More than One Century of History for Photocatalysis, from Past, Present and Future Perspectives
Abstract
:1. Introduction
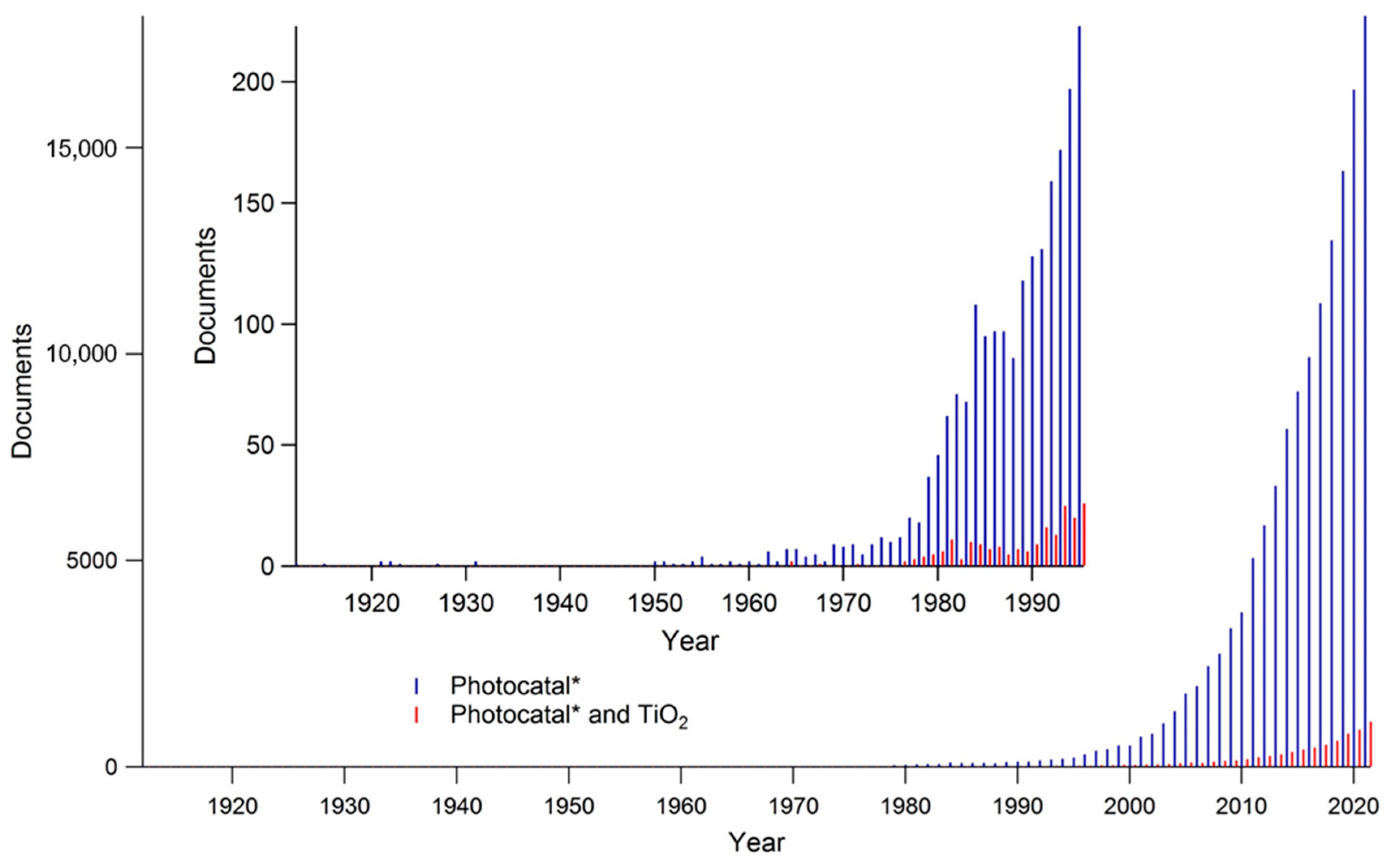
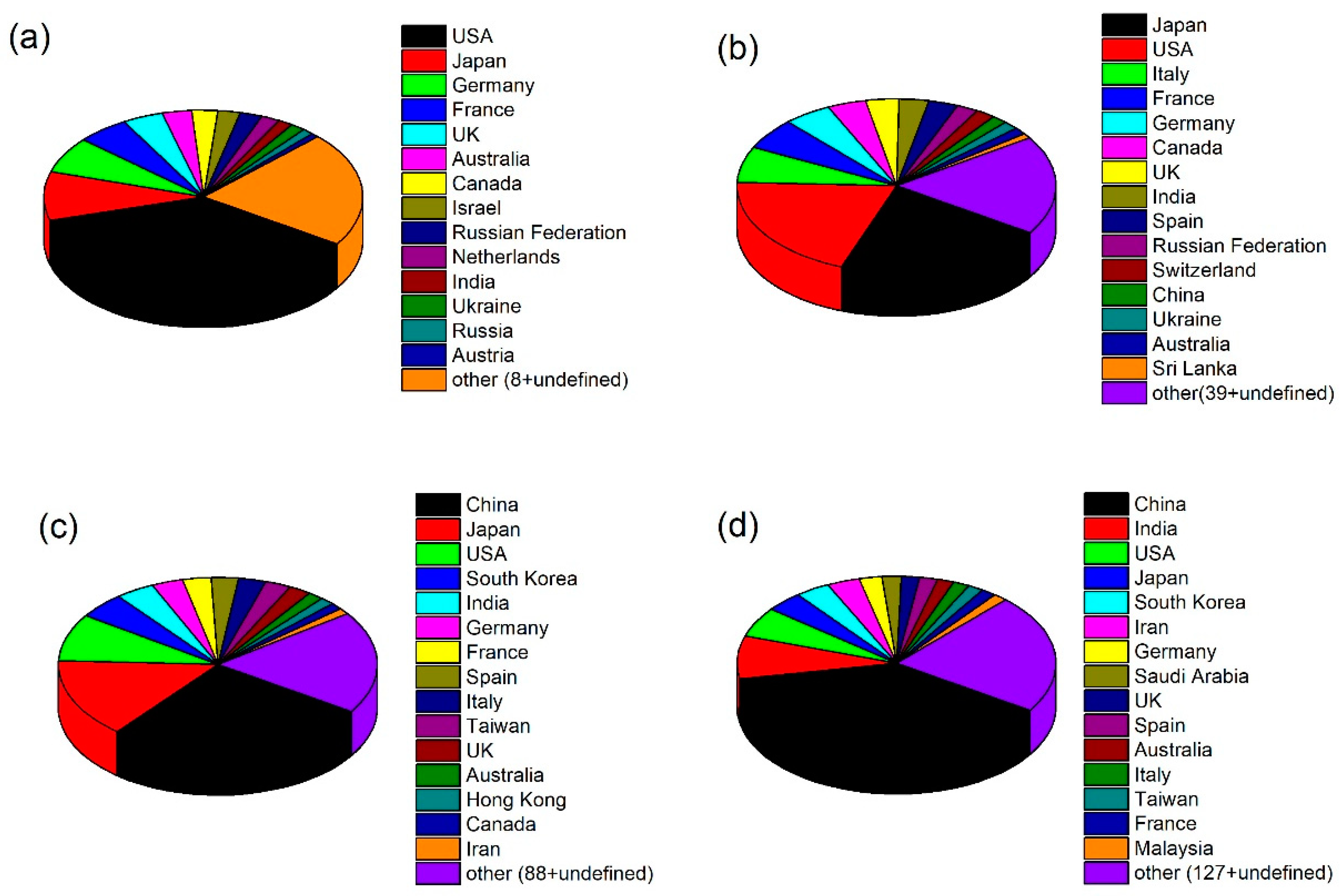
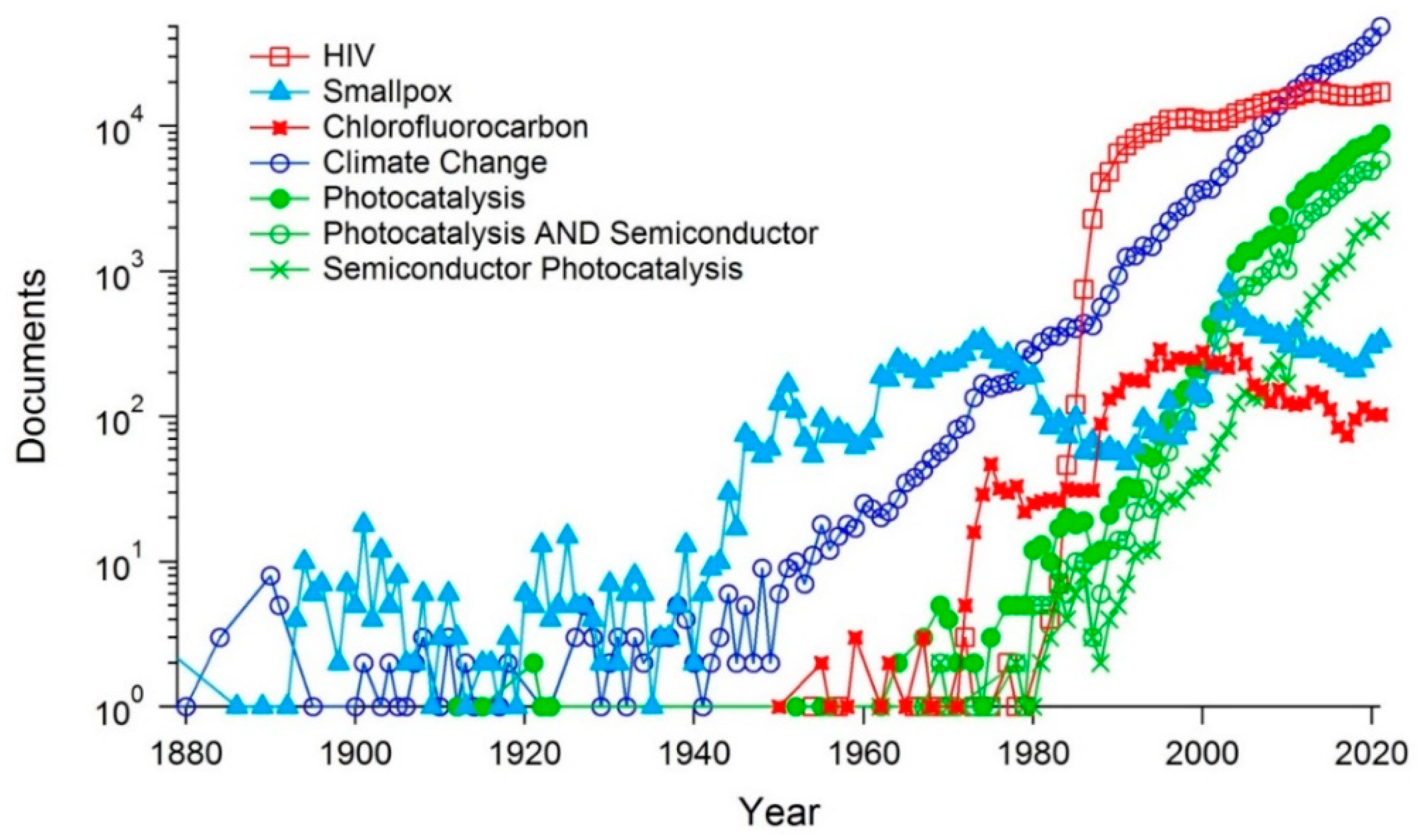
2. Bibliometric Analysis of Photocatalysis as a Scientific Topic: Temporal and Geographic Analysis
3. Evolution of the Main Topics in the Field of Photocatalysis
1—Until 1980, the Pioneering Years. In this first period of time, 0.07% of the documents were published. The main research interests concerned the mechanism, the demonstration of functionality and the addition of co-catalysts such as metals and RuO2. The global picture was dominated by TiO2, but SrTiO3, CdS and ZnO were also investigated, and they appear also in the top-cited papers. Researchers’ attention was devoted also to the production of H2, CH4 (photo-Kolbe) and to CO2 and N2 reduction. Review papers were general and focused on principles and the mechanism of the process.
2—1980–1995, Maturity. In total, 0.49% of the documents were published in this period. The research was directed towards the kinetic analysis, metal doping, pollutant removal and structural studies regarding quantum effects on semiconductors. TiO2 gained a much more prominent role than in the previous period of time. General reviews were joined by more specific reviews considering sub-fields such as environmental applications, water purification and energy applications.
3—1996–2010, Explosion. In total, 16.6% of the documents appeared in that period of time. The research concerned non-metal doping (C, N, F), heterostructures, the fabrication of Z-schemes with multiple materials and CO2 utilization. TiO2 predominance started to be challenged by C3N4 and ternary semiconductors such as BiVO4. Reviews started to represent the majority of top-cited papers, and they were focused on specific aspects (e.g., surface properties, disinfection, degradation of specific classes of compounds) and on recent advances.
