Abstract
Compared to the Claus process, selective H2S catalytic oxidation to sulfur is a promising reaction, as it is not subject to thermodynamic limitations and could theoretically achieve ~100% H2S conversion to sulfur. In this study, we investigated the effects of Co and Fe co-doping in ABO3 perovskite on H2S selective catalytic oxidation. A series of LaFexCo1−xO3 (x = 0, 0.2, 0.4, 0.6, 0.8, 1.0) perovskites were synthesized by the sol-gel method. Compared to LaFeO3 and LaCoO3, co-doped LaFexCo1−xO3 significantly improved the H2S conversion and sulfur selectivity at a lower reaction temperature. Nearly 100% sulfur yield was achieved on LaFe0.4Co0.6O3 under 220 °C with exceptional catalyst stability (above 95% sulfur yield after 77 h). The catalysts were characterized by XRD, BET, FTIR, XPS, and H2-TPR. The characterization results showed that the structure of LaFexCo1−xO3 changed from the rhombic phase of LaCoO3 to the cubic phase of LaFeO3 with Fe substitution. Doping with appropriate iron (x = 0.4) facilitates the reduction of Co ions in the catalyst, thereby promoting the H2S selective oxidation. This study demonstrates a promising approach for low-temperature H2S combustion with ~100% sulfur yield.
1. Introduction
Natural gas processing/utilizing processes, petroleum refining processes, and coal chemistry produce H2S-containing waste gas [1,2,3].The removal of H2S from industry waste gas is crucial as H2S is a toxic and corrosive gas, leading to severe pollution and equipment/pipeline corrosion [4]. Numerous efforts have been conducted to remove H2S and the most widely used technology is called the Claus process, which recovers elemental sulfur from H2S-containing gas [5,6]. However, due to thermodynamic limitations, the Claus process cannot achieve 100% H2S conversion, leaving 2–5% unconverted H2S gas remaining after the treatment [7]. Selective H2S catalytic oxidation could be added as a secondary process after the Claus process apparatus to oxidize the residual, low-concentration H2S-containing gas to elemental sulfur with increased overall sulfur yield. The design of a catalyst with high H2S conversion and high sulfur selectivity is the key to the selective catalytic oxidation. So far, H2S selective oxidation catalysts mainly include active carbon (AC)-based catalysts [8], carbon nanotube-based catalysts [9], clay-supported catalysts [10], and metal oxide-based catalysts [11]. Nevertheless, most of these H2S selective oxidation catalysts face challenge on the needs of excessive oxygen feed, which is produced from economically expensive air separation units. For instance, Fang et al. [8] found that the Mn/AC catalyst can achieve optimal catalytic activity at 180 °C, but it needs excess amounts of oxygen to participate in the H2S oxidation process. Ba et al. [9] found that the H2S conversion over nitrogen-doped carbon nanotubes dropped from 100% to 92% as the O2/H2S ratio decreased from 2.5 to 0.6. Soriano et al. [11] observed that VNa-0.1 had high sulfur selectivity at 200 °C under the H2S/air/He molar ratio of 1.2/5.0/93.8, with an excess of O2. Moreover, the durations of the catalyst stability in the literature were usually not long, less than 20 h [12,13,14]. Thus, a catalyst with remarkable catalytic activity, excellent selectivity, outstanding stability, and desired stoichiometric H2S/O2 operating conditions is urgently needed.
Perovskite oxides such as ABO3 have been extensively investigated. The typical structure of perovskite oxides is cubic, with one A-site cation coordinated by 12 anions and eight B-site cations (belonging to 1/8 to one specific unit cell) coordinated by six anions [15,16]. For (distorted) perovskites with lower symmetry, the first shell coordination numbers are smaller. It is well-recognized that the tuning of A and B site cations can affect the catalytic activity and stability of perovskite oxides. Moreover, structural modifications related to the generation of the oxygen vacancies and/or changes in the valence states of the original cations can be achieved by the partial substitution of the ions at A site or B site [17,18,19]. Therefore, the flexibility and chemical versatility of ABO3 perovskite oxides can be used to design highly active, selective, and stable catalysts [20]. Among these, LaCoO3 and LaFeO3 stand out as redox catalysts with satisfactory catalytic performance reported for CO oxidation [21] and NO oxidation [22]. Moreover, Yang et al. [23] found that LaCoO3 showed high H2S conversion and high selectivity to sulfur (98.2%) at 260 °C. Zhang et al. [24] found that LaFeO3 exhibited outstanding selectivity (100%) under relatively low reaction temperature and various H2S/O2 ratios. It is desired to obtain catalysts with ~100% H2S conversion and ~100% sulfur selectivity, and the influence of co-doping Fe and Co into the B-site of perovskite could be further explored.
This work investigates the influence of Fe substitution on LaCoO3 in H2S selective oxidation. LaFexCo1−xO3 (x = 0, 0.2, 0.4, 0.6, 0.8, 1) perovskite oxides were synthesized by the sol-gel method and their catalytic behaviors in the H2S selective oxidation were investigated. It was observed that the incorporation of iron enhanced the catalytic activity of the LaFexCo1−xO3 catalysts. In addition, the crystal structure and redox ability were comprehensively investigated by various characterization techniques such as XRD, BET, FTIR, XPS, and H2-TPR. On the basis of these results, the catalytic and deactivation mechanisms of H2S selective oxidation over LaFexCo1−xO3 are discussed.
2. Experiment
2.1. Catalyst Preparation
Powder samples of LaFexCo1−xO3 (x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0) perovskite oxides were synthesized by the sol-gel method using citric acid as a complexing agent. The starting materials were La(NO3)3∙6H2O (AR, Macklin, Shanghai, China), Fe(NO3)3∙9H2O (AR, Macklin, Shanghai, China), and Co(NO3)2∙6H2O (AR, Macklin, Shanghai, China). The required amounts of nitrate samples were first dissolved in distilled water. Citric acid (AR, Aladdin, Shanghai, China) was added so that the number of moles of citric acid was equal to the number of moles of total metal cations. The obtained solution was evaporated at 80 °C and then PEG-20000 (AR, Macklin, Shanghai, China) was added into the solution. Stirring was continued until a viscous gel was formed. Finally, the gel was dried in an oven at 105 °C overnight and calcined at 650 °C for 5 h in a furnace to obtain the catalyst samples.
2.2. Catalyst Characterization
XRD (X-ray diffraction) patterns were determined using the X’Pert PRO powder diffraction system (Empyrean, San Jose, California, USA) with Cu Kα radiation (λ = 0.15418 nm, 40 kV/40 mA) in the 2θ range of 10–80°.
