Copper-Containing Catalysts for Azide–Alkyne Cycloaddition in Supercritical CO2
Abstract
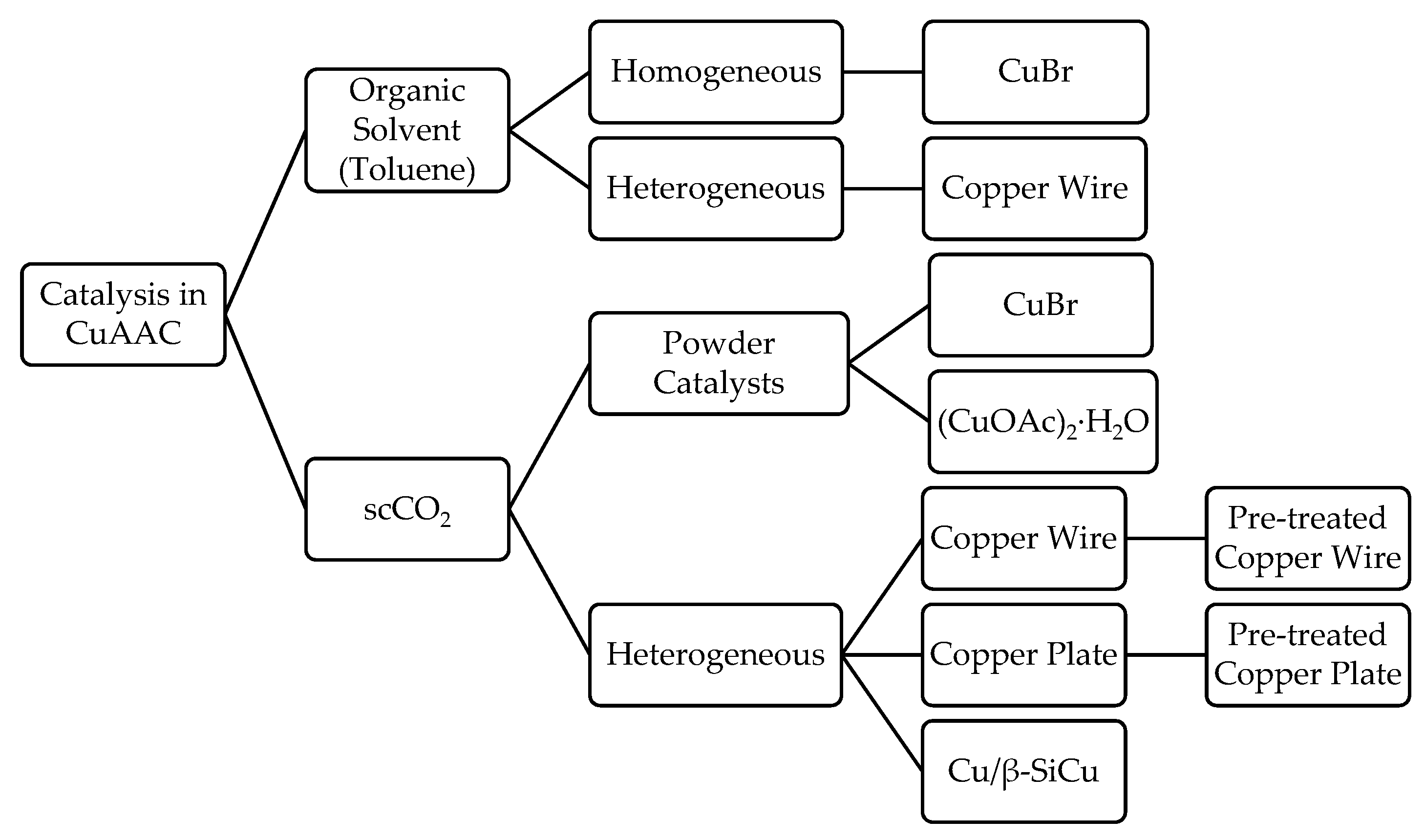
:1. Introduction
2. Results and Discussion
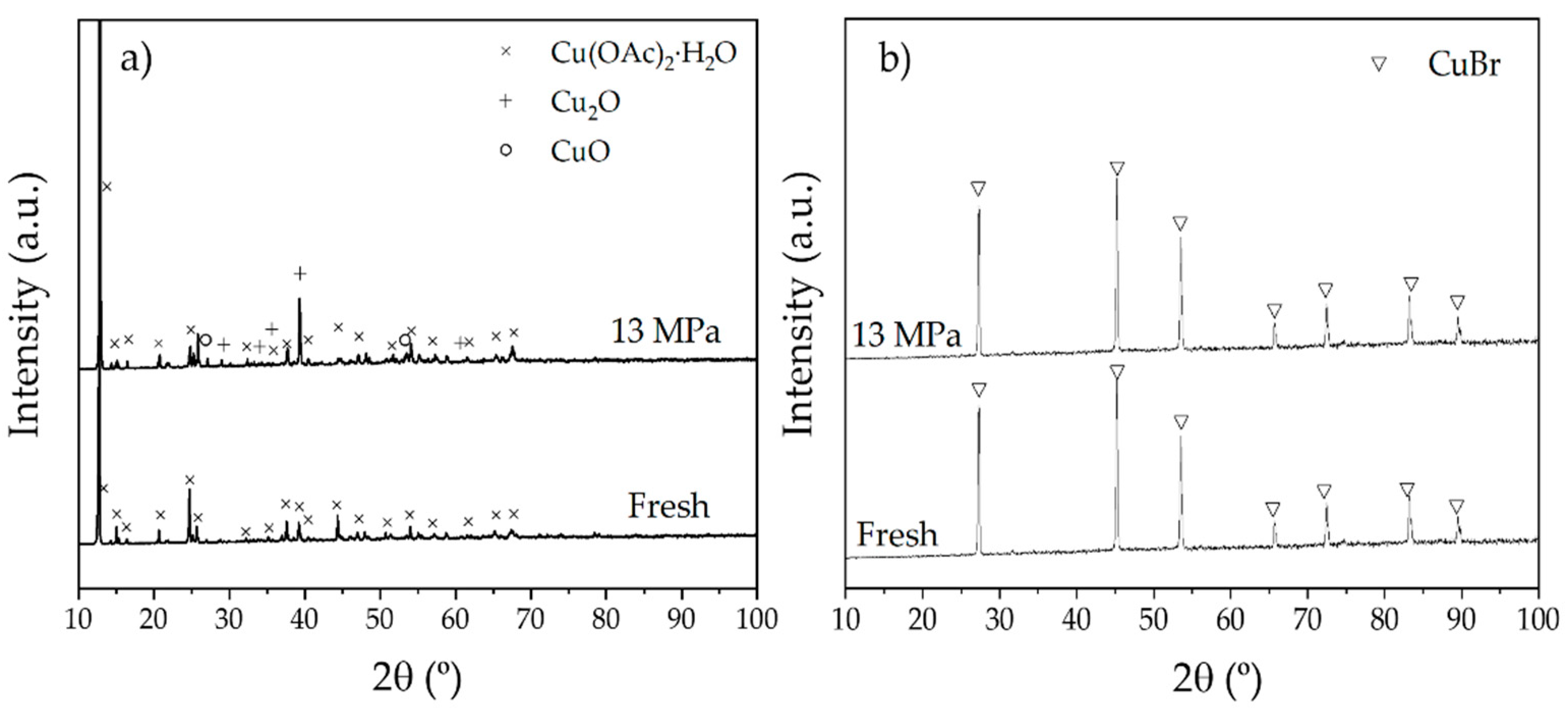
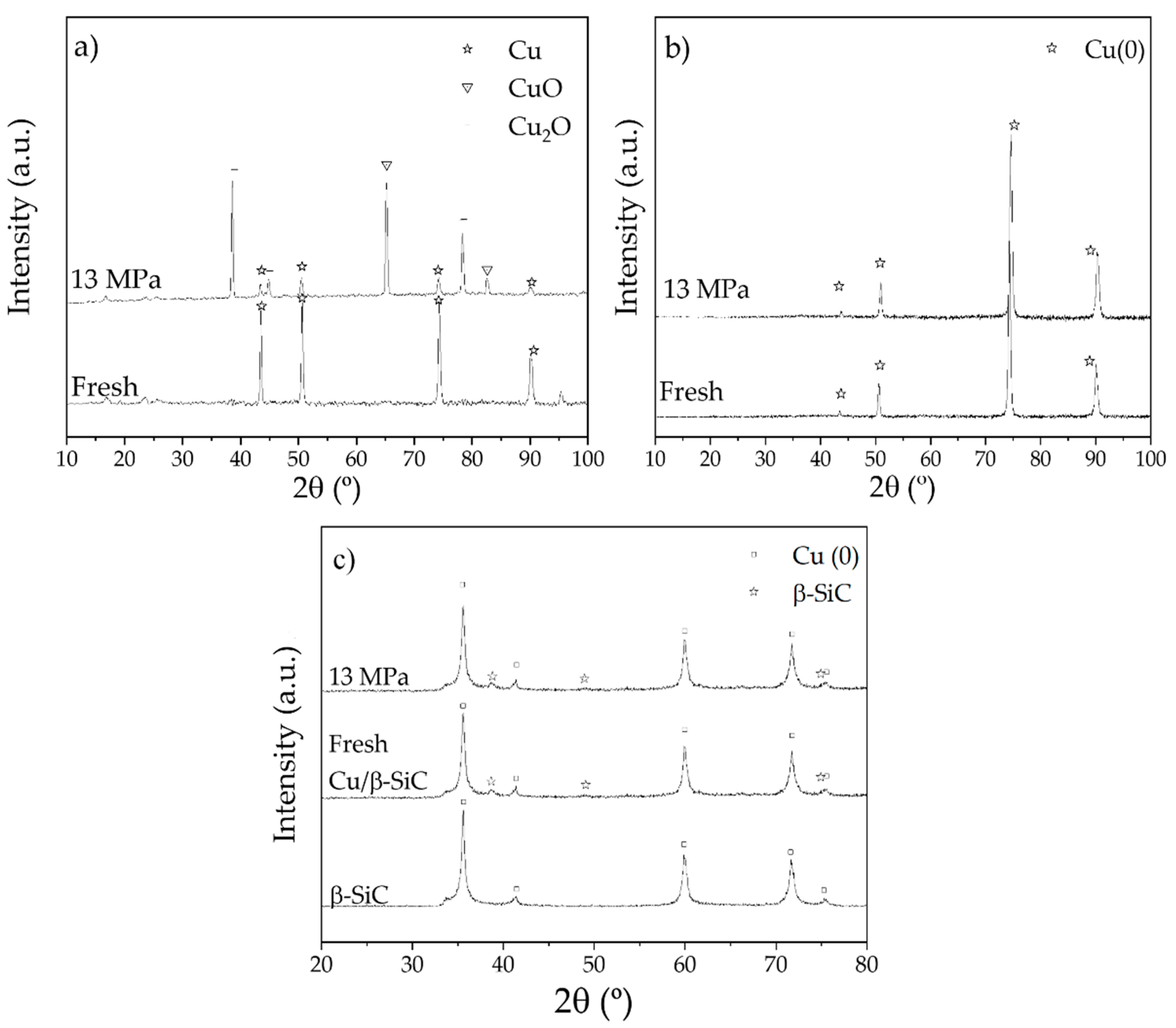
2.1. Behaviour of Copper Catalyst in scCO2
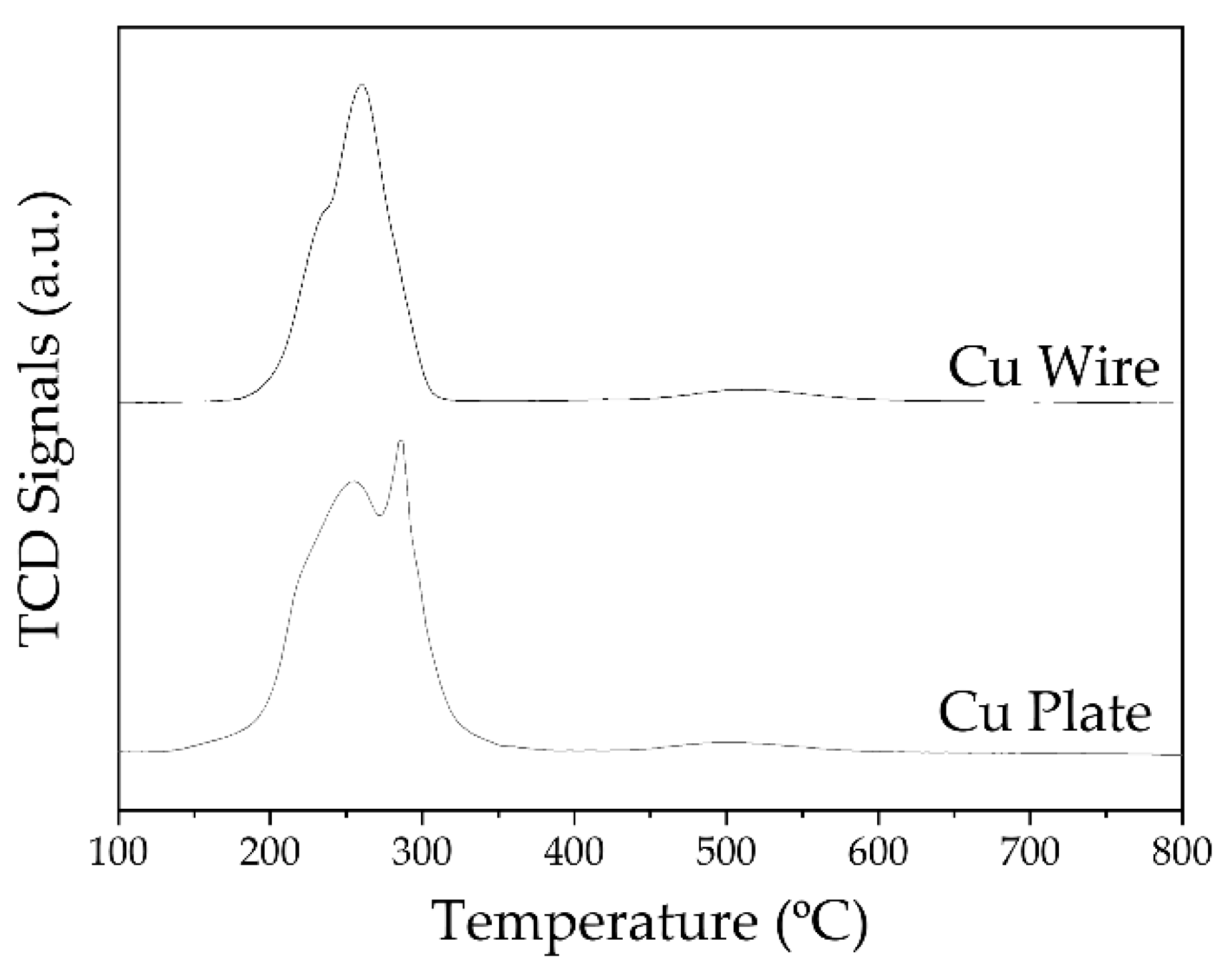
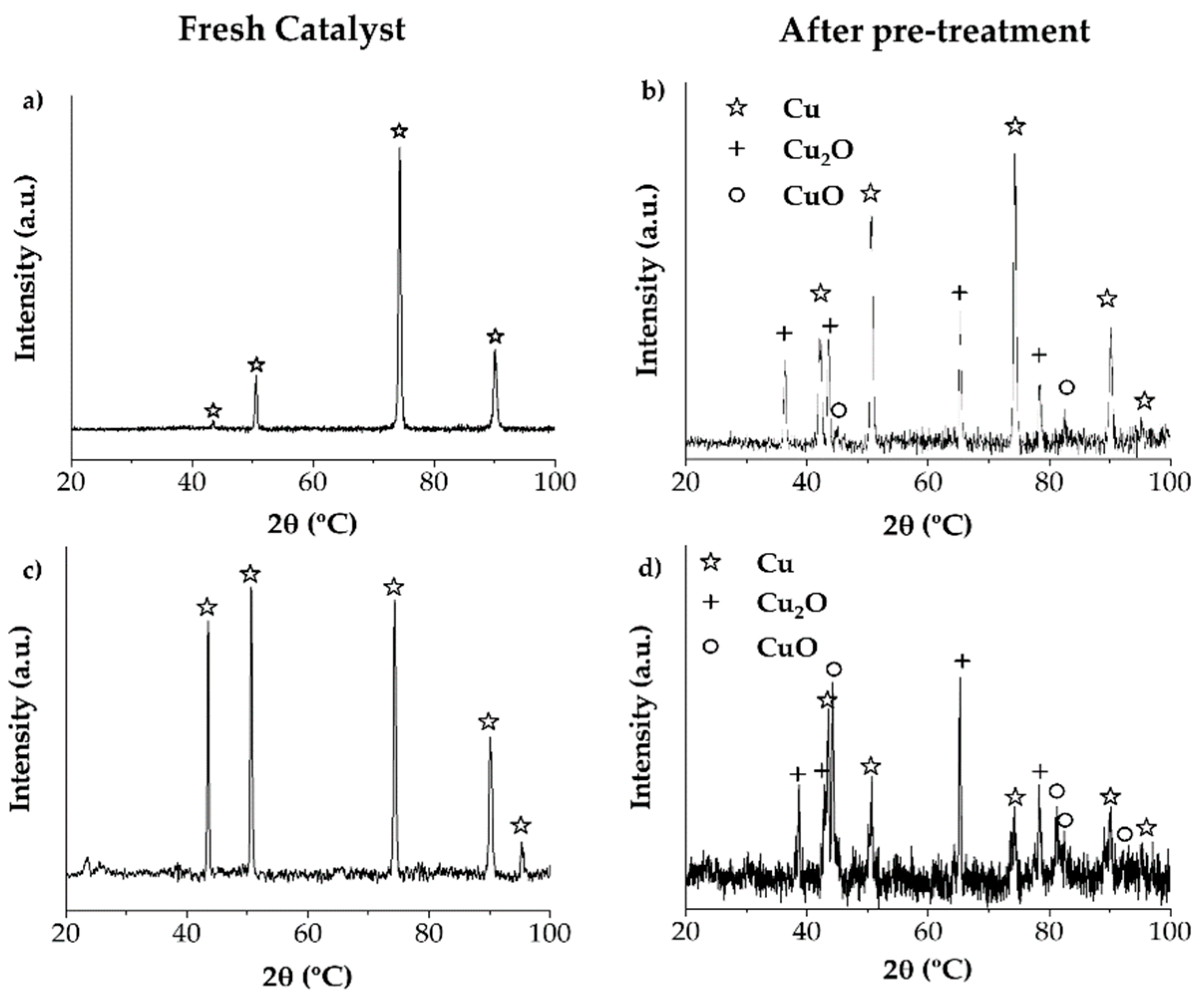
2.2. Pre-Treament of Cu Wire and the Cu Plate
2.3. CuAAC Model Reaction with Different Copper Structures
2.4. Reusability of Heterogeneous Copper Structures in scCO2
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Click Product in scCO2
3.3. Synthesis of Click Product at Atmospheric Pressure
3.4. Click Product Characterization
3.5. Synthesis Cu/β-SiC
3.6. Pre-Treatment of Cu Wire and Cu Plate
3.7. Catalyst Characterization
3.8. Purification Steps
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huisgen, R.; Mloston, G.; Langhals, E. The first two-step 1,3-dipolar cycloadditions: Non-stereospecificity. J. Am. Chem. Soc. 1986, 108, 6401–6402. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Meng, G.; Guo, T.; Ma, T.; Zhang, J.; Shen, Y.; Sharpless, K.B.; Dong, J. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature 2019, 574, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Van Dijk, M.; Rijkers, D.T.S.; Liskamp, R.M.J.; van Nostrum, C.F.; Hennink, W.E. Synthesis and Applications of Biomedical and Pharmaceutical Polymers via Click Chemistry Methodologies. Bioconjug. Chem. 2009, 20, 2001–2016. [Google Scholar] [CrossRef]
- Crescenzi, V.; Cornelio, L.; di Meo, C.; Nardecchia, S.; Lamanna, R. Novel Hydrogels via Click Chemistry: Synthesis and Potential Biomedical Applications. Biomacromolecules 2007, 8, 1844–1850. [Google Scholar] [CrossRef]