4—2010–present, Inflation. In total, 82.8% of the documents were published in this most-recent period of time. The research expanded towards plasmon resonance, and carbon-based materials (graphene, C3N4, C quantum dots) became predominant, both alone and in composites. TiO2 is still the most studied among oxide materials, usually in combination with other materials. Many elements are now considered Mo, Cd, In, Ga, Ag, Ce, W, C, N, F, S, O, Bi, V, Zn, Cu, Fe, …, even if crustal abundance considerations would suggest limiting the research on abundant and inexpensive elements (vide infra). Reviews represent more than 75% of top-cited papers, and they are mainly focused on recent advances, on sub-topics (Z-scheme, WPS, CO2…) and on particular material classes (e.g., specific metal oxides).[139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219]
- In periods one and two, more than 60% of the most-cited documents were published in the last 5 years considered. This is also due to the exponential growth of the production with time, because there are more recent papers to cite.
- During the Inflation period, the median year of publication is 2012.5, very close to the beginning of the period considered (2011). Even though the time available for the most recent articles is limited, the consideration made before is still valid, and the papers published in 2011–2013 are much less than in 2018–2020, as an example. Another important factor could be the difficulty to find, read and cite a specific paper in the gigantic and increasing scientific production. Recent papers could be less cited than expected because they are not known by the whole community
- Citations before 2018 highlight that, besides the obvious trend for the most recent period, papers from the Pioneering Years are still popular and reasonably cited, as ¼ of their citations arrived in the last 5 years, a trend shared with the Explosion (1996–2010). Conversely, only 14% of the citations arrived in the last 5 years of the Maturity period. Those documents were already very modern, and it is the authors’ opinion that they should receive more recognition in the present literature. Unfortunately, it is sometimes preferred to cite more recent papers to give the idea of cutting-edge research, with few references to the noble and pioneering investigations, completely neglecting instead a whole body of relevant and rigorous research, whose size—in terms of published documents—still allows its almost complete knowledge.