BET (Brunauer–Emmett–Teller) surface areas and textural properties of the catalysts were determined by nitrogen adsorption–desorption isotherms using an ASAP-2020 apparatus (Micromeritics, Norcross, GA, USA). The adsorption process was carried out at liquid nitrogen temperature. The BET method was adopted to calculate the specific surface area.
FTIR (Fourier transform infrared) spectra were measured using a Nicolet Model iS-50 instrument (Thermo Scientific, Waltham, MA, USA) in the region from 400 to 4000 cm−1. The sample was taken in pellet form in the KBr matrix for measurement.
X-ray photoelectron spectroscopy (XPS) was performed on an ESCALAB 250Xi (Thermo Scientific, USA) with Al Kα radiation as the radiation source at 300 W. The spectra of C 1s, O 1s, La 3d, Co 2p, and Fe 2p were recorded. The binding energies were calibrated using the C 1s peak of contaminant carbon (B.E. = 284.8 eV) as the standard.
H2-TPR (H2 temperature-programmed reduction) experiment was measured on an Auto Chem 2920 chemical adsorption instrument (Micromeritics, Norcross, GA, USA). The TPR profiles were obtained by passing a 10% H2/Ar flow (50 mL/min) through the pretreated catalyst (about 60 mg). The temperature was increased from room temperature to 700 °C with a rate of 10 °C/min, and the H2 concentration was measured by a thermal conductivity detector. Before the determination, the catalyst was heated to 300 °C under He atmosphere for 60 min.
2.3. Catalytic Performance Tests
All catalytic tests were performed in a continuous flow fixed-bed quartz reactor with an internal diameter of 10 mm at atmospheric pressure, as shown in Figure 1. A certain amount of catalyst was placed in the reactor. A simulant gas was flown into the reactor, with 0.5% H2S, 0.25% O2, and balance gas of N2. The total gas flow rate was fixed at 150 mL/min and the reaction temperature ranged from 180 °C to 260 °C. A sulfur condenser was attached to the bottom of the fixed-bed quartz reactor. The tail gas was analyzed by gas chromatography (GC2060, Ruimin, China) equipped with an FPD (flame photometric detector) and a TCD (thermal conductivity detector). We note that in H2S oxidation, the formation of S is considered a selective reaction and the formation of SO2 is considered an unselective reaction. The conversion of H2S, sulfur selectivity, and sulfur yield were determined by the following equations:
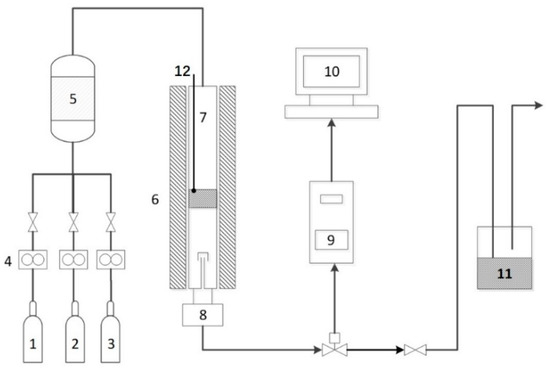
Figure 1.
Schematic diagram of H2S catalytic process: (1) 1% H2S gas cylinder; (2) N2 gas cylinder; (3)O2 gas cylinder; (4) mass flow controllers; (5) gas mixer; (6) tube furnace; (7) fixed bed reactor; (8) condenser; (9) gas chromatograph; (10) computer; (11) NaOH wash bottle; (12) thermocouple.
3. Results and Discussion
3.1. Catalytic Performances of LaFexCo1−xO3 Catalysts
3.1.1. Effect of Reaction Temperature
The effect of reaction temperature on the catalytic performance of the LaFexCo1−xO3 catalysts for H2S selective catalytic oxidation is investigated in Figure 2. Figure 2A shows the H2S conversion for LaCoO3, LaFeO3, and Fe-doped LaCoO3. At 180 °C, LaCoO3 can only achieve H2S conversion of 75%, while LaFeO3 achieved slightly higher H2S conversion of 83%. With Fe co-doping in the B-site, H2S conversions were significantly higher, and all samples achieved conversions higher than 88%. This indicates that B-site Fe doping significantly increases the activity of the pristine perovskite catalysts. Temperature effects were also investigated. For LaCoO3, it was shown that H2S conversion increased with temperature and eventually reached 93% at 260 °C. For co-doped LaFe0.4Co0.6O3, LaFe0.6Co0.4O3, and LaFe0.8Co0.2O3, H2S conversion increased to 99% at only 200 °C. This again shows that LaFexCo1−xO3 with Fe doping has notably higher activities. For LaFeO3, the H2S conversion increased and then decreased with the further increasing temperature. This could be ascribed to lattice oxygen uncoupling from the perovskite structure at higher temperatures, which caused a loss of activity of perovskite [25].
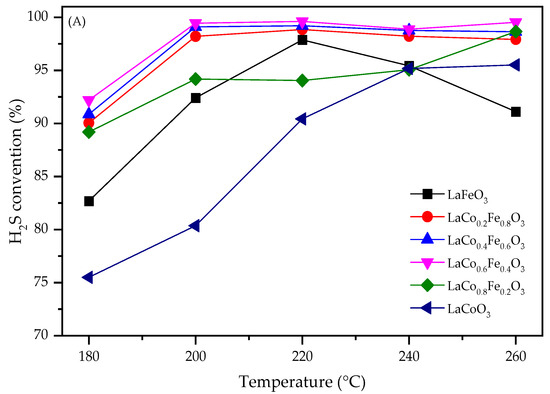
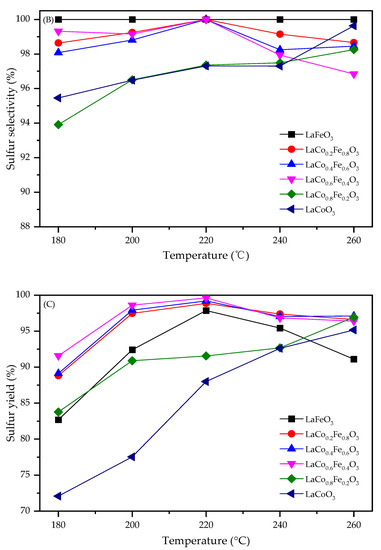
Figure 2.
Effect of temperature on (A) H2S conversion, (B) sulfur selectivity, and (C) sulfur yield for LaFexCo1−xO3 catalysts.