- Nwe, K.; Brechbiel, M. Growing Applications of “Click Chemistry” for Bioconjugation in Contemporary Biomedical Research. Cancer Biother. Radiopharm. 2009, 24, 289–302. [Google Scholar] [CrossRef]
- Sokolova, N.V.; Nenajdenko, V.G. Recent advances in the Cu (i)-catalyzed azide–alkyne cycloaddition: Focus on functionally substituted azides and alkynes. RSC Adv. 2013, 3, 16212–16242. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R. ‘Click’ Chemistry in Polymer and Material Science: An Update. Macromol. Rapid Commun. 2008, 29, 952–981. [Google Scholar] [CrossRef]
- Ellanki, A.R.; Islam, A.; Rama, V.S.; Pulipati, R.P.; Rambabu, D.; Krishna, G.R.; Reddy, C.M.; Mukkanti, K.; Vanaja, G.R.; Kalle, A.M.; et al. Solvent effect on copper-catalyzed azide-alkyne cycloaddition (CuAAC): Synthesis of novel triazolyl substituted quinolines as potential anticancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 3455–3459. [Google Scholar] [CrossRef] [PubMed]
- Kazarian, S. Polymer Processing with Supercritical Fluids. Polym. Sci. 2000, 42, 78–101. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Su, J.-H.; Manivannan, G.; Lee, P.; Sawan, S.; Spall, W. Interaction of supercritical carbon dioxide with polymers. I. Crystalline polymers. J. Appl. Polym. Sci. 1996, 59, 695–705. [Google Scholar] [CrossRef]
- Prajapati, D.; Gohain, M. Recent Advances in the Application of Supercritical Fluids for Carbon—Carbon Bond Formation in Organic Synthesis. Tetrahedron 2004, 60, 815–833. [Google Scholar] [CrossRef]
- Oakes, R.S.; Clifford, A.A.; Rayner, C.M. The use of supercritical fluids in synthetic organic chemistry. J. Chem. Soc. Perkin Trans. 1 2001, 9, 917–941. [Google Scholar] [CrossRef]
- Grignard, B.; Schmeits, S.; Riva, R.; Detrembleur, C.; Lecomte, P.; Jérôme, C. First example of “click” copper(i) catalyzed azide-alkyne cycloaddition in supercritical carbon dioxide: Application to the functionalization of aliphatic polyesters. Green Chem. 2009, 11, 1525–1529. [Google Scholar] [CrossRef]
