; Loh, J.M.; Chan, E.-S.; Song, C.P. Towards rapid and sustainable synthesis of biodiesel: A review of effective parameters and scale-up potential of intensification technologies for enzymatic biodiesel production. J. Ind. Eng. Chem. 2022, 114, 1–18. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Mubashir, M.; Asif, S.; Waseem, A.; Mukhtar, A.; Saqib, S.; Siti Halimatul Munawaroh, H.; Lam, M.K.; Khoo, K.S.; et al. A practical approach for synthesis of biodiesel via non-edible seeds oils using trimetallic based montmorillonite nano-catalyst. Bioresour. Technol. 2021, 328, 124859. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Rehan, M.; Saeed, M.; Lam, S.S.; Nizami, A.S.; Waseem, A.; Sultana, S.; Zafar, M. Production of high quality biodiesel from novel non-edible Raphnus raphanistrum L. seed oil using copper modified montmorillonite clay catalyst. Environ. Res. 2021, 193, 110398. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Saeed, M.; Waseem, A.; Nizami, A.-S.; Sultana, S.; Zafar, M.; Rehan, M.; Srinivasan, G.R.; Ali, A.M.; et al. Biodiesel production from novel non-edible caper (Capparis spinosa L.) seeds oil employing Cu–Ni doped ZrO2 catalyst. Renew. Sustain. Energy Rev. 2021, 138, 110558. [Google Scholar] [CrossRef]
- Ma, T.; Liu, D.; Liu, Z.; Xu, J.; Dong, Y.; Chen, G.; Yun, Z. 12-Tungstophosphoric acid-encapsulated metal-organic framework UiO-66: A promising catalyst for the esterification of acetic acid with n-butanol. J. Taiwan Inst. Chem. Eng. 2022, 133, 104277. [Google Scholar] [CrossRef]
- Mohiddin, M.N.B.; Tan, Y.H.; Seow, Y.X.; Kansedo, J.; Mubarak, N.M.; Abdullah, M.O.; Chan, Y.S.; Khalid, M. Evaluation on feedstock, technologies, catalyst and reactor for sustainable biodiesel production: A review. J. Ind. Eng. Chem. 2021, 98, 60–81. [Google Scholar] [CrossRef]
- Imtenan, S.; Varman, M.; Masjuki, H.H.; Kalam, M.A.; Sajjad, H.; Arbab, M.I.; Fattah, I.M.R. Impact of low temperature combustion attaining strategies on diesel engine emissions for diesel and biodiesels: A review. Energy Convers. Manag. 2014, 80, 329–356. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Shahir, S.A.; Masjuki, H.H.; Kalam, M.A.; Imran, A.; Rizwanul Fattah, I.M.; Sanjid, A. Feasibility of diesel–biodiesel–ethanol/bioethanol blend as existing CI engine fuel: An assessment of properties, material compatibility, safety and combustion. Renew. Sustain. Energy Rev. 2014, 32, 379–395. [Google Scholar] [CrossRef]
- Hazrat, M.A.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Fattah, I.M.R.; Ong, H.C.; Mahlia, T.M.I. Biodiesel production from transesterification of Australian Brassica napus L. oil: Optimisation and reaction kinetic model development. Environ. Dev. Sustain. 2022, 1–26. [Google Scholar] [CrossRef]
- Raheem, I.; Mohiddin, M.N.B.; Tan, Y.H.; Kansedo, J.; Mubarak, N.M.; Abdullah, M.O.; Ibrahim, M.L. A review on influence of reactor technologies and kinetic studies for biodiesel application. J. Ind. Eng. Chem. 2020, 91, 54–68. [Google Scholar] [CrossRef]
- Moradi, G.R.; Mohadesi, M.; Ghanbari, M.; Moradi, M.J.; Hosseini, S.; Davoodbeygi, Y. Kinetic comparison of two basic heterogenous catalysts obtained from sustainable resources for transesterification of waste cooking oil. Biofuel Res. J. 2015, 2, 236–241. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification kinetics of soybean oil. J. Am. Oil Chem. Soc. 1986, 63, 1375–1380. [Google Scholar] [CrossRef]
- Gaurav, A.; Dumas, S.; Mai, C.T.Q.; Ng, F.T.T. A kinetic model for a single step biodiesel production from a high free fatty acid (FFA) biodiesel feedstock over a solid heteropolyacid catalyst. Green Energy Environ. 2019, 4, 328–341. [Google Scholar] [CrossRef]
- Hsiao, M.-C.; Kuo, J.-Y.; Hsieh, P.-H.; Hou, S.-S. Improving Biodiesel Conversions from Blends of High- and Low-Acid-Value Waste Cooking Oils Using Sodium Methoxide as a Catalyst Based on a High Speed Homogenizer. Energies 2018, 11, 2298. [Google Scholar] [CrossRef] [Green Version]
- Mićić, R.; Tomić, M.; Martinović, F.; Kiss, F.; Simikić, M.; Aleksic, A. Reduction of free fatty acids in waste oil for biodiesel production by glycerolysis: Investigation and optimization of process parameters. Green Process. Synth. 2019, 8, 15–23. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Maleki, M.; Ansari, A. Production of biodiesel from waste cooking oil using a homogeneous catalyst: Study of semi-industrial pilot of microreactor. Renew. Energy 2019, 136, 677–682. [Google Scholar] [CrossRef]
- Biki, Z. Australia: Biofuels Annual; AS2020-0020; United States Department of Agriculture—Foreign Agriculture Service (USDA-FAS): Washington, DC, USA, 2020.
