A Parametric Study of the Crystal Phases on Au/TiO2 Photocatalysts for CO2 Gas-Phase Reduction in the Presence of Water
Abstract
:1. Introduction
2. Results
2.1. Structural Characterization (XRD)
2.2. Surface Characterizations
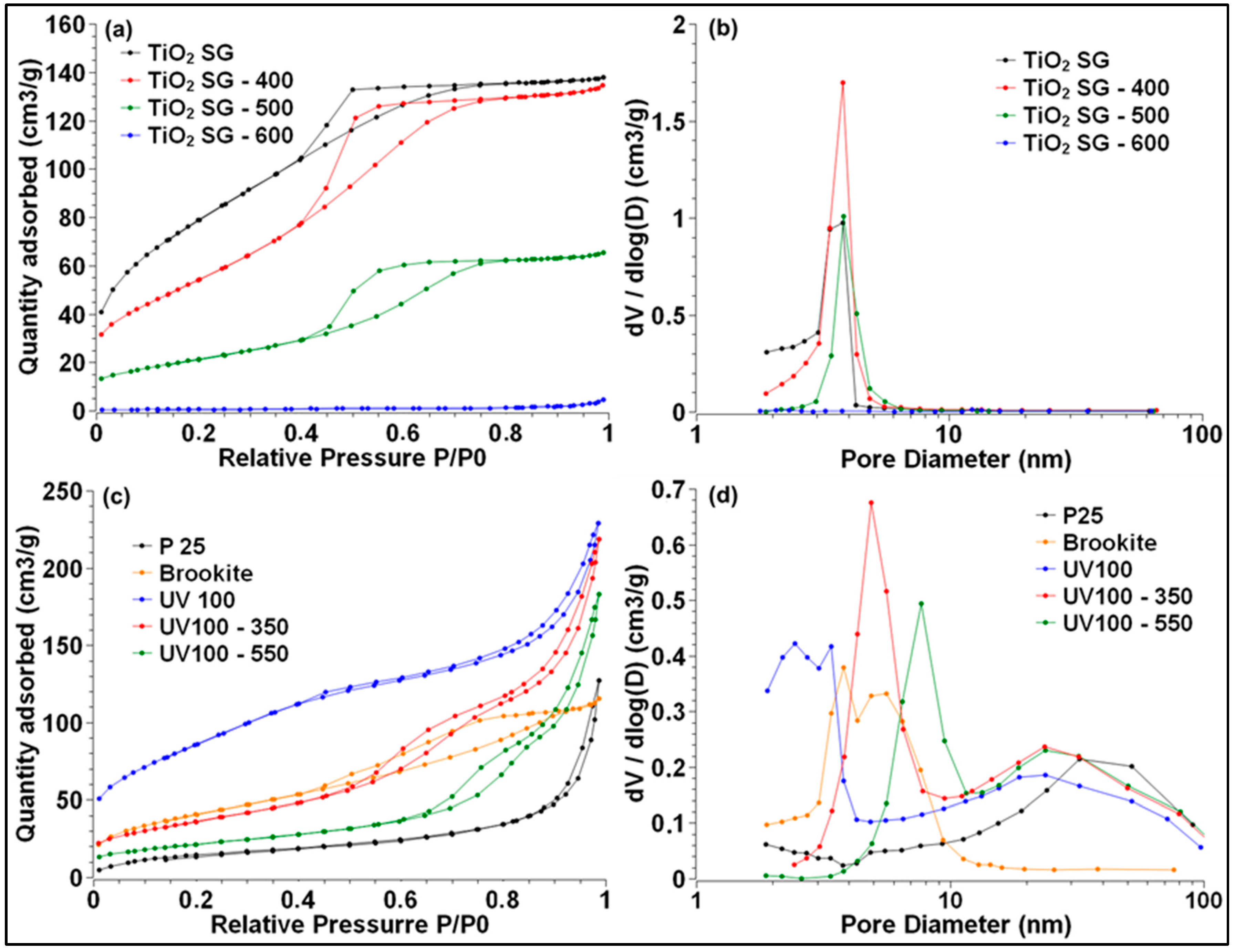
2.2.1. BET Surface Area and Porosity Measurements
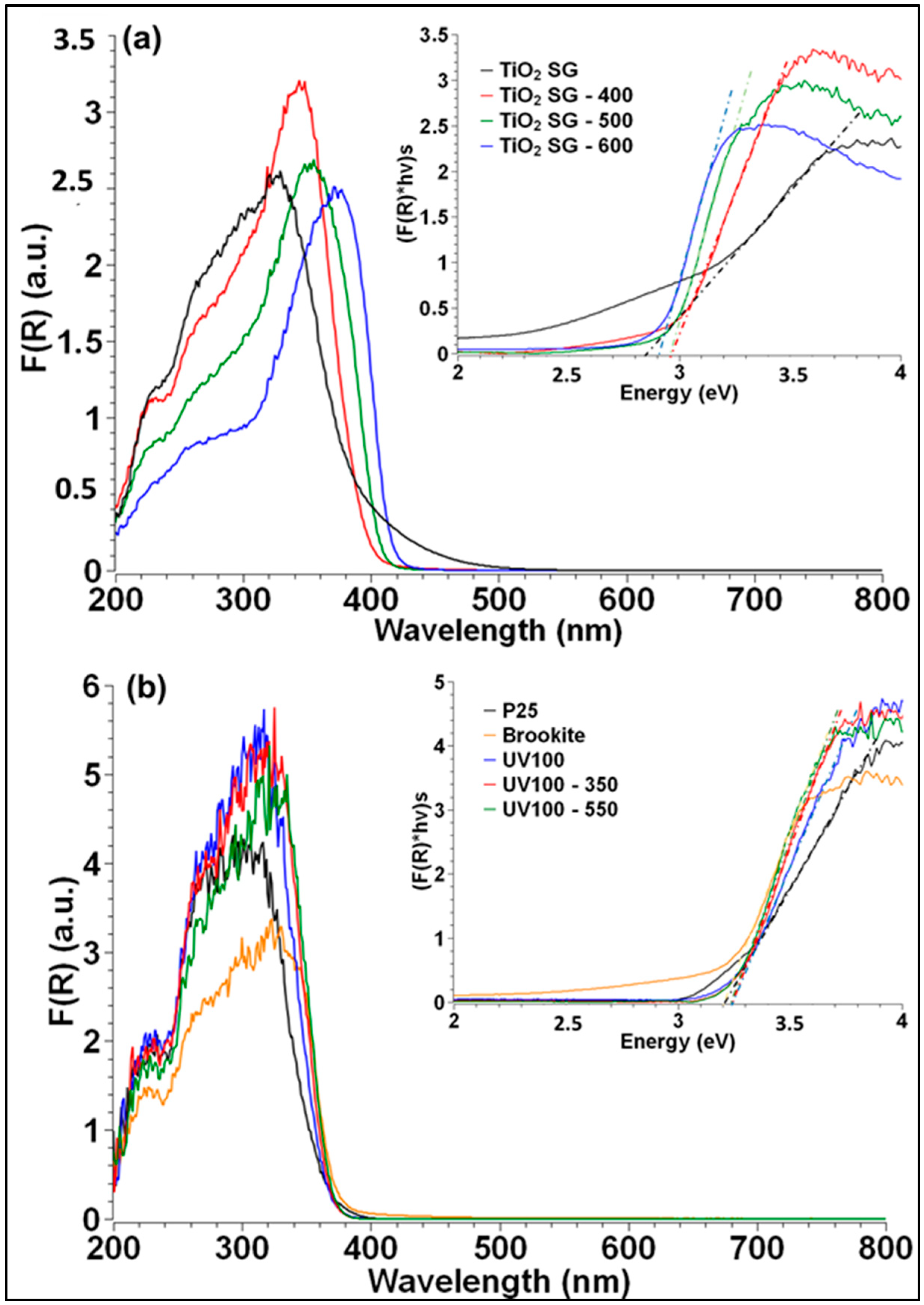
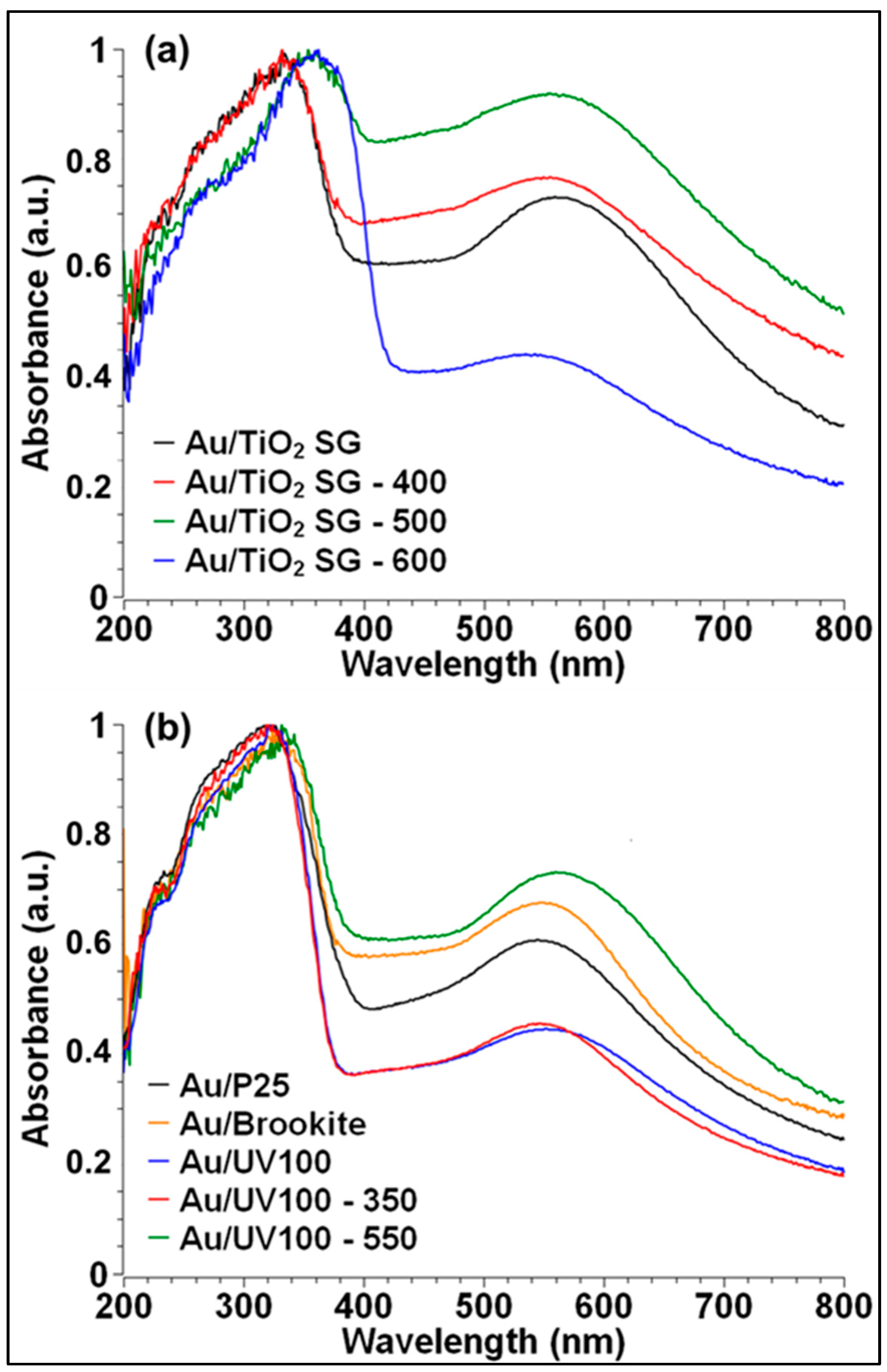
2.2.2. Au NPs Deposition and UV-Vis Absorption Properties
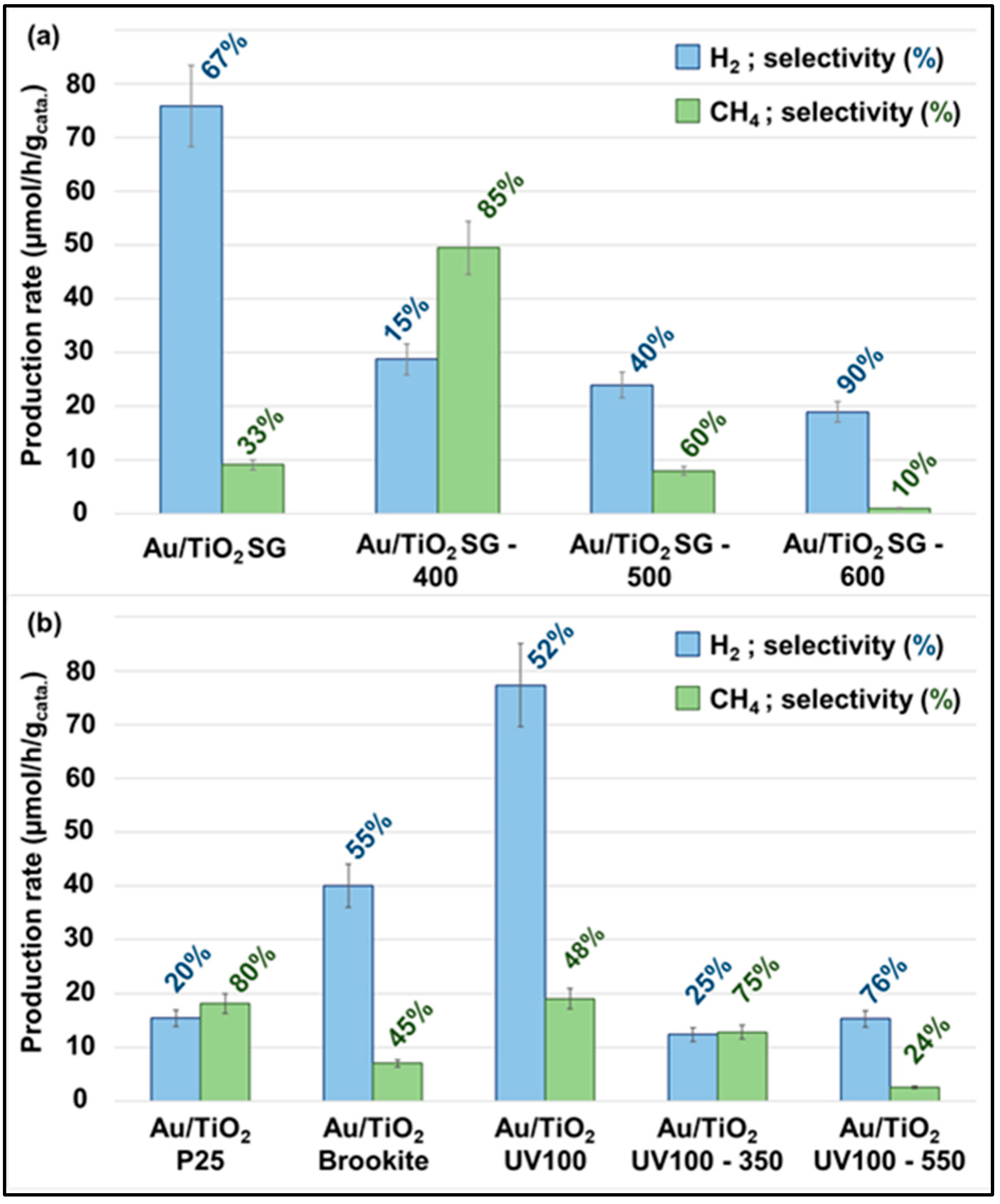
2.3. Gas-Phase CO2 Photoreduction Activity
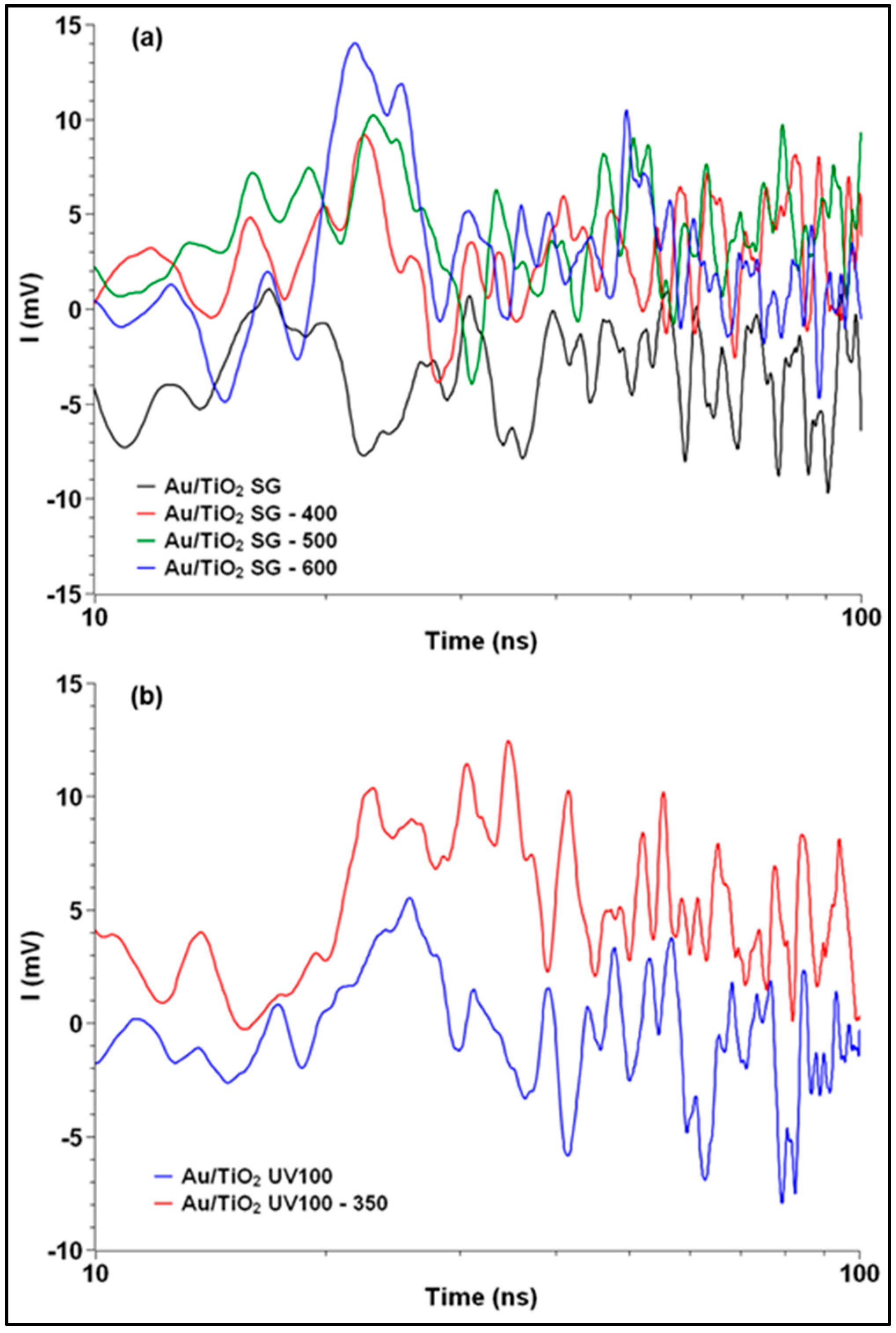
2.4. TRMC Measurements
2.5. EPR Measurements
3. Discussion
4. Materials and Methods
4.1. TiO2 Sol Gel (SG) Synthesis and Hombikat UV-100 Treatment
4.2. Au Nanoparticles (NPs) Deposition
4.3. Characterization Methods
4.4. Photocatalytic Tests
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic Reduction of Carbon Dioxide in aqueous Suspensions of Semiconductor Powders. Nature 1979, 277, 637. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernandez-Garcia, M.; Colon, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar]
- Neatu, S.; Macia-Agullo, J.A.; Garcia, H. Solar Light Photocatalytic CO2 Reduction: General Considerations and Selected Bench-Mark Photocatalysts. Int. J. Mol. Sci. 2014, 15, 5246–5262. [Google Scholar] [CrossRef] [Green Version]
- LianJun, L.; Ying, L. Understanding the Reaction Mechanism of Photocatalytic Reduction of CO2 with H2O on TiO2-Based Photocatalysts: A Review. Aerosol Air Qual. Res. 2014, 14, 453–469. [Google Scholar]
- Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.-H.; Le, T.-H.; Vo, D.N.; Trinh, Q.T.; Bae, S.-R.; Chae, S.Y.; Kim, S.Y.; Le, Q.V. Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels. Nanomaterials 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, Z.; Li, W.; Pan, H. Photocatalytic reduction of CO2 over noble metal-loaded and nitrogen-doped mesoporous TiO2. Appl. Catal. A. 2012, 429–430, 31–38. [Google Scholar] [CrossRef]
- Linic, S.; Aslam, U.; Boerigter, C.; Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 2015, 14, 567–576. [Google Scholar]
- Jia, J.; Wang, H.; Lu, Z.; O’Brien, P.G.; Ghoussoub, M.; Duchesne, P.; Zheng, Z.; Li, P.; Qiao, Q.; Wang, L.; et al. Photothermal Catalyst Engineering: Hydrogenation of Gaseous CO2 with High Activity and Tailored Selectivity. Adv. Sci. 2017, 4, 1700252. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-E.; Kim, D.J.; Devthade, V.; Jo, W.-K.; Tonda, S. Size-dependent selectivity and activity of highly dispersed sub-nanometer Pt clusters integrated with P25 for CO2 photoreduction into methane fuel. Appl. Surf. Sci. 2022, 584, 152532. [Google Scholar] [CrossRef]
- Barrocas, B.T.; Ambrozova, N.; Koci, K. Photocatalytic Reduction of Carbon Dioxide on TiO2 Heterojunction Photocataysts—A review. Materials 2022, 15, 967. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar]
- Wang, L.; Zhang, Z.; Han, Q.; Liu, Y.; Zhong, J.; Chen, J.; Huang, J.; She, H.; Wang, Q. Preparation of CdS-P25/ZIF-67 composite material and its photocatalytic CO2 reduction performance. Appl. Surf. Sci. 2022, 584, 152645. [Google Scholar] [CrossRef]