- Grignard, B.; Calberg, C.; Jerome, C.; Detrembleur, C. “One-pot” dispersion ATRP and alkyne-azide Huisgen’s 1,3-dipolar cycloaddition in supercritical carbon dioxide: Towards the formation of functional microspheres. J. Supercrit. Fluids 2010, 53, 151–155. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Ren, B.; Jiang, Y.; Hu, Z. Cu(OAc)2·H2O—An efficient catalyst for Huisgen-click reaction in supercritical carbon dioxide. Tetrahedron Lett. 2015, 56, 2472–2475. [Google Scholar] [CrossRef]
- Gracia, E.; García, M.T.; Borreguero, A.M.; de Lucas, A.; Gracia, I.; Rodríguez, J.F. Functionalization and optimization of PLA with coumarin via click chemistry in supercritical CO2. J. CO2 Util. 2017, 20, 20–26. [Google Scholar] [CrossRef]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-catalysed azide-alkyne cycloadditions (CuAAC): An update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef]
- Chassaing, S.; Bénéteau, V.; Pale, P. When CuAAC ’Click Chemistry’ goes heterogeneous. Catal. Sci. Technol. 2016, 6, 923–957. [Google Scholar] [CrossRef]
- Neumann, S.; Biewend, M.; Rana, S.; Binder, W.H. The CuAAC: Principles, Homogeneous and Heterogeneous Catalysts, and Novel Developments and Applications. Macromol. Rapid Commun. 2020, 41, 190–359. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Chen, J.; Fan, Y.; Zhou, H.; Guo, K.; Fang, Z.; Xie, L.; Wang, L.; Wang, Y. A promoted copper-catalysed Azide-alkyne cycloaddition (CuAAC) for broad spectrum peptide-engineered implants. Chem. Eng. J. 2022, 427, 130918. [Google Scholar] [CrossRef]
- Meldal, M.; Diness, F. Recent Fascinating Aspects of the CuAAC Click Reaction. Trends Chem. 2020, 2, 569–584. [Google Scholar] [CrossRef]
