Hydrogen-Rich Gas Production from Two-Stage Catalytic Pyrolysis of Pine Sawdust with Nano-NiO/Al2O3 Catalyst
Abstract
:1. Introduction
2. Results and Discussion
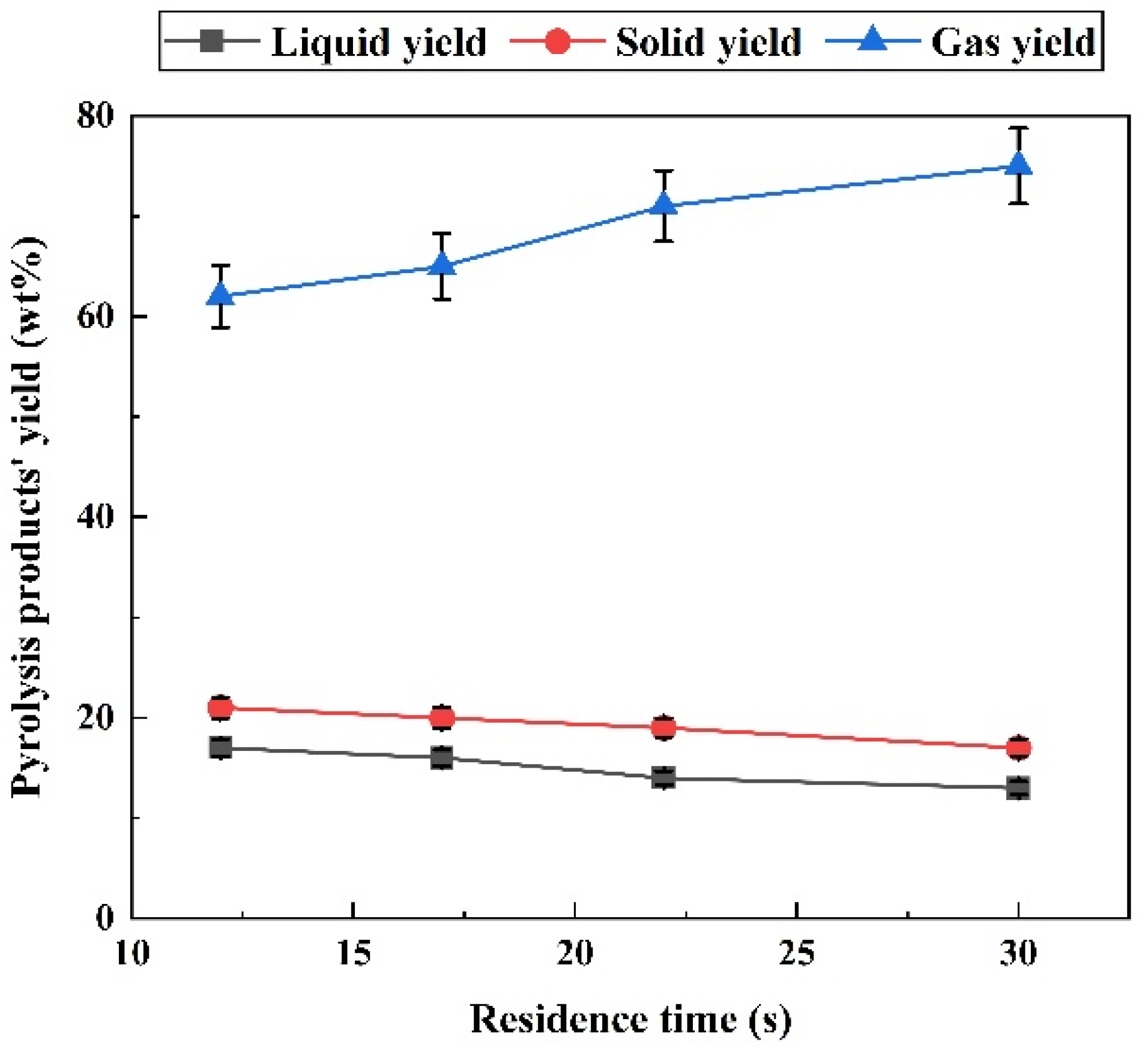
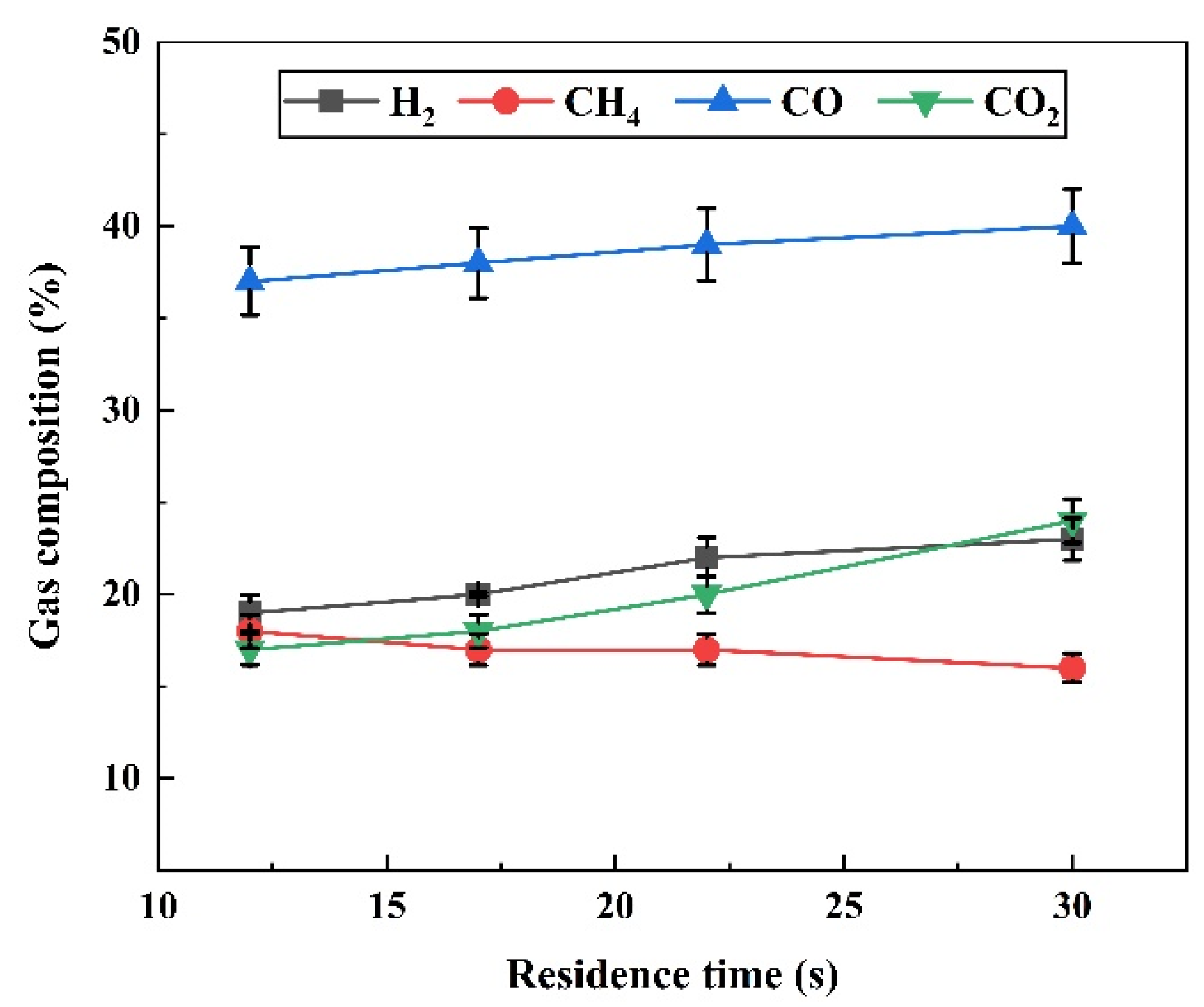
2.1. Effect of Residence Time on Pyrolysis
2.1.1. Effect of Residence Time on Pyrolysis Products Distribution
2.1.2. Effect of Residence Time on Gas Composition
2.1.3. Effect of Residence Time on Gas Products Properties
