Applying Hydrogenation to Stabilize N-TiO2 and Enhance Its Visible Light Photocatalytic Activity
Abstract
:1. Introduction
2. Calculation Method
3. Results and Discussion
3.1. Crystal Structure and Formation Energy
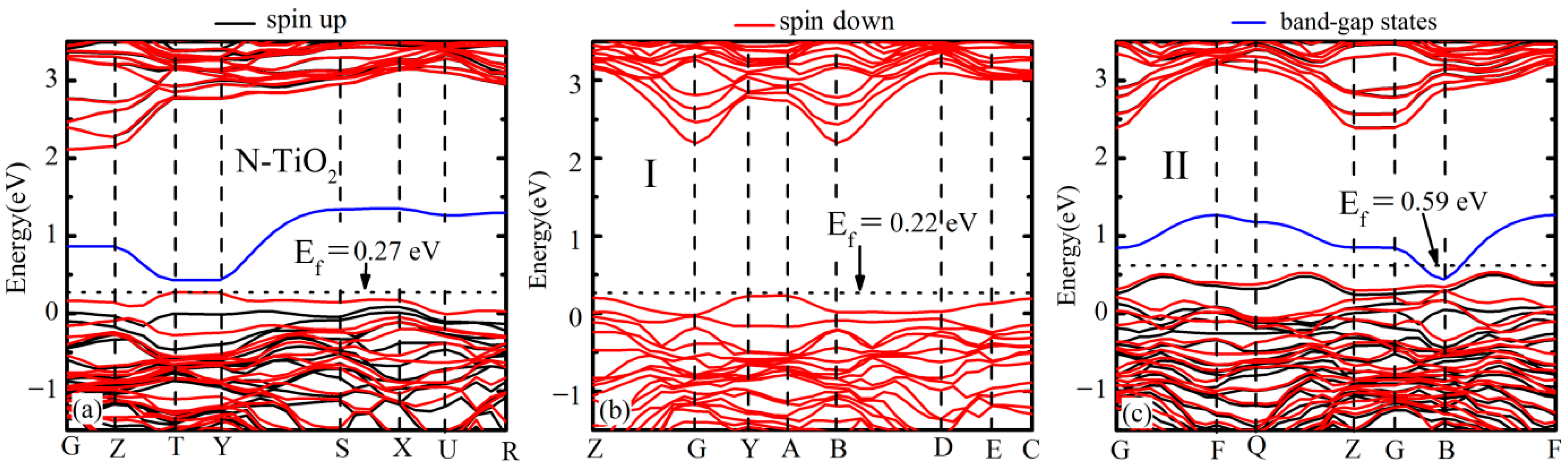
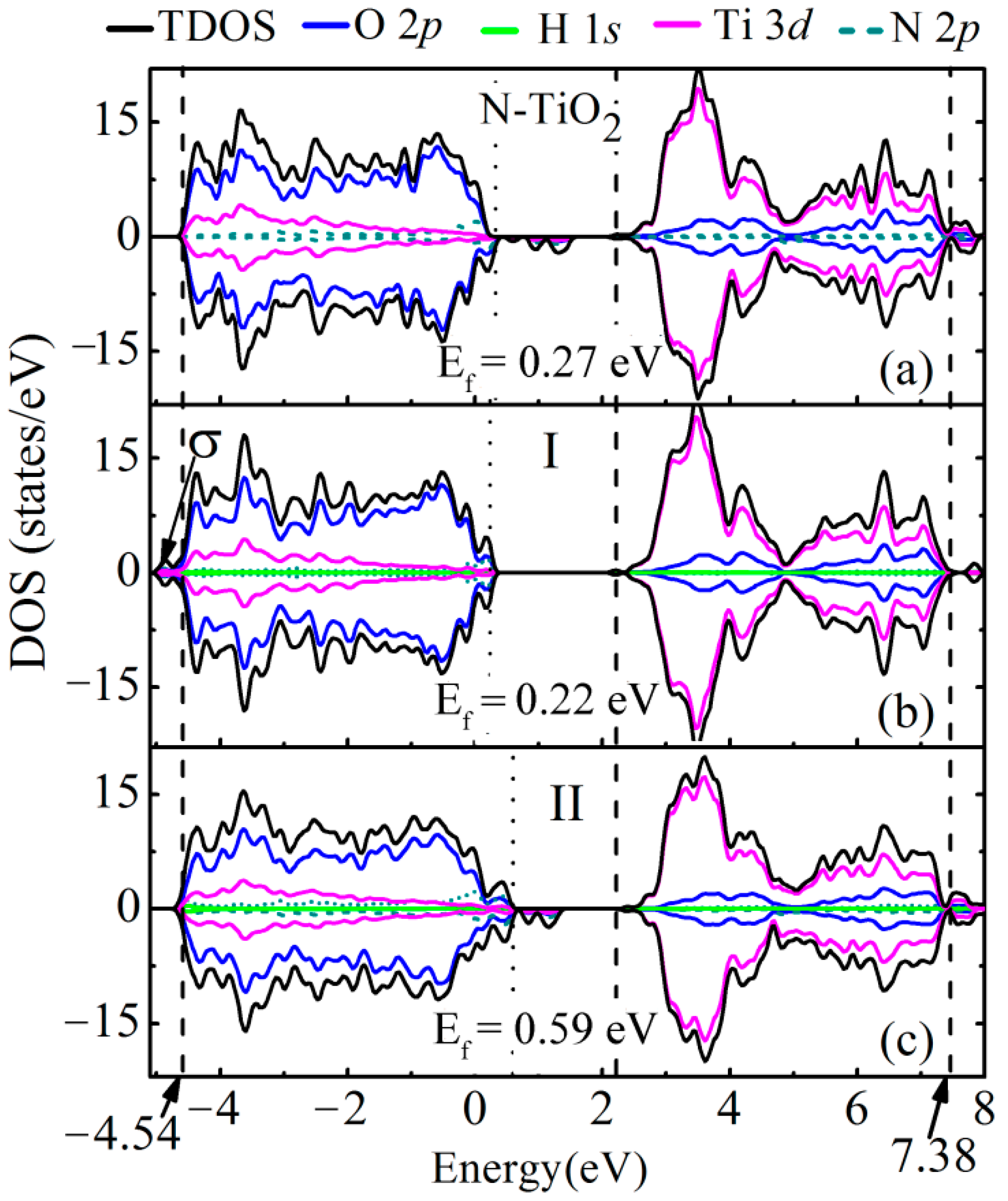
3.2. Electronic Band Structure and Density of States
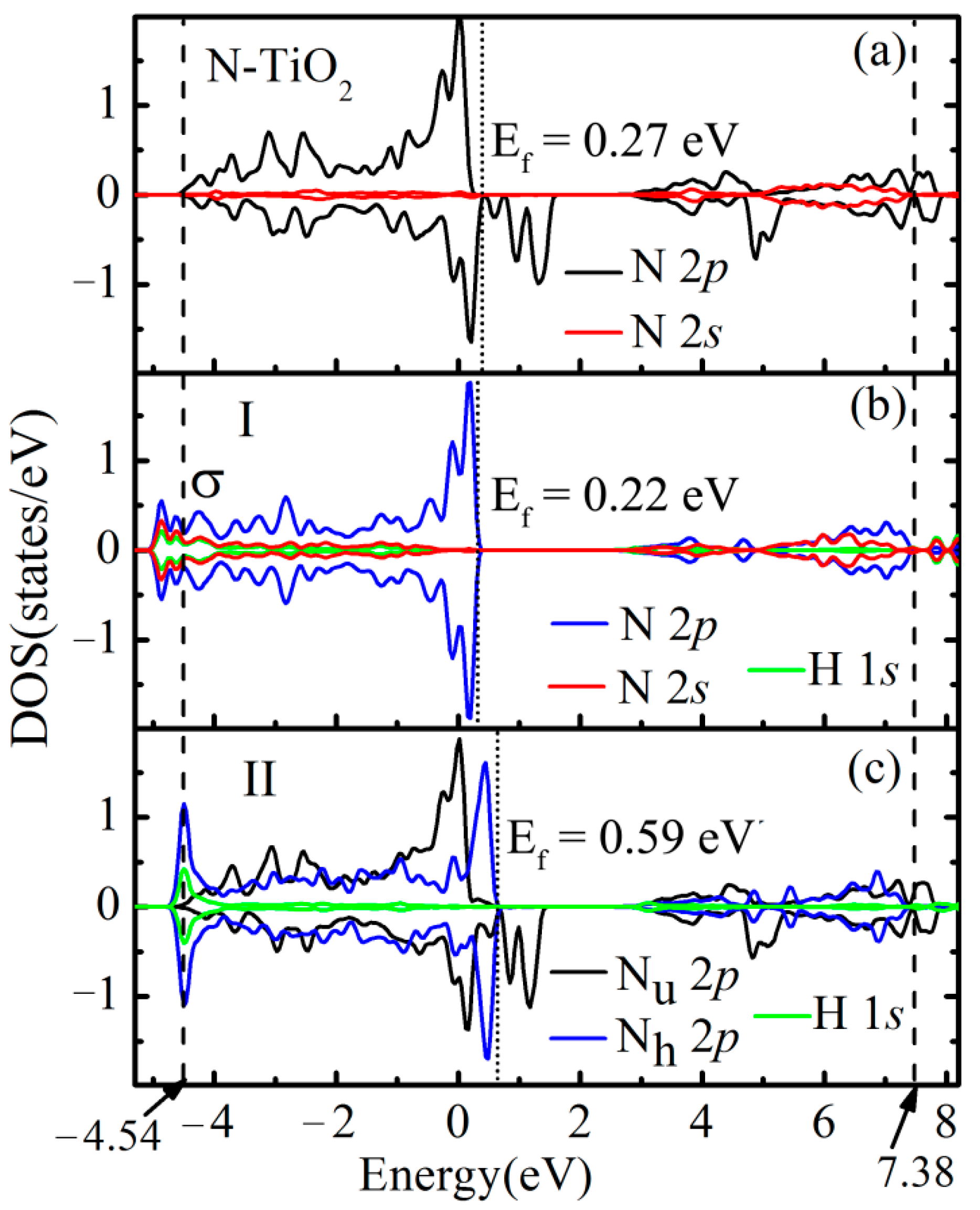
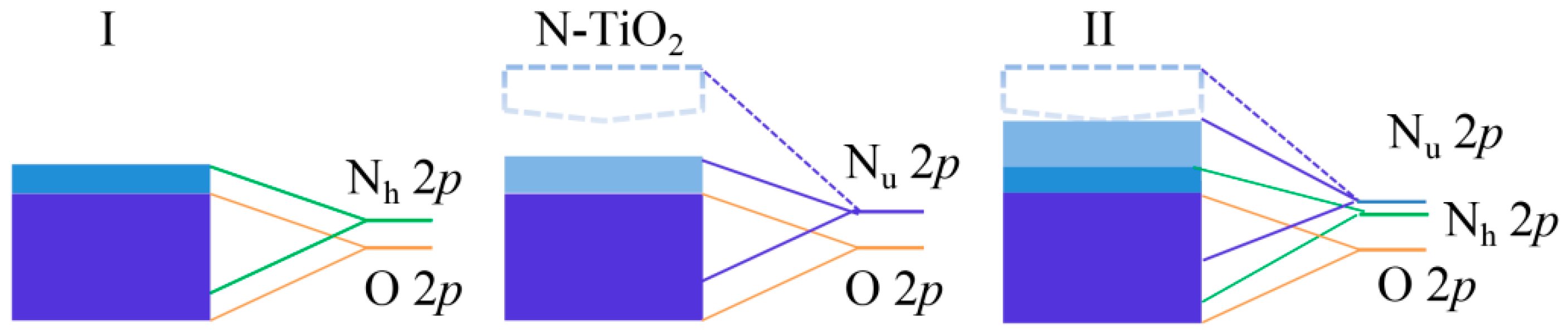
3.3. Orbital Analysis
3.4. Mulliken Population Analysis
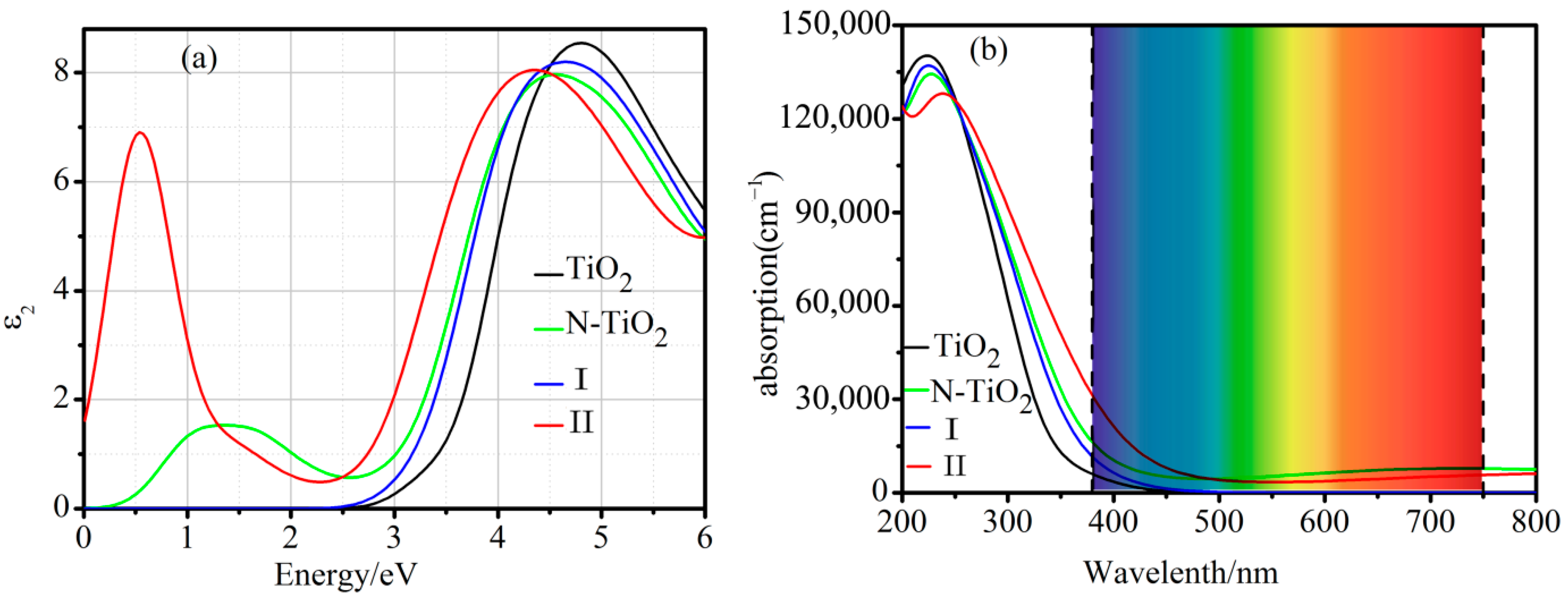
3.5. Optical Properties
3.6. Mechanism of Electronic Structure Change
3.7. Photocatalytic Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Shang, J.; Tang, X.; Kou, L. Tunable Photocatalytic Water Splitting by the Ferroelectric Switch in a 2D AgBiP2Se6 Monolayer. J. Am. Chem. Soc. 2020, 142, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Qin, J.; Shi, L.; Yang, G.; Zhang, J.; Sun, L. Rolling the WSSe Bilayer into Double-Walled Nanotube for the Enhanced Photocatalytic Water-Splitting Performance. Nanomaterials 2021, 11, 705. [Google Scholar] [CrossRef]
- Cai, J.; Shen, J.; Zhang, X.; Ng, Y.H.; Huang, J.; Guo, W.; Lin, C.; Lai, Y. Light-Driven Sustainable Hydrogen Production Utilizing TiO2 Nanostructures: A Review. Small Methods 2019, 3, 1800184. [Google Scholar] [CrossRef] [Green Version]