- Wang, Q.; Chan, T.R.; Hilgraf, R.; Fokin, V.V.; Sharpless, K.B.; Finn, M.G. Bioconjugation by Copper(I)-Catalyzed Azide-Alkyne [3 + 2] Cycloaddition. J. Am. Chem. Soc. 2003, 125, 3192–3193. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.R.; Hilgraf, R.; Sharpless, K.B.; Fokin, V.V. Polytriazoles as Copper(I)-Stabilizing Ligands in Catalysis. Org. Lett. 2004, 6, 2853–2855. [Google Scholar] [CrossRef]
- Golas, P.L.; Tsarevsky, N.V.; Sumerlin, B.S.; Matyjaszewski, K. Catalyst Performance in “Click” Coupling Reactions of Polymers Prepared by ATRP: Ligand and Metal Effects. Macromolecules 2006, 39, 6451–6457. [Google Scholar] [CrossRef]
- Fauché, K.; Cisnetti, F. Magnetic Fe3O4 nanoparticles bearing CuI-NHC complexes by an “auto-click” strategy. Inorg. Chim. Acta 2021, 520, 120312. [Google Scholar] [CrossRef]
- Chassaing, S.; Kumarraja, M.; Sido, A.S.S.; Pale, P.; Sommer, J. Click Chemistry in CuI-zeolites: The Huisgen [3 + 2]-Cycloaddition. Org. Lett. 2007, 9, 883–886. [Google Scholar] [CrossRef]
- Parveen, S.; Gurjaspreet, G.K.; Jandeep, S.; Harminder, S. Robust and Versatile Cu(I) metal frameworks as potential catalysts for azide-alkyne cycloaddition reactions. Rev. Mol. Catal. 2021, 504, 111432. [Google Scholar] [CrossRef]
- Gurjaspreet, S.; Jasbhinder, S.; Akshpreet, S.; Jandeep, S.; Manoj, K.; Kshitiz, G.; Sanjay, C. Synthesis, characterization and antibacterial studies of schiff based 1,2,3-triazole bridged silatranes. J. Organomet. Chem. 2018, 871, 21–27. [Google Scholar] [CrossRef]
- Jiang, Y.; He, X.; Zhang, W.; Li, X.; Guo, N.; Zhao, Y.; Xu, G.; Li, W. Metallic copper wire: A simple, clear and reusable catalyst for the CuAAC reaction in supercritical carbon dioxide. RSC Adv. 2015, 5, 73340–73345. [Google Scholar] [CrossRef]