2.2. Catalytic Effect of Nano-NiO/γ-A12O3
2.2.1. Effect of Catalyst on Pyrolysis Products Yield
2.2.2. Effect of Catalyst on Gas Composition
2.2.3. Effect of Catalyst on Properties of Gas Products
3. Experimental Section
3.1. Sample Preparation
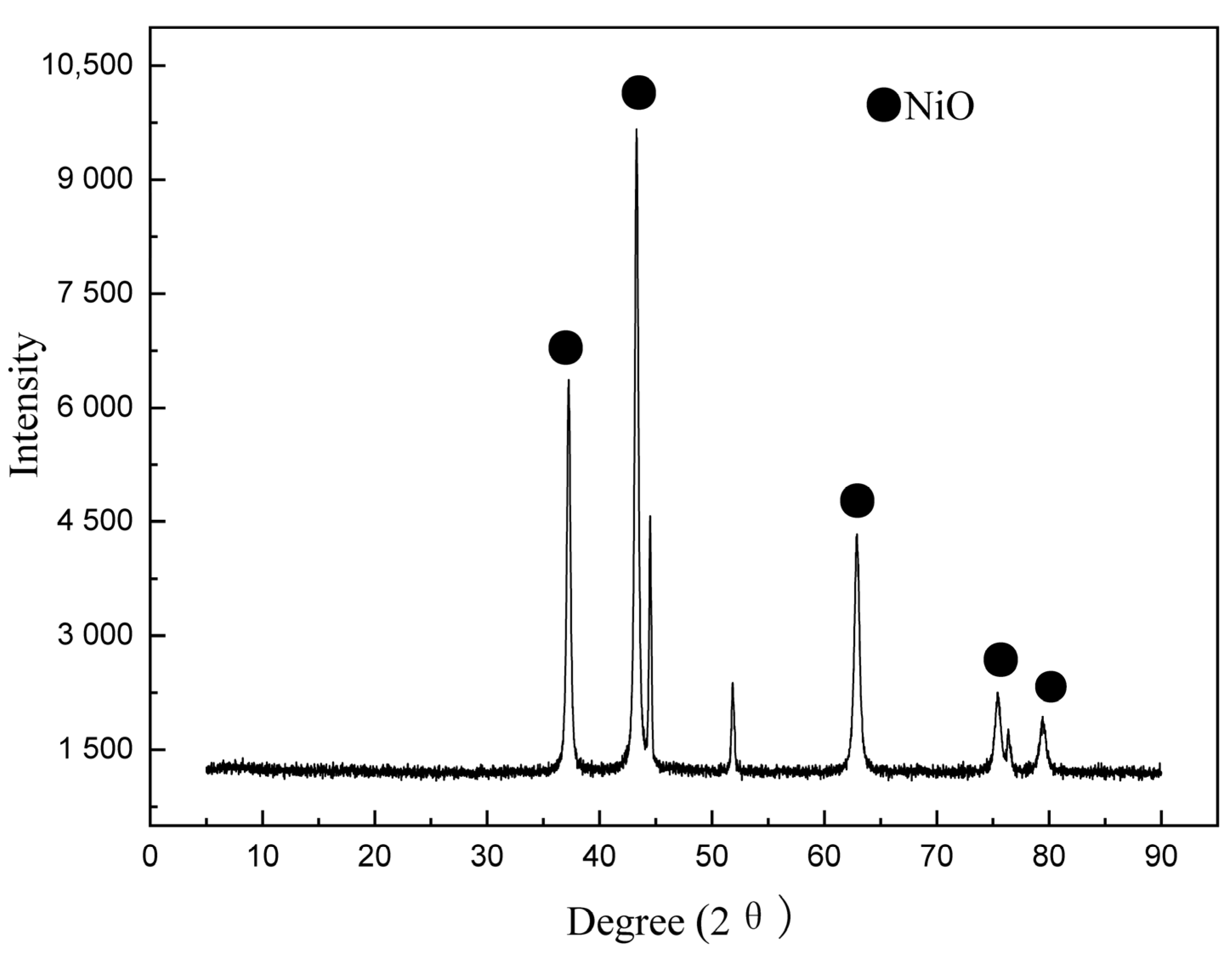
3.2. Catalyst Preparation and Characterization
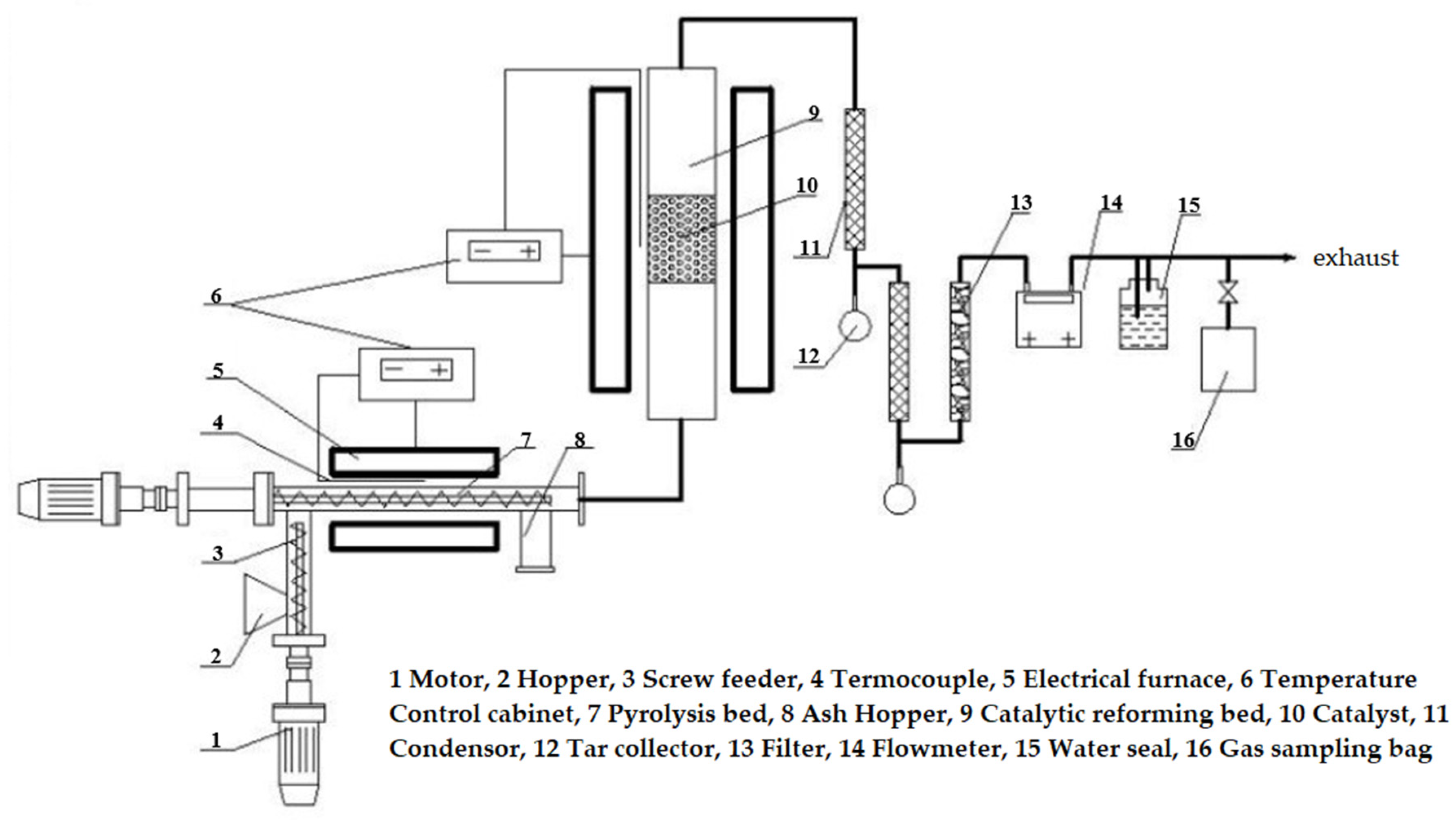
3.3. Experimental Apparatus and Procedures