- Ju, L.; Liu, P.; Yang, Y.; Shi, L.; Yang, G.; Sun, L. Tuning the photocatalytic water-splitting performance with the adjustment of diameter in an armchair WSSe nanotube. J. Energy Chem. 2021, 61, 228–235. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Kidkhunthod, P.; Chanlek, N.; Soontaranon, S.; Wantala, K. High anatase purity of nitrogen-doped TiO2 nanorice particles for the photocatalytic treatment activity of pharmaceutical wastewater. Appl. Surf. Sci. 2019, 478, 1–14. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Khan, M.; Yi, Z.; Fawad, U.; Muhammad, W.; Niaz, A.; Zaman, M.I.; Ullah, A. Enhancing the photoactivity of TiO2 by codoping with silver and molybdenum: The effect of dopant concentration on the photoelectrochemical properties. Mater. Res. Express 2017, 4, 045023. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao Samuel, S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, X. The synergetic effect of V and Fe-co-doping in TiO2 studied from the DFT + U first-principle calculation. Appl. Surf. Sci. 2017, 399, 654–662. [Google Scholar] [CrossRef]
- Niu, M.; Zhang, J.; Cao, D. I, N-Codoping Modification of TiO2 for Enhanced Photoelectrochemical H2O Splitting in Visible-Light Region. J. Phys. Chem. C 2017, 121, 26202–26208. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, Y.; Tian, J.; Jing, T.; Kan, W.; Hu, Y. Theoretical calculation and experiment study on the electronic structure, microstructures and photocatalytic activity of N–Al codoped TiO2. RSC Adv. 2017, 7, 52105–52110. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, W.; Li, C.; He, L. Electronic and photocatalytic properties of N/F co-doped anatase TiO2. RSC Adv. 2017, 7, 55282–55287. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Liu, S.; Zhang, S.; Wang, R.; Wang, K. Preparation and visible-light photocatalytic activity of N-doped TiO2 by plasma-assisted sol-gel method. Catal. Today 2019, 337, 37–43. [Google Scholar] [CrossRef]
- Avilés-García, O.; Espino-Valencia, J.; Romero-Romero, R.; Rico-Cerda, J.L.; Arroyo-Albiter, M.; Solís-Casados, D.A.; Natividad-Rangel, R. Enhanced Photocatalytic Activity of Titania by Co-Doping with Mo and W. Catalysts 2018, 8, 631. [Google Scholar] [CrossRef] [Green Version]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-,N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Assadi, M.H.N.; Hanaor, D.A. The effects of copper doping on photocatalytic activity at (101) planes of anatase TiO2: A theoretical study. Appl. Surf. Sci. 2016, 387, 682–689. [Google Scholar] [CrossRef] [Green Version]
