Mixed Oxides Derived from Hydrotalcites Mg/Al Active in the Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalysts
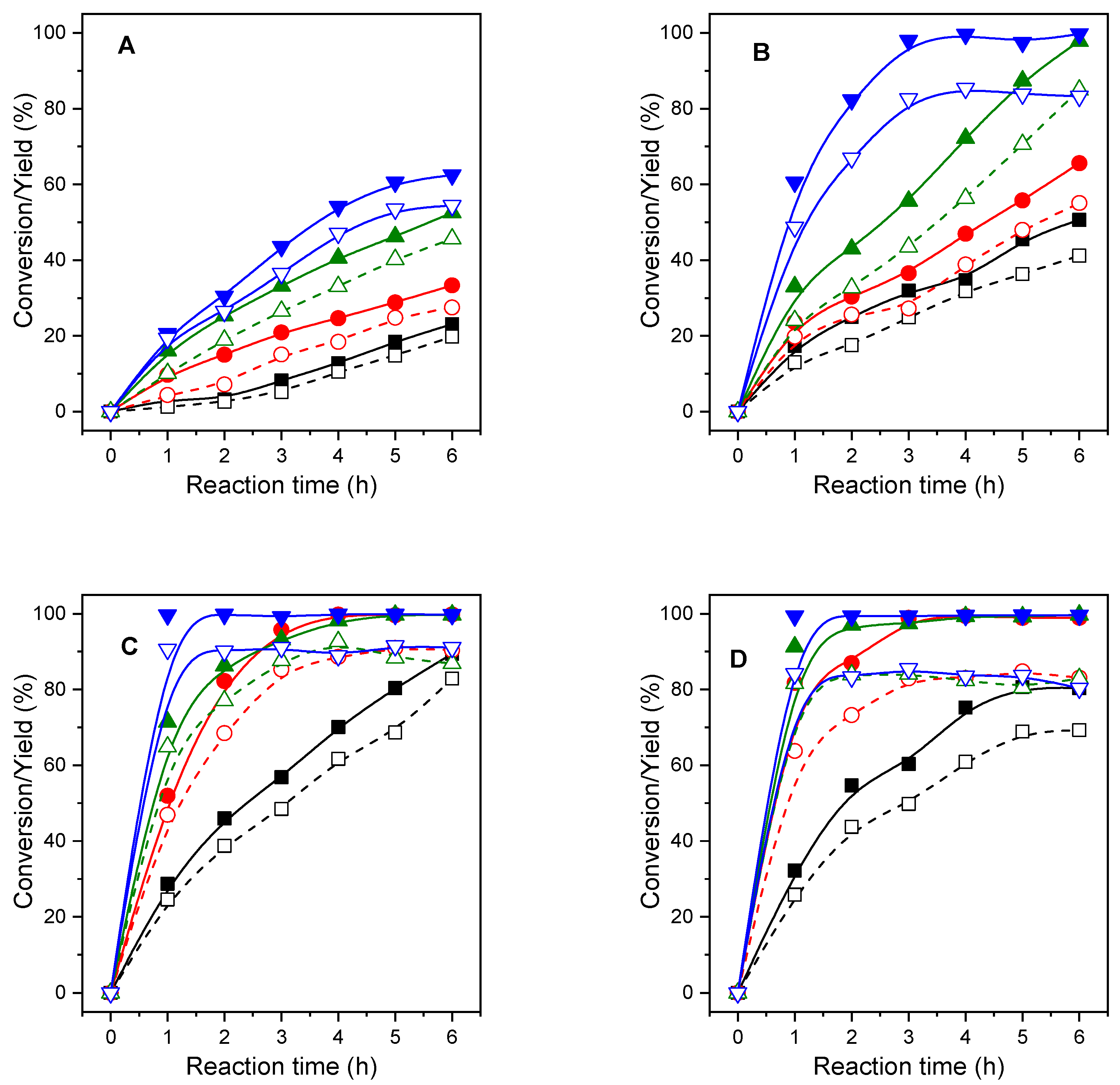
2.2. Catalytic Activity of MgAl-x Catalysts
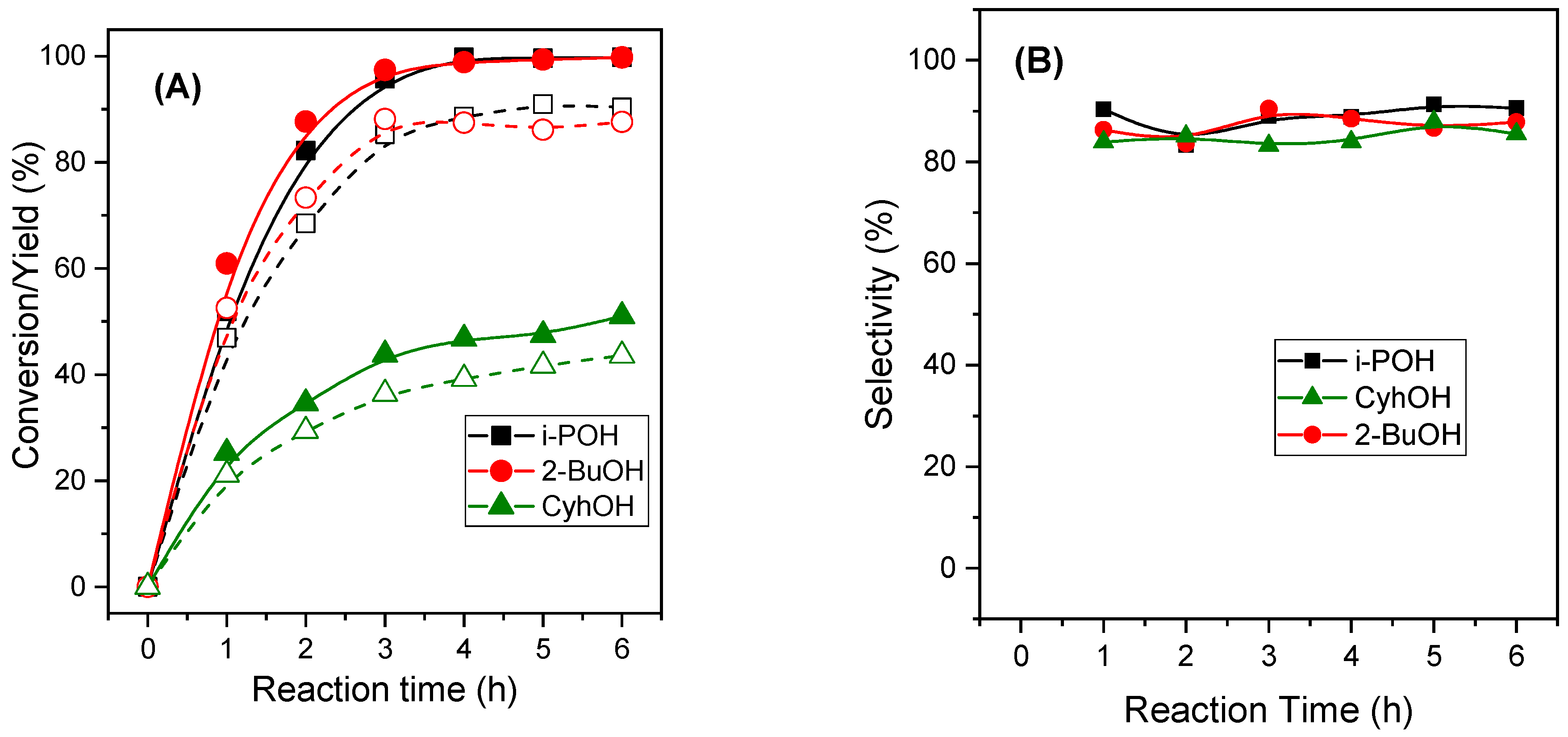
2.2.1. Influence of the Alcohol in the Catalytic Activity of MgAl-x Catalysts
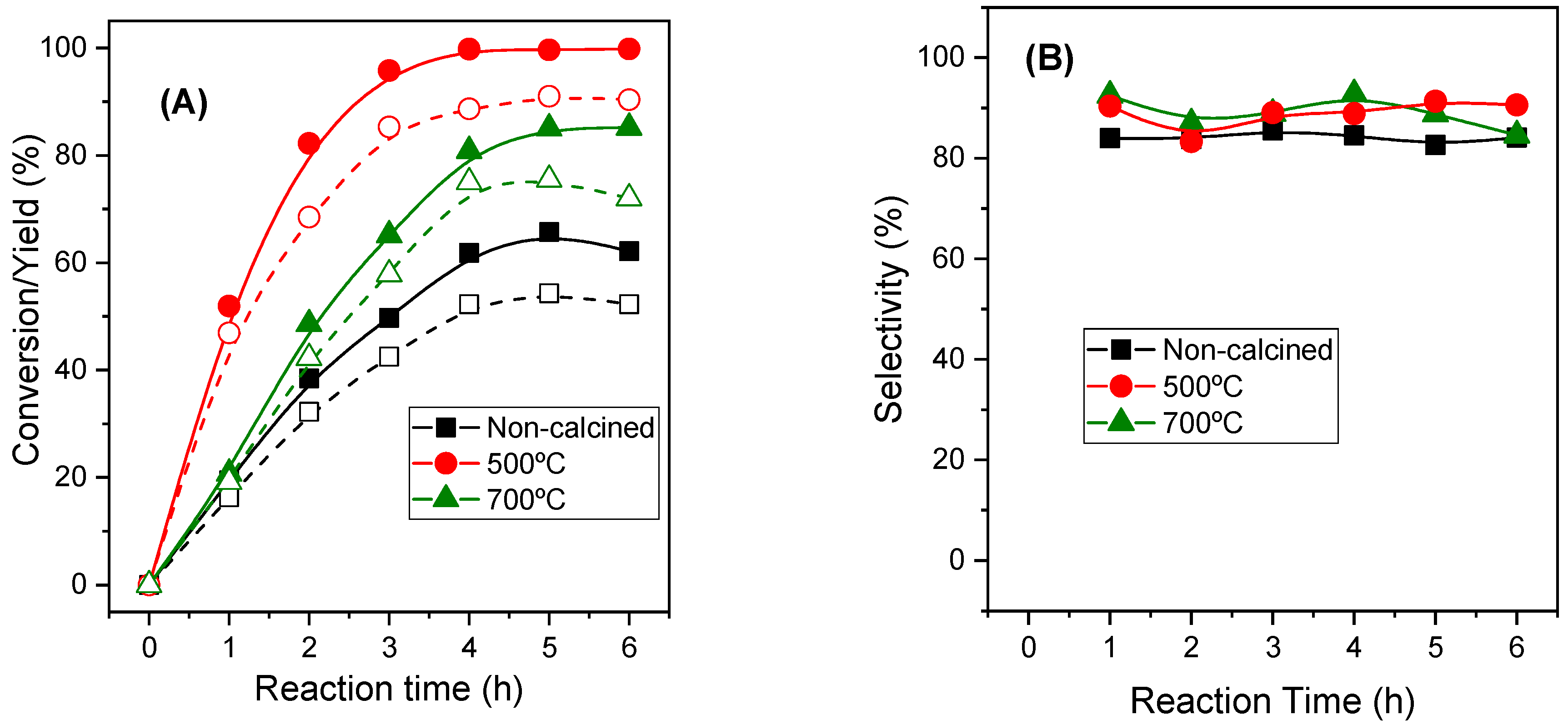
2.2.2. Influence of the Calcination Temperature of MgAl-3 Catalysts
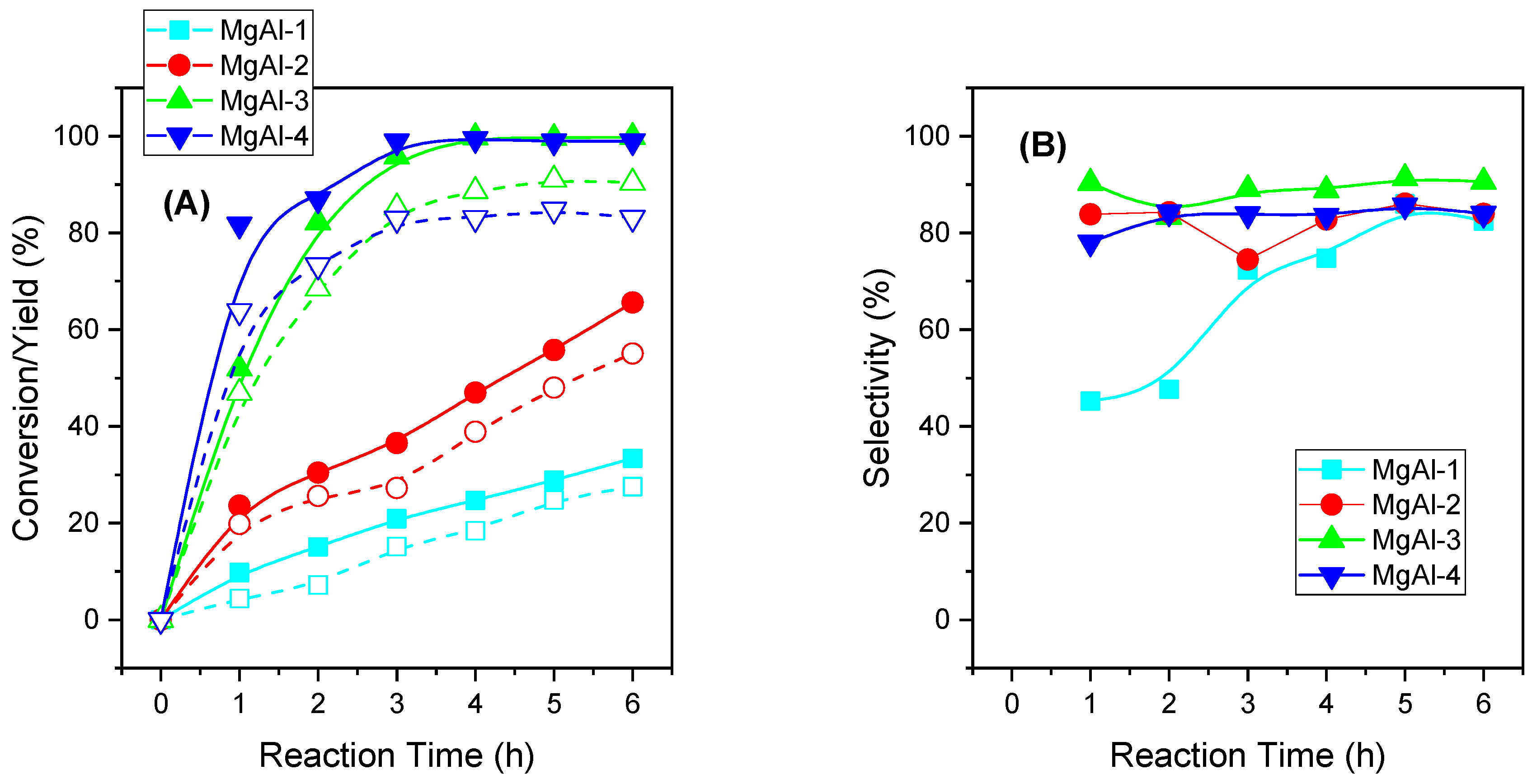
2.2.3. Influence of the Mg/Al Molar Ratio
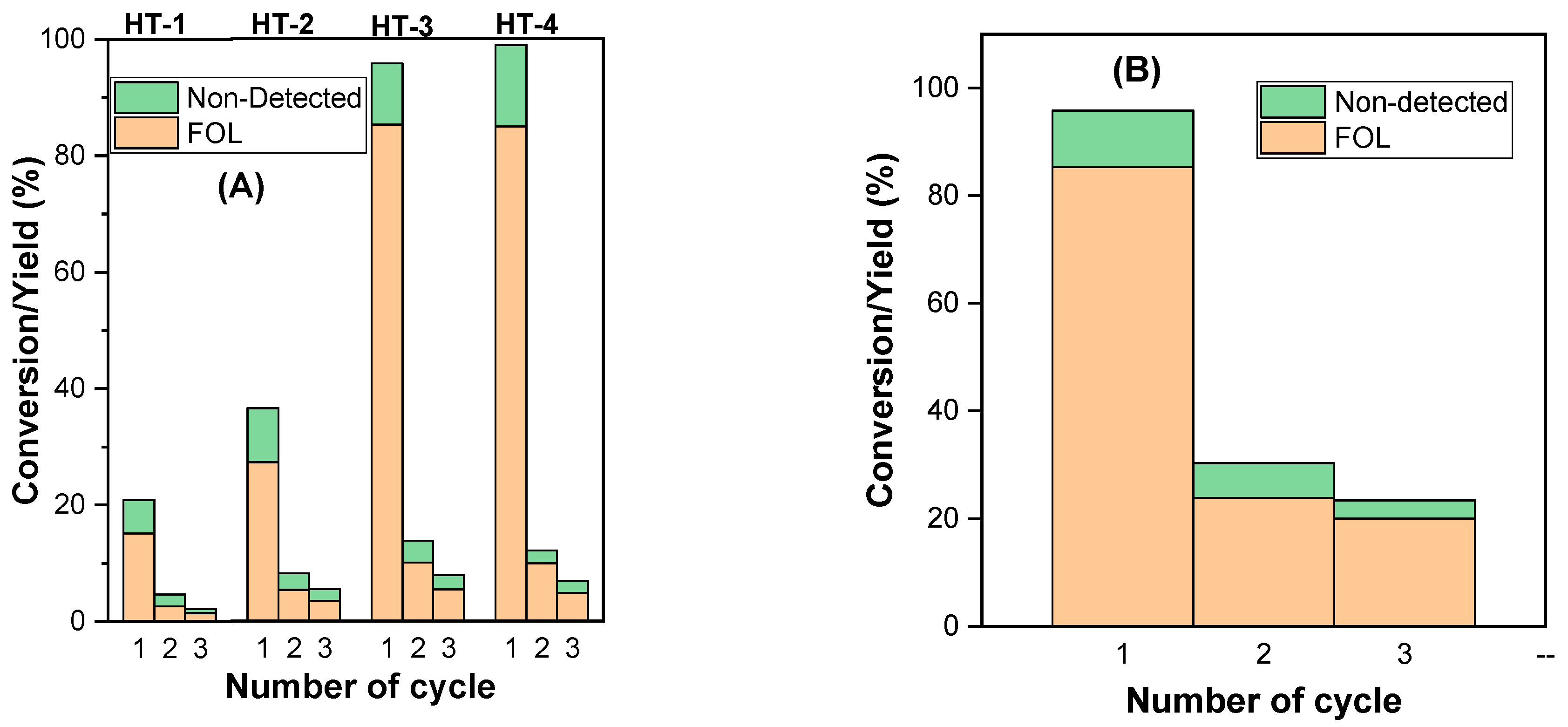
2.2.4. Reutilization of the MgAl-x Catalysts
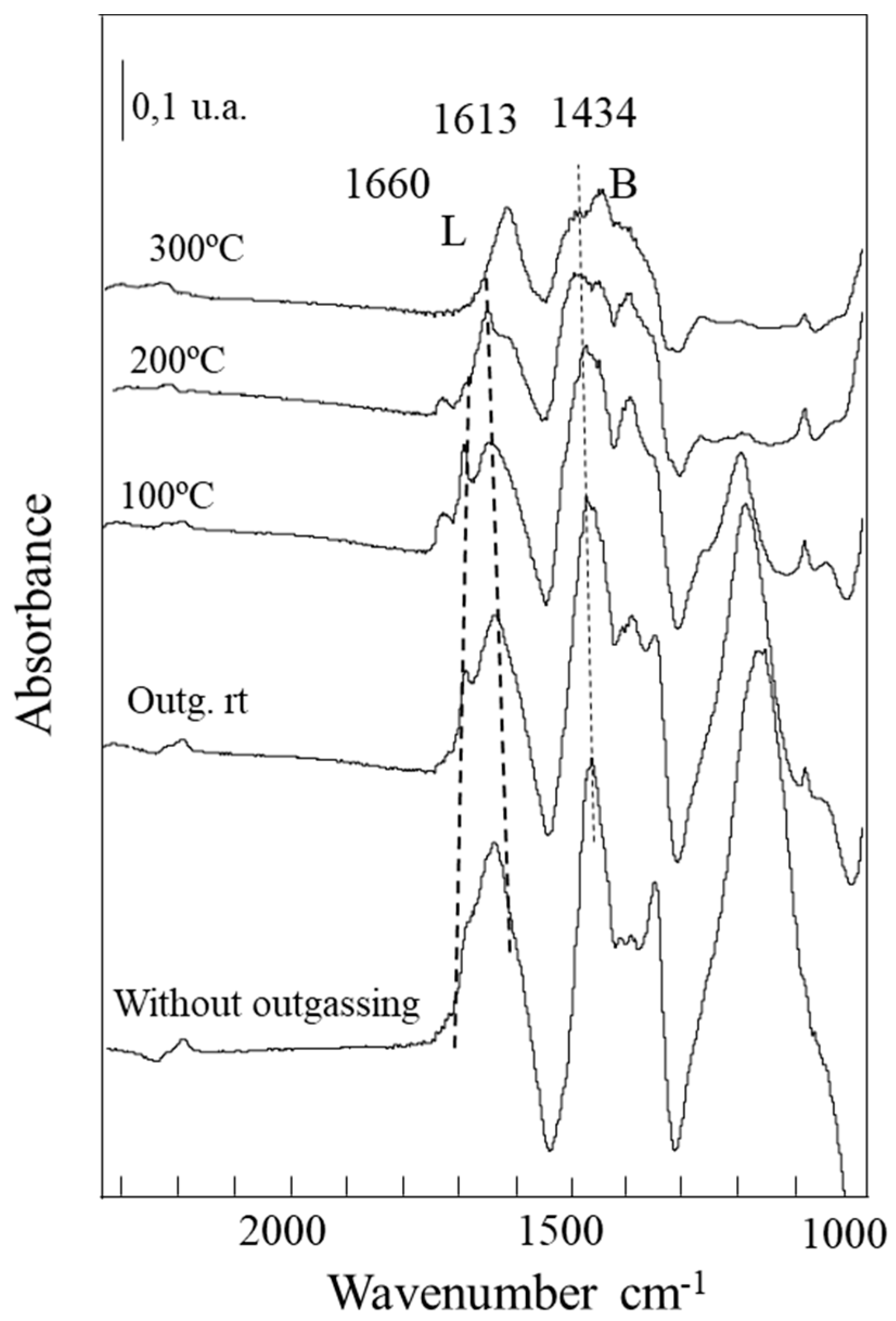
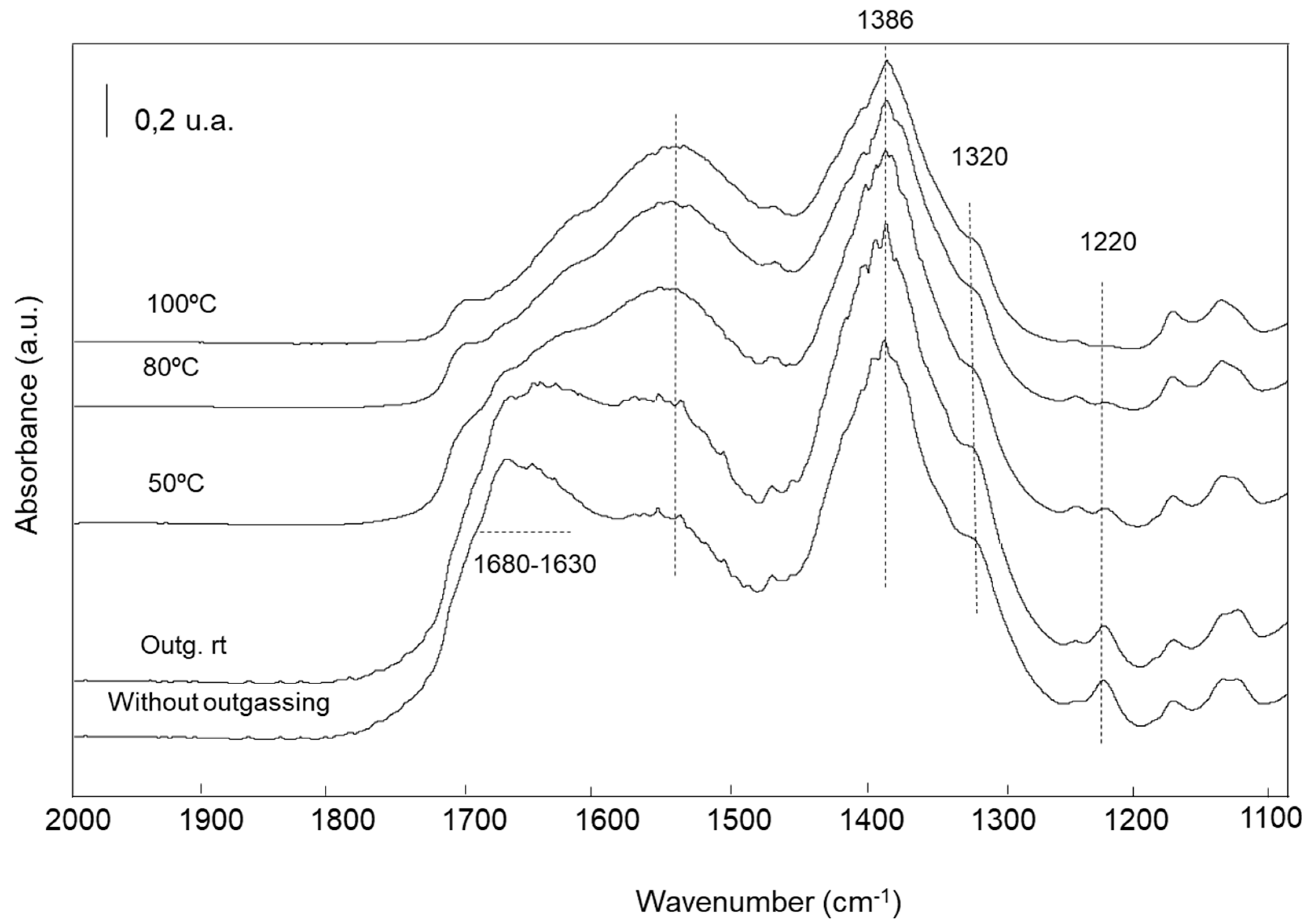
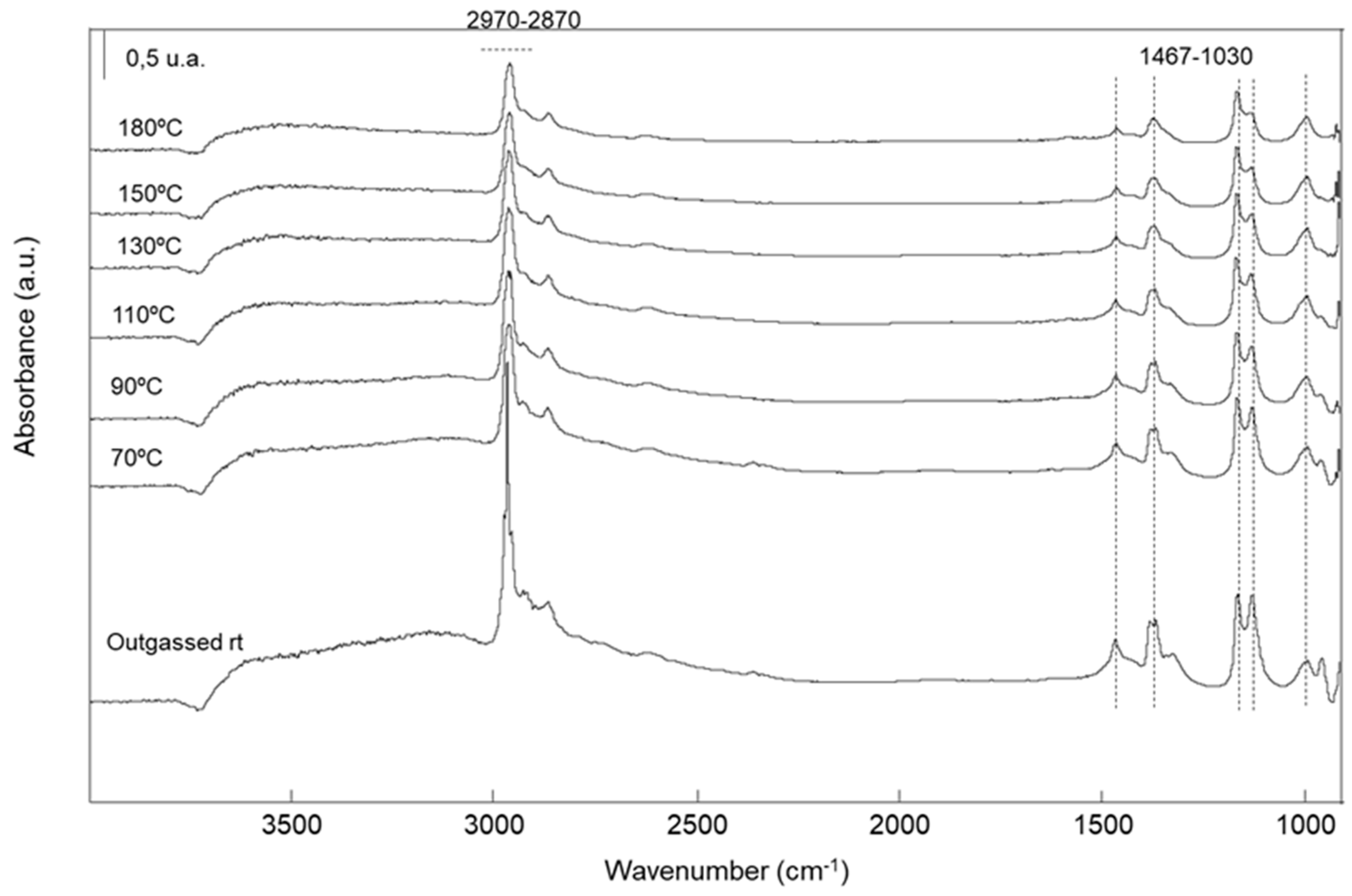
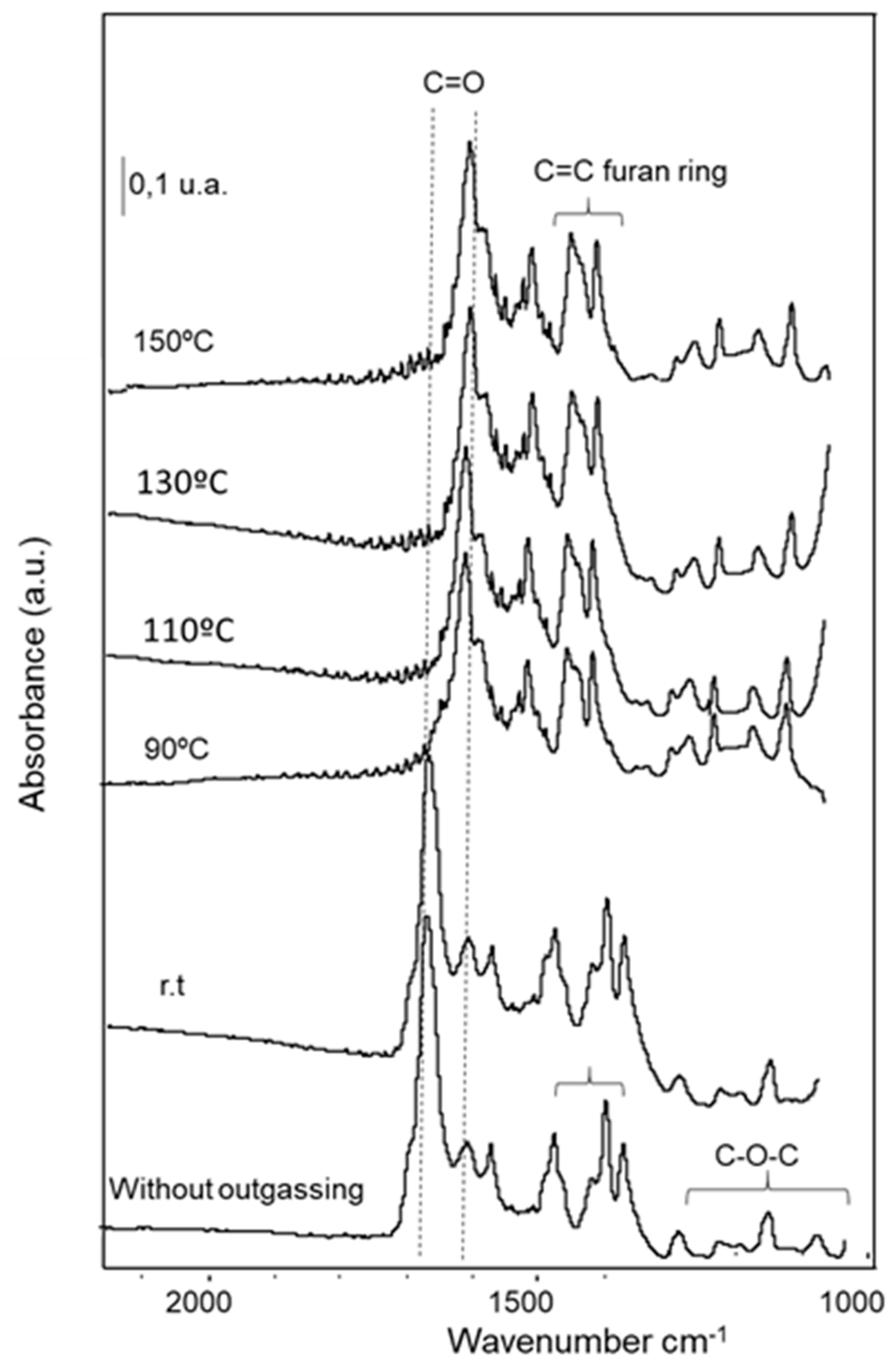
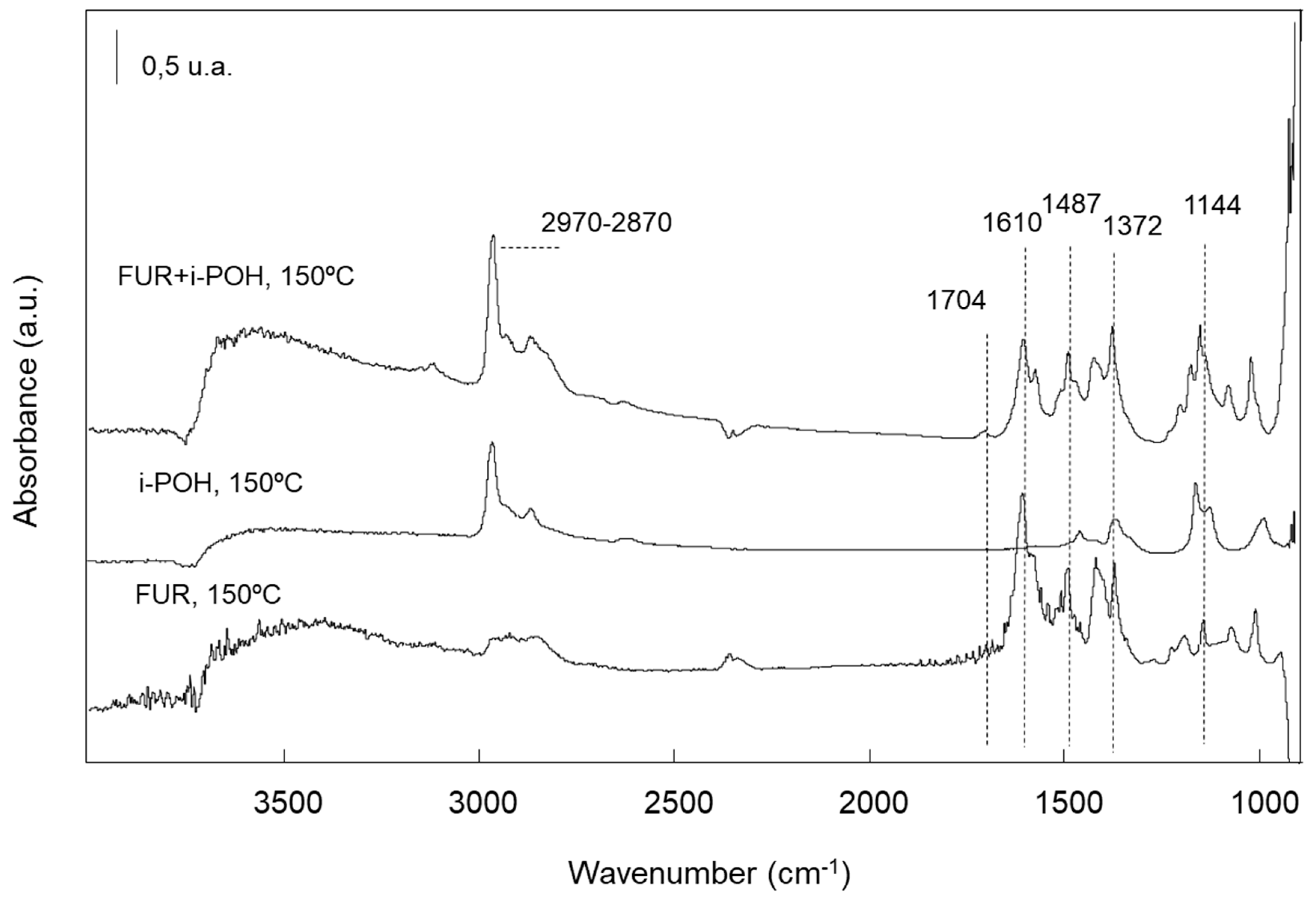
2.2.5. Characterization of the Adsorbed Species by FTIR Spectroscopy
2.2.6. Influence of the Reaction Temperature in the Catalytic Activity of MgAl-x Catalysts
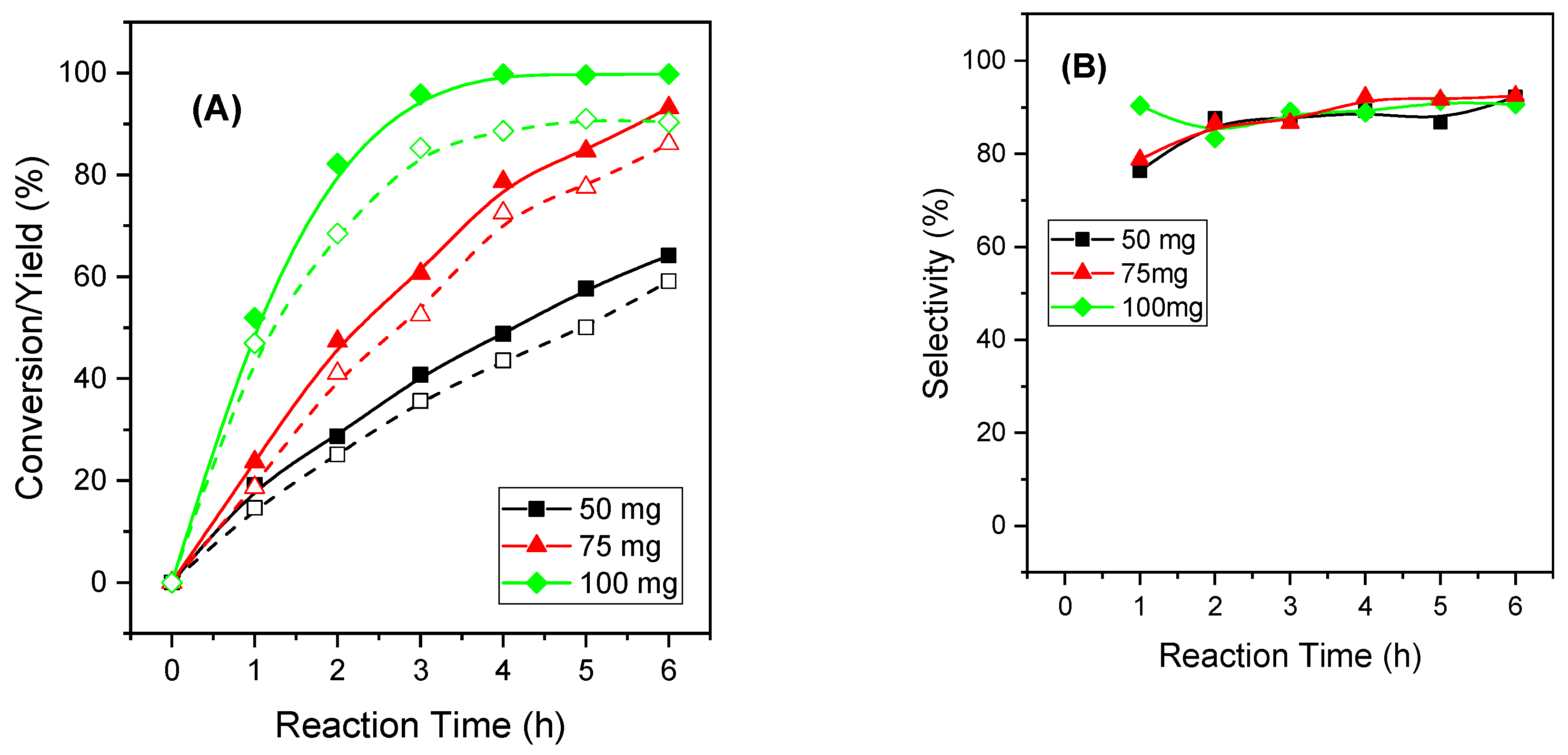
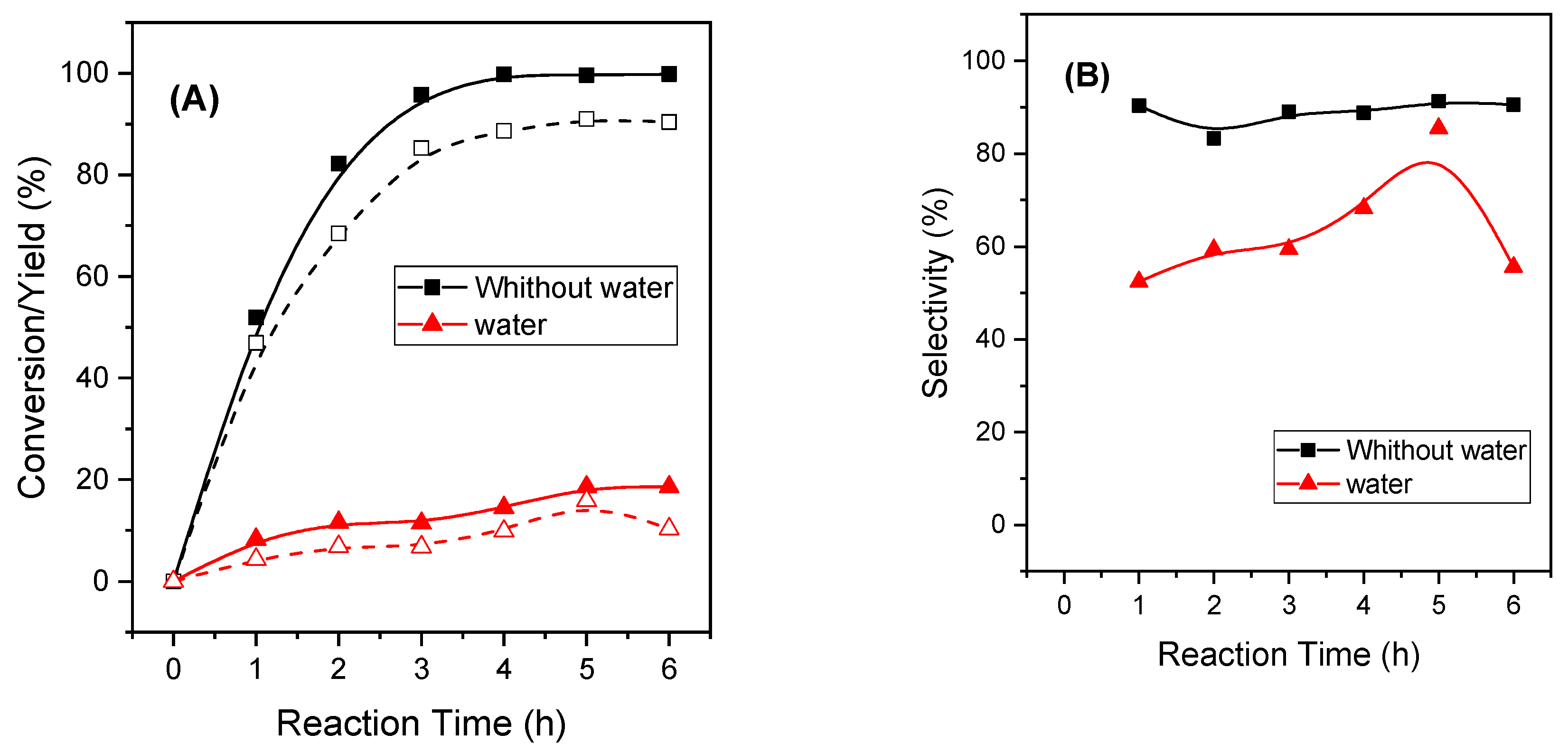
2.2.7. Influence of the Catalytic Parameters in the Activity of MgAl-3 Catalyst
3. Materials and Methods
3.1. Synthesis of Catalysts
3.2. Characterization of the Catalysts
3.3. Catalytic Reaction and Measurement of the Reaction Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kamm, B.; Gruber, P.R.; Kamm, M. Biorefineries-Industrial Processes and Products: Status Quo and Future Directions. Biorefineries-Ind. Process. Prod. Status Quo Futur. Dir. 2008, 1–2, 1–959. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sá Daba, I.; Ló Pez Granados, M. Furfural: A Renewable and Versatile Platform Molecule for the Synthesis of Chemicals and Fuels. Energy Environ. Sci. 2016, 9, 1144. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many By-Products 10. Furfural Processes. Sugar Ser. 2000, 13, 36–74. [Google Scholar]
- Dalvand, K.; Rubin, J.; Gunukula, S.; Clayton Wheeler, M.; Hunt, G. Economics of Biofuels: Market Potential of Furfural and Its Derivatives. Biomass Bioenergy 2018, 115, 56–63. [Google Scholar] [CrossRef]
- Petersen, T.W.G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab.: Golden, CO, USA, 2004. [Google Scholar]
- Lange, J.-P.; Van Der Heide, E.; Van Buijtenen, J.; Price, R. Furfural-A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, Properties and Catalytic Hydrogenation of Furfural to Fuel Additives and Value-Added Chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Sitthisa, S.; Resasco, D.E. Hydrodeoxygenation of Furfural over Supported Metal Catalysts: A Comparative Study of Cu, Pd and Ni. Catal. Lett. 2011, 141, 784–791. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Durán-Martín, D.; Moreno-Tost, R.; Santamaría-González, J.; Mérida-Robles, J.; Mariscal, R.; Maireles-Torres, P. Gas-Phase Hydrogenation of Furfural to Furfuryl Alcohol over Cu/ZnO Catalysts. J. Catal. 2016, 336, 107–115. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakazawa, H.; Watanabe, H.; Tomishige, K. Total Hydrogenation of Furfural over a Silica-Supported Nickel Catalyst Prepared by the Reduction of a Nickel Nitrate Precursor. ChemCatChem 2012, 4, 1791–1797. [Google Scholar] [CrossRef]