3.4. Data Analysis
- (1)
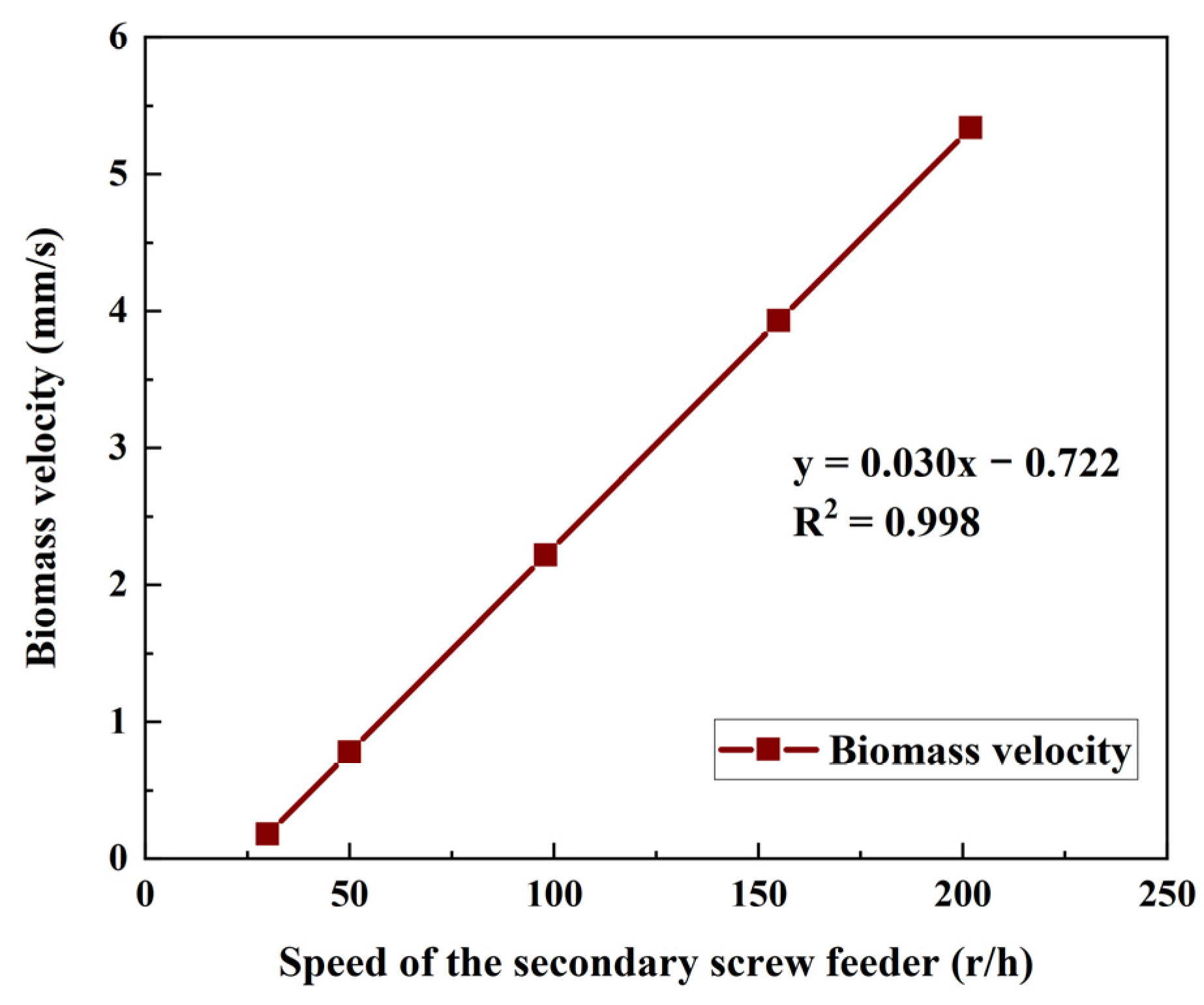
- Residence time, t (s), was calculated by the ratio of the length of the pyrolysis reaction zone (250 mm) to the biomass moving velocity (Vfeed) in the reactor.
- (2)
- Dry gas yield, GY (Nm3/kg), refers to the volume of gas that can be generated per kilogram of dried biomass, which was calculated by the following equation:GY = Produced gas volume (Nm3) ÷ Dry biomass weight (kg)
- (3)
- Carbon conversion rate, ηc (%), refers to the ratio of carbon content in gas products to the carbon content in biomass, which was calculated by the following equation:where C means the mass percentage of carbon in the ultimate analysis of samples, GY is the dry gas yield, CO, CO2, and CH4 are the volume ratio of CO, CO2, and CH4 in gas products.
- (4)
- The lower heating value of gas products (LHV, MJ/Nm3) was calculated by the following equation [36]:where CO, H2, and CH4 are their corresponding gas composition by volume in gas products.LHV = CO × 12.63 + H2 × 10.78 + CH4 × 35.81
4. Conclusions
- The longer residence time resulted in fewer solid and liquid products but more gas products. Longer residence time could promote tar cracking and gas-phase conversion reactions, improve the syngas yield, H2/CO ratio, and improve carbon conversion.