- Trinh, H.; Yusup, S.; Uemura, Y. Optimization and kinetic study of ultrasonic assisted esterification process from rubber seed oil. Bioresour. Technol. 2018, 247, 51–57. [Google Scholar] [CrossRef]
- Zeng, D.; Yang, L.; Fang, T. Process optimization, kinetic and thermodynamic studies on biodiesel production by supercritical methanol transesterification with CH3ONa catalyst. Fuel 2017, 203, 739–748. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; García-Martínez, N.; Durán-del-Amor, M.d.M.; Quesada-Medina, J. Advances on kinetics and thermodynamics of non-catalytic supercritical methanol transesterification of some vegetable oils to biodiesel. Energy Convers. Manag. 2018, 173, 187–196. [Google Scholar] [CrossRef]
- Zhou, L. Reaction Kinetics of Biodiesel Production by Using Low Quality Feedstock; University of Regina: Regina, SK, Canada, 2013. [Google Scholar]
- Permsuwan, A.; Tippayawong, N.; Kiatsiriroat, T.; Thararux, C.; Wangkarn, S. Reaction Kinetics of Transesterification Between Palm Oil and Methanol under Subcritical Conditions. Energy Sci. Technol. 2011, 2, 35–42. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sindhanaiselvan, S.; Gandhi, N.N.; Devi, S.S.; Renganathan, S. Optimization and kinetic studies on biodiesel production from underutilized Ceiba Pentandra oil. Fuel 2013, 103, 693–698. [Google Scholar] [CrossRef]
- Kurhade, A.; Dalai, A.K. Kinetic modeling, mechanistic, and thermodynamic studies of HPW-MAS-9 catalysed transesterification reaction for biodiesel synthesis. Fuel Process. Technol. 2019, 196, 106164. [Google Scholar] [CrossRef]
- Banchero, M.; Gozzelino, G. A Simple Pseudo-Homogeneous Reversible Kinetic Model for the Esterification of Different Fatty Acids with Methanol in the Presence of Amberlyst-15. Energies 2018, 11, 1843. [Google Scholar] [CrossRef] [Green Version]
- Zuccaro, G.; Pirozzi, D.; Yousuf, A. Chapter 4—Lignocellulosic biomass to biodiesel. In Lignocellulosic Biomass Liquid Biofuels; Yousuf, A., Pirozzi, D., Sannino, F., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 127–167. [Google Scholar] [CrossRef]
- Kusumo, F.; Mahlia, T.M.I.; Pradhan, S.; Ong, H.C.; Silitonga, A.S.; Fattah, I.M.R.; Nghiem, L.D.; Mofijur, M. A framework to assess indicators of the circular economy in biological systems. Environ. Technol. Innov. 2022, 28, 102945. [Google Scholar] [CrossRef]
- Mercy Nisha Pauline, J.; Sivaramakrishnan, R.; Pugazhendhi, A.; Anbarasan, T.; Achary, A. Transesterification kinetics of waste cooking oil and its diesel engine performance. Fuel 2021, 285, 119108. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Alagumalai, A.; Mathimani, T.; Atabani, A.E. Optimization, kinetic and thermodynamic studies on sustainable biodiesel production from waste cooking oil: An Indian perspective. Fuel 2020, 273, 117725. [Google Scholar] [CrossRef]
- Al-Saadi, A.; Mathan, B.; He, Y. Biodiesel production via simultaneous transesterification and esterification reactions over SrO–ZnO/Al2O3 as a bifunctional catalyst using high acidic waste cooking oil. Chem. Eng. Res. Des. 2020, 162, 238–248. [Google Scholar] [CrossRef]
- Putra, M.D.; Irawan, C.; Udiantoro; Ristianingsih, Y.; Nata, I.F. A cleaner process for biodiesel production from waste cooking oil using waste materials as a heterogeneous catalyst and its kinetic study. J. Clean. Prod. 2018, 195, 1249–1258. [Google Scholar] [CrossRef]
- Roy, T.; Sahani, S.; Chandra Sharma, Y. Study on kinetics-thermodynamics and environmental parameter of biodiesel production from waste cooking oil and castor oil using potassium modified ceria oxide catalyst. J. Clean. Prod. 2020, 247, 119166. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Cd 3d-63: Acid Value of Fats and Oils; American Oil Chemists’ Society (AOCS): Urbana, IL, USA, 2017. [Google Scholar]
- Aricetti, J.A.; Tubino, M. A Visual Titration Method for the Determination of the Acid Number of Oils and Fats: A Green Alternative. J. Am. Oil Chem. Soc. 2012, 89, 2113–2115. [Google Scholar] [CrossRef]
- Gumahin, A.C.; Galamiton, J.M.; Allerite, M.J.; Valmorida, R.S.; Laranang, J.-R.L.; Mabayo, V.I.F.; Arazo, R.O.; Ido, A.L. Response surface optimization of biodiesel yield from pre-treated waste oil of rendered pork from a food processing industry. Bioresour. Bioprocess. 2019, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Minitab. What Are Response Surface Designs, Central Composite Designs, and Box-Behnken Designs? Available online: https://support.minitab.com/en-us/minitab/18/help-and-how-to/modeling-statistics/doe/supporting-topics/response-surface-designs/response-surface-central-composite-and-box-behnken-designs/ (accessed on 1 February 2020).
