Research Progress and Reaction Mechanism of CO2 Methanation over Ni-Based Catalysts at Low Temperature: A Review
Abstract
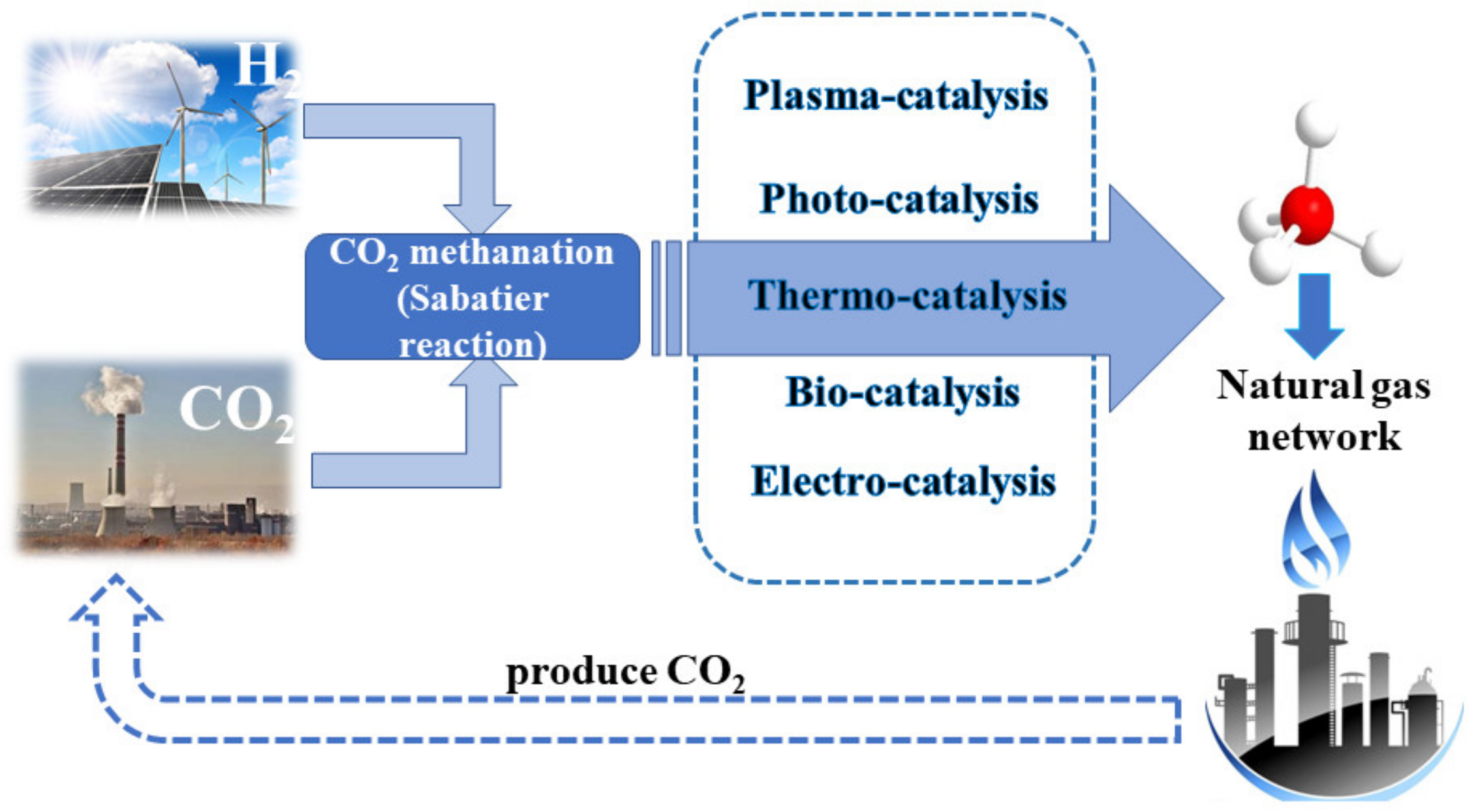
:1. Introduction
2. CO2 Methanation at Low Temperature
2.1. The Nature of Active Sites on CO2 Methanation
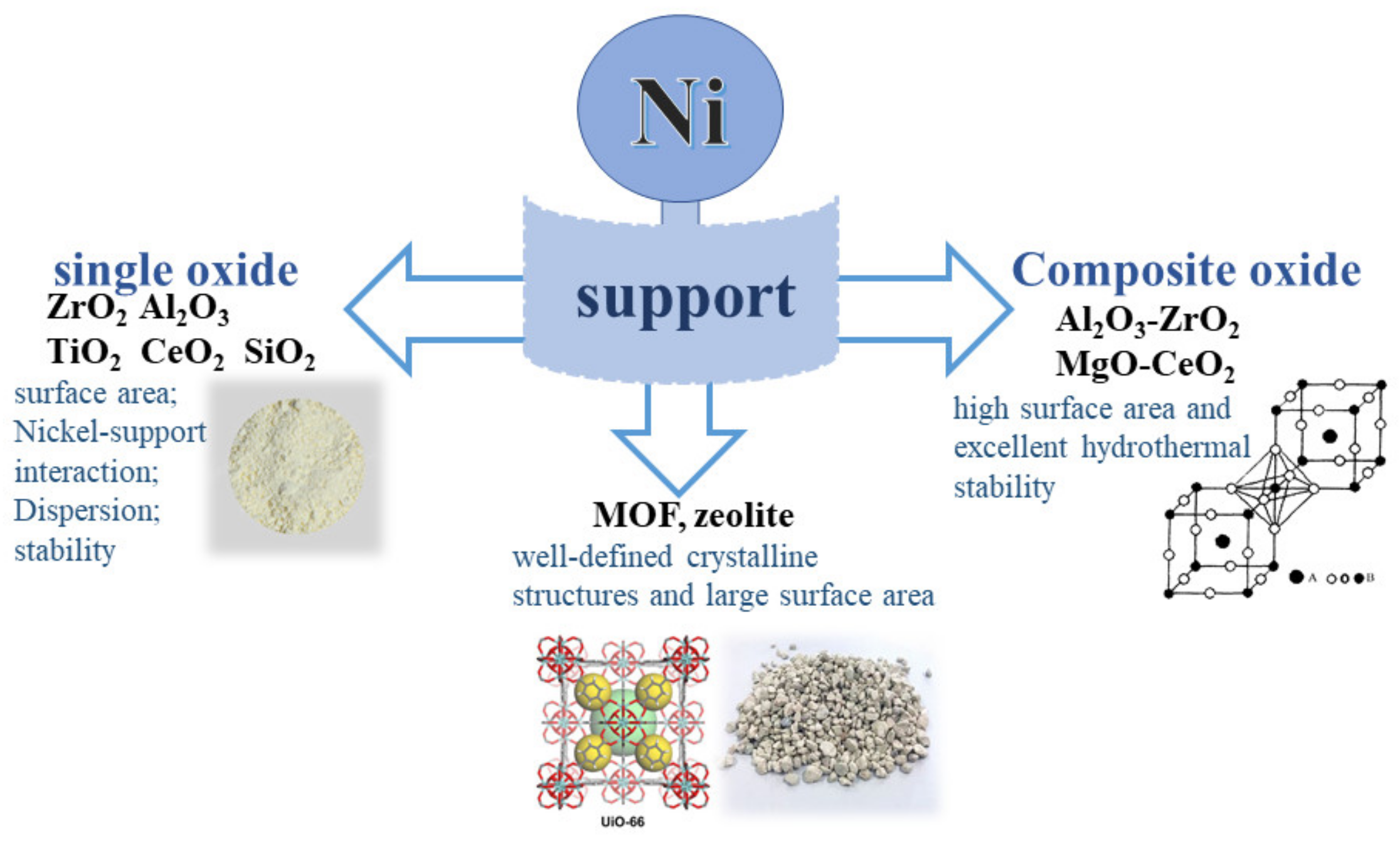
2.2. The Support on CO2 Methanation
2.2.1. Ni-Based Catalysts Supported on Single Oxide Supports
2.2.2. Ni-Based Catalysts Supported on Composite Oxide Supports
2.2.3. Ni-Based Catalysts Supported on Other Supports
2.3. Promoter Effect on CO2 Methanation
2.3.1. Alkaline Earth Oxides Promoted Ni-Based Catalysts
2.3.2. Noble Metals Promoted Ni-Based Catalysts
2.3.3. Rare-Earth Metal Promoted Ni-Based Catalysts
2.3.4. Other Transition Metals and Non-Metallic Elements Promoted Ni-Based Catalysts
2.4. The Effect of Preparation Methods on CO2 Methanation
3. The Reaction Mechanism of CO2 Methanation
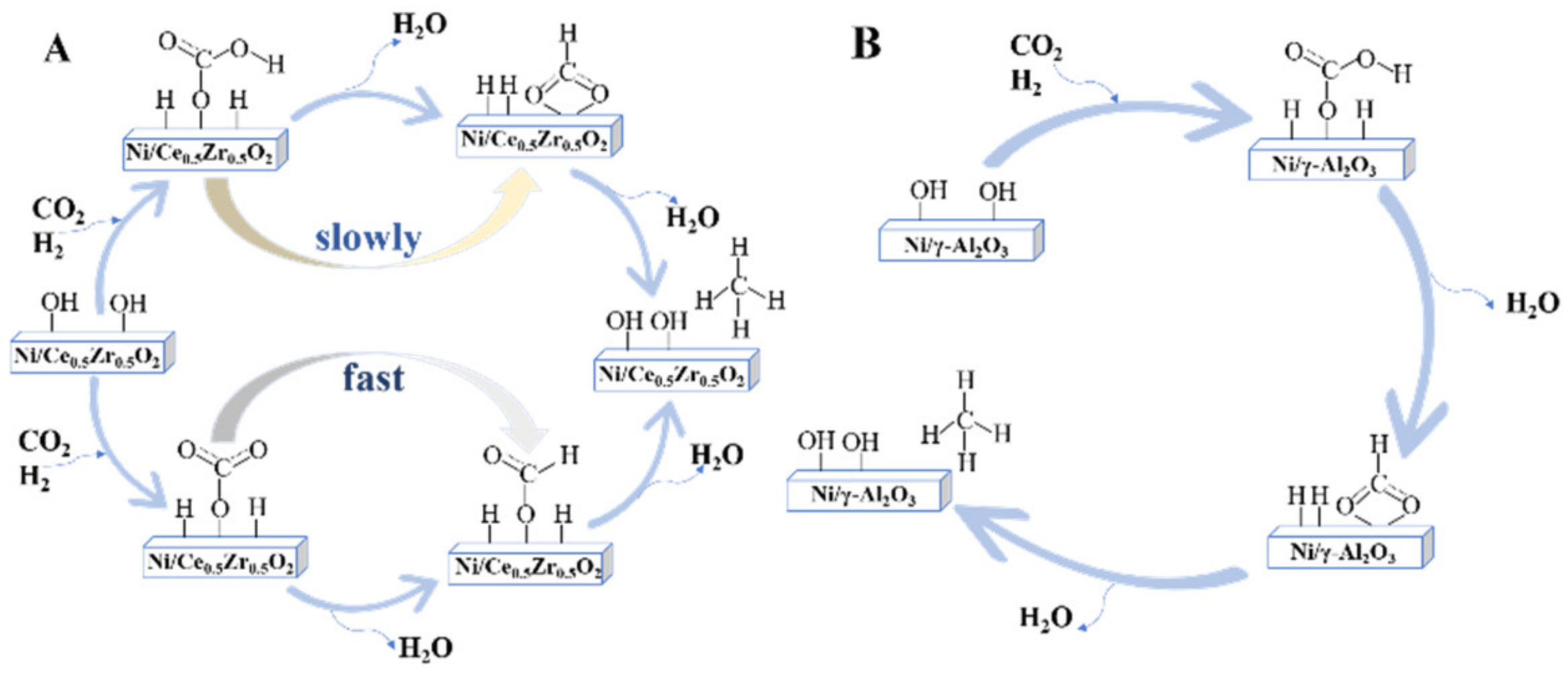
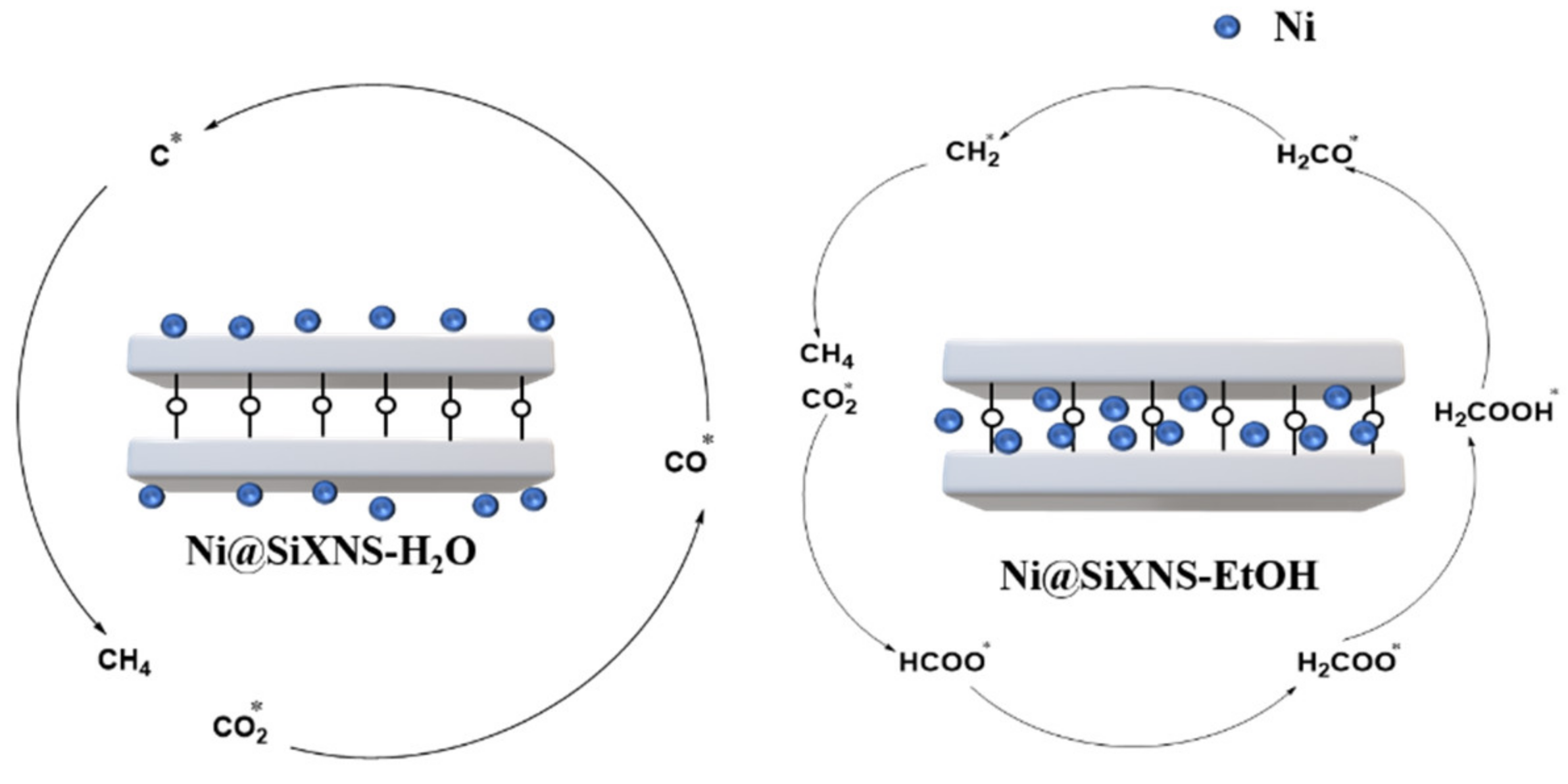
3.1. The Formate Pathway
3.2. The CO Pathway
3.3. The Key Factors of CO2 Methanation Reaction Route
4. Summary and Perspective
- (1)
- Design of CO2 methanation catalysts with high activity at low temperature simultaneously with high carbon deposition resistance and anti-sintering properties;
- (2)
- Try new materials and technologies such as MOF, alloy material, and plasma assisted technology for the design and preparation of CO2 methanation catalysts;
- (3)
- Combine photo-catalysis, electro-catalysis, and plasma-catalysis with traditional thermo-catalysis to integrate their advantages;
- (4)
- Investigate the mechanism of the activation and cleavage of C–O in CO2, and the relationship between CO2 activation and H2 activation as well as provide deep insights into the CO2 methanation reaction pathways and the key factors in the reaction mechanism; and
- (5)
- Combine the theoretical calculations with experiments to explore the role of the active metal, support, and the nickel–support surface in the CO2 methanation reaction process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anwar, M.N.; Fayyaz, A.; Sohail, N.F.; Khokhar, M.F.; Baqar, M.; Yasar, A.; Rasool, K.; Nazir, A.; Raja, M.U.F.; Rehan, M.; et al. CO2 utilization: Turning greenhouse gas into fuels and valuable products. J. Environ. Manag. 2020, 260, 110059. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, J.; Liu, F.; Wu, D. Reaction mechanism of CO2 methanation over Rh/TiO2 catalyst. Fuel 2020, 276, 118093. [Google Scholar] [CrossRef]
- Burger, T.; Koschany, F.; Thomys, O.; Köhler, K.; Hinrichsen, O. CO2 methanation over Fe- and Mn-promoted co-precipitated Ni-Al catalysts: Synthesis, characterization and catalysis study. Appl. Catal. A Gen. 2018, 558, 44–54. [Google Scholar] [CrossRef]
- Ahadzi, E.; Ramyashree, M.; Priya, S.S.; Sudhakar, K.; Tahir, M. CO2 to green fuel: Photocatalytic process optimization study. Sustain. Chem. Pharm. 2021, 24, 100533. [Google Scholar] [CrossRef]
- Silva, T.C.D.; Isha, A.; Chandra, R.; Vijay, V.K.; Subbarao, P.M.V.; Kumar, R.; Chaudhary, V.P.; Singh, H.; Khan, A.A.; Tyagi, V.K.; et al. Enhancing methane production in anaerobic digestion through hydrogen assisted pathways—A state-of-the-art review. Renew. Sustain. Energy Rev. 2021, 151, 111536. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, X.; Zhao, Y.; Zhang, W. Preparation and photocatalytic properties of Cu2ZnSnS4 for H2 production. Mater. Res. Express 2020, 7, 095902. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, B.; Shi, J.; Tan, D.; Zhang, F.; Liu, L.; Tan, X.; Shao, D.; Yang, G.; Zhang, J. An NH2-MIL-125 (Ti)/Pt/g-C3N4 catalyst promoting visible-light photocatalytic H2 production. Sustain. Energy Fuels 2019, 3, 1233–1238. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Y.; Hu, X.; Liu, E.; Fan, J. Enhanced photocatalytic H2 production over dual-cocatalyst-modified g-C3N4 heterojunctions. Chin. J. Catal. 2019, 40, 434–445. [Google Scholar] [CrossRef]
- Chen, J.-L.; Liu, M.-M.; Xie, S.-Y.; Yue, L.-J.; Gong, F.-L.; Chai, K.-M.; Zhang, Y.-H. Cu2O-loaded TiO2 heterojunction composites for enhanced photocatalytic H2 production. J. Mol. Struct. 2021, 1247, 131294. [Google Scholar] [CrossRef]
- Tong, M.; Lan, Y.; Yang, Q.; Zhong, C. High-throughput computational screening and design of nanoporous materials for methane storage and carbon dioxide capture. Green Energy Environ. 2018, 3, 107–119. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, E.A.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2011, 112, 724–781. [Google Scholar] [CrossRef]
- Ray, K.; Deo, G. A potential descriptor for the CO2 hydrogenation to CH4 over Al2O3 supported Ni and Ni-based alloy catalysts. Appl. Catal. B Environ. 2017, 218, 525–537. [Google Scholar] [CrossRef]
- Alonso, A.; Vico, J.M.; Markeb, A.A.; Busquets-Fité, M.; Komilis, D.; Puntes, V.; Sánchez, A.; Font, X. Critical review of existing nanomaterial adsorbents to capture carbon dioxide and methane. Sci. Total Environ. 2017, 595, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Akhter, P.; Russo, N.; Saracco, G. Novel Ti-KIT-6 material for the photocatalytic reduction of carbon dioxide to methane. Catal. Commun. 2013, 36, 58–62. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Li, L.; Hu, C.; Da Costa, P. Dry reforming of methane over Ni–ZrOx catalysts doped by manganese: On the effect of the stability of the structure during time on stream. Appl. Catal. A Gen. 2021, 617, 118120. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, Y.; Costa, P.D.; Hu, C. Highly Carbon-Resistant Y Doped NiO–ZrOm Catalysts for Dry Reforming of Methane. Catalysts 2019, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Deng, G.; Zhang, G.; Zhu, X.; Guo, Q.; Liao, X.; Chen, X.; Li, K. Optimized Ni-based catalysts for methane reforming with O2-containing CO2. Appl. Catal. B Environ. 2021, 289, 120033. [Google Scholar] [CrossRef]
- Charisiou, N.; Siakavelas, G.; Papageridis, K.; Baklavaridis, A.; Tzounis, L.; Avraam, D.; Goula, M. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Bereketidou, O.; Goula, M. Biogas reforming for syngas production over nickel supported on ceria–alumina catalysts. Catal. Today 2012, 195, 93–100. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Wang, Y.; Li, L.; Zeng, W.; Li, G.; Hu, C. Steam reforming of CH4 at low temperature on Ni/ZrO2 catalyst: Effect of H2O/CH4 ratio on carbon deposition. Int. J. Hydrogen Energy 2020, 45, 14281–14292. [Google Scholar] [CrossRef]
- Chen, S.; Pei, C.; Gong, J. Insights into interface engineering in steam reforming reactions for hydrogen production. Energy Environ. Sci. 2019, 12, 3473–3495. [Google Scholar] [CrossRef]
- Cai, L.; He, T.; Xiang, Y.; Guan, Y. Study on the reaction pathways of steam methane reforming for H2 production. Energy 2020, 207, 118296. [Google Scholar] [CrossRef]
- Fan, L.; Mokhov, A.; Saadabadi, S.A.; Brandon, N.; Aravind, P.V. Methane steam reforming reaction in solid oxide fuel cells: Influence of electrochemical reaction and anode thickness. J. Power Sources 2021, 507, 230276. [Google Scholar] [CrossRef]
- Li, S.; Guo, S.; Gong, D.; Kang, N.; Fang, K.-G.; Liu, Y. Nano composite composed of MoOx-La2O3-Ni on SiO2 for storing hydrogen into CH4 via CO2 methanation. Int. J. Hydrogen Energy 2019, 44, 1597–1609. [Google Scholar] [CrossRef]