- The catalyst of nano-NiO/γ-A12O3 had catalytic activity for tar removal, which could decrease tar yield to near zero at 800 °C.
- The increasing catalytic temperature of nano-NiO/γ-A12O3 could significantly increase H2 and CO yields by improving the tar decomposition and the secondary gas reactions.
- Increasing catalytic temperature improved dry gas yield and carbon conversion but decreased the H2/CO ratio and the low heating value.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, L.; Sun, L.; Xu, L.; Wan, C.; An, Y.; Ye, M. Recent Developments of Effective Catalysts for Hydrogen Storage Technology Using N-Ethylcarbazole. Catalysts 2020, 10, 648. [Google Scholar] [CrossRef]
- Yin, H.; Yip, A.C.K. A Review on the Production and Purification of Biomass-Derived Hydrogen Using Emerging Membrane Technologies. Catalysts 2017, 7, 297. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; He, J.; Yang, G.; Sun, G.; Sage, V. A Review of the Enhancement of Bio-Hydrogen Generation by Chemicals Addition. Catalysts 2019, 9, 353. [Google Scholar] [CrossRef] [Green Version]
- Tareen, W.U.K.; Dilbar, M.T.; Farhan, M.; Nawaz, M.A.; Durrani, A.W.; Memon, K.A.; Mekhilef, S.; Seyedmahmoudian, M.; Horan, B.; Amir, M. Present Status and Potential of Biomass Energy in Pakistan Based on Existing and Future Renewable Resources. Sustainability 2020, 12, 249. [Google Scholar] [CrossRef] [Green Version]
- Nie, Y.; Li, J.; Wang, C.; Huang, G.; Fu, J.; Chang, S.; Li, H.; Ma, S.; Yu, L.; Cui, X.; et al. A fine-resolution estimation of the biomass resource potential across China from 2020 to 2100. Resour. Conserv. Recycl. 2022, 176, 105944. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2020, 312, 123614. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Q.; Bartocci, P.; Wei, H.; Liu, S.S.; Wu, Z.; Zhou, H.; Yang, H.; Fantozzi, F.; Chen, H. Bioenergy in China: Evaluation of domestic biomass resources and the associated greenhouse gas mitigation potentials. Renew. Sustain. Energy Rev. 2020, 127, 109842. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Cortese, M.; Martino, M. Bioalcohol Reforming: An Overview of the Recent Advances for the Enhancement of Catalyst Stability. Catalysts 2020, 10, 665. [Google Scholar] [CrossRef]
- Ng, F.T.T.; Dalai, A.K. Catalysis for the Production of Sustainable Fuels and Chemicals. Catalysts 2020, 10, 388. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Bhattacharya, S. Direct and two-step gasification behaviour of Victorian brown coals in an entrained flow reactor. Energy Convers. Manag. 2019, 195, 1044–1055. [Google Scholar] [CrossRef]
- Xu, T.; Bhattacharya, S. Entrained flow gasification behaviour of Victorian brown coal char at low temperature. Fuel 2018, 234, 549–557. [Google Scholar] [CrossRef]
- Xu, T.; Wu, Y.; Bhattacharya, S. Gasification kinetic modelling of Victorian brown coal chars and validity for entrained flow gasification in CO2. Int. J. Min. Sci. Technol. 2021, 31, 473–481. [Google Scholar] [CrossRef]
- Liu, J.; Hou, Q.; Ju, M.; Ji, P.; Sun, Q.; Li, W. Biomass Pyrolysis Technology by Catalytic Fast Pyrolysis, Catalytic Co-Pyrolysis and Microwave-Assisted Pyrolysis: A Review. Catalysts 2020, 10, 742. [Google Scholar] [CrossRef]