- Gracia, E.; García, M.; De Lucas, A.; Rodríguez, J.; Gracia, I. Copper wire as a clean and efficient catalyst for click chemistry in supercritical CO2. Catal. Today 2018, 346, 65–68. [Google Scholar] [CrossRef]
- Behl, G.; Sikka, M.; Chhikara, A.; Chopra, M. PEG-coumarin based biocompatible self-assembled fluorescent nanoaggregates synthesized via click reactions and studies of aggregation behavior. J. Colloid Interface Sci. 2014, 416, 151–160. [Google Scholar] [CrossRef]
- López, S.; Gracia, I.; García, M.T.; Rodríguez, J.F.; Ramos, M.J. Synthesis and Operating Optimization of the PEG Conjugate via CuAAC in scCO2. ACS Omega 2021, 6, 6163–6171. [Google Scholar] [CrossRef]
- Alterovitz, S.A.; Woollam, J.A. Cubic Silicon Carbide (β-SiC). In Handbook of Optical Constants of Solids; Palik, E.D., Ed.; Academic Press: Burlington, MA, USA, 1997; pp. 705–707. [Google Scholar] [CrossRef]
- García-Vargas, J.M.; Valverde, J.L.; Díez, J.; Sánchez, P.; Dorado, F. Preparation of Ni-Mg/β-SiC catalysts for the methane tri-reforming: Effect of the order of metal impregnation. Appl. Catal. B Environ. 2015, 164, 316–323. [Google Scholar] [CrossRef]
- Nguyen, D.L.; Leroi, P.; Ledoux, M.J.; Pham-Huu, C. Influence of the oxygen pretreatment on the CO2 reforming of methane on Ni/β-SiC catalyst. Catal. Today 2009, 141, 393–396. [Google Scholar] [CrossRef]
- de Meester, P.; Fletcher, S.R.; Skapski, A.C. Refined crystal structure of tetra-mu-acetato-bisaquodicopper(II). J. Chem. Soc. Dalton Trans. 1973, 1973, 2575–2578. [Google Scholar] [CrossRef]
- Herbert, M.; Montilla, F.; Galindo, A. Supercritical carbon dioxide, a new medium for aerobic alcohol oxidations catalysed by copper-TEMPO. Dalton Trans. 2010, 39, 900–907. [Google Scholar] [CrossRef]