- Alarcón, A.; Guilera, J.; Díaz-López, J.A.; Andreu, T. Optimization of nickel and ceria catalyst content for synthetic natural gas production through CO2 methanation. Fuel Process. Technol. 2019, 193, 114–122. [Google Scholar] [CrossRef]
- Xing, Y.; Ma, Z.; Su, W.; Wang, Q.; Wang, X.; Zhang, H. Analysis of Research Status of CO2 Conversion Technology Based on Bibliometrics. Catalysts 2020, 10, 370. [Google Scholar] [CrossRef] [Green Version]
- Panagiotopoulou, P. Methanation of CO2 over alkali-promoted Ru/TiO2 catalysts: II. Effect of alkali additives on the reaction pathway. Appl. Catal. B Environ. 2018, 236, 162–170. [Google Scholar] [CrossRef]
- Rasheed, T.; Shafi, S.; Anwar, M.T.; Rizwan, K.; Ahmad, T.; Bilal, M. Revisiting Photo and Electro-catalytic Modalities for Sustainable Conversion of CO2. Appl. Catal. A Gen. 2021, 623, 118248. [Google Scholar] [CrossRef]
- Chang, K.; Wang, T.; Chen, J.G. Hydrogenation of CO2 to methanol over CuCeTiOx catalysts. Appl. Catal. B Environ. 2017, 206, 704–711. [Google Scholar] [CrossRef]
- Dubey, A.; Nencini, L.; Fayzullin, R.R.; Nervi, C.; Khusnutdinova, J.R. Bio-Inspired Mn(I) Complexes for the Hydrogenation of CO2 to Formate and Formamide. ACS Catal. 2017, 7, 3864–3868. [Google Scholar] [CrossRef]
- Frei, M.S.; Mondelli, C.; Garcia-Muelas, R.; Morales-Vidal, J.; Philipp, M.; Safonova, O.V.; Lopez, N.; Stewart, J.A.; Ferre, D.C.; Perez-Ramirez, J. Nanostructure of nickel-promoted indium oxide catalysts drives selectivity in CO2 hydrogenation. Nat. Commun. 2021, 12, 1960. [Google Scholar] [CrossRef]
- García-Trenco, A.; Regoutz, A.; White, E.R.; Payne, D.; Shaffer, M.S.; Williams, C.K. PdIn intermetallic nanoparticles for the Hydrogenation of CO2 to Methanol. Appl. Catal. B Environ. 2018, 220, 9–18. [Google Scholar] [CrossRef]
- Ramyashree, M.S.; Priya, S.S.; Freudenberg, N.C.; Sudhakar, K.; Tahir, M. Metal-organic framework-based photocatalysts for carbon dioxide reduction to methanol: A review on progress and application. J. CO2 Util. 2020, 43, 101374. [Google Scholar] [CrossRef]
- Francis, A.; Kumar, H.; Sudhakar, K.; Tahir, M. A review on recent developments in solar photoreactors for carbon dioxide conversion to fuels. J. CO2 Util. 2021, 47, 101515. [Google Scholar] [CrossRef]
- Göbel, C.; Schmidt, S.; Froese, C.; Fu, Q.; Chen, Y.-T.; Pan, Q.; Muhler, M. Structural evolution of bimetallic Co-Cu catalysts in CO hydrogenation to higher alcohols at high pressure. J. Catal. 2020, 383, 33–41. [Google Scholar] [CrossRef]
- Goryachev, A.; Pustovarenko, A.; Shterk, G.; Alhajri, N.S.; Jamal, A.; Albuali, M.; van Koppen, L.; Khan, I.S.; Russkikh, A.; Ramirez, A.; et al. A Multi-Parametric Catalyst Screening for CO2 Hydrogenation to Ethanol. ChemCatChem 2021, 13, 3324–3332. [Google Scholar] [CrossRef]
- Lian, Y.; Fang, T.; Zhang, Y.; Liu, B.; Li, J. Hydrogenation of CO2 to alcohol species over Co@Co3O4/C-N catalysts. J. Catal. 2019, 379, 46–51. [Google Scholar] [CrossRef]
- Yang, C.; Mu, R.; Wang, G.; Song, J.; Tian, H.; Zhao, Z.-J.; Gong, J. Hydroxyl-mediated ethanol selectivity of CO2 hydrogenation. Chem. Sci. 2019, 10, 3161–3167. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Porosoff, M.D. Development of Tandem Catalysts for CO2 Hydrogenation to Olefins. ACS Catal. 2019, 9, 2639–2656. [Google Scholar] [CrossRef]
- Aldana, P.U.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyk, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar] [CrossRef]
- Aziz, M.; Jalil, A.; Triwahyono, S.; Sidik, S. Methanation of carbon dioxide on metal-promoted mesostructured silica nanoparticles. Appl. Catal. A Gen. 2014, 486, 115–122. [Google Scholar] [CrossRef]
- Budi, C.S.; Wu, H.-C.; Chen, C.-S.; Saikia, D.; Kao, H.-M. Ni Nanoparticles Supported on Cage-Type Mesoporous Silica for CO2 Hydrogenation with High CH4 Selectivity. ChemSusChem 2016, 9, 2326–2331. [Google Scholar] [CrossRef]
- Chen, H.; Liu, P.; Liu, J.; Feng, X.; Zhou, S. Mechanochemical in-situ incorporation of Ni on MgO/MgH2 surface for the selective O-/C-terminal catalytic hydrogenation of CO2 to CH4. J. Catal. 2021, 394, 397–405. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G.; Jiang, X.; Wang, J.; Song, C.; Guo, X. Insight into the role of Fe5C2 in CO2 catalytic hydrogenation to hydrocarbons. Catal. Today 2020, 371, 162–170. [Google Scholar] [CrossRef]
- Fan, W.K.; Tahir, M. Recent trends in developments of active metals and heterogenous materials for catalytic CO2 hydrogenation to renewable methane: A review. J. Environ. Chem. Eng. 2021, 9, 105460. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Wiese, K.; Parlinska-Wojtan, M.; Rabeah, J.; Brückner, A.; Behm, R.J. Encapsulation of Ru nanoparticles: Modifying the reactivity toward CO and CO2 methanation on highly active Ru/TiO2 catalysts. Appl. Catal. B Environ. 2020, 270, 118846. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. Mechanistic study of low temperature CO2 methanation over Rh/TiO2 catalysts. J. Catal. 2013, 301, 141–153. [Google Scholar] [CrossRef]
- Wang, X.; Hong, Y.; Shi, H.; Szanyi, J. Kinetic modeling and transient DRIFTS–MS studies of CO2 methanation over Ru/Al2O3 catalysts. J. Catal. 2016, 343, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Ashok, J.; Pati, S.; Hongmanorom, P.; Tianxi, Z.; Junmei, C.; Kawi, S. A review of recent catalyst advances in CO2 methanation processes. Catal. Today 2020, 356, 471–489. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Zhu, S.; Liang, S.; Tong, Y.; An, X.; Long, J.; Fu, X.; Wang, X. Photocatalytic reduction of CO2 with H2O to CH4 on Cu(I) supported TiO2 nanosheets with defective {001} facets. Phys. Chem. Chem. Phys. 2015, 17, 9761–9770. [Google Scholar] [CrossRef]
- He, Z.; Tang, J.; Shen, J.; Chen, J.; Song, S. Enhancement of photocatalytic reduction of CO2 to CH4 over TiO2 nanosheets by modifying with sulfuric acid. Appl. Surf. Sci. 2016, 364, 416–427. [Google Scholar] [CrossRef]
- Yan, Y.; Yu, Y.; Huang, S.; Yang, Y.; Yang, X.; Yin, S.; Cao, Y. Adjustment and Matching of Energy Band of TiO2-Based Photocatalysts by Metal Ions (Pd, Cu, Mn) for Photoreduction of CO2 into CH4. J. Phys. Chem. C 2017, 121, 1089–1098. [Google Scholar] [CrossRef]
- Tan, D.; Zhang, J.; Shi, J.; Li, S.; Zhang, B.; Tan, X.; Zhang, F.; Liu, L.; Shao, D.; Han, B. Photocatalytic CO2 Transformation to CH4 by Ag/Pd Bimetals Supported on N-Doped TiO2 Nanosheet. ACS Appl. Mater. Interfaces 2018, 10, 24516–24522. [Google Scholar] [CrossRef]
- Han, C.; Wang, B.; Wu, N.; Shen, S.; Wang, Y. Deep and selective photoreduction of CO2 to CH4 over ultrafine Pt nanoparticles-decorated SiC nanosheets. Appl. Surf. Sci. 2020, 515, 145952. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Fan, J.-S.; Tan, R.-Q.; Yao, H.-C.; Peng, Y.; Liu, Q.-C.; Li, Z.-J. Selective Photocatalytic Reduction of CO2 to CH4 Modulated by Chloride Modification on Bi2WO6 Nanosheets. ACS Appl. Mater. Interfaces 2020, 12, 54507–54516. [Google Scholar] [CrossRef]
- Tahir, M. Hierarchical 3D VO2/ZnV2O4 microspheres as an excellent visible light photocatalyst for CO2 reduction to solar fuels. Appl. Surf. Sci. 2019, 467–468, 1170–1180. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B. Constructing a Stable 2D/2D Heterojunction of Oxygen-Cluster-Modified Ti3AlC2 MAX Cocatalyst with Proton-Rich C3N4 for Highly Efficient Photocatalytic CO2 Methanation. Ind. Eng. Chem. Res. 2020, 59, 9841–9857. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M. Synergistic Effect of Co/La in Oxygen Vacancy Rich Ternary CoAlLa Layered Double Hydroxide with Enhanced Reductive Sites for Selective Photoreduction of CO2 to CH4. Energy Fuels 2021, 35, 8922–8943. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R.; Tahir, M. Indirect Z-scheme heterojunction of NH2-MIL-125(Ti) MOF/g-C3N4 nanocomposite with RGO solid electron mediator for efficient photocatalytic CO2 reduction to CO and CH4. J. Environ. Chem. Eng. 2021, 9, 105600. [Google Scholar] [CrossRef]