- Pang, S.H.; Schoenbaum, C.A.; Schwartz, D.K.; Medlin, J.W. ARTICLE Directing Reaction Pathways by Catalyst Active-Site Selection Using Self-Assembled Monolayers. Nat. Commun. 2013, 4, 2448. [Google Scholar] [CrossRef] [Green Version]
- Baker, L.R.; Kennedy, G.; Van Spronsen, M.; Hervier, A.; Cai, X.; Chen, S.; Wang, L.W.; Somorjai, G.A. Furfuraldehyde Hydrogenation on Titanium Oxide-Supported Platinum Nanoparticles Studied by Sum Frequency Generation Vibrational Spectroscopy: Acid-Base Catalysis Explains the Molecular Origin of Strong Metal-Support Interactions. J. Am. Chem. Soc. 2012, 134, 14208–14216. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, Z.; An, Z.; Zhou, J.; Li, J. Active Metal Oxide-Nitrogen-Doped Carbon Hybrid Catalysts towards Selective Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol. Appl. Catal. A Gen. 2022, 636, 118574. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, J.; Yang, J.H. Bimetallic Cu-Ni/MCM-41 Catalyst for Efficiently Selective Transfer Hydrogenation of Furfural into Furfural Alcohol. Mol. Catal. 2022, 517, 112065. [Google Scholar] [CrossRef]
- Meerwein, H.; Schmidt, R. Ein Neues Verfahren Zur Reduktion von Aldehyden Und Ketonen. Justus Liebigs Ann. Chem. 1925, 444, 221–238. [Google Scholar] [CrossRef]
- Verley, M. Sur l’échange de Groupements Fonctionnels Entre Deux Molécules. Passage Des Cétones Aux Alcohols et Inversement. Bull. Soc. Chim. Fr. 1925, 37, 871–874. [Google Scholar]
- Ponndorf, W. Der Reversible Austausch Der Oxydationsstufen Zwischen Aldehyden Oder Ketonen Einerseits Und Primären Oder Sekundären Alkoholen Anderseits. Angew. Chem. 1926, 39, 138–143. [Google Scholar] [CrossRef]
- Graves, C.R.; Zhou, H.; Stern, C.L.; Nguyen, S.B.T. A Mechanistic Investigation of the Asymmetric Meerwein-Schmidt-Ponndorf- Verley Reduction Catalyzed by BINOL/AlMe3—Structure, Kinetics, and Enantioselectivity. J. Org. Chem. 2007, 72, 9121–9133. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Graves, C.R.; Nguyen, S.B.T.; Martin, J.M.L.; Ratner, M.A. The Mechanism of Aluminum-Catalyzed Meerwein-Schmidt-Ponndorf-Verley Reduction of Carbonyls to Alcohols. J. Am. Chem. Soc. 2004, 126, 14796–14803. [Google Scholar] [CrossRef]
- Liu, Y.C.; Ko, B.T.; Huang, B.H.; Lin, C.C. Reduction of Aldehydes and Ketones Catalyzed by a Novel Aluminum Alkoxide: Mechanistic Studies of Meerwein-Ponndorf-Verley Reaction. Organometallics 2002, 21, 2066–2069. [Google Scholar] [CrossRef]