- Minitab. Interpret All Statistics and Graphs for Simple Regression. Available online: https://support.minitab.com/en-us/minitab-express/1/help-and-how-to/modeling-statistics/regression/how-to/simple-regression/interpret-the-results/all-statistics-and-graphs/#vif (accessed on 8 March 2020).
- Berrios, M.; Siles, J.; Martin, M.; Martin, A. A kinetic study of the esterification of free fatty acids (FFA) in sunflower oil. Fuel 2007, 86, 2383–2388. [Google Scholar] [CrossRef]
- Prasanna Rani, K.N.; Ramana Neeharika, T.S.V.; Kumar, T.P.; Satyavathi, B.; Sailu, C. Kinetics of Non-Catalytic Esterification of Free Fatty Acids Present in Jatropha Oil. J. Oleo Sci. 2016, 65, 441–445. [Google Scholar] [CrossRef] [Green Version]
- Su, C.-H. Kinetic study of free fatty acid esterification reaction catalyzed by recoverable and reusable hydrochloric acid. Bioresour. Technol. 2013, 130, 522–528. [Google Scholar] [CrossRef]
- Krishnamurthy, K.N.; Sridhara, S.N.; Ananda Kumar, C.S. Optimization and kinetic study of biodiesel production from Hydnocarpus wightiana oil and dairy waste scum using snail shell CaO nano catalyst. Renew. Energy 2020, 146, 280–296. [Google Scholar] [CrossRef]
- Cheng, J.; Li, Y.; He, S.; Shen, W.; Liu, Y.; Song, Y. Reaction Kinetics of Transesterification between Vegetable Oil and Methanol under Supercritical Conditions. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 30, 681–688. [Google Scholar] [CrossRef]
- Lieu, T.; Yusup, S.; Moniruzzaman, M. Kinetic study on microwave-assisted esterification of free fatty acids derived from Ceiba pentandra Seed Oil. Bioresour. Technol. 2016, 211, 248–256. [Google Scholar] [CrossRef]
- Ataya, F.; Dubé, M.; Ternan, M. Acid-Catalyzed Transesterification of Canola Oil to Biodiesel under Single- and Two-Phase Reaction Conditions. Energy Fuels 2007, 21, 2450–2459. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Janulis, P.; Kitrys, S. Kinetics of free fatty acids esterification with methanol in the production of biodiesel fuel. Eur. J. Lipid Sci. Technol. 2004, 106, 831–836. [Google Scholar] [CrossRef]
- Kocsisová, T.; Cvengroš, J.; Lutišan, J. High-temperature esterification of fatty acids with methanol at ambient pressure. Eur. J. Lipid Sci. Technol. 2005, 107, 87–92. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Neves, S.C.G.; Da Silva, M.J. Esterification of Oleic Acid for Biodiesel Production Catalyzed by SnCl2: A Kinetic Investigation. Energies 2008, 1, 79–92. [Google Scholar] [CrossRef]
























| T (°C) | T (K) | n (Order) | lnk’ | k’ | k = k’*CA0 | 1/T | lnk | Average Reaction Order, n |
|---|---|---|---|---|---|---|---|---|
| 60 | 333 | 1.1768 | −2.2923 | 0.1010338 | 0.101034 | 0.003003 | −2.2923 | 1.151 |
| 55 | 328 | 1.1612 | −3.4144 | 0.0328961 | 0.032896 | 0.003049 | −3.4144 | |
| 50 | 323 | 1.115 | −3.7529 | 0.0234496 | 0.02345 | 0.003096 | −3.7529 |