- Geppert, F.; Liu, D.; van Eerten-Jansen, M.; Weidner, E.; Buisman, C.; Ter Heijne, A. Bioelectrochemical Power-to-Gas: State of the Art and Future Perspectives. Trends Biotechnol. 2016, 34, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Peng, X.; Liu, X.; Sun, X.; Shi, J.; Han, L.; Li, G.; Luo, J. Efficient and stable electroreduction of CO2to CH4on CuS nanosheet arrays. J. Mater. Chem. A 2017, 5, 20239–20243. [Google Scholar] [CrossRef]
- Ghaib, K.; Nitz, K.; Ben-Fares, F.-Z. Katalytische Methanisierung von Kohlenstoffdioxid. Chem. Ing. Tech. 2016, 88, 1435–1443. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas projects review: Lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sustain. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Aryal, N.; Kvist, T.; Ammam, F.; Pant, D.; Ottosen, L.D. An overview of microbial biogas enrichment. Bioresour. Technol. 2018, 264, 359–369. [Google Scholar] [CrossRef]
- Azzolina-Jury, F. Novel boehmite transformation into γ-alumina and preparation of efficient nickel base alumina porous extrudates for plasma-assisted CO2 methanation. J. Ind. Eng. Chem. 2018, 71, 410–424. [Google Scholar] [CrossRef]
- Azzolina-Jury, F.; Bento, D.; Henriques, C.; Thibault-Starzyk, F. Chemical engineering aspects of plasma-assisted CO2 hydrogenation over nickel zeolites under partial vacuum. J. CO2 Util. 2017, 22, 97–109. [Google Scholar] [CrossRef]
- Neyts, E.; Bogaerts, A. Understanding plasma catalysis through modelling and simulation—A review. J. Phys. D Appl. Phys. 2014, 47. [Google Scholar] [CrossRef]
- Kho, E.T.; Tan, T.H.; Lovell, E.; Wong, R.J.; Scott, J.; Amal, R. A review on photo-thermal catalytic conversion of carbon dioxide. Green Energy Environ. 2017, 2, 204–217. [Google Scholar] [CrossRef]
- Baysal, Z.; Kureti, S. CO2 methanation on Mg-promoted Fe catalysts. Appl. Catal. B Environ. 2019, 262, 118300. [Google Scholar] [CrossRef]
- Dębek, R.; Azzolina-Jury, F.; Travert, A.; Maugé, F. A review on plasma-catalytic methanation of carbon dioxide—Looking for an efficient catalyst. Renew. Sustain. Energy Rev. 2019, 116, 109427. [Google Scholar] [CrossRef]
- Renda, S.; Ricca, A.; Palma, V. Precursor salts influence in Ruthenium catalysts for CO2 hydrogenation to methane. Appl. Energy 2020, 279, 115767. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Zhao, Q.; Hu, C. The Effect of Si on CO2 Methanation over Ni-xSi/ZrO2 Catalysts at Low Temperature. Catalysts 2021, 11, 67. [Google Scholar] [CrossRef]
- Martínez, J.; Hernández, E.; Alfaro, S.; Medina, R.L.; Aguilar, G.V.; Albiter, E.; Valenzuela, M.A. High Selectivity and Stability of Nickel Catalysts for CO2 Methanation: Support Effects. Catalysts 2018, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Tu, J.; Wu, H.; Qian, Q.; Han, S.; Chu, M.; Jia, S.; Feng, R.; Zhai, J.; He, M.; Han, B. Low temperature methanation of CO2 over an amorphous cobalt-based catalyst. Chem. Sci. 2021, 12, 3937–3943. [Google Scholar] [CrossRef]
- Mateo, D.; Albero, J.; García, H. Titanium-Perovskite-Supported RuO2 Nanoparticles for Photocatalytic CO2 Methanation. Joule 2019, 3, 1949–1962. [Google Scholar] [CrossRef]
- Falbo, L.; Martinelli, M.; Visconti, C.G.; Lietti, L.; Bassano, C.; Deiana, P. Kinetics of CO2 methanation on a Ru-based catalyst at process conditions relevant for Power-to-Gas applications. Appl. Catal. B Environ. 2018, 225, 354–363. [Google Scholar] [CrossRef]
- Liu, Q.; Bian, B.; Fan, J.; Yang, J. Cobalt doped Ni based ordered mesoporous catalysts for CO2 methanation with enhanced catalytic performance. Int. J. Hydrogen Energy 2018, 43, 4893–4901. [Google Scholar] [CrossRef]
- Xu, X.; Liu, L.; Tong, Y.; Fang, X.; Xu, J.; Jiang, D.-E.; Wang, X. Facile Cr3+-Doping Strategy Dramatically Promoting Ru/CeO2 for Low-Temperature CO2 Methanation: Unraveling the Roles of Surface Oxygen Vacancies and Hydroxyl Groups. ACS Catal. 2021, 11, 5762–5775. [Google Scholar] [CrossRef]
- Dreyer, J.; Li, P.; Zhang, L.; Beh, G.K.; Zhang, R.; Sit, P.; Teoh, W.Y. Influence of the oxide support reducibility on the CO2 methanation over Ru-based catalysts. Appl. Catal. B Environ. 2017, 219, 715–726. [Google Scholar] [CrossRef]
- Iqbal, M.M.A.; Abu Bakar, W.A.W.; Toemen, S.; Razak, F.I.A.; Azelee, N.I.W. Optimization study by Box-Behnken design (BBD) and mechanistic insight of CO2 methanation over Ru-Fe-Ce/γ-Al2O3 catalyst by in-situ FTIR technique. Arab. J. Chem. 2020, 13, 4170–4179. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.M.; Park, J.-N. Bifunctional Mechanism of CO2 Methanation on Pd-MgO/SiO2 Catalyst: Independent Roles of MgO and Pd on CO2 Methanation. J. Phys. Chem. C 2010, 114, 7128–7131. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, Q.; Wang, S.; Chen, Y.; Zhang, M. The synergistic effect of Pd NPs and UiO-66 for enhanced activity of carbon dioxide methanation. J. CO2 Util. 2019, 31, 167–172. [Google Scholar] [CrossRef]
- Tada, S.; Ochieng, O.J.; Kikuchi, R.; Haneda, T.; Kameyama, H. Promotion of CO2 methanation activity and CH4 selectivity at low temperatures over Ru/CeO2/Al2O3 catalysts. Int. J. Hydrog. Energy 2014, 39, 10090–10100. [Google Scholar] [CrossRef]
- Chai, S.; Men, Y.; Wang, J.; Liu, S.; Song, Q.; An, W.; Kolb, G. Boosting CO2 methanation activity on Ru/TiO2 catalysts by exposing (001) facets of anatase TiO2. J. CO2 Util. 2019, 33, 242–252. [Google Scholar] [CrossRef]
- Abe, T.; Tanizawa, M.; Watanabe, K.; Taguchi, A. CO2 methanation property of Ru nanoparticle-loaded TiO2 prepared by a polygonal barrel-sputtering method. Energy Environ. Sci. 2009, 2, 315–321. [Google Scholar] [CrossRef]
- Lippi, R.; Howard, S.C.; Barron, H.; Easton, C.D.; Madsen, I.C.; Waddington, L.J.; Vogt, C.; Hill, M.R.; Sumby, C.J.; Doonan, C.J.; et al. Highly active catalyst for CO2 methanation derived from a metal organic framework template. J. Mater. Chem. A 2017, 5, 12990–12997. [Google Scholar] [CrossRef]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; González-Marcos, J.A.; Bueno-López, A.; González-Velasco, J.R. Effect of metal loading on the CO2 methanation: A comparison between alumina supported Ni and Ru catalysts. Catalysis Today 2020, 356, 419–432. [Google Scholar] [CrossRef]
- Bando, K.; Soga, K.; Kunimori, K.; Ichikuni, N.; Okabe, K.; Kusama, H.; Sayama, K.; Arakawa, H. CO2 hydrogenation activity and surface structure of zeolite-supported Rh catalysts. Appl. Catal. A Gen. 1998, 173, 47–60. [Google Scholar] [CrossRef]
- Botzolaki, G.; Goula, G.; Rontogianni, A.; Nikolaraki, E.; Chalmpes, N.; Zygouri, P.; Karakassides, M.; Gournis, D.; Charisiou, N.; Goula, M.; et al. CO2 Methanation on Supported Rh Nanoparticles: The combined Effect of Support Oxygen Storage Capacity and Rh Particle Size. Catalysts 2020, 10, 944. [Google Scholar] [CrossRef]