Figure 2B compares the selectivities towards sulfur. It was observed that LaFeO3 exhibited almost perfect selectivities (~100%) at all temperatures investigated, while LaCoO3 exhibited lower selectivity, with ~95% at 180 °C. A small amount of Fe doping did not increase the selectivity, and 94% sulfur selectivity was achieved for LaFe0.2Co0.8O3 at 180 °C. However, a further increase in Fe contents significantly increased the selectivities, and >98% selectivities were achieved for LaFe0.4Co0.6O3, LaFe0.6Co0.4O3, and LaFe0.2Co0.8O3. This again shows that larger Fe co-doping is beneficial. Temperature effects were also investigated. For LaCoO3 and LaFe0.2Co0.8O3, the selectivities increased with respect to temperature. For LaFe0.4Co0.6O3, LaFe0.6Co0.4O3, and LaFe0.2Co0.8O3, a volcanic shape was present and the highest selectivities (~100%) were obtained at 220 °C. Sulfur yield was further compared in Figure 2C. As expected, LaFe0.4Co0.6O3, LaFe0.6Co0.4O3, and LaFe0.2Co0.8O3 achieved ~100% sulfur yield at 220 °C, higher than LaFe0.2Co0.8O3, LaFeO3, and LaCoO3. This shows that perovskites with Co and Fe co-doping are highly effective in H2S selective oxidation to sulfur.
3.1.2. Effect of GHSV (Gas Hourly Space Velocity)
LaFe0.4Co0.6O3 was selected as one of the best catalysts for selective H2S oxidation. The influence of GHSV on the performance of the LaFe0.4Co0.6O3 catalyst was investigated at 220 °C. As shown in Figure 3, the H2S conversion, sulfur selectivity, and sulfur yield were all above 99% when the GHSV was in the range of 3000 h−1–6000 h−1. However, it was found that as the GHSV further increased, the catalytic activity of LaFe0.4Co0.6O3 catalyst was greatly reduced. H2S conversion decreased from 99% to 82% with the increase in GHSV from 6000 h−1 to 15,000 h−1. Therefore, LaFe0.4Co0.6O3 presented excellent catalytic performance in the GHSV range of 3000 h−1–6000 h−1.
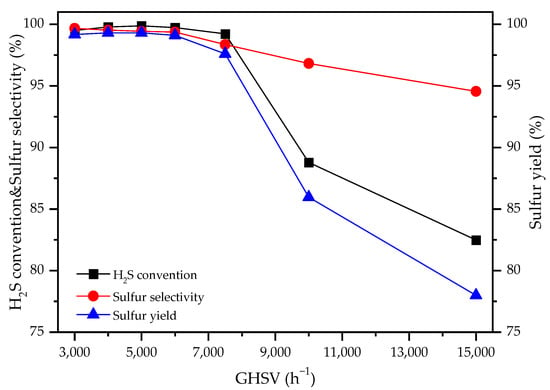
Figure 3.
Effect of GHSV on catalytic performance for LaFe0.4Co0.6O3 catalysts at 220 °C.
3.1.3. Effect of the H2S/O2 Molar Ratio
Figure 4 depicts the effect of the H2S/O2 molar ratio on H2S selective catalytic oxidation over the LaFe0.4Co0.6O3 catalyst at 220 °C. As shown in Figure 4, as the H2S/O2 molar ratio decreased from 3:1 to 2:1 (i.e., from oxygen-deficient to stoichiometric), H2S conversion increased from 75% to nearly 100% with sulfur selectivity close to 100%. As H2S/O2 further reduced to 1 (i.e., oxygen-excess), H2S conversion decreased slightly, but sulfur selectivity decreased drastically to ~70%. This is attributed to the further oxidation of the generated sulfur to sulfur dioxide under excess oxygen. Therefore, as opposed to previous studies that required an oxygen-excess environment [9,11], co-doped LaFe0.4Co0.6O3 catalyst only requires stoichiometric H2S/O2 condition, showing process advantages.
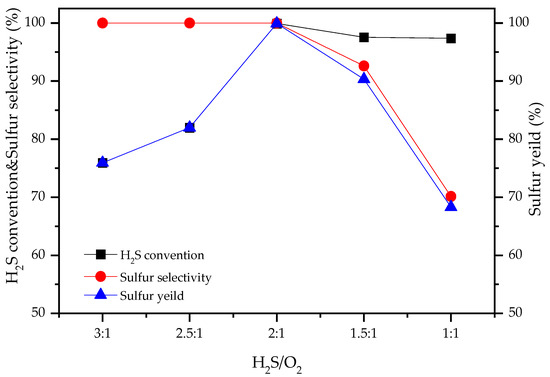
Figure 4.
Effect of the H2S/O2 molar ratio on the catalytic performance of LaFe0.4Co0.6O3 catalysts. Reaction conditions: H2S/O2/N2 = 0.5/0.25/99.25, T = 220 °C, GHSV = 5000 h−1.
3.1.4. Durability of LaFexCo1−xO3 Catalysts
Catalyst stability is an important criterion for industrial applications. Figure 5 illustrates the durability behavior of the LaFe0.4Co0.6O3 catalyst at 220 °C. The selectivity of sulfur can be maintained at ~100% with the reaction proceeding within 77 h. For catalyst activity, during the first 64 h, the H2S conversion of the LaFe0.4Co0.6O3 catalyst decreased slightly from 99.9% to 98.1%. The deactivation accelerated after 64 h; however, H2S conversion was still above 95% at 77 h. Longer reaction time was not attempted given the limitation of the lab equipment. Although deactivation was still observed, the co-doped LaFe0.4Co0.6O3 catalyst exhibited excellent stability when compared with several other reported catalysts using a 5% decrease in sulfur yield as a criterion. The comparisons with previously reported carbon materials, pillared clay materials, and metal oxides are shown in Figure 6 [2,7,13,26,27].
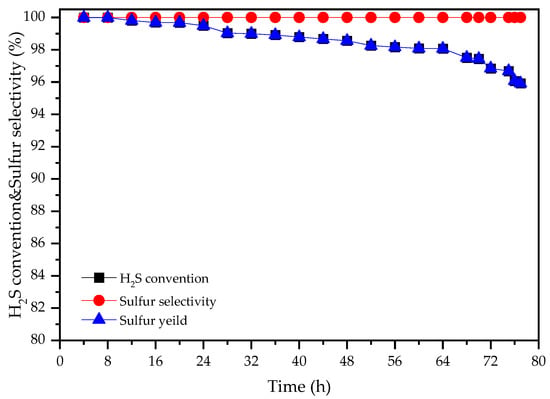
Figure 5.
Time-on-stream behavior of the LaFe0.4Co0.6O3 catalysts. Reaction conditions: H2S/O2/N2 = 0.5/0.25/99.25, T = 220 °C, GHSV = 5000 h−1.