| T (°C) | T (k) | Rate Const. k1 (1/mol·min) | R-sq Value | 1/T | lnk1 |
|---|---|---|---|---|---|
| 60 | 333 | 0.0469 | 0.9982 | 0.003003 | −3.059737604 |
| 55 | 328 | 0.0353 | 0.9929 | 0.003049 | −3.343872315 |
| 50 | 323 | 0.0247 | 0.9828 | 0.003096 | −3.700952035 |
| T (°C) | T (k) | Rate Const. k1 (1/mol·min) | R-sq Value | 1/T | lnk1 |
|---|---|---|---|---|---|
| 60 | 333 | 0.0394 | 0.987 | 0.003003 | −3.233989463 |
| 55 | 328 | 0.0256 | 0.9698 | 0.003049 | −3.665162927 |
| 50 | 323 | 0.0197 | 0.968 | 0.003096 | −3.927136643 |
| T (°C) | T (K) | n (Order) | lnk’ | k’ | k = k’*CB0 | 1/T | lnk | Average Reaction Order, n |
|---|---|---|---|---|---|---|---|---|
| 60 | 333 | 1.0095 | −2.8348 | 0.05873 | 0.05873 | 0.003003 | −2.8348 | 1.088133 |
| 55 | 328 | 1.0962 | −3.4466 | 0.031854 | 0.031854 | 0.003049 | −3.4466 | |
| 50 | 323 | 1.1587 | −3.7215 | 0.024198 | 0.024198 | 0.003096 | −3.7215 |
| Physicochemical Properties | WCO Biodiesel | Test Standards | ASTM D6751/EN 14214 Limits |
|---|---|---|---|
| Kinematic viscosity (mm2/s, at 40 °C) | 5.31 | ASTM D445 | 1.9–6.0 (ASTM) |
| Density (kg/m3, at 15 °C) | 889 | EN ISO 3675 | 860–900 (EN) |
| Higher heating value (MJ/kg, HHV) | 40.16 | ASTM D4868 | - |
| Lower heating value (MJ/kg, LHV) | 39.51 | ASTM D4868 | - |
| Oxidation stability (hours, at 110 °C) | 8.88 | EN 14112 | >8 h (EN) |
| Flash point (°C) | 172.1 | ASTM D93 | 100–170 (ASTM) |
| Pour point (°C) | −3.2 | ASTM D97 | - |
| Cloud point (°C) | 3.5 | ASTM D2500 | Location and season dependent |
| Cold filter plugging point (CFPP, °C) | −1.3 | EN 116 | Location and season dependent |
| Cetane number (CN) | 57.98 | ASTM D613 | >47 (ASTM) |
| Iodine value (IV, g I2/100 g oil) | 91.11 | EN 14111 | <120 (EN) |
| Saponification value (SV, mg KOH/g oil) | 204.12 | ASTM D5558 | - |
| Acid value (AV, mg KOH/g oil) | 0.24 | EN 14104 | <0.50 (ASTM/EN) |
| Feedstock | Methanol: Oil Molar Ratio | Catalyst | Biodiesel Yield (%) | Temp. (°C) | Time (min) | Kinetic Parameters | Ref. |
|---|---|---|---|---|---|---|---|
| Waste cooking oil (bubble washing before transesterification) | 10.6:1 | 0.6% (w/w) | 90 ± 0.25 | 63 | 63 | First-order kinetics Ea = 57.82 kJ/mol A = 74 × 106 min−1 | [44] |
| Waste cooking oil (Transesterification) | 6:1 | NaOH 1% (w/v) | 90 | 60 | 120 | Irreversible pseudo-second-order Ea = 27.24 kJ/mol | [43] |
| Waste cooking oil (Transesterification) | 14:1 | CaO/SiO2 8% w/w | 91 | 60 | 90 | Adsorption, surface reaction and desorption A = 5.44 × 108 min−1 Ea = 66.27 kJ/mol | [46] |
| Waste cooking oil (batch reaction) | 14:1 | K–CeO2 1.5 wt.% | 99.09 (conversion) | 65 ± 0.5 | 75 | Adsorption, surface reaction and desorption Ea = 50.1 kJ mol−1 A = 35.4 × 105 min−1 | [47] |
| Waste cooking oil (Simultaneous esterification and transesterification) | 10:1 (ethanol) | SrO–ZnO/Al2O3 as bifunctional catalyst 15 wt.% | 95.7 (conversion) | 75 | 300 | - | [45] |