- Ooi, T.; Ichikawa, H.; Maruoka, K. Practical Approach to the Meerwein ± Ponndorf ± Verley Reduction of Carbonyl Substrates with New Aluminum Catalysts. Angew. Chem. Int. Ed. 2001, 40, 3610–3612. [Google Scholar] [CrossRef]
- Nandi, P.; Solovyov, A.; Okrut, A.; Katz, A. AlIII-Calix[4]Arene Catalysts for Asymmetric Meerwein-Ponndorf-Verley Reduction. ACS Catal. 2014, 4, 2492–2495. [Google Scholar] [CrossRef]
- Graves, C.R.; Joseph Campbell, E.; Nguyen, S.B.T. Aluminum-Based Catalysts for the Asymmetric Meerwein–Schmidt–Ponndorf–Verley–Oppenauer (MSPVO) Reaction Manifold. Tetrahedron Asymmetry 2005, 16, 3460–3468. [Google Scholar] [CrossRef]
- Creyghton, E.J.; Ganeshie, S.D.; Downing, R.S.; Van Bekkum, H. Stereoselective Meerwein–Ponndorf–Verley and Oppenauer Reactions Catalysed by Zeolite BEA. J. Mol. Catal. A Chem. 1997, 115, 457–472. [Google Scholar] [CrossRef]
- Gao, Z.K.; Hong, Y.C.; Hu, Z.; Xu, B.Q. Transfer Hydrogenation of Cinnamaldehyde with 2-Propanol on Al2O3 and SiO2 –Al2O3 Catalysts: Role of Lewis and Brønsted Acidic Sites. Catal. Sci. Technol. 2017, 7, 4511–4519. [Google Scholar] [CrossRef]
- Gonell, F.; Boronat, M.; Corma, A. Structure–Reactivity Relationship in Isolated Zr Sites Present in Zr-Zeolite and ZrO2 for the Meerwein–Ponndorf–Verley Reaction. Catal. Sci. Technol. 2017, 7, 2865–2873. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.Y.; Consoli, D.F.; Gunther, W.R.; Román-Leshkov, Y. Investigation of the Reaction Kinetics of Isolated Lewis Acid Sites in Beta Zeolites for the Meerwein–Ponndorf–Verley Reduction of Methyl Levulinate to γ-Valerolactone. J. Catal. 2014, 320, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Assary, R.S.; Curtiss, L.A.; Dumesic, J.A. Exploring Meerwein-Ponndorf-Verley Reduction Chemistry for Biomass Catalysis Using a First-Principles Approach. ACS Catal. 2013, 3, 2694–2704. [Google Scholar] [CrossRef]
- Iglesias, J.; Melero, J.A.; Morales, G.; Moreno, J.; Segura, Y.; Paniagua, M.; Cambra, A.; Hernández, B. Zr-SBA-15 Lewis Acid Catalyst: Activity in Meerwein Ponndorf Verley Reduction. Catalysts 2015, 5, 1911–1927. [Google Scholar] [CrossRef] [Green Version]
- De Bruyn, M.; Limbourg, M.; Denayer, J.; Baron, G.V.; Parvulescu, V.; Grobet, P.J.; De Vos, D.E.; Jacobs, P.A. Mesoporous Zr and Hf Catalysts for Chemoselective MPV Reductions of Unsaturated Ketones. Appl. Catal. A Gen. 2003, 254, 189–201. [Google Scholar] [CrossRef]
- Boronat, M.; Corma, A.; Renz, M. Mechanism of the Meerwein-Ponndorf-Verley-Oppenauer (MPVO) Redox Equilibrium on Sn- and Zr—Beta Zeolite Catalysts. J. Phys. Chem. B 2006, 110, 21168–21174. [Google Scholar] [CrossRef]
- Corma, A.; Domine, M.E.; Valencia, S. Water-Resistant Solid Lewis Acid Catalysts: Meerwein–Ponndorf–Verley and Oppenauer Reactions Catalyzed by Tin-Beta Zeolite. J. Catal. 2003, 215, 294–304. [Google Scholar] [CrossRef]