- Martin, N.M.; Hemmingsson, F.; Schaefer, A.; Ek, M.; Merte, L.R.; Hejral, U.; Gustafson, J.; Skoglundh, M.; Dippel, A.-C.; Gutowski, O.; et al. Structure–function relationship for CO2 methanation over ceria supported Rh and Ni catalysts under atmospheric pressure conditions. Catal. Sci. Technol. 2019, 9, 1644–1653. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shi, H.; Kwak, J.H.; Szanyi, J. Mechanism of CO2 Hydrogenation on Pd/Al2O3 Catalysts: Kinetics and Transient DRIFTS-MS Studies. ACS Catal. 2015, 5, 6337–6349. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Huang, J.; Huang, J.; Zhan, G.; Li, Q. Enhanced active site extraction from perovskite LaCoO3 using encapsulated PdO for efficient CO2 methanation. J. Energy Chem. 2020, 53, 9–19. [Google Scholar] [CrossRef]
- Park, J.-N.; McFarland, E.W. A highly dispersed Pd–Mg/SiO2 catalyst active for methanation of CO2. J. Catal. 2009, 266, 92–97. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, T.; Zhang, H.; Xie, H.; Feng, Y. Carbon dioxide methanation on ordered mesoporous CO/KIT-6 catalyst. Chem. Eng. Commun. 2013, 201, 233–240. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, T.; Xie, H.; Zheng, X. Effects of structure on the carbon dioxide methanation performance of Co-based catalysts. Int. J. Hydrogen Energy 2013, 38, 10012–10018. [Google Scholar] [CrossRef]
- Li, W.; Nie, X.; Jiang, X.; Zhang, A.; Ding, F.; Liu, M.; Liu, Z.; Guo, X.; Song, C. ZrO2 support imparts superior activity and stability of Co catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 220, 397–408. [Google Scholar] [CrossRef]
- Nam, H.; Kim, J.H.; Kim, H.; Kim, M.J.; Jeon, S.-G.; Jin, G.-T.; Won, Y.; Hwang, B.W.; Lee, S.-Y.; Baek, J.-I.; et al. CO2 methanation in a bench-scale bubbling fluidized bed reactor using Ni-based catalyst and its exothermic heat transfer analysis. Energy 2020, 214, 118895. [Google Scholar] [CrossRef]
- Ye, R.-P.; Liao, L.; Reina, T.R.; Liu, J.; Chevella, D.; Jin, Y.; Fan, M.; Liu, J. Engineering Ni/SiO2 catalysts for enhanced CO2 methanation. Fuel 2020, 285, 119151. [Google Scholar] [CrossRef]
- Lin, X.; Wang, S.; Tu, W.; Hu, Z.; Ding, Z.; Hou, Y.; Xu, R.; Dai, W. MOF-derived hierarchical hollow spheres composed of carbon-confined Ni nanoparticles for efficient CO2 methanation. Catal. Sci. Technol. 2019, 9, 731–738. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Jia, A.; Hu, G.; Jiao, Z.; Liu, Y.; Zhang, X. Role of surface Ni and Ce species of Ni/CeO2 catalyst in CO2 methanation. Appl. Surf. Sci. 2016, 383, 248–252. [Google Scholar] [CrossRef]
- Kirchner, J.; Anolleck, J.K.; Lösch, H.; Kureti, S. Methanation of CO2 on iron based catalysts. Appl. Catal. B Environ. 2018, 223, 47–59. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent trend in thermal catalytic low temperature CO2 methanation: A critical review. Catal. Today 2020, 368, 2–19. [Google Scholar] [CrossRef]
- He, F.; Zhuang, J.; Lu, B.; Liu, X.; Zhang, J.; Gu, F.; Zhu, M.; Xu, J.; Zhong, Z.; Xu, G.; et al. Ni-based catalysts derived from Ni-Zr-Al ternary hydrotalcites show outstanding catalytic properties for low-temperature CO2 methanation. Appl. Catal. B Environ. 2021, 293, 120218. [Google Scholar] [CrossRef]
- Siakavelas, G.; Charisiou, N.; AlKhoori, S.; AlKhoori, A.; Sebastian, V.; Hinder, S.; Baker, M.; Yentekakis, I.; Polychronopoulou, K.; Goula, M. Highly selective and stable nickel catalysts supported on ceria promoted with Sm2O3, Pr2O3 and MgO for the CO2 methanation reaction. Appl. Catal. B Environ. 2020, 282, 119562. [Google Scholar] [CrossRef]
- Sholeha, N.A.; Jannah, L.; Rohma, H.N.; Widiastuti, N.; Prasetyoko, D.; Jalil, A.A.; Bahruji, H. Synthesis of zeolite NaY from dealuminated metakaolin as Ni support for CO2 hydrogenation to methane. Clays Clay Miner. 2020, 68, 513–523. [Google Scholar] [CrossRef]
- Do, J.Y.; Park, N.-K.; Seo, M.W.; Lee, D.; Ryu, H.-J.; Kang, M. Effective thermocatalytic carbon dioxide methanation on Ca-inserted NiTiO3 perovskite. Fuel 2020, 271, 117624. [Google Scholar] [CrossRef]
- Everett, O.E.; Zonetti, P.C.; Alves, O.C.; de Avillez, R.R.; Appel, L.G. The role of oxygen vacancies in the CO2 methanation employing Ni/ZrO2 doped with Ca. Int. J. Hydrogen Energy 2020, 45, 6352–6359. [Google Scholar] [CrossRef]
- Hongmanorom, P.; Ashok, J.; Zhang, G.; Bian, Z.; Wai, M.H.; Zeng, Y.; Xi, S.; Borgna, A.; Kawi, S. Enhanced performance and selectivity of CO2 methanation over phyllosilicate structure derived Ni-Mg/SBA-15 catalysts. Appl. Catal. B Environ. 2020, 282, 119564. [Google Scholar] [CrossRef]
- Lin, J.; Ma, C.; Luo, J.; Kong, X.; Xu, Y.; Ma, G.; Wang, J.; Zhang, C.; Li, Z.; Ding, M. Preparation of Ni based mesoporous Al2O3 catalyst with enhanced CO2 methanation performance. RSC Adv. 2019, 9, 8684–8694. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, S.N.; Chong, C.C.; Setiabudi, H.D.; Cheng, Y.W.; Teh, L.P.; Jalil, A.A. Ni/Fibrous type SBA-15: Highly active and coke resistant catalyst for CO2 methanation. Chem. Eng. Sci. 2020, 229, 116141. [Google Scholar] [CrossRef]
- Ye, R.-P.; Li, Q.; Gong, W.; Wang, T.; Razink, J.J.; Lin, L.; Qin, Y.-Y.; Zhou, Z.; Adidharma, H.; Tang, J.; et al. High-performance of nanostructured Ni/CeO2 catalyst on CO2 methanation. Appl. Catal. B Environ. 2019, 268, 118474. [Google Scholar] [CrossRef]
- Gnanakumar, E.S.; Chandran, N.; Kozhevnikov, I.V.; Grau-Atienza, A.; Ramos-Fernandez, E.V.; Sepulveda-Escribano, A.; Shiju, N.R. Highly efficient nickel-niobia composite catalysts for hydrogenation of CO2 to methane. Chem. Eng. Sci. 2018, 194, 2–9. [Google Scholar] [CrossRef]
- Moghaddam, S.V.; Rezaei, M.; Meshkani, F.; Daroughegi, R. Synthesis of nanocrystalline mesoporous Ni/Al2O3SiO2 catalysts for CO2 methanation reaction. Int. J. Hydrogen Energy 2018, 43, 19038–19046. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, T.; Dong, L.; Qin, Z.; Ji, H. Ni/bentonite catalysts prepared by solution combustion method for CO2 methanation. Chin. J. Chem. Eng. 2018, 26, 2361–2367. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, T.; Dong, L.; Su, T.; Li, B.; Luo, X.; Xie, X.; Qin, Z.; Xu, C.; Ji, H. Mn Modified Ni/Bsentonite for CO2 Methanation. Catalysts 2018, 8, 646. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Ma, C.; Wang, Q.; Xu, Y.; Ma, G.; Wang, J.; Wang, H.; Dong, C.; Zhang, C.; Ding, M. Enhanced low-temperature performance of CO2 methanation over mesoporous Ni/Al2O3-ZrO2 catalysts. Appl. Catal. B Environ. 2018, 243, 262–272. [Google Scholar] [CrossRef]
- Shang, X.; Deng, D.; Wang, X.; Xuan, W.; Zou, X.; Ding, W.; Lu, X. Enhanced low-temperature activity for CO2 methanation over Ru doped the Ni/CexZr(1−x)O2 catalysts prepared by one-pot hydrolysis method. Int. J. Hydrog. Energy 2018, 43, 7179–7189. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Liu, Y.; Wang, S. CO2 methanation on the catalyst of Ni/MCM-41 promoted with CeO2. Sci. Total Environ. 2018, 625, 686–695. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Orooji, Y. Promoted nickel-based catalysts on modified mesoporous silica support: The role of yttria and magnesia on CO2 methanation. Microporous Mesoporous Mater. 2020, 306, 110455. [Google Scholar] [CrossRef]
- Wang, W.; Chu, W.; Wang, N.; Yang, W.; Jiang, C. Mesoporous nickel catalyst supported on multi-walled carbon nanotubes for carbon dioxide methanation. Int. J. Hydrogen Energy 2015, 41, 967–975. [Google Scholar] [CrossRef]
- Muroyama, H.; Tsuda, Y.; Asakoshi, T.; Masitah, H.; Okanishi, T.; Matsui, T.; Eguchi, K. Carbon dioxide methanation over Ni catalysts supported on various metal oxides. J. Catal. 2016, 343, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Men, Y.; Liu, S.; Wang, J.; Wang, K.; Tang, Y.; An, W.; Pan, X.; Li, L. Remarkably efficient and stable Ni/Y2O3 catalysts for CO2 methanation: Effect of citric acid addition. Appl. Catal. B Environ. 2021, 293, 120206. [Google Scholar] [CrossRef]
