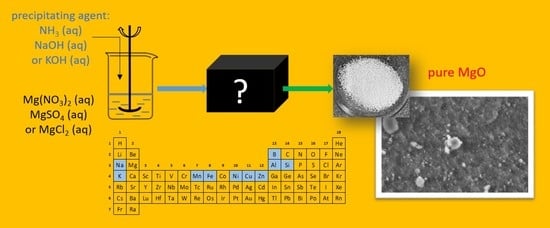
A Hands-on Guide to the Synthesis of High-Purity and High-Surface-Area Magnesium Oxide
Abstract
1. Introduction
- Developing a procedure for the synthesis of Mg(OH)2 by a wet method on a laboratory scale, the formation of the obtained hydroxide dust grains and the thermal decomposition of hydroxide grains to MgO;
- Determining the effect of the operation of obtaining magnesium hydroxide, its formation into grains and its subsequent calcination to the oxide on the final purity of the MgO catalyst;
- Performing a large-scale (of the order of 1500 g of the product) synthesis of very pure Mg(OH)2 as a MgO precursor on the basis of the conditions optimized in point 1.
2. Results
2.1. Properties of Mg(OH)2 Obtained from Commercial MgO
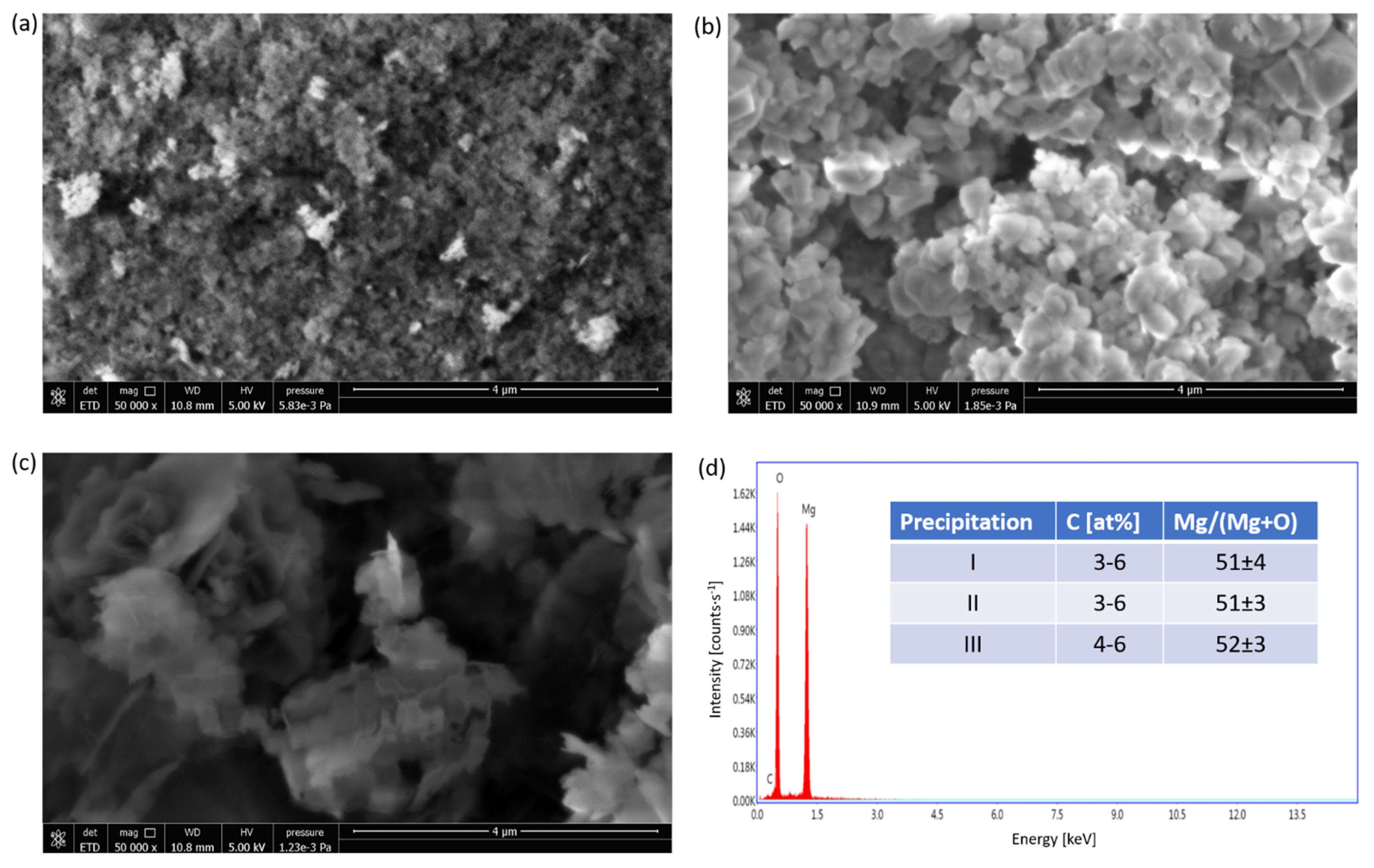