- Mathew, K.; Zheng, C.; Winston, D.; Chen, C.; Dozier, A.; Rehr, J.J.; Ong, S.P.; Persson, K.A. High-throughput computational X-ray absorption spectroscopy. Sci. Data 2018, 5, 180151. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, J.J.; Li, J.J. Cubic assembly composed of CuBr nanoparticles. J. Cryst. Growth 2004, 267, 283–287. [Google Scholar] [CrossRef]
- Sahle-Demessie, E.; Gonzalez, M.A.; Enriquez, J.; Zhao, Q. Selective Oxidation in Supercritical Carbon Dioxide Using Clean Oxidants. Ind. Eng. Chem. Res. 2000, 39, 4858–4864. [Google Scholar] [CrossRef]
- Meldal, M. Polymer “Clicking” by CuAAC Reactions. Macromol. Rapid Commun. 2008, 29, 1016–1051. [Google Scholar] [CrossRef]
- ICH Q3D Elemental Impurities|European Medicines Agency. Available online: https://www.ema.europa.eu/en/ich-q3d-elemental-impurities (accessed on 18 November 2021).
- Díaz, D.; Punna, S.; Holzer, P.; McPherson, A.K.; Sharpless, K.B.; Fokin, V.V.; Finn, M.G. Click chemistry in materials synthesis. 1. Adhesive polymers from copper-catalyzed azide-alkyne cycloaddition. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 4392–4403. [Google Scholar] [CrossRef]
- Bell, C.A.; Jia, Z.; Perrier, S.; Monteiro, M.J. Modulating catalytic activity of polymer-based cuAAC “click” reactions. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4539–4548. [Google Scholar] [CrossRef]
- López, S.; Ramos, M.J.; García-Vargas, J.M.; García, M.T.; Rodríguez, J.F.; Gracia, I. Carbon dioxide sorption and melting behaviour of mPEG-alkyne. J. Supercrit. Fluids 2021, 171, 105182. [Google Scholar] [CrossRef]
- Oh, C.; Yi, I.; Park, K.P. Nucleophilic vinylic substitution of halocoumarins and halo-l,4-naphthoquinones with morpholine. J. Heterocycl. Chem. 1994, 31, 841–844. [Google Scholar] [CrossRef]
- Sivakumar, K.; Xie, F.; Cash, B.M.; Long, S.; Barnhill, H.N.; Wang, Q. A Fluorogenic 1,3-Dipolar Cycloaddition Reaction of 3-Azidocoumarins and Acetylenes. Org. Lett. 2004, 6, 4603–4606. [Google Scholar] [CrossRef]