- Indrakanti, V.P.; Kubicki, J.D.; Schobert, H.H. Photoinduced activation of CO2 on Ti-based heterogeneous catalysts: Current state, chemical physics-based insights and outlook. Energy Environ. Sci. 2009, 2, 745–758. [Google Scholar] [CrossRef]
- Ghuman, K.K.; Singh, C.C. Effect of doping on electronic structure and photocatalytic behavior of amorphous TiO2. J. Phys. Condens. Matter 2013, 25, 475501. [Google Scholar] [CrossRef] [Green Version]
- Naik, B.; Kim, S.M.; Jung, C.H.; Moon, S.Y.; Kim, S.H.; Park, J.Y. Enhanced H2 Generation of Au-Loaded, Nitrogen-Doped TiO2 Hierarchical Nanostructures under Visible Light. Adv. Mater. Interfaces 2014, 1, 1300018. [Google Scholar] [CrossRef]
- Cottineau, T.; Béalu, N.; Gross, P.-A.; Pronkin, S.N.; Keller, N.; Savinova, E.R.; Keller, V. One step synthesis of niobium doped titania nanotube arrays to form (N,Nb) co-doped TiO2 with high visible light photoelectrochemical activity. J. Mater. Chem. 2013, 1, 2151. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Lu, Y.; Xu, J.; Wang, Y.; Zhu, Y. Effect of Compensated Codoping on the Photoelectrochemical Properties of Anatase TiO2 Photocatalyst. J. Phys. Chem. C. 2011, 115, 16963–16969. [Google Scholar] [CrossRef]
- Almaev, A.V.; Yakovlev, N.N.; Kushnarev, B.O.; Kopyev, V.V.; Novikov, V.A.; Zinoviev, M.M.; Yudin, N.N.; Podzivalov, S.N.; Erzakova, N.N.; Chikiryaka, A.V.; et al. Gas Sensitivity of IBSD Deposited TiO2 Thin Films. Coatings 2022, 12, 1565. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction-Pyrolytic Method for TiO2 polymorphs Production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Devi, A.D.; Pushpavanam, S.; Singh, N.; Verma, J.; Kaur, M.P.; Roy, S.C. Enhanced methane yield by photoreduction of CO2 at moderate temperature and pressure using Pt coated, graphene oxide wrapped TiO2 nanotubes. Results Eng. 2022, 14, 100441. [Google Scholar] [CrossRef]