- Corma, A.; Domine, M.E.; Nemeth, L.; Valencia, S. Al-Free Sn-Beta Zeolite as a Catalyst for the Selective Reduction of Carbonyl Compounds (Meerwein-Ponndorf-Verley Reaction). J. Am. Chem. Soc. 2002, 124, 3194–3195. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Z. Catalytic Transfer Hydrogenation of Furfural into Furfuryl Alcohol over Magnetic γ-Fe2O3@HAP Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 942–947. [Google Scholar] [CrossRef]
- Hidalgo, J.M.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Delaminated Layered Double Hydroxides as Catalysts for the Meerwein–Ponndorf–Verley Reaction. Appl. Catal. A Gen. 2014, 470, 311–317. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Borau, V.; Jiménez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Influence of the Preparation Method on the Structural and Surface Properties of Various Magnesium Oxides and Their Catalytic Activity in the Meerwein–Ponndorf–Verley Reaction. Appl. Catal. A Gen. 2003, 244, 207–215. [Google Scholar] [CrossRef]
- Xiao, Z. Insight into the Meerwein-Ponndorf-Verley Reduction of Cinnamaldehyde over MgAl Oxides Catalysts. Mol. Catal. 2017, 436, 1–9. [Google Scholar] [CrossRef]
- Kumbhar, P.S. Jaime Sanchez-Valente; Joseph Lopez; François Figueras Meerwein–Ponndorf–Verley Reduction of Carbonyl Compounds Catalysed by Mg–Al Hydrotalcite. Chem. Commun. 1998, 0, 535–536. [Google Scholar] [CrossRef]
- Axpuac, S.; Aramendía, M.A.; Hidalgo-Carrillo, J.; Marinas, A.; Marinas, J.M.; Montes-Jiménez, V.; Urbano, F.J.; Borau, V. Study of Structure–Performance Relationships in Meerwein–Ponndorf–Verley Reduction of Crotonaldehyde on Several Magnesium and Zirconium-Based Systems. Catal. Today 2012, 187, 183–190. [Google Scholar] [CrossRef]
- Wang, Q.; Ohare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Antunes, M.M.; Lima, S.; Neves, P.; Magalhães, A.L.; Fazio, E.; Fernandes, A.; Neri, F.; Silva, C.M.; Rocha, S.M.; Ribeiro, M.F.; et al. One-Pot Conversion of Furfural to Useful Bio-Products in the Presence of a Sn,Al-Containing Zeolite Beta Catalyst Prepared via Post-Synthesis Routes. J. Catal. 2015, 329, 522–537. [Google Scholar] [CrossRef] [Green Version]
- Koehle, M.; Lobo, R.F. Lewis Acidic Zeolite Beta Catalyst for the Meerwein–Ponndorf–Verley Reduction of Furfural. Catal. Sci. Technol. 2016, 6, 3018–3026. [Google Scholar] [CrossRef]
- Bui, L.; Luo, H.; Gunther, W.R.; Romµn-Leshkov, Y.; Bui, L.; Luo, H.; Gunther, W.R.; Romµn-Leshkov, Y. Domino Reaction Catalyzed by Zeolites with Brønsted and Lewis Acid Sites for the Production of γ-Valerolactone from Furfural. Angew. Chem. Int. Ed. 2013, 52, 8022–8025. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.M.; Lima, S.; Neves, P.; Magalhães, A.L.; Fazio, E.; Neri, F.; Pereira, M.T.; Silva, A.F.; Silva, C.M.; Rocha, S.M.; et al. Integrated Reduction and Acid-Catalysed Conversion of Furfural in Alcohol Medium Using Zr,Al-Containing Ordered Micro/Mesoporous Silicates. Appl. Catal. B Environ. 