- Sekar, M.; Mathimani, T.; Alagumalai, A.; Chi, N.T.L.; Duc, P.A.; Bhatia, S.K.; Brindhadevi, K.; Pugazhendhi, A. A review on the pyrolysis of algal biomass for biochar and bio-oil—Bottlenecks and scope. Fuel 2021, 283, 119190. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Xu, T.; Srivatsa, S.C.; Bhattacharya, S. In-situ synchrotron IR study on surface functional group evolution of Victorian and Thailand low-rank coals during pyrolysis. J. Anal. Appl. Pyrolysis 2016, 122, 122–130. [Google Scholar] [CrossRef]
- Park, K.-B.; Jeong, Y.-S.; Guzelciftci, B.; Kim, J.-S. Two-stage pyrolysis of polystyrene: Pyrolysis oil as a source of fuels or benzene, toluene, ethylbenzene, and xylenes. Appl. Energy 2020, 259, 114240. [Google Scholar] [CrossRef]
- Guzelciftci, B.; Park, K.-B.; Kim, J.-S. Production of phenol-rich bio-oil via a two-stage pyrolysis of wood. Energy 2020, 200, 117536. [Google Scholar] [CrossRef]
- Muhammad, I.; Manos, G. Improving the Conversion of Biomass in Catalytic Pyrolysis via Intensification of Biomass-Catalyst Contact by Co-Pressing. Catalysts 2021, 11, 805. [Google Scholar] [CrossRef]
- Xu, Y.; Zhai, M.; Guo, H.; Qi, H.; Zou, X.; Jin, S.; Dong, P. High-Temperature Pyrolysis Characteristics for a Single Biomass Particle. Energy Fuels 2019, 33, 11153–11162. [Google Scholar] [CrossRef]
- Safdari, M.-S.; Amini, E.; Weise, D.R.; Fletcher, T.H. Heating rate and temperature effects on pyrolysis products from live wildland fuels. Fuel 2019, 242, 295–304. [Google Scholar] [CrossRef]
- Mazlan, M.A.F.; Uemura, Y.; Osman, N.B.; Yusup, S. Fast pyrolysis of hardwood residues using a fixed bed drop-type pyrolyzer. Energy Convers. Manag. 2015, 98, 208–214. [Google Scholar] [CrossRef]
- Eri, Q.; Wang, B.; Peng, J.; Zhao, X.; Li, T. Detailed CFD modelling of fast pyrolysis of different biomass types in fluidized bed reactors. Can. J. Chem. Eng. 2018, 96, 2043–2052. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, T.; Gao, B.; Meng, R.; Zhou, P.; Chen, G.; Zhan, Y.; Lu, W.; Wang, H. Comparison between hydrogen-rich biogas production from conventional pyrolysis and microwave pyrolysis of sewage sludge: Is microwave pyrolysis always better in the whole temperature range? Int. J. Hydrogen Energy 2021, 46, 23322–23333. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Z.; Chen, L.; Yang, S.; Xie, X.; Gao, M.; Zhao, B.; Si, H.; Li, J.; Hua, D. Catalytic Fast Pyrolysis of Biomass into Aromatic Hydrocarbons over Mo-Modified ZSM-5 Catalysts. Catalysts 2020, 10, 1051. [Google Scholar] [CrossRef]
- Rahman, M.; Liu, R.; Cai, J. Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil—A review. Fuel Processing Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Mihalcik, D.J.; Mullen, C.A.; Boateng, A.A. Screening acidic zeolites for catalytic fast pyrolysis of biomass and its components. J. Anal. Appl. Pyrolysis 2011, 92, 224–232. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Uemura, Y.; Yusup, S.; Sugiura, Y.; Nishiyama, N. In situ catalytic fast pyrolysis of paddy husk pyrolysis vapors over MCM-22 and ITQ-2 zeolites. J. Anal. Appl. Pyrolysis 2015, 114, 32–39. [Google Scholar] [CrossRef]
- Li, Q.; Faramarzi, A.; Zhang, S.; Wang, Y.; Hu, X.; Gholizadeh, M. Progress in catalytic pyrolysis of municipal solid waste. Energy Convers. Manag. 2020, 226, 113525. [Google Scholar] [CrossRef]