- Cho, E.H.; Park, Y.-K.; Park, K.Y.; Song, D.; Koo, K.Y.; Jung, U.; Yoon, W.R.; Ko, C.H. Simultaneous impregnation of Ni and an additive via one-step melt-infiltration: Effect of alkaline-earth metal (Ca, Mg, Sr, and Ba) addition on Ni/γ-Al2O3 for CO2 methanation. Chem. Eng. J. 2021, 428, 131393. [Google Scholar] [CrossRef]
- Hongmanorom, P.; Ashok, J.; Chirawatkul, P.; Kawi, S. Interfacial synergistic catalysis over Ni nanoparticles encapsulated in mesoporous ceria for CO2 methanation. Appl. Catal. B Environ. 2021, 297, 120454. [Google Scholar] [CrossRef]
- Quindimil, A.; Bacariza, M.C.; González-Marcos, J.A.; Henriques, C.; González-Velasco, J.R. Enhancing the CO2 methanation activity of γ-Al2O3 supported mono- and bi-metallic catalysts prepared by glycerol assisted impregnation. Appl. Catal. B Environ. 2021, 296, 120322. [Google Scholar] [CrossRef]
- Zhu, M.; Tian, P.; Cao, X.; Chen, J.; Pu, T.; Shi, B.; Xu, J.; Moon, J.; Wu, Z.; Han, Y.-F. Vacancy engineering of the nickel-based catalysts for enhanced CO2 methanation. Appl. Catal. B Environ. 2020, 282, 119561. [Google Scholar] [CrossRef]
- Ho, P.H.; de Luna, G.S.; Angelucci, S.; Canciani, A.; Jones, W.; Decarolis, D.; Ospitali, F.; Aguado, E.R.; Rodríguez-Castellón, E.; Fornasari, G.; et al. Understanding structure-activity relationships in highly active La promoted Ni catalysts for CO2 methanation. Appl. Catal. B Environ. 2020, 278, 119256. [Google Scholar] [CrossRef]
- Czuma, N.; Zarębska, K.; Motak, M.; Gálvez, M.E.; Da Costa, P. Ni/zeolite X derived from fly ash as catalysts for CO2 methanation. Fuel 2020, 267, 117139. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, M.; Wang, Q.; Huang, R.; Jin, Y.; Bian, B.; Tumurbaatar, C.; Ishtsog, B.; Bold, T.; Yang, Y. Enhanced activity and stability of Ni/La2O2CO3 catalyst for CO2 methanation by metal-carbonate interaction. Appl. Catal. B Environ. 2020, 277, 119271. [Google Scholar] [CrossRef]
- Rui, N.; Zhang, X.; Zhang, F.; Liu, Z.; Cao, X.; Xie, Z.; Zou, R.; Senanayake, S.D.; Yang, Y.; Rodriguez, J.A.; et al. Highly active Ni/CeO2 catalyst for CO2 methanation: Preparation and characterization. Appl. Catal. B Environ. 2020, 282, 119581. [Google Scholar] [CrossRef]
- Zhang, L.; Bian, L.; Zhu, Z.; Li, Z. La-promoted Ni/Mg-Al catalysts with highly enhanced low-temperature CO2 methanation performance. Int. J. Hydrogen Energy 2018, 43, 2197–2206. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Liu, C.-J. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl. Catal. B Environ. 2018, 244, 159–169. [Google Scholar] [CrossRef]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; Marcos, J.A.G.; González-Velasco, J.R. Ni catalysts with La as promoter supported over Y- and BETA- zeolites for CO2 methanation. Appl. Catal. B Environ. 2018, 238, 393–403. [Google Scholar] [CrossRef]
- Mihet, M.; Lazar, M.D. Methanation of CO2 on Ni/γ-Al2O3: Influence of Pt, Pd or Rh promotion. Catal. Today 2018, 306, 294–299. [Google Scholar] [CrossRef]
- Ashok, J.; Ang, M.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2016, 281, 304–311. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Bacariza, M.C.; Graça, I.; Ribeiro, M.F.; Lopes, J.M.; Henriques, C. Insight into CO2 methanation mechanism over NiUSY zeolites: An operando IR study. Appl. Catal. B Environ. 2015, 174–175, 120–125. [Google Scholar] [CrossRef]
- Mutz, B.; Carvalho, H.W.; Mangold, S.; Kleist, W.; Grunwaldt, J.-D. Methanation of CO2: Structural response of a Ni-based catalyst under fluctuating reaction conditions unraveled by operando spectroscopy. J. Catal. 2015, 327, 48–53. [Google Scholar] [CrossRef]
- Song, H.; Yang, J.; Zhao, J.; Chou, L. Methanation of Carbon Dioxide over a Highly Dispersed Ni/La2O3 Catalyst. Chin. J. Catal. 2010, 31, 21–23. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Pino, L.; Frontera, P.; Ferraro, M.; Antonucci, V. Activity and stability of powder and monolith-coated Ni/GDC catalysts for CO2 methanation. Appl. Catal. B: Environ. 2018, 226, 384–395. [Google Scholar] [CrossRef]
- Beierlein, D.; Häussermann, D.; Pfeifer, M.; Schwarz, T.; Stöwe, K.; Traa, Y.; Klemm, E. Is the CO2 methanation on highly loaded Ni-Al2O3 catalysts really structure-sensitive? Appl. Catal. B Environ. 2019, 247, 200–219. [Google Scholar] [CrossRef]
- Feng, K.; Tian, J.; Guo, M.; Wang, Y.; Wang, S.; Wu, Z.; Zhang, J.; He, L.; Yan, B. Experimentally unveiling the origin of tunable selectivity for CO2 hydrogenation over Ni-based catalysts. Appl. Catal. B Environ. 2021, 292, 120191. [Google Scholar] [CrossRef]
- Cárdenas-Arenas, A.; Cortés, H.S.; Bailón-García, E.; Davó-Quiñonero, A.; Lozano-Castelló, D.; Bueno-López, A. Active, selective and stable NiO-CeO2 nanoparticles for CO2 methanation. Fuel Process. Technol. 2021, 212, 106637–106644. [Google Scholar] [CrossRef]
- Lin, L.; Gerlak, C.A.; Liu, C.; Llorca, J.; Yao, S.; Rui, N.; Zhang, F.; Liu, Z.; Zhang, S.; Deng, K.; et al. Effect of Ni particle size on the production of renewable methane from CO2 over Ni/CeO2 catalyst. J. Energy Chem. 2021, 61, 602–611. [Google Scholar] [CrossRef]
- Hao, Z.; Shen, J.; Lin, S.; Han, X.; Chang, X.; Liu, J.; Li, M.; Ma, X. Decoupling the effect of Ni particle size and surface oxygen deficiencies in CO2 methanation over ceria supported Ni. Appl. Catal. B Environ. 2021, 286, 119922. [Google Scholar] [CrossRef]
- Aljishi, A.; Veilleux, G.; Lalinde, J.A.H.; Kopyscinski, J. The effect of synthesis parameters on ordered mesoporous nickel alumina catalyst for CO2 methanation. Appl. Catal. A Gen. 2018, 549, 263–272. [Google Scholar] [CrossRef]
- Tada, S.; Ikeda, S.; Shimoda, N.; Honma, T.; Takahashi, M.; Nariyuki, A.; Satokawa, S. Sponge Ni catalyst with high activity in CO2 methanation. Int. J. Hydrogen Energy 2017, 42, 30126–30134. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, B.; Liu, Y.; Zhang, Y. An active and stable nickel-based catalyst with embedment structure for CO2 methanation. Appl. Catal. B Environ. 2020, 269, 118801. [Google Scholar] [CrossRef]
- Hu, C.; Yao, J.; Yang, H.; Chen, Y.; Tian, A.M. On the Inhomogeneity of Low Nickel Loading Methanation Catalyst. J. Catal. 1997, 166, 1–7. [Google Scholar] [CrossRef]
- Wen, X.; Xu, L.; Chen, M.; Shi, Y.; Lv, C.; Cui, Y.; Wu, X.; Cheng, G.; Wu, C.-E.; Miao, Z.; et al. Exploring the influence of nickel precursors on constructing efficient Ni-based CO2 methanation catalysts assisted with in-situ technologies. Appl. Catal. B Environ. 2021, 297, 120486. [Google Scholar] [CrossRef]
- Yan, X.; Sun, W.; Fan, L.; Duchesne, P.N.; Wang, W.; Kübel, C.; Wang, D.; Kumar, S.G.H.; Li, Y.F.; Tavasoli, A.; et al. Nickel@Siloxene catalytic nanosheets for high-performance CO2 methanation. Nat. Commun. 2019, 10, 2608–2618. [Google Scholar] [CrossRef] [Green Version]
- Vrijburg, W.L.; van Helden, J.W.A.; Parastaev, A.; Groeneveld, E.; Pidko, E.A.; Hensen, E.J.M. Ceria–zirconia encapsulated Ni nanoparticles for CO2 methanation. Catal. Sci. Technol. 2019, 9, 5001–5010. [Google Scholar] [CrossRef] [Green Version]
- Swalus, C.; Jacquemin, M.; Poleunis, C.; Bertrand, P.; Ruiz, P. CO2 methanation on Rh/γ-Al2O3 catalyst at low temperature: “In situ” supply of hydrogen by Ni/activated carbon catalyst. Appl. Catal. B Environ. 2012, 125, 41–50. [Google Scholar] [CrossRef]