2016, 182, 485–503. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Urbaneja, P.; García-Sancho, C.; Moreno-Tost, R.; Mérida-Robles, J.; Santamaría-González, J.; Jiménez-López, A.; Maireles-Torres, P. Glycerol Valorization by Etherification to Polyglycerols by Using Metal Oxides Derived from MgFe Hydrotalcites. Appl. Catal. A Gen. 2014, 470, 199–207. [Google Scholar] [CrossRef]
- Nogueira, K.A.B.; Cecilia, J.A.; Santos, S.O.; Aguiar, J.E.; Vilarrasa-García, E.; Rodríguez-Castellón, E.; Azevedo, D.C.S.; Silva, I.J. Adsorption Behavior of Bovine Serum Albumin on Zn–Al and Mg–Al Layered Double Hydroxides. J. Sol-Gel Sci. Technol. 2016, 80, 748–758. [Google Scholar] [CrossRef]
- Ulibarri, M.A.; Hernandez, M.J.; Cornejo, J. Changes in Textural Properties Derived from the Thermal Decomposition of Synthetic Pyroaurite. Thermochim. Acta 1987, 113, 79–86. [Google Scholar] [CrossRef]
- Valente, J.S.; Hernandez-Cortez, J.; Cantu, M.S.; Ferrat, G.; López-Salinas, E. Calcined Layered Double Hydroxides Mg–Me–Al (Me: Cu, Fe, Ni, Zn) as Bifunctional Catalysts. Catal. Today 2010, 150, 340–345. [Google Scholar] [CrossRef]
- León, M.; Díaz, E.; Vega, A.; Ordóñez, S.; Auroux, A. Consequences of the Iron–Aluminium Exchange on the Performance of Hydrotalcite-Derived Mixed Oxides for Ethanol Condensation. Appl. Catal. B Environ. 2011, 102, 590–599. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Gursky, J.A.; Blough, S.D.; Luna, C.; Gomez, C.; Luevano, A.N.; Gardner, E.A. Particle-Particle Interactions between Layered Double Hydroxide Nanoparticles. J. Am. Chem. Soc. 2006, 128, 8376–8377. [Google Scholar] [CrossRef]
- Zhao, W.; Veerappan Vaithilingam, B.; Ghosh, S.; Li, X.; Geuzebroek, F.; El Nasr, A.S.; Khan, I.; Dara, S.; Mittal, N.; Daoutidis, P.; et al. High-Capacity Regenerable H2S Sorbent for Reducing Sulfur Emissions. Ind. Eng. Chem. Res. 2021, 60, 14779–14787. [Google Scholar] [CrossRef]
- Li, J.; Fan, H.; Jia, X. Multilayered ZnO Nanosheets with 3D Porous Architectures: Synthesis and Gas Sensing Application. J. Phys. Chem. C 2010, 114, 14684–14691. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and Surface and Catalytic Properties of Mg-Al Basic Oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Padmasri, A.H.; Venugopal, A.; Durga Kumari, V.; Rama Rao, K.S.; Kanta Rao, P. Calcined Mg-Al, Mg-Cr and Zn-Al Hydrotalcite Catalysts for Tert-Butylation of Phenol with Iso-Butanol—A Comparative Study. J. Mol. Catal. A Chem. 2002, 188, 255–265. [Google Scholar] [CrossRef]