- Khaselev, O.; Turner John, A. A Monolithic Photovoltaic-Photoelectrochemical Device for Hydrogen Production via Water Splitting. Science 1998, 280, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.; Li, J.; Li, S.-S.; Xia, J.-B.; Wei, S.-H. Design of Narrow-Gap TiO2: A Passivated Codoping Approach for Enhanced Photoelectrochemical Activity. Phys. Rev. Lett. 2009, 102, 036402. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-Concentration Dependence on Photocatalytic Activity of TiO2-xNx Powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, X.; Tian, B.; Zhou, S.; Li, Y.; Du, Z. The effect of electronegative difference on the electronic structure and visible light photocatalytic activity of N-doped anatase TiO2 by first-principles calculations. Appl. Phys. Lett. 2011, 98, 162107. [Google Scholar] [CrossRef]
- Yang, K.; Dai, Y.; Huang, B. Study of the Nitrogen Concentration Influence on N-Doped TiO2 Anatase from First-Principles Calculations. J. Phys. Chem. C 2007, 111, 12086–12090. [Google Scholar] [CrossRef]
- Esmat, M.; El-Hosainy, H.; Tahawy, R.; Jevasuwan, W.; Tsunoji, N.; Fukata, N.; Ide, Y. Nitrogen doping-mediated oxygen vacancies enhancing co-catalyst-free solar photocatalytic H2 production activity in anatase TiO2 nanosheet assembly. Appl. Catal. B Environ. 2021, 285, 119755. [Google Scholar] [CrossRef]