- Goto, H.; Masegi, H.; Sadale, S.B.; Noda, K. Intricate behaviors of gas phase CO2 photoreduction in high vacuum using Cu2O loaded TiO2 nanotube arrays. J. CO2 Util. 2022, 59, 101964. [Google Scholar] [CrossRef]
- Butburee, T.; Kotchasarn, P.; Hirunsit, P.; Sun, Z.; Tang, Q.; Khemthong, P.; Sangkhun, W.; Thongsuwan, W.; Kumnorkaew, P.; Wang, H.; et al. New understanding of crystal control and facet selectivity of titanium dioxide ruling photocatalytic performance. J. Mater. Chem. A 2019, 7, 8156–8166. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Zhao, H.; Chen, J.; Cheng, J.; Yang, K.; Li, Y. Engineering Coexposed {001} and {101} Facets in Oxygen-Deficient TiO2 Nanocrystals for Enhanced CO2 Photoreduction under Visible Light. ACS Catal. 2016, 6, 1097–1108. [Google Scholar] [CrossRef]
- Scholes, D.T.; Yee, P.Y.; Lindemuth, J.R.; Kang, H.; Onorato, J.; Ghosh, R.; Luscombe, C.K.; Spano, F.C.; Tolbert, S.H.; Schwartz, B.J. The Effects of Crystallinity on Charge Transport and the Structure of Sequentially Processed F4TCNQ-Doped Conjugated Polymer Films. Adv. Funct. Mater. 2017, 27, 1702654. [Google Scholar] [CrossRef]
- Rodriguez, M.M.; Peng, X.H.; Liu, L.J.; Li, Y.; Andino, J.M. A Density Functional Theory and Experimental Study of CO2 Interaction with Brookite TiO2. J. Phys. Chem. C. 2012, 116, 19755–19764. [Google Scholar] [CrossRef]