- Reduction reactions promoted through photocatalysis [220,221]. This field could regain popularity in the near future because of the emerging concern on PFAS pollution, which has recently been assessed as beyond planetary boundary [222]. PFAS are not emitted in significantly larger amounts compared with other pollutants; nevertheless, their inertness makes them extremely persistent in the environment, and their environmental impact and mitigation costs are therefore relevant [223]. Reduction of the C-F bond through semiconductor photocatalysis could represent an effective strategy for their removal from the environment and especially to prevent their dispersion. Furthermore, a better comprehension of the reductive photoactivated processes could be essential for a better comprehension of processes potentially important for energetics, such as the water photosplitting, the production of hydrogen through reforming of organic by-products or residual biomasses, the CO2 photo-reduction and the artificial photosynthesis [214,224,225]. Moreover, reductive processes activated by irradiated semiconductors have been proposed both in the Maturity period and nowadays for the recovery of precious or critical metals from diluted solutions [60,88,226,227,228,229,230].
- Surface modification by fluorination [231,232] was found to deeply modify TiO2 behavior in F–-containing solutions, [233] because of its strong adsorption on {001} facets and defective sites [234]. This led to a new field of investigation concerning the engineering of TiO2 nanoparticles, as fluorides could act as powerful shape controllers during hydrothermal synthesis [235]. The resulting nanoplatelets now find applications in different fields, e.g., nanometrology [236].
- Different crystalline facets’ reactivity demonstrated by Ohno and coworkers [237], which elegantly demonstrated how {101} facets in anatase and {110} facets in rutile are able to more efficiently trap electrons and therefore promote reduction reactions, whereas {001} facets in anatase and {011} in rutile preferentially trap photoholes and therefore promote oxidation reactions, e.g., PbO2 deposition from aqueous Pb2+ in the original work. This concept has been confirmed and exploited at several reprises, and it represents one of the main strategies to improve the efficiency of the photocatalytic process [238,239].
- Mechanistic studies of semiconductor photocatalysis. Even nowadays, several decades after the pioneering investigations in the field, errors and misconceptions around the working mechanism behind semiconductor photocatalysis are still present and widespread in the specialized literature. One of the most common examples is the interpretation in terms of substrate adsorption of the non-linear growth of substrate removal rate as a function of its concentration. Even if the Langmuir–Hinshelwood isotherm is still frequently reported to justify such a behavior, this explanation has no real physical significance, as elegantly observed by Emeline et al. [240] and formally demonstrated through quantitative kinetic modeling at several reprises [241,242,243,244,245,246].
4. Present Situation and Future Challenges
5. Conclusions and Perspectives
- Reduced involvement and reduced scientific production as a consequence of the development of alternative technologies. Examples could be (i) the production of hybrid technologies able to very efficiently convert the sunlight into electricity through photovoltaics and to store this energy in super electrochemical capacitors (and/or other energy storage systems), making useless the photocatalytic production of high energetic vectors such as H2; (ii) the development of very efficient and scarce energy-demand membrane technologies able to efficiently remove pollutants from water, putting the photocatalytic technologies for the removal of biorecalcitrant pollutants out of business; (iii) for the specific application of effluents decontamination and disinfection, the exponential growing of renewables energies for the production of electricity would impulse other technologies based on ozonation, UVC and other electricity-based processes that have substantially reduced their environmental impact.
- Almost constant involvement of the researchers, as the research on photocatalysis continues without any major breakthrough and the sector maintains good relevance in the fields of chemistry and material science, reaching a physiological limit in the number of publications mainly related to the limits and rules of the editorial scientific markets.
- Continued increase in the involvement of new research groups attracted by one or more breakthroughs within the traditional research lines in photocatalysis (e.g., synthesis of catalysts with a quantum yield near one operative in the visible spectrum) or outside it (e.g., production of UV irradiation systems at dramatically low cost and with very low energy requests or increment of the cost of competitor technologies of photocatalysis). In this way the photocatalytic technologies could reach a high technology readiness level (TRL), paying back the impressive scientific efforts carried out—since the beginning—on this topic from generations of scientists.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Breeze, P. Solar Power. In Power Generation Technologies, 3rd ed.; Newnes: London, UK, 2019; p. 462. [Google Scholar]
- Ciamician, G. Sur le actions chimiques de lumière. Bull. Soc. Chim. Fr. 1908, IV, i–xxvii. [Google Scholar]
- Lemoine, G. Etudes quantitatives sur l’action chimique de la lumière pour òa décomposition mutuelle de l’acide oxalique et du chlorure ferrique. Ann. Chim. Phys. (Paris) 1895, VI, 433–540. [Google Scholar]
- Plotnikow, J. Textbook of Photochemistry; Verlag von Willhelm Knapp: Berlin, Germany, 1910; p. 72. [Google Scholar]
- Landau, M. Le phénomène de la photocatalyse. Compt. Rend. 1913, 156, 1894–1896. [Google Scholar]
- Landau, M. Action of ultraviolet rays on lactic acid. Compt. Rend. 1912, 152, 1308–1309. [Google Scholar]