- Vogt, C.; Groeneveld, E.; Kamsma, G.; Nachtegaal, M.; Lu, L.; Kiely, C.; Berben, P.H.; Meirer, F.; Weckhuysen, B.M. Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat. Catal. 2018, 1, 127–134. [Google Scholar] [CrossRef]
- Iglesias, I.; Quindimil, A.; Mariño, F.; De-La-Torre, U.; González-Velasco, J.R. Zr promotion effect in CO2 methanation over ceria supported nickel catalysts. Int. J. Hydrogen Energy 2018, 44, 1710–1719. [Google Scholar] [CrossRef]
- Zhou, R.; Rui, N.; Fan, Z.; Liu, C.-J. Effect of the structure of Ni/TiO2 catalyst on CO2 methanation. Int. J. Hydrogen Energy 2016, 41, 22017–22025. [Google Scholar] [CrossRef]
- Du, Y.; Qin, C.; Xu, Y.; Xu, D.; Bai, J.; Ma, G.; Ding, M. Ni nanoparticles dispersed on oxygen vacancies-rich CeO2 nanoplates for enhanced low-temperature CO2 methanation performance. Chem. Eng. J. 2021, 418, 129402. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Zhu, M.; Han, Y.-F. Essential Role of the Support for Nickel-Based CO2 Methanation Catalysts. ACS Catal. 2020, 10, 14581–14591. [Google Scholar] [CrossRef]
- Siang, T.; Jalil, A.; Fatah, N.; Chung, M. Tailoring Rh content on dendritic fibrous silica alumina catalyst for enhanced CO2 capture in catalytic CO2 methanation. J. Environ. Chem. Eng. 2020, 9, 104616. [Google Scholar] [CrossRef]
- Italiano, C.; Llorca, J.; Pino, L.; Ferraro, M.; Antonucci, V.; Vita, A. CO and CO2 methanation over Ni catalysts supported on CeO2, Al2O3 and Y2O3 oxides. Appl. Catal. B Environ. 2019, 264, 118494. [Google Scholar] [CrossRef]
- Daroughegi, R.; Meshkani, F.; Rezaei, M. Enhanced low-temperature activity of CO2 methanation over ceria-promoted Ni-Al2O3 nanocatalyst. Chem. Eng. Sci. 2020, 230, 116194. [Google Scholar] [CrossRef]
- Garbarino, G.; Wang, C.; Cavattoni, T.; Finocchio, E.; Riani, P.; Flytzani-Stephanopoulos, M.; Busca, G. A study of Ni/La-Al2O3 catalysts: A competitive system for CO2 methanation. Appl. Catal. B Environ. 2018, 248, 286–297. [Google Scholar] [CrossRef]
- Guilera, J.; del Valle, J.; Alarcón, A.; Díaz, J.A.; Andreu, T. Metal-oxide promoted Ni/Al2O3 as CO2 methanation micro-size catalysts. J. CO2 Util. 2019, 30, 11–17. [Google Scholar] [CrossRef]
- Vrijburg, W.L.; Moioli, E.; Chen, W.; Zhang, M.; Terlingen, B.J.P.; Zijlstra, B.; Filot, I.A.W.; Züttel, A.; Pidko, E.A.; Hensen, E.J.M. Efficient Base-Metal NiMn/TiO2 Catalyst for CO2 Methanation. ACS Catal. 2019, 9, 7823–7839. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Urakawa, A. Continuous CO2 capture and reduction in one process: CO2 methanation over unpromoted and promoted Ni/ZrO2. J. CO2 Util. 2018, 25, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Atzori, L.; Cutrufello, M.G.; Meloni, D.; Monaci, R.; Cannas, C.; Gazzoli, D.; Sini, M.; Deiana, P.; Rombi, E. CO2 methanation on hard-templated NiO CeO2 mixed oxides. Int. J. Hydrogen Energy 2017, 42, 20689–20702. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Kim, T.W.; Park, E.D. CO and CO2 methanation over supported Ni catalysts. Catal. Today 2016, 293–294, 89–96. [Google Scholar] [CrossRef]
- Da Silva, D.C.; Letichevsky, S.; Borges, L.E.; Appel, L.G. The Ni/ZrO2 catalyst and the methanation of CO and CO2. Int. J. Hydrogen Energy 2012, 37, 8923–8928. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Mu, M.; Ding, F.; Liu, Z.; Guo, X.; Song, C. Organic acid-assisted preparation of highly dispersed Co/ZrO2 catalysts with superior activity for CO2 methanation. Appl. Catal. B Environ. 2019, 254, 531–540. [Google Scholar] [CrossRef]
- Halder, A.; Lenardi, C.; Timoshenko, J.; Mravak, A.; Yang, B.; Kolipaka, L.K.; Piazzoni, C.; Seifert, S.; Bonačić-Koutecký, V.; Frenkel, A.I.; et al. CO2 Methanation on Cu-Cluster Decorated Zirconia Supports with Different Morphology: A Combined Experimental In Situ GIXANES/GISAXS, Ex Situ XPS and Theoretical DFT Study. ACS Catal. 2021, 11, 6210–6224. [Google Scholar] [CrossRef]
- Mikhail, M.; Da Costa, P.; Amouroux, J.; Cavadias, S.; Tatoulian, M.; Gálvez, M.E.; Ognier, S. Tailoring physicochemical and electrical properties of Ni/CeZrOx doped catalysts for high efficiency of plasma catalytic CO2 methanation. Appl. Catal. B Environ. 2021, 294, 120233. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Bebiano, S.S.; Lopes, J.M.; Henriques, C. Magnesium as Promoter of CO2 Methanation on Ni-Based USY Zeolites. Energy Fuels 2017, 31, 9776–9789. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Wang, Y.; Li, Z.; Song, Y.; Zhang, J.; Wang, J.; He, X.; Wang, C.; Lin, W. Highly Dispersed Ni Catalyst on Metal–Organic Framework-Derived Porous Hydrous Zirconia for CO2 Methanation. ACS Appl. Mater. Interfaces 2020, 12, 17436–17442. [Google Scholar] [CrossRef]
- Wang, X.; Yang, M.; Zhu, X.; Zhu, L.; Wang, S. Experimental study and life cycle assessment of CO2 methanation over biochar supported catalysts. Appl. Energy 2020, 280, 115919. [Google Scholar] [CrossRef]
- Liang, C.; Hu, X.; Wei, T.; Jia, P.; Zhang, Z.; Dong, D.; Zhang, S.; Liu, Q.; Hu, G. Methanation of CO2 over Ni/Al2O3 modified with alkaline earth metals: Impacts of oxygen vacancies on catalytic activity. Int. J. Hydrogen Energy 2019, 44, 8197–8213. [Google Scholar] [CrossRef]
- Xu, L.; Yang, H.; Chen, M.; Wang, F.; Nie, D.; Qi, L.; Lian, X.; Chen, H.; Wu, M. CO2 methanation over Ca doped ordered mesoporous Ni-Al composite oxide catalysts: The promoting effect of basic modifier. J. CO2 Util. 2017, 21, 200–210. [Google Scholar] [CrossRef]
- Wang, W.; Hu, C.; Chen, Y. Methanation of carbon dioxide over a low nickel catalyst. Chem. Res. Appl. 1990, 2, 100–103. [Google Scholar]
- Li, S.; Liu, G.; Zhang, S.; An, K.; Ma, Z.; Wang, L.; Liu, Y. Cerium-modified Ni-La2O3/ZrO2 for CO2 methanation. J. Energy Chem. 2020, 43, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Alarcón, A.; Guilera, J.; Soto, R.; Andreu, T. Higher tolerance to sulfur poisoning in CO2 methanation by the presence of CeO2. Appl. Catal. B Environ. 2019, 263, 118346. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Baran, R.; Dębek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Da Costa, P.; Glatzel, P. Examination of the influence of La promotion on Ni state in hydrotalcite-derived catalysts under CO2 methanation reaction conditions: Operando X-ray absorption and emission spectroscopy investigation. Appl. Catal. B Environ. 2018, 232, 409–419. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Chen, M.; Nie, D.; Lian, X.; Lu, Z.; Chen, H.; Zhang, K.; Ge, P. CO2 methanation over rare earth doped Ni based mesoporous catalysts with intensified low-temperature activity. Int. J. Hydrogen Energy 2017, 42, 15523–15539. [Google Scholar] [CrossRef]
- Serrer, M.-A.; Gaur, A.; Jelic, J.; Weber, S.; Fritsch, C.; Clark, A.H.; Saraçi, E.; Studt, F.; Grunwaldt, J.-D. Structural dynamics in Ni–Fe catalysts during CO2 methanation—role of iron oxide clusters. Catal. Sci. Technol. 2020, 10, 7542–7554. [Google Scholar] [CrossRef]
- Xu, L.; Lian, X.; Chen, M.; Cui, Y.; Wang, F.; Li, W.; Huang, B. CO2 methanation over Co Ni bimetal-doped ordered mesoporous Al2O3 catalysts with enhanced low-temperature activities. Int. J. Hydrogen Energy 2018, 43, 17172–17184. [Google Scholar] [CrossRef]