- Liu, L.J.; Zhao, H.L.; Andino, J.M.; Li, Y. Photocatalytic CO2 Reduction with H2O on TiO2 Nanocrystals: Comparison of Anatase, Rutile, and Brookite Polymorphs and Exploration of Surface Chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Pan, H.; Gu, B.H.; Zhang, Z.Y. Phase-Dependent Photocatalytic Ability of TiO2: A First-Principles Study. J. Chem. Theory Comput. 2009, 5, 3074–3078. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with Oxygen Vacancies: Synthesis, Properties and Photocatalytic Applications. Nanoscale 2013, 5, 3601. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, J.; You, Z. A facile TiO2 containing oxygen vacancies and hydroxyl as a Ru-loaded underlay for CO2 hydrogenation to CH4. Appl. Surf. Sci. 2022, 587, 152856. [Google Scholar] [CrossRef]
- Elbana, O.; Fujitsuka, M.; Kim, S.; Majima, T. Charge Carrier Dynamics in TiO2 Mesocrystals with Oxygen Vacancies for Photocatalytic Hydrogen Generation under Solar Light Irradiation. J. Phys. Chem. C. 2018, 122, 15163–15170. [Google Scholar] [CrossRef]
- Chen, R.; Fan, F.; Dittrich, T.; Li, C. Imaging photogenerated charge carriers on surfaces and interfaces of photocatalysts with surface photovoltage microscopy. Chem. Soc. Rev. 2018, 47, 8238–8262. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.; Quesada-Cabrera, R.; Bardey, S.; Promdet, P.; Sapienza, R.; Keller, V.; Meier, S.A.; Caps, V.; Parkin, I.P.; Cortés, E. Probing the Role of Atomic Defects in Photocatalytic Systems through Photoinduced Enhanced Raman Scattering. ACS Energy Lett. 2021, 6, 4273–4281. [Google Scholar] [CrossRef]
- Schweke, D.; Mordehovitz, Y.; Halabi, M.; Shelly, L.; Hayrun, S. Defect Chemistry of Oxides for Energy Applications. Adv. Mater. 2018, 30, 1706300. [Google Scholar]