2.1.1. Composition Determination
2.1.2. MgO-T Samples: XRD Measurements Results and Textural Properties
2.1.3. Temperature-Programmed Desorption (TPD) Measurements of MgO-T Samples
2.1.4. Surface Acidity and Basicity of MgO-T Samples
2.2. Magnesium Nitrate as a Starting Material
2.3. Impact of Post-Synthesis Treatment on Properties of Mg(OH)2 and MgO
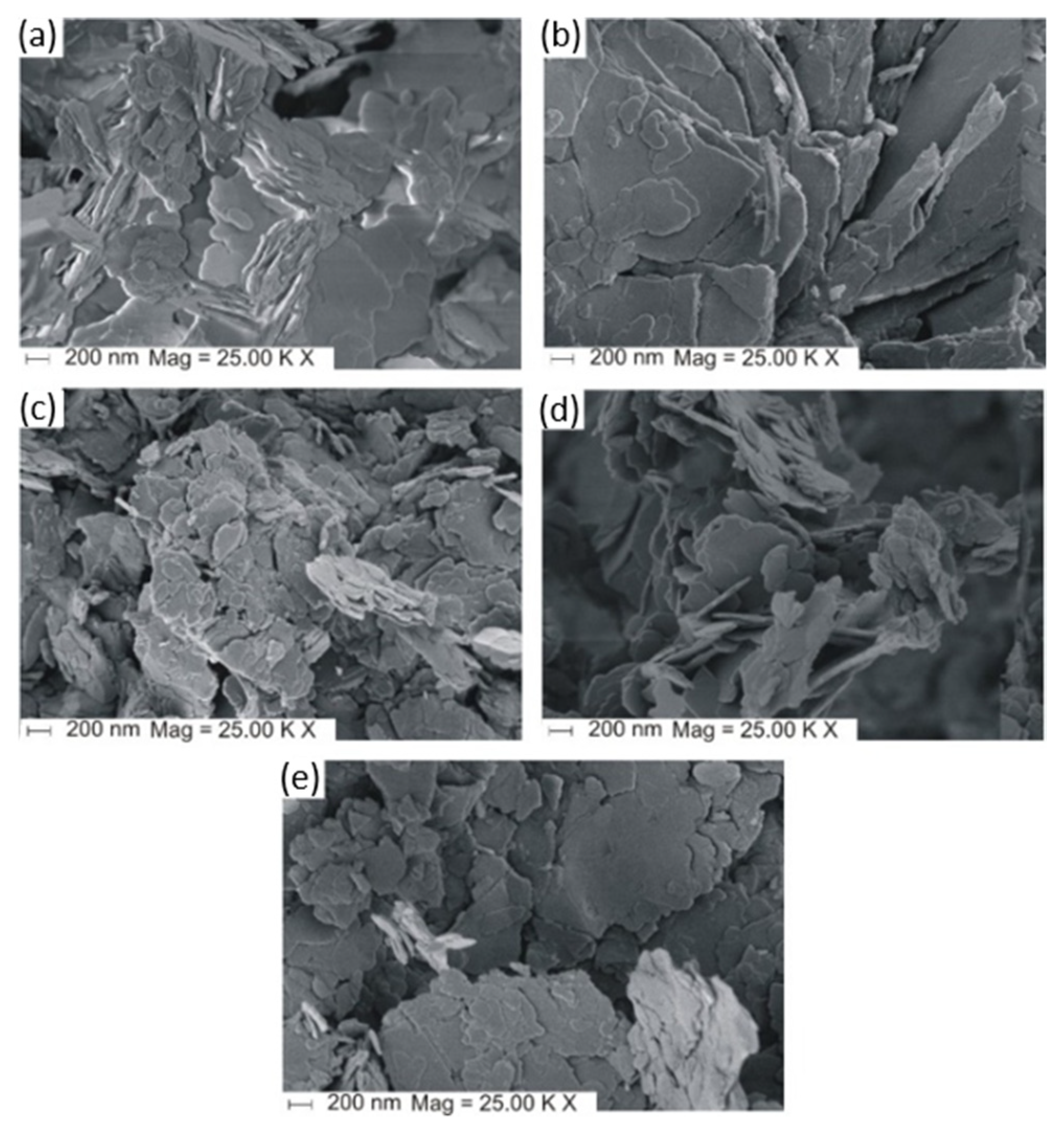
2.3.1. Impact of Counter Ion and Precipitating Agent
2.3.2. Influence of the Formation of Mg(OH)2 Grains and Their Thermal Decomposition on the Final Purity of MgO
- Pressing pure magnesium hydroxide dust into pellets;
- Crushing the obtained pellets;
- Separating the proper fraction of magnesium hydroxide using sieves;
- The thermal decomposition of the separated hydroxide fraction to magnesium oxide.