| Waste cooking oil (Batch reaction) Esterification then transesterification | 8.12:1 then 6.1:1 | 1.9 wt.% of H2SO4 1.2 wt.% of KOH | 99.77 | 60 (for both) | 90 Then 110 | Pseudo-homogeneous first-order irreversible Esterification: Ea = 57.36909 kJ/mol A = 4.73 107 min−1 Transesterification: Ea = 61.90272 kJ/mol A = 1.43 1011 min−1 | This work |
| Design Summary (Face-Centred CCD, α = 1): | |||||
|---|---|---|---|---|---|
| Factors: | 4 | Replicates: | 1 | Base blocks: | 1 |
| Base runs: | 31 | Total runs: | 31 | Total blocks: | 1 |
| Two-level factorial (Full factorial) Point Types: | |||||
| Cube points | Centre points in cube | Axial points | Centre points in axial | ||
| 16 | 7 | 8 | 0 | ||
| CCD Factors and limits: | |||||
| Factors | Coded Factor | Symbol | Low | High | |
| Methanol: Oil (M) | A | M | 6 | 12 | |
| H2SO4 (wt.%) | B | A | 1.5 | 2.5 | |
| Temperature (°C) | C | T | 55 | 65 | |
| Time (minutes) | D | D | 90 | 180 | |
| Run Order | M | A | T | D | %E Removal (Exp) | %Ep Removal (Predicted) |
|---|---|---|---|---|---|---|
| 1 | 6 | 1.5 | 55 | 90 | 92.85 | 92.87 |
| 3 | 6 | 2.5 | 55 | 90 | 94.46 | 94.51 |
| 5 | 6 | 1.5 | 65 | 90 | 91.94 | 91.81 |
| 7 | 6 | 2.5 | 65 | 90 | 94.29 | 94.33 |
| 9 | 6 | 1.5 | 55 | 180 | 89.41 | 89.45 |
| 11 | 6 | 2.5 | 55 | 180 | 90.81 | 90.76 |
| 13 | 6 | 1.5 | 65 | 180 | 90.63 | 90.69 |
| 15 | 6 | 2.5 | 65 | 180 | 92.79 | 92.91 |
| 17 | 6 | 2 | 60 | 135 | 95.69 | 95.58 |
| 19 | 9 | 1.5 | 60 | 135 | 97.15 | 97.21 |
| 20 | 9 | 2.5 | 60 | 135 | 94.62 | 94.56 |
| 21 | 9 | 2 | 55 | 135 | 94.66 | 94.59 |
| 22 | 9 | 2 | 65 | 135 | 94.89 | 94.96 |
| 23 | 9 | 2 | 60 | 90 | 98.37 | 98.34 |
| 24 | 9 | 2 | 60 | 180 | 97.11 | 97.19 |
| 25 | 9 | 2 | 60 | 135 | 97.29 | 97.33 |
| 26 | 9 | 2 | 60 | 135 | 97.23 | 97.28 |
| 27 | 9 | 2 | 60 | 135 | 97.35 | 97.28 |
| 28 | 9 | 2 | 60 | 135 | 97.45 | 97.28 |
| 29 | 9 | 2 | 60 | 135 | 97.01 | 97.28 |
| 30 | 9 | 2 | 60 | 135 | 97.19 | 97.28 |
| 31 | 9 | 2 | 60 | 135 | 97.33 | 97.28 |
| 2 | 12 | 1.5 | 55 | 90 | 92.49 | 92.51 |
| 4 | 12 | 2.5 | 55 | 90 | 85.16 | 85.20 |
| 6 | 12 | 1.5 | 65 | 90 | 91.21 | 91.20 |
| 8 | 12 | 2.5 | 65 | 90 | 84.61 | 84.65 |
| 10 | 12 | 1.5 | 55 | 180 | 91.49 | 91.51 |
| 12 | 12 | 2.5 | 55 | 180 | 83.99 | 84.09 |
| 14 | 12 | 1.5 | 65 | 180 | 92.38 | 92.43 |
| 16 | 12 | 2.5 | 65 | 180 | 85.63 | 85.66 |
| 18 | 12 | 2 | 60 | 135 | 91.89 | 91.93 |
| Variables | M | A | T | D |
|---|---|---|---|---|
| Optimal Values | 8.12121 | 1.89394 | 59.6465 | 90 |
| Response | Fit | SE Fit | 95% CI | 95% PI |
| Acidity removed | 98.8479 | 0.062 | (98.7164, 98.9794) | (98.6140, 99.0818) |
| Source | DF | Seq SS | Contribution | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Model | 14 | 508.107 | 99.97% | 508.107 | 36.2934 | 4359.11 | 0 |