- Kovacic, Z.; Likozar, B.; Hus, M. Photocatalytic CO2 Reduction: A Review of Ab Initio Mechanism, Kinetics, and Multiscale Modeling Simulations. ACS Catal. 2020, 10, 14984–15007. [Google Scholar] [CrossRef]
- Marchal, C.; Piquet, A.; Behr, M.; Cottineau, T.; Papaefthimiou, V.; Keller, V.; Caps, V. Activation of solid grinding-derived Au/TiO2 photocatalysts for solar H2 production from water-methanol mixtures with low alcohol content. J. Catal. 2017, 352, 22–34. [Google Scholar] [CrossRef]
- Bardey, S.; Bonduelle-Skrzypczak, A.; Fécant, A.; Zhznpznq, C.; Colbeau-Justin, C.; Caps, V.; Keller, V. Plasmonic photocatalysis applied to solar fuels. Faraday Discuss. 2019, 214, 417–439. [Google Scholar] [CrossRef] [Green Version]
- Haruta, M.; Bobayashi, T.; Sano, H.; Yamada, N. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature far Below 0°C. Chem. Lett. 1987, 16, 405–408. [Google Scholar]
- Mohamed, I.M.A.; Dao, V.-D.; Yasin, A.S.; Barakat, N.A.M.; Choi, H.-S. Design of ultrafine nickel oxide nanostructured material for enhanced electrocatalytic oxidation of urea: Physicochemical and electrochemical analyses. Appl. Surf. Sci. 2017, 400, 355–364. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Andino, J.M.; Li, Y. Bicrystalline TiO2 with controllable anatase-brookite phase content for enhanced CO2 photoreduction to fuels. J. Mater. Chem. A 2013, 1, 8209–8216. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Yu, X.; Li, B.; Yan, L.; Xing, Z.; Li, J. Enhanced visible light activity and mechanism of TiO2 codoped with molybdenum and nitrogen. Mater. Sci. Eng. B 2013, 178, 425–430. [Google Scholar] [CrossRef]