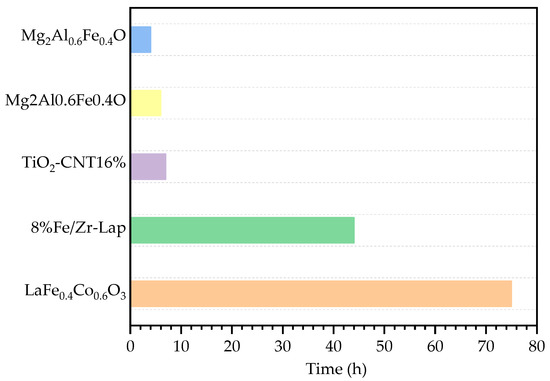
Figure 6.
Time-on-stream behavior of different catalysts using sulfur yield decreased by 5% as a criterion.
3.2. Characterization of LaFexCo1−xO3 Catalysts
3.2.1. Phase Identifications
The phase structures of all catalysts were examined by XRD. Figure 7 shows the XRD patterns of LaFeO3, LaCoO3, and LaFexCo1−xO3 catalysts. It was observed that co-doped LaFexCo1−xO3 generally maintained a perovskite-phase structure. With increasing amounts of Fe (III) dopant, a small shift to lower 2-theta angles was observed, indicating that Fe3+ is substituted into the perovskite structure. For x = 0, 0.2, and 0.4, there is a clear reflection peak splitting at 59.4°, consistent with the spitting of the main peak at 33°. For x = 0.6 and x = 0.8, the peak splitting effect has become less apparent, but the peak shape is still asymmetric. It is noted that the peak splitting effect is minimal on LaFeO3, with no Co-doping. This behavior is mainly due to the transformation of the phase structure from a rhombohedral structure of LaCoO3 (PDF 84-0848) to a cubic structure of LaFeO3 (PDF 75-0541) with Fe3+ dopant.
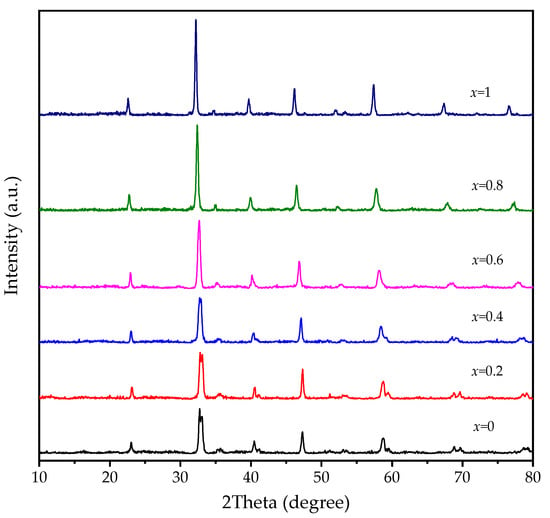
Figure 7.
XRD patterns of LaFexCo1−xO3 catalysts calcined at 650 °C.
3.2.2. FTIR Spectrum
FTIR has the advantage of being very sensitive to structural distortions [28]. Figure 8 shows the FTIR spectra of LaFeO3, LaCoO3, and LaFexCo1−xO3 catalysts. The ~3400 cm−1 band may be the water vapor background error caused by the instrument state. Moreover, the catalysts were calcined at 650 °C and any remaining CHX shall not appear. LaCoO3 has the characteristic bands at 418 cm−1 (stretching La-O vibrations) [29], 553 cm−1 (stretching Co-O vibrations) [30], and 595 cm−1 (bending O-Co-O vibrations) [31] that ascribed to the vibration of the metal–oxygen band. As a comparison, the FTIR spectrum of LaFeO3 shows stretching Fe–O vibration at 555 cm−1, which corresponds to the stretching Fe–O vibration in FeO6 octahedra [32,33]. For LaFexCo1−xO3, with the increase in Fe doping, two bands at 553 cm−1 and 595 cm−1 gradually changed to a single band at 555 cm−1, and no new band was found. This indicates that the structure distortion of LaFexCo1−xO3 changed from LaCoO3-like to LaFeO3-like with an increasing amount of Fe doping. This result is consistent with XRD.
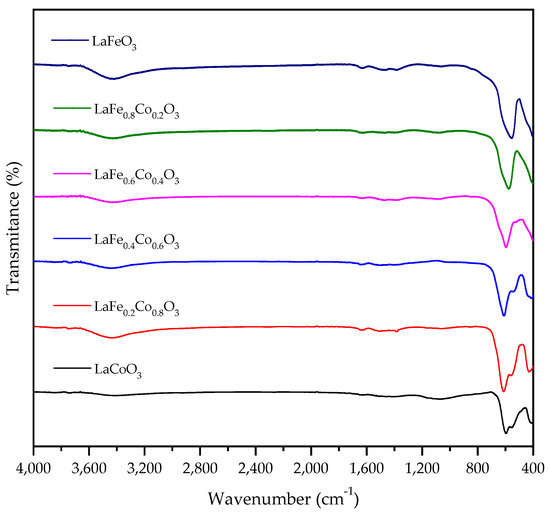
Figure 8.
FTIR spectra of fresh LaFexCo1−xO3 catalysts (x = 0, 0.2, 0.4, 0.6, 0.8, 1.0).
3.2.3. Textural Properties
The specific surface area and pore structure of the catalyst were studied by N2-physisorption analysis. Table 1 summarizes the BET surface area, pore volume, and average pore diameter of all investigated catalysts. The specific surface area increased from 9.26 m2/g of LaCoO3 to 11.39 m2/g of LaFe0.4Co0.6O3 with the increase in Fe doping amount, and further decreased to 8.02 m2/g of LaFeO3 with excess Fe in the B-site. This trend is also consistent with the increase in H2S conversion, indicating that the increasing specific surface area of the catalyst may be favorable for the exposure of active sites, leading to higher catalytic activity [24].

Table 1.
Textural properties of the LaFexCo1−xO3 catalysts.
The low temperature data at 180 °C are used to illustrate this effect, as all high temperature points have close to 100% H2S conversion, which cannot reflect the intrinsic activity of the oxygen vacancies. We set the lowest surface area as 1 and scale other samples based on that. It is shown in Figure 9 that after normalization, except for LaFeO3 and LaFe0.8Co0.2O3, all other samples have similar conversions between 64% and 68%. This reflects that the intrinsic nature of oxygen vacancies is similar and a larger surface area with more oxygen vacancies exposed can lead to higher catalyst activity. This is consistent with the conclusion of this manuscript, where oxygen vacancy is an important factor to determine catalyst activity. As for the increased intrinsic activity of oxygen vacancies for LaFeO3 and LaFe0.8Co0.2O3, this could be due to Fe-enriched perovskite having oxygen vacancies with higher activity. It would be an interesting topic in the future to synthesize Fe-enriched perovskite with higher surface areas to further boost catalyst activity.