- Bing, W.; Zheng, L.; He, S.; Rao, D.; Xu, M.; Zheng, L.; Wang, B.; Wang, Y.; Wei, M. Insights on Active Sites of CaAl-Hydrotalcite as a High-Performance Solid Base Catalyst toward Aldol Condensation. ACS Catal. 2018, 8, 656–664. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Panagiotopoulou, P.; Mironenko, A.V.; Jenness, G.R.; Vlachos, D.G.; Xu, B. Mechanistic Insights into Metal Lewis Acid-Mediated Catalytic Transfer Hydrogenation of Furfural to 2-Methylfuran. ACS Catal. 2015, 5, 3988–3994. [Google Scholar] [CrossRef] [Green Version]
- Panagiotopoulou, P.; Martin, N.; Vlachos, D.G. Effect of Hydrogen Donor on Liquid Phase Catalytic Transfer Hydrogenation of Furfural over a Ru/RuO2/C Catalyst. J. Mol. Catal. A Chem. 2014, 392, 223–228. [Google Scholar] [CrossRef]
- Mironenko, A.V.; Vlachos, D.G. Conjugation-Driven “Reverse Mars-van Krevelen”-Type Radical Mechanism for Low-Temperature C-O Bond Activation. J. Am. Chem. Soc. 2016, 138, 8104–8113. [Google Scholar] [CrossRef]
- He, J.; Schill, L.; Yang, S.; Riisager, A. Catalytic Transfer Hydrogenation of Bio-Based Furfural with NiO Nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 17220–17229. [Google Scholar] [CrossRef]
- Feng, L.; Jiang, S.; Wang, Y.; Huang, J.; Li, C. Catalytic Transfer Hydrogenation of Furfural over CuNi@C Catalyst Prepared from Cu–Ni Metal-Organic Frameworks. Russ. J. Phys. Chem. A 2021, 95, 68–79. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway in Biomass Upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Kim, K.; Ahn, W.S. Catalytic Transfer Hydrogenation of Bio-Based Furfural by Palladium Supported on Nitrogen-Doped Porous Carbon. Catal. Today 2019, 324, 49–58. [Google Scholar] [CrossRef]
- García-Sancho, C.; Moreno-Tost, R.; Mérida-Robles, J.M.; Santamaría-González, J.; Jiménez-López, A.; Torres, P.M. Etherification of Glycerol to Polyglycerols over MgAl Mixed Oxides. Catal. Today 2011, 167, 84–90. [Google Scholar] [CrossRef]
- Bocanegra, S.A.; Ballarini, A.D.; Scelza, O.A.; de Miguel, S.R. The Influence of the Synthesis Routes of MgAl2O4 on Its Properties and Behavior as Support of Dehydrogenation Catalysts. Mater. Chem. Phys. 2008, 111, 534–541. [Google Scholar] [CrossRef]
- Gyngazova, M.S.; Grazia, L.; Lolli, A.; Innocenti, G.; Tabanelli, T.; Mella, M.; Albonetti, S.; Cavani, F. Mechanistic Insights into the Catalytic Transfer Hydrogenation of Furfural with Methanol and Alkaline Earth Oxides. J. Catal. 2019, 372, 61–73. [Google Scholar] [CrossRef]
- Chang, X.; Liu, A.F.; Cai, B.; Luo, J.Y.; Pan, H.; Huang, Y.B. Catalytic Transfer Hydrogenation of Furfural to 2-Methylfuran and 2-Methyltetrahydrofuran over Bimetallic Copper–Palladium Catalysts. ChemSusChem 2016, 9, 3330–3337. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Fan, R.; Zhang, H.; Wang, G.; Zhao, H. Transfer-Hydrogenation of Furfural and Levulinic Acid over Supported Copper Catalyst. Fuel 2018, 231, 165–171. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Shao, S.; Yan, J.; Zhang, H.; Cai, Y. Catalytic Transfer Hydrogenation of Biomass-Derived Oxygenated Chemicals over Hydrotalcite-like Copper Catalyst Using Methanol as Hydrogen Donor. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Ramos, R.; Peixoto, A.F.; Arias-Serrano, B.I.; Soares, O.S.G.P.; Pereira, M.F.R.; Kubička, D.; Freire, C. Catalytic Transfer Hydrogenation of Furfural over Co3O4−Al2O3 Hydrotalcite-Derived Catalyst. ChemCatChem 2020, 12, 1467–1475. [Google Scholar] [CrossRef]