- Lignier, P.; Comotti, M.; Schüth, F.; Rousset, J.-L.; Caps, V. Effect of the titania morphology on the Au/TiO2 catalyzed aerobic epoxidation of stilbene. Catal. Today 2009, 141, 355–360. [Google Scholar] [CrossRef]
- Hammoud, L.; Streibel, C.; Toufaily, J.; Hamieh, T.; Keller, V.; Caps, V. The role of the gold-platinum interface in AuPt/TiO2-catalyzed plasmon-induced reduction of CO2 with water. Faraday Discuss. 2022. [Google Scholar] [CrossRef]
- Colbeau-Justin, C.; Kunst, M.; Huguenin, D. Structural influence on charge-carrier lifetimes in TiO2 powders studied by microwave absorption. J. Mater. Sci. 2003, 38, 24. [Google Scholar]
- Livraghi, S.; Chierotti, M.R.; Giamello, E.; Magnacca, G.; Paganini, M.C.; Cappelletti, G.; Bianchi, C.L. Nitrogen-Doped Titanium Dioxide Active in Photocatalytic Reactions with Visible Light: A Multi-Technique Characterization on Differently Prepared Materials. J. Phys. Chem. C 2008, 112, 17244–17252. [Google Scholar] [CrossRef]
- Hensling, F.V.E.; Xu, C.; Gunkel, F.; Dittmann, R. UV radiation enhanced oxygen vacancy formation caused by the PLD plasma plume. Sci. Rep. 2017, 7, 39953. [Google Scholar]
- Deml, A.M.; Holder, A.M.; O’Hayre, R.P.; Musgrave, C.B.; Stevanović, V. Intrinsic Material Properties Dictating Oxygen Vacancy Formation Energetics in Metal Oxides. J. Phys. Chem. Lett. 2015, 6, 1948–1953. [Google Scholar] [CrossRef]
- Zhang, Z.; Long, J.; Xie, X.; Lin, H.; Zhou, Y.; Yuan, R.; Dai, W.; Ding, Z.; Wang, X.; Fu, X. ChemPhysChem, 2012; 13, 1542–1550.