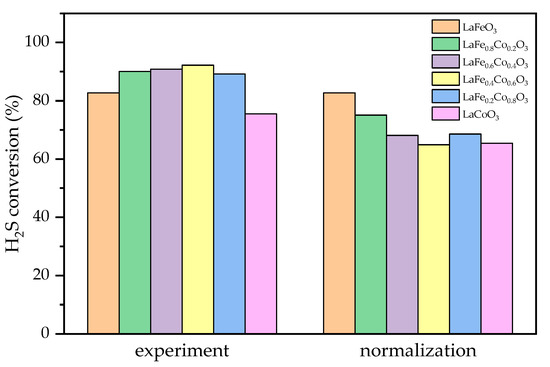
Figure 9.
Experimental and normalized H2S conversion of LaFexCo1−xO3 catalysts at 180 °C.
3.2.4. H2-TPR Spectrum
H2-TPR was conducted to determine the reducibility of the catalysts, as shown in Figure 10. It can be seen that the LaCoO3 sample has reduction peaks at 380 °C and 405 °C, which can be attributed to the reduction of Co3+ to Co2+ [34], and a higher-temperature reduction peak at 546 °C belonging to the reduction of Co2+ to Co0 [35,36]. The addition of Fe in the LaCoO3 leads to reduction peaks with lower temperatures. For example, LaFe0.2Co0.8O3 catalyst has reduction peaks at 367 °C and 390 °C. With the further increase in the Fe doping amount, LaFe0.4Co0.6O3, LaFe0.6Co0.4O3, LaFe0.8Co0.2O3, and LaFeO3 show reduction peaks in the range of 300 °C–400 °C. These indicate that the addition of Fe promotes the reduction of Co3+. As the reduction of Co3+ to Co2+ is also associated with the creation of lattice oxygen vacancies, it can be concluded that Co and Fe co-doping facilitates oxygen vacancy formation in perovskite. This is consistent with the trend of H2S conversion, which indicates that the ease of oxygen vacancy formation leads to increased catalyst activities for H2S oxidation. This result is also consistent with other literature reports [37,38]. The high-temperature reduction peak (546 °C in LaCoO3) was pushed to a much higher temperature (660 °C in LaFe0.2Co0.8O3), and completely eliminated in LaFe0.4Co0.6O3, LaFe0.6Co0.4O3, and LaFe0.8Co0.2O3 [39,40]. The higher-temperature reduction peak is associated with further Co2+ reduction to Co0, which indicates that the Co and Fe co-doped samples are highly stable even under a high-temperature reducing environment, explaining the exceptional catalyst stability observed in experiments. The reduction peak area also increased with the increase in Fe doping amount, which also indicates that Fe-doped LaCoO3 has stronger oxidation ability and a higher amount of active lattice oxygen [41].
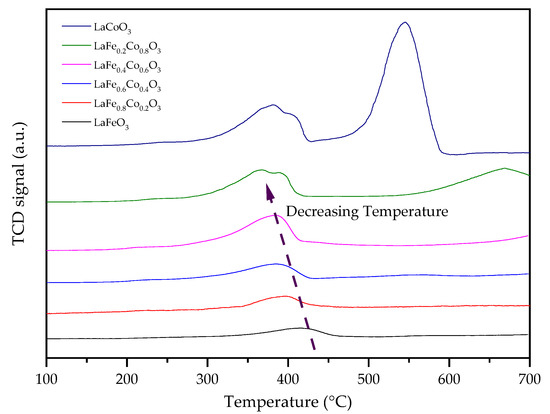
Figure 10.
H2-TPR patterns of LaFexCo1−xO3 catalysts calcined at 650 °C.
3.2.5. The Surface Chemical States Revealed by XPS
XPS analysis was conducted to elucidate the chemical states of surface Co, Fe, O, and S species of the fresh and used LaFe0.4Co0.6O3 catalysts, shown in Figure 11. From the high-resolution spectrum of Co 2p (Figure 11a), it can be seen that there are peaks at bonding energies of 795.1 eV and 780.0 eV, corresponding to 2p1/2 and 2p3/2 of Co3+, respectively [42,43,44]. Two peaks appeared at 710.4 eV and 723.8 eV in Figure 11b and can be ascribed to Fe 2p3/2 and Fe 2p1/2 levels, respectively. These binding energies agreed well with an Fe3+ oxidation state assignment [45,46]. The O 1s spectrum was shown in Figure 11c, which showed two peaks at 531.2 eV and 529.0 eV. The peak at 529.0 eV is attributed to lattice oxygen (Olat), while the peak at 531.2 eV corresponds to adsorbed oxygen (Oads) on oxygen vacancies [42,47,48]. It was reported that oxygen vacancy plays an important role in the reaction, and can not only accelerate the decomposition of oxygen molecules, but also increase the mobility of lattice oxygen [49]. It can be seen that the content of lattice oxygen decreased sharply, which confirms that the lattice oxygen of the catalyst may participate in the H2S oxidation reaction to form sulfate species. Simultaneously, the oxygen peaks were shifted towards higher B.E., with a shift value of 0.3 eV and 0.9 eV for lattice oxygen species and oxygen vacancies. As a shift to higher B.E. indicates lower electron density, this shows that the oxygen vacancies in used samples have much lower electron density. It was reported that oxygen vacancies with lower electron density lead to significantly lower ethylene yield in oxidative dehydrogenation of ethane [50]. The shift could also be caused by the formation of OH− and SO42− groups during the catalytic reaction. In this study, it is hypothesized that the deactivation of the H2S selective oxidation reaction is also mainly related to the lower electron density of oxygen vacancies. Figure 11d shows the S 2p XPS spectrum of the used LaFe0.4Co0.6O3 catalyst to investigate the deactivation process of the catalyst. Tiny peaks around 163.3 eV were detected, which can be ascribed to the presence of the conjugated sulfur (Sn) [51], indicating a sulfur deposit on the sample. The main peaks of S 2p1/2 and S 2p3/2 at binding energies of 170.3 eV and 169.0 eV indicate that S exists as a sulfate species [52]. It should be noted that deposited sulfur could also lead to catalyst deactivations.
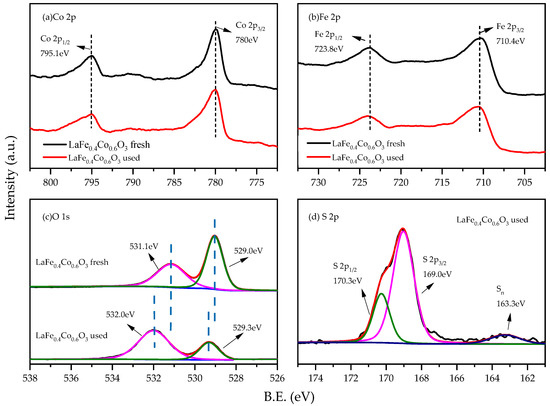
Figure 11.