- Baly, E.C.C.; Heilbron, I.M.; Barker, W.F. Phtocatalysis. Part I. The Synthesis of Fmmaldehyde and Carbohydrates from Carbon Dioxide and Water. J. Chem. Soc. Trans. 1921, 119, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Baly, E.C.C.; Heilbron, I.M.; Hudson, D.P. Photocatalysis. Part II. The Photosynthesis of Nitrogen Compounds from Nitrates and Carbon Dioxide. J. Chem. Soc. Trans. 1922, 121, 1078–1088. [Google Scholar] [CrossRef]
- Baly, E.C.C.; Heilbron, I.M.; Stern, H.J. Photocatalysis. Part III. The Photosynthesis of Naturally Occurring Xitrogen Compounds from Carbon Dioxide and Ammonia. J. Chem. Soc. Trans. 1923, 123, 185–197. [Google Scholar] [CrossRef]
- Markham, M.C.; Hannan, M.C.; Paternostro, R.M.; Rose, C.B. Oxidation of Alcohols Catalyzed by Zinc Oxide and Light. J. Am. Chem. Soc. 1958, 80, 5394–5397. [Google Scholar] [CrossRef]
- Markham, M.C.; Hannan, M.C.; Evans, S.W. Factors Influencing the Oxidation of Phenols, Catalyzed by Zinc Oxide and Light. J. Am. Chem. Soc. 1954, 76, 820–823. [Google Scholar] [CrossRef]
- Schwab, G.-M.; Noller, H.; Steinbach, F.; Venugopalan, M. Oxidation of Carbon Monoxide and Methyl Alcohol photosensitized in the Gas Phase by Zinc Oxide. Nature 1962, 193, 774–775. [Google Scholar] [CrossRef]
- Markham, M.C.; Hannan, M.C.; Lin, L.; Coffey, C.; Jones, B. Photochemical properties of antimony trioxide. J. Phys. Chem. 1958, 62, 989–992. [Google Scholar] [CrossRef]
- Cohn, G.; Goodeve, C.F. The photochemistry of antimony trioxide. Trans. Faraday Soc. 1940, 35, 433–440. [Google Scholar] [CrossRef]
- Weyl, W.A.; Förland, T. Photochemistry of rutile. Ind. Eng. Chem. 1950, 42, 257–263. [Google Scholar] [CrossRef]
- Jacobsen, A.E. Titanium dioxide pigments Correlation between Photochemical Reactivity and Chalking. Ind. Eng. Chem. 1949, 41, 523–526. [Google Scholar] [CrossRef]
- Vail, C.B.; Holmquist, J.P.; White, L., Jr. Function of Organic Material in the Photochemical Formation of Hydrogen Peroxide at Zinc Oxide Surfaces. J. Am. Chem. Soc. 1954, 76, 624–625. [Google Scholar] [CrossRef]
- Rubin, T.R.; Calvert, J.G.; Rankin, G.T.; MacNevin, W. Photochemical Synthesis of Hydrogen Peroxide at Zinc Oxide Surface. J. Am. Chem. Soc. 1953, 75, 2850–2853. [Google Scholar] [CrossRef]
- Calvert, J.G.; Theurer, K.; Rankin, G.T.; MacNevin, W.M. A Study of the Mechanism of the Photochemical Synthesis of Hydrogen Peroxide at Zinc Oxide Surfaces. J. Am. Chem. Soc. 1954, 76, 2575–2578. [Google Scholar] [CrossRef]
- Markham, M.C. Photocatalytic properties of oxides. J. Chem. Educ. 1955, 32, 540–543. [Google Scholar] [CrossRef]
- Braslavsky, S.E.; Braun, A.M.; Cassano, A.E.; Emeline, A.V.; Litter, M.I.; Palmisano, L.; Parmon, V.N.; Serpone, N. Glossary of terms used in photocatalysis and radiation catalysis (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 931–1014. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Carey, J.H.; Lawrence, J.; Tosine, H.M. Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Pruden, A.L.; Ollis, D.F. Degradation of Chloroform by Photoassisted Heterogeneous Catalysis in Dilute Aqueous Suspensions of Titanium Dioxide. Environ. Sci. Technol. 1983, 17, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Barbeni, M.; Pramauro, E.; Pelizzetti, E.; Borgarello, E.; Serpone, N. Photodegradation of pentachlorophenol catalyzed by semiconductor particles. Chemosphere 1985, 14, 195–208. [Google Scholar] [CrossRef]
- Peral, J.; Ollis, D.F. Heterogeneous photocatalytic oxidation of gas-phase organics for air purification: Acetone, 1-butanol, butyraldehyde, formaldehyde, and m-xylene oxidation. J. Catal. 1992, 136, 554–565. [Google Scholar] [CrossRef]
- Ireland, J.C.; Klostermann, P.; Rice, E.W.; Clark, R.M. Inactivation of Escherichia coli by Titanium Dioxide Photocatalytic Oxidation. Appl. Environ. Microbiol. 1993, 59, 1668–1670. [Google Scholar] [CrossRef] [Green Version]
- Serpone, N.; Emeline, A.V. Semiconductor Photocatalysis—Past, Present, and Future Outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Sato, S. Photocatalytic activity of NOx-doped TiO2 in the visible light region. Chem. Phys. Lett. 1986, 123, 126–128. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Biedrzycki, J.; Livraghi, S.; Giamello, E.; Agnoli, S.; Granozzi, G. Fluorine- and niobium-doped TiO2: Chemical and spectroscopic properties of polycrystalline n-type-doped anatase. J. Phys.Chem. C 2014, 118, 8462–8473. [Google Scholar] [CrossRef]
- Czoska, A.M.; Livraghi, S.; Chiesa, M.; Giamello, E.; Agnoli, S.; Granozzi, G.; Finazzi, E.; Di Valentiny, C.; Pacchioni, G. The nature of defects in fluorine-doped TiO2. J. Phys. Chem. C 2008, 112, 8951–8956. [Google Scholar] [CrossRef]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Liu, N.; Schneider, C.; Freitag, D.; Hartmann, M.; Venkatesan, U.; Müller, J.; Spiecker, E.; Schmuki, P. Black TiO2 Nanotubes: Cocatalyst-Free Open-Circuit Hydrogen Generation. Nano Lett. 2014, 14, 3309–3313. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Muñoz, P.; Fresno, F.; Ivanez, J.; Robert, D.; Keller, N. Activity enhancement pathways in LaFeO3@TiO2 heterojunction photocatalysts for visible and solar light driven degradation of myclobutanil pesticide in water. J. Hazard. Mater. 2020, 400, 123099. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Yue, C.; Yuan, R.; Qiu, J.; Liu, F.Q.; Zhu, J.J. Enhanced removal of sulfamethoxazole by a novel composite of TiO2 nanocrystals in situ wrapped-Bi2O4 microrods under simulated solar irradiation. Chem. Eng. J. 2020, 384, 123278. [Google Scholar] [CrossRef]
- Tobajas, M.; Belver, C.; Rodriguez, J.J. Degradation of emerging pollutants in water under solar irradiation using novel TiO2-ZnO/clay nanoarchitectures. Chem. Eng. J. 2017, 309, 596–606. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Minella, M.; Sordello, F.; Minero, C. Graphitic carbon nitride-based metal-free photocatalyst. In Material Science in Photocatalysis; García-López, E.I., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 449–484. [Google Scholar]