3. Materials and Methods
3.1. Synthesis of Mg(OH)2
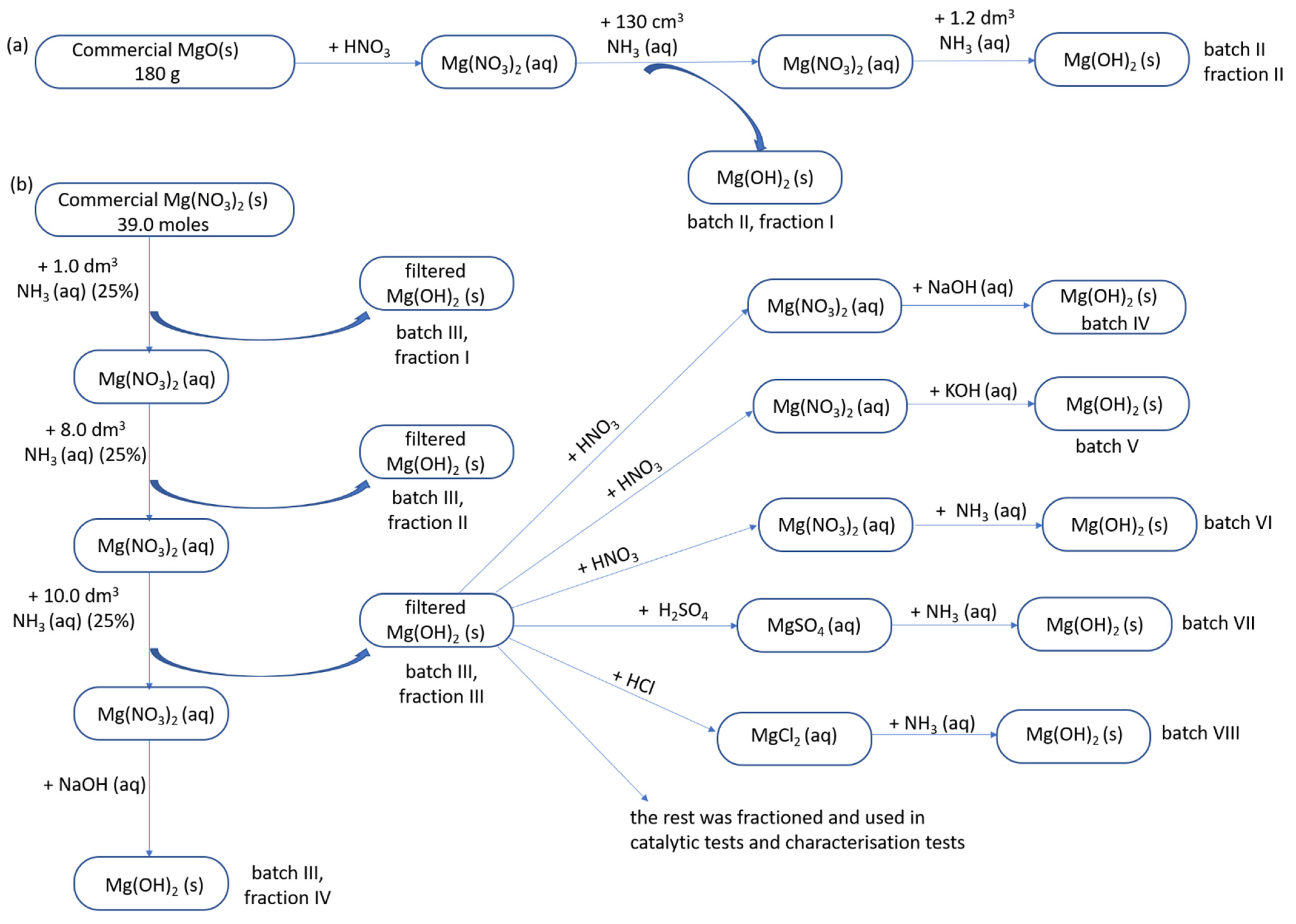
- Starting materials: There were two starting materials for the large-scale synthesis of MgO: commercial MgO for only batch II (Scheme 1a), details provided in Section 3.1.2, and commercial magnesium nitrate for all other batches (Scheme 1b).
- Precipitating agents: Three precipitating agents were used to obtain Mg(OH)2: aqueous ammonia (Scheme 1a,b), sodium hydroxide and potassium hydroxide (details provided in Section 3.1.3).
- Counter ions: There were three precursors of magnesium hydroxide used to test how much residual counter ions from the salt used was present in the obtained precipitate and hence for establishing the proper washing procedure (details provided in Section 3.1.4).
3.1.1. Large-Scale Preparation of Mg(OH)2 from Commercial Mg(NO3)2
3.1.2. Large Scale Preparation of Mg(OH)2 from Commercial MgO
3.1.3. Synthesis of Mg(OH)2 from Mg(NO3)2 and Alkali Metal Hydroxides
3.1.4. Synthesis of Mg(OH)2 from Mg(NO3)2, MgSO4 and MgCl2, and NH3 (aq)
3.2. Formation of Grains of Mg(OH)2
- Pressing Mg(OH)2 powder in a steel die (20 mm i.d.) under pressure of 10 MPa into thin wafers (weight 317 ± 15 mg, the average of 10 weight measurements);
- Crushing the wafers with a pestle in a mortar (porcelain or agate) by weak striking;
- Sieving the grains of Mg(OH)2 through a set of sieves made of phosphorus bronze (94% Cu, 5% Sn and 0.4% P); the 0.4–0.5 mm fraction was selected for further investigations.
3.3. Preparation of MgO by Calcination of Mg(OH)2
- At 873 or 1873 K (6 h) in static air (muffle furnace);
- At selected temperatures (473–1073 K) in a flow of air (3 dm3·h−1) for 1 h and flow of nitrogen (3 dm3·h−1) for 5 h (vertical tubular quartz reactor with a glass-wool plug), heating rate 10 deg·min−1;
- Under vacuum (pressure 0.013 hPa (rotary pump) or 27 hPa (water pump) in horizontal tubular quartz reactor, heating rate 2 and 10 deg min−1.