XPS spectra of the fresh and used LaFe0.4Co0.6O3 catalysts calcined at 650 °C. (a) Co 2p (b) Fe 2p (c) S 1s (d) S 2p.
3.3. Catalytic and Deactivation Mechanisms
Based on the results of experiments and characterizations, the possible reaction mechanism of H2S selective oxidation over LaFe0.4Co0.6O3 can be proposed as follows: H2S was first adsorbed on the oxygen vacancies on the LaFe0.4Co0.6O3 catalyst surface, then the adsorbed H2S dissociated into HS- or S2− ions. Subsequently, the HS− or S2− ions were oxidized by the surface-active oxygen and/or lattice oxygen of the LaFe0.4Co0.6O3 catalyst, and Fe3+ was reduced to Fe2+ or Co3+ to Co2+ and oxygen vacancies were formed. Then, O2 diffused and adsorbed on the catalyst surfaces, and oxidized Fe2+ to Fe3+ or Co2+ to Co3+, thereby replenishing the lattice oxygen. The possible reaction mechanism of H2S selective oxidation over the LaFe0.4Co0.6O3 is proposed as illustrated in Figure 12. Compared to LaFeO3 and LaCoO3, co-doped LaFe0.4Co0.6O3 has high oxygen vacancy concentration, leading to increased catalyst activities.
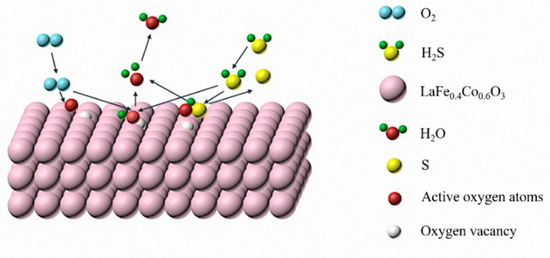
Figure 12.
Schematic of H2S selective oxidation over the LaFe0.4Co0.6O3.
The S 2p XPS spectrum of used LaFe0.4Co0.6O3 revealed the deactivation process of the catalyst. The sulfate accounted for 92% of the total molar amount of sulfur and the elemental sulfur accounted for 8%. The Fe 2p energy value of the used catalyst shifted slightly to a higher value, an index of an increase in positive polarity, indicating that the interaction between sulfate anion and iron cation generates Fe2(SO4)3 [24]. It can be concluded that the catalyst deactivation is mainly due to the formation of iron sulfate, which will destroy the redox process of Fe3+/Fe2+, resulting in a decrease in catalytic activity. The deposition of elemental sulfur also leads to a reduction in catalytic activity.
4. Conclusions
In summary, a series of LaFexCo1−xO3 catalysts were synthesized by the sol-gel method and investigated for H2S selective oxidation. XRD, N2-adsorption, H2-TPR, and XPS analyses were conducted. The conclusions of this study can be summarized as follows:
- (1)
- The incorporation of Fe into LaCoO3 had a great influence on the conversion and selectivity of H2S selective oxidation reaction. It was shown that Fe doping not only enhanced catalytic activity for the H2S selective oxidation reaction, but also reduced the optimal reaction temperature.
- (2)
- LaFe0.4Co0.6O3 had the highest catalytic activity and the catalyst deactivation was not obvious before 77 h, and its stability is better than some types of catalysts. Analysis of XRD and FTIR showed that the LaFexCo1−xO3 catalyst existed in a single perovskite structure. H2-TPR indicates that oxygen vacancy creation is more feasible on a co-doped LaFexCo1−xO3 catalyst.
- (3)
- The reaction mechanism and inactivation mechanism were predicted. The presence of lattice oxygen in the LaFe0.4Co0.6O3 catalyst and the oxygen vacancies generated in the reaction are beneficial to the H2S selective oxidation reaction. In addition, the deposition of elemental sulfur and the formation of oxygen vacancies with lower electron density could lead to catalyst deactivation.
Author Contributions
Conceptualization, X.Y., Y.G. and F.W.; formal analysis, X.Y. and X.T.; investigation, X.Y., X.T. and Y.G.; data curation, X.Y.; writing—original draft preparation, X.Y.; writing—review and editing, Y.G., L.D. and Y.W.; supervision, G.Y.; project administration, F.W.; funding acquisition, F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 21978092.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds LaFexCo1−xO3 are available from the authors per requested.