- Malato, S.; Miralles-Cuevas, S.; Cabrera-Reina, A. Solar photocatalysis for water decontamination and disinfection (2017–2020). Photochemistry 2022, 49, 236–269. [Google Scholar]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-Scheme Photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C-Photochem. Rev. 2000, 1, 2000. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Som, I.; Roy, M. Recent development on titania-based nanomaterial for photocatalytic CO2 reduction: A review. J. Alloys Compd. 2022, 918, 165533. [Google Scholar] [CrossRef]
- Nasir, A.; Khalid, S.; Yasin, T.; Mazare, A. A Review on the Progress and Future of TiO2/Graphene Photocatalysts. Energies 2022, 15, 6248. [Google Scholar] [CrossRef]
- Morrison, S.R.; Freund, T. Chemical role of holes and electrons in ZnO photocatalysis. J. Chem. Phys. 1967, 47, 1543–1551. [Google Scholar] [CrossRef]
- Gerischer, H. Charge transfer processes at semiconductor-electrolyte interfaces in connection with problems of catalysis. Surf. Sci. 1969, 18, 97–122. [Google Scholar] [CrossRef]
- Bickley, R.I.; Stone, F.S. Photoadsorption and photocatalysis at rutile surfaces. I. Photoadsorption of oxygen. J. Catal. 1973, 31, 389–397. [Google Scholar] [CrossRef]
- Bickley, R.I.; Munuera, G.; Stone, F.S. Photoadsorption and photocatalysis at rutile surfaces. II. Photocatalytic oxidation of isopropanol. J. Catal. 1973, 31, 398–407. [Google Scholar] [CrossRef]
- Mollers, F.; Tolle, H.J.; Memming, R. On the Origin of the Photocatalytic Deposition of Noble Metals on TiO2. J. Electrochem. Soc. 1974, 121, 1160–1167. [Google Scholar] [CrossRef]
- Frank, S.N.; Bard, A.J. Heterogeneous photocatalytic oxidation of cyanide and sulfite in aqueous solutions at semiconductor powders. J. Phys. Chem. 1977, 81, 1484–1488. [Google Scholar] [CrossRef]
- Frank, S.N.; Bard, A.J. Heterogeneous Photocatalytic Oxidation of Cyanide Ion in Aqueous Solutions at TiO2 Powder. J. Am. Chem. Soc. 1977, 99, 303–304. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Guth, T.D. Photolysis of Water and Photoreduction of Nitrogen on Titanium Dioxide. J. Am. Chem. Soc. 1977, 99, 7189–7193. [Google Scholar] [CrossRef]
- Watanabe, T.; Takizawa, T.; Honda, K. Photocatalysis through excitation of adsorbates. 1. Highly efficient N-deethylation of rhodamine B adsorbed to CdS. J. Phys. Chem. 1977, 81, 1845–1851. [Google Scholar] [CrossRef]
- Janusz, J.M.; Berson, J.A. Heterogeneous Photocatalytic Synthesis of Methane from Acetic Acid—New Kolbe Reaction Pathway. J. Am. Chem. Soc. 1978, 100, 2239–2240. [Google Scholar]
- Kraeutler, B.; Bard, A.J. Heterogeneous Photocatalytic Preparation of Supported Catalysts. Photodeposition of Platinum on TiO2 Powder and Other Substrates. J. Am. Chem. Soc. 1978, 100, 4317–4318. [Google Scholar] [CrossRef]
- Kraeutler, B.; Bard, A.J. Heterogeneous Photocatalytic Decomposition of Saturated Carboxylic Acids on TiO2 Powder. Decarboxylative Route to Alkanes. J. Am. Chem. Soc. 1978, 100, 5985–5992. [Google Scholar] [CrossRef]
- Maruska, H.P.; Ghosh, A.K. Photocatalytic decomposition of water at semiconductor electrodes. Sol. Energy 1978, 20, 443–458. [Google Scholar] [CrossRef]
- Takizawa, T.; Watanabe, T.; Honda, K. Photocatalysis through excitation of adsorbates. 2. A comparative study of rhodamine B and methylene blue on cadmium sulfide. J. Phys. Chem. 1978, 82, 1391–1396. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Jaeger, C.D.; Bard, A.J. Spin trapping and electron spin resonance detection of radical intermediates in the photodecomposition of water at TiO2 particulate systems. J. Phys. Chem. 1979, 83, 3146–3152. [Google Scholar] [CrossRef]
- Reiche, H.; Dunn, W.W.; Bard, A.J. Heterogeneous photocatalytic and photosynthetic deposition of copper on TiO2 and WO3 powders. J. Phys. Chem. 1979, 83, 2248–2251. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry. Science 1980, 207, 139–144. [Google Scholar] [CrossRef]
- Domen, K.; Naito, S.; Soma, M.; Onishi, T.; Tamaru, K. Photocatalytic decomposition of water vapour on an NiO-SrTiO3 catalyst. J. Chem. Soc. Chem. Commun. 1980, 12, 543–544. [Google Scholar] [CrossRef]
- Izumi, I.; Dunn, W.W.; Wilbourn, K.O.; Fan, F.R.F.; Bard, A.J. Heterogeneous photocatalytic oxidation of hydrocarbons on platinized TiO2 powders. J. Phys. Chem. 1980, 84, 3207–3210. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Conversion of carbohydrate into hydrogen fuel by a photocatalytic process. Nature 1980, 286, 474–476. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Photocatalytic hydrogen production from liquid methanol and water. J. Chem. Soc. Chem. Commun. 1980, 15, 694–695. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Photocatalytic decomposition of gaseous water over TiO2 and TiO2–RuO2 surfaces. Chem. Phys. Lett. 1980, 72, 87–89. [Google Scholar] [CrossRef]
- Watanabe, T.; Honda, K. Measurement of the extinction coefficient of the methyl viologen cation radical and the efficiency of its formation by semiconductor photocatalysis. J. Phys. Chem. 1982, 86, 2617–2619. [Google Scholar] [CrossRef]
- Shilov, A.E. Energy Resources through Photochemistry and Catalysis; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Anpo, M.; Shima, T.; Kodama, S.; Kubokawa, Y. Photocatalytic hydrogenation of CH3CCH with H2O on small-particle TiO2: Size quantization effects and reaction intermediates. J. Phys. Chem. 1987, 91, 4305–4310. [Google Scholar] [CrossRef]
- Matthews, R.W. Photooxidation of organic impurities in water using thin films of titanium dioxide. J. Phys. Chem. 1987, 91, 3328–3333. [Google Scholar] [CrossRef]
- Al-Ekabi, H.; Serpone, N. Kinetic studies in heterogeneous photocatalysis. 1. Photocatalytic degradation of chlorinated phenols in aerated aqueous solutions over TiO2 supported on a glass matrix. J. Phys. Chem. 1988, 92, 5726–5731. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Preparation and characterization of quantum-size titanium dioxide. J. Phys. Chem. 1988, 92, 5196–5201. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photocatalytic production of H2O2 and organic peroxides in aqueous suspensions of TiO2, ZnO, and desert sand. Environ. Sci. Technol. 1988, 22, 798–806. [Google Scholar] [CrossRef]