| Linear | 4 | 101.585 | 19.99% | 101.585 | 25.3964 | 3050.3 | 0 |
| M | 1 | 64.298 | 12.65% | 64.298 | 64.2978 | 7722.65 | 0 |
| A | 1 | 29.876 | 5.88% | 29.876 | 29.8764 | 3588.39 | 0 |
| T | 1 | 0.517 | 0.10% | 0.517 | 0.5168 | 62.07 | 0 |
| D | 1 | 6.894 | 1.36% | 6.894 | 6.8944 | 828.07 | 0 |
| Square | 4 | 315.337 | 62.04% | 315.337 | 78.8342 | 9468.58 | 0 |
| M2 | 1 | 280.065 | 55.10% | 32.021 | 32.0212 | 3845.99 | 0 |
| A2 | 1 | 18.408 | 3.62% | 5.216 | 5.2157 | 626.44 | 0 |
| T2 | 1 | 16.367 | 3.22% | 16.581 | 16.5807 | 1991.47 | 0 |
| D2 | 1 | 0.496 | 0.10% | 0.496 | 0.4964 | 59.62 | 0 |
| 2-Way Interaction | 6 | 91.185 | 17.94% | 91.185 | 15.1975 | 1825.34 | 0 |
| MA | 1 | 79.656 | 15.67% | 79.656 | 79.6556 | 9567.25 | 0 |
| MT | 1 | 0.126 | 0.02% | 0.126 | 0.126 | 15.14 | 0.001 |
| MD | 1 | 6.15 | 1.21% | 6.15 | 6.1504 | 738.71 | 0 |
| AT | 1 | 0.555 | 0.11% | 0.555 | 0.555 | 66.66 | 0 |
| AD | 1 | 0.032 | 0.01% | 0.032 | 0.0324 | 3.89 | 0.066 |
| TD | 1 | 4.666 | 0.92% | 4.666 | 4.6656 | 560.37 | 0 |
| Error | 16 | 0.133 | 0.03% | 0.133 | 0.0083 | ||
| Lack-of-Fit | 10 | 0.015 | 0.00% | 0.015 | 0.0015 | 0.08 | 1 |
| Pure Error | 6 | 0.118 | 0.02% | 0.118 | 0.0197 | ||
| Total | 30 | 508.24 | 100.00% | ||||
| S | R-sq | R-sq (adj) | PRESS | R-sq (pred) | |||
| 0.0912462 | 99.97% | 99.95% | 0.201949 | 99.96% | |||
| Term | Coefficients | Standard Error | 95% CI | T-Value | p-Value | VIF |
|---|---|---|---|---|---|---|
| Constant | 97.282 | 0.0271 | (97.2246, 97.3394) | 3593.99 | 0 | |
| M | −1.89 | 0.0215 | (−1.9356, −1.8444) | −87.88 | 0 | 1 |
| A | −1.2883 | 0.0215 | (−1.3339, −1.2427) | −59.9 | 0 | 1 |
| T | 0.1694 | 0.0215 | (0.1239, 0.2150) | 7.88 | 0 | 1 |
| D | −0.6189 | 0.0215 | (−0.6645, −0.5733) | −28.78 | 0 | 1 |
| M2 | −3.5127 | 0.0566 | (−3.6327, −3.3926) | −62.02 | 0 | 2.91 |
| A2 | −1.4177 | 0.0566 | (−1.5377, −1.2976) | −25.03 | 0 | 2.91 |
| T2 | −2.5277 | 0.0566 | (−2.6477, −2.4076) | −44.63 | 0 | 2.91 |
| D2 | 0.4373 | 0.0566 | (0.3173, 0.5574) | 7.72 | 0 | 2.91 |
| MA | −2.2312 | 0.0228 | (−2.2796, −2.1829) | −97.81 | 0 | 1 |
| MT | −0.0888 | 0.0228 | (−0.1371, −0.0404) | −3.89 | 0.001 | 1 |
| MD | 0.62 | 0.0228 | (0.5716, 0.6684) | 27.18 | 0 | 1 |
| AT | 0.1863 | 0.0228 | (0.1379, 0.2346) | 8.16 | 0 | 1 |
| AD | −0.045 | 0.0228 | (−0.0934, 0.0034) | −1.97 | 0.066 | 1 |
| TD | 0.54 | 0.0228 | (0.4916, 0.5884) | 23.67 | 0 | 1 |
| Design Summary (Face-Centred CCD, α = 1) | |||||
|---|---|---|---|---|---|
| Factors: | 4 | Replicates: | 1 | Base blocks: | 2 |
| Base runs: | 30 | Total runs: | 30 | Total blocks: | 2 |
| Two-level factorial (Full factorial) Point Types | |||||
| Cube points | Centre points in cube | Axial points | Centre points in axial | ||
| 16 | 4 | 8 | 2 | ||
| Factors | Coded Factor | Symbol | Low | High | |
| Methanol: Oil (M) | A | M | 5 | 7 | |
| KOH (wt.%) | B | B | 1 | 2 | |