3.4. Reagents
3.5. Characterization of Mg(OH)2 and MgO
3.5.1. Composition Determination
3.5.2. Specific Surface Area
3.5.3. Thermogravimetric Analysis
3.5.4. Temperature Programmed Desorption Studies
3.5.5. Secondary Emission Microscopy—Energy Dispersive X-ray Spectroscopy
3.5.6. Basic/Acidic Site Determination and Quantification
4. Conclusions
- Compacting pure magnesium hydroxide dust into pellets;
- Crushing the obtained pellets;
- Separation of the proper fraction of magnesium hydroxide on sieves;
- Thermal decomposition of the hydroxide to magnesium oxide
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramlee, N.N.; Md Illias, R.; Rahman, R.A.; Toemen, S.; Selvasembian, R.; Ahmad, R.A.; Abdul Manas, N.H.; Wan Azelee, N.I. Biochemical and Physical Characterization of Immobilized Candida rugosa Lipase on Metal Oxide Hybrid Support. Catalysts 2022, 12, 854. [Google Scholar] [CrossRef]
- Zigla, A.A.; Kox, T.; Mevoa, D.; Assaouka, H.T.; Nsangou, I.N.; Daawe, D.M.; Kenmoe, S.; Kouotou, P.M. Magnesium-Modified Co3O4 Catalyst with Remarkable Performance for Toluene Low Temperature Deep Oxidation. Catalysts 2022, 12, 411. [Google Scholar] [CrossRef]
- Bagabas, A.; Al-Fatesh, A.S.; Kasim, S.O.; Arasheed, R.; Ibrahim, A.A.; Ashamari, R.; Anojaidi, K.; Fakeeha, A.H.; Abu-Dahrieh, J.K.; Abasaeed, A.E. Optimizing MgO Content for Boosting γ-Al2O3-Supported Ni Catalyst in Dry Reforming of Methane. Catalysts 2021, 11, 1233. [Google Scholar] [CrossRef]
- Saied, E.; Eid, A.M.; Hassan, S.E.-D.; Salem, S.S.; Radwan, A.A.; Halawa, M.; Saleh, F.M.; Saad, H.A.; Saied, E.M.; Fouda, A. The Catalytic Activity of Biosynthesized Magnesium Oxide Nanoparticles (MgO-NPs) for Inhibiting the Growth of Pathogenic Microbes, Tanning Effluent Treatment, and Chromium Ion Removal. Catalysts 2021, 11, 821. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Bhanja, P.; Salam, N.; Bhaumik, A.; Islam, S.M. Magnesium oxide as an efficient catalyst for CO2 fixation and N-formylation reactions under ambient conditions. Mol. Catal. 2018, 450, 46–54. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S. Magnesium oxide as a heterogeneous catalyst support. Rev. Inorg. Chem. 2016, 36, 1–41. [Google Scholar] [CrossRef]
- Leofanti, G.; Solari, M.; Tauszik, G.; Garbassi, F.; Galvagno, S.; Schwank, J. Magnesium oxide as a catalyst support: The influence of chlorine. Appl. Catal. 1982, 3, 131–139. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Kumar, R.; Fakeeha, A.H.; Kasim, S.O.; Khatri, J.; Ibrahim, A.A.; Arasheed, R.; Alabdulsalam, M.; Lanre, M.S.; Osman, A.; et al. Promotional effect of magnesium oxide for a stable nickel-based catalyst in dry reforming of methane. Sci. Rep. 2020, 10, 13861. [Google Scholar] [CrossRef] [PubMed]
- Di Cosimo, J.I.; Díez, V.K.; Ferretti, C.; Apesteguía, C.R. Chapter 1. Basic catalysis on MgO: Generation, characterization and catalytic properties of active sites. Catalysis 2014, 26, 1–28. [Google Scholar] [CrossRef]