- Mi, L.; Xu, P.; Shen, H.; Wang, P.-N.; Shen, W. First-principles calculation of N:H codoping effect on energy gap narrowing of TiO2. Appl. Phys. Lett. 2007, 90, 171909. [Google Scholar] [CrossRef]
- Xu, P.; Mi, L.; Wang, P.-N. Improved optical response for N-doped anatase TiO2 films prepared by pulsed laser deposition in N2/NH3/O2 mixture. J. Cryst. Growth 2006, 289, 433–439. [Google Scholar] [CrossRef]
- Mi, L.; Zhang, Y.; Wang, P.-N. First-principles study of the hydrogen doping influence on the geometric and electronic structures of N-doped TiO2. Chem. Phys. Lett. 2008, 458, 341–345. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, Y.-W.; Shenoy, V.B.; Gao, H. Effects of H-, N-, and (H, N)-Doping on the Photocatalytic Activity of TiO2. J. Phys. Chem. C 2011, 115, 12224–12231. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Guo, D.; Zhang, Y. Band gap engineering of compensated (N, H) and (C, 2H) codoped anatase TiO2: A first-principles calculation. Chem. Phys. Lett. 2012, 539–540, 175–179. [Google Scholar] [CrossRef]
- Russo, S.P.; Grey, I.E.; Wilson, N.C. Nitrogen/Hydrogen Codoping of Anatase: A DFT Study. J. Phys. Chem. C 2008, 112, 7653–7664. [Google Scholar] [CrossRef]
- Hongzhiwei Technology, D.S. Version 2021A, China. Available online: https://iresearch.net.cn/cloudSoftware (accessed on 1 November 2021).