References
- Pongthawornsakun, B.; Phatyenchuen, S.; Panpranot, J.; Praserthdam, P. The low temperature selective oxidation of H2S to elemental sulfur on TiO2 supported V2O5 catalysts. J. Environ. Chem. Eng. 2018, 6, 1414–1423. [Google Scholar] [CrossRef]
- Yang, C.; Ye, H.; Byun, J.; Hou, Y.; Wang, X. N-Rich Carbon Catalysts with Economic Feasibility for the Selective Oxidation of Hydrogen Sulfide to Sulfur. Environ. Sci. Technol. 2020, 54, 12621–12630. [Google Scholar] [CrossRef]
- Wiheeb, A.D.; Shamsudin, I.K.; Ahmad, M.A.; Murat, M.N.; Kim, J.; Othman, M.R. Present technologies for hydrogen sulfide removal from gaseous mixtures. Rev. Chem. Eng. 2013, 29, 449–470. [Google Scholar] [CrossRef]
- Garcia-Arriaga, V.; Alvarez-Ramirez, J.; Amaya, M.; Sosa, E. H2S and O2 influence on the corrosion of carbon steel immersed in a solution containing 3 M diethanolamine. Corros. Sci. 2010, 52, 2268–2279. [Google Scholar] [CrossRef]
- Hao, Z. H2S selective catalytic oxidation: Catalysts and processes. ACS Catal. 2015, 5, 1053–1067. [Google Scholar]
- Huang, H.; Shen, L.; Yang, S.; Hu, W.; Zhang, L.; Feng, J.; Jiang, L.; Yu, T.; Li, Z.; Zou, Z. Exploring N-Containing Compound Catalyst for H2S Selective Oxidation: Case Study of TaON and Ta3N5. Catal. Lett. 2021, 151, 1728–1737. [Google Scholar] [CrossRef]
- Liu, Y.; Song, C.; Wang, Y.; Cao, W.; Lei, Y.; Feng, Q.; Chen, Z.; Liang, S.; Xu, L.; Jiang, L. Rational designed Co@ N-doped carbon catalyst for high-efficient H2S selective oxidation by regulating electronic structures. Chem. Eng. J. 2020, 401, 126038. [Google Scholar] [CrossRef]
- Fang, H.-B.; Zhao, J.-T.; Fang, Y.-T.; Huang, J.-J.; Wang, Y. Selective oxidation of hydrogen sulfide to sulfur over activated carbon-supported metal oxides. Fuel 2013, 108, 143–148. [Google Scholar] [CrossRef]
- Ba, H.; Duong-Viet, C.; Liu, Y.; Nhut, J.-M.; Granger, P.; Ledoux, M.J.; Pham-Huu, C. Nitrogen-doped carbon nanotube spheres as metal-free catalysts for the partial oxidation of H2S. Comptes Rendus Chim. 2016, 19, 1303–1309. [Google Scholar] [CrossRef] [Green Version]
- Bineesh, K.V.; Kim, D.-K.; Kim, D.-W.; Cho, H.-J.; Park, D.-W. Selective catalytic oxidation of H2S to elemental sulfur over V2O5/Zr-pillared montmorillonite clay. Energy Environ. Sci. 2010, 3, 302–310. [Google Scholar] [CrossRef]
- Soriano, M.; Nieto, J.L.; Ivars, F.; Concepcion, P.; Rodríguez-Castellón, E. Alkali-promoted V2O5 catalysts for the partial oxidation of H2S to sulphur. Catal. Today 2012, 192, 28–35. [Google Scholar] [CrossRef]
- Phatyenchuen, S.; Pongthawornsakun, B.; Panpranot, J.; Praserthdam, P. Effect of transition metal dopants (M= Nb, La, Zr, and Y) on the M-TiO2 supported V2O5 catalysts in the selective oxidation of H2S to elemental sulfur. J. Environ. Chem. Eng. 2018, 6, 5655–5661. [Google Scholar] [CrossRef]
- Daraee, M.; Baniadam, M.; Rashidi, A.; Maghrebi, M. Synthesis of TiO2-CNT hybrid nanocatalyst and its application in direct oxidation of H2S to S. Chem. Phys. 2018, 511, 7–19. [Google Scholar] [CrossRef]
- Ghasemy, E.; Emrooz, H.B.M.; Rashidi, A.; Hamzehlouyan, T. Highly uniform molybdenum oxide loaded N-CNT as a remarkably active and selective nanocatalyst for H2S selective oxidation. Sci. Total. Environ. 2020, 711, 134819. [Google Scholar] [CrossRef] [PubMed]
- Varandili, S.B.; Babaei, A.; Ataie, A. Characterization of B site codoped LaFeO3 nanoparticles prepared via co-precipitation route. Rare Met. 2018, 37, 181–190. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, C.; Zhu, J.; Wang, L.; Gao, S.; Xia, X. Enhanced degradation of atrazine by nanoscale LaFe1−xCuxO3-δ perovskite activated peroxymonosulfate: Performance and mechanism. Sci. Total. Environ. 2019, 673, 565–575. [Google Scholar] [CrossRef]
- Madoui, N.; Omari, M. Synthesis and electrochemical properties of LaCr1−xCoxO3 (0≤x≤0.5) via co-precipitation method. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1005–1013. [Google Scholar] [CrossRef]
- Pena, M.; Fierro, J. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Omari, E.; Omari, M.; Barkat, D. Oxygen evolution reaction over copper and zinc co-doped LaFeO3 perovskite oxides. Polyhedron 2018, 156, 116–122. [Google Scholar] [CrossRef]
- Da, Y.; Lirong, Z.; Caiyun, W.; Teng, M.; Rui, C.; Cairong, G.; Guoliang, F. Catalytic Oxidation of diesel soot particulates over Pt substituted LaMn1−xPtxO3 perovskite oxides. Catal. Today 2019, 327, 73–80. [Google Scholar] [CrossRef]
- Wang, S.; Xu, X.; Zhu, J.; Tang, D.; Zhao, Z. Effect of preparation method on physicochemical properties and catalytic performances of LaCoO3 perovskite for CO oxidation. J. Rare Earths 2019, 37, 970–977. [Google Scholar] [CrossRef]
- Zhou, C.; Feng, Z.; Zhang, Y.; Hu, L.; Chen, R.; Shan, B.; Yin, H.; Wang, W.G.; Huang, A. Enhanced catalytic activity for NO oxidation over Ba doped LaCoO3 catalyst. RSC Adv. 2015, 5, 28054–28059. [Google Scholar] [CrossRef]
- Yang, X.; Park, D.-W.; Kim, M.-I. Selective oxidation of hydrogen sulfide over LaCoO3 and LaSrCoO4 mixed oxides. Korean J. Chem. Eng. 2007, 24, 592–595. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Jiang, G.; Li, N.; Hao, Z.; Qu, S. H2S selective catalytic oxidation over Ce substituted La1−xCexFeO3 perovskite oxides catalyst. Chem. Eng. J. 2018, 348, 831–839. [Google Scholar] [CrossRef]
- Ahmad, A.; Al Mamun, M.; Al-Mamun, M.; Huque, S.; Ismail, M. LFO Perovskites as Oxygen Carriers for Chemical Looping Oxygen Uncoupling (CLOU). J. Therm. Anal. Calorim. 2021, 1–9. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Jiang, G.; Sun, Y.; Qu, S. Selective oxidation of H2S over Fe supported on Zr-intercalated Laponite clay mesoporous composite catalysts at low temperature. Catal. Today 2020, 355, 366–374. [Google Scholar] [CrossRef]
- Xin, Z.; Zhuo, W.; Tang, Y.; Qiao, N.; Hao, Z. Catalytic Behaviors of Combined Oxides Derived from Mg/AlxFe1−x-Cl Layered Double Hydroxides for H2S Selective Oxidation. Catal. Sci. Technol. 2015, 5, 4991–4999. [Google Scholar]