- Montero, J.; Isaacs, M.; Lee, A.; Lynam, J.; Wilson, K. The surface chemistry of nanocrystalline MgO catalysts for FAME production: An in situ XPS study of H2O, CH3OH and CH3OAc adsorption. Surf. Sci. 2015, 646, 170–178. [Google Scholar] [CrossRef]
- Mohammadi, L.; Rahdar, A.; Bazrafshan, E.; Dahmardeh, H.; Thysiadou, A.; Kyzas, G.Z. Benzene Removal from Aqueous Solutions by Heterogeneous Catalytic Ozonation Process with Magnesium Oxide Nanoparticles. J. Int. Ozone Assoc. 2020, 43, 147–162. [Google Scholar] [CrossRef]
- Du, L.; Lia, Z.; Ding, S.; Chen, C.; Qu, S.; Yi, W.; Lu, J.; Ding, J. Synthesis and characterization of carbon-based MgO catalysts for biodiesel production from castor oil. Fuel 2019, 258, 116122. [Google Scholar] [CrossRef]
- Yousefi, S.; Haghighi, M.; Vahid, B.R. Facile and efficient microwave combustion fabrication of Mg-spinel as support for MgO nanocatalyst used in biodiesel production from sunflower oil: Fuel type approach. Chem. Eng. Res. Des. 2018, 138, 506–518. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S. Optimization of Alkaline Earth Metal Oxide and Hydroxide Catalysts for Base-Catalyzed Reactions. Adv. Catal. 2006, 49, 239–302. [Google Scholar] [CrossRef]
- Baird, M.J.; Lunsford, J.H. Catalytic sites for the isomerization of 1-butene over magnesium oxide. J. Catal. 1972, 26, 440–450. [Google Scholar] [CrossRef]
- Xiang, W.; Moa, X.; Feng, S.; Xu, F.; Zhou, G.; Zhou, H.; Xua, C.; Chen, B. Effect of MgO on WO3/SiO2-catalyzed light olefin metathesis using different feedstocks. Mol. Catal. 2017, 442, 49–56. [Google Scholar]
- Tanabe, K.; Holderich, W.F. ChemInform Abstract: Industrial Application of Solid Acid-base Catalysts. Appl. Catal. A Gen. 2010, 30, 199938249. [Google Scholar] [CrossRef]
- Margellou, A.; Koutsouki, A.; Petrakis, D.; Vaimakis, T.; Manos, G.; Kontominas, M.; Pomonis, P. Enhanced production of biodiesel over MgO catalysts synthesized in the presence of Poly-Vinyl-Alcohol (PVA). Ind. Crop. Prod. 2018, 114, 146–153. [Google Scholar] [CrossRef]
- Kampars, V.; Kampare, R.; Krumina, A. MgO Catalysts for FAME Synthesis Prepared Using PEG Surfactant during Precipitation and Calcination. Catalysts 2022, 12, 226. [Google Scholar] [CrossRef]
- Hung, C.-H.; Chen, C.-S.; Sheu, H.-S.; Chang, J.-R. Deactivation and rejuvenation of pellet MgO/SiO2 catalysts for trans-esterification of soybean oil with methanol to bio-diesel: Roles of MgO morphology change in catalysis. Ind. Eng. Chem. Res. 2018, 57, 456–469. [Google Scholar] [CrossRef]
- Matsuda, T.; Tanabe, J.; Hayashi, N.; Sasaki, Y.; Miura, H.; Sugiyama, K. Properties of magnesium oxides prepared from various salts and their catalytic activity in 1-butene isomerisation. Bull. Chem. Soc. Jpn. 1982, 55, 990–994. [Google Scholar] [CrossRef]