| Temperature (OC) | C | T | 50 | 60 | |
| Time (minutes) | D | D | 60 | 120 | |
| Run Order | M | B | T | D | Biodiesel Yield, Y (%) | Predicted Yield, YP (%) |
|---|---|---|---|---|---|---|
| 1 | 7 | 1 | 60 | 120 | 95.14 | 95.11 |
| 2 | 7 | 2 | 50 | 60 | 80.71 | 80.57 |
| 4 | 7 | 2 | 50 | 120 | 80.99 | 81.07 |
| 7 | 7 | 1 | 50 | 120 | 82.15 | 82.08 |
| 10 | 7 | 1 | 60 | 60 | 93.54 | 93.59 |
| 12 | 7 | 1 | 50 | 60 | 82.58 | 82.66 |
| 13 | 7 | 2 | 60 | 120 | 92.91 | 93.03 |
| 20 | 7 | 2 | 60 | 60 | 90.36 | 90.43 |
| 23 | 7 | 1.5 | 55 | 90 | 90.49 | 90.32 |
| 6 | 6 | 1.5 | 55 | 90 | 96.13 | 96.19 |
| 8 | 6 | 2 | 60 | 90 | 96.01 | 96.05 |
| 14 | 6 | 2 | 60 | 120 | 98.21 | 97.87 |
| 15 | 6 | 1.5 | 55 | 90 | 96.22 | 96.19 |
| 21 | 6 | 1.5 | 60 | 90 | 98.92 | 98.82 |
| 22 | 6 | 1.5 | 55 | 60 | 94.95 | 94.84 |
| 24 | 6 | 1.5 | 55 | 90 | 95.05 | 95.02 |
| 25 | 6 | 1.5 | 55 | 120 | 95.88 | 96.04 |
| 26 | 6 | 1 | 55 | 90 | 91.99 | 91.95 |
| 27 | 6 | 1.5 | 55 | 90 | 94.79 | 95.02 |
| 28 | 6 | 2 | 55 | 90 | 91.28 | 91.34 |
| 29 | 6 | 1.5 | 50 | 90 | 88.62 | 88.74 |
| 3 | 5 | 2 | 60 | 60 | 81.41 | 81.45 |
| 5 | 5 | 1 | 60 | 60 | 81.72 | 81.65 |
| 9 | 5 | 1 | 60 | 120 | 83.42 | 83.54 |
| 11 | 5 | 2 | 60 | 120 | 84.33 | 84.43 |
| 16 | 5 | 2 | 50 | 120 | 75.29 | 75.22 |
| 17 | 5 | 2 | 50 | 60 | 74.30 | 74.34 |
| 18 | 5 | 1 | 50 | 120 | 73.34 | 73.27 |
| 19 | 5 | 1 | 50 | 60 | 73.44 | 73.47 |
| 30 | 5 | 1.5 | 55 | 90 | 81.55 | 81.43 |
| Variables | M | B | T | D |
|---|---|---|---|---|
| Optimal values | 6.1 | 1.2 | 60 | 110 |
| Response | Fit | SE Fit | 95% CI | 95% PI |
| WCO biodiesel yield | 99.7718 | 0.0789517 | (99.6025, 99.9411) | (99.4524, 100.091) |
| Source | DF | Seq SS | Contribution | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Model | 15 | 1864.91 | 99.99% | 1864.91 | 124.327 | 7797.7 | 0 |
| Blocks | 1 | 304.65 | 16.33% | 3.49 | 3.489 | 218.82 | 0 |
| Linear | 4 | 1019.23 | 54.65% | 843.52 | 210.881 | 13,226.28 | 0 |
| M | 1 | 356.18 | 19.10% | 356.18 | 356.178 | 22,339.18 | 0 |
| B | 1 | 14.49 | 0.78% | 1.82 | 1.823 | 114.35 | 0 |
| T | 1 | 632.74 | 33.92% | 477.8 | 477.799 | 29,967.16 | 0 |
| D | 1 | 15.83 | 0.85% | 6.42 | 6.418 | 402.51 | 0 |
| Square | 4 | 517.84 | 27.76% | 505.8 | 126.45 | 7930.86 | 0 |
| M2 | 1 | 471.63 | 25.29% | 274.06 | 274.062 | 17,188.96 | 0 |
| B2 | 1 | 41.83 | 2.24% | 29.69 | 29.691 | 1862.2 | 0 |
| T2 | 1 | 3.62 | 0.19% | 4.03 | 4.033 | 252.92 | 0 |
| D2 | 1 | 0.76 | 0.04% | 0.44 | 0.445 | 27.9 | 0 |
| 2-Way Interaction | 6 | 23.19 | 1.24% | 23.19 | 3.864 | 242.37 | 0 |
| MB | 1 | 8.78 | 0.47% | 8.78 | 8.776 | 550.45 | 0 |
| MT | 1 | 7.58 | 0.41% | 7.58 | 7.576 | 475.18 | 0 |
| MD | 1 | 0.14 | 0.01% | 0.14 | 0.143 | 8.94 | 0.01 |
| BT | 1 | 1.1 | 0.06% | 1.3 | 1.302 | 81.67 | 0 |
| BD | 1 | 1.27 | 0.07% | 1.09 | 1.09 | 68.34 | 0 |
| TD | 1 | 4.32 | 0.23% | 4.32 | 4.321 | 270.99 | 0 |