- Yan, Y.; Dai, Y.; He, H.; Yu, Y.; Yang, Y. A novel W-doped Ni-Mg mixed oxide catalyst for CO2 methanation. Appl. Catal. B Environ. 2016, 196, 108–116. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Papageridis, K.; Delimitis, A.; Pachatouridou, E.; Iliopoulou, E.F. Nickel on alumina catalysts for the production of hydrogen rich mixtures via the biogas dry reforming reaction: Influence of the synthesis method. Int. J. Hydrogen Energy 2015, 40, 9183–9200. [Google Scholar] [CrossRef]
- Liu, H.; Zou, X.; Wang, X.; Lu, X.; Ding, W. Effect of CeO2 addition on Ni/Al2O3 catalysts for methanation of carbon dioxide with hydrogen. J. Nat. Gas Chem. 2012, 21, 703–707. [Google Scholar] [CrossRef]
- Zhi, G.; Guo, X.; Wang, Y.; Jin, G.; Guo, X. Effect of La2O3 modification on the catalytic performance of Ni/SiC for methanation of carbon dioxide. Catal. Commun. 2011, 16, 56–59. [Google Scholar] [CrossRef]
- Graça, I.; González, L.V.; Bacariza, M.C.; Fernandes, A.; Henriques, C.; Lopes, J.M.; Ribeiro, M.F. CO2 hydrogenation into CH4 on NiHNaUSY zeolites. Appl. Catal. B Environ. 2014, 147, 101–110. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Bartlett, S.A.; Trunschke, A.; Sun, H.; Scott, J.; Lee, A.F.; Wilson, K.; Maschmeyer, T.; Schlögl, R.; et al. Inducing synergy in bimetallic RhNi catalysts for CO2 methanation by galvanic replacement. Appl. Catal. B Environ. 2020, 277, 119029. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zhao, G.; Yang, H.; Yuan, M.; An, X.; Zhou, H.; Qiao, Y.; Tian, Y. CO2 methanation over ordered mesoporous NiRu-doped CaO-Al2O3 nanocomposites with enhanced catalytic performance. Int. J. Hydrog. Energy 2018, 43, 239–250. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, W.; Li, Z. Highly efficient Ni/ZrO2 catalysts prepared via combustion method for CO2 methanation. J. CO2 Util. 2016, 16, 236–244. [Google Scholar] [CrossRef]
- Bukhari, S.N.; Chong, C.C.; Teh, L.P.; Vo, D.-V.N.; Ainirazali, N.; Triwahyono, S.; Jalil, A.A.; Setiabudi, H.D. Promising hydrothermal technique for efficient CO2 methanation over Ni/SBA-15. Int. J. Hydrog. Energy 2018, 44, 20792–20804. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Wang, S.; Wang, S. In situ FTIR spectroscopic study of the CO2 methanation mechanism on Ni/Ce0.5Zr0.5O2. Catal. Sci. Technol. 2014, 4, 502–509. [Google Scholar] [CrossRef]
- Xu, X.; Tong, Y.; Huang, J.; Zhu, J.; Fang, X.; Xu, J.; Wang, X. Insights into CO2 methanation mechanism on cubic ZrO2 supported Ni catalyst via a combination of experiments and DFT calculations. Fuel 2020, 283, 118867. [Google Scholar] [CrossRef]
- Miao, B.; Ma, S.S.K.; Wang, X.; Su, H.; Chan, S.H. Catalysis mechanisms of CO2 and CO methanation. Catal. Sci. Technol. 2016, 6, 4048–4058. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Wang, X.; Fang, X.; Wang, H.; Xu, X. New insights into CO2 methanation mechanisms on Ni/MgO catalysts by DFT calculations: Elucidating Ni and MgO roles and support effects. J. CO2 Util. 2019, 33, 55–63. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, J.; Kang, J.K.; Park, E.D. CO and CO2 methanation over M (M Mn, Ce, Zr, Mg, K, Zn, or V)-promoted Ni/Al@Al2O3 catalysts. Catal. Today 2019, 348, 80–88. [Google Scholar] [CrossRef]
- Hasan, M.; Asakoshi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. CO2 methanation mechanism over Ni/Y2O3: An in situ diffuse reflectance infrared Fourier transform spectroscopic study. Phys. Chem. Chem. Phys. 2021, 23, 5551–5558. [Google Scholar] [CrossRef]
- Solis-Garcia, A.; Hernandez, J.F.; Almendarez-Camarillo, A.; Fierro-Gonzalez, J. Participation of surface bicarbonate, formate and methoxy species in the carbon dioxide methanation catalyzed by ZrO2-supported Ni. Appl. Catal. B Environ. 2017, 218, 611–620. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Sun, T.; Wang, S.; Wang, S. Insight into the reaction route of CO2 methanation: Promotion effect of medium basic sites. Catal. Commun. 2013, 45, 74–78. [Google Scholar] [CrossRef]
- Younas, M.; Kong, L.L.; Bashir, M.J.K.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent Advancements, Fundamental Challenges, and Opportunities in Catalytic Methanation of CO2. Energy Fuels 2016, 30, 8815–8831. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Liang, B.; Duan, H.; Hou, B.; Huang, Y. Catalytic carbon dioxide hydrogenation to methane: A review of recent studies. J. Energy Chem. 2016, 25, 553–565. [Google Scholar] [CrossRef]
- Jacquemin, M.; Beuls, A.; Ruiz, P. Catalytic production of methane from CO2 and H2 at low temperature: Insight on the reaction mechanism. Catal. Today 2010, 157, 462–466. [Google Scholar] [CrossRef]
- Cerdá-Moreno, C.; Chica, A.; Keller, S.; Rautenberg, C.; Bentrup, U. Ni-sepiolite and Ni-todorokite as efficient CO2 methanation catalysts: Mechanistic insight by operando DRIFTS. Appl. Catal. B Environ. 2019, 264, 118546. [Google Scholar] [CrossRef]


| Catalysts | Metal Loading (%) | Synthesis Method | Reaction Conditions | CO2 Con. (%) | CH4 Yie. (%) | CH4 Sel. (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | H2: CO2 | GHSV/h−1 | |||||||
| Ru-CeO2/Al2O3 | 2 | Impregnation | 300 | 5:1 | 1000 | 60 | nd | 99 | [86] |
| Ru/TiO2 | 2.5 | Impregnation | 350 | 4:1 | 6000 | 90 | nd | ~99 | [87] |
| Ru/TiO2 | 0.8 | “Dry” modification | 180 | 4:1 | nd | nd | nd | 100 | [88] |
| Ru/UiO-66 | 1 | Impregnation | 250 | 4:1 | nd | 60 | nd | 100 | [89] |
| Ru/Al2O3 | 4 | Impregnation | 375 | 5:1 | 10,000 | 85 | nd | nd | [90] |
| RhY | 6 | Ion-exchange | 150 | 3:1 | 6000 | 5.9 | nd | 99.8 | [91] |
| Rh/MSN | 5 | Impregnation | 350 | 4:1 | 5000 | 99 | nd | 100 | [42] |
| Rh/ACZ | 10 | Impregnation | 402 | 4:1 | nd | ~50 | 46.8 | nd | [92] |
| Rh/CeO2 | 3 | Impregnation | 350 | 4:1 | nd | ~46 | ~41 | ~100 | [93] |
| Pd/UiO-66 | 6 | Sol–gel | 340 | 4:1 | 15,000 | 56 | nd | 97.3 | [85] |
| Pd/Al2O3 | 5 | Impregnation | 280 | 4:1 | 45,000 | nd | nd | 40 | [94] |
| PdO@LaCoO3 | 3 | One-pot | 300 | 3:1 | 18,000 | 62.3 | nd | >99 | [95] |
| PdO/LaCoO3 | 3 | Impregnation | 300 | 3:1 | 18,000 | 31.8 | nd | 87.4 | [95] |
| Pd–Mg/SiO2 | 6.2 | Microemulsion | 450 | 4:1 | 7320 | 59.2 | 56.4 | 95.3 | [96] |
| Catalysts | Synthesis Method | Reaction Conditions | CO2 Con. (%) | CH4 Yie. (%) | CH4 Sel. (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | H2: CO2 | GHSV/h−1 | ||||||
| Ni/Pr2O3-CeO2 | Impregnation | 350 | 4:1 | 25,000 | 54.5 | 54.5 | 100 | [107] |
| Ni-CeO2/γ-Al2O3 | Impregnation | 300 | 4:1 | 36,000 | 79 | nd | 100 | [26] |
| Ni/NaY | Impregnation | 500 | 4:1 | nd | 67 | nd | 94 | [108] |
| Ca-NiTiO3/γ-Al2O3 | Precipitation | 350 | 4:1 | 2000 | 84.73 | 78.84 | 99.95 | [109] |
| Ni/CaZrO2 | Impregnation | 350 | 4:1 | 24,000 | ~75 | nd | 99 | [110] |
| Ni-5Mg/SBA-15 | Ammonia evaporation (AE) | 400 | 4:1 | 30,000 | 75 | nd | 100 | [111] |
| Ni/γ-Al2O3 | Impregnation | 500 | 4:1 | 6000 | 77.2 | nd | 99.9 | [112] |
| Ni/F-SBA-15 | Impregnation | 450 | 4:1 | 24,900 | 99.7 | 98.2 | nd | [113] |
| Ni/CeO2 | Sol–gel | 250 | 4:1 | 10,000 | 80.5 | nd | 95.8 | [114] |
| Ni-Nb2O5 | Impregnation | 350 | 4:1 | 20,600 | 92 | nd | 99 | [115] |
| Ni/Al2O3-SiO2 | Sol–gel | 350 | 3.5:1 | 12,000 | 82.38 | nd | 98.19 | [116] |
| Ni/bentonite | Solution combustion | 300 | 4:1 | 3600 | 85 | nd | 100 | [117] |
| Ni-Mn/Bn | Impregnation | 270 | 4:1 | 3600 | 85.2 | nd | 99.8 | [118] |
| Ni/Al2O3-ZrO2 | Sol-gel | 300 | 4:1 | 6000 | 77 | nd | ~100 | [119] |
| Ru-Ni/Ce0.9Zr0.1O2 | One-pot hydrolysis | 300 | 4:1 | 2400 | 98.2 | nd | 100 | [120] |
| Ni-CeO2/MCM-41 | Deposition precipitation | 380 | 4:1 | 9000 | 85.6 | nd | 99.8 | [121] |