- Kusanur, R.A.; Kulkarni, M.V.; Kulkarni, G.M.; Nayak, S.K.; Row, T.N.G.; Ganesan, K.; Sun, C.-M. Unusual anisotropic effects from 1,3-dipolar cycloadducts of 4-azidomethyl coumarins. J. Heterocycl. Chem. 2010, 47, 91–97. [Google Scholar] [CrossRef]
- Chaurasia, C.S.; Kauffman, J.M. Synthesis and fluorescent properties of a new photostable thiol reagent “BACM”. J. Heterocycl. Chem. 1990, 27, 727–733. [Google Scholar] [CrossRef]



| Entry | Copper Source | PMDTA (mmol) | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | CuBr | - | 6 | 0 |
| 2 | CuBr | - | 12 | 0 |
| 3 | CuBr | - | 24 | 0 |
| 4 | CuBr | - | 48 | 0 |
| 5 | CuBr | 0.05 | 6 | 33.6 |
| 6 | CuBr | 0.05 | 12 | 34.2 |
| 7 | CuBr | 0.05 | 24 | 43.18 |
| 8 | CuBr | 0.05 | 48 | 63.7 |
| 9 | CuBr | 0.025 | 24 | 11.48 |
| 10 | Cu Wire | - | 6 | 0 |
| 11 | Cu Wire | - | 12 | 0 |
| 12 | Cu Wire | - | 24 | 0 |
| 13 | Cu Wire | - | 48 | 0 |
| 14 | Cu Wire | 0.05 | 6 | 32.55 |
| 15 | Cu Wire | 0.05 | 12 | 42.91 |
| 16 | Cu Wire | 0.05 | 24 | 78.76 |
| 17 | Cu Wire | 0.05 | 48 | 91.92 |
| 18 | Cu Wire | 0.025 | 24 | 33.63 |
| Entry | Copper Source | C/A Molar Ratio a | Catalyst Loading (mg) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | CuBr | 1 | 7.17 | 24 | 0 |
| 2 | CuBr | 1 | 7.17 | 48 | 0 |
| 3 | Cu(CH3COO)2·H2O | 0.5 | 5 | 6 | 11.74 |
| 4 | Cu(CH3COO)2·H2O | 0.5 | 5 | 12 | 14.20 |
| 5 | Cu(CH3COO)2·H2O | 0.5 | 5 | 24 | 82.32 |
| 6 | Cu(CH3COO)2·H2O | 0.5 | 5 | 48 | 90.12 |
| 7 | Cu Wire | 1 | 3.177 | 24 | 11.60 |
| 8 | Cu Wire | 10 | 31.77 | 24 | 84.54 |
| 9 | Cu Wire | 100 | 317.73 | 6 | 18.26 |
| 10 | Cu Wire | 100 | 317.73 | 12 | 56.78 |
| 11 | Cu Wire | 100 | 317.73 | 24 | 95.96 |
| 12 | Cu Wire | 100 | 317.73 | 48 | 97.80 |
| 13 | Cu Plate | 10 | 31.77 | 24 | 15.70 |