- Li, S.; Sanna, A.; Andrésen, J.M. Influence of temperature on pyrolysis of recycled organic matter from municipal solid waste using an activated olivine fluidized bed. Fuel Processing Technol. 2011, 92, 1776–1782. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Liu, J.; Chen, D. Catalytic characteristics of innovative Ni/slag catalysts for syngas production and tar removal from biomass pyrolysis. Int. J. Hydrogen Energy 2019, 44, 11848–11860. [Google Scholar] [CrossRef]
- Karcz, A.; Porada, S. Formation of C1-C3 hydrocarbons during pressure pyrolysis and hydrogasification in relation to structural changes in coal. Fuel 1995, 74, 806–809. [Google Scholar] [CrossRef]
- Li, J.; Yan, R.; Xiao, B.; Liang, D.T.; Lee, D.H. Preparation of Nano-NiO Particles and Evaluation of Their Catalytic Activity in Pyrolyzing Biomass Components. Energy Fuels 2008, 22, 16–23. [Google Scholar] [CrossRef]
- Li, J.; Yan, R.; Xiao, B.; Liang, D.T.; Du, L. Development of Nano-NiO/Al2O3 Catalyst to be Used for Tar Removal in Biomass Gasification. Environ. Sci. Technol. 2008, 42, 6224–6229. [Google Scholar] [CrossRef]
- Xu, T.; Xu, J.; Wu, Y. Hydrogen-Rich Gas Production from Two-Stage Catalytic Pyrolysis of Pine Sawdust with Calcined Dolomite. Catalysts 2022, 12, 131. [Google Scholar] [CrossRef]
- Sripada, P.; Xu, T.; Kibria, M.; Bhattacharya, S. Comparison of entrained flow gasification behaviour of Victorian brown coal and biomass. Fuel 2017, 203, 942–953. [Google Scholar] [CrossRef]



| Residence Time (s) | 12 | 17 | 22 | 30 |
|---|---|---|---|---|
| Syngas (H2 + CO) (%) | 56.31 | 58.10 | 59.64 | 62.27 |
| H2/CO (−) | 0.51 | 0.53 | 0.56 | 0.58 |
| LHV (MJ/Nm3) | 13.40 | 13.29 | 13.53 | 13.43 |
| Dry gas yield (Nm3/kg) | 0.77 | 0.84 | 0.88 | 1.12 |
| Carbon conversion (wt%) | 65.48 | 72.57 | 78.03 | 85.25 |
| Catalytic Temperature (°C) | No Catalyst | 500 | 600 | 700 | 800 |
|---|---|---|---|---|---|
| H2/CO (−) | 0.47 | 1.00 | 0.86 | 0.77 | 0.74 |
| Syngas (H2 + CO) (%) | 45.48 | 67.34 | 76.81 | 81.84 | 84.28 |
| Dry gas yield (Nm3/kg) | 0.36 | 0.58 | 0.62 | 0.78 | 0.97 |
| Carbon conversion (wt%) | 34.23 | 43.57 | 48.03 | 60.67 | 73.06 |
| LHV (MJ/Nm3) | 12.4 | 14.59 | 14.20 | 13.73 | 12.70 |
| Sample | Proximate Analysis (wt%) | Ultimate Analysis (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Moisture | Volatile | Fixed Carbon | Ash | C | H | N | S | |
| Pine sawdust | 9.18 | 62.23 | 15.77 | 11.82 | 46.36 | 5.75 | 2.26 | 0.35 |
| Samples | BET Surface Area (m2/g) | Micropore Surface Area (m2/g) | External Surface Area (m2/g) | Pore Volume (cm2/g) |
|---|---|---|---|---|
| Support, γ-A12O3 | 130.2 | 13.6 | 117.2 | 0.42 |
| Nano-NiO/γ-A12O3 | 125.2 | 10.3 | 114.9 | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Zheng, X.; Xu, J.; Wu, Y. Hydrogen-Rich Gas Production from Two-Stage Catalytic Pyrolysis of Pine Sawdust with Nano-NiO/Al2O3 Catalyst. Catalysts 2022, 12, 256. https://doi.org/10.3390/catal12030256
Xu T, Zheng X, Xu J, Wu Y. Hydrogen-Rich Gas Production from Two-Stage Catalytic Pyrolysis of Pine Sawdust with Nano-NiO/Al2O3 Catalyst. Catalysts. 2022; 12(3):256. https://doi.org/10.3390/catal12030256
Chicago/Turabian StyleXu, Tao, Xiuren Zheng, Jue Xu, and Yongping Wu. 2022. "Hydrogen-Rich Gas Production from Two-Stage Catalytic Pyrolysis of Pine Sawdust with Nano-NiO/Al2O3 Catalyst" Catalysts 12, no. 3: 256. https://doi.org/10.3390/catal12030256