- Carriazo, D.; Martín, C.; Rives, V. An FT-IR Study of the Adsorption of Isopropanol on Calcined Layered Double Hydroxides Containing Isopolymolybdate. Catal. Today 2007, 126, 153–161. [Google Scholar] [CrossRef]
- Martín, C.; Martín, I.; Rives, V.; Grzybowska, B.; Gressel, I. A FTIR Spectroscopy Study of Isopropanol Reactivity on Alkali-Metal-Doped MoO3/TiO2 Catalysts. Spectrochim. Acta Part A Mol. Spectrosc. 1996, 52, 733–740. [Google Scholar] [CrossRef]
- Del Arco, M.; Gutiérrez, S.; Martín, C.; Rives, V. FTIR Study of Isopropanol Reactivity on Calcined Layered Double Hydroxides. Phys. Chem. Chem. Phys. 2001, 3, 119–126. [Google Scholar] [CrossRef]
- Mavrikakis, M.; Barteau, M.A. Oxygenate Reaction Pathways on Transition Metal Surfaces. J. Mol. Catal. A Chem. 1998, 131, 135–147. [Google Scholar] [CrossRef]
- Sitthisa, S.; Sooknoi, T.; Ma, Y.; Balbuena, P.B.; Resasco, D.E. Kinetics and Mechanism of Hydrogenation of Furfural on Cu/SiO2 Catalysts. J. Catal. 2011, 277, 1–13. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Niu, S.; Li, Y. A Study of Furfural Decarbonylation on K-Doped Pd/Al2O3 Catalysts. J. Mol. Catal. A Chem. 2011, 335, 71–81. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, Y.; Chen, L.; Wang, L.; Shi, Y.; Yin, P.; Wang, W.; Shao, M.; Zhang, X.; Wei, M. Synergetic Effect of Cu0 − Cu+ Derived from Layered Double Hydroxides toward Catalytic Transfer Hydrogenation Reaction. Appl. Catal. B Environ. 2022, 314, 121515. [Google Scholar] [CrossRef]
- López-Asensio, R.; Cecilia, J.A.; Jiménez-Gómez, C.P.; García-Sancho, C.; Moreno-Tost, R.; Maireles-Torres, P. Selective Production of Furfuryl Alcohol from Furfural by Catalytic Transfer Hydrogenation over Commercial Aluminas. Appl. Catal. A Gen. 2018, 556, 1–9. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-Ray Line Broadening from Filed Aluminium and Wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]

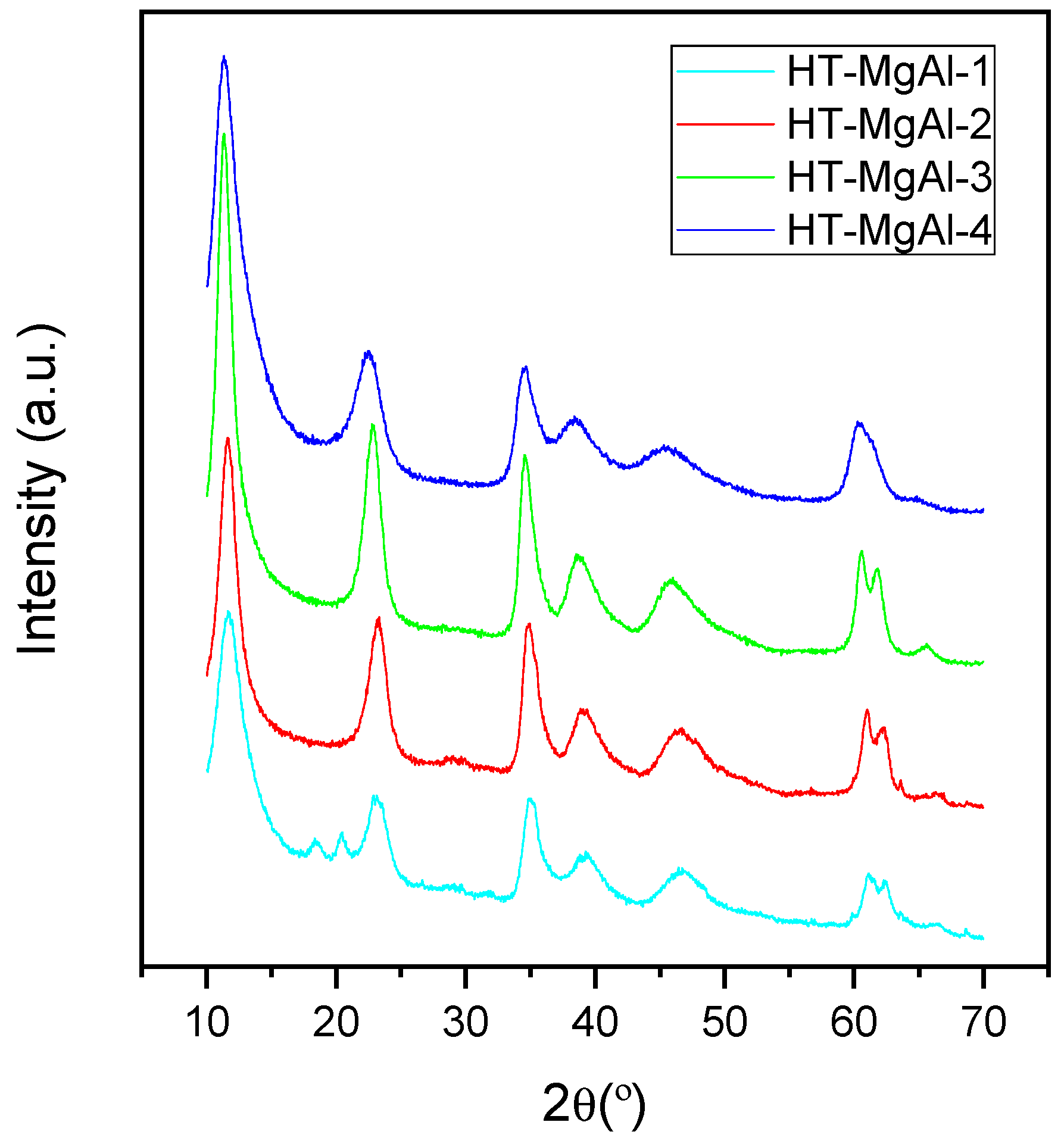



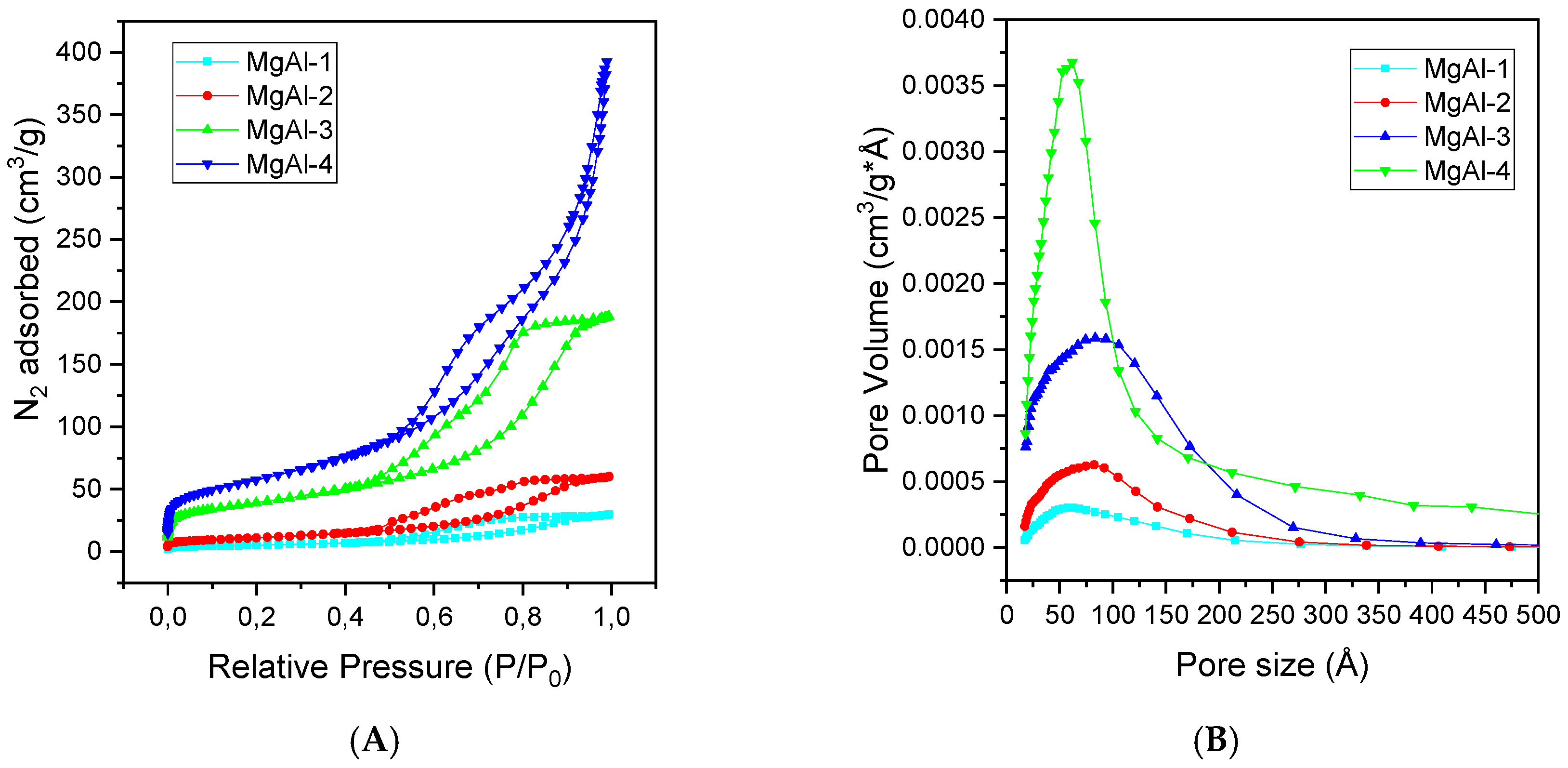




| Hydrotalcite | d(003) (Å) | c * (Å) | a * (Å) |
|---|---|---|---|
| HT-MgAl-1 | 7.59 | 23.34 | 3.03 |
| HT-MgAl-2 | 7.62 | 23.45 | 3.04 |
| HT-MgAl-3 | 7.80 | 23.14 | 3.06 |
| HT-MgAl-4 | 7.80 | 22.92 | 3.07 |
| Hydrotalcite | 1er Peak (%) | 2° Peak (%) | 3er Peak (%) | Total (%) |
|---|---|---|---|---|
| HT-MgAl-1 | * 25.4 (186 °C) | 17.3 (374 °C) | - | 42.7 |
| HT-MgAl-2 | 17.4 (180 °C) | 24.2 (391 °C) | 4.1 (481 °C) | 45.7 |