| 14 | Cu Plate | 100 | 317.73 | 6 | 0 |
| 15 | Cu Plate | 100 | 317.73 | 12 | 13.20 |
| 16 | Cu Plate | 100 | 317.73 | 24 | 23.07 |
| 17 | Cu Plate | 100 | 317.73 | 48 | 32.66 |
| 18 | Cu/β-SiC | 0.1 | 0.32 | 24 | 16.15 |
| 19 | Cu/β-SiC | 0.5 | 1.59 | 6 | 35.20 |
| 20 | Cu/β-SiC | 0.5 | 1.59 | 12 | 82.30 |
| 21 | Cu/β-SiC | 0.5 | 1.59 | 24 | 82.59 |
| 22 | Cu/β-SiC | 0.5 | 1.59 | 48 | 90.90 |
| 23 | Cu/β-SiC | 1 | 3.2 | 24 | 91.94 |
| 24 | Pre-treated Cu Wire | 100 | 317.73 | 24 | 97.14 |
| 25 | Pre-treated Cu Plate | 100 | 317.73 | 24 | 89.70 |
| Cycle | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Copper Wire | Yield (%) | 95.9 | 94.7 | 95.1 | 95.4 | 94.2 |
| Copper Leaching (%) | - | 1.2 | 6 | 5 | 3.4 | |
| Cu/β-SiC | Yield (%) | 82.9 | 79.6 | - | - | - |
| Copper Leaching (%) | - | 2 | ||||
| Pre-treated CW | Yield (%) | 97.1 | 86.6 | 85.2 | 25.1 | - |
| Copper Leaching (%) | - | 2.4 | 5.8 | 7 | ||
| Pre-treated CP | Yield (%) | 89.7 | 90.1 | 73.3 | 13.4 | - |
| Copper Leaching (%) | - | 7.9 | 12.1 | 5.6 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, S.; García-Vargas, J.M.; García, M.T.; Rodríguez, J.F.; Gracia, I.; Ramos, M.J. Copper-Containing Catalysts for Azide–Alkyne Cycloaddition in Supercritical CO2. Catalysts 2022, 12, 194. https://doi.org/10.3390/catal12020194
López S, García-Vargas JM, García MT, Rodríguez JF, Gracia I, Ramos MJ. Copper-Containing Catalysts for Azide–Alkyne Cycloaddition in Supercritical CO2. Catalysts. 2022; 12(2):194. https://doi.org/10.3390/catal12020194
Chicago/Turabian StyleLópez, Sonia, Jesús Manuel García-Vargas, María Teresa García, Juan Francisco Rodríguez, Ignacio Gracia, and María Jesús Ramos. 2022. "Copper-Containing Catalysts for Azide–Alkyne Cycloaddition in Supercritical CO2" Catalysts 12, no. 2: 194. https://doi.org/10.3390/catal12020194