- Gaikwad, V.M.; Sheikh, J.R.; Acharya, S.A. Investigation of photocatalytic and dielectric behavior of LaFeO3 nanoparticles prepared by microwave-assisted sol–gel combustion route. J. Sol-Gel Sci. Technol. 2015, 76, 27–35. [Google Scholar] [CrossRef]
- Andoulsi, R.; Horchani-Naifer, K.; Férid, M. Effect of the preparation route on the structure and microstructure of LaCoO3. Chem. Pap. 2014, 68, 608–613. [Google Scholar] [CrossRef]
- Chumakova, V.; Marikutsa, A.; Rumyantseva, M.; Fasquelle, D.; Gaskov, A. Nanocrystalline LaCoO3 modified by Ag nanoparticles with improved sensitivity to H2S. Sens. Actuators B Chem. 2019, 296, 126661. [Google Scholar] [CrossRef]
- Sarker, A.R. Synthesis of high quality LaCoO3 crystals using water based sol-gel method. Int. J. Mater. Sci. Appl. 2015, 4, 159–164. [Google Scholar]
- Noroozifar, M.; Khorasani-Motlagh, M.; Ekrami-Kakhki, M.-S.; Khaleghian-Moghadam, R. Enhanced electrocatalytic properties of Pt–chitosan nanocomposite for direct methanol fuel cell by LaFeO3 and carbon nanotube. J. Power Sources 2014, 248, 130–139. [Google Scholar] [CrossRef]
- Li, J.; Pan, X.; Xu, Y.; Jia, L.; Yi, X.; Fang, W. Synergetic effect of copper species as cocatalyst on LaFeO3 for enhanced visible-light photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2015, 40, 13918–13925. [Google Scholar] [CrossRef]
- Merino, N.A.; Barbero, B.P.; Grange, P.; Cadús, L.E. La1−xCaxCoO3 perovskite-type oxides: Preparation, characterisation, stability, and catalytic potentiality for the total oxidation of propane. J. Catal. 2005, 231, 232–244. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, X.; Wu, C.; Wen, Y.; Xue, Y.; Chen, R.; Zhang, Z.; Shan, B.; Yin, H.; Wang, W.G. NO oxidation catalysis on copper doped hexagonal phase LaCoO3: A combined experimental and theoretical study. Phys. Chem. Chem. Phys. 2014, 16, 5106–5112. [Google Scholar] [CrossRef] [PubMed]
- Wachowski, L.; Zielinski, S.; Burewicz, A. Preparation, stability and oxygen stoichiometry in perovskite-type binary oxides. Acta Chim. Acad. Sci. Hung. 1981, 106, 217–225. [Google Scholar]
- Zheng, X.; Li, Y.; You, W.; Lei, G.; Cao, Y.; Zhang, Y.; Jiang, L. Construction of Fe-doped TiO2−x ultrathin nanosheets with rich oxygen vacancies for highly efficient oxidation of H2S. Chem. Eng. J. 2022, 430, 132917. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Zheng, Y.; Shen, L.; Xiao, Y.; Cao, Y.; Zhang, Y.; Au, C.; Jiang, L. Highly Efficient Porous FexCe1–xO2−δ with Three-Dimensional Hierarchical Nanoflower Morphology for H2S-Selective Oxidation. ACS Catal. 2020, 10, 3968–3983. [Google Scholar] [CrossRef]
- Merino, N.A.; Barbero, B.P.; Ruiz, P.; Cadús, L.E. Synthesis, characterisation, catalytic activity and structural stability of LaCo1-yFeyO3±λ perovskite catalysts for combustion of ethanol and propane. J. Catal. 2006, 240, 245–257. [Google Scholar] [CrossRef]
- Escalona, N.; Fuentealba, S.; Pecchi, G. Fischer–Tropsch synthesis over LaFe1−xCoxO3 perovskites from a simulated biosyngas feed. Appl. Catal. A Gen. 2010, 381, 253–260. [Google Scholar] [CrossRef]
- Yu, S.; Xu, S.; Sun, B.; Lu, Y.; Li, L.; Zou, W.; Wang, P.; Gao, F.; Tang, C.; Dong, L. Synthesis of CrOx/C catalysts for low temperature NH3-SCR with enhanced regeneration ability in the presence of SO2. RSC Adv. 2018, 8, 3858–3868. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.B.; Gao, L.; Long, Z.; Liu, D.T.; Lin, L. Studies on catalysis of Cu-doped Mn-based perovskite-type oxide in wet oxidation of lignin to produce aromatic aldehydes. Energy Fuels 2010, 24, 4797–4802. [Google Scholar] [CrossRef]
- Ding, J.C.; Li, H.Y.; Cai, Z.X.; Wang, X.X.; Guo, X. Near room temperature CO sensing by mesoporous LaCoO3 nanowires functionalized with Pd nanodots. Sens. Actuators B Chem. 2016, 222, 517–524. [Google Scholar] [CrossRef]
- Liu, H.; Sun, H.; Xie, R.; Zhang, X.; Zheng, K.; Peng, T.; Wu, X.; Zhang, Y. Substrate-dependent structural and CO sensing properties of LaCoO3 epitaxial films. Appl. Surf. Sci. 2018, 442, 742–749. [Google Scholar] [CrossRef]
- Koyuncu, D.D.E.; Yasyerli, S. Selectivity and Stability Enhancement of Iron Oxide Catalyst by Ceria Incorporation for Selective Oxidation of H2S to Sulfur. Ind. Eng. Chem. Res. 2009, 48, 5223–5229. [Google Scholar] [CrossRef]
- Yong, Z.; Jiang, B.; Yuan, M.; Li, P.; Wei, L.; Zheng, X. Formaldehyde-sensing properties of LaFeO3 particles synthesized by citrate sol–gel method. J. Sol-Gel Sci. Technol. 2016, 79, 167–175. [Google Scholar]
- Bhargav, K.K.; Maity, A.; Ram, S.; Majumder, S.B. Low temperature butane sensing using catalytic nano-crystalline lanthanum ferrite sensing element. Sens. Actuators B Chem. 2014, 195, 303–312. [Google Scholar] [CrossRef]
- Lee, W.Y.; Yun, H.J.; Yoon, J.W. Characterization and magnetic properties of LaFeO3 nanofibers synthesized by electrospinning. J. Alloy. Compd. 2014, 583, 320–324. [Google Scholar] [CrossRef]
- Barbero, B.P.; Gamboa, J.A.; Cadús, L.E. Synthesis and characterisation of La1xCaxFeO3 perovskite-type oxide catalysts for total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2006, 65, 21–30. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Zhao, K.; Li, H.; Huang, Z. Mg-doped La1.6Sr0.4FeCoO6 for anaerobic oxidative dehydrogenation of ethane using surface-absorbed oxygen with tuned electronic structure. Fuel Process. Technol. 2021, 216, 106771. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Hao, Z.; Jiang, G.; Yang, H.; Qu, S. Insight into the H2S selective catalytic oxidation performance on well-mixed Ce-containing rare earth catalysts derived from MgAlCe layered double hydroxides. J. Hazard. Mater. 2018, 342, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.J.; Yabusaki, S.B.; Engelhard, M.H.; Mitroshkov, A.V.; Thornton, E.C. Oxidation of H2S by iron oxides in unsaturated conditions. Environ. Sci. Technol. 2003, 37, 2192–2199. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).