| HT-MgAl-3 | 14.9 (146 °C) | 24.5 (379 °C) | 5.3 (486 °C) | 44.7 |
| HT-MgAl-4 | 14.8 (102 °C) | 24.2 (352 °C) | 5.9 (484 °C) | 44.9 |
| Catalyst | BET (m2/g) | a t-Plot (m2/g) | Vp (cm3/g) | dp (nm) | Basicity (µmol CO2/g) |
|---|---|---|---|---|---|
| MgAl-1 | 18 | 1 | 0.044 | 8.3 | 23.6 |
| MgAl-2 | 39 | 1 | 0.088 | 7.9 | 57.2 |
| MgAl-3 | 139 | 19 | 0.265 | 8.2 | 117.4 |
| MgAl-4 | 205 | 9 | 0.575 | 10.3 | 146.7 |
| MgAl-3R b | 160 | 3 | 0.079 | 5.7 |
| Catalyst | C (%) | O (%) | Mg (%) | Al (%) | Mg/Al Surface Atomic Ratio |
|---|---|---|---|---|---|
| MgAl-1 | 1.2 | 67.8 | 15.13 | 15.87 | 0.95 |
| MgAl-2 | 4.25 | 65.37 | 19.61 | 10.77 | 1.82 |
| MgAl-3 | 9.52 | 65.19 | 18.60 | 6.68 | 2.78 |
| MgAl-4 | 2.03 | 65.55 | 26.66 | 5.76 | 4.62 |
| Catalyst | C1s (eV) | O1s (eV) | Mg2p (eV) | Al2p (eV) |
|---|---|---|---|---|
| MgAl-1 | 284.6 (55.7%) 290.0 (44.3%) | 530.4 (66.0%) 532.0 (34.0%) | 49.8 | 73.9 |
| MgAl-2 | 284.7 (71.4%) 289.7 (28.6%) | 530.4 (79.4%) 532.3 (20.6%) | 49.6 | 73.8 |
| MgAl-3 | 285.2 (60.9%) 289.3 (39.1%) | 530.6 (94.7%) 531.9 (5.3%) | 49.9 | 74.2 |
| MgAl-4 | 284.8 (63.4%) 289.3 (36.6%) | 529.9 (45.9%) 531.6 (54.1%) | 49.3 | 73.7 |
| Catalyst | C1s (eV) | O1s (eV) | Mg2p (eV) | Al2p (eV) | Mg/Al Surface Atomic Ratio |
|---|---|---|---|---|---|
| MgAl-3 | 284.7 (56.4%) 285.8 (18.8%) 288.4 (24.8%) | 530.1 (53.7%) 531.7 (46.3%) | 49.5 (92.7%) 50.8 (7.3%) | 73.8 (95.6%) 75.5 (4.4%) | 2.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Asensio, R.; Cecilia-Buenestado, J.A.; Herrera-Delgado, C.; Larrubia-Vargas, M.Á.; García-Sancho, C.; Maireles-Torres, P.J.; Moreno-Tost, R. Mixed Oxides Derived from Hydrotalcites Mg/Al Active in the Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol. Catalysts 2023, 13, 45. https://doi.org/10.3390/catal13010045
López-Asensio R, Cecilia-Buenestado JA, Herrera-Delgado C, Larrubia-Vargas MÁ, García-Sancho C, Maireles-Torres PJ, Moreno-Tost R. Mixed Oxides Derived from Hydrotalcites Mg/Al Active in the Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol. Catalysts. 2023; 13(1):45. https://doi.org/10.3390/catal13010045
Chicago/Turabian StyleLópez-Asensio, Raquel, Juan Antonio Cecilia-Buenestado, Concepción Herrera-Delgado, María Ángeles Larrubia-Vargas, Cristina García-Sancho, Pedro Jesús Maireles-Torres, and Ramón Moreno-Tost. 2023. "Mixed Oxides Derived from Hydrotalcites Mg/Al Active in the Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol" Catalysts 13, no. 1: 45. https://doi.org/10.3390/catal13010045
APA StyleLópez-Asensio, R., Cecilia-Buenestado, J. A., Herrera-Delgado, C., Larrubia-Vargas, M. Á., García-Sancho, C., Maireles-Torres, P. J., & Moreno-Tost, R. (2023). Mixed Oxides Derived from Hydrotalcites Mg/Al Active in the Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol. Catalysts, 13(1), 45. https://doi.org/10.3390/catal13010045