- Tanabe, K.; Zhang, G.; Hattori, H. Addition of metal cations to magnesium oxide catalysts for the aldol condensation of ace-tone. Appl. Catal. 1989, 48, 63–70. [Google Scholar] [CrossRef]
- Matsuda, T.; Sugimoto, M. High activity of MgO catalyst prepared from magnesium oxalate for the hydrogenation of butadiene. React. Kinet. Catal. Lett. 1991, 44, 69–73. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Fadoni, M.; Vercelli, B. Magnesium salts and oxide: An XPS overview. Appl. Surf. Sci. 1997, 119, 253–259. [Google Scholar] [CrossRef]
- Holt, T.E.; Logan, A.D.; Chakraborti, S.; Datye, A.K. The effect of catalyst preparation conditions on the morphology of MgO catalyst supports. Appl. Catal. 1987, 34, 199–213. [Google Scholar] [CrossRef]
- Wanke, S.; Fiedorow, R. The Influence of Preparation Methods on Surface Area, Porosity and Crystallinity of Magnesium Oxide. Stud. Surf. Sci. Catal. 1987, 39, 601–609. [Google Scholar] [CrossRef]
- Choudhary, V.; Pandit, M. Surface properties of magnesium oxide obtained from magnesium hydroxide: Influence on preparation and calcination conditions of magnesium hydroxide. Appl. Catal. 1991, 71, 265–274. [Google Scholar] [CrossRef]
- Mu, J.; Pearlmutter, D.D. Thermal decomposition of carbonates, carboxylates, oxalates, acetates, formates, and hydroxides. Thermochim. Acta 1981, 49, 207–218. [Google Scholar] [CrossRef]
- Vahid, B.R.; Haghighi, M. Biodiesel production from sunflower oil over MgO/MgAl2O4 nanocatalyst: Effect of fuel type on catalyst nanostructure and performance. Energy Convers. Manag. 2017, 134, 290–300. [Google Scholar] [CrossRef]
- Pearson, R.G. Ionization potentials and electron affinities in aqueous solution. J. Am. Chem. Soc. 1986, 108, 6109–6114. [Google Scholar] [CrossRef]
- Martin, D.; Brause, W.; Radeglia, R. Struktur-Reaktivitätsuntersuchungen mit heterosubstituierten Nitrilen. II. H-Brückenwechselwirkungen zwischen OH-Protonendonatoren und Cyanverbindungen Korrelationen. J. Prakt. Chem. 1970, 312, 797–811. [Google Scholar] [CrossRef]
- Busca, G. Bases and Basic Materials in Chemical and Environmental Processes. Liquid versus Solid Basicity. Chem. Rev. 2010, 110, 2217–2249. [Google Scholar] [CrossRef] [PubMed]
- Bryantsev, V.S.; Diallo, M.S.; Goddard, W.A. pKa calculations of aliphatic amines, diamines, and aminoamides via Density Functional Theory with a Poisson−Boltzmann Continuum Solvent Model. J. Phys. Chem. A 2007, 111, 4422–4430. [Google Scholar] [CrossRef] [PubMed]
- Juskelis, M.V.; Slanga, J.P.; Roberie, T.G.; Peters, A.W. A comparison of CaO, beta, and a dealuminated Y by ammonia TPD and by temperature programmed 2-propylamine cracking. J. Catal. 1992, 138, 391–394. [Google Scholar] [CrossRef]
- Gorte, R.J.; Crossley, S.P. A perspective on catalysis in solid acids. J. Catal. 2019, 375, 524–530. [Google Scholar] [CrossRef]