- Matthews, R.W. Kinetics of photocatalytic oxidation of organic solutes over titanium dioxide. J. Catal. 1988, 111, 264–272. [Google Scholar] [CrossRef]
- Abdullah, M. Effects of common inorganic anions on rates of photocatalytic oxidation of organic carbon over illuminated titanium dioxide. J. Phys. Chem. 1990, 94, 6820–6825. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ollis, D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Bickley, R.I.; Gonzalez-Carreno, T.; Lees, J.S.; Palmisano, L.; Tilley, R.J.D. A structural investigation of titanium dioxide photocatalysts. J. Solid State Chem. 1991, 92, 178–190. [Google Scholar] [CrossRef]
- Gerischer, H.; Heller, A. The role of oxygen in photooxidation of organic molecules on semiconductor particles. J. Phys. Chem. 1991, 95, 5261–5267. [Google Scholar] [CrossRef]
- Kormann, C.; Hoffmann, M.R.; Bahnemann, D.W. Photolysis of Chloroform and Other Organic Molecules in Aqueous TiO2 Suspensions. Environ. Sci. Technol. 1991, 25, 494–500. [Google Scholar] [CrossRef]
- Ollis, D.F.; Pelizzetti, E.; Serpone, N. Destruction of water contaminants. Environ. Sci. Technol. 1991, 25, 1522–1529. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous Photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Mills, A.; Davies, R.H.; Worsley, D. Water purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Hotchandani, S.; Kamat, P.V. Electrochemically assisted photocatalysis. TiO2 particulate film electrodes for photocatalytic degradation of 4-chlorophenol. J. Phys. Chem. 1993, 97, 9040–9044. [Google Scholar] [CrossRef]
- Weller, H. Colloidal Semiconductor Q-Particles: Chemistry in the Transition Region Between Solid State and Molecules. Angew. Chem. Int. Ed. Engl. 1993, 32, 32–41. [Google Scholar] [CrossRef]
- Wold, A. Photocatalytic Properties of TiO2. Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Yamaguchi, T. Application of ZrO2 as a catalyst and a catalyst support. Catal. Today 1994, 20, 199–217. [Google Scholar] [CrossRef]
- Bamwenda, G.R.; Tsubota, S.; Nakamura, T.; Haruta, M. Photoassisted hydrogen production from a water-ethanol solution: A comparison of activities of AuTiO2 and PtTiO2. J. Photochem. Photobiol. A 1995, 89, 177–189. [Google Scholar] [CrossRef]
- Fernández, A.; Lassaletta, G.; Jiménez, V.M.; Justo, A.; González-Elipe, A.R.; Herrmann, J.M.; Tahiri, H.; Ait-Ichou, Y. Preparation and characterization of TiO2 photocatalysts supported on various rigid supports (glass, quartz and stainless steel). Comparative studies of photocatalytic activity in water purification. Appl. Catal. B Environ. 1995, 7, 49–63. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Obee, T.N.; Brown, R.T. TiO2 Photocatalysis for Indoor Air Applications: Effects of Humidity and Trace Contaminant Levels on the Oxidation Rates of Formaldehyde, Toluene, and 1,3-Butadiene. Environ. Sci. Technol. 1995, 29, 1223–1231. [Google Scholar] [CrossRef]
- Serpone, N.; Maruthamuthu, P.; Pichat, P.; Pelizzetti, E.; Hidaka, H. Exploiting the interparticle electron transfer process in the photocatalysed oxidation of phenol, 2-chlorophenol and pentachlorophenol: Chemical evidence for electron and hole transfer between coupled semiconductors. J. Photochem. Photobiol. A 1995, 85, 247–255. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Kamat, P.V. Enhanced Rates of Photocatalytic Degradation of an Azo Dye Using SnO2/TiO2 Coupled Semiconductor Thin Films. Environ. Sci. Technol. 1995, 29, 841–845. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A-Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Herrmann, J.M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Yong, X.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 3–4, 543–556. [Google Scholar]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J. Phys. Chem. B 2002, 106, 7729–7744. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Ho, W.; Jiang, Z.; Zhang, L. Effects of F- doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-concentration dependence on photocatalytic activity of TiO2−xNx powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight Photocatalysis by Carbon-Modified Titanium Dioxide. Angew. Chem. Int. Ed. Engl. 2003, 42, 4908–4911. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/Gold Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.; Bard, A.J. Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting. Nano Lett. 2006, 6, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Kamat, P.V. Meeting the clean energy demand: Nanostructure architectures for solar energy conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C-Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Seger, B.; Kamt, P.V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. Engl. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.; Smith, S.C.; Chen, Z.; Lu, G.Q.; Cheng, H.M. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic water splitting: Recent progress and future challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z.; et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Ouyang, S.; Umezawa, N.; Cao, J.; Ye, J. Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties. J. Am. Chem. Soc. 2011, 133, 6490–6492. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Henderson, M.A. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination-A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. Engl. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and enhanced visible-light photocatalytic H2- production activity of graphene/C3N4 composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C-Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: From photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. Engl. 2012, 51, 68–89. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO 2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Xuan, J.; Xiao, W.J. Visible-light photoredox catalysis. Angew. Chem. Int. Ed. Engl. 2012, 51, 6828–6838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yates, J.T. Band bending in semiconductors: Chemical and physical consequences at surfaces and interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S.Z. Graphitic carbon nitride materials: Controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. Engl. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Zhang, F.; Wang, D.; Yang, J.; Li, M.; Zhu, J.; Zhou, X.; Han, H.; Li, C. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat. Commun. 2013, 4, 1432. [Google Scholar] [CrossRef] [Green Version]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.Q.; Fu, X.; Zhang, N.; Xu, Y.J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. technological use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2014, 40, 11094–11111. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide-from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef]
- Schultz, D.M.; Yoon, T.P. Solar synthesis: Prospects in visible light photocatalysis. Science 2014, 343, 6174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.C.; Li, J.R.; Lv, X.L.; Zhang, Y.Q.; Guo, G. Photocatalytic organic pollutants degradation in metal-organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2-Reduction activity of anatase TiO2 by Coexposed {001} and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, W. Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.; Jaroniec, M. All-solid-state Z-scheme photocatalytic systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 6217. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two–electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting-a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Gawande, M.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [Green Version]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T. Alkali-Assisted Synthesis of Nitrogen Deficient Graphitic Carbon Nitride with Tunable Band Structures for Efficient Visible-Light-Driven Hydrogen Evolution. Adv. Mater. 2017, 29, 1605148. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal-Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Wu, H.; Tan, H.L.; Toe, C.Y.; Scott, J.; Wang, L.; Amal, R.; Ng, Y.H. Photocatalytic and Photoelectrochemical Systems: Similarities and Differences. Adv. Mater. 2020, 32, 1904717. [Google Scholar] [CrossRef]
- Minero, C.; Piccinini, P.; Calza, P.; Pelizzetti, E. Photocatalytic reduction/oxidation processes occurring at the carbon and nitrogen of tetranitromethane. New J. Chem. 1996, 20, 1159–1164. [Google Scholar]
- Calza, P.; Minero, C.; Pelizzetti, E. Photocatalytically Assisted Hydrolysis of Chlorinated Methanes under Anaerobic Conditions. Environ. Sci. Technol. 1997, 31, 2198–2203. [Google Scholar] [CrossRef]
- Cousins, I.T.; Johansson, J.H.; Salter, M.E.; Sha, B.; Scheringer, M. Outside the Safe Operating Space of a New Planetary Boundary for Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Technol. 2022, 56, 11172–11179. [Google Scholar] [CrossRef]
- Obsekov, V.; Kahn, L.G.; Trasande, L. Leveraging. Systematic Reviews to Explore Disease Burden and Costs of Per- and Polyfluoroalkyl Substance Exposures in the United States. Expo. Health 2022. [Google Scholar] [CrossRef]
- Kärkäs, M.D.; Verho, O.; Johnston, E.V.; Åkermark, B. Artificial Photosynthesis: Molecular Systems for Catalytic Water Oxidation. Chem. Rev. 2014, 114, 11863–12001. [Google Scholar] [CrossRef]
- Tao, X.; Zhao, Y.; Wang, S.; Li, C.; Li, R. Recent advances and perspectives for solar-driven water splitting using particulate photocatalysts. Chem. Soc. Rev. 2022, 51, 3561–3608. [Google Scholar] [CrossRef] [PubMed]
- Baba, R.; Nakabayashi, S.; Fujishima, A.; Honda, K. Investigation of the mechanism of hydrogen evolution during photocatalytic water decomposition on metal-loaded semiconductor powders. J. Phys. Chem. 1985, 89, 1902–1905. [Google Scholar] [CrossRef]
- Borgarello, E.; Serpone, N.; Emo, G.; Harris, R.; Pelizzetti, E.; Minero, C. Light-Induced Reduction of Rhodium(III) and Palladium(II) on Titanium Dioxide Dispersions and the Selective Photochemical Separation and Recovery of Gold(III), Platinum(IV), and Rhodium(III) in Chloride Media. Inorg. Chem. 1986, 25, 4499–4503. [Google Scholar] [CrossRef]
- Curran, J.S.; Domenech, J.; Jaffrezic-Renault, N.; Philippe, R. Kinetics and Mechanism of Platinum Deposition by Photoelectrolysis in Illuminated Suspensions of Semiconducting Titanium Dioxide. J. Phys. Chem. 1985, 89, 957–963. [Google Scholar] [CrossRef]
- Litter, M.I. Heterogeneous photocatalysis Transition metal ions in photocatalytic systems. Appl. Catal. B Environ. 1999, 23, 89–114. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, M.; Wen, J.; Wan, Y.; Zhao, Q.; Cao, X.; Ding, Y.; Wang, Z.L.; Li, H.; Bian, Z. Selective recovery of precious metals through photocatalysis. Nat. Sustain. 2021, 4, 618–626. [Google Scholar] [CrossRef]
- Minero, C.; Mariella, G.; Maurino, V.; Pelizzetti, E. Photocatalytic Transformation of Organic Compounds in the Presence of Inorganic Anions. 1. Hydroxyl-Mediated and Direct Electron-Transfer Reactions of Phenol on a Titanium Dioxide–Fluoride System. Langmuir 2000, 16, 2632–2641. [Google Scholar] [CrossRef]
- Minero, C.; Mariella, G.; Maurino, V.; Vione, D.; Pelizzetti, E. Photocatalytic transformation of organic compounds in the presence of inorganic ions. 2. Competitive reactions of phenol and alcohols on a titanium dioxide-fluoride system. Langmuir 2000, 16, 8964–8972. [Google Scholar] [CrossRef]
- Monllor-Satoca, D.; Gómez, R. Electrochemical Method for Studying the Kinetics of Electron Recombination and Transfer Reactions in Heterogeneous Photocatalysis: The Effect of Fluorination on TiO2 Nanoporous Layers. J. Phys. Chem. C 2007, 112, 139–147. [Google Scholar] [CrossRef]