| Error | 14 | 0.22 | 0.01% | 0.22 | 0.016 | ||
| Lack-of-Fit | 12 | 0.19 | 0.01% | 0.19 | 0.015 | 0.82 | 0.672 |
| Pure Error | 2 | 0.04 | 0.00% | 0.04 | 0.019 | ||
| Total | 29 | 1865.13 | 100.00% | ||||
| Model Summary | |||||||
| S | R-sq | R-sq(adj) | PRESS | R-sq(pred) | |||
| 0.12627 | 99.99% | 99.98% | 1.02028 | 99.95% | |||
| Term | Coef | SE Coef | 95% CI | T-Value | p-Value | VIF |
|---|---|---|---|---|---|---|
| Constant | 95.5939 | 0.0544 | (95.4773, 95.7105) | 1758.53 | 0 | |
| Blocks | ||||||
| 1 | 0.5795 | 0.0392 | (0.4955, 0.6635) | 14.79 | 0 | 2.57 |
| 2 | −0.5795 | 0.0392 | (−0.6635, −0.4955) | −14.79 | 0 | |
| M | 4.4483 | 0.0298 | (4.3845, 4.5122) | 149.46 | 0 | 1 |
| B | −0.3105 | 0.029 | (−0.3728, −0.2482) | −10.69 | 0 | 1.05 |
| T | 5.0267 | 0.029 | (4.9644, 5.0890) | 173.11 | 0 | 1.05 |
| D | 0.587 | 0.0293 | (0.5242, 0.6497) | 20.06 | 0 | 1.02 |
| M2 | −9.0713 | 0.0692 | (−9.2197, −8.9229) | −131.11 | 0 | 2.16 |
| B2 | −3.381 | 0.0783 | (−3.5491, −3.2130) | −43.15 | 0 | 2.57 |
| T2 | −1.246 | 0.0783 | (−1.4141, −1.0780) | −15.9 | 0 | 2.57 |
| D2 | 0.3865 | 0.0732 | (0.2296, 0.5435) | 5.28 | 0 | 2.34 |
| MB | −0.7406 | 0.0316 | (−0.8083, −0.6729) | −23.46 | 0 | 1 |
| MT | 0.6881 | 0.0316 | (0.6204, 0.7558) | 21.8 | 0 | 1 |
| MD | −0.0944 | 0.0316 | (−0.1621, −0.0267) | −2.99 | 0.01 | 1 |
| BT | −0.2775 | 0.0307 | (−0.3433, −0.2116) | −9.04 | 0 | 1.06 |
| BD | 0.256 | 0.031 | (0.1896, 0.3224) | 8.27 | 0 | 1.02 |
| TD | 0.5097 | 0.031 | (0.4433, 0.5761) | 16.46 | 0 | 1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazrat, M.A.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Silitonga, A.S.; Fattah, I.M.R.; Mahlia, T.M.I. Kinetic Modelling of Esterification and Transesterification Processes for Biodiesel Production Utilising Waste-Based Resource. Catalysts 2022, 12, 1472. https://doi.org/10.3390/catal12111472
Hazrat MA, Rasul MG, Khan MMK, Ashwath N, Silitonga AS, Fattah IMR, Mahlia TMI. Kinetic Modelling of Esterification and Transesterification Processes for Biodiesel Production Utilising Waste-Based Resource. Catalysts. 2022; 12(11):1472. https://doi.org/10.3390/catal12111472
Chicago/Turabian StyleHazrat, M. A., Mohammad G. Rasul, Mohammad M. K. Khan, Nanjappa Ashwath, Arridina S. Silitonga, I. M. R. Fattah, and T. M. Indra Mahlia. 2022. "Kinetic Modelling of Esterification and Transesterification Processes for Biodiesel Production Utilising Waste-Based Resource" Catalysts 12, no. 11: 1472. https://doi.org/10.3390/catal12111472
APA StyleHazrat, M. A., Rasul, M. G., Khan, M. M. K., Ashwath, N., Silitonga, A. S., Fattah, I. M. R., & Mahlia, T. M. I. (2022). Kinetic Modelling of Esterification and Transesterification Processes for Biodiesel Production Utilising Waste-Based Resource. Catalysts, 12(11), 1472. https://doi.org/10.3390/catal12111472