- Coluccia, S.; Garrone, E.; Borello, E. Infrared spectroscopic study of molecular and dissociative adsorption of ammonia on magnesium oxide, calcium oxide and strontium oxide. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1983, 79, 607–613. [Google Scholar] [CrossRef]
- Borello, E.; Coluccia, S.; Zecchina, A. Infrared emission study of the reaction of CO with ammonia preadsorbed on MgO. J. Catal. 1985, 93, 331–339. [Google Scholar] [CrossRef]
- Kijeński, J.; Malinowski, S. Influence of sodium on the physico-chemical and catalytic properties of magnesium oxide. JCS Faraday Trans. I 1978, 74, 250–261. [Google Scholar] [CrossRef]
- Gliński, M.; Ulkowska, U. Reactivity of Alcohols in Chemoselective Transfer Hydrogenation of Acrolein over Magnesium Oxide as the Catalyst. Catal. Lett. 2010, 141, 293–299. [Google Scholar] [CrossRef]
- Tanabe, K.; Misono, M.; Ono, Y.; Hattori, H. Acid and base centers: Structure and acid-base property. Stud. Surf. Sci. Catal. 1989, 51, 27–213. [Google Scholar]
- Gregg, S.J.; Ramsay, J.D. Adsorption of carbon dioxide by magnesia studied by use of infrared and isotherm measurements. J. Chem. Soc. A 1970, 2784–2787. [Google Scholar] [CrossRef]
- Coluccia, S.; Lavagnino, S.; Marchese, L. The hydroxylated surface of MgO powders and the formation of surface sites. Mater. Chem. Phys. 1988, 18, 445–464. [Google Scholar] [CrossRef]
- Pinto, P.; Lanza, G.; Ardisson, J.; Lago, R. Controlled Dehydration of Fe(OH)3 to Fe2O3: Developing Mesopores with Complexing Iron Species for the Adsorption of β-Lactam Antibiotics. J. Braz. Chem. Soc. 2019, 30, 310–317. [Google Scholar] [CrossRef]
- Sato, T.; Nakamura, T.; Ozawa, F. Thermal decomposition of nickel hydroxide. J. Appl. Chem. Biotechnol. 1975, 25, 583–590. [Google Scholar] [CrossRef]
- Tamanaka, T.; Tanabe, K. New determination of acid-base strength distribution of a common scale on solid surfaces. J. Phys. Chem. 1975, 75, 2409–2411. [Google Scholar] [CrossRef]




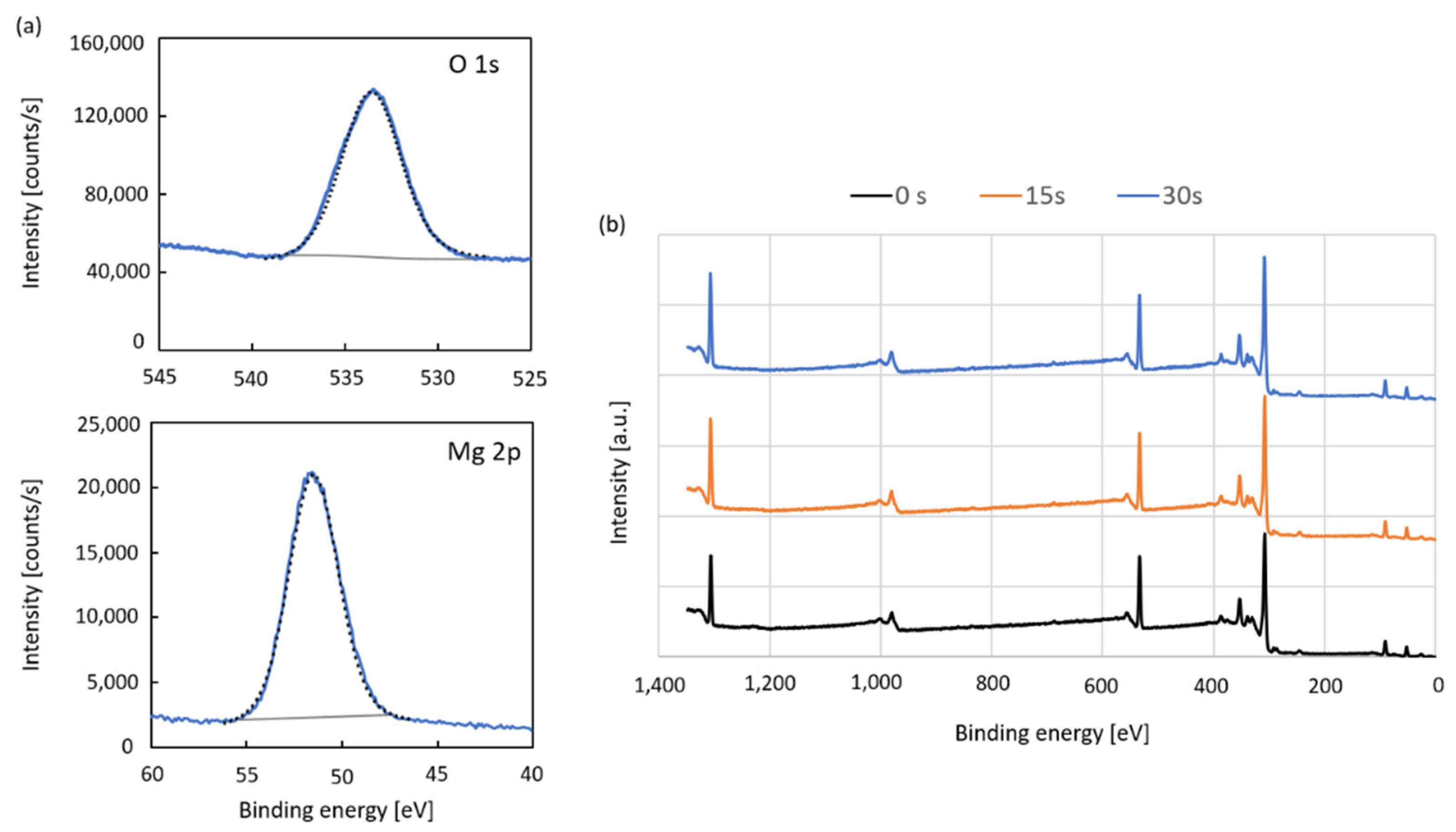
| T [K] | SBET [m2·g−1] | Pore Volume [cm3·g−1] | Av. Pore Diameter [nm] | D 2 [nm] | Lattice Parameter [nm] |
|---|---|---|---|---|---|
| 473 | 27 1 | 0.200 | 24.1 | 12.7 | a = 0.3153 c = 0.4744 |
| 573 | 36 | - | - | 12.0 | a = 0.3132 c = 0.4664 |
| 673 | 222 | 0.510 | 11.0 | 6.6 | a = 0.4222 |
| 773 | 134 | - | - | 8.7 | a = 0.4197 |
| 873 | 100 | 0.529 | 17.2 | 9.9 | a = 0.4191 |
| 973 | 69 | - | - | 11.4 | a = 0.4190 |
| 1073 | 47 | - | - | 15.5 | a = 0.4188 |
| 1873 | 5 | 0.059 | 42.1 | 38.5 | a = 0.4175 |
| MgO-T | SBET [m2·g−1] | Conc. of Acidic Sites [μmol·g−1] | Conc. of Basic Sites [μmol·g−1] | |
|---|---|---|---|---|
| n-BuNH2 | Et3N | PhCOOH | ||
| MgO-473 | 27 | 30 1 | 0 2 | 490 3 |
| MgO-873 | 100 | 115 4 | 5 2 | 1785 5 |
| MgO-1873 | 5 | 5 2 | 2 2 | 190 4 |
| MgO-T 1 | H0 | H- | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.8 | 4.8 | 7.2 | 9.3 | 15.0 | 18.4 | 22.3 | 26.5 | 33.0 | |
| MgO-473 | + | + | + | ||||||