| Y2O3-Ni/MgO-MCM-41 | Direct synthesis | 400 | 4:1 | 9000 | 65.55 | nd | 84.44 | [122] |
| Ni-Ce/CNT | Ultrasonic-assisted co-impregnation | 350 | 4:1 | 30,000 | 83.8 | nd | ~100 | [123] |
| Ni/MSN | Impregnation | 350 | 4:1 | 50,000 | 85.4 | nd | 99.9 | [42] |
| Catalysts | Synthesis Method | Reaction Conditions | CO2 Con. (%) | CH4 Yie. (%) | CH4 Sel. (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | H2:CO2:Ar (N2, He) | GHSV/h−1 | ||||||
| Ni/Y2O3 | Impregnation | 300 | 4:1:5 | 20,000 | 77 | 80 | 99.5 | [124] |
| CA-Ni/Y2O3 | Impregnation | 350 | 4:1:5 | 6000 | 92 | ~90 | 100 | [125] |
| Ca/Ni/Al2O3 | Impregnation | 275 | 4:1:5 | 160,000 | 93 | nd | 99 | [126] |
| Ni/mpCeO2 | Precipitation | 350 | 16:4:5 | 60,000 | 81 | nd | 99 | [127] |
| Ni-RuAl | Glycerol Assisted Impregnation (GAI) | 400 | 16:4:5 | 30,000 | 60 | nd | 99.5 | [128] |
| NiCeY | Precipitation | 450 | 12:3:5 | 30,000 | nd | 80 | 95 | [129] |
| NiLaAl-HT | Precipitation | 450 | 4:1:1 | 480,000 | 88 | nd | 98 | [130] |
| Ni/zeolite X | Fusion method | 450 | 12:3:5 | 12,000 | 53 | nd | >90 | [131] |
| Ni/La2O2CO3 | Impregnation | 450 | 12:3:5 | 20,000 | 91 | nd | 99.9 | [132] |
| Ni/CeO2 | Gas discharge plasma | 275 | 16:4:5 | 56,000 | 84.2 | nd | 99.5 | [133] |
| Ni/ZrO2 | Impregnation | 400 | 4:1:5 | 43,500 | 50 | nd | 100 | [76] |
| Ni-La/Mg-Al | Urea hydrolysis | 200 | 36:9:5 | 45,000 | 61 | nd | ~100 | [134] |
| Ni/ZrO2 | Plasma decomposition | 350 | 16:4:5 | 60,000 | 79.1 | 76.5 | 100 | [135] |
| Ni-La2O3/Na-BETA | Impregnation | 350 | 4:1:1.25 | 10,000 | 65 | nd | 99 | [136] |
| Ni-Pd/γ-Al2O3 | Impregnation | 300 | 4:1:8.5 | 5700 | 90.5 | nd | 98.7 | [137] |
| NiCo/Al2O3 | Evaporation-induced self-assembly | 400 | 12:3:5 | 10,000 | 78 | nd | 99 | [80] |
| Ni/CeO2-ZrO2 | Ammonia evaporation | 275 | 8:2:15 | 20,000 | 55 | nd | 99.8 | [138] |
| Ni/USY | Impregnation | 450 | 4:1:15 | nd | 72.6 | nd | 95 | [139] |
| Ni/CaO–Al2O3 | nd | 400 | 12:3:10 | 15,000 | 81 | 80 | nd | [140] |
| Ni/La2O3 | Impregnation | 320 | 4:1:1 | 3250 | 97.1 | nd | 100 | [141] |
| Catalysts | Ni Loading (%) | Reaction Temp. (°C) | CO2 Con. (%) | CH4 Yie. (%) | CH4 Sel. (%) | Ref. |
|---|---|---|---|---|---|---|
| Ni/CeZrO4 | 2 | 350 | 63 | nd | 100 | [154] |
| Ni/CaZrO2 | 5 | 350 | ~75 | nd | 99 | [110] |
| Ni/NaY | 5 | 500 | 67 | nd | 94 | [108] |
| Ni/Ce0.85Zr0.15O2 | 5 | 500 | 70 | nd | ~100 | [157] |
| Ni/MSN | 5 | 350 | 85.4 | nd | 99.9 | [42] |
| Ni/F-SBA-15 | 5 | 450 | 99.7 | 98.2 | nd | [113] |
| Ni/TiO2 | 6.17 | 350 | 73.2 | nd | nd | [158] |
| Ni/MgO- MgH2 | 7.9 | 300 | 85.2 | nd | 99.5 | [44] |
| Ni/CeO2 | 10 | 275 | 84.2 | nd | 99.5 | [133] |
| Ni/SiO2 | 10 | 310 | 77.2 | nd | ~100 | [101] |
| Ni/Y2O3 | 10 | 300 | 77 | 80 | 99.5 | [124] |
| 12CA-Ni/Y2O3 | 10 | 350 | 92 | ~90 | 100 | [125] |
| Ni/CeO2 | 10 | 300 | 84 | nd | 100 | [159] |
| Ni/mpCeO2 | 10 | 350 | 81 | nd | 99 | [127] |
| Ni-10La2O3/Na-BETA | 10 | 350 | 65 | nd | 99 | [136] |
| Ni-Pd/γ-Al2O3 | 10 | 300 | 90.5 | nd | 98.7 | [137] |
| Ni3Co/Al2O3 | 10 | 400 | 78 | nd | 99 | [80] |
| Ni/CeO2-ZrO2 | 10 | 275 | 55 | nd | 99.8 | [138] |
| Ni/CeO2 | 10 | 340 | 91.1 | nd | 100 | [103] |
| Ni-5Mg/SBA-15 | 10 | 400 | 75 | nd | 100 | [111] |
| Ni/La2O3 | 10 | 320 | 97.1 | nd | 100 | [141] |
| Catalysts | Synthesis Method | Reaction Temp. (°C) | CO2 Con. (%) | CH4 Sel. (%) | Ref. |
|---|---|---|---|---|---|
| Ni-La2O3/Na-BETA | Impregnation | 350 | 65 | 99 | [136] |
| Ni/Pr2O3-CeO2 | Impregnation | 350 | 54.5 | 100 | [107] |
| Ni/Y2O3 | Impregnation | 300 | 77 | 99.5 | [124] |
| Ni/CeO2 | Impregnation | 300 | 84 | 100 | [159] |
| Ni/MSN | Impregnation | 350 | 85.4 | 99.9 | [42] |
| Ni-CeO2/Al2O3 | Impregnation | 350 | 85 | nd | [188] |
| Ni–La/SiC | Impregnation | 250 | 39.6 | 99.6 | [189] |
| NiO-CeO2/SBA-15 | Impregnation | 300 | 76 | 93 | [168] |
| Ni/ZrO2 | Impregnation | 400 | 50 | 100 | [76] |
| Ni/CeZrO4 | Impregnation | 350 | 63 | 100 | [154] |
| Ni-Mn/Bn | Impregnation | 270 | 85.2 | 99.8 | [118] |
| Ni/TiO2 | Impregnation | 350 | 73.2 | nd | [158] |
| Ni-Pd/γ-Al2O3 | Impregnation | 300 | 90.5 | 98.7 | [137] |
| NiCeUSY | Impregnation | 400 | 68.3 | 95.1 | [190] |
| Ni/La2O3 | Impregnation | 320 | 97.1 | 100 | [141] |
| Ni-CeO2/γ-Al2O3 | Impregnation | 300 | 79 | 100 | [26] |
| Ni/NaY | Impregnation | 500 | 67 | 94 | [108] |
| Ni/CaZrO2 | Impregnation | 350 | ~75 | 99 | [110] |
| Ni/γ-Al2O3 | Impregnation | 500 | 77.2 | 99.9 | [112] |
| Ni/F-SBA-15 | Impregnation | 450 | 99.7 | nd | [113] |
| Ni/Ce-ABC | Impregnation | 360 | 88.6 | 92.3 | [176] |
| Ni-La2O3/γ-Al2O3 | Impregnation | 300 | 97 | >99 | [165] |
| Ni/USY | Impregnation | 450 | 72.6 | 95 | [139] |
| NiCe/CNT | Ultrasonic-assisted co-impregnation | 350 | 83.8 | ~100 | [123] |
| Ni-RuAl | Glycerol Assisted Impregnation (GAI) | 400 | 60 | 99.5 | [128] |
| Ni/mpCeO2 | Precipitation | 350 | 81 | 99 | [127] |
| NiCeY | Precipitation | 450 | nd | 95 | [129] |
| NiLaAl-HT | Precipitation | 450 | 88 | 98 | [130] |
| Ca-NiTiO3/γ-Al2O3 | Precipitation | 350 | 84.73 | 99.95 | [109] |
| Ni-Ce-Al2O3 | Precipitation | 350 | 73.2 | 99.1 | [163] |
| Ni/Ce0.85Zr0.15O2 | Precipitation | 500 | 70 | ~100 | [157] |
| Ni-CeO2/MCM-41 | Deposition precipitation | 380 | 85.6 | 99.8 | [121] |
| Ni/CeO2 | Sol-gel | 250 | 80.5 | 95.8 | [114] |
| Ni/Al2O3-SiO2 | Sol-gel | 350 | 82.38 | 98.19 | [116] |
| Ni/SiO2 | Sol-gel | 310 | 77.2 | ~100 | [101] |
| Ru-Ni/Ce0.9Zr0.1O2 | One-pot hydrolysis | 300 | 98.2 | 100 | [120] |
| Ni-La/Mg-Al | Urea hydrolysis | 200 | 61 | ~100 | [134] |
| Ni/bentonite | Solution combustion | 300 | 85 | 100 | [117] |
| Ni-Mg/SBA-15 | Ammonia evaporation (AE) | 400 | 75 | 100 | [111] |
| Ni/CeO2-ZrO2 | Ammonia evaporation (AE) | 275 | 55 | 99.8 | [138] |
| Y2O3-Ni/MgO-MCM-41 | Direct synthesis | 400 | 65.55 | 84.44 | [122] |
| Ni/zeolite X | Fusion method | 450 | 53 | >90 | [131] |
| RhNi/Al2O3 | Galvanic replacement (GR) | 250 | 97 | >90 | [191] |
| Ni/CeO2 | Gas discharge plasma | 275 | 84.2 | 99.5 | [133] |
| Ni/MgO- MgH2 | Mechanochemical ball-milling method | 300 | 85.2 | 99.5 | [44] |
| Ni/ZrO2 | Plasma decomposition | 350 | 79.1 | 100 | [135] |
| NiCo/Al2O3 | Evaporation-induced self-assembly | 400 | 78 | 99 | [80] |
| NiRu/CaO-Al2O3 | Facile evaporation-induced self-assembly method | 380 | 83.8 | 100 | [192] |
| Ni/CeO2 | Hard template method | 340 | 91.1 | 100 | [103] |
| Ni/ZrO2 | Combustion method | 300 | 60 | ~97.5 | [193] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zeng, W.; Song, M.; Wu, X.; Li, G.; Hu, C. Research Progress and Reaction Mechanism of CO2 Methanation over Ni-Based Catalysts at Low Temperature: A Review. Catalysts 2022, 12, 244. https://doi.org/10.3390/catal12020244
Li L, Zeng W, Song M, Wu X, Li G, Hu C. Research Progress and Reaction Mechanism of CO2 Methanation over Ni-Based Catalysts at Low Temperature: A Review. Catalysts. 2022; 12(2):244. https://doi.org/10.3390/catal12020244
Chicago/Turabian StyleLi, Li, Wenqing Zeng, Mouxiao Song, Xueshuang Wu, Guiying Li, and Changwei Hu. 2022. "Research Progress and Reaction Mechanism of CO2 Methanation over Ni-Based Catalysts at Low Temperature: A Review" Catalysts 12, no. 2: 244. https://doi.org/10.3390/catal12020244
APA StyleLi, L., Zeng, W., Song, M., Wu, X., Li, G., & Hu, C. (2022). Research Progress and Reaction Mechanism of CO2 Methanation over Ni-Based Catalysts at Low Temperature: A Review. Catalysts, 12(2), 244. https://doi.org/10.3390/catal12020244