- Segall, M.D.; Lindan, P.J.D.; Probert, M.; Pickard, C.J.; Hasnip, P.; Clark, S.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Shi, A.J.; Li, B.X.; Wan, C.R.; Leng, D.C.; Lei, E.Y. Hybrid density functional studies of C-anion-doped anatase TiO2. Chem. Phys. Lett. 2016, 650, 19–28. [Google Scholar] [CrossRef]
- Bagwasi, S.; Tian, B.; Zhang, J.; Nasir, M. Synthesis, characterization and application of bismuth and boron Co-doped TiO2: A visible light active photocatalyst. Chem. Eng. J. 2013, 217, 108–118. [Google Scholar] [CrossRef]
- Ibrahim, H.H.; Mohamed, A.A.; Ibrahim, I.A. Electronic and optical properties of mono and co-doped anatase TiO2: First principles calculations. Mater. Chem. Phys. 2020, 252, 123285. [Google Scholar] [CrossRef]
- Doustkhah, E.; Assadi, M.H.N.; Komaguchi, K.; Tsunoji, N.; Esmat, M.; Fukata, N.; Tomita, O.; Abe, R.; Ohtani, B.; Ide, Y. In situ Blue titania via band shape engineering for exceptional solar H2 production in rutile TiO2. Appl. Catal. B Environ. 2021, 297, 120380. [Google Scholar] [CrossRef]
- Zhu, H.X.; Liu, J.-M. First principles calculations of electronic and optical properties of Mo and C co-doped anatase TiO2. Appl. Phys. A 2014, 117, 831–839. [Google Scholar] [CrossRef]



| Eform | Configuration | ||
|---|---|---|---|
| N-TiO2 | Sample Ⅰ | Sample Ⅱ | |
| Eform (Ti-rich) | 0.023 | −2.175 | −2.454 |
| Eform (O-rich) | 5.002 | 2.805 | 7.504 |
| System | Ti | O | Nu | Nh | H |
|---|---|---|---|---|---|
| N-TiO2 | +1.33 (4) +1.29 (2) +1.32 (1) +1.31 (1) | −0.67 (7) −0.66 (7) −0.68 (1) | −0.58 | - | - |
| sample Ⅰ | +1.33 (5) +1.32 (3) | −0.67 (9) −0.66 (6) | - | −0.86 | +0.26 |
| sample Ⅱ | +1.33 (2) +1.32 (1) +1.30 (2) +1.31 (1) +1.29 (1) +1.28 (1) | −0.67 (2) −0.66 (8) −0.68 (3) −0.65 (1) | −0.59 | −0.84 | +0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Tang, X.; Qin, X.; Tang, Z.; Yuan, D.; Ju, L. Applying Hydrogenation to Stabilize N-TiO2 and Enhance Its Visible Light Photocatalytic Activity. Catalysts 2022, 12, 178. https://doi.org/10.3390/catal12020178
Zhao D, Tang X, Qin X, Tang Z, Yuan D, Ju L. Applying Hydrogenation to Stabilize N-TiO2 and Enhance Its Visible Light Photocatalytic Activity. Catalysts. 2022; 12(2):178. https://doi.org/10.3390/catal12020178
Chicago/Turabian StyleZhao, Dongqiu, Xiao Tang, Xuming Qin, Zhenjie Tang, Di Yuan, and Lin Ju. 2022. "Applying Hydrogenation to Stabilize N-TiO2 and Enhance Its Visible Light Photocatalytic Activity" Catalysts 12, no. 2: 178. https://doi.org/10.3390/catal12020178