| MgO-573 | + | + | + | + | |||||
| MgO-673 | + | + | + | + | + | + | |||
| MgO-873 | + | + | + | + | + | + | |||
| MgO-1073 | + | + | + | + | + | ||||
| MgO-1873 | + | + | + | + | + | ||||
| Sample | Fe [%] | Ni [%] | Mn [%] |
|---|---|---|---|
| Mg(OH)2 (I) | 0.1683 | 0.3804 | 0.082 |
| Mg(OH)2 (II) | 0.0127 | 0.0296 | 0.0107 |
| Mg(OH)2 (III) | 0.00171 | 0.0012 1 | 0.00111 |
| Element | [E] [%] 1 | Element | [E] [%] 1 |
|---|---|---|---|
| Si | 0.0040 | Ca | 0.0010 |
| B | 0.0019 | Na | 0.0008 |
| Fe | 0.0017 | Zn | 0.0007 |
| Ni | 0.0012 | Al | 0.0005 |
| Mn | 0.0011 | Cu | 0.0003 |
| No. of Wash Portions | T max [K] 1 | ||||
|---|---|---|---|---|---|
| NO3− | SO42− | Cl− | NO3− | NO3− | |
| NH3 | NH3 | NH3 | KOH | NaOH | |
| 5 | 689 | 687 | 676 | 674 | 675 |
| 15 | 690 | 688 | 681 | 674 | 673 |
| 25 | 692 | 688 | 686 | 673 | 673 |
| T [K] | Conditions | SBET [m2∙g−1] |
|---|---|---|
| 473 | Stream of N2 1 | 29 2 |
| 573 | Stream of N2 1 | 27 2 |
| Vacuum 3 | 143 4 | |
| 673 | Stream of N2 1 | 222 5 |
| Vacuum 2 | 326 | |
| Vacuum 3,6 | 337 | |
| 873 | Static air | 84 |
| Stream of N2 1 | 129 | |
| Vacuum 3 | 280 7 |
| Origin of Mg(OH)2 | SBET [m2∙g−1] | |
|---|---|---|
| After 15 Washings | After 25 Washings | |
| MgCl2 + NH3 ∙ aq | 32.1 | 38.6 |
| MgSO4 + NH3 ∙ aq | 40.3 | 54.2 |
| Mg(NO3)2 + NaOH | 65.0 | 70.2 |
| Mg(NO3)2 + KOH | 60.3 | 66.1 |
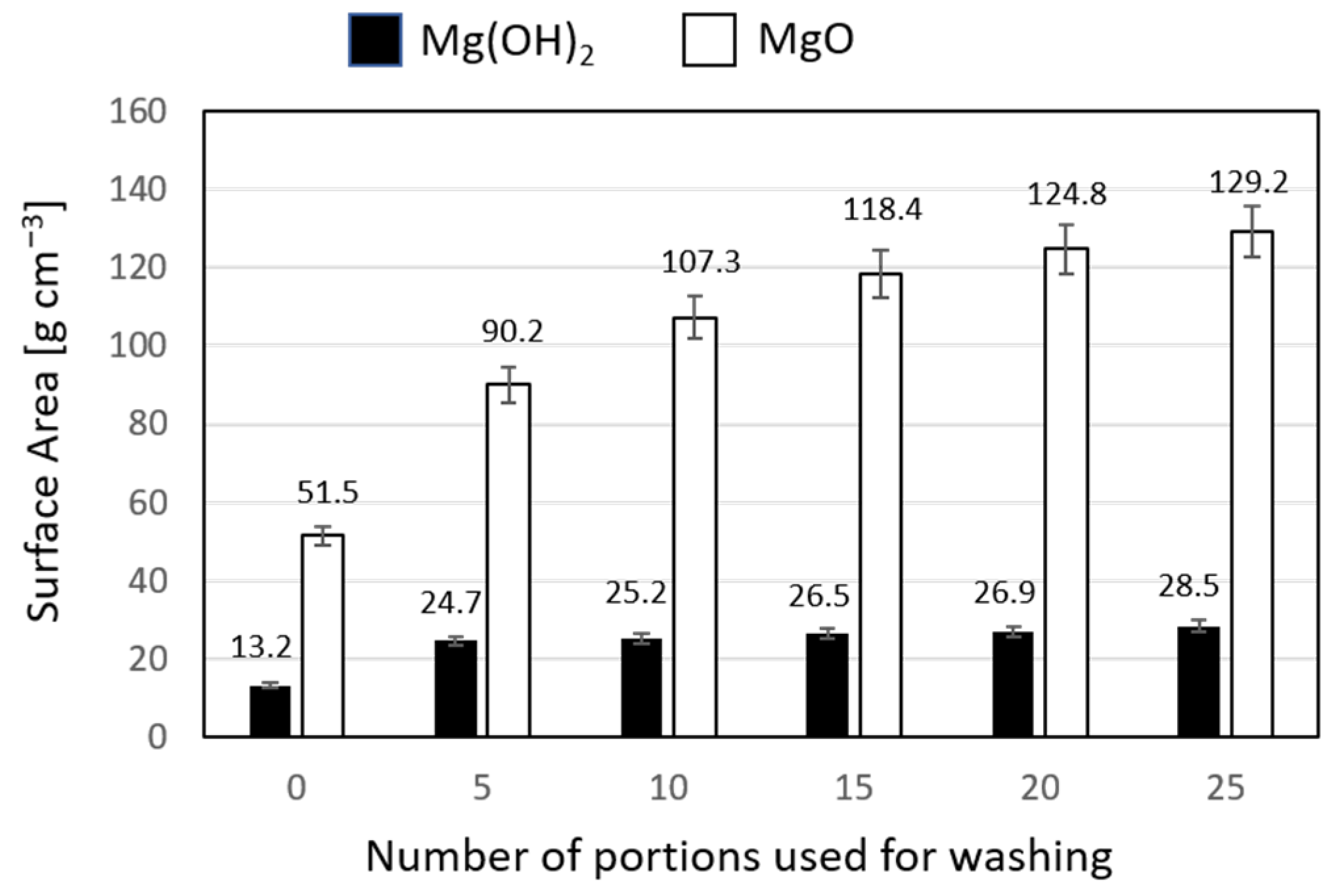
| Mg(NO3)2 + NH3 ∙ aq | 118.4 | 129.2 |
| Sample | Tmax [K] | SBET of MgO [m2 g−1] |
|---|---|---|
| Mg(OH)2 (I) | 635 | 12.8 |
| Mg(OH)2 (II) | 681 | 62.9 |
| Mg(OH)2 (III) | 692 | 129.2 |
| Element | Concentration of Impurities 1 [μg·g−1] | |||
|---|---|---|---|---|
| Mg(OH)2 | Mg(OH)2 2 | Mg(OH)2 3 | MgO 4 | |
| Fe | 17 | 19 | - | - |
| Si | 40 | 51 5 | - | 59 |
| Al | 5 | 25 | - | - |
| Cu | 3 | 3 | 10 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gliński, M.; Czajka, A.; Ulkowska, U.; Iwanek, E.M.; Łomot, D.; Kaszkur, Z. A Hands-on Guide to the Synthesis of High-Purity and High-Surface-Area Magnesium Oxide. Catalysts 2022, 12, 1595. https://doi.org/10.3390/catal12121595
Gliński M, Czajka A, Ulkowska U, Iwanek EM, Łomot D, Kaszkur Z. A Hands-on Guide to the Synthesis of High-Purity and High-Surface-Area Magnesium Oxide. Catalysts. 2022; 12(12):1595. https://doi.org/10.3390/catal12121595
Chicago/Turabian StyleGliński, Marek, Agnieszka Czajka, Urszula Ulkowska, Ewa M. Iwanek (nee Wilczkowska), Dariusz Łomot, and Zbigniew Kaszkur. 2022. "A Hands-on Guide to the Synthesis of High-Purity and High-Surface-Area Magnesium Oxide" Catalysts 12, no. 12: 1595. https://doi.org/10.3390/catal12121595
APA StyleGliński, M., Czajka, A., Ulkowska, U., Iwanek, E. M., Łomot, D., & Kaszkur, Z. (2022). A Hands-on Guide to the Synthesis of High-Purity and High-Surface-Area Magnesium Oxide. Catalysts, 12(12), 1595. https://doi.org/10.3390/catal12121595