- Minella, M.; Faga, M.G.; Maurino, V.; Minero, C.; Pelizzetti, E.; Coluccia, S.; Martra, G. Effect of fluorination on the surface properties of titania P25 powder: An FTIR study. Langmuir 2010, 26, 2521–2527. [Google Scholar] [CrossRef]
- Taguchi, T.; Saito, Y.; Sarukawa, K.; Ohno, T.; Matsumura, M. Formation of new crystal faces on TiO2 particles by treatment with aqueous HF solution or hot sulfuric acid. New J. Chem. 2003, 27, 1304–1306. [Google Scholar] [CrossRef] [Green Version]
- Maurino, V.; Pellegrino, F.; Picotto, G.B.; Ribotta, L. Quantitative three-dimensional characterization of critical sizes of non-spherical TiO2 nanoparticles by using atomic force microscopy. Ultramicroscopy 2022, 234, 113480. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 2002, 26, 1167–1170. [Google Scholar] [CrossRef] [Green Version]
- D’Arienzo, M.; Dozzi, M.V.; Redaelli, M.; Di Credico, B.; Morazzoni, F.; Scotti, R.; Polizzi, S. Crystal Surfaces and Fate of Photogenerated Defects in Shape-Controlled Anatase Nanocrystals: Drawing Useful Relations to Improve the H2 Yield in Methanol Photosteam Reforming. J. Phys. Chem. C 2015, 119, 12385–12393. [Google Scholar] [CrossRef]
- Pellegrino, F.; Sordello, F.; Mino, L.; Minero, C.; Hodoroaba, V.-D.; Martra, G.; Maurino, V. Formic Acid Photoreforming for Hydrogen Production on Shape-Controlled Anatase TiO2 Nanoparticles: Assessment of the Role of Fluorides, {101}/{001} Surfaces Ratio, and Platinization. ACS Catal. 2019, 9, 6692–6697. [Google Scholar] [CrossRef]
- Emeline, A.V.; Ryabchuk, V.K.; Serpone, N. Dogmas and Misconceptions in Heterogeneous Photocatalysis. Some Enlightened Reflections. J. Phys. Chem. B 2005, 109, 18515–18521. [Google Scholar] [CrossRef]
- Minero, C. A rigorous kinetic approach to model primary oxidative steps of photocatalytic degradations. Sol. Energy Mater. Sol. Cells 1995, 38, 421–430. [Google Scholar] [CrossRef]
- Minero, C. Kinetic analysis of photoinduced reactions at the water semiconductor interface. Catal. Today 1999, 54, 205–216. [Google Scholar] [CrossRef]
- Minero, C.; Vione, D. A quantitative evalution of the photocatalytic performance of TiO2 slurries. Appl. Catal. B Environ. 2006, 67, 257–269. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Augugliaro, V.; Cardillo, A.G.; Loddo, V.; Palmisano, L.; Parrino, F.; Santarelli, F. A reaction engineering approach to kinetic analysis of photocatalytic reactions in slurry systems. Catal. Today 2016, 259, 87–96. [Google Scholar] [CrossRef]
- Brandi, R.J.; Alfano, O.M.; Cassano, A.E. Evaluation of Radiation Absorption in Slurry Photocatalytic Reactors. 1. Assessment of Methods in Use and New Proposal. Environ. Sci. Technol. 2000, 34, 2623–2630. [Google Scholar] [CrossRef]
- Brandi, R.J.; Alfano, O.M.; Cassano, A.E. Evaluation of Radiation Absorption in Slurry Photocatalytic Reactors. 2. Experimental Verification of the Proposed Method. Environ. Sci. Technol. 2000, 34, 2631–2639. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Hamdany, A.H.; Satyanaga, A.; Zhang, D.; Kim, Y.; Kim, J.R. Photocatalytic Cementitious Material for Eco-Efficient Construction—A Systematic Literature Review. Appl. Sci. 2022, 12, 8741. [Google Scholar] [CrossRef]
- Dudek, D.; Janus, M. Photoactive Cements: A Review. Materials 2022, 15, 5407. [Google Scholar] [CrossRef] [PubMed]
- Minella, M.; Minero, C. Evaluation of gas / solid photocatalytic performance for the removal of VOCs at ppb and sub-ppb levels. Chemosphere 2021, 272, 129636. [Google Scholar] [CrossRef] [PubMed]
- Zacarías, S.M.; Manassero, A.; Pirola, S.; Alfano, O.M.; Satuf, M.L. Design and performance evaluation of a photocatalytic reactor for indoor air disinfection. Environ. Sci. Pollut. Res. 2021, 28, 23859–23867. [Google Scholar] [CrossRef]
- Available online: https://www.nobelprize.org/uploads/2019/10/advanced-chemistryprize2019.pdf (accessed on 16 September 2022).
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef]
- Suess, H.E.; Urey, H.C. Abundances of the Elements. Rev. Mod. Phys. 1956, 28, 1956. [Google Scholar] [CrossRef]
- Rhodes, C.J. Endangered elements, critical raw materials and conflict minerals. Sci. Prog. 2019, 102, 304–350. [Google Scholar] [CrossRef]
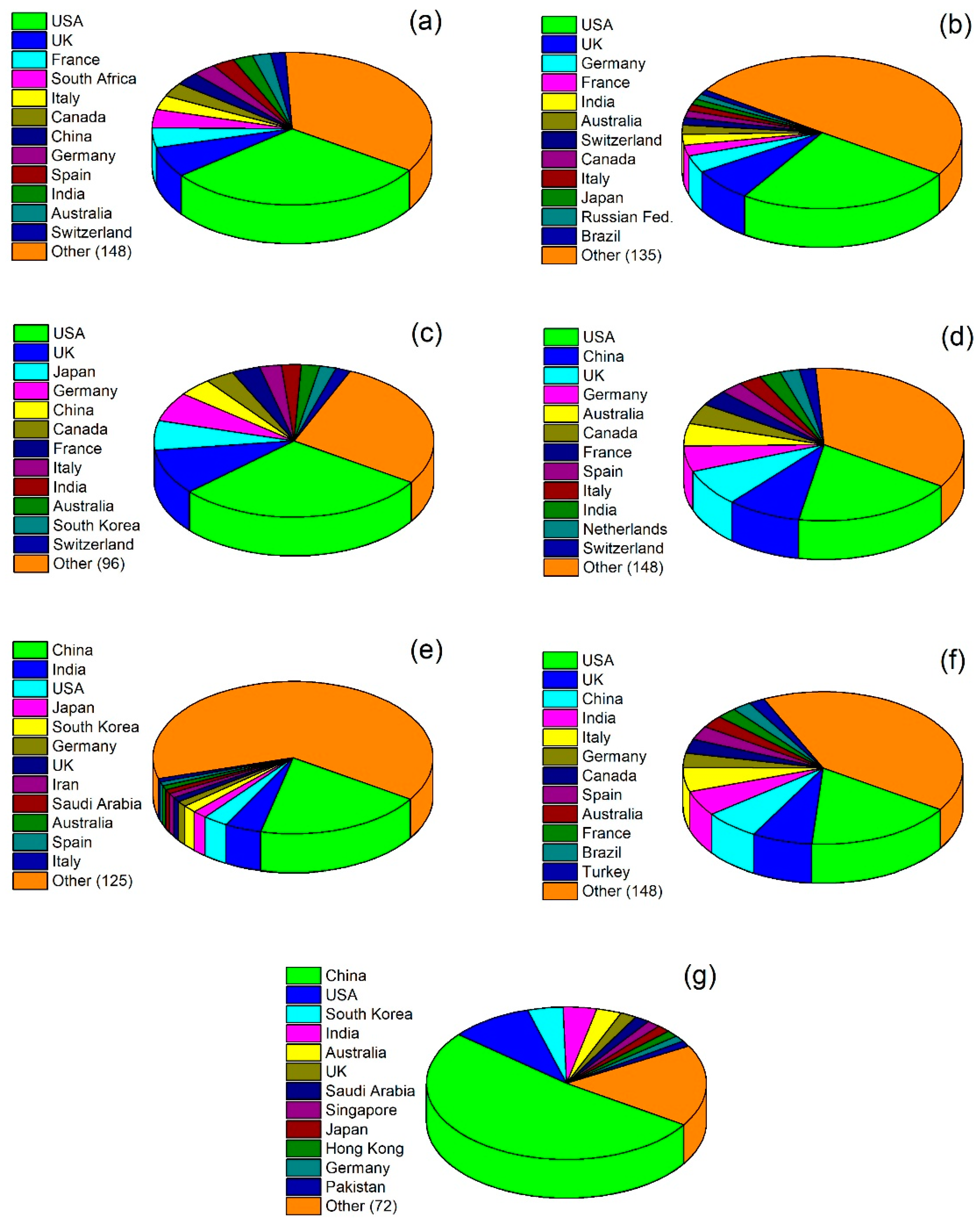
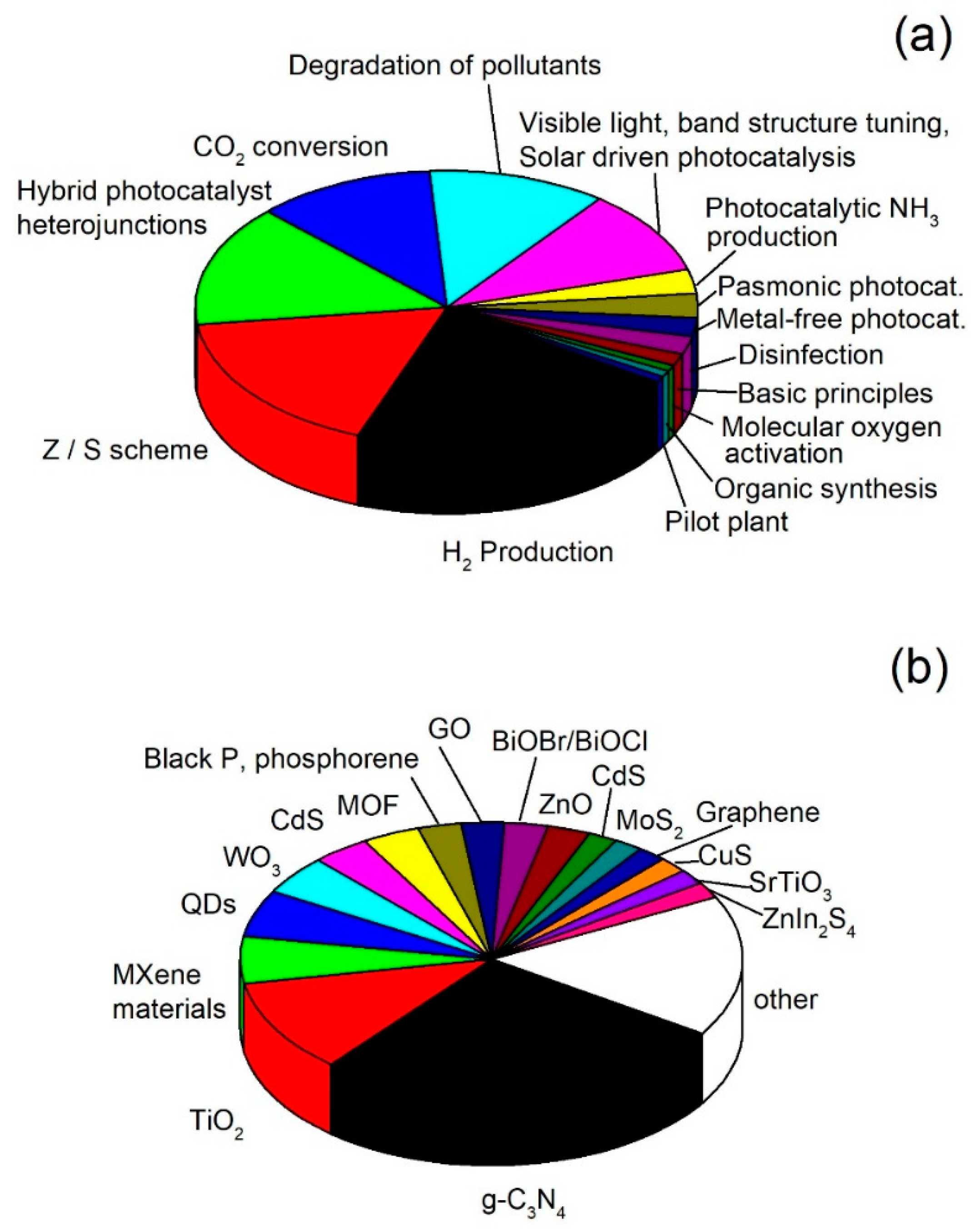
| Years | 1800–1980 | 1981–1995 | 1996–2010 | 2011–Present |
|---|---|---|---|---|
| Name | Pioneering Years | Maturity | Explosion | Inflation |
| Citation trend last 5 years | Increasing | Decreasing | Decreasing | Increasing |
| Relevant reviews | 4 | 9 | 24 | 61 |
| Citations (% before 2018) | 9149 (75%) | 13,889 (89%) | 61,836 (73%) | 36,235 (40%) |
| Citations/Papers | 457 | 694 | 3092 | 1812 |
| Citations a (% before 2018) | 11,112 (74%) | 51,517 (84%) | 143,139 (66%) | 156,429 (32%) |
| Citations/Papers a | 463 | 1776 | 3328 | 1931 |
| Citations 1st | 1993 | 1947 | 11,488 | 4816 |
| Citations 20th | 145 | 501 | 1658 | 1187 |
| Median citations | 350 | 589 | 2053 | 1461 |
| Median of the year of publication | 1978 | 1991 | 2004.5 | 2012.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sordello, F.; Calza, P.; Minero, C.; Malato, S.; Minella, M. More than One Century of History for Photocatalysis, from Past, Present and Future Perspectives. Catalysts 2022, 12, 1572. https://doi.org/10.3390/catal12121572
Sordello F, Calza P, Minero C, Malato S, Minella M. More than One Century of History for Photocatalysis, from Past, Present and Future Perspectives. Catalysts. 2022; 12(12):1572. https://doi.org/10.3390/catal12121572
Chicago/Turabian StyleSordello, Fabrizio, Paola Calza, Claudio Minero, Sixto Malato, and Marco Minella. 2022. "More than One Century of History for Photocatalysis, from Past, Present and Future Perspectives" Catalysts 12, no. 12: 1572. https://doi.org/10.3390/catal12121572
APA StyleSordello, F., Calza, P., Minero, C., Malato, S., & Minella, M. (2022). More than One Century of History for Photocatalysis, from Past, Present and Future Perspectives. Catalysts, 12(12), 1572. https://doi.org/10.3390/catal12121572