- Martsinovich, N.; Troisi, A. How TiO2 crystallographic surfaces influence charge injection rates from a chemisorbed dye sensitiser. Phys. Chem. Chem. Phys. 2012, 38, 13392–13401. [Google Scholar] [CrossRef]
- Guillois, K.; Burel, L.; Tuel, A.; Caps, V. Gold-catalyzed aerobic epoxidation of trans-stilbene in methylcyclohexane. Part I: Design of a reference catalyst. Appl. Catal. A 2012, 415–416, 1–9. [Google Scholar]
- Beltram, A.; Romero-Ocana, I.; Delgado-Jaen, J.J.; Montini, T.; Fornasiero, P. Photocatalytic valorization of ethanol and glycerol over TiO2 polymorphs for sustainable hydrogen production. Appl. Catal. Gen. 2016, 518, 167–175. [Google Scholar] [CrossRef]
- Vigneron, F.; Piquet, A.; Baaziz, W.; Ronot, P.; Boos, A.; Janowska, I.; Pham-Huu, C.; Petit, C.; Caps, V. Hydrophobic gold catalysts: From synthesis on passivated silica to synthesis on few-layer graphene. Catal. Today 2014, 235, 90–97. [Google Scholar] [CrossRef]




| Mean Crystallite Size (nm) a | SBET (m2/g) b | Vpore (cm3/g) b | Mean Pore Diameter (nm) b | Eg (eV) c | |||
|---|---|---|---|---|---|---|---|
| Anatase | Brookite | Rutile | |||||
| TiO2 P25 | 17 | / | 23 | 55 | 0.20 | ≈30 | 3.20 |
| TiO2 brookite | / | 19 | / | 150 | 0.18 | 3–5 | 3.20 |
| TiO2 UV100 | 11 | / | / | 315 | 0.33 | ˂3 | 3.25 |
| TiO2 UV100-350 | 17 | / | / | 135 | 0.24 | 4.5 | 3.25 |
| TiO2 UV100-550 | 19 | / | / | 77 | 0.28 | 8.0 | 3.25 |
| TiO2SG | 6 | 7 | n.d | 295 | 0.19 | <4 | 2.85 |
| TiO2SG-400 | 6 | 10 | n.d | 200 | 0.22 | <4 | 2.96 |
| TiO2SG-500 | 10 | 11 | 19 | 75 | 0.11 | <4 | 2.95 |
| TiO2SG-600 | / | / | 29 | n.d | n.d | n.d. | 2.90 |
| Support | Au(Th.) (wt.%) | Au(Real) (wt.%) | Au Deposition Yield (%) | λSPR (nm) |
|---|---|---|---|---|
| TiO2 P25 | 0.86 | 0.79 | 92 | 548 |
| TiO2 Brookite | 0.86 | 0.82 | 95 | 550 |
| TiO2 UV100 | 556 | |||
| TiO2 UV100-350 | 0.86 | 0.81 | 94 | 548 |
| TiO2 UV100-550 | 562 | |||
| TiO2 SG | 561 | |||
| TiO2 SG-400 | 0.86 | 0.84 | 98 | 556 |
| TiO2 SG-500 | 559 | |||
| TiO2 SG-600 | 541 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchal, C.; Mary, C.; Hammoud, L.; Xi, Q.; Toufaily, J.; Hamieh, T.; Suhadolnik, L.; Fornasiero, P.; Colbeau-Justin, C.; Caps, V.; et al. A Parametric Study of the Crystal Phases on Au/TiO2 Photocatalysts for CO2 Gas-Phase Reduction in the Presence of Water. Catalysts 2022, 12, 1623. https://doi.org/10.3390/catal12121623
Marchal C, Mary C, Hammoud L, Xi Q, Toufaily J, Hamieh T, Suhadolnik L, Fornasiero P, Colbeau-Justin C, Caps V, et al. A Parametric Study of the Crystal Phases on Au/TiO2 Photocatalysts for CO2 Gas-Phase Reduction in the Presence of Water. Catalysts. 2022; 12(12):1623. https://doi.org/10.3390/catal12121623
Chicago/Turabian StyleMarchal, Clément, Caroline Mary, Leila Hammoud, Qingyang Xi, Joumana Toufaily, Tayssir Hamieh, Luka Suhadolnik, Paolo Fornasiero, Christophe Colbeau-Justin, Valérie Caps, and et al. 2022. "A Parametric Study of the Crystal Phases on Au/TiO2 Photocatalysts for CO2 Gas-Phase Reduction in the Presence of Water" Catalysts 12, no. 12: 1623. https://doi.org/10.3390/catal12121623
APA StyleMarchal, C., Mary, C., Hammoud, L., Xi, Q., Toufaily, J., Hamieh, T., Suhadolnik, L., Fornasiero, P., Colbeau-Justin, C., Caps, V., Cottineau, T., & Keller, V. (2022). A Parametric Study of the Crystal Phases on Au/TiO2 Photocatalysts for CO2 Gas-Phase Reduction in the Presence of Water. Catalysts, 12(12), 1623. https://doi.org/10.3390/catal